Abstract
Cyclin D1 is frequently overexpressed in esophageal squamous cell carcinoma (ESCC) and is considered a key driver of this disease. Mutations in FBXO4, F-box specificity factor that directs SCF-mediated ubiquitylation of cyclin D1, occur in ESCC with concurrent overexpression of cyclin D1 suggesting a potential tumor suppressor role for FBXO4. To evaluate the contribution of FBXO4-dependent regulation cyclin D1 in esophageal squamous cell homeostasis, we exposed FBXO4 knockout mice to N-nitrosomethylbenzylamine (NMBA), an esophageal carcinogen. Our results revealed that loss of FBXO4 function facilitates NMBA induced papillomas in FBXO4 het (+/−) and null (−/−) mice both by numbers and sizes 11 months after single dose NMBA treatment at 2mg/kg by gavage when compared to that in wt (+/+) mice (P < 0.01). No significant difference was noted between heterozygous or nullizygous mice consistent with previous work. To assess cyclin D1/CDK4 dependence, mice were treated with the CDK4/6 specific inhibitor, PD0332991, for 4 weeks. PD0332991 treatment (150mg/kg daily), reduced tumor size and tumor number. Collectively, our data support a role for FBXO4 as a suppressor of esophageal tumorigenesis.
Keywords: cyclin D1, CDK4, ESCC, FBXO4, PD0332991
Abbreviations
- BrdU
Bromodeoxyuridine
- CDK
Cyclin Dependent Kinase
- DMSO
Dimethyl Sulfoxide
- EGFR
Epidermal Growth Factor Receptor
- ESCC
Esophageal Squamous Cell Carcinoma
- FBXO4
F box only protein 4
- GI
Gastrointestinal tract
- H&E
Hematoxylin and Eosin
- Het
Heterozygous
- NMBA
N-nitrosomethylbenzylamine
- PE
Preneoplastic Esophagus
- PI
Propidium Iodide
- PBS
Phosphate Buffered Saline
- Rb
Retinoblastoma Protein
- SCF
Skp1-Cul1-F box protein
- SCC
Squamous Cell Carcinoma
- TNFa
Tumor Necrosis Factor alpha
- Wt
Wild Type
Introduction
Cyclin D1 functions as a rate limiting allosteric regulator of cyclin dependent kinase 4. The cyclin D1/CDK4 kinase initiates the inactivation of the retinoblastoma protein through direct phosphorylation in response to growth factor stimulation.1 The growth factor dependence of cyclin D1/CDK4 activity uniquely positions this kinase as an integrator of growth factor signaling and cell division. Not surprisingly, cyclin D1/CDK4 activity is frequently dysregulated in human cancer. Overexpression of nuclear cyclin D1 is a transforming event that contributes directly to neoplastic manifestation.1 The oncogenic functions of cyclin D1 emphasize the importance of the regulation of cyclin D1 accumulation in normal cells.
While cyclin D1 overexpression can result from gene amplification, its overexpression and consequent nuclear accumulation is perhaps more frequently associated with decreased protein degradation. Cyclin D1 protein degradation is regulated by the SCF-FBXO4/αB-crystallin E3 ligase.2 FBXO4 mutations have been identified in human cancers including carcinomas of the esophagus and breast as well as metastatic melanoma3,4 and such mutations result in dysregulation of cyclin D1 and cyclin D1/CDK4 activity. Likewise targeted knockout of FBXO4 in mice results in spontaneous tumors in multiple tissues including haematopoietic and mammary epithelium that are associated with cyclin D1 overexpression.5,6 Additional work has revealed that FBXO4 is a haplo-insufficient tumor suppressor such that loss of a single FBXO4 allele results in tumorigenesis with no selection for loss of heterozygocity.
