Abstract
Recombinant Secretory IgA (SIgA) complexes have the potential to improve antibody-based passive immunotherapeutic approaches to combat many mucosal pathogens. In this report, we describe the expression, purification and characterization of a human SIgA format of the broadly neutralizing anti-HIV monoclonal antibody (mAb) 2G12, using both transgenic tobacco plants and transient expression in Nicotiana benthamiana as expression hosts (P2G12 SIgA). The resulting heterodecameric complexes accumulated in intracellular compartments in leaf tissue, including the vacuole. SIgA complexes could not be detected in the apoplast. Maximum yields of antibody were 15.2 μg/g leaf fresh mass (LFM) in transgenic tobacco and 25 μg/g LFM after transient expression, and assembly of SIgA complexes was superior in transgenic tobacco. Protein L purified antibody specifically bound HIV gp140 and neutralised tier 2 and tier 3 HIV isolates. Glycoanalysis revealed predominantly high mannose structures present on most N-glycosylation sites, with limited evidence for complex glycosylation or processing to paucimannosidic forms. O-glycan structures were not identified. Functionally, P2G12 SIgA, but not IgG, effectively aggregated HIV virions. Binding of P2G12 SIgA was observed to CD209 / DC-SIGN, but not to CD89 / FcalphaR on a monocyte cell line. Furthermore, P2G12 SIgA demonstrated enhanced stability in mucosal secretions in comparison to P2G12 IgG mAb.
Keywords: antibody stability, glycosylation, HIV microbicide, molecular farming, secretory IgA
Abbreviations
- DC-SIGN
dendritic cell – specific intercellular adhesion molecule 3 grabbing non-integrin
- SC
secretory component
- SEM
standard error of the mean; SIgA, secretory IgA
- LFM
leaf fresh mass
Introduction
Due to their prevalence in mucosal secretions, secretory IgA antibodies (SIgA) are frequently described as the first line of defense against invasive microorganisms at the mucosal barrier (for a recent review see refs. 1–2). In comparison to IgG, SIgA is well suited to function on mucosal surfaces; it is non-inflammatory, resistant to proteolysis, tetravalent, and capable of unique interactions with structural and functional components of the mucosa. As a result of these features, SIgA has been proposed as an advantageous format for antibody-based prophylactics aimed at preventing infection at mucosal surfaces.3
SIgA is a heterodecameric antibody complex composed of 2 IgA antibodies linked together by the J chain and associated with the secretory component (SC); a structure for which high resolution models have been published.4,5 SIgA complex assembly normally requires 2 cell types, class-switched plasma cells co-expressing dimeric IgA and J chain and epithelial cells expressing polymeric Ig receptor (pIgR), from which SC is derived during transcytosis.6,7 Ex vivo production of recombinant SIgA has been described through expression of dimeric IgA and secretory component in separate cell lines and the combination of the 2 through in vitro association.8 SIgA produced in this fashion is therefore a product of 3 separate processes, which inevitably has an effect on the cost of manufacture and an increased burden of regulatory compliance. These factors are therefore significant limitations for the production of pharmaceutical biologics based on SIgA. Reconstituting the assembly of SIgA in single recombinant mammalian cells has proved technically challenging, with poor yields and inconsistent assembly frequently encountered.9,10 Although SIgA complexes have previously been produced in Chinese hamster ovary (CHO) cells9 and murine Sp2/0 transfectomas,11 plant cells have shown considerably more promise in this area.12,13 We have previously described the production and purification of a secretory IgA antibody with a chimeric heavy chain in plants.12,14,15
2G12 IgG was originally isolated from peripheral lymphocytes isolated from human immunodeficiency virus (HIV) infected donors,16 and it neutralizes a broad range of HIV virus isolates from clades A and B. MAb 2G12 belongs to a small but growing group of broadly neutralizing anti-HIV antibodies (HIV bnAb) that have potential as passive immunotherapeutics. mAb 2G12 binds an epitope defined by the high mannose glycan cluster of HIV gp120.17 This cluster of glycans typically prevents effective antibody responses to this region of gp120, and mAb 2G12 relies on a unique ‘domain-exchanged’ conformation to bind this region with high affinity.18 mAb 2G12 has been shown to protect non-human primates from vaginal challenge with R5-tropic SHIV when applied systemically,19,20 or from rectal challenge when applied topically to the same surface.21 In clinical trials with acutely infected volunteers, mAb 2G12 administered systemically was able to exert selective pressure on the virus and delay viral rebound when antiretroviral therapy was suspended.22,23 As a microbicidal prophylactic, mAb 2G12 IgG produced using CHO cells and formulated as a gel was found to be generally well tolerated, although it was found to be less stable in the vagina than 2 other antibodies present in the formulation.24
Plant production platforms offer a unique range of advantages over existing eukaryote production paradigms that can facilitate the commercial development of products that rely on low-cost high-volume biologic APIs, such as antibodies. The potential of plant systems for the production of biologics has been reviewed elsewhere.25-28 As part of an effort to establish proof-of-concept for plant-made antibodies in clinical applications, the Pharma-Planta consortium developed processes for the production of 2G12 IgG in maize,29 and a cGMP compliant process in Nicotiana tabacum. A subsequent Phase 1 safety trial of P2G12 IgG in a microbicide formulation demonstrated that the preparation was well tolerated and remained detectable in the vagina for 8 hours after administration (manuscript submitted).
In this report, we describe the production of a recombinant SIgA format of mAb 2G12 in 2 plant expression systems, transgenic N. tabacum and transient expression in N. benthamiana. We investigated the subcellular localization of P2G12 SIgA in transgenic tobacco and compared it to previous observations on the trafficking of Guy's 13 SIgA/G based on protein biochemistry.30 Following purification, we compared P2G12 SIgA with P2G12 IgG functionally, in respect to their ability to bind gp120, aggregate and neutralize HIV isolates. The glycosylation profile of P2G12 SIgA was elucidated, and the ability of plant-produced SIgA to interact with 2 cellular receptors with reported roles in SIgA biology, FcalphaR (CD89) and DC-SIGN (CD209) was investigated. Finally, persistence of P2G12 SIgA with P2G12 IgG in fresh vaginal mucosal secretions was compared.
Results
Assembly and expression of human 2G12 secretory IgA (P2G12 SIgA) in transgenic N. tabacum and agroinfiltrated N. benthamiana leaves
Two systems were compared for the production of recombinant secretory IgA (SIgA): transgenic N. tabacum, for which correct assembly of a chimeric murine/rabbit SIgA has previously been demonstrated,15 and transient expression in N. benthamiana via agroinfiltration. The second approach was investigated due to the potential for increased yield per unit biomass and the ability to circumvent time-consuming plant breeding and screening programmes.
Transgenic lines expressing 2G12 IgA complexes were created by sequential sexually crossing of T1 generation plants transgenic for 2G12 α, kappa, human J-chain and human secretory component (SC) to stack 2, 3 and 4 transgenes incrementally, as described previously.12 Combinations of 4 Agrobacterium lines harbouring binary expression vectors for each constituent chain were used to induce the transient expression of 2G12 IgA complexes. Extracts from the leaves of mature transgenic plants or N. benthamiana 5 d after infiltration were analyzed by SDS-PAGE and western blotting with anti-α chain antisera. Bands consistent in size with ‘monomeric’ IgA (IgA, Mr 150 kDa), dimerized IgA (IgA × J, Mr ∼300 kDa) and secretory IgA (IgA × J × SC, Mr ∼370 kDa) were detected in both transgenic (Fig. 1, panel A) and transient (Fig. 1, panel B) systems. Few unassembled or degradation fragments were detected, particularly in the quadruple transgenic plant sample, where the predominant molecular species was SIgA. The Mr 70 kDA species observed in the transient quadruple infiltrant ‘α κ J SC’ sample is consistent in size with free SC, which indicated that excess SC may have been produced in this approach.
Figure 1.
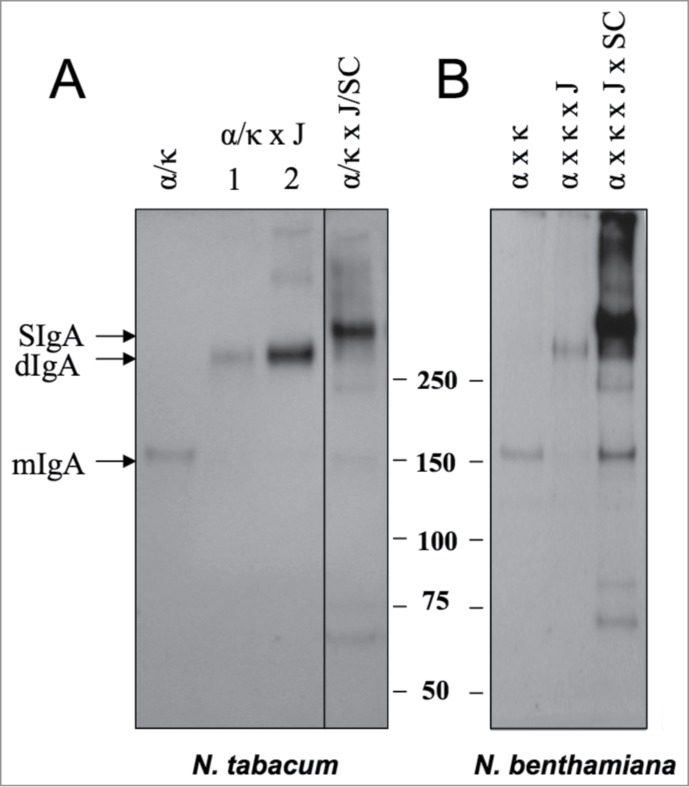
Expression of secretory IgA in stably transformed and transiently transfected plants. (A) Leaf samples from N. tabacum plants transgenic for mAb 2G12 α and kappa chains (lane α/κ), plants resulting from a cross between α/κ and J chain transgenic plants (lane α/κ x J) and plants resulting from a cross between α/κ plants and a J/SC hybrid (lane α/κ x J x SC) (B) N. benthamiana plants infiltrated with combinations of Agrobacterium preparations harbouring T-DNAs for the constituent chains of the SIgA complex. All samples were subjected to non-reducing SDS-PAGE and immunoblotting with anti-human IgA antisera. Bands corresponding to the expected sizes relative to the marker are labeled as monomeric IgA ‘mIgA’, dimeric IgA ‘dIgA’, and secretory IgA ‘SIgA’ and apply to both panels.
