Abstract
Study Objectives:
Idiopathic rapid eye movement (REM) sleep behavior disorder (RBD) is a harbinger of synuclein-mediated neurodegenerative diseases. It is unknown if this also applies to isolated REM sleep without atonia (RWA). We performed a long-term follow-up investigation of subjects with isolated RWA.
Methods:
Participants were recruited from 50 subjects with isolated RWA who were identified at the sleep laboratory of the Department of Neurology at the Medical University of Innsbruck between 2003 and 2005. Eligible subjects underwent follow-up clinical examination, polysomnography, and assessment of neurodegenerative biomarkers (cognitive impairment, finger speed deficit, impaired color vision, olfactory dysfunction, orthostatic hypotension, and substantia nigra hyperechogenicity).
Results:
After a mean of 8.6 ± 0.9 y, 1 of 14 participating subjects (7.3%) progressed to RBD. Ten of 14 RWA subjects (71.4%) were positive for at least one neurodegenerative biomarker. Substantia nigra hyperechogenicity and presence of mild cognitive impairment were both present in 4 of 14 subjects with isolated RWA. Electromyographic activity measures increased significantly from baseline to follow-up polysomnography (“any” mentalis and both anterior tibialis muscles: 32.5 ± 9.4 versus 52.2 ± 16.6%; p = 0.004).
Conclusion:
This study provides first evidence that isolated RWA is an early biomarker of synuclein-mediated neurodegeneration. These results will have to be replicated in larger studies with longer observational periods. If confirmed, these disease findings have implications for defining at-risk cohorts for Parkinson disease.
Citation:
Stefani A, Gabelia D, Högl B, Mitterling T, Mahlknecht P, Stockner H, Poewe W, Frauscher B. Long-term follow-up investigation of isolated rapid eye movement sleep without atonia without rapid eye movement sleep behavior disorder: a pilot study. J Clin Sleep Med 2015;11(11):1273–1279.
Keywords: biomarker, EMG activity, polysomnography, REM sleep behavior disorder, SINBAR
Rapid eye movement (REM) sleep without atonia (RWA) is the polysomnographic (PSG) hallmark of REM sleep behavior disorder (RBD), a condition characterized by repeated dream-enactment behaviors potentially resulting in injury of the patient or the bedpartner.1 A correct diagnosis of RBD has prognostic implications as long-term follow-up investigations of idiopathic RBD (iRBD) cohorts have shown, that up to 80% of subjects will eventually develop a synuclein-mediated neurodegenerative disease.2–4
RWA is defined as sustained muscle activity in REM sleep in the chin electromyography (EMG) and/or excessive transient muscle activity during REM sleep in the chin or limb EMG.5 Previous studies quantified RWA in order to define cutoff values and to allow a correct diagnosis of RBD.6–8 The SINBAR (Sleep Innsbruck Barcelona) group proposed cutoff values of 15% for phasic, 18% for “any”, and 9.6% for tonic chin EMG activity.7
RWA can also be incidentally observed in PSGs of patients with no history of dream-enacting behavior or relevant visible movements in video-PSG (v-PSG). Whether the proposed EMG cutoff values7 are also suitable for the definition of RWA is not definitely resolved. Normative values exceeding the 90th percentile resulted in similar theoretical cutoff values for RWA.8 In addition, a recent study of our group demonstrated that at least some of the subjects with isolated RWA meet the quantitative EMG criteria for RBD diagnosis.9
BRIEF SUMMARY
Current Knowledge/Study Rationale: Isolated rapid eye movement (REM) sleep without atonia (RWA) might be an even earlier stage of synuclein-mediated neurodegeneration than REM sleep behavior disorder (RBD). To date, no data on isolated RWA progression over time are available.
Study Impact: This is the first long-term follow-up study in subjects with isolated RWA. We provide first evidence for neurodegeneration in isolated RWA, as shown by a progression of RWA over time, a positivity in neurodegenerative biomarkers, and a reported conversion rate to RBD in isolated RWA subjects.
Although it has been proposed that RWA is a precursor of RBD and—by implication—a potential prodromal manifestation of neurodegenerative synucleinopathies, no study so far investigated its long-term course. The hypothesis that RWA is indeed a potential neurodegenerative biomarker is further supported by its progressive course in patients with RBD,10 as well as the finding that the loss of REM atonia predicts the occurrence of future neurodegenerative disease.11 It is unknown, however, whether neurodegenerative biomarkers such as olfactory dysfunction,2,12–16 orthostatic hypotension,13,17–20 impaired color vision,13,15 neurocognitive deficits,21–24 or substantia nigra (SN) hyperechogenicity25 also associate with isolated RWA.
The aim of this study was to perform the first long-term follow-up evaluation of patients with an isolated finding of RWA, in order to examine the proportion of RWA subjects who will develop de novo RBD with or without parkinsonism, perform an investigation of neurodegenerative biomarkers in RWA, and assess RWA progression.