N-nitrosomethylbenzylamine (NMBA) is a carcinogen of n-nitrosamine compounds that induces squamous cancer in rat esophagus and fore stomach proliferation/dysplasia in mice.7-9 Importantly, NMBA contamination is thought to be one of the factors in human esophageal squamous carcinoma. NMBA is not carcinogenic per se. It requires in vivo transformation and production of intermediate reactive products to form stable adducts such as alkylated purine and pyrimidines. Human and rat esophagi can fully convert NMBA into metabolites that alkylate DNA at the N7 and O6 position of guanine. The persistence and accumulation of these adducts lead to DNA mutations and carciogenesis in rats.10-14 Ethanol can enhance the mutagenic activation of NMBA by cytochrome P450 2A in rats.15 Differential expression of 4807 genes in preneoplastic esophagus (PE) and 17 846 genes in esophageal papillomas in rat was reported by Wang et al.16
Given the occurrence of inactivating mutations in human esophageal squamous carcinoma (ESCC), we wished to directly assess whether FBXO4 loss would contribute to upper GI malignancy in a model system. In this paper, we report that the loss of FBXO4 facilitates NMBA induced carcinogenesis; administration of PD0332991, a highly specific small molecule inhibitor of the CDK4/6 kinase17 reduced tumor burden consistent with cyclin D1 as a key downstream target of FBXO4.
Results
FBXO4 loss sensitizes mice to NMBA induced papilloma formation
NMBA treatment can trigger dysplastic growth in epithelium of the upper GI of rats and mice.7-9 NMBA treatment of cyclin D1 transgenic mice triggered increased epithelial proliferation and more severe dysplasia relative to non-transgenic controls consitent with the oncogenic potential of cyclin D1.8 To ascertain the affect of FBXO4-dependent tumor suppression in the upper gastrointestinal tract (GI), we exposed wild type, FBXO4 heterozygous (+/−) and nullizygous (−/−) mice to a single oral dose of NMBA, 2mg/kg body weight or vehicle control. Mice were then monitored for up to 11 months for overt signs of malignancy. Mice were sacrificed at 8 and 11 months and upper gastrointestinal tissue including both the esophagus and fore stomach were assessed for neoplastic manifestations.
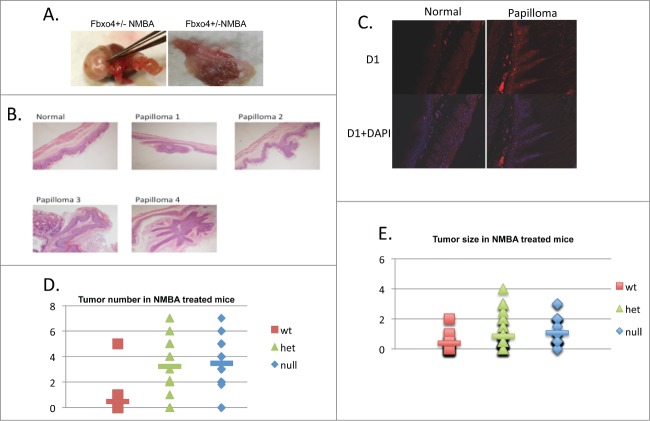
In total, 21 FBXO4 wild type (wt), 32 FBXO4 hetrozygote (het) and 22 FBXO4 homozygote (null) mice were treated with single dose NMBA. Eleven months post NMBA exposure papilloma formation was observed in all 3 genotypes (Fig. 1; Table 1). While papillomas were observed in all genotypes, there were significant increases in papilloma number when comparing wt/het and wt/null groups (Fig. 1; Table 1; P < 0.01). We also noted a significant increases in papilloma size in both het and null groups with respect to the wild type group (Fig. 1; Table 1; P < 0.01). No significant difference was observed between het/null groups (P > 0.05) consistent FBXO4 haploinsufficiency. Cyclin D1 overexpression was noted in papillomas independent of genotype consistent with cyclin D1/CDK4 regulating proliferation in this tissue (Fig. 1C).
Figure 1.
The gross and pathological findings of NMBA treated mice. (A) Representative papillomas recovered from fore stomach. Enlarged lymph nodes, not pictured, were also observed. (B) H&E staining of normal fore stomach and papillomas. (C) Immunofluoresent staining of normal fore stomach or papilloma from NMBA treated FBXO4+/− mice. (D) Quantified tumor numbers from NMBA treated mice of the indicated genotypes. P < 0.01 between wt/het and wt/null groups, respectively. The P > 0.05 between het/null groups. (E) Quantified tumor size in NMBA treated groups. P < 0.01 between wt/het and wt/null groups, respectively. The P > 0.05 between het/null groups.