Yields of assembled IgA complexes were calculated using a sandwich ELISA against a human IgA standard. The proportion of decameric SIgA complexes was then inferred from densitometry of Coomassie stained SDS-PAGE gels. In transgenic plants, crude yields ranged between 6 and 15.2 μg per gram leaf fresh mass (LFM), with 76% (standard error of the mean (SEM) = 3.6, 3 independent batches analyzed) present in SIgA complexes. In transient expression, crude yields were higher, typically reaching 25 μg/g LFM, although the proportion of decamers was significantly reduced (48%, SEM 8.2, 3 independent batches analyzed).
P2G12 SIgA accumulates in intracellular compartments in the leaves of transgenic plants
A hybrid SIgA/G complex has previously been shown to accumulate in the vacuole of tobacco mesophyll protoplasts.30 We examined the deposition of 2G12 human IgA antibody formats in transgenic tobacco leaves by means of confocal and electron microscopy.
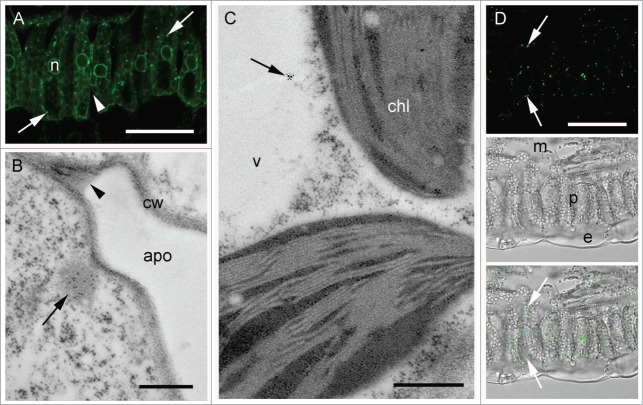
The subcellular localization of monomeric 2G12 IgA in tobacco leaves was examined first. Some labeling of the endoplasmic reticulum (ER) network was observed, likely reflecting the trafficking of the recombinant antibody within the secretory pathway. However, no significant signal was detected in the apoplast (Fig. 2A). Instead, punctate structures immunoreactive with anti-α chain antiserum were observed, both when examined by immunohistochemistry (IHC) and by immunogold labeling and electron microscopy (Fig. 2A and B). These structures were approximately 400 nm in size and morphologically similar to ER-derived protein storage compartments.31 Incubation of isolated leaf protoplasts with ER-TrackerTM Green confirmed the ER-derived origin of the protein body (PB)-like compartments that were induced in the IgA-expressing lines (Supplemental Fig. 1). Labeling of the PB-like structures was also observed when an anti-mAb 2G12 idiotype reagent was used for IHC (Fig. 2C), indicating that they are the likely site of deposition of at least a proportion of correctly folded and assembled IgA complexes. Overlay panels show the position of signal within the leaf structure under transmitted differential interference contrast light (Fig. 2C lower panels).
Figure 2.
Localization of monomeric IgA in plant leaf sections. (A) Transverse section of palisade mesophyll, after immunohistochemistry (IHC) with anti-α chain antiserum and Alexa Fluor® 488-conjugated secondary antibody. Arrows indicate PB-like structures (B) Immunogold labeling with anti-α chain antiserum. Arrow indicates a PB-like structure enriched with Ig α chains, chl = chloroplast (C) IHC with an anti-2G12 idiotype antibody and an Alexa Fluor® 488-conjugated secondary antibody. Arrows indicate deposition sites of antibody complexes containing the 2G12 idiotype. Lower panels, phase contrast images of a transverse section of palisade mesophyll with (lower) and without (middle) fluorescence overlay. Scale bars: 20 μm (A), 0.25 μm (B), 200 μm (C).
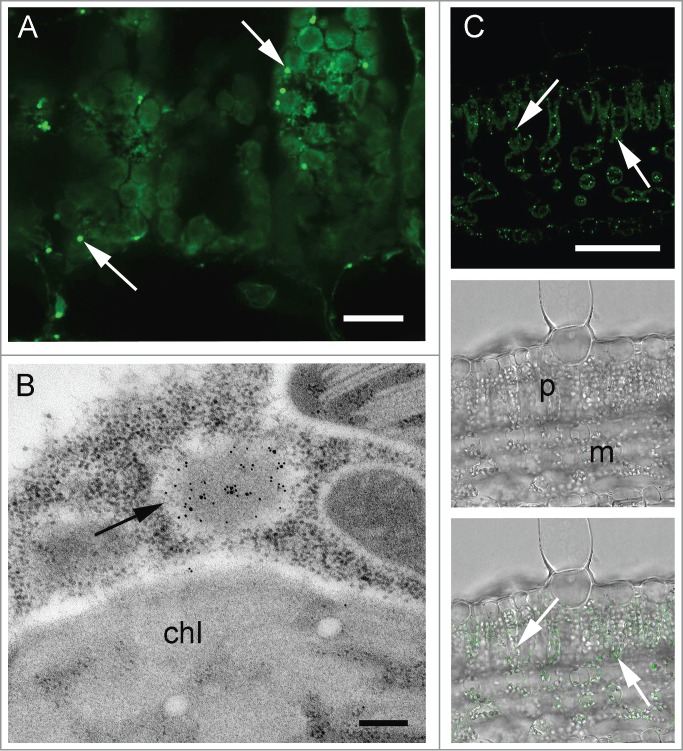
Similarly, in SIgA-expressing leaf samples, no significant signal was detected within the apoplast (Fig. 3A and B). Again, IgA chains could be detected mainly in punctuate structures spread within the cytoplasm (Fig. 3A) and similar in size and appearance to those observed previously for 2G12 monomeric IgA (Fig. 2B). In addition, some labeling of the vacuolar space was also detected (Fig. 3C). Punctate structures, similar to those labeled with anti- α-chain antiserum were observed with the anti-2G12 idiotype monoclonal antibody (mAb), indicating that they contain assembled heavy and light chains (Fig. 3D). No signal within the ER could be detected with the anti-idiotype serum in either monomeric IgA or SIgA lines, suggesting the ER labeling obtained with the anti-α chain serum corresponds to an accumulation of antibody chains prior to assembly and that assembled complexes are rapidly trafficked from the ER.
Figure 3.
Localization of SIgA complexes in plant leaf sections. (A) Transverse section of palisade mesophyll, after IHC with anti-α chain antiserum and Alexa Fluor® 488-conjugated secondary antibody. Arrows indicate PB-like structures (B) Immunogold labeling with anti-α chain antiserum, of the membrane-proximal region of a mesophyll cell showing apoplast (apo) and cell wall (cw) structures. The labeling is concentrated in the PB-like structures (arrow), whereas no labeling is visible in the apoplast (arrowhead) (C) Immunogold labeling in the vacuole (arrow) with anti-α chain and anti-kappa chain antisera in combination. Vacuole (v) and chloroplast (chl) structures are indicated (D) IHC with an anti-2G12 idiotype antibody and an Alexa Fluor® 488-conjugated secondary antibody. Arrows indicate deposition sites of antibody complexes containing the 2G12 idiotype; Lower panels, phase contrast images of a transverse section of palisade mesophyll with (lower) and without (middle) fluorescence overlay. Scale bars: 100 μm (A), 0.5 μm (B and C), 200 μm (D).
Affinity purification ligands for SIgA
We observed that P2G12 IgA complexes were not bound by protein A, despite belonging to the Vh3 germline family that has been described as protein A ligands.32 We tested the ability of several other commercial ligands to purify assembled 2G12 SIgA complexes by performing affinity chromatography of plant extracts against the ligands immobilized as the solid phase. A peptide from the staphylococcal M protein (Peptide M33) and Peptostreptococcus magnus protein L were capable of binding SIgA, dimeric IgA (dIgA, consisting of 2 IgA molecules dimerized by the J chain) and mIgA forms of mAb 2G12 through interactions with IgA-Fc and kappa light chain, respectively. SSL7 (Staphylococcus aureus superantigen-like protein 7) has been reported to bind IgA at a site near the Cα2-Cα3 domain boundary that is also involved in interactions with both FcαRI (CD89) and SC. Accordingly, SSL7 selectively purified 2G12 IgA monomers from transfected N. benthamiana extract. Protein L affinity was selected for the preparation of purified protein stocks for subsequent experiments. Recovery of assembled antibodies (α-kappa reactive) after purification was approximately 80% for 100–500 g batches (data not shown).
Glycosylation profile of transiently-expressed P2G12 SIgA
Next, the glycosylation profile of all 4 constituent chains of the SIgA complex was determined. Protein-L purified complexes produced in N. benthamiana were separated by SDS-PAGE followed by colloidal Coomassie staining, and individual bands were excised from the gel, digested with trypsin, and analyzed by LC-ESI-MS. There are 2 predicted N-glycosylation sites in the α heavy chain, one in the J chain and 7 in secretory component (Fig. 4).34,35 All but one of the predicted glycosylation sites in SIgA were found to be occupied in the plant antibody samples (Table 1, Supplemental Fig. 2). Both predicted N-glycosylation sites in the α chains were occupied by high mannose glycans (Man5-Man9) and in the case of N459, the typical plant complex glycan GnGnXF was also detected. There was no evidence for paucimannosidic structures characteristic of a vacuolar fate. The single N-glycosylation site in the J chain was occupied by heterologous glycan structures including GnMXF. Six of the 7 potential glycosylation sites in SC were found to contain glycan structures, the exception being N168, for which no predicted peptide mass was detected. Sites N65 and N72 were analyzed as one peptide. This was confirmed in 2 biological replicate assays. The other potential N-glycosylation sites were occupied by high mannose glycans, and complex glycan structures were detected in 2 out of the 6 identified glycopeptides, N403 and N451. The results suggest that at least a proportion of the α chain, J chain and secretory component must have been exposed to the glycosylation enzymes present in the Golgi apparatus.