METHODS
Selection of the Cohort of Subjects with Isolated RWA
All subjects were recruited from an existing cohort of 50 subjects with an isolated finding of qualitative RWA diagnosed at the sleep laboratory, Department of Neurology, Medical University of Innsbruck, between the years 2003 and 2005. Information on the original cohort as well as detailed inclusion and exclusion criteria are described elsewhere.9 RWA was defined according to current diagnostic criteria1 as an increased amount of phasic or tonic EMG activity in either the chin or extremities in the absence of both a history suggestive for RBD and RBD-specific behavioral manifestations as documented in the v-PSG.
The major inclusion criterion was a follow-up interval ≥ 7 y. Exclusion criteria were presence of any relevant psychiatric or neurological disease, and use of medication acting on the central nervous system or potentially inducing RBD. Eligible patients were invited for a follow-up visit. Patients who had at follow-up PSG an apnea-hypopnea index (AHI) > 15/h were excluded. Patients with sleep-related breathing disorders (SRBD) under effective treatment with nasal continuous positive airway pressure (nCPAP) therapy were included.
This study was approved by the local ethical committee of Innsbruck Medical University. All participants granted written informed consent prior to study participation.
Clinical Follow-Up Visit
All eligible RWA subjects underwent a clinical visit between 2012 and 2014. It comprised a detailed history including RBD-specific questionnaires (RBD single question screen, Innsbruck RBD inventory),26,27 and questions focused on environmental RBD risk factors.28 Neurological examination, including the unified Parkinson disease (PD) rating scale motor score (UPDRS III), was performed by two trained raters.
Assessment of Neurodegenerative Biomarkers
All participants underwent a test battery of neurodegenerative biomarkers including the Montreal Cognitive Assessment scale (MoCA),22 the Purdue pegboard test,13 the Farnsworth-Munsell 100 (FM-100) Hue color vision test,29 the University of Pennsylvania smell identification test (UPSIT),30 the orthostatic standing test, and a transcranial sonography.
MoCA
This test is a validated scale demonstrated to be more sensitive for cognitive impairment in iRBD than the Mini-Mental State Examination.22 For assessment of mild cognitive impairment, the cutoff was set at 26.22
Purdue Pegboard Test
This test is one of hand dexterity, motor speed, and fingereye coordination. Subjects performed separately for each hand and then bimanually different items, consisting of transferring a series of pins from a dish into corresponding holes, with three 30-sec trials for each item. The outcome measure was the average number of pins placed in the three conditions.13
FM-100 Hue Color Vision Test
Each subject was first tested for color blindness using 24 pseudoisochromatic cards. Only subjects without color vision blindness can perform the FM-100 Hue test.29 Both color blindness and color vision tests were performed under a standard light of D65 (UnityColor Light2go, Torso-Verlag, Wertheim and Herbert Waldmann GmbH & Co. KG, Villingen-Schwenningen). The color vision test consists of 85 sortable colored discs spanning the color spectrum, divided into four components (red-yellow, yellow-green, green-blue, and blue-red). For each component, there are two anchor discs of different colors. The examiner scrambled the discs, and asked the subjects to place them in the correct order. The total error score was calculated using the Farnsworth-Munsell test scoring software. An error score > 125% of the age-expected average was considered indicative of color vision deficit.15
UPSIT
Odor discrimination was assessed with the 40-item UPSIT (UPSIT-40),30 consisting of 40 scratch-and-sniff pads. Patients were instructed to choose the correct odor from four options. The cutoff score for olfactory dysfunction was set at 80% of the sex- and age-expected average.13
Orthostatic Standing Test
After a 10 min resting time in the supine position, the participants were asked to stand up and remain in standing position for 5 min. Orthostatic hypotension was defined as drop of systolic blood pressure ≥ 20 mmHg and/or diastolic blood pressure ≥ 10 mmHg.31
Transcranial Sonography
This procedure was performed using a 2.5-MHz transducer (Logiq 7; General Electric, Milwaukee, WI, USA). The penetration depth was 16 cm, the dynamic range 45–50 dB. Image brightness and time gain were adapted as needed for best visualization. The examination was performed from both sides using the temporal approach. Areas of echogenicity were manually encircled on digitally stored images, and the total area of echogenic signals in the SN region was measured as described elsewhere.32 Hyperechogenicity was defined as an area of echogenic signal ≥ 0.18 cm2 on at least one side.33
Baseline and Follow-Up V-PSG
All subjects underwent v-PSG according to current standards.5 V-PSG consisted of electrooculography, electroencephalography (F3, F4, C3, C4, O1, O2, M1 and M2 electrodes), cardiorespiratory recording [single channel electrocardiography, recording of nasal air flow (thermocouple), nasal pressure cannula, tracheal microphone, thoracic and abdominal respiratory movements (piezo), transcutaneous oxygen saturation], EMG of the mental, submental, both anterior tibialis (AT), and both flexor digitorum superficialis (FDS) muscles, and time-synchronized digital videography. The video was recorded with an infrared camera (Elbex Inc. EX series, Regensburg, Germany).
