Abstract
Heavy metal pollution has became one of the realistic matters of globality. Previous reports indicated that heavy metals could significantly inhibit pollen germination and tube growth. In the present study, comparative studies on the effects of different heavy metals (As, Hg, Cd, Cr and Cu) on in-vitro picea wilsonii pollen gernimation and tube growth were carried out. Microscopic evaluation revealed that different heavy metals had various degree of toxicity on P. wilsonii pollen tube development. As showed the most toxic effects on pollen germination, which was followed by Hg and Cd, while Cr and Cu showed relatively lower toxicity. Besides, pollentubes showed varying shapes in response to different heavy metal stress. Pollen tubes treated with Cd, Hg and As were usually characterized by irregularly increasing diameters and swelling tips with distinct cytoplasimic vacuolation. On the other hand, except for the slightly increased diameters, no obvious abnormal shape were observed in tubes treated with Cr or Cu. Lyso-Tracker Green staining indicated that only Cd-treated pollen tubes showed numerous vacuole-like acidic organelles, though cytoplasmic vacuolization were also observed in pollen tubes treated with Hg and A. In brief, our data indicated that different heavy metals have various effects on Picea wilsonii pollen germination and tube growth, and that in-vitro pollen culture might be used as a competent system for biomonitoring of air pollution.
Keywords: heavy metal, Picea wilsonii, pollen, pollen tube
During the past few decades, heavy metal pollution has became one of the realistic matters of globality. Therefore, the effects of heavy metals on both animal and plant system have received considerable attention. It was reported that pollen grains of cross-pollination species were easy to accumulate high levels of heavy metals during their trips through the polluted air, and thus were proposed as a biomonitor of heavy metal pollution.1,2 Given that pollen grains of a large number of species can steadily germinate on a simple medium, and that heavy metals, such as Hg (mercury),3-5 Pb (plumbum),6 and Cd (cadmium)7,8 significantly inhibited pollen germination and tube growth, even at very low concentration, it was reasonable for us to speculate that in-vitro pollen germination and tube growth might be a more competent system for biomonitoring of air pollution. To further validate this speculation, the effects of different heavy metals, including Na2HAs4O.12H2O, HgCl2, CdCl2, CrCl3, and CuCl2, on in-vitro picea wilsonii pollen gernimation and tube growth were carried out in the present study.6,9
Microscopic evaluation revealed that different heavy metals had various degree of toxicity on P. wilsonii pollen tube development. As (arsenic), which is technically not a true metal, but a semi-metal, showed the most toxic effects on P. wilsonii pollen tube development. In fact, the germination rate of pollen grains cultured in standard germination medium for 20 h was nearly 75%. While the data of 2 μM As-treated samples was only 58.6% (p < 0.01). Previous reports indicated that Hg3-5 and Cd,8,10-12 strongly inhibited pollen germination and tube growth. In the present study, the germination rates of samples treated with 5 μM Hg and 10 μM Cd were 61.2% and 61.9%, respectively (p < 0.01), confirming the high toxicity of the 2 metals on pollen tube development. Gur and Topdemir10 reported that, when compared with Cd and Hg, Cu (copper) had the least effects on pollen germination and tube growth of Cydonia oblonga M and Prunus domestica L. Besides, Speranza et al.13 reported that effects of Cr (chromium) on pollen tubes of kiwifruit could be observed when 25 or 50 μM Cr were used. Here, our data indicated that, more than 60% pollen grains could still germinate when were treated with as high as 20 μM chromium and copper, respectively (p < 0.01), indicating that Cr and Cu did have relatively lower toxicity.
What more interesting, though pollen grains treated with different concentration of metals (2 μM As, 5 μM Hg, 10 μM Cd, 20 μM Cr, and 20 μM Cu) had similar germination rate, pollen tubes showed varying shapes in response to different heavy metal stress. As shown in Fig. 1, pollen tubes treated with Cd (Fig. 1B), As (Fig. 1D), and Hg (Fig. 1F) were usually characterized by irregularly increasing diameters and swelling tips with distinct cytoplasimic vacuolation, which were seldon observed in untreated tubes (Fig. 1A). These data is similar with previous studies,8,11 revealing the metal-induced disruption of tip growth in pollen tubes. On the other hand, though diameters of tubes treated with Cu or Cr slightly increased, neither obvious vacuoles, nor swelling tips could be observed in these tubes (Fig. 1C and E), indicating the maintaining of the tip growth in these samples.
Figure 1.

Effects of metals on the tube morphology. Pollen tubes were cultured in standard medium (A), or treated with 10 μM CdCl2 (B), 20 μM CuCl2 (C), 2 μM Na2HAs4O.12H2O (D), 20 μM CrCl3 (E), and 5 μM HgCl2 (F), respectively. DIC (Differential Interference Contrast) pictures were obtained using a ZEISS Axiovert 200 M microscope equipped with a Q imaging RETIGA-SRV CCD. Bars = 10 μm.
