Highlight
The diversity of L-type lectin receptor kinases (LecRKs) was examined in tomato and Nicotiana benthamiana together with their role in resistance to Phytophthora pathogens.
Key words: Immune receptors, LecRKs, phylogenetic analysis, Phytophthora pathogens, plant resistance, RLK.
Abstract
Membrane-bound receptors play crucial roles as sentinels of plant immunity against a large variety of invading microbes. One class of receptors known to be involved in self/non-self-surveillance and plant resistance comprises the L-type lectin receptor kinases (LecRKs). Previously, we reported that several Arabidopsis LecRKs play a role in resistance to Phytophthora pathogens. In this study, we determined whether homologues of these LecRKs from the Solanaceous plants Nicotiana benthamiana and tomato (Solanum lycopersicum) play similar roles in defence against Phytophthora. In genome-wide screenings, a total of 38 (Nb)LecRKs were identified in N. benthamiana and 22 (Sl)LecRKs in tomato, each consisting of both a lectin and a kinase domain. Phylogenetic analysis revealed that, in contrast to Arabidopsis, which has a LecRK family comprising nine clades, Solanaceous species have just five of these nine clades (i.e. IV, VI, VII, VIII, and IX), plus four additional clades that lack Arabidopsis homologues. Several of the Solanaceous LecRKs were selected for functional analysis using virus-induced gene silencing. Infection assays with Phytophthora capsici and Phytophthora infestans on LecRK-silenced plants revealed that N. benthamiana and tomato homologues in clade IX play a role in Phytophthora resistance similar to the two Arabidopsis LecRKs in this clade, suggesting conserved functions of clade IX LecRKs across different plant families. This study provides a first insight into the diversity of Solanaceous LecRKs and their role in plant immunity, and shows the potential of LecRKs for Phytophthora resistance breeding.
Introduction
Plant diseases caused by Phytophthora pathogens are a major constraint to the production of a large variety of Solanaceous crops (Kroon et al., 2012). Renowned are Phytophthora infestans, the causal agent of late blight disease on potato and tomato, and Phytophthora capsici, which is highly destructive to multiple Solanaceous crops, including tomato, aubergine, and pepper (Fry, 2008; Bouwmeester et al., 2009; Lamour et al., 2012). Breeding for Phytophthora resistance has been focused largely on the introgression of resistance (R) genes encoding the intracellular nucleotide-binding leucine-rich repeat receptors (NLRs), which mediate effector-triggered immunity (ETI) upon recognition of cognate effectors (Vleeshouwers et al., 2011). Since Phytophthora pathogens can quickly adapt, NLR-mediated resistance is often not durable. In the pathogen population, new races emerge that have inactivated or modified effector genes and can thus circumvent R gene recognition (Vleeshouwers et al., 2011; Kasuga and Gijzen, 2013).
Besides ETI, plants rely on defence mediated by plasma membrane-localized receptor-like kinases (RLKs), which play pivotal roles in the surveillance of ‘non-self’ (e.g. microbe-associated molecular patterns; MAMPs) and ‘modified self’ (e.g. damage-associated molecular patterns; DAMPs) molecules as exogenous stress signals to initiate non-race-specific immunity. These so-called pattern recognition receptors (PRRs) have been suggested to mediate basal defence, which is thought to have a high potential to confer broad-spectrum disease resistance in plants (Boller and Felix, 2009). So far, PRRs have received limited attention in resistance breeding.