Figure 4.
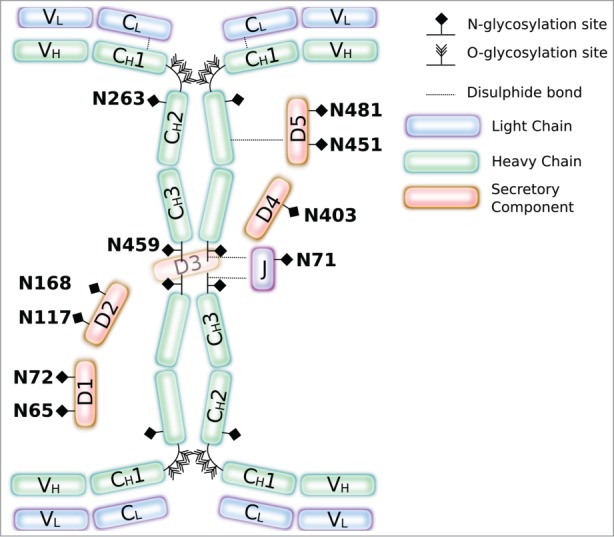
Glycosylation of SIgA. Illustration of SIgA showing potential glycosylation sites in SIgA. Numbering of glycosites matches that used in Table 1.
Table 1.
Glycoanalysis of 2G12 SIgA. Detected glycan structures on each chain of the SIgA complex. MS profiles are shown in supplemental Figure 2 Predominant glycan structures are marked with a +. The predicted glycan site SC4 was not detected in either of 2 assays. Glycan structure nomenclature available at http://www.proglycan.com/
| Chain | Glycosite | Peptide sequence | Structures |
|---|---|---|---|
| α | 263 | ANLTCTLTGLR | Man6 |
| Man7 | |||
| Man8+ | |||
| Man9 | |||
| α | 459 | LAGKPTHVNVSVVMAE | Man7 |
| Man8+ | |||
| Man9+ | |||
| GnGnXF | |||
| J | 71 | ENISDPTSPLR | Man6 |
| Man7 | |||
| Man8 | |||
| Man9 | |||
| MMXF+ | |||
| GnMXF+ | |||
| SC | 65 | ANLTNFPENGTFVVNIAQLSQDDSGR | Man8+ |
| Man9+ | |||
| SC | 72 | ANLTNFPENGTFVVNIAQLSQDDSGR | Man8+ |
| Man9+ | |||
| SC | 117 | VSQGPGLLNDTK | Man6 |
| Man7+ | |||
| Man8 | |||
| Man9 | |||
| SC | 168 | QIGLYPVLVIDSSGYVNPNYTGR | ND |
| SC | 403 | LSLLEEPGNGTFTVILNQLTSR | Man7 |
| Man8+ | |||
| Man9 | |||
| MMXF | |||
| MGnXF | |||
| GnGnXF | |||
| SC | 451 | VPGNVTAVLGETLK | Man8 |
| Man9 | |||
| MMXF | |||
| MGnXF | |||
| GnGnXF | |||
| SC | 481 | WNNTGCQALPSQDEGPSK | Man7 |
| Man8+ | |||
| Man9 |
The hinge region of the alpha1 chains in SIgA1 also contains 9 potential O-glycosylation sites (Fig. 4) which are known to be modified with extensive and diverse sugar moieties in human colostrum.36 The hinge peptide derived from P2G12 SIgA1 was detected at a mass consistent with partial hydroxyproline formation (5 conversions), a modification that is associated with certain plant-specific O-glycosylation events. However, no masses corresponding to additional sugar moieties could be detected (data not shown).
P2G12 SIgA binds HIV envelope protein, neutralizes resistant HIV isolates and aggregates HIV virions
We tested the ability of P2G12 SIgA to bind baculovirus-expressed gp120 using an ELISA and compared binding with P2G12 IgG and CHO 2G12 IgG. All 3 preparations bound to antigen (Fig. 5A). We then proceeded to test P2G12 SIgA for its ability to neutralize 3 HIV isolates that have been previously characterized as possessing moderate and low sensitivity to neutralization by antibodies (Table 2).37,38 P2G12 SIgA had equivalent neutralizing activity against the TRO.11 pseudovirus as the IgG format. PVO.4 and SC422661.8 envelopes were also neutralized by P2G12 SIgA, although with reduced potency (IC50 titer 2.44 vs 0.48 μg/ml and 10.82 vs 0.79 μg/ml, respectively).
Figure 5.
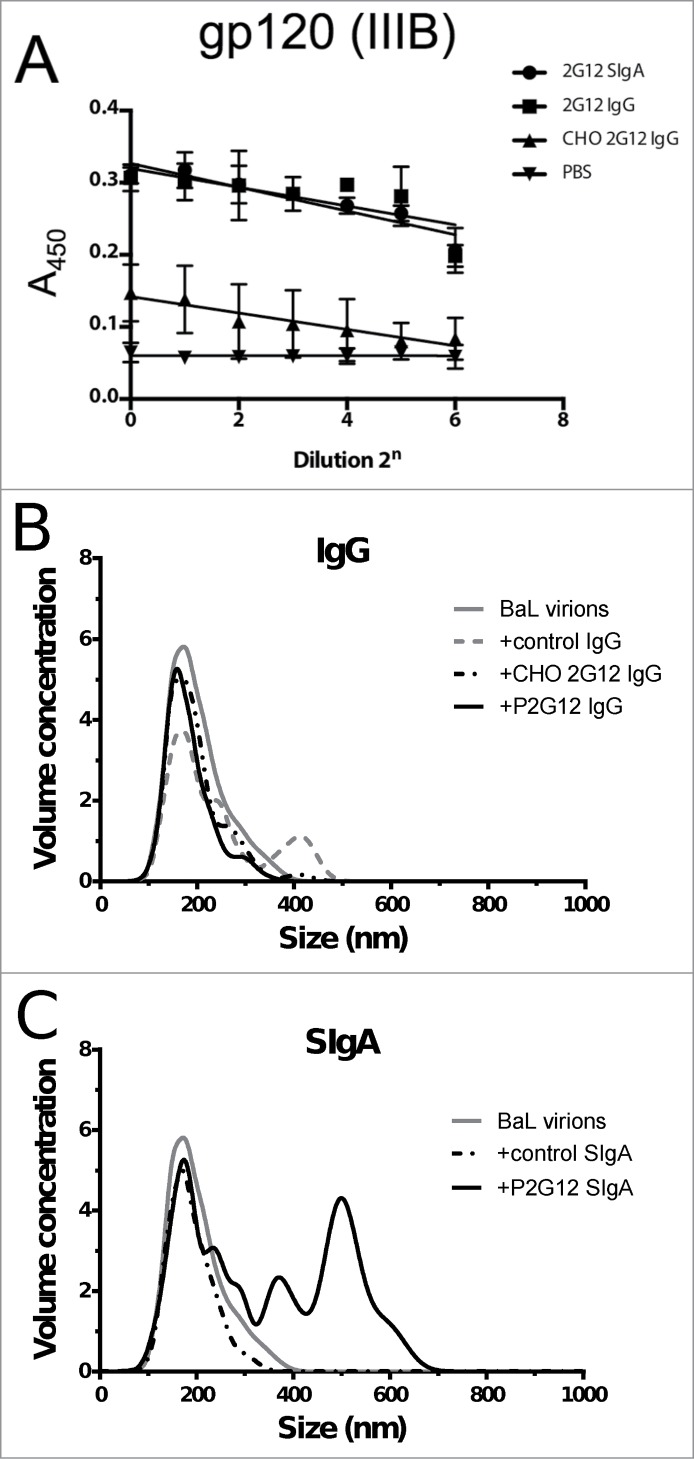
Plant 2G12 SIgA retains antigen binding and is capable of aggregating lab-adapted strains of HIV. (A) Functional ELISA showing binding of 2G12 antibodies expressed in plants and CHO cells to baculovirus-expressed HIV gp120 (clade IIIB) (B) Calculated particle size and relative volume concentration for BaL virions (gray line) in the presence of specific and non-specific control IgGs; CHO 2G12 IgG (black dots and dashes), Nt-derived 2G12 IgG (black line) and polyclonal IgG from human sera (gray dashes). Volume concentration is given by (# particles × 106) / ml (C) As (B), comparing aggregation in the presence of P2G12 SIgA (black line) and non-specific SIgA from human colostrum (black dots and dashes).
Table 2.
Neutralization potency of plant derived 2G12 IgG and SIgA against HIV pseudotype viruses. Calculated 50% Inhibitory Dose (ID50 titres) of P2G12 SIgA against 3 clade B HIV pseudotypes, TRO.11, SC422661.8 and PVO.4, categorized as moderately or unusually resistant to antibody-mediated neutralization.38 Murine leukemia virus (MuLV) pseudotyped virions were included as a control
| ID50 Titer (μg/ml) |
||
|---|---|---|
| Virus | 2G12 IgG | 2G12 SIgA |
| TRO.11 | 0.20 | 0.19 |
| SC422661.8 | 0.79 | 10.82 |
| PVO.4 | 0.48 | 2.44 |
| MuLV(neg.) | >20 | >20 |
Aggregation of pathogens by IgA complexes may contribute to effective microbicide activity by decreasing the diffusion coefficient of the resulting complexes. To test the ability of plant-produced 2G12 SIgA to aggregate HIV virions in vitro, we used nanoparticle tracking analysis (NTA), a technique that relates the Brownian motion of particles observed using an ultramicroscope to their size. A preparation of inactivated HIV virions (clade B, strain BaL) gave a monodispersed population with a mean Stokes radius (RH) of ∼200 nm (Fig. 5B). This population was not significantly affected by the addition of a control (non-HIV specific) monoclonal IgG or 2G12 IgG, produced in CHO cells or in transgenic tobacco. The standard deviation of the measurements taken for all samples was 40 nm. Control SIgA derived from human colostrum did not produce a measureable size shift, while plant-produced 2G12 SIgA preparations produced aggregates with a mean Stokes radius of ∼700 nm, Fig. 5C). Two independent preparations of 2G12 SIgA were tested, and in both cases the mean size of aggregates observed exceeded that of the 2G12 IgG preparation.