Sleep was scored according to American Academy of Sleep Medicine (AASM) criteria5 with the allowance to score REM sleep despite excessive chin EMG activity.7,8 REM sleep onset was determined by the occurrence of the first REM in the electrooculographic channel, and the end of REM sleep when either no REM was detected in 3 consecutive min or an arousal, K-complexes, or spindles were observed.7,8 V-PSG during REM sleep was carefully looked through for the presence of movement or vocalizations suggestive for RBD.1
Periodic leg movements in sleep (PLMS) were scored according to AASM criteria5 and indices were calculated for NREM, REM, and total sleep. EMG activity during REM sleep in RWA subjects at baseline and follow-up PSG and in controls was manually analyzed by one trained scorer, who was blind to patient status and time point of investigation. Before analysis, EMG activity during REM sleep was accurately checked for artifacts; all mini epochs containing artifacts preventing correct scoring were excluded from analysis. PLMS during REM sleep were identified by their characteristic morphology and periodicity, and excluded from quantitative analysis of phasic EMG activity.7,8 EMG activity during REM sleep was manually quantified according to SINBAR criteria7 in the mentalis and both AT muscles at baseline and also in both FDS muscles at follow-up PSG. Each EMG channel was analyzed separately. Percentages of phasic, tonic, and “any” EMG activities were calculated.
Statistics
IBM SPSS 21 (SPSS, Inc., Chicago, IL, USA) was used for all statistical analysis. Data were tested for normal distribution using the Shapiro-Wilk test. Descriptive statistics are given as numbers (percentages) as well as means ± standard deviations, as data are normally distributed. Independent and dependent t-tests, as applicable, were performed. Any p values less than 0.05 were considered significant. In case of multiple comparisons, Bonferroni correction was performed, and p values were set accordingly.
RESULTS
RWA Subjects
Fourteen subjects (13 men) of the initial isolated RWA sample of 50 patients participated in this study; 36 subjects did not participate, as they fulfilled exclusion criteria (n = 15), denied study participation (n = 13), or were lost to follow-up (n = 8). Baseline RWA percentages for “any”, phasic and tonic mentalis EMG activity, phasic EMG activity in both AT, and their combination did not differ between RWA subjects who participated and those who did not participate (data not shown, all p > 0.05).
The mean age of the investigated isolated RWA subjects at follow-up was 62.5 ± 12.8 y, and the mean follow-up interval was 8.6 ± 0.9 y. The primary reasons for baseline PSG examination were suspected SRBD (n = 5, 35.7%), control of nCPAP therapy in SRBD (n = 5, 35.7%), excessive daytime sleepiness (n = 3, 21.4%) and insomnia (n = 1, 7.1%).
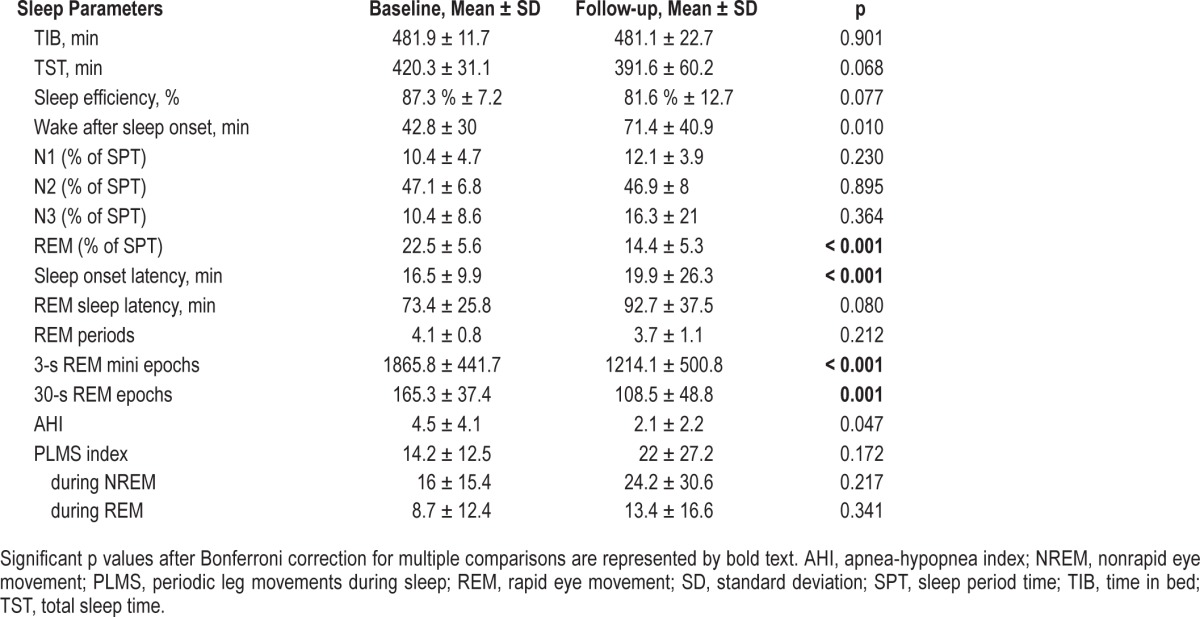
Table 1 provides the sleep parameters at baseline and follow-up PSG. AHI and PLMS indices did not differ across both investigations after Bonferroni correction (AHI: p = 0.047, PLMS: all p > 0.05).