Table 1.
NMBA treated mice; gross finding and pathology.
| Fbxo4 genotype | Mice | Papilloma (% of mice with papilloma) | |
|---|---|---|---|
| Wt | 21 | 6 (28.6) | |
| Het | 32 | 27 (84.1) | |
| Null |
20 |
22 (90.9) |
|
|
Fbxo4 genotype |
Total # of papillomas |
Average size (mm) |
Average no./mouse |
| Wt | 10 | 0.38+/−0.59 | 0.48+/−1.1 |
| Het | 103 | 0.86+/−0.78* | 3.22+/−2.1* |
| Null | 76 | 1.08+/−0.84*@ | 3.45+/−1.8*@ |
Compared with wt P value <0.01; @compared with Het, P>0.05.
CDK4 inhibition reduces tumor number and size
FBXO4 comprises the specificity component of an SCF E3 ligase that regulates cyclin D1 accumulation. To address whether the susceptibility of FBXO4+/− and −/− reflected a dependence on cyclin D1/CDK4 catalytic function, 10 FBXO4 (+/−) mice were divided into 2 groups of 5 each 11 months post NMBA treatment. One group was treated with PD0332991 daily (150 mg/kg) for 4 weeks and the second group was provided with vehicle only. PD0332991 treatment significantly reduced tumor number (P < 0.01) and size (P < 0.05) as shown in (Fig. 2; Table 2).
Figure 2.

The gross pathological findings from PD0332991 and vehicle treated mice. (A-B) Papillomas from PD0332991 treated mice were smaller and fewer in number relative to control mice. (C-D) Quantified tumor number and size following treatment of NMBA induced mice with PD0332991 for 4 weeks (PD0332991 treatment started at 11 months post NMBA); C, (P < 0.05) and D, (P < 0.01), respectively.
Table 2.
Papilloma number in PD0332991 treated mice.
| Total no. of papilloma | Average size (mm) | Average no./mouse |
|---|---|---|
| PD0332991 | ||
| 7 | 0.44+/−0.28** | 1.75+/−2.05* |
| DMSO | ||
| 24 | 1.02+/−0.84 | 4.8+/−1.32 |
P value <0.05; **P value <0.01
PD0332991 treatment is associated with reduced Rb phosphorylation and reduced proliferation in vivo
PD0332991 treatment is expected to inhibit CDK4/6 catalytic activity resulting in reduced phosphorylation of Rb at sights targeted by the cyclin D1/CDK4 kinase and consequently with reduced cell proliferation. To address this, mice were injected with BrdU 6 hours prior to euthanasia. BrdU incorporation was assessed by indirect immunofluorescent staining using a BrdU specific antibody in parallel with antiserum specific for p-Ser780-Rb phosphorylation in PD0332991 treated versus vehicle treated papillomas. In the normal fore stomach tissue, PD0332991 treatment caused no significant changes in Phospho-Rbs780 or BrdU staining when compared to DMSO control group (data not shown). In contrast, reduced BrdU incorporation and reduced p-Ser-780 was noted in hyperplastic papillomas harvested from mice treated with PD0332991 (Fig. 3A). As independent confirmation, we also stained papillomas from PD0332991 vs. vehicle treated mice with Ki67. Consistently, we noted a significant reduction in Ki67 reactive cells in PD0332991 treated mice.
Figure 3.
