Abstract
Volatile organic compounds (VOCs) are secondary metabolites acting as a language for the communication of plants with the environment. In orange fruits, the monoterpene D-limonene accumulates at very high levels in oil glands from the peel. Drastic down-regulation of D-limonene synthase gene expression in the peel of transgenic oranges harboring a D-limonene synthase transgene in antisense (AS) configuration altered the monoterpene profile in oil glands, mainly resulting in reduced accumulation of D-limonene. This led to fruit resistance against Penicillium digitatum (Pd), Xanthomonas citri subsp. citri (Xcc) and other specialized pathogens. Here, we analyze resistance to pathogens in independent AS and empty vector (EV) lines, which have low, medium or high D-limonene concentrations and show that the level of resistance is inversely related to the accumulation of D-limonene in orange peels, thus explaining the need of high D-limonene accumulation in mature oranges in nature for the efficient attraction of specialized microorganism frugivores.
Keywords: citrus, defense, D-limonene, monoterpene, necrotroph, Penicillium digitatum, secondary metabolism, volatiles, Xanthomonas citri subsp citri
Higher plants produce a wide diversity of chemical compounds, traditionally known as secondary metabolites; many of them are volatiles that defend them against herbivores and pathogens and influence the feeding behavior of pollinators, seed dispersers, and herbivore predators.1-5
These secondary metabolites, including terpenoids, offer great potential for biotechnological applications, mainly with the aim of achieving resistance to pest and pathogens in crops. An improvement of our knowledge beyond general phytochemical cataloging of these compounds is needed, by performing specific experiments raised to identify their mode of action within the plant and on plant interactions with other organisms.6
Plants with either down- or up-regulated volatile isoprenoid synthesis are excellent tools to dissect the biological role of specific plant VOCs. In the same way that the up-regulation of some terpenoids have been associated generally with plant defense properties,7-11 the downregulation of their production may sometimes reduce the susceptibility to specific pests or microorganisms, as it has been shown in plants such as tobacco or poplar.12,13
In a previous report, we showed that transgenic oranges (Citrus sinensis L. Osb. cv. Navelina and Pineapple) accumulating highly reduced levels of the monoterpene D-limonene in the fruit peel became resistant to the bacterium Xanthomonas citri subsp citri (Xcc), to the fungus Penicillium digitatum (Pd) and to other specialized fungi.5,14,15 D-limonene synthase down-regulation was associated with constitutive upregulation of genes involved in plant innate immune response to pathogens and to increased accumulation of jasmonic acid upon challenge by the pathogen.5 Therefore, we concluded that D-limonene is required for specialized pathogens to establish infections in mature oranges. To assess whether different D-limonene concentrations in the fruit would affect infection rate and/or symptom intensity, we have now compared the responses to either Pd or Xcc inoculation displayed by transgenic oranges with very low and medium levels of D-limonene accumulation and EV transgenic oranges with very high levels of D-limonene accumulation (comparable to wild-type (WT) oranges).
Overexpression of the full-length cDNA from a D-limonene synthase gene from Satsuma mandarin (CitMTSE1) in antisense (AS) configuration in transgenic oranges generally resulted in a drastic reduction in the accumulation of D-limonene and increased amounts of monoterpene alcohols such as nerol, geraniol and citronellol in fruit peels.14 We have now identified independent transformants AS2, AS4 and AS6 harboring several insertions of the transgene (Fig. 1), which accumulated intermediate levels of these terpene compounds (Fig. S1).
Figure 1.
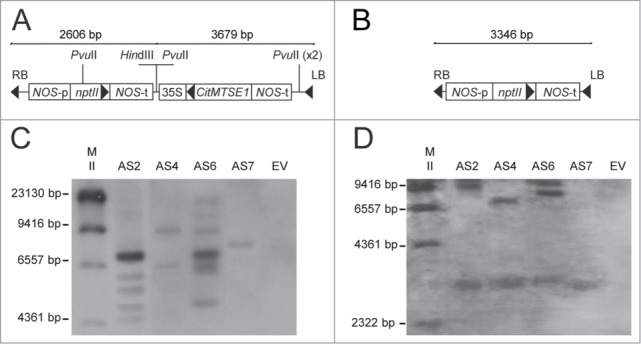
Molecular analysis of DNA isolated from orange leaves of antisense (AS) and empty vector control (EV) Navelina sweet orange transgenic plants. (A, B) Map of the T-DNA region of the binary vector used to transform AS (A) and EV (B) plants. LB, left T-DNA border region; RB, right T-DNA border region; nptII, neomycin phosphotransferase II transgene conferring kanamycin resistance, under the control of the nopaline synthase (NOS) promoter and terminator regions; CitMTSE1, limonene synthase gene in antisense orientation under control of the Cauliflower mosaic virus (CaMV) 35S promoter and the NOS terminator. (C, D) Southern blot analysis of independent AS transgenic lines (AS2, AS4 and AS6 and AS7) and the EV control line. The DNA was digested with the enzymes HindIII for testing loci number integrations (C) or PvuII for assessing integrity of the D-limonene transgene (D). The 35S promoter was used as a probe. M: DNA molecular weight marker II from Roche Applied Science.
