Abstract
The protective efficacy of a human monoclonal antibody directed against the a determinant of hepatitis B virus (HBV) surface antigen was studied in a chimpanzee. A single high dose of 5 mg/kg (body weight) of monoclonal antibody SDZ OST 577 was intravenously administered to a chimpanzee, followed by intravenous challenge with 10(3.5) chimpanzee infectious doses of a wild-type HBV, the MS-2 strain (ayw subtype). The passively acquired antibody to HBV surface antigen could be detected for 40 weeks. Serum HBV DNA tested by a "nested" polymerase chain reaction assay was negative through the 36th week after virus challenge but became positive by the 38th week. The chimpanzee subsequently developed acute hepatitis B approximately 1 year after challenge. The nucleotide sequence of the a determinant of the surface gene of the replicated virus was identical with that of the inoculated wild-type virus. Thus, a human monoclonal antibody directed against the a determinant of HBV surface antigen delayed but did not prevent experimental infection of HBV and hepatitis in the chimpanzee. Our results indicate an incomplete ability of this antibody to protect against HBV infection in vivo after a single infusion.
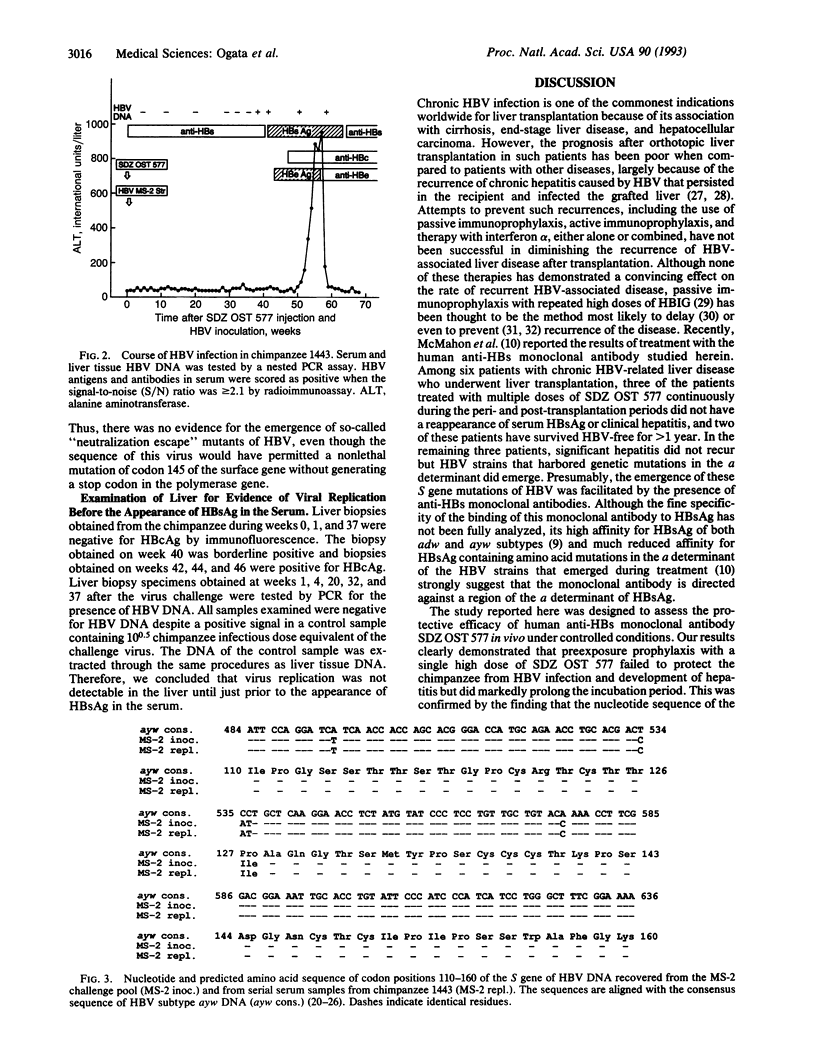
Full text
PDF
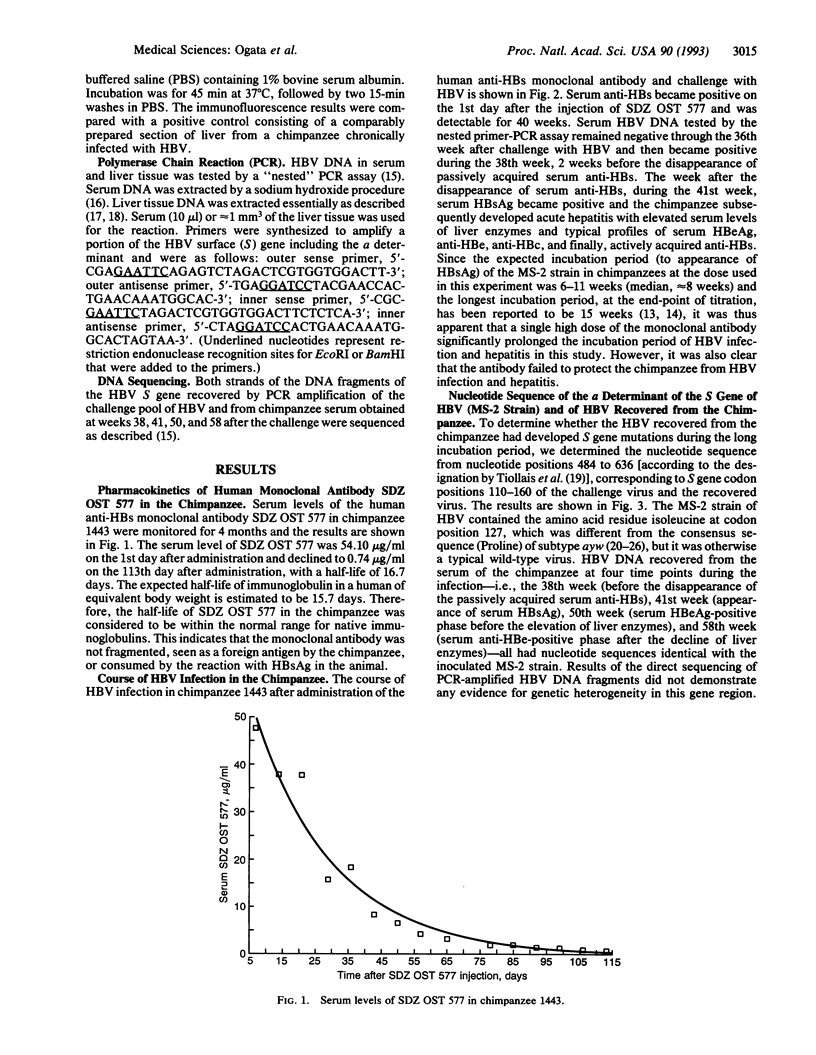


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker L. F., Maynard J. E., Purcell R. H., Hoofnagle J. H., Berquist K. R., London W. T., Gerety R. J., Krushak D. H. Hepatitis B virus infection in chimpanzees: titration of subtypes. J Infect Dis. 1975 Oct;132(4):451–458. doi: 10.1093/infdis/132.4.451. [DOI] [PubMed] [Google Scholar]
- Barker L. F., Maynard J. E., Purcell R. H., Hoofnagle J. H., Berquist K. R., London W. T. Viral hepatitis, type B, in experimental animals. Am J Med Sci. 1975 Jul-Aug;270(1):189–195. doi: 10.1097/00000441-197507000-00026. [DOI] [PubMed] [Google Scholar]
- Beasley R. P., Hwang L. Y., Lee G. C., Lan C. C., Roan C. H., Huang F. Y., Chen C. L. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983 Nov 12;2(8359):1099–1102. doi: 10.1016/s0140-6736(83)90624-4. [DOI] [PubMed] [Google Scholar]
- Beasley R. P., Hwang L. Y., Stevens C. E., Lin C. C., Hsieh F. J., Wang K. Y., Sun T. S., Szmuness W. Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double-blind, placebo-controlled trial. Hepatology. 1983 Mar-Apr;3(2):135–141. doi: 10.1002/hep.1840030201. [DOI] [PubMed] [Google Scholar]
- Bhatnagar P. K., Papas E., Blum H. E., Milich D. R., Nitecki D., Karels M. J., Vyas G. N. Immune response to synthetic peptide analogues of hepatitis B surface antigen specific for the a determinant. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4400–4404. doi: 10.1073/pnas.79.14.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichko V., Pushko P., Dreilina D., Pumpen P., Gren E. Subtype ayw variant of hepatitis B virus. DNA primary structure analysis. FEBS Lett. 1985 Jun 3;185(1):208–212. doi: 10.1016/0014-5793(85)80771-7. [DOI] [PubMed] [Google Scholar]