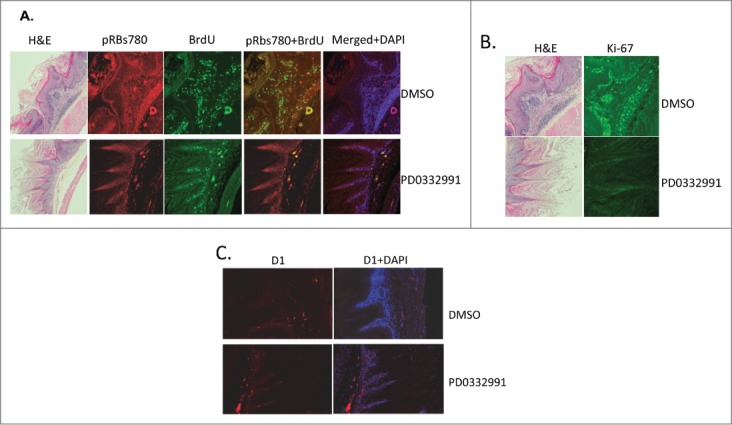
Immunufluorescence staining of fore stomach in PD0332991 versus vehicle control treated mice. (A) Phospho-Rb, ser-780 (pRBs780) and BrdU staining of fore stomach containing papilloma. (B) Total Rb and Ki-67 staining of papillomas from vehicle or PD0332991 treated mice. (C) Cyclin D1 staining of papillomas from vehicle or PD0332991 treated mice.
Human ESCC cell lines are sensitive to PD0332991
Our results suggest that the upper gastrointestinal proliferative disease wherein cyclin D1/CDK4 function as key driver molecules, such as esophageal carcinomas,18 might be responsive to PD0332991. We therefore evaluated the response of 3 independently derived esophageal cancer cell lines, TE7, TE8, TE10 for their response to PD0332991 treatment. These three lines were chosen based upon the status of cyclin D1 regulation with TE7 harboring a nuclear, stabilized cyclin D1 allele, P287A; TE10 is hemizygous at the FBXO4 locus, FBXO4-S8R, which results in increase nuclear cyclin D1/CDK4 activity; TE8 exhibits high levels of Akt activity.3 We initially evaluated the ability of PD0332991 to inhibit CDK4-dependent Rb phosphorylation. Cells were treated with vehicle control or PD0332991 for 4 days following which cells were washed and refed with complete media lacking PD0332991. Treatment efficiently reduce phosphorylation of Ser-780 (Fig. 4A). Two days post washout, cells had restored Rb phosphorylation suggesting cell cycle reentry. To confirm this interpretation, we assessed the cell cycle status of cells following PD0332991 for 4 days followed by a period of washout for 2 or 4 days. PD0332991 effectively triggered an accumulation of cells with a 2N DNA content at 4 days post treatment consistent with G1 cell cycle arrest (Fig. 4B). By two and 4 days post-treatment, cells were exhibited increased DNA content consistent with S-phase entry. These data demonstrate that PD0332991 can trigger G1 arrest or quiescence in esophageal tumor cells harboring mutations that directly increase cyclin D1/CDK4 activity and in tumor derived cells harboring wild type cyclin D1-signaling.
Figure 4.
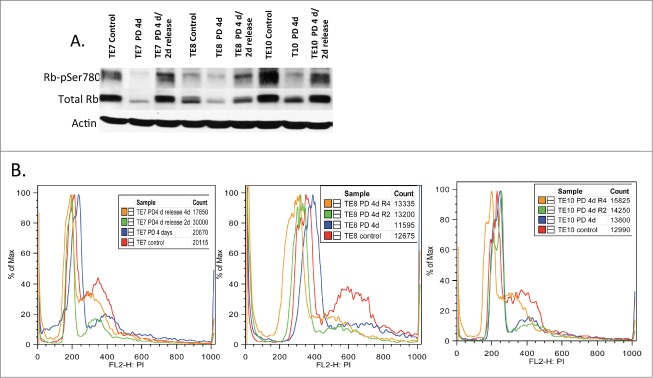
The biological effects of PD0332991 on human esophageal SCC derived cell lines. (A) Cell lysates were prepared from the indicated ESCC cell lines that were treated with vehicle (control) or PD0332991 for 4 days followed by release into complete medium lacking drug for 2 days. Lysates were probed with antibodies specific for total Rb, pRBs780 and actin. (B) Flow cytometry analysis of cells treated with vehicle or PD0332991 for 4 days followed by release into complete medium lacking PD0332991 for 2 or 4 d.