Attempts to alter the concentration of D-limonene in citrus fruits may be counter-productive, as the modification of the flux of isoprenoids by metabolic engineering potentially risks the production of other isoprenoid derivatives and thus normal fruit growth and development. To test this possibility, we measured the number of oil glands and their size in green and mature peel of AS2, AS4, AS6 and EV fruits and found no significant differences between them (Fig. 2). Previously, we found no differences between fruit peel of AS lines with highly reduced levels of D-limonene and EV controls.5 Therefore, the decrease of D-limonene concentrations, either high or medium, did not cause morphological alterations or other pleiotropic effects in the AS transgenic fruits.
Figure 2.
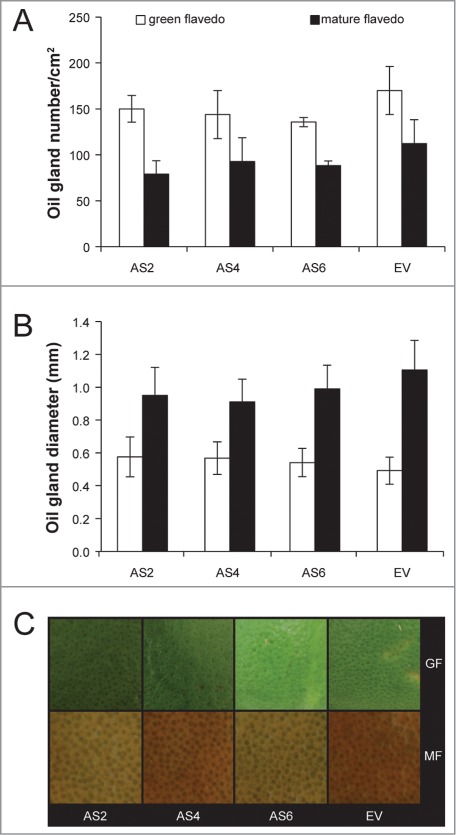
Characteristics of green (70 mm diameter) and mature (90 mm diameter) peels (flavedo) from AS and EV Navelina sweet orange fruits. (A, B) Oil gland number and size in green and mature flavedo, respectively. Data represent means ± SE and are derived from analysis of 10 fruits per plant. No significant differences were found at P ≤ 0.05 using Fisher's protected LSD test at each stage. (C) Magnification of oil glands in 4 cm2 peel pieces of AS and EV fruits in green (GF) and mature flavedo (MF).
To compare the effect of medium, low and high (WT) levels of D-limonene accumulation on resistance to specialized pathogens of orange fruits, we chose AS6, AS7 and EV lines, respectively. Volatile terpene contents were analyzed by GC-MS as reported before for mature fruits of the 3 transgenic lines (Fig. 3).14 Challenge inoculations of Pd and Xcc were performed as reported before.14 We observed that AS6 was resistant to both pathogens compared to the EV control line, but less than AS7, both in term of percentage of infected wounds and in symptom intensity (Fig. 4). The experiments were repeated with AS2 and AS4, obtaining results comparable to those of AS6 (data not shown). The resistance phenotype was co-related to the decrease in D-limonene concentration in the transgenic fruits. However, we cannot rule out that changes in the accumulation of other monoterpene compounds in peel oil glands or activation of defense responses derived from such constitutive changes may also contribute to the different levels of resistance observed in AS fruits.
Figure 3.
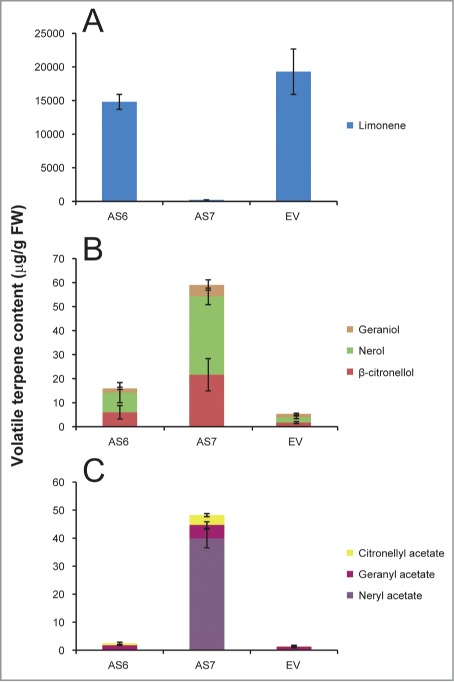
Comparison of the volatile terpene content (μg/g FW) of mature flavedo from AS6, AS7 and EV transgenic fruits. (A) D-limonene; (B) monoterpene alcohols; (C) alcohol derivatives. Data represent means ± SE and are derived from analysis of at least 5 fruits per plant. Quantification was achieved using calibration curves constructed for each volatile with linear regression equations of commercially available synthetic compounds. The volatiles which were not available commercially were quantified with standard curves obtained from similar structure available compounds.