In the previous report, our data indicated that Cd markdly induced the formation of acidic vacuoles in pollen tubes.8 To further elucidate the possible relation between metal-induced cytoplasmic vacuolization and acidic organelles, Lyso-Tracker Green was utilized according to previous studies.8,14 Confocal observation indicated that Lyso-Tracker in untreated tubes was mainly accumulated in some small isolated punctate structures, indicating the existence of some small vacuoles with acid hydrolase (Fig. 2A). Similar results were observed in tubes treated with Cu (Fig. 2C) and Cr (Fig. 2E), indicating that both of the 2 metals did not significantly influence the quantity and distribution of acidic organelles in pollen tubes. On the other hand, numerous vacuole-like organelles with various diameters were labeled with Lyso-Tracker in Cd-treated tubes (Fig. 2B). While hardly any signal were detected in pollen tubes treated with As (Fig. 2D) and Hg (Fig. 2F), though cytoplasmic vacuolization were usually observed in both samples. These data indicated that Cd, but not all kinds of metals, promoted the formation of acidic vacuoles in pollen tubes. We might further speculate that pollen tubes might have multiform mechanisms to survive different metal stress. Given the mutisource of vacuole biogenisis,15 further researches on the mechanism of metal-induced cytoplasmic vacuolization were required.
Figure 2.
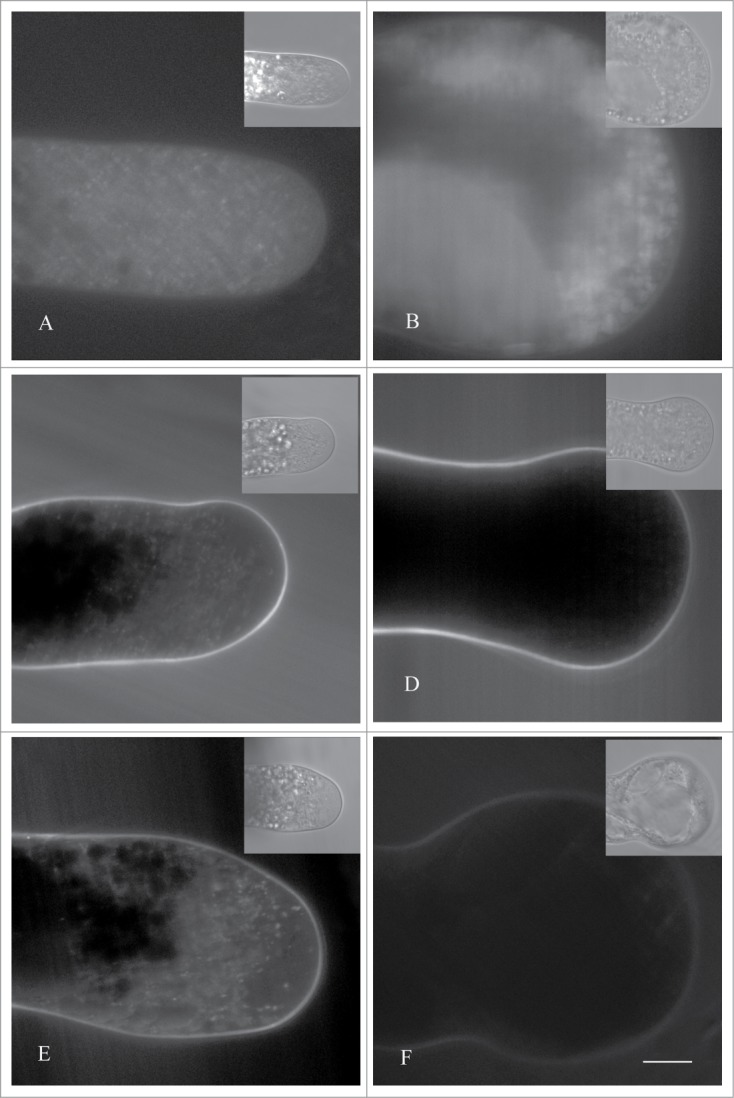
Effects of metals on the formation of acid organelles. Pollen tubes were cultured in standard medium (A), or treated with 10 μM CdCl2 (B), 20 μM CuCl2 (C), 2 μM Na2HAs4O.12H2O (D), 20 μM CrCl3 (E), and 5 μM HgCl2 (F), respectively, for 20 h. All samples were stained with 10 μM Lyso-Tracker Green (Invitrogen, final concentration) for 30 min, and then were examined using a Zeiss 5 Live laser scanning confocal microscope, with an excitation wavelength of 488 nm and emitted wavelength of BP 494–555 nm. Bars = 10 μm.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by Excellent Talent Training Project of Beijing (Grant No. 2012D005016000006), and the Natural Science Foundation of China (Grant No. 31371387).