Discussion
Primarily squamous cell cancer accounts for approximately 90–95% of all esophageal cancer worldwide, arising from the cells that line the upper part of the esophagus.19-21 The incidence of esophageal carcinoma is approximately 3–6 cases per 100,000 persons. Small and localized tumors are treated surgically with curative intent. Larger tumors tend not to be operable and hence are treated with palliative care, but with poor prognosis. New therapeutic interventions and a mouse model mimic of human esophageal SCC are much needed for this malignancy to improve the current situation. Mutation and/or overexpression of key oncogenes and tumor suppressors such as H-ras,22 p53,23 c-myc,24 TNF-α/EGFR25 and cyclin D126,27 are associated with human ESCC and have been reported in rat SCC induced by NMBA.
In this study, we established a mouse model based on FBXO4 loss of function and NMBA induced papillomas in the fore stomach. Previous work demonstrated that FBXO4 loss triggers cyclin D1 overexpression and predisposes mice to spontaneous tumors in multiple tissue types as well as BRAF-driven melanoma.2,5 Importantly, tumorigenesis is dependent upon increased cyclin D1 dosage, triggered by FBXO4 loss.2,4 Because FBXO4+/− and -/− mice do not develop upper GI tumors at a measurable frequency, we utilized NMBA exposure as a relevant carcinogenic challenge. NMBA is a potent carcinogen, which induces esophageal SCC in rat and fore stomach proliferation and dysplasia in mouse. NMBA contamination in food was reported and listed as one of the risk factors in human esophageal SCC. Both FBXO4 heterozygous and nullizygous mice exhibited increased papilloma burden in the fore stomach relative to wild type mice exposed to the same carcinogenic regime. During the time course of observation, we did not find evidence of progression to overt carcinoma suggesting the need for the acquisition of additional genetic events for its manifestation.
PD0332991 is a specific inhibitor of CDK4/6 kinases, which phosphorylates Rb to promote cell cycle progression. PD0332991 has low toxicity and high tolerance in humans as well as in animals and is in phase II/III clinical trials. Because loss of FBXO4 function results in cyclin D1 dysregulation, we used this model system to test the utility of PD0332991 as a potential therapeutic entity and to assess the contribution of cyclin D1/CDK4 in disease progression. Four weeks of PD0332991 treatment significantly reduced tumor number and size. PD0332991 treatment was associated with reduced Rb phosphorylation and decreased mitogenic indices consistent with on target effects.
To determine whether CDK4 inhibition might be of utility in the treatment of human ESCC, we tested its impact on proliferation of ESCC-derived cell lines. Consistent with ESCC exhibiting dependence upon cyclin D1-dependent CDK4 activation, PD0332991 treatment triggered G1 cell cycle arrest. There was no evidence of senescence following treatments (data not shown). Rather, arrest was reversible suggesting that treatment induced a state of quiescence. As such, this would imply extended treatment would be required to manage ESCC using CDK4/6 inhibitors. Because PD0332991 exhibits a low toxicity profile, extended treatment could be achieved and based upon these results may represent an avenue for continued evaluation as a therapeutic modality of human ESCC.
Materials and Methods
Experimental animals and genotyping all animal experiments were carried out at BRB-II/III facility of the University of Pennsylvania in accordance with IACUC protocols and University Laboratory Animal Research (ULAR) guidelines. FBXO4 mice and genotyping protocols were maintained and used as described previously.4,5 FBXO4 wild type (wt), heterozygote (het) and homozygote (null) mice of 3 months old were treated with single dose NMBA (N-Benzyl-N-methylnitrosamine (Ash Stevens Inc.., Detroit, MI, USA) at 2mg/kg body weight in 20%DMSO by gavage in experiment groups.8,28 Control mice were given 20%DMSO. Eleven months after NMBA or DMSO, fore stomach, esophagus and other tissues were collected and processed as previously described.4,5 Hematoxylin and eosin (H&E) staining was performed for pathology analyses. For treatment with PD0332991 (Palbociclib, ChemieTek, Prod. No. CT-PD2991, Indianapolis, IN, USA), 10 FBXO4 het mice 11 months after NMBA treatment were divided into 2 groups of 5 each. The treatment group was given PD0332991 at 150 mg/kg body weight in 20%DMSO by gavage daily for 28 days. The control group was given 20%DMSO only. Before harvest, each mouse was given BrdU (Sigma) at 50mg/kg body weight in PBS by IP for 6 hours.