Interaction of P2G12 SIgA with cellular receptors
IgA interacts with the immune system through specific receptors, including CD89 / FcalphaR1 and Fcα/μR, and also through receptors with other characterized roles, including galectin-3 and CD71 / transferrin receptor.39 As the binding site on the α heavy chain for CD89 is typically occluded by the highly glycosylated secretory component, SIgA is considered to interact primarily with lectin receptors. For example, CD209 / DC-SIGN has been proposed as a sampling receptor for SIgA in Payer's Patches.40
To begin to build a picture of the interactions between P2G12 SIgA and receptors, we tested binding of P2G12 SIgA to CD89 and DC-SIGN. U937 cells positive for CD89 (CD89 panel, Fig. 6A) were incubated with 10 μg/ml aggregated, monomeric IgA (aIgA), 12.5 μg/ml human colostrum SIgA (SIgA) or 12.5 μg/ml P2G12 SIgA (PSIgA). The profile of a monomeric human IgA (10 μg/ml) is overlaid on each histogram for comparison. Different concentrations were used to provide the same molar amount of CD89 binding sites for each sample. Although aggregated monomeric IgA demonstrated binding to U937 cells (Fig. 6A top right panel), no significant binding of either colostrum or plant SIgA was observed (lower panels). DC-SIGN binding was tested using a receptor enzyme immunoassay (Fig. 6B). Here, human colostrum SIgA and P2G12 SIgA at 2 μg/ml gave a robust signal, while monomeric IgA produced a very weak signal. A human IgG did not bind DC-SIGN at this concentration.
Figure 6.
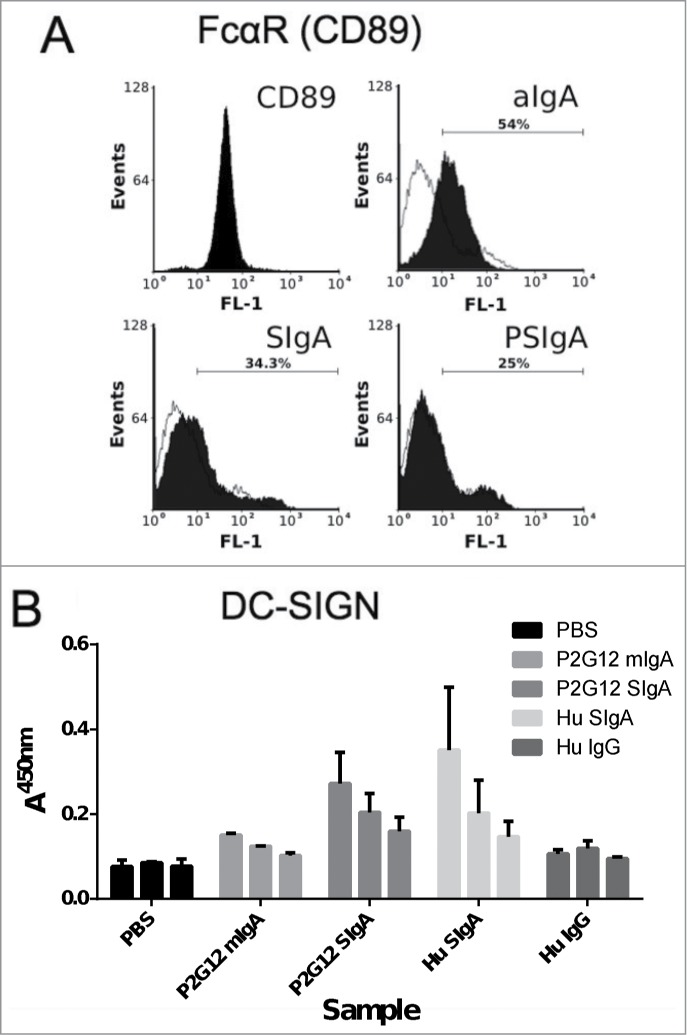
Interactions of P2G12 antibody with CD89 and CD209. (A) FACS histograms showing binding of IgA preparations to CD89/FcαRI expressed on U937 human monocytes. CD89 panel, staining with anti-human CD-89-FITC antibody; aIgA, aggregated human monomeric IgA; SIgA, human colostrum SIgA; PSIgA, mAb 2G12 SIgA. Stipled line, binding profile of monomeric IgA to U937 cells (B) Binding of plant-expressed 2G12 antibodies (starting concentration 2 μg/ml, 2-fold dilutions) and human colostrum derived SIgA (2 μg/ml) to DC-SIGN immobilised on EIA-optimised 96-well plates. Data shown is the mean (+sd) of 3 repeat experiments.
Stability of P2G12 SIgA in mucosal secretions
We compared the stability of P2G12 SIgA with an IgG preparation of the same antibody, using an in vitro spike-in experiment based on the addition of heterologous Ab to centrifuge-clarified human cervicovaginal mucus (CVM) (Fig. 7). P2G12 SIgA (50 μg) or IgG (50 μg) was added to 100 μl aliquots of a single mucus supernatant sample and incubated at 37°C before sampling at the times indicated. Time-point samples for both antibodies were then analyzed by SDS-PAGE and protein gel blotting using anti-human gamma and anti-human α antisera as appropriate. Data from 3 experiments (using cervico-vaginal secretions from 3 volunteers) is shown. As expected, some endogenous IgG was observed in the samples (IgG label, ‘SIgA’ lanes), but no endogenous SIgA could be detected (Fig. 7A ‘IgG’ lanes). Although the rates of degradation for both IgG and P2G12 SIgA varied between experiments when different mucus samples were used, the signal corresponding to intact IgG was lost at a consistently faster rate over the experimental time-course than that corresponding to intact P2G12 SIgA (Fig. 7B). The half-life of IgG was approximately 100 minutes, in contrast to P2G12 SIgA, which had a half-life of approximately 1000 minutes.
Figure 7.
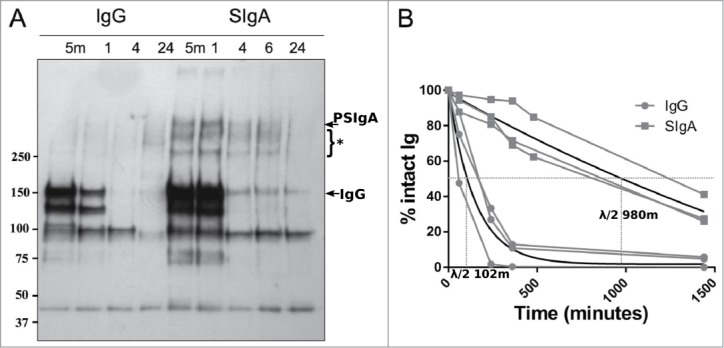
Stability of P2G12 SIgA in vaginal fluid. (A) Human cervicovaginal fluid was spiked with 50 μg P2G12 IgG (left) or P2G12 SIgA (right) and incubated at 37°C for the indicated time. Samples were analyzed by SDS-PAGE and immunoblotting using anti-kappa antisera. Intact PSIgA and total IgG are indicated by arrows. Bands labeled with an asterisk indicate degraded PSIgA detected in these samples. Three individual experiments were performed, and representative results from one experiment are shown (B) ‘% Intact Ig’ was calculated by expressing the intensity of the band corresponding to the intact antibody complex as a percentage of the intensity of the same band at the 0 minute time-point. Results from all 3 experiments are shown. Each individual experiment is shown (black lines). Gray lines, trendlines fitted using linear regression (GraphPad Prism, GraphPad Software). Half-lives for IgG and P2G12 SIgA calculated from the regression curve are shown.
Discussion
Formatting pathogen-neutralizing antibodies as SIgA complexes could confer several potential advantages to their use as mucosal passive immunotherapeutics and prophylactics in human disease.3 SIgA predominates at most mucosal surfaces in humans and is uniquely adapted to the mucosal environment, deriving multivalency from the dimer-promoting properties of the obligate J chain and enhanced protease resistance and reduced inflammatory properties from the subsequent association of the secretory component.4,41,42 Efficiently reconstituting the assembly of SIgA in heterologous mammalian cells using recombinant DNA has been challenging, leading to limited yields and ultimately prohibitive cost of goods for any envisaged application.43 Expression in plants has been proposed as an alternative system for the production of therapeutic SIgAs, as such platforms demonstrate the ability to correctly fold and assemble SIgA complexes and many approaches are cheap and readily scalable.12,27 Proof of concept for secretory antibody assembly was previously demonstrated in transgenic N. tabacum,12 but the relatively high proportion of assembly intermediates or degradation products was a significant barrier to clinical development. In that example, the antibody was a complex containing a chimeric murine gamma and α heavy chain, murine J chain and rabbit secretory component. In the present study, a human SIgA complex (P2G12 SIgA) was expressed. The assembly of this complex was much improved, and intact SIgA was the predominant form in all preparations. This compares to our previous report where an estimated 50% of Guy's13 SIgA/G was not assembled as a SIgA complex.12
A significant proportion of 2G12 SIgA recovered from the leaves of transgenic N. tabacum and agroinfiltrated N. benthamiana was fully assembled when analyzed by SDS-PAGE and western blot. This proportion was higher in the transgenic plant samples. This may reflect a physiological difference between the plant species, an effect of the time-course of expression, or a stress response in the infiltrated plants. During these experiments, it was noted anecdotally that plants that exhibited less signs of stress produced a greater proportion of assembled SIgA complexes. The development of binary vectors and cloning techniques that permit transgenesis of multiple genes at a single locus has the potential to greatly simplify the generation of these stable lines.13,44 The maximum yield of P2G12 SIgA observed here for transgenic plants was 15.2 μg/g leaf fresh weight, which is less than that reported for Guy's 13 SIgA/G (10–80 μg/g)14 and for a human SIgA against rotavirus VP8* produced in transiently-transfected plants using a combinatorial approach (32.5 μg/g leaf fresh weight).13 However, the proportion of fully assembled SIgA decamers was significantly higher in transgenics when compared to transient expression (76% vs 48%, P < 0.05). This is consistent with previous reports of ∼70% assembly for Guy's 13 SIgA (transgenic)30 and 33% for anti-VP8* SIgA (transient).13 The yields reported here are also competitive with than those previously reported for the production of SIgA in CHO cells.9,43,45
We previously observed that recombinant mAb 2G12 IgG is efficiently secreted from tobacco mesophyll protoplasts.46 Accordingly, 2G12 IgG has recently been observed to accumulate primarily within the intercellular spaces of tobacco leaf tissue.47 The subcellular localization of tobacco expressed mAb 2G12 IgA and SIgA complexes observed here is strikingly different to IgG. Both complexes were observed to accumulate in ∼400 nm vesicular structures in the cytosol, and to some extent in the vacuole, but not in the apoplast. The protein body-like structures were also labeled with the anti-2G12 idiotype reagent, indicating that they contain assembled and functional mAb 2G12 Fab or F(ab’)2 domains. The secretion of a chimeric secretory IgA/G complex, as well as the corresponding dimeric and monomeric forms (G13 IgA/G) have previously been observed to be retarded in comparison to the original IgG format,30 although the rate of secretion can be restored to equivalent or greater levels in tobacco protoplasts by the deletion of the 18 residue Ig α tailpiece essential for J chain binding.48 The results shown here for 2G12 SIgA are consistent with these previous findings. The retention of SIgA complexes within plant cells may require some consideration of appropriate DSP protocols to optimize yield and recovery of functional SIgA complexes.