Table 1.
Polysomnographic parameters of the study sample with isolated RWA at baseline and follow-up.
Clinical Visit
At follow-up, RBD developed in 1 of 14 isolated RWA subjects (7.1%) with an RBD suggestive history, a positive Innsbruck RBD-Inventory score of 0.6, and observed violent movements during REM sleep in the v-PSG. None of the isolated RWA subjects developed manifest parkinsonism. The mean UPDRS III score was 0.6 ± 0.9. None of the risk factors for RBD (head injury, smoking, farming, welding, pesticide exposure) were frequent among the subjects with isolated RWA.
Biomarkers of Neurodegeneration
Neurodegenerative biomarkers results are provided in Table 2. Ten subjects with isolated RWA (71.4%) were positive for at least one biomarker. The subject who developed RBD had hyposmia.
Table 2.
Assessment of neurodegenerative biomarkers in the subjects with isolated RWA.
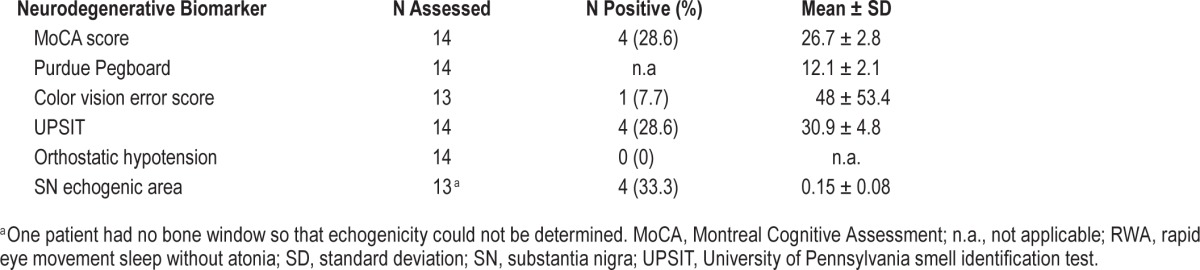
With respect to the individual biomarkers, four of 14 cases of the isolated RWA group (28.6%) had mild cognitive dysfunction, and four of 12 cases (33.3%) had SN hyperechogenicity. The eight cases in the isolated RWA group who exceeded the SINBAR cutoff values for RBD at baseline showed comparable results (see Table S1, supplemental material).
Quantitative Assessment of EMG Activity
Table 3 and Figure 1 provide the baseline and follow-up EMG activity measures as well as the number of subjects exceeding SINBAR cutoff values for RBD.7
Table 3.
REM-related EMG activity measures in the subjects with isolated RWA at baseline and follow-up.
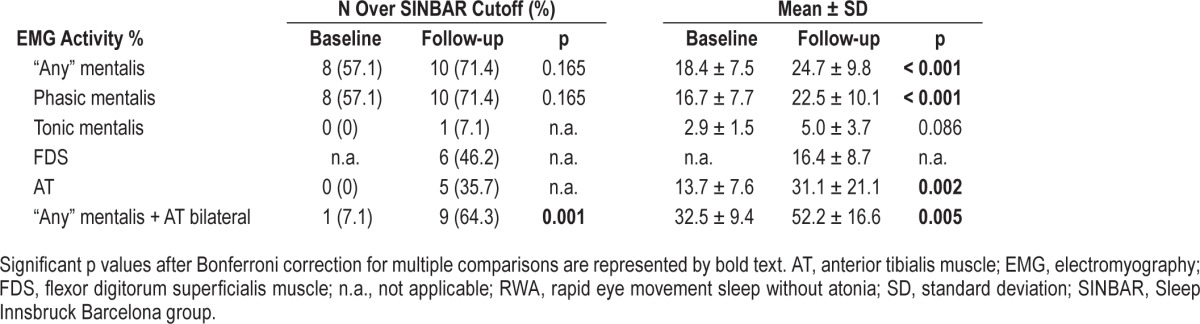
Figure 1.