Immunohistochemistry
Briefly, paraffin-embedded tissues went through serial xylene and ethanol process. Antigens were retrieved with Antigen Retrieval Citra Plus (HK-080-9K, BioGenex, Fremont CA, USA) by boiling for 10 min, cooling down RT for 30 min; washed in PBS, incubated with primary antibodies, washed with PBS, incubated with fluorescence labeled second antibodies, washed with PBS again. The slides were sealed with Vectashield mounting medium (H-1200, Vector Laboratories) and cover glass for microscopy (Olympus IX81).
Cell lines, western blot and cell cycle analysis
Human esophageal squamous cancer derived cell lines TE7 (cyclin D1 T287A mutation), TE10 (FBXO4 S8R mutation), TE 15 (FBXO4 and cyclin D1 wild types) and TE8 (high AKT expression, FBXO4 and cyclin D1 wild types) were maintained in Dulbecco's modified Eagle's medium (DMEM) containing glutamine, penicillin-streptomycin, and 10% fetal bovine serum (FBS) (Gemini). For PD0332991 treatment, cells were incubated with DMEM medium containing 1 μm PD 0332991 and the medium was changed every 2 days to keep PD0332991 activity. Cells were harvested, processed, SDS-paged and immunoblotted as previously described. For cell cycle analyses, cells were trypsinized, wash 2 times with PBS and fixed in 70% ethanol at −20°C till use. Then, fixed cell were washed 3 times with PBS, stained with PI 50μg/ml in PBS containing 100ug RNAse A/ml for 30 min at RT and analyzed using BD Caliber.
Other reagents
Cyclin D1 mouse monoclonal antibody D1-72-13G; cyclinD1 mouse anti-human antibody AB3 (Calbiochem); phospho-Rb (sc-12901-R,) total Rb (sc-74562); Ki-67 antibody (VP-K451; Vector Laboratories); Goat-anti-rabbit Alexa-Fluor 488 (A11008) and Goat-anti-mouse Alexa-fluor 546(A11003).
Statistical analyses
Statistical analyses utilized a 2-tailed Student t test, with P values of <0.05 indicating statistical significance. Error bars represent the means ± the standard deviations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by P01-CA098101 (JAD, AKR, AJPK, AJB, KKW). We thank NIH-P30-DK050306, the Molecular Pathology and Imaging, Molecular Biology and Cell Culture Core Facilities.
References
- 1.Diehl JA. Cycling to Cancer with Cyclin Dl. Cancer Biol Ther 2002; 1:226-231; PMID:12432268; http://dx.doi.org/ 10.4161/cbt.72 [DOI] [PubMed] [Google Scholar]
- 2.Lin DI, Barbash O, Kumar KG, Weber JD, Harper JW, Klein-Szanto AJ, Rustgi A, Fuchs SY, Diehl JA. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex. Mol Cell 2006; 24:355-366; PMID:17081987; http://dx.doi.org/ 10.1016/j.molcel.2006.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbash O, Zamfirova P, Lin DI, Chen X, Yang K, Nakagawa H, Lu F, Rustgi AK, Diehl JA. Mutations in Fbx4 inhibit dimerization of the SCF(Fbx4) ligase and contribute to cyclin D1 overexpression in human cancer. Cancer Cell 2008; 14:68-78; PMID:18598945; http://dx.doi.org/ 10.1016/j.ccr.2008.