We found P2G12 SIgA to be extensively glycosylated with a mixture of Golgi-modified and unmodified N-linked glycans. This is consistent with the observed accumulation of SIgA in PB-like structures derived from the ER. The N-glycan structures differ from those isolated from a preparation of SIgA from human colostrum,49 and are devoid of certain epitopes that have been implicated in binding to pathogenic microorganisms, for example sialic acid moieties that interact with S-fimbriated E. coli,50 Lewisb epitopes with Helicobacter pylori51 and Lewisx epitopes with Clostridium difficile.52
In the mammalian immune system, the proline-rich hinge region of IgA1 produced by most plasma cells is extensively modified with O-linked glycan structures. For P2G12 SIgA, there was evidence of conversion of proline to hydroxyproline in the hinge region. Hydroxyproline conversion is typical of plant cell wall proteins, and indicates exposure of P2G12 SIgA to prolyl hydroxylase, an enzyme enriched in the Golgi apparatus and protein bodies.53 Hydroxyproline conversion is often associated with the subsequent addition of galactose and arabinose sugar moieties,54 although such plant-specific glycans were not identified here.
Apart from interactions with the microbiome, glycans present on mammalian SIgA have established roles in the localization of the SIgA complex within the glycocalyx and in the interaction of SIgA with cellular receptors. SC glycans are known to mediate interactions with a component of the glycocalyx, as glycosylated, but not deglycosylated, SC reconstituted with dIgA was predominately found in the mucus layer of the nasal cavity after intranasal administration.55 Plant recombinant SIgA production, in combination with well-developed glycoengineering approaches available in plants, may help in the future to elucidate specific requirements for this interaction. We observed binding of plant SIgA to DC-SIGN/CD209 in a solid phase assay. DC-SIGN has been proposed as a sampling receptor for immune surveillance of the gut by SIgA.40,56 DC-SIGN is expressed on most myeloid cells, including a population of CX3CR1+ transepithelial DC resident in the lamina propria. SIgA bound to monocytes is typically endocytosed,40 but is not sufficient to activate the cell.57 In contrast, we did not observe binding to CD89/FcαRI on U937 monocytes. Although SC binding typically reduces the binding of dIgA for this receptor, interactions between SC glycans and Mac1/CD11b have previously been reported to stabilize SIgA binding to CD89 on granulocytes.58 Unstimulated U937 cells do not express high levels of the Mac1 complex,59 which may have restricted the binding of P2G12 SIgA observed here.
The glycosylation profile of P2G12 SIgA also has implications for the downstream purification of plant SIgA complexes. Purification of SIgA has often proved challenging, as SIgA does not typically bind commonly used antibody affinity matrices (protein A and protein G). Jacalin, a lectin capable of binding O-glycans, has previously been used to purify SIgA from endogenous sources.60 However, in view of the lack of O-glycosylation observed here, this approach is not suitable for plant-produced SIgAs. Protein L, as used in this work, represents a robust purification strategy for the subset of SIgAs containing binding determinants. Binding to protein L can be conferred to any IgA through a framework engineering approach.61
In a pseudovirus-neutralization assay, P2G12 SIgA retained its ability to neutralize resistant clade B envelopes (TRO.11, PVO.4 and SC422661.8); however, the potency of neutralization appeared to be reduced (compared with IgG) in 2 cases. As we observed equivalent neutralization potency for both antibody formats against the TRO.11 envelope, but reduced potency against PVO.4 and SC422661.8, it is possible that the formation of SIgA complexes might selectively interfere with binding of the antibody to certain envelopes. An IgM class-switched version of 2G12 produced in CHO cells produced uniformly lower IC50 values than the equivalent IgG, an effect ascribed to greater functional affinity for the epitope,62 but this effect was not observed here. The relative neutralization potency of SIgA and IgG formats of the same antibody will be an important area for future study.
We tested the potential of P2G12 SIgA as a passive immunotherapeutic by comparing its ability to aggregate HIV and persist in human CVM against P2G12 IgG. HIV aggregation is potentially desirable for a microbicide or prophylactic formulation as aggregated virions have a reduced diffusion coefficient through the mucus layer of the genital tract, and consequently are afforded fewer opportunities to initial infection.63 Specific antibodies of the SIgA format have been shown to impede transcytosis of HIV (BaL) virions more effectively than the equivalent IgG.64 Theoretically up to 4 virions may be bound by a single SIgA complex, as IgA displays spatially separated antigen-binding sites.65 We analyzed the Brownian motion of particles in antibody-virion mixtures, and identified multimerized virions in all samples containing P2G12 SIgA. These multimers were not seen in 2G12 IgG preparations. Importantly, we also did not observe any virion aggregation in the presence of a control SIgA purified from human colostrum. As non-specific SIgA failed to aggregate HIV virions, the observed aggregation is likely to be effected by paratope-mediated cross-linking rather than glycan-pathogen or glycan-mucin interactions.
Previous work has indicated that the addition of SC to dIgA confers increased resistance to trypsin66 and to intestinal proteases.41 We have previously shown that a plant-produced chimeric secretory antibody, Guy's 13 PSIgA/G, persisted in the human oral cavity for 3 times as long as IgG of equivalent specificity.14 Here, we observed that bands corresponding to fully assembled P2G12 SIgA persisted in human CVM for substantially longer than P2G12 IgG and endogenous IgG (approximately 10-fold improvement). CVM is typically acidic due to the presence of lactobacilli in healthy vaginal flora.67 We have previously observed that plant-produced mAb 2G12 IgG is lost after incubation at low pH.46 The stability of P2G12 SIgA in this experiment may reflect a lower sensitivity to pH extremes.
Antibody-based therapy and prophylaxis has an enormous potential for the treatment and management of infectious diseases that is yet to be realized due to the high cost of developing and producing these drugs, and the high doses that are required. Plant production platforms are well suited to meet the challenge of producing these agents, as they have the potential to reach the required scale while controlling manufacturing costs.68 Here, we show that plant-derived mAb 2G12 SIgA persists longer in CVM, retains the ability to neutralize HIV and, furthermore, effectively aggregates HIV virions. We tested 2 plant platforms for the expression of SIgA and found stable N. tabacum transgenics to accumulate greater quantities of fully assembled SIgA than transient expression in N. benthamiana leaves. Produced in this way, recombinant SIgA-based passive immunotherapies or prophylactics could represent extremely effective tools for the control of mucosal infection in the future.
Materials and Methods
DNA constructs
Class-switching of the human anti-HIV mAb 2G12 from IgG1 to IgA1 was achieved genetically, using splicing by overlap extension (SOE) PCR with 2 plasmid DNAs encoding either mAb 2G12 Ig gamma1 or an alpha1 chain and overlapping internal primers.
For the generation of transgenic plants, the DNA fragment encoding 2G12 alpha1 was amplified by PCR and inserted between the XhoI and EcoRI sites of the pMON530-based pL32 vector (conferring kanamycin resistance in plants). The pL32 vector contains a CaMV 35S promoter sequence and encodes for a signal peptide derived from a murine IgG1 gene upstream of the gene of interest (GenBank: BC018535.1). A nos terminator is present downstream of the GOI ORF. No further targeting sequences were added. A DNA fragment encoding the 2G12 kappa chain was cloned in a similar fashion. ORFs corresponding to human secretory component (GenBank accession BC110495, bases 81–1891) and human J chain (GenBank accession BC038982, bases 58–537) were inserted between the XhoI and EcoRI sites of either the pL32 vector or the pGREENII-based pNUTBAR vector (conferring bialaphos resistance in plants).
For transient expression of 2G12 SIgA1, ORFs encoding all 4 chains were inserted into the pEAQ-HT-DEST3 vector69 using Gateway® recombination methodology (Life Technologies, Paisley, Scotland).
Cultivation, transformation and crossing of N. tabacum plants
N. tabacum var. Petit Havana plants were cultivated in growth rooms at 28°C with a 16 h/8 h day/night cycle. Leaf disc transformations were performed on sterilized leaf samples as previously described70 using Agrobacterium tumefaciens strain GV3101 harboring either a pL32 expression vector or pNUTBAR supplemented with the pSOUP helper vector. Antibiotic selection of transgenic calli induced through hormone treatment was achieved by the addition of 200 μg/ml kanamycin (for pL32) or 5 μg/ml bialaphos (for pNUTBAR) to the plant growth media. Following the emergence of substantial leaf and root structures, transgenic plantlets (T0) were transferred to greenhouse conditions and self-crossed. The presence of the transgene in the T1 generation was confirmed by protein gel blot (α chain, kappa chain and SC) or PCR (J chain).