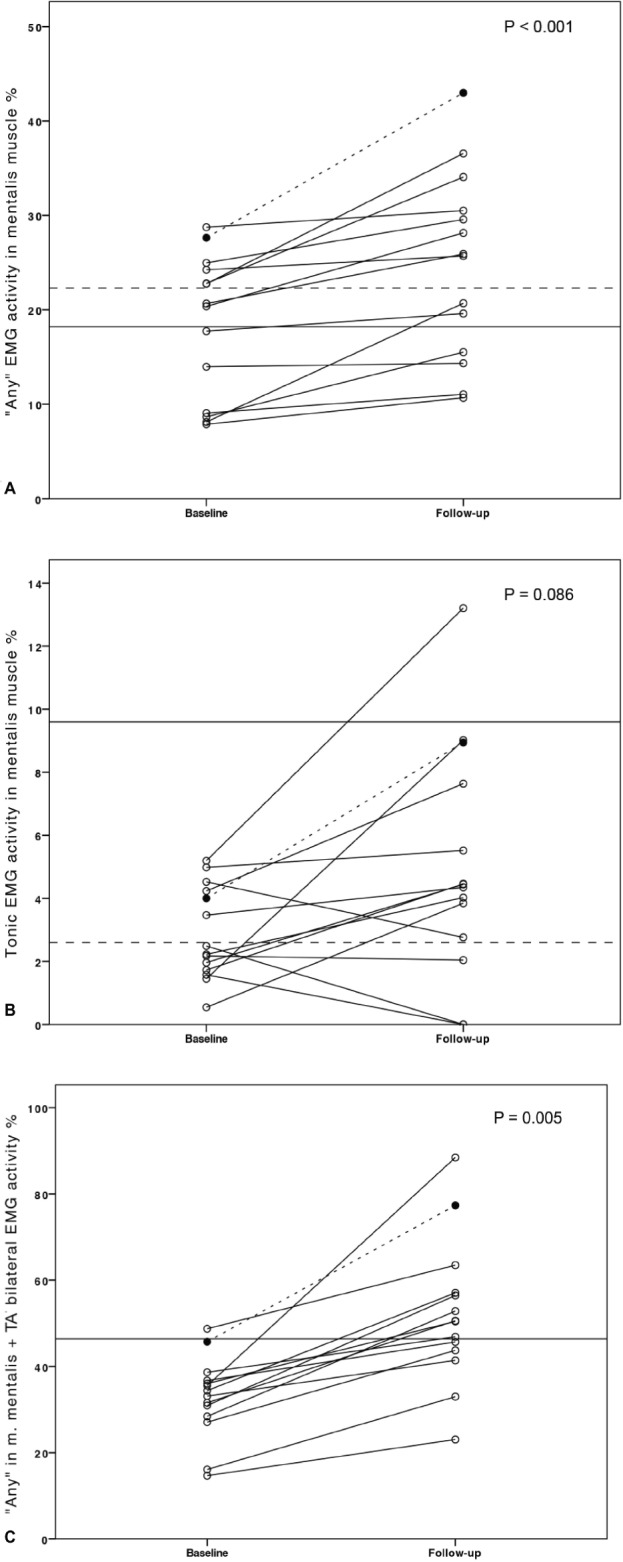
Individual “any” (A) and tonic (B) mentalis EMG activity indices and EMG activity indices for the combination of “any” in mentalis muscle and both anterior tibialis muscles (C) in the isolated RWA subjects at baseline and follow-up. Circles represent individuals. The subject in whom RBD developed is presented as a filled black circle, with dotted line. Horizontal solid lines: SINBAR RBD cutoff-values;7 dotted lines 90th percentile of normative values.8 AT, anterior tibialis muscle; EMG, electromyography; SINBAR, Sleep Innsbruck Barcelona group; RBD, rapid eye movement sleep behavior disorder; RWA, rapid eye movement sleep without atonia.
Except for tonic EMG activity, all measured types of EMG activity in the mentalis and both AT muscles increased over the observational period. Eight of the subjects with isolated RWA exceeded the SINBAR cutoff values for RBD at baseline. Overall, the number of subjects exceeding the cutoff values also increased over the observational period. This comparison reached statistical significance for the combination of “any” mentalis and phasic in both AT muscles EMG activity (p = 0.004), but not for EMG activities in the single muscles (all p > 0.05). Similar results were observed when regarding only the eight subjects exceeding the SINBAR cutoff values for RBD at baseline (see Table S2, supplemental material). The subject in whom RBD developed was over the SINBAR cutoff for phasic and “any” in mentalis muscle at baseline, and for all indices except tonic EMG activity in the mentalis muscle at follow-up.
DISCUSSION
This is the first long-term follow-up investigation in subjects with an isolated finding of RWA, including assessment of bio-markers for synuclein-mediated neurodegenerative disease and v-PSG. Major findings are that neurodegenerative biomarkers are positive in a substantial number of subjects with isolated RWA, and that REM-related EMG activity measures increase with time in subjects with isolated RWA.
RWA as Precursor of RBD
In this study 1 of 14 subjects with isolated RWA (7.1%) met criteria for RBD at follow-up. This percentage would be higher than the reported RBD prevalence of 0.38%–2.1% in the general population,34,35 and would support that RWA is a precursor of RBD. Nevertheless, the small sample size in this study precludes firm conclusions. Interestingly, in one additional patient of the isolated RWA cohort, RBD developed after the follow-up visit. This patient also complained about memory impairment.
Neurodegenerative Biomarkers are Positive in a Substantial Number of Subjects with Isolated RWA
We found a positivity in at least one neurodegenerative bio-marker in most of the subjects with isolated RWA, although none of the neurodegenerative biomarkers alone seemed to predict a higher risk of conversion to RBD. This could be explained by the protean manifestation of synuclein-mediated neurodegenerative disorders, with different structures involved in different patients and over time. Therefore, a varied association with neurodegenerative biomarkers should be expected.