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee EK, Lian Z, D'Andrea K, Letrero R, Sheng W, Liu S, Diehl JN, Pytel D, Barbash O, Schuchter L, et al.. The FBXO4 tumor suppressor functions as a barrier to BRAFV600E-dependent metastatic melanoma. Mol Cell Biol 2013; 33:4422-4433; PMID:24019069; http://dx.doi.org/ 10.1128/MCB.00706-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaites LP, Lee EK, Lian Z, Barbash O, Roy D, Wasik M, Klein-Szanto AJ, Rustgi AK, Diehl JA. The Fbx4 tumor suppressor regulates cyclin D1 accumulation and prevents neoplastic transformation. Mol Cell Biol 2011; 31:4513-4523; PMID:21911473; http://dx.doi.org/ 10.1128/MCB.05733-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaites LP, Lian Z, Lee EK, Yin B, DeMicco A, Bassing CH, Diehl JA. ATM deficiency augments constitutively nuclear cyclin D1-driven genomic instability and lymphomagenesis. Oncogene 2014; 33:129-133; PMID:23318439; http://dx.doi.org/ 10.1038/onc.2012.577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Autrup H, Stoner GD. Metabolism of N-nitrosamines by cultured human and rat esophagus. Cancer Res 1982; 42:1307-1311; PMID:7060009 [PubMed] [Google Scholar]
- 8.Jenkins TD, Mueller A, Odze R, Shahsafaei A, Zukerberg LR, Kent R, Stoner GD, Rustgi AK. Cyclin D1 overexpression combined with N-nitrosomethylbenzylamine increases dysplasia and cellular proliferation in murine esophageal squamous epithelium. Oncogene 1999; 18:59-66; PMID:9926920; http://dx.doi.org/ 10.1038/sj.onc.1202296 [DOI] [PubMed] [Google Scholar]
- 9.Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis 2001; 22:1737-1746; PMID:11698334; http://dx.doi.org/ 10.1093/carcin/22.11.1737 [DOI] [PubMed] [Google Scholar]
- 10.Doniger J, Day RS, DiPaolo JA. Quantitative assessment of the role of O6-methylguanine in the initiation of carcinogenesis by methylating agents. Proc Natl Acad Sci U S A 1985; 82:421-425; PMID:3855560; http://dx.doi.org/ 10.1073/pnas.82.2.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong LY, Lin HJ, Lee CL. Methylation of DNA in target and non-target organs of the rat with methylbenzylnitrosamine and dimethylnitrosamine. IntJ Cancer 1979; 23:679-682; PMID:457309; http://dx.doi.org/ 10.1002/ijc.2910230514 [DOI] [PubMed] [Google Scholar]
- 12.Harris CC, Autrup H, Stoner GD, Trump BF, Hillman E, Schafer PW, Jeffrey AM. Metabolism of benzo(a)pyrene, N-nitrosodimethylamine, and N-nitrosopyrrolidine and identification of the major carcinogen-DNA adducts formed in cultured human esophagus. Cancer research 1979; 39:4401-4406; PMID:498072 [PubMed] [Google Scholar]
- 13.Siglin JC, Morse MA, Schut HA, Geil RG, Conran PB, Stoner GD. O6-methylguanine levels and histopathological changes in the rat esophagus and liver following single and repeated administration of N-nitrosomethylbenzylamine. Carcinogenesis 1996; 17:1135-1140; PMID:8640924; http://dx.doi.org/ 10.1093/carcin/17.5.1135 [DOI] [PubMed] [Google Scholar]
- 14.Van Benthem J, Vermeulen E, Winterwerp HH, Wild CP, Scherer E, Den Engelse L. Accumulation of O6- and 7-methylguanine in DNA of N-nitroso-N-methylbenzylamine-treated rats is restricted to non-target organs for N-nitroso-N-methylbenzylamine-induced carcinogenesis. Carcinogenesis 1992; 13:2101-2105; PMID:1423882; http://dx.doi.org/ 10.1093/carcin/13.11.2101 [DOI] [PubMed] [Google Scholar]
- 15.Tatematsu K, Koide A, Morimura K, Fukushima S, Mori Y. The enhancing effect of ethanol on the mutagenic activation of N-nitrosomethylbenzylamine by cytochrome P450 2A in the rat oesophagus. Mutagenesis 2013; 28:161-169; PMID:23325793; http://dx.doi.org/ 10.1093/mutage/ges066 [DOI] [PubMed] [Google Scholar]