In order to breed a plant line containing 4 transgenic loci, T1 plants positive for α or kappa chain expression were sexually crossed and the progeny (T2) screened for the production of intact IgA by western blot. T2 plants expressing IgA were subsequently crossed with a T1 generation plant transgenic for SC and J chain genes, and the resulting T3 progeny screened again for the segregation of all 4 transgenes.
Transient transfection of N. benthamiana plants
N. benthamiana plants were germinated in Jiffy pots (Jiffy Products Int., Holland) and seedlings transferred to compost under the growth room conditions indicated above. Plants were allowed to grow for a period of 4–6 weeks before being used for agroinfiltration. Plants were infiltrated in batches using a vacuum chamber filled with an admixed Agrobacterium suspension containing equal proportions of bacteria harbouring pEAQ-HT plasmids corresponding to each chain of the SIgA complex. The final density of the bacterium in suspension was at an OD600 of 0.5. Infiltrated plants were returned to an isolated area of the growth room and cultivated for a period of 5 d.
Immunolocalization and microscopy
Tobacco leaves were fixed in 4% paraformaldehyde and 0.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4), and then embedded in resin as previously described.71 Ultrathin sections mounted on grids for electron microscopy or thin (1 μm) sections mounted on glass slides for fluorescence microscopy were pre-incubated in 5% (w/v) BSA in phosphate buffer (0,1M, pH 7.4) and then incubated with polyclonal antisera against the Ig α or kappa chain, respectively. An anti-2G12 idiotype mAb (Polymun, Vienna, Austria) was used to detect assembled 2G12 antibody moieties. For electron microscopy, sections showing silver interference were collected on copper grids and immunolocalization was carried out as previously described.71 The primary antisera were detected with secondary antibodies labeled with Alexa Fluor 488 for fluorescence microscopy. For electron microscopy secondary antibodies labeled with 10-nm or 15 nm gold particles were used, respectively. Following immunolocalization, ultra-thin sections were stained with 2 % (w/v) aqueous uranyl acetate. Observations were made using a FEI Tecnai G2 (FEI Europe B.V., Eindhoven, The Netherlands) and a Leica DM5500B (Leica Microsystems Handelsges.m.b.H, Vienna, Austria), respectively. For ER-staining, protoplasts were released from transgenic tobacco leaves and incubated with the fluorescent dye ER-TrackerTM (Invitrogen) at a concentration of 3.3 μM. Protoplasts were observed using the Leica SP5 CLSM.
Purification of Secretory IgA from plant extracts
N. tabacum and N. benthamiana leaf tissues were homogenized using a blender for 2× 45-second periods at high speed. The homogenate was subjected to an initial clarification step, being passed through 2 layers of Miracloth (Millipore UK Ltd.) and centrifuged at 20,000 g for 20 minutes at 10°C. The supernatant was then filtered through a 0.22 μm syringe filter before being applied to a 1 ml bed volume protein L-sepharose column at a flow rate of 1.5 ml/min. Bound antibody was eluted with 0.1 M glycine pH 2.5 and dialysed against 3 changes of ≥1000-fold excess PBS buffer over a minimum period of 48 hours before use in assays.
Immunoassays
SDS-PAGE and immunoblotting were performed using NuPAGE Novex® apparatus and 3–8% pre-cast tris-acetate or 4–12% bis-tris gels (Life Technologies, Paisley, Scotland). For SIgA detection, blots were probed with horseradish peroxidase (HRP)-labeled goat polyclonal anti-human IgA antiserum (A0295; Sigma, Poole, UK). For IgG detection, HRP labeled goat polyclonal anti-human IgG, Fcγ-specific antiserum was used (109–035–008; Jackson ImmunoResearch, Newmarket, UK).
Immunosorbant assays (indirect ELISA) used Nunc Maxisorp 96-well microtitre plates (Thermo Fisher Scientific, Waltham, MA, USA) coated with gp120 (NIBSC CFAR 0607) or CD209/DC-SIGN (161-DC-050, R&D Systems) at 1 μg/ml in carbonate buffer pH10. Binding of sample antibodies was detected using HRP-labeled sheep anti-human α chain antiserum, anti-human kappa antiserum or anti-human gamma antiserum (AP010, AP015 & AP004, The Binding Site, Birmingham, UK) as appropriate.
Binding of antibodies to FcalphaR expressed on U937 cells (human monocytes) was assessed by FACS using a FACScalibur (Becton Dickenson, Oxford, UK) instrument and a FITC-conjugated anti-human IgA antibody (F5259, Sigma).
Yield determination
For yield determination (expressed as μg protein per g leaf fresh mass), samples of pre-affinity chromatography filtrates were analyzed by sandwich ELISA using anti-α and HRP-conjugated anti-kappa antisera (as above). Signal corresponding to the concentration of assembled heavy and light chain complexes was compared to a standard curve derived from a quantified IgA standard (human colostrum IgA, I2636, Sigma), which had been observed to migrate at a size consistent with SIgA complexes in SDS-PAGE. The ELISA sensitivity for SIgA (human colostrum) was 5 ng/ml, and the assay gave linear responses over the range 100 ng/ml to 3500 ng/ml. Plant samples were titrated against diluent buffer (PBS) to ensure concentrations were calculated from within the linear range of the standard curve.
The proportion of the measured concentration consisting of SIgA decamers from 3 independent batches was then determined using SDS-PAGE (as above) followed by Coomassie staining and densitometric analysis (ChemiGenius2 and GeneTools, Syngene, Cambridge, UK).
Analysis of glycan structures by LC-ESI-MS
Purified SIgA complexes were reduced using 5 mM DTT in a 0.1 M ammonium bicarbonate buffer at 56°C for 45 min followed by S-carbamidomethylation using 25 mM iodoacetamide in a 0.1 M ammonium bicarbonate buffer pH 8.0 at 25°C for 30 min. After precipitation using 80% acetone at 20°C for 45 min, the protein was digested with 0.2 g trypsin in 0.1 M ammonium bicarbonate buffer overnight at 37°C. After deactivation of trypsin at 96°C for 6 min, the protein was digested with 0.2 g endoproteinase GluC in 0.1 M ammonium bicarbonate buffer overnight at 37°C. Glycopeptide analysis was performed using reverse phase liquid chromatography–electrospray ionization–mass spectrometry (LC-ESI-MS) as described previously.70,72
HIV-1 Env Pseudovirus Neutralization Assay
Virus neutralisation using a luciferase-based assay in TZM.bl cells was measured as previously described.38 The 50% inhibitory dose (ID50) was calculated using regression analysis and corresponds to the concentration of antibody that caused a 50% reduction in the bioluminescence of the target cells in comparison to control cells receiving only virus (200 TCID50).
Nanoparticle tracking analysis
A suspension of HIV virions (BaL strain, clade B, inactivated and prepared as described by Stieh et al.)73 at a dilution of approx. 1 μg/ml (corresponding to between 1–25 × 108 particles/ml) was monitored for aggregation in antibody solution of between 2 and 20 μg/ml. Aggregation was measured in 1× PBS using nanoparticle tracking analysis (NTA; Nanosight LM10-HS, Nanosight Ltd., Amesbury, Wiltshire). 60-second sample videos were analyzed using the “Nanoparticle Tracking Analysis 2.0 Analysis” software version 0125.
Collection of vaginal fluid samples and antibody spike-in experiments
The trial was conducted at the Vaccine Institute, St George's, University of London (SGUL). Ethical approval was gained from NRES committee London- City and East REC ref: 13/LO/0400. All volunteers gave fully informed written consent, and the trial was conducted according to the UK Clinical Trials Regulations and Good Clinical Practice guidelines. Vaginal mucus was collected from mid-menstrual cycle healthy volunteers using an Instead® SoftCup™ (Evofem Inc., San Diego, CA) menstrual cup which was worn for one day. Mucus was removed from the cup by centrifugation at 3,000× g in a 50 ml Falcon™ tube (BD Biosciences), and clarified by a further centrifugation at 5,000× g. Supernatants were collected and aliquoted into equal volumes before being mixed with 50 μg plant 2G12 SIgA or 50 μg IgG from human plasma in a volume of less than 10 μl of 0.1× PBS. Following the immediate collection of a 15 μl sample (the 0-minute time-point), antibody/mucus solutions were incubated at 37°C and sampled at 60, 150, 240 and 1440 minutes. Samples were analyzed by protein gel blotting under non-reducing conditions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The trial was conducted at the Vaccine Institute, St George's, University of London (SGUL). Ethical approval was gained from NRES committee London- City and East REC ref: 13/LO/0400. We gratefully acknowledge Polymun Scientific GmbH for providing access to 2G12 mAb, and reagents for this study. We are grateful to Dr. Mike Seaman, Beth Israel Deaconess Medical Center, Boston, through the Collaboration for AIDS Vaccine Discovery (CAVD) for performing HIV neutralisation assays.