References
- 1. Kalbande DM, Dhadse SN, Chaudhari PR, Wate SR. Biomonitoring of heavy metals by pollen in urban environment. Environ Monit Assess 2008. 138: 233-8; PMID:17593535; http://dx.doi.org/ 10.1007/s10661-007-9793-0 [DOI] [PubMed] [Google Scholar]
- 2. Calzoni GL, Antognoni F, Pari E, Fonti P, Gnes A, Speranza A. Active biomonitoring of heavy metal pollution using Rosa rugosa plants. Environ Pollut 2007. 149: 239-45; PMID:17321656; http://dx.doi.org/ 10.1016/j.envpol.2006.12.023 [DOI] [PubMed] [Google Scholar]
- 3. Munzuroglu O, Gur N. The effects of heavy metals on the pollen germination and pollen tube growth of apples (Malus sylvestris Miller cv. Golden). Turk J Biol 2000. 24:677-84. [DOI] [PubMed] [Google Scholar]
- 4. Tuna AL, Burun B, Yokas I, Coban E. The effects of heavy metals on pollen germination and pollen tube length in the tobacco plant. Turk J Biol 2002. 26: 109-113. [Google Scholar]
- 5. Sawidis T, Reiss HD. Effects of heavy-metals on pollen-tube growth and ultrastructure. Protoplasma 1995. 185: 113-122; http://dx.doi.org/10.1007/BF01272851 [Google Scholar]
- 6. Sheng X, Zhang S, Jiang L, Li K, Gao Y, Li X. Lead stress disrupts the cytoskeleton organization and cell wall construction during Picea wilsonii pollen germination and tube growth. Biol Trace Elem Res 2012. 146: 86-93; PMID:21947795; http://dx.doi.org/ 10.1007/s12011-011-9212-9 [DOI] [PubMed] [Google Scholar]
- 7. Xiong ZT, Peng YH. Response of pollen germination and tube growth to cadmium with special reference to low concentration exposure. Ecotox EnvironSafe 2001. 48:51-55; http://dx.doi.org/ 10.1006/eesa.2000.2002 [DOI] [PubMed] [Google Scholar]
- 8. Wang X, Gao Y, Feng Y, Li X, Wei Q, Sheng X. Cadmium stress disrupts the endomembrane organelles and endocytosis during Picea wilsonii pollen germination and tube growth. PLoS ONE 2014. 9: e94721; PMID:24722362; http://dx.doi.org/ 10.1371/journal.pone.0094721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheng XY, Hu ZH, Lu HF, Wang XH, Baluska F, Samaj J, Lin JX. Roles of the ubiquitin/proteasome pathway in pollen tube growth with emphasis on MG132-induced alterations in ultrastructure, cytoskeleton, and cell wall components. Plant Physiol 2006. 141: 1578-90; PMID:16778013; http://dx.doi.org/ 10.1104/pp.106.081703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gur N, Topdemir A. Effects of heavy metals (Cd, Cu, Pb, Hg) on pollen germination and tube growth of quince (Cydonia oblonga M.) and plum (Prunus domestica L.). Fresen Environ Bull 2005. 14:36-39. [Google Scholar]
- 11. Sawidis T. Effect of cadmium on pollen germination and tube growth in Lilium longiflorum and Nicotiana tabacum. Protoplasma 2008. 233:95-106; PMID:18709476; http://dx.doi.org/ 10.1007/s00709-008-0306-y [DOI] [PubMed] [Google Scholar]
- 12. Strickland RC. Cadmium uptake by Pinus resinosa Ait. Pollen and the effect on cation release and membrane permeability. Plant Physiol 1979. 64: 366-70; PMID:16660967; http://dx.doi.org/ 10.1104/pp.64.3.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Speranza A, Taddei AR, Gambellini G, Ovidi E, Scoccianti V. The cell wall of kiwifruit pollen tubes is a target for chromium toxicity: alterations to morphology, callose pattern and arabinogalactan protein distribution. Plant Biol 2009. 11:179-193; PMID:19228325; http://dx.doi.org/ 10.1111/j.1438-8677.2008.00129.x [DOI] [PubMed] [Google Scholar]
- 14. Sheng X, Wei Q, Jiang L, Li X, Gao Y, Wang L. Different degree in proteasome malfunction has various effects on root growth possibly through preventing cell division and promoting autophagic vacuolization. PLoS ONE 2012. 7:e45673; PMID:23029176; http://dx.doi.org/ 10.1371/journal.pone.0045673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marty F. Plant vacuoles. Plant Cell 1999. 11: 587-600; PMID:10213780; http://dx.doi.org/ 10.1105/tpc.11.4.587 [DOI] [PMC free article] [PubMed] [Google Scholar]