- Brown S. E., Howard C. R., Zuckerman A. J., Steward M. W. Determination of the affinity of antibodies to hepatitis B surface antigen in human sera. J Immunol Methods. 1984 Aug 3;72(1):41–48. doi: 10.1016/0022-1759(84)90431-9. [DOI] [PubMed] [Google Scholar]
- Carman W. F., Zanetti A. R., Karayiannis P., Waters J., Manzillo G., Tanzi E., Zuckerman A. J., Thomas H. C. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990 Aug 11;336(8711):325–329. doi: 10.1016/0140-6736(90)91874-a. [DOI] [PubMed] [Google Scholar]
- Ehrlich P. H., Moustafa Z. A., Justice J. C., Harfeldt K. E., Kelley R. L., Ostberg L. Characterization of human monoclonal antibodies directed against hepatitis B surface antigen. Hum Antibodies Hybridomas. 1992 Jan;3(1):2–7. [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Gerin J. L., Alexander H., Shih J. W., Purcell R. H., Dapolito G., Engle R., Green N., Sutcliffe J. G., Shinnick T. M., Lerner R. A. Chemically synthesized peptides of hepatitis B surface antigen duplicate the d/y specificities and induce subtype-specific antibodies in chimpanzees. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2365–2369. doi: 10.1073/pnas.80.8.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady G. F., Lee V. A., Prince A. M., Gitnick G. L., Fawaz K. A., Vyas G. N., Levitt M. D., Senior J. R., Galambos J. T., Bynum T. E. Hepatitis B immune globulin for accidental exposures among medical personnel: final report of a multicenter controlled trial. J Infect Dis. 1978 Nov;138(5):625–638. doi: 10.1093/infdis/138.5.625. [DOI] [PubMed] [Google Scholar]
- Harrison T. J., Hopes E. A., Oon C. J., Zanetti A. R., Zuckerman A. J. Independent emergence of a vaccine-induced escape mutant of hepatitis B virus. J Hepatol. 1991;13 (Suppl 4):S105–S107. doi: 10.1016/0168-8278(91)90037-c. [DOI] [PubMed] [Google Scholar]
- Iwarson S., Tabor E., Thomas H. C., Goodall A., Waters J., Snoy P., Shih J. W., Gerety R. J. Neutralization of hepatitis B virus infectivity by a murine monoclonal antibody: an experimental study in the chimpanzee. J Med Virol. 1985 May;16(1):89–96. doi: 10.1002/jmv.1890160112. [DOI] [PubMed] [Google Scholar]
- Iwarson S., Wahl M., Ruttimann E., Snoy P., Seto B., Gerety R. J. Successful postexposure vaccination against hepatitis B in chimpanzees. J Med Virol. 1988 Aug;25(4):433–439. doi: 10.1002/jmv.1890250407. [DOI] [PubMed] [Google Scholar]