Funding
Funding for this work was provided by the EU Pharma-Planta project, the ERC Future-Pharma project and the Hotung Foundation.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Corthesy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol 2013; 4:185; PMID:23874333; http://dx.doi.org/ 10.3389/fimmu.2013.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaetzel CS. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism. Immunology letters 2014; S0165-2478:00100-X; PMID:24877874; http://dx.doi.org/ 10.1016/j.imlet.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corthesy B. Recombinant secretory immunoglobulin A in passive immunotherapy: linking immunology and biotechnology. Curr Pharm Biotechnol 2003; 4:51-67; PMID:12570682; http://dx.doi.org/ 10.2174/1389201033378020 [DOI] [PubMed] [Google Scholar]
- 4.Bonner A, Almogren A, Furtado PB, Kerr MA, Perkins SJ. Location of secretory component on the Fc edge of dimeric IgA1 reveals insight into the role of secretory IgA1 in mucosal immunity. Mucosal Immunol 2009; 2:74-84; PMID:19079336; http://dx.doi.org/ 10.1038/mi.2008.68 [DOI] [PubMed] [Google Scholar]
- 5.Bonner A, Almogren A, Furtado PB, Kerr MA, Perkins SJ. The nonplanar secretory IgA2 and near planar secretory IgA1 solution structures rationalize their different mucosal immune responses. J Biol Chem 2009; 284:5077-87; PMID:19109255; http://dx.doi.org/ 10.1074/jbc.M807529200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandtzaeg P. Transport models for secretory IgA and secretory IgM. Clin Exp Immunol 1981; 44:221-32; PMID:6118214 [PMC free article] [PubMed] [Google Scholar]
- 7.Mostov KE, Blobel G. A transmembrane precursor of secretory component. The receptor for transcellular transport of polymeric immunoglobulins. J Biol Chem 1982; 257:11816-21; PMID:7118912 [PubMed] [Google Scholar]
- 8.Moldt B, Saye-Francisco K, Schultz N, Burton DR, Hessell AJ. Simplifying the synthesis of SIgA: combination of dIgA and rhSC using affinity chromatography. Methods 2013; 65:127-32; PMID:23811333; http://dx.doi.org/ 10.1016/j.ymeth.2013.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berdoz J, Blanc CT, Reinhardt M, Kraehenbuhl JP, Corthesy B. In vitro comparison of the antigen-binding and stability properties of the various molecular forms of IgA antibodies assembled and produced in CHO cells. Proc Nat Acad Sci U S A 1999; 96:3029-34; PMID:10077631; http://dx.doi.org/ 10.1073/pnas.96.6.3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chintalacharuvu KR, Morrison SL. Production of secretory immunoglobulin A by a single mammalian cell. Proc Nat Acad Sci U S A 1997; 94:6364-8; PMID:9177223; http://dx.doi.org/ 10.1073/pnas.94.12.6364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chintalacharuvu KR, Morrison SL. Residues critical for H-L disulfide bond formation in human IgA1 and IgA2. J Immunol 1996; 157:3443-9; PMID:8871643 [PubMed] [Google Scholar]
- 12.Ma JK, Hiatt A, Hein M, Vine ND, Wang F, Stabila P, van Dolleweerd C, Mostov K, Lehner T. Generation and assembly of secretory antibodies in plants. Science 1995; 268:716-9; PMID:7732380; http://dx.doi.org/ 10.1126/science.7732380 [DOI] [PubMed] [Google Scholar]
- 13.Juarez P, Huet-Trujillo E, Sarrion-Perdigones A, Falconi EE, Granell A, Orzaez D. Combinatorial analysis of secretory immunoglobulin a (sIgA) expression in plants. Int J Mol Sci 2013; 14:6205-22; PMID:23507755; http://dx.doi.org/ 10.3390/ijms14036205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma JK, Hikmat BY, Wycoff K, Vine ND, Chargelegue D, Yu L, Hein MB, Lehner T. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat Med 1998; 4:601-6; PMID:9585235; http://dx.doi.org/ 10.1038/nm0598-601 [DOI] [PubMed] [Google Scholar]
- 15.Ma JK, Lehner T, Stabila P, Fux CI, Hiatt A. Assembly of monoclonal antibodies with IgG1 and IgA heavy chain domains in transgenic tobacco plants. Eur J Immunol 1994; 24:131-8; PMID:8020548; http://dx.doi.org/ 10.1002/eji.1830240120 [DOI] [PubMed] [Google Scholar]
- 16.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A, et al. . Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retrov 1994; 10:359-69; PMID:7520721; http://dx.doi.org/ 10.1089/aid.1994.10.359 [DOI] [PubMed] [Google Scholar]
- 17.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol 1996; 70:1100-8; PMID:8551569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Roux KH, et al. . Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 2003; 300:2065-71; PMID:12829775; http://dx.doi.org/ 10.1126/science.1083182 [DOI] [PubMed] [Google Scholar]
- 19.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. Antibody protects macaques against vaginal challenge with a pathogenic R5 simianhuman immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol 2001; 75:8340-7; PMID:11483779; http://dx.doi.org/ 10.1128/JVI.75.17.8340-8347.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog 2009; 5:e1000433; PMID:19436712; http://dx.doi.org/ 10.1371/journal.ppat.1000433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watkins JD, Sholukh AM, Mukhtar MM, Siddappa NB, Lakhashe SK, Kim M, Reinherz EL, Gupta S, Forthal DN, Sattentau QJ, et al. . Anti-HIV IgA isotypes: differential virion capture and inhibition of transcytosis are linked to prevention of mucosal R5 SHIV transmission. AIDS 2013; 27:F13-F20; PMID:23775002; http://dx.doi.org/ 10.1097/QAD.0b013e328360eac6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehandru S, Vcelar B, Wrin T, Stiegler G, Joos B, Mohri H, Boden D, Galovich J, Tenner-Racz K, Racz P, et al. . Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J Virol 2007; 81:11016-31; PMID:17686878; http://dx.doi.org/ 10.1128/JVI.01340-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trkola A, Kuster H, Rusert P, Joos B, Fischer M, Leemann C, Manrique A, Huber M, Rehr M, Oxenius A, et al. . Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med 2005; 11:615-22; PMID:15880120; http://dx.doi.org/ 10.1038/nm1244 [DOI] [PubMed] [Google Scholar]
- 24.Morris GC. MABGEL 1: C2F5, C4E10 & C2G12 as a vaginal microbicide. PhD Thesis, University of Hull, 2012; https://hydra.hull.ac.uk/resources/hull:7126 [Google Scholar]
- 25.Virdi V, Depicker A. Role of plant expression systems in antibody production for passive immunization. Int J Dev Biol 2013; 57:587-93; PMID:24166441; http://dx.doi.org/ 10.1387/ijdb.130266ad [DOI] [PubMed] [Google Scholar]
- 26.Melnik S, Stoger E. Green factories for biopharmaceuticals. Curr Med Chem 2013; 20:1038-46; PMID:23210788 [PubMed] [Google Scholar]
- 27.Paul M, Ma JK. Plant-made pharmaceuticals: leading products and production platforms. Biotechnol Appl Biochem 2011; 58:58-67; PMID:21446960; http://dx.doi.org/ 10.1002/bab.6 [DOI] [PubMed] [Google Scholar]
- 28.Fischer R, Stoger E, Schillberg S, Christou P, Twyman RM. Plant-based production of biopharmaceuticals. Curr Opin Plant Biol 2004; 7:152-8; PMID:15003215; http://dx.doi.org/ 10.1016/j.pbi.2004.01.007 [DOI] [PubMed] [Google Scholar]
- 29.Ramessar K, Rademacher T, Sack M, Stadlmann J, Platis D, Stiegler G, Labrou N, Altmann F, Ma J, Stoger E, et al. . Cost-effective production of a vaginal protein microbicide to prevent HIV transmission. Proc Natl Acad Sci U S A 2008; 105:3727-32; PMID:18316741; http://dx.doi.org/ 10.1073/pnas.0708841104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frigerio L, Vine ND, Pedrazzini E, Hein MB, Wang F, Ma JK, Vitale A. Assembly, secretion, and vacuolar delivery of a hybrid immunoglobulin in plants. Plant Physiol 2000; 123:1483-94; PMID:10938364; http://dx.doi.org/ 10.1104/pp.123.4.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mainieri D, Rossi M, Archinti M, Bellucci M, De Marchis F, Vavassori S, Pompa A, Arcioni S, Vitale A. Zeolin. A new recombinant storage protein constructed using maize gamma-zein and bean phaseolin. Plant Physiol 2004; 136:3447-56; PMID:15502013; http://dx.doi.org/ 10.1104/pp.104.046409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roben PW, Salem AN, Silverman GJ. VH3 family antibodies bind domain D of staphylococcal protein A. J Immunol 1995; 154:6437-45; PMID:7759880 [PubMed] [Google Scholar]
- 33.Sandin C, Linse S, Areschoug T, Woof JM, Reinholdt J, Lindahl G. Isolation and detection of human IgA using a streptococcal IgA-binding peptide. J Immunol 2002; 169:1357-64; PMID:12133959; http://dx.doi.org/ 10.4049/jimmunol.169.3.1357 [DOI] [PubMed] [Google Scholar]
- 34.Eiffert H, Quentin E, Wiederhold M, Hillemeir S, Decker J, Weber M, Hilschmann N. Determination of the molecular structure of the human free secretory component. Biol Chem Hoppe-Seyler 1991; 372:119-28; PMID:1859628; http://dx.doi.org/ 10.1515/bchm3.1991.372.1.119 [DOI] [PubMed] [Google Scholar]
- 35.Field MC, Amatayakul-Chantler S, Rademacher TW, Rudd PM, Dwek RA. Structural analysis of the N-glycans from human immunoglobulin A1: comparison of normal human serum immunoglobulin A1 with that isolated from patients with rheumatoid arthritis. Biochem J 1994; 299 (Pt 1):261-75; PMID:8166649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novak J, Tomana M, Kilian M, Coward L, Kulhavy R, Barnes S, Mestecky J. Heterogeneity of O-glycosylation in the hinge region of human IgA1. Mol Immunol 2000; 37:1047-56; PMID:11399322; http://dx.doi.org/ 10.1016/S0161-5890(01)00019-0 [DOI] [PubMed] [Google Scholar]