One class of RLKs that has been suggested to function as PRRs in recognition of stress signals and subsequent initiation of plant defence comprises the L-type lectin receptor kinases (LecRKs). Arabidopsis has 45 LecRK genes that are distributed over nine clades (clades I–IX) and seven singletons, with several showing induced expression upon pathogen attack and in response to pathogen-associated elicitors and MAMPs (Bouwmeester and Govers, 2009). One of these is LecRK-I.9, which functions in maintaining cell wall integrity and plays a crucial role in Phytophthora resistance in Arabidopsis (Gouget et al., 2006; Bouwmeester et al., 2011). Interfamily gene transfer of Arabidopsis LecRK-I.9 to the Solanaceous plants Nicotiana benthamiana and potato conferred enhanced resistance to P. infestans (Bouwmeester et al., 2014), suggesting a conserved functionality in stress signal recognition and immunity among plant species. Recently, it was shown that LecRK-I.9 (also known as DORN1) also functions as a receptor of extracellular ATP (eATP) (Choi et al., 2014). Possibly, eATP is released upon pathogen attack or wounding and as such may function as a DAMP (Choi et al., 2014). Two other Arabidopsis LecRKs that have been studied for their roles in plant defence are LecRK-V.5 and LecRK-VI.2. Both were found to play a role in bacterial resistance by mediating stomatal immunity (Desclos-Theveniau et al., 2012; Singh et al., 2012). Ectopic expression of LecRK-VI.2 in N. benthamiana primes MAMP-mediated defence and increases resistance against various hemibiotrophic and necrotrophic bacterial pathogens (Huang and Zimmerli, 2014). Another example is Arabidopsis LecRK-I.8, which is required for proper PR-1 induction upon treatment with egg-derived elicitors of the cabbage butterfly Pieris brassicae (Gouhier-Darimont et al., 2013). Recently, we published a study that was aimed at investigating the role of Arabidopsis LecRKs in defence against a variety of plant pathogens. This revealed that,next to LecRK-I.9, there are 14 other LecRKs that have a putative role in resistance against Phytophthora pathogens in Arabidopsis (Wang et al., 2014). Arabidopsis lines with T-DNA insertions in these 14 LecRK genes showed altered susceptibility when challenged with P. capsici and Phytophthora brassicae, suggesting that LecRK family members collectively build up a basal level of Phytophthora resistance.
Analysis of LecRKs for a potential role in defence in plant species other than Arabidopsis has so far been limited. NbLRK1 from N. benthamiana was reported to interact with INF1, an elicitin secreted by P. infestans, and suggested to play a role in mediating INF1-induced cell death (Kanzaki et al., 2008). In addition, several LecRKs were indicated to be involved in plant defence since they are induced upon treatment with pathogens or pathogen-derived elicitors. Multiple cucumber (Cucumis sativus) LecRKs were found to be induced upon infection by P. capsici and Phytophthora melonis (Wu et al., 2014), and a cotton (Gossypium hirsutum) LecRK, GhLecRK-2, was shown to be upregulated upon treatment with a cell-wall-derived fraction of the vascular wilt fungus Verticillium dahliae (Phillips et al., 2013).
Characterization of defence mechanisms in model plants, such as Arabidopsis, paves the way to study similar processes in crops (Koornneef and Meinke, 2010). Disrupting homologous genes encoding proteins with a conserved physiological function often results in similar phenotypes in different plant species. For example, the role of the PRR FLS2 in perception of bacterial flagellin was first discovered in Arabidopsis and thereafter found to be largely conserved in various plant lineages (Gomez-Gomez and Boller, 2000; Hann and Rathjen, 2007; Robatzek et al., 2007; Takai et al., 2008).
To determine whether LecRKs are functionally conserved in Solanaceous species, we set out to investigate the function of Solanaceous LecRKs in Phytophthora resistance. In this study, we performed a genome-wide identification of LecRKs in N. benthamiana and tomato (Solanum lycopersicum) and analysed the phylogenetic relationship of these LecRKs with Arabidopsis LecRKs. Subsequently, several Solanaceous LecRKs were selected for functional analysis using virus-induced gene silencing and infection assays to pinpoint their role in Phytophthora resistance.
Materials and methods
Sequence identification and gene analysis
Protein sequences of Arabidopsis LecRKs analysed by Bouwmeester and Govers (2009) were retrieved from the TAIR website (http://www.arabidopsis.org, last accessed 27 July 2015). Protein sequences were used as queries for reciprocal BLAST searches via the Sol Genomic Network (SGN) website (http://solgenomics.net; last accessed 27 July 2015) against the genomic databases of N. benthamiana and tomato. Obtained LecRK sequences were further analysed by comparative analysis using publicly available expressed sequence tags (ESTs), RNA sequencing (RNA-seq) data derived from Nicotiana benthamiana Genome Page of the University of Sydney (http://sydney.edu.au/science/molecular_bioscience/sites/benthamiana/, last accessed July 27, 2015; Naim et al., 2012; Nakasugi et al., 2013) and tomato RNA-seq data (L. Faino, personal communication). NbS00026192g0010.1 was verified by sequencing after amplification of the entire cDNA sequence using Pfu DNA polymerase (Promega) and gene-specific primers (Supplementary Table S1, available at JXB online). All the retrieved cDNA sequences were compared with the genomic DNA sequences, followed by manual validation of the open reading frame and presence of introns (Table 1). Amino acid sequences were subjected to the protein domain and motif annotation webtools SMART (http://smart.embl-heidelberg.de; last accessed July 27, 2015), SignalP 3.0 (http://www.cbs.dtu.dk/services/SignalP-3.0; last accessed 27 July 2015) and TMHMM Server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM; last accessed 27 July 2015). Predicted kinase domain sequences were aligned by ClustalW and manually checked for subdomains according to those defined based on LecRK-VI.2 and NtCPK5 (Wang et al., 2005; Singh et al., 2013).