- Kaneko S., Feinstone S. M., Miller R. H. Rapid and sensitive method for the detection of serum hepatitis B virus DNA using the polymerase chain reaction technique. J Clin Microbiol. 1989 Sep;27(9):1930–1933. doi: 10.1128/jcm.27.9.1930-1933.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugman S., Giles J. P., Hammond J. Viral hepatitis, type B (MS-2 strain) prevention with specific hepatitis B immune serum globulin. JAMA. 1971 Dec 13;218(11):1665–1670. [PubMed] [Google Scholar]
- Krugman S., Giles J. P., Hammond J. Viral hepatitis, type B (MS-2 strain). Studies on active immunization. JAMA. 1971 Jul 5;217(1):41–45. [PubMed] [Google Scholar]
- Lai M. E., Melis A., Mazzoleni A. P., Farci P., Balestrieri A. Sequence analysis of hepatitis B virus genome of a new mutant of ayw subtype isolated in Sardinia. Nucleic Acids Res. 1991 Sep 25;19(18):5078–5078. doi: 10.1093/nar/19.18.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauchart W., Müller R., Pichlmayr R. Immunoprophylaxis of hepatitis B virus reinfection in recipients of human liver allografts. Transplant Proc. 1987 Feb;19(1 Pt 3):2387–2389. [PubMed] [Google Scholar]
- McAleer W. J., Buynak E. B., Maigetter R. Z., Wampler D. E., Miller W. J., Hilleman M. R. Human hepatitis B vaccine from recombinant yeast. Nature. 1984 Jan 12;307(5947):178–180. doi: 10.1038/307178a0. [DOI] [PubMed] [Google Scholar]
- McMahon G., Ehrlich P. H., Moustafa Z. A., McCarthy L. A., Dottavio D., Tolpin M. D., Nadler P. I., Ostberg L. Genetic alterations in the gene encoding the major HBsAg: DNA and immunological analysis of recurrent HBsAg derived from monoclonal antibody-treated liver transplant patients. Hepatology. 1992 May;15(5):757–766. doi: 10.1002/hep.1840150503. [DOI] [PubMed] [Google Scholar]
- Mora N. P., Klintmalm G. B., Poplawski S. S., Cofer J. B., Husberg B. S., Gonwa T. A., Goldstein R. M. Recurrence of hepatitis B after liver transplantation: does hepatitis-B-immunoglobulin modify the recurrent disease? Transplant Proc. 1990 Aug;22(4):1549–1550. [PubMed] [Google Scholar]
- Moriyama K., Nakajima E., Hohjoh H., Asayama R., Okochi K. Immunoselected hepatitis B virus mutant. Lancet. 1991 Jan 12;337(8733):125–125. doi: 10.1016/0140-6736(91)90793-o. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Strick N. Specificity of antibodies elicited by a synthetic peptide having a sequence in common with a fragment of a virus protein, the hepatitis B surface antigen. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7871–7875. doi: 10.1073/pnas.79.24.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norder H., Hammas B., Löfdahl S., Couroucé A. M., Magnius L. O. Comparison of the amino acid sequences of nine different serotypes of hepatitis B surface antigen and genomic classification of the corresponding hepatitis B virus strains. J Gen Virol. 1992 May;73(Pt 5):1201–1208. doi: 10.1099/0022-1317-73-5-1201. [DOI] [PubMed] [Google Scholar]