- 16.Wang LS, Dombkowski AA, Seguin C, Rocha C, Cukovic D, Mukundan A, Henry C, Stoner GD. Mechanistic basis for the chemopreventive effects of black raspberries at a late stage of rat esophageal carcinogenesis. Mol Carcinog 2011; 50:291-300; PMID:21465577; http://dx.doi.org/ 10.1002/mc.20634 [DOI] [PubMed] [Google Scholar]
- 17.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK, et al.. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 2004; 3:1427-1438; PMID:15542782 [PubMed] [Google Scholar]
- 18.Okano J, Snyder L, Rustgi AK. Genetic alterations in esophageal cancer. Methods Mol Biol 2003; 222:131-145; PMID:12710684 [DOI] [PubMed] [Google Scholar]
- 19.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003; 349:2241-2252; PMID:14657432; http://dx.doi.org/ 10.1056/NEJMra035010 [DOI] [PubMed] [Google Scholar]
- 20.http://www.cancer.gov/cancertopics/types/esophageal. [Google Scholar]
- 21.Peiffer DS, Zimmerman NP, Wang LS, Ransom BW, Carmella SG, Kuo CT, Siddiqui J, Chen JH, Oshima K, Huang YW, et al.. Chemoprevention of Esophageal Cancer with Black Raspberries, Their Component Anthocyanins, and a Major Anthocyanin Metabolite, Protocatechuic Acid. Cancer Prev Res 2014; 7:574-84; PMID:24667581; http://dx.doi.org/ 10.1158/1940-6207.CAPR-14-0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, You M, Reynolds SH, Stoner GD, Anderson MW. Mutational activation of the cellular Harvey ras oncogene in rat esophageal papillomas induced by methylbenzylnitrosamine. Cancer Res 1990; 50:1591-1595; PMID:2406014 [PubMed] [Google Scholar]
- 23.Wang D, Weghorst CM, Calvert RJ, Stoner GD. Mutation in the p53 tumor suppressor gene in rat esophageal papillomas induced by N-nitrosomethylbenzylamine. Carcinogenesis 1996; 17:625-630; PMID:8625469; http://dx.doi.org/ 10.1093/carcin/17.4.625 [DOI] [PubMed] [Google Scholar]
- 24.Xie Y, Tang E, Ren B, Feng L. Overexpression of S-adenosylhomocysteine hydrolase and down-regulation of prohibitin and c-Myc binding protein in the human esophageal squamous cell line HEEC exposed to N-nitrosomethylbenzylamine. Mol Med Rep 2010; 3:503-508; PMID:21472270 [DOI] [PubMed] [Google Scholar]
- 25.Wang QS, Sabourin CL, Bijur GN, Robertson FM, Stoner GD. Alterations in transforming growth factor-alpha and epidermal growth factor receptor expression during rat esophageal tumorigenesis. Mol Carcinog 1996; 15:144-153; PMID:8599581; http://dx.doi.org/ 10.1002/(SICI)1098-2744(199602)15:2%3c144::AID-MC7%3e3.0.CO;2-J [DOI] [PubMed] [Google Scholar]
- 26.Wang QS, Sabourin CL, Wang H, Stoner GD. Overexpression of cyclin D1 and cyclin E in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis. Carcinogenesis 1996; 17:1583-1588; PMID:8761413; http://dx.doi.org/ 10.1093/carcin/17.8.1583 [DOI] [PubMed] [Google Scholar]
- 27.Youssef EM, Hasuma T, Morishima Y, Takada N, Osugi H, Higashino M, Otani S, Fukushima S. Overexpression of cyclin D1 in rat esophageal carcinogenesis model. JapJ Cancer Res 1997; 88:18-25; PMID:9045891; http://dx.doi.org/ 10.1111/j.1349-7006.1997.tb00296.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanesi N, Fidanza V, Fong LY, Mancini R, Druck T, Valtieri M, Rüdiger T, McCue PA, Croce CM, Huebner K. The tumor spectrum in FHIT-deficient mice. Proc Natl Acad Sci U S A 2001; 98:10250-10255; PMID:11517343; http://dx.doi.org/ 10.1073/pnas.191345898 [DOI] [PMC free article] [PubMed] [Google Scholar]