Table 1.
Overview of LecRKs in Arabidopsis, N. benthamiana and tomato
| Cladea | No. of introns | Protein length (aa) | SPb | Lectin | TM | Kinase | RD motifc | Remarks | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | AtLecRK-I.1–11 | ||||||||||
| II | AtLecRK-II.1–2 | ||||||||||
| III | AtLecRK-III.1–2 | ||||||||||
| IV | AtLecRK-IV.1–4 | ||||||||||
| IV | NbS00015931g0001.1 | 0 | 666 | 1–19 | 20–257 | 285–307 | 342–612 | + | |||
| NbS00029393g0102.1 | 0 | 669 | 1–21 | 22–259 | 286–308 | 342–612 | + | ||||
| Solyc09g012000.1.1d | 0 | 669 | 1–21 | 22–260 | 285–307 | 342–612 | + | ||||
| Solyc09g011990.1.1d | 0 | 668 | 1–21 | 23–260 | 286–308 | 342–612 | + | ||||
| Solyc09g011060.2.1e | 1 | 832 | 1–21 | 22–259 | 284–306 | 341–611 | + | Contains a OB-NTP-binding domain | |||
| V | AtLecRK-V.1–9 | ||||||||||
| VI | AtLecRK-VI.1–4 | ||||||||||
| VI | NbS00010453g0003.1 | 0 | 679 | 1–21 | 22–265 | 297–319 | 351–622 | + | Corrected ORF | ||
| NbS00005128g0014.1 | 0 | 678 | 1–20 | 21–264 | 296–318 | 350–621 | + | Corrected ORF | |||
| Solyc09g005000.1.1 | 0 | 674 | 1–22 | 23–262 | 294–313 | 345–616 | + | ||||
| VII | AtLecRK-VII.1–2 | ||||||||||
| VII | NbS00024573g0008.1 | 0 | 698 | 1–26 | 27–271 | 303–325 | 362–632 | + | Corrected ORF | ||
| NbS00025337g0001.1 | 0 | 695 | 1–23 | 24–268 | 300–322 | 359–629 | + | ||||
| NbS00032834g0007.1 | 1 | 678 | 1–25 | 26–271 | 303–325 | 362–631 | + | Corrected ORF | |||
| Solyc02g078170.1.1 | 0 | 698 | 1–25 | 26–271 | 303–325 | 362–632 | + | Corrected ORF | |||
| VIII | LecRK-VIII.1–2 | ||||||||||
| VIII | NbS00026087g0010.1 | 0 | 716 | 1–29 | 30–257 | 325–347 | 384–657 | + | Corrected ORF | ||
| NbS00026192g0010.1 | 0 | 716 | 1–23 | 25–257 | 326–348 | 384–657 | + | Corrected ORF; alias NbLRK1 | |||
| NbS00008527g0008.1 | 0 | 709 | 1–22 | 23–249 | 318–340 | 377–650 | + | Corrected ORF | |||
| NbS00016101g0013.1 | 0 | 710 | 1–18 | 23–250 | 320–342 | 378–651 | + | Corrected ORF | |||
| Solyc10g084250.1.1 | 0 | 721 | 1–30 | 31–257 | 326–348 | 386–658 | + | ||||
| Solyc09g007510.1.1 | 0 | 709 | 1–20 | 21–236 | 313–335 | 373–645 | + | Corrected ORF | |||
| IX | LecRK-IX.1–2 | ||||||||||
| IX | NbS00034752g0003.1 | 2f | 485 | 1–18 | 19–196 | – | 220–441 | + | Lacking STK subdomains V, VIa | ||
| NbS00059538g0001.1 | 0 | 649 | 1–26 | 27–251 | 269–291 | 336–605 | + | Corrected ORF | |||
| Solyc03g043710.1.1 | 0 | 647 | 1–22 | 23–249 | 267–289 | 333–602 | + | ||||
| X | AtLecRK-S.1 | ||||||||||
| X | NbS00001559g0001.1 | 0 | 671 | 1–19 | 20–264 | 295–317 | 353–626 | + | |||