- Ogata N., Alter H. J., Miller R. H., Purcell R. H. Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3392–3396. doi: 10.1073/pnas.88.8.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N., Kamimura T., Asakura H. Point mutation, allelic loss and increased methylation of c-Ha-ras gene in human hepatocellular carcinoma. Hepatology. 1991 Jan;13(1):31–37. [PubMed] [Google Scholar]
- Ogata N., Tokino T., Kamimura T., Asakura H. A comparison of the molecular structure of integrated hepatitis B virus genomes in hepatocellular carcinoma cells and hepatocytes derived from the same patient. Hepatology. 1990 Jun;11(6):1017–1023. doi: 10.1002/hep.1840110617. [DOI] [PubMed] [Google Scholar]
- Ohnuma H., Takai E., Machida A., Tsuda F., Okamoto H., Tanaka T., Naito M., Munekata E., Miki K., Miyakawa Y. Synthetic oligopeptides bearing a common or subtypic determinant of hepatitis B surface antigen. J Immunol. 1990 Oct 1;145(7):2265–2271. [PubMed] [Google Scholar]
- Okamoto H., Tsuda F., Sakugawa H., Sastrosoewignjo R. I., Imai M., Miyakawa Y., Mayumi M. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J Gen Virol. 1988 Oct;69(Pt 10):2575–2583. doi: 10.1099/0022-1317-69-10-2575. [DOI] [PubMed] [Google Scholar]
- Ostberg L., Pursch E. Human X (mouse X human) hybridomas stably producing human antibodies. Hybridoma. 1983;2(4):361–367. doi: 10.1089/hyb.1983.2.361. [DOI] [PubMed] [Google Scholar]
- Pasek M., Goto T., Gilbert W., Zink B., Schaller H., MacKay P., Leadbetter G., Murray K. Hepatitis B virus genes and their expression in E. coli. Nature. 1979 Dec 6;282(5739):575–579. doi: 10.1038/282575a0. [DOI] [PubMed] [Google Scholar]
- Perrillo R. P., Campbell C. R., Strang S., Bodicky C. J., Costigan D. J. Immune globulin and hepatitis B immune globulin. Prophylactic measures for intimate contacts exposed to acute type B hepatitis. Arch Intern Med. 1984 Jan;144(1):81–85. doi: 10.1001/archinte.144.1.81. [DOI] [PubMed] [Google Scholar]
- Prince A. M., Ikram H., Hopp T. P. Hepatitis B virus vaccine: identification of HBsAg/a and HBsAg/d but not HBsAg/y subtype antigenic determinants on a synthetic immunogenic peptide. Proc Natl Acad Sci U S A. 1982 Jan;79(2):579–582. doi: 10.1073/pnas.79.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell R. H., Gerin J. L. Hepatitis B subunit vaccine: a preliminary report of safety and efficacy tests in chimpanzees. Am J Med Sci. 1975 Sep-Oct;270(2):395–399. [PubMed] [Google Scholar]
- Redeker A. G., Mosley J. W., Gocke D. J., McKee A. P., Pollack W. Hepatitis B immune globulin as a prophylactic measure for spouses exposed to acute type B hepatitis. N Engl J Med. 1975 Nov 20;293(21):1055–1059. doi: 10.1056/NEJM197511202932101. [DOI] [PubMed] [Google Scholar]
- Rossi G., Grendele M., Colledan M., Gridelli B., Fassati L. R., Maggi U., Reggiani P., Gatti S., Piazzini A., Lunghi G. Prevention of hepatitis B virus reinfection after liver transplantation. Transplant Proc. 1991 Jun;23(3):1969–1969. [PubMed] [Google Scholar]