Concerning individual biomarkers, the most frequently positive were mild cognitive impairment and SN hyperechogenicity. Cognitive impairment has been demonstrated in patients with iRBD, including impairment in attention, verbal memory, and executive functions.21 In our cohort, we found scores below the cutoff on the MoCA, which has been validated in patients with RBD.22
Abnormal hyperechogenicity of the SN pars compacta is present in 80% to 90% of patients with PD.36 It is present early in the disease, and shows no progression with time.37 We found SN hyperechogenicity in 33.3% of subjects with isolated RWA. This number is increased compared to that of healthy normals.36
The subject in whom RBD developed at follow-up had hyposmia. Olfactory dysfunction has been demonstrated in 40% to 60% of patients with RBD.13,14 A significant difference in ol-factory performance has been reported between patients with iRBD in whom parkinsonism developed compared to those in whom parkinsonism did not develop in a 5-year follow-up.16 In the current study, we did not find an increased prevalence of olfactory dysfunction among subjects with isolated RWA. Further studies investigating a combination of biomarkers in larger groups of RWA subjects are needed.
Loss of REM Sleep Atonia Progresses Over Time
We found a significant increase in all REM sleep EMG activity measures except for tonic chin EMG activity over the observational period. An increase in both tonic and phasic EMG activity with time was already described in patients with iRBD10 and an increase in tonic but not phasic chin EMG activity was reported in patients with RBD in whom a neurodegenerative disease later developed.11 Different pathophysiological mechanisms may underlie the presence of excessive tonic and phasic EMG activity during REM sleep. Tonic EMG activity seems to reflect degeneration of the sublaterodorsal nucleus in the brainstem, with subsequent disinhibition of spinal motoneurons eventually resulting in increased EMG activity during REM sleep.38 Phasic EMG activity seems to depend on activation of brainstem locomotor generators during REM sleep and on an alteration of pathways in the intermediate ventromedial medulla.39 Degeneration of the sublaterodorsal nucleus, resulting in increased tonic EMG activity during REM sleep, could be more closely linked with an increased risk of PD.11 In our study, the patient in whom RBD developed showed a marked increase in tonic chin EMG activity over time (from 4% to 8.9%). However, also other subjects in whom RBD or parkinsonism did not develop showed a marked increase of tonic chin EMG activity. Further investigations with a higher number of subjects and longer follow-up periods are needed to clarify this question. We do not think that this increase in REM-related EMG activity is related to the reduction of REM sleep as observed in our study; indeed the latter is most likely explained by the known physiological decrease of REM sleep with age.40
Potential Limitations
The investigated sample size of 14 subjects is small, particularly considering that our baseline cohort included 50 patients. The high attrition rate is a limitation of the current study. It was only in part due to loss to follow-up; more important factors were denial of study participation and the presence of exclusion criteria at follow-up. The neurodegenerative biomarkers were assessed only at follow-up. Prospective studies investigating their association with isolated RWA over time could be more informative. The duration of the follow-up period was less than 10 y. Based on the results of the current study one might expect that longer follow-up periods of 15–20 y, for example, would have been preferable. Almost all RWA subjects have mild SRBD, or had SRBD under nCPAP treatment, which might have had an influence on RWA itself. Nevertheless, AHI was stable, whereas RWA worsened over the observational period. Moreover, a recent study by McCarter et al.41 showed that nCPAP treatment does not affect RWA.41 Confirmation of our findings in healthy normal sleepers with an isolated finding of RWA is a necessary next step.
CONCLUSION
This is the first long-term follow-up study in subjects with an isolated finding of RWA. The relevance of RWA as early sign of synuclein-mediated neurodegeneration needs to be further investigated; however, we demonstrated in RWA subjects a significant progression of EMG activity during REM sleep with time, probably reflecting ongoing degeneration of structures that control REM sleep atonia. The possible role of RWA as an early marker of neurodegeneration is further supported by the high frequency of neurodegenerative biomarkers in subjects with isolated RWA. If these findings will be replicated in larger studies with longer observational periods, the finding of isolated RWA will have implications for defining at-risk cohorts for PD.