- 37.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, et al. . Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 2005; 79:10108-25; PMID:16051804; http://dx.doi.org/ 10.1128/JVI.79.16.10108-10125.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, et al. . Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol 2010; 84:1439-52; PMID:19939925; http://dx.doi.org/ 10.1128/JVI.02108-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reljic R, Williams A, Ivanyi J. Mucosal immunotherapy of tuberculosis: is there a value in passive IgA? Tuberculosis 2006; 86:179-90; PMID:16510311; http://dx.doi.org/ 10.1016/j.tube.2006.01.011 [DOI] [PubMed] [Google Scholar]
- 40.Baumann J, Park CG, Mantis NJ. Recognition of secretory IgA by DC-SIGN: implications for immune surveillance in the intestine. Immunol Lett 2010; 131:59-66; PMID:20362001; http://dx.doi.org/ 10.1016/j.imlet.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crottet P, Corthesy B. Secretory component delays the conversion of secretory IgA into antigen-binding competent F(ab’)2: a possible implication for mucosal defense. J Immunol 1998; 161:5445-53; PMID:9820520 [PubMed] [Google Scholar]
- 42.Lindh E. Increased risistance of immunoglobulin A dimers to proteolytic degradation after binding of secretory component. J Immunol 1975; 114:284-6; PMID:1090649 [PubMed] [Google Scholar]
- 43.Johansen FE, Natvig Norderhaug I, Roe M, Sandlie I, Brandtzaeg P. Recombinant expression of polymeric IgA: incorporation of J chain and secretory component of human origin. Eur J Immunol 1999; 29:1701-8; PMID:10359125; http://dx.doi.org/ 10.1002/(SICI)1521-4141(199905)29:05%3c1701::AID-IMMU1701%3e3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- 44.Weber E, Engler C, Gruetzner R, Werner S, Marillonnet S. A modular cloning system for standardized assembly of multigene constructs. PloS one 2011; 6:e16765; PMID:21364738; http://dx.doi.org/ 10.1371/journal.pone.0016765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chintalacharuvu KR, Morrison SL. Production and characterization of recombinant IgA. Immunotechnol: Int J Immunol Eng 1999; 4:165-74; PMID:10231086; http://dx.doi.org/ 10.1016/S1380-2933(98)00012-8 [DOI] [PubMed] [Google Scholar]
- 46.Hehle VK, Paul MJ, Drake PM, Ma JK, van Dolleweerd CJ. Antibody degradation in tobacco plants: a predominantly apoplastic process. BMC Biotechnol 2011; 11:128; PMID:22208820; http://dx.doi.org/ 10.1186/1472-6750-11-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arcalis E, Stadlmann J, Rademacher T, Marcel S, Sack M, Altmann F, Stoger E. Plant species and organ influence the structure and subcellular localization of recombinant glycoproteins. Plant Mol Biol 2013; 83:105-17; PMID:23553222; http://dx.doi.org/ 10.1007/s11103-013-0049-9 [DOI] [PubMed] [Google Scholar]
- 48.Hadlington JL, Santoro A, Nuttall J, Denecke J, Ma JK, Vitale A, Frigerio L. The C-terminal extension of a hybrid immunoglobulin AG heavy chain is responsible for its Golgi-mediated sorting to the vacuole. Mol Biol Cell 2003; 14:2592-602; PMID:12808054; http://dx.doi.org/ 10.1091/mbc.E02-11-0771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Royle L, Roos A, Harvey DJ, Wormald MR, van Gijlswijk-Janssen D, Redwan el RM, Wilson IA, Daha MR, Dwek RA, Rudd PM. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem 2003; 278:20140-53; PMID:12637583; http://dx.doi.org/ 10.1074/jbc.M301436200 [DOI] [PubMed] [Google Scholar]
- 50.Schroten H, Stapper C, Plogmann R, Kohler H, Hacker J, Hanisch FG. Fab-independent antiadhesion effects of secretory immunoglobulin A on S-fimbriated Escherichia coli are mediated by sialyloligosaccharides. Infect Immun 1998; 66:3971-3; PMID:9673289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boren T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 1993; 262:1892-5; PMID:8018146; http://dx.doi.org/ 10.1126/science.8018146 [DOI] [PubMed] [Google Scholar]
- 52.Dallas SD, Rolfe RD. Binding of Clostridium difficile toxin A to human milk secretory component. J Med Microbiol 1998; 47:879-88; PMID:9788811; http://dx.doi.org/ 10.1099/00222615-47-10-879 [DOI] [PubMed] [Google Scholar]
- 53.Cohen PB, Schibeci A, Fincher GB. Biosynthesis of arabinogalactan-protein in lolium multiflorum (Ryegrass) endosperm cells: III. Subcellular distribution of prolyl hydroxylase. Plant Physiol 1983; 72:754-8; PMID:16663080; http://dx.doi.org/ 10.1104/pp.72.3.754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shpak E, Barbar E, Leykam JF, Kieliszewski MJ. Contiguous hydroxyproline residues direct hydroxyproline arabinosylation in Nicotiana tabacum. J Biol Chem 2001; 276:11272-8; PMID:11154705; http://dx.doi.org/ 10.1074/jbc.M011323200 [DOI] [PubMed] [Google Scholar]
- 55.Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthesy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 2002; 17:107-15; PMID:12150896; http://dx.doi.org/ 10.1016/S1074-7613(02)00341-2 [DOI] [PubMed] [Google Scholar]
- 56.Rochereau N, Drocourt D, Perouzel E, Pavot V, Redelinghuys P, Brown GD, Tiraby G, Roblin X, Verrier B, Genin C, et al. . Dectin-1 is essential for reverse transcytosis of glycosylated SIgA-antigen complexes by intestinal M cells. PLoS Biol 2013; 11:e1001658; PMID:24068891; http://dx.doi.org/ 10.1371/journal.pbio.1001658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heystek HC, Moulon C, Woltman AM, Garonne P, van Kooten C. Human immature dendritic cells efficiently bind and take up secretory IgA without the induction of maturation. J Immunol 2002; 168:102-7; PMID:11751952; http://dx.doi.org/ 10.4049/jimmunol.168.1.102 [DOI] [PubMed] [Google Scholar]
- 58.Van Spriel AB, Leusen JH, Vile H, Van De Winkel JG. Mac-1 (CD11bCD18) as accessory molecule for Fc alpha R (CD89) binding of IgA. J Immunol 2002; 169:3831-6; PMID:12244179; http://dx.doi.org/ 10.4049/jimmunol.169.7.3831 [DOI] [PubMed] [Google Scholar]
- 59.Miller LJ, Schwarting R, Springer TA. Regulated expression of the Mac-1, LFA-1, p150,95 glycoprotein family during leukocyte differentiation. J Immunol 1986; 137:2891-900; PMID:2428876 [PubMed] [Google Scholar]
- 60.Roque-Barreira MC, Campos-Neto A. Jacalin: an IgA-binding lectin. J Immunol 1985; 134:1740-3 [PubMed] [Google Scholar]
- 61.Boes A, Spiegel H, Delbruck H, Fischer R, Schillberg S, Sack M. Affinity purification of a framework 1 engineered mousehuman chimeric IgA2 antibody from tobacco. Biotechnol Bioeng 2011; 108:2804-14; PMID:21755499; http://dx.doi.org/ 10.1002/bit.23262 [DOI] [PubMed] [Google Scholar]
- 62.Wolbank S, Kunert R, Stiegler G, Katinger H. Characterization of human class-switched polymeric (immunoglobulin M [IgM] and IgA) anti-human immunodeficiency virus type 1 antibodies 2F5 and 2G12. J Virol 2003; 77:4095-103; PMID:12634368; http://dx.doi.org/ 10.1128/JVI.77.7.4095-4103.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, Hope TJ, Hanes J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J Virol 2009; 83:11196-200; PMID:19692470; http://dx.doi.org/ 10.1128/JVI.01899-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hocini H, Bomsel M. Infectious human immunodeficiency virus can rapidly penetrate a tight human epithelial barrier by transcytosis in a process impaired by mucosal immunoglobulins. J Infect Dis 1999; 179 Suppl 3:S448-53; PMID:10099117; http://dx.doi.org/ 10.1086/314802 [DOI] [PubMed] [Google Scholar]
- 65.Boehm MK, Woof JM, Kerr MA, Perkins SJ. The Fab and Fc fragments of IgA1 exhibit a different arrangement from that in IgG: a study by X-ray and neutron solution scattering and homology modelling. J Mol Biol 1999; 286:1421-47; PMID:10064707; http://dx.doi.org/ 10.1006/jmbi.1998.2556 [DOI] [PubMed] [Google Scholar]
- 66.Renegar KB, Jackson GD, Mestecky J. In vitro comparison of the biologic activities of monoclonal monomeric IgA, polymeric IgA, and secretory IgA. J Immunol 1998; 160:1219-23; PMID:9570537 [PubMed] [Google Scholar]
- 67.O’Hanlon DE, Moench TR, Cone RA. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PloS One 2013; 8:e80074; PMID:24223212; http://dx.doi.org/ 10.1371/journal.pone.0080074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Twyman RM, Schillberg S, Fischer R. Optimizing the yield of recombinant pharmaceutical proteins in plants. Curr Pharm Des 2013; 19:5486-94; PMID:23394567; http://dx.doi.org/ 10.2174/1381612811319310004 [DOI] [PubMed] [Google Scholar]
- 69.Sainsbury F, Thuenemann EC, Lomonossoff GP. pEAQ: versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol J 2009; 7:682-93; PMID:19627561; http://dx.doi.org/ 10.1111/j.1467-7652.2009.00434.x [DOI] [PubMed] [Google Scholar]
- 70.Teh AY, Maresch D, Klein K, Ma JK. Characterization of VRC01, a potent and broadly neutralizing anti-HIV mAb, produced in transiently and stably transformed tobacco. Plant Biotechnol J 2014; 12:300-11; PMID:24256218; http://dx.doi.org/ 10.1111/pbi.12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arcalis E, Marcel S, Altmann F, Kolarich D, Drakakaki G, Fischer R, Christou P, Stoger E. Unexpected deposition patterns of recombinant proteins in post-endoplasmic reticulum compartments of wheat endosperm. Plant Physiol 2004; 136:3457-66; PMID:15489278; http://dx.doi.org/ 10.1104/pp.104.050153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pabst M, Chang M, Stadlmann J, Altmann F. Glycan profiles of the 27 N-glycosylation sites of the HIV envelope protein CN54gp140. Biol Chem 2012; 393:719-30; PMID:22944675; http://dx.doi.org/ 10.1515/hsz-2012-0148 [DOI] [PubMed] [Google Scholar]
- 73.Stieh DJ, Phillips JL, Rogers PM, King DF, Cianci GC, Jeffs SA, Gnanakaran S, Shattock RJ. Dynamic electrophoretic fingerprinting of the HIV-1 envelope glycoprotein. Retrovirology 2013; 10:33; PMID:23514633; http://dx.doi.org/ 10.1186/1742-4690-10-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.