Figure 4.
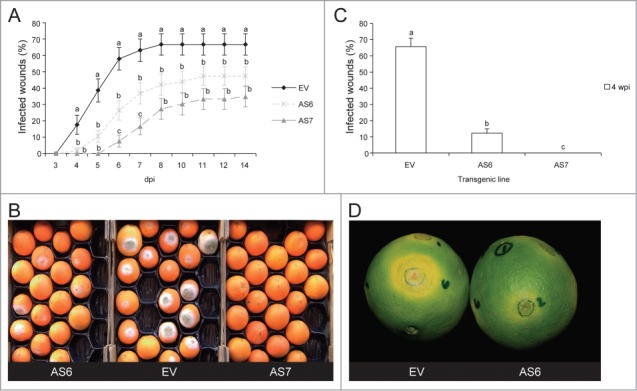
Transgenic expression of CitMTSE1 in antisense orientation in orange fruits confers different levels of resistance against fungal and bacterial specialized pathogens. (A, B) Progress of the disease caused by the fungus Penicillium digitatum in mature orange fruits inoculated with 1×104 spores mL−1. (A) Percentage of infected wounds in orange fruits from AS6, AS7 and EV lines. The results are the average ± SEM (n ≥ 20). dpi, days postinoculation. (B) AS and EV fruits at 6 dpi. (C, D) Progress of the disease caused by the bacterium Xanthomonas citri subsp citri in green orange fruits inoculated with 106 CFU mL−1. (C) Percentage of infected wounds in orange fruits from AS6, AS7 and EV lines at 4 weeks postinoculation (wpi). The results are the average ± SEM (n ≥ 10). (D) AS6 and EV fruits at 4 wpi. We repeated all experiments several times during 2 consecutive seasons and obtained similar results. For each time point, means with different letter are significantly different according to Fisher's Protected LSD test (P ≤ 0.05) applied after an ANOVA to arcsine-transformed data.
Terpenoids represent one of the largest and diverse classes of metabolites in the plant kingdom and are involved in many physiological and ecological processes.16 Plants during their life cycles interact with a vast range of different microbial species. The ways by which plants recognize, coordinate and regulate the exchange of resources and information with the myriads of potentially interacting microbes are not yet completely understood.17
Metabolic engineering may create great opportunities to study the ecological importance of terpenoids in the interactions of plants with other organisms, including microbes.18,19 D-limonene accumulates at huge levels in mature oranges, representing more than 95% of total terpene compounds found in the oil glands from their fruit peel, and it is produced at a very high metabolic cost. We show here that reduced levels of D-limonene as those found in AS6 fruits are sufficient to generate good levels of resistance against Pd and Xcc, though lower than those found in AS lines with very low concentrations of D-limonene. Therefore, high levels of D-limonene are required for efficient interactions of the fruit with specialized microorganisms, which may be involved in seed dispersal by vertebrate frugivores.