DISCLOSURE STATEMENT
This study was supported by funds of the Oesterreichische Nationalbank (Austria's central bank, Anniversary Fund, project number:15127). Dr. Stefani was supported by funds of the Oesterreichische Nationalbank (Austria's central bank, Anniversary Fund, project number: 15127). Dr. Gabelia has received travel support from Habel Medizintechnik and Vivisol. Dr. Högl reports personal fees from UCB, Mundipharma, Respironics, and Sanofioutside the submitted work and travel support from Otsuka. Dr. Mitterling has received travel support from AOP Orphan. Dr. Poewe has received personal fees from AbbVie, Allergan, Astra-Zeneca, BIAL, Boehringer-Ingelheim, Boston Scientific, GlaxoSmithKline, Ipsen, Lund-beck, Medtronic, MSD, Merck-Serono, Merz, Novartis, OrionPharma, Teva, UCB, Zamban and reports royalties of Thieme, Wiley Blackwell, Oxford University Press, and Cambridge University Press, all outside of the submitted work. The other authors have indicated no financial conflicts of interest. The work for this study was performed at the Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
ACKNOWLEDGMENTS
The authors are grateful for the assistance of the staff and technicians of the sleep laboratory at the Department of Neurology at Innsbruck Medical University, particularly Mr. Heinz Hackner.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- AT
anterior tibialis
- EMG
electromyography
- FDS
flexor digitorum superficialis
- FM-100
Farnsworth-Munsell 100
- iRBD
idiopathic RBD
- MoCA
Montreal Cognitive Assessment Scale
- nCPAP
nasal continuous positive airway pressure
- PD
Parkinson disease
- PLMS
periodic leg movements in sleep
- PSG
polysomnography
- RBD
REM sleep behavior disorder
- RWA
REM sleep without atonia
- SINBAR
Sleep Innsbruck Barcelona group
- SN
substantia nigra
- SRBD
sleep-related breathing disorders
- UPDRS III
Unified Parkinson's disease rating scale motor score
- UPSIT
University of Pennsylvania smell identification test
- v-PSG
video-PSG
SUPPLEMENTAL MATERIAL
Assessment of neurodegenerative biomarkers in subjects with rapid eye movement sleep without atonia above SINBAR cutoffs at baseline polysomnography.
REM-related EMG activity measures for the subjects with rapid eye movement sleep without atonia over SINBAR cutoffs at baseline polysomnography, comparing baseline and follow-up.
REFERENCES
- 1.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and postmortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12:443–53. doi: 10.1016/S1474-4422(13)70056-5. [DOI] [PubMed] [Google Scholar]
- 3.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14:744–8. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behaviour disorder. Neurology. 2009;72:1296–300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iber C, Ancoli-Israel C, Chesson A, Quan SF for the American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st ed. [Google Scholar]
- 6.Montplaisir J, Gagnon JF, Fantini ML, et al. Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Mov Disord. 2010;25:2044–51. doi: 10.1002/mds.23257. [DOI] [PubMed] [Google Scholar]
- 7.Frauscher B, Iranzo A, Gaig C, et al. Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep. 2012;35:835–47. doi: 10.5665/sleep.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frauscher B, Gabelia D, Mitterling T, et al. Motor events during healthy sleep: a quantitative polysomnographic study. Sleep. 2014;37:763–73. doi: 10.5665/sleep.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasai-Sakuma T, Frauscher B, Mitterling T, et al. Quantitative assessment of isolated rapid eye movement (REM) sleep without atonia without clinical REM sleep behavior disorder: clinical and research implications. Sleep Med. 2014;15:1009–15. doi: 10.1016/j.sleep.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Iranzo A, Ratti PL, Casanova-Molla J, Serradell M, Vilaseca I, Santamaria J. Excessive muscle activity increases over time in idiopathic REM sleep behavior disorder. Sleep. 2009;32:1149–53. doi: 10.1093/sleep/32.9.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Postuma RB, Gagnon JF, Rompre S, Montplaisir JY. Severity of REM atonia loss in idiopathic REM sleep behavior disorder predicts Parkinson disease. Neurology. 2010;74:239–44. doi: 10.1212/WNL.0b013e3181ca0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stiasny-Kolster K, Doerr Y, Möller JC, et al. Combination of “idiopathic” REM sleep behavior disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT_ SPECT. Brain. 2005;128:126–37. doi: 10.1093/brain/awh322. [DOI] [PubMed] [Google Scholar]
- 13.Postuma RB, Lang AE, Massicotte-Marquez J, Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology. 2006;66:845–51. doi: 10.1212/01.wnl.0000203648.80727.5b. [DOI] [PubMed] [Google Scholar]
- 14.Fantini ML, Postuma RB, Montplaisir J, Ferini-Strambi L. Olfactory deficit in idiopathic rapid eye movements sleep behavior disorder. Brain Res Bull. 2006;70:386–90. doi: 10.1016/j.brainresbull.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir JY. Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder. Ann Neurol. 2011;69:811–8. doi: 10.1002/ana.22282. [DOI] [PubMed] [Google Scholar]
- 16.Mahlknecht P, Iranzo A, Högl B, et al. Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology. 2015;84:654–8. doi: 10.1212/WNL.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 17.Ferini-Strambi L, Oldani A, Zucconi M, Smirne S. Cardiac autonomic activity during wakefulness and sleep in REM sleep behavior disorder. Sleep. 1996;19:367–9. doi: 10.1093/sleep/19.5.367. [DOI] [PubMed] [Google Scholar]