- Samuel D., Bismuth A., Serres C., Arulnaden J. L., Reynes M., Benhamou J. P., Brechot C., Bismuth H. HBV infection after liver transplantation in HBsAg positive patients: experience with long-term immunoprophylaxis. Transplant Proc. 1991 Feb;23(1 Pt 2):1492–1494. [PubMed] [Google Scholar]
- Schellekens H., de Reus A., Peetermans J. H., van Eerd P. A. The protection of chimpanzees against hepatitis B viral infection using a recombinant yeast-derived hepatitis B surface antigen. Postgrad Med J. 1987;63 (Suppl 2):93–96. [PubMed] [Google Scholar]
- Seeff L. B., Wright E. C., Zimmerman H. J., Alter H. J., Dietz A. A., Felsher B. F., Finkelstein J. D., Garcia-Pont P., Gerin J. L., Greenlee H. B. Type B hepatitis after needle-stick exposure: prevention with hepatitis B immune globulin. Final report of the Veterans Administration Cooperative Study. Ann Intern Med. 1978 Mar;88(3):285–293. doi: 10.7326/0003-4819-88-3-285. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Demetris A. J., Van Thiel D. Liver transplantation (2). N Engl J Med. 1989 Oct 19;321(16):1092–1099. doi: 10.1056/NEJM198910193211606. [DOI] [PubMed] [Google Scholar]
- Stevens C. E., Toy P. T., Tong M. J., Taylor P. E., Vyas G. N., Nair P. V., Gudavalli M., Krugman S. Perinatal hepatitis B virus transmission in the United States. Prevention by passive-active immunization. JAMA. 1985 Mar 22;253(12):1740–1745. [PubMed] [Google Scholar]
- Steward M. W., Sisley B. M., Stanley C., Brown S. E., Howard C. R. Immunity to hepatitis B: analysis of antibody and cellular responses in recipients of a plasma-derived vaccine using synthetic peptides mimicking S and pre-S regions. Clin Exp Immunol. 1988 Jan;71(1):19–25. [PMC free article] [PubMed] [Google Scholar]
- Tabor E., Purcell R. H., London W. T., Gerety R. J. Use of and interpretation of results using inocula of hepatitis B virus with known infectivity titers. J Infect Dis. 1983 Mar;147(3):531–534. doi: 10.1093/infdis/147.3.531. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Todo S., Demetris A. J., Van Thiel D., Teperman L., Fung J. J., Starzl T. E. Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology. 1991 Apr;13(4):619–626. [PMC free article] [PubMed] [Google Scholar]
- Wahl M., Iwarson S., Snoy P., Gerety R. J. Failure of hepatitis B immune globulin to protect against exp infection in chimpanzees. J Hepatol. 1989 Sep;9(2):198–203. doi: 10.1016/0168-8278(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Wong V. C., Ip H. M., Reesink H. W., Lelie P. N., Reerink-Brongers E. E., Yeung C. Y., Ma H. K. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin. Double-blind randomised placebo-controlled study. Lancet. 1984 Apr 28;1(8383):921–926. doi: 10.1016/s0140-6736(84)92388-2. [DOI] [PubMed] [Google Scholar]