For biotechnological purposes, our results indicate that AS lines with the highest reduction of D-limonene concentrations in fruit peel would be more promising ones for generating field resistance against citrus pathogens.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Ministry of Science and Innovation of Spain (grant no. AGL2009–08052) and by the Fundo de Defesa da Citricultura, (FUNDECITRUS), Brazil.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Pichersky E, Gershenzon J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr Opin Plant Biol 2002; 5:237-243; PMID:11960742; http://dx.doi.org/ 10.1016/S1369-5266(02)00251-0 [DOI] [PubMed] [Google Scholar]
- 2.Dudareva N, Negre F, Nagegowda DA, Orlova I. Plant volatiles: Recent advances and future perspectives. Crit Rev Plant Sci 2006; 25:417-440; http://dx.doi.org/ 10.1080/07352680600899973 [DOI] [Google Scholar]
- 3.Bednarek P, Osbourn A. Plant-microbe interactions: Chemical diversity in plant defense. Science 2009; 324:746-748; PMID:19423814; http://dx.doi.org/ 10.1126/science.1171661 [DOI] [PubMed] [Google Scholar]
- 4.Vickers CE, Gershenzon J, Lerdau MT, Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol 2009; 5:283-291; PMID:19377454; http://dx.doi.org/ 10.1038/nchembio.158 [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez A, Shimada T, Cervera M, Alquézar B, Gadea J, Gómez-Cadenas A, De Ollas CJ, Rodrigo MJ, Zacarías L, Peña L. Terpene downregulation triggers defense responses in transgenic orange leading to resistance against fungal pathogens. Plant Physiol 2014; 164:321-339; http://dx.doi.org/ 10.1104/pp.113.224279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrissey J. Biological activity of defence-related plant secondary metabolites In: Osbourn AE, Lanzotti V, eds. Plant-Derived Natural Products. Springer; US; 2009:283-299 [Google Scholar]
- 7.Aharoni A, Giri AP, Deuerlein S, Griepink F, de Kogel W, Verstappen FWA, Verhoeven HA, Jongsma MA, Schwab W, Bouwmeester HJ. Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell 2003; 15:2866-2884; PMID:14630967; http://dx.doi.org/ 10.1105/tpc.016253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kappers IF, Aharoni A, van Herpen TWJM, Luckerhoff LLP, Dicke M, Bouwmeester HJ. Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science 2005; 309:2070-2072; PMID:16179482; http://dx.doi.org/ 10.1126/science.1116232 [DOI] [PubMed] [Google Scholar]
- 9.Schnee C, Köllner TG, Held M, Turlings TCJ, Gershenzon J, Degenhardt J. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci U S A 2006; 103:1129-1134; PMID:16418295; http://dx.doi.org/ 10.1073/pnas.0508027103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu S, Schalk M, Clark A, Miles RB, Coates R, Chappell J. Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants. Nat Biotech 2006; 24:1441-1447; http://dx.doi.org/ 10.1038/nbt1251 [DOI] [PubMed] [Google Scholar]
- 11.Degenhardt J, Hiltpold I, Köllner TG, Frey M, Gierl A, Gershenzon J, Hibbard BE, Ellersieck MR, Turlings TCJ. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc Natl Acad Sci U S A 2009; 106:13213-13218; PMID:19666594; http://dx.doi.org/ 10.1073/pnas.0906365106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang E, Wang R, DeParasis J, Loughrin JH, Gan S, Wagner GJ. Suppression of a P450 hydroxylase gene in plant trichome glands enhances natural-product-based aphid resistance. Nat Biotechnol 2001; 19:371-374; PMID:11283597; http://dx.doi.org/ 10.1038/86770 [DOI] [PubMed] [Google Scholar]
- 13.Behnke K, Grote R, Brüggemann N, Zimmer I, Zhou G, Elobeid M, Janz D, Polle A, Schnitzler J. Isoprene emission-free poplars - a chance to reduce the impact from poplar plantations on the atmosphere. New Phytol 2012; 194:70-82; PMID:22142198; http://dx.doi.org/ 10.1111/j.1469-8137.2011.03979.x [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez A, San Andrés V, Cervera M, Redondo A, Alquézar B, Shimada T, Gadea J, Rodrigo MJ, Zacarías L, Palou L, López MM, Castañera P, Peña L. Terpene down-regulation in orange reveals the role of fruit aromas in mediating interactions with insect herbivores and pathogens. Plant Physiol 2011; 156:793-802; http://dx.doi.org/ 10.1104/pp.111.176545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez A, San Andrés V, Cervera M, Redondo A, Alquézar B, Shimada T, Gadea J, Rodrigo M, Zacarías L, Palou L, Lopez MM, Castañera P, Peña L. The monoterpene limonene in orange peels attracts pests and microorganisms. Plant Signal Behav 2011; 6:1820-1823; http://dx.doi.org/ 10.4161/psb.6.11.16980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutensohn M, Nguyen TTH, McMahon RD III, Kaplan I, Pichersky E, Dudareva N. Metabolic engineering of monoterpene biosynthesis in tomato fruits via introduction of the non-canonical substrate neryl diphosphate. Metab Eng 2014; 24:107-116; PMID:24831707; http://dx.doi.org/ 10.1016/j.ymben.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 17.Bonanomi G, Vinale F, Scala F. The role of natural products in plant-microbe interactions In: Osbourn AE, Lanzotti V, eds. Springer; US; 2009:301-320 [Google Scholar]
- 18.Lücker J, Bouwmeester H, Aharoni A. Metabolic engineering of terpenoid biosynthesis in plants In: Verpoorte R, Alfermann AW, Johnson TS, eds. Springer; Netherlands; 2007:219-236 [Google Scholar]
- 19.Yu F, Utsumi R. Diversity, regulation, and genetic manipulation of plant mono- and sesquiterpenoid biosynthesis. Cell Mol Life Sci 2009; 66:3043-3052; PMID:19547916; http://dx.doi.org/ 10.1007/s00018-009-0066-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