- 18.Lanfranchi PA, Fradette L, Gagnon JF, Colombo R, Montplaisir J. Cardiac autonomic regulation during sleep in idiopathic REM sleep behavior disorder. Sleep. 2007;30:1019–25. doi: 10.1093/sleep/30.8.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frauscher B, Nomura T, Duerr S, et al. Investigation of autonomic function in idiopathic REM sleep behavior disorder. J Neurol. 2012;259:1056–61. doi: 10.1007/s00415-011-6298-0. [DOI] [PubMed] [Google Scholar]
- 20.Ferini-Strambi L, Oertel W, Dauvilliers Y, et al. Autonomic symptoms in idiopathic REM behavior disorder: a multicentre case-control study. J Neurol. 2014;261:1112–8. doi: 10.1007/s00415-014-7317-8. [DOI] [PubMed] [Google Scholar]
- 21.Massicotte-Marquez J, Decary A, Gagnon JF, et al. Executive dysfunction and memory impairment in idiopathic REM sleep behavior disorder. Neurology. 2008;70:1250–7. doi: 10.1212/01.wnl.0000286943.79593.a6. [DOI] [PubMed] [Google Scholar]
- 22.Gagnon JF, Postuma RB, Joncas S, Desjardins C, Latreille V. The Montreal cognitive assessment: a screening tool for mild cognitive impairment in REM sleep behavior disorder. Mov Disord. 2010;25:936–40. doi: 10.1002/mds.23079. [DOI] [PubMed] [Google Scholar]
- 23.Delazer M, Zamarian L, Wenter J, et al. Decision making and executive functions in REM sleep behavior disorder. Sleep. 2012;35:667–73. doi: 10.5665/sleep.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terzaghi M, Zucchella C, Rustioni V, Sinforiani E, Manni R. Cognitive performances and mild cognitive impairment in idiopathic rapid eye movement sleep behavior disorder: results of a longitudinal follow-up study. Sleep. 2013;36:1527–32. doi: 10.5665/sleep.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stockner H, Iranzo A, Seppi K, et al. Midbrain hyperechogenicity in idiopathic REM sleep behavior disorder. Mov Disord. 2009;24:1906–9. doi: 10.1002/mds.22483. [DOI] [PubMed] [Google Scholar]
- 26.Postuma RB, Arnulf I, Högl B, et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord. 2012;27:913–6. doi: 10.1002/mds.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frauscher B, Ehrmann L, Zamarian L, et al. Validation of the Innsbruck REM sleep behavior disorder inventory. Mov Disord. 2012;27:1673–8. doi: 10.1002/mds.25223. [DOI] [PubMed] [Google Scholar]
- 28.Postuma RB, Montplaisir JY, Pelletier A, et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology. 2012;79:428–34. doi: 10.1212/WNL.0b013e31825dd383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farnsworth D. The Farnsworth-Munsell 100-hue and dichotomous tests for color vision. J Opt Soc Am. 1943;33:568–78. [Google Scholar]
- 30.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 31.The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- 32.Stockner H, Sojer M, Seppi K, et al. Midbrain sonography in patients with essential tremor. Mov Disord. 2007;22:414–7. doi: 10.1002/mds.21344. [DOI] [PubMed] [Google Scholar]
- 33.Mahlknecht P, Seppi K, Stockner H, et al. Substantia nigra hyperechogenicity as a marker for parkinson's disease: a population-based study. Neurodegener Dis. 2013;12:212–8. doi: 10.1159/000348595. [DOI] [PubMed] [Google Scholar]
- 34.Chiu HFK, Wing YK, Lam LCW, et al. Sleep-related injury in the elderly - an epidemiological study in Hong Kong. Sleep. 2000;23:513–7. [PubMed] [Google Scholar]
- 35.Kang SH, Yoon IY, Lee SD, Han JW, Kim TH, Kim KW. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep. 2013;36:1147–52. doi: 10.5665/sleep.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaenslen A, Unmuth B, Godau J, et al. The specificity and sensitivity of transcranial ultrasound in the differential diagnosis of Parkinson's disease: a prospective blinded study. Lancet Neurol. 2008;7:417–24. doi: 10.1016/S1474-4422(08)70067-X. [DOI] [PubMed] [Google Scholar]
- 37.Berg D, Merz B, Reiners K, Naumann M, Becker G. Five-year follow-up study of hyperechogenicity of the substantia nigra in Parkinson's disease. Mov Disord. 2005;20:383–5. doi: 10.1002/mds.20311. [DOI] [PubMed] [Google Scholar]
- 38.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–94. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 39.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behavior disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–88. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 40.Mitterling T, Högl B, Schönwald SW, et al. Sleep and respiration in 100 healthy caucasian sleepers - a polysomnographic study according to AASM standards. Sleep. 2015;38:867–75. doi: 10.5665/sleep.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarter SJ, St Louis EK, Duwell EJ, et al. Diagnostic thresholds for quantitative REM sleep phasic burst duration, phasic and tonic muscle activity, and REM atonia index in REM sleep behavior disorder with and without comorbid obstructive sleep apnea. Sleep. 2014;37:1649–62. doi: 10.5665/sleep.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Assessment of neurodegenerative biomarkers in subjects with rapid eye movement sleep without atonia above SINBAR cutoffs at baseline polysomnography.
REM-related EMG activity measures for the subjects with rapid eye movement sleep without atonia over SINBAR cutoffs at baseline polysomnography, comparing baseline and follow-up.