| Solyc03g112310.1.1 | 0 | 662 | 1–21 | 22–266 | 296–318 | 352–622 | + | ||||
| XI | AtLecRK-S.4 | ||||||||||
| XI | NbS00015570g0009.1 | 0 | 691 | 1–23 | 24–263 | 291–313 | 345–615 | + | Corrected ORF | ||
| NbS00002771g0001.1 | 0 | 691 | 1–23 | 24–263 | 291–313 | 345–615 | + | Corrected ORF | |||
| NbS00043874g0006.1 | 1 | 424 | – | 1–24 | 50–72 | 106–351 | + | Truncated lectin domain; lacking STK subdomains III, IV | |||
| NbS00000505g0005.1 | 2f | 557 | – | 7–204 | – | 267–484 | + | Corrected ORF; lacking STK subdomain X | |||
| NbS00056619g0001.1 | 0 | 681 | 1–23 | 25–263 | 289–311 | 345–615 | + | Corrected ORF | |||
| NbS00027351g0001.1 | 0 | 674 | 1–16 | 18–256 | 282–304 | 338–608 | + | Corrected ORF | |||
| NbS00029393g0011.1 | 0 | 681 | 1–23 | 25–263 | 289–311 | 345–615 | + | Corrected ORF | |||
| Solyc05g053010.1.1 | 0 | 690 | 1–23 | 24–262 | 287–309 | 344–614 | + | ||||
| Solyc10g084860.1.1 | 1f | 667 | 1–20 | 21–260 | 286–308 | 342–593 | + | Lacking STK subdomain X | |||
| Solyc09g011070.1.1e | 0 | 681 | 1–21 | 23–261 | 287–309 | 343–613 | + | ||||
| XII | AtLecRK-S.5 | ||||||||||
| XII | NbS00003611g0313.1 | 0 | 679 | 1–28 | 29–268 | 282–304 | 348–625 | + | Corrected ORF | ||
| NbS00005288g0011.1 | 3f | 540 | 1–23 | 24–253 | – | 309–528 | + | Corrected ORF; lacking STK subdomains VIa, X | |||
| Solyc10g080510.1.1 | 0 | 670 | 1–29 | 30–268 | 282–304 | 348–623 | + | ||||
| XIII | AtLecRK-S.6 | ||||||||||
| XIII | NbS00006201g0004.1 | 0 | 669 | 1–25 | 26–238 | 286–308 | 348–618 | + | Corrected ORF | ||
| NbS00021029g0001.1 | 0 | 669 | 1–25 | 26–238 | 286–308 | 348–618 | + | Corrected ORF | |||
| Solyc04g071000.1.1 | 0 | 677 | 1–25 | 26–241 | 294–316 | 356–626 | + | ||||
| XIV | AtLecRK-S.7 | ||||||||||
| XIV | NbS00007030g0016.1 | 0 | 671 | 1–22 | 23–251 | 291–313 | 350–622 | + | Corrected ORF | ||
| NbS00020348g0007.1 | 0 | 671 | 1–22 | 23–251 | 291–313 | 350–622 | + | Corrected ORF | |||
| Solyc07g065610.1.1 | 0 | 666 | 1–20 | 21–249 | 289–311 | 346–618 | + | ||||
| XV | Solyc03g080060.1.1 | 0 | 663 | 1–16 | 29–279 | 317–339 | 379–626 | ‒/KN | Corrected ORF; lacking STK subdomain VIII | ||
| NbS00029224g0003.1 | 0 | 661 | 1–20 | 29–279 | 317–339 | 379–626 | –/KN | Lacking STK subdomain VIII | |||
| NbS00001395g0006.1 | 1 | 662 | 1–20 | 29–279 | 317–339 | 379–626 | –/KN | Lacking STK subdomain VIII | |||
| XVI | NbS00007832g0008.1 | 1 | 676 | 1–24 | 27–276 | 289–311 | 350–621 | + | |||
| NbS00001007g0015.1 | 4f | 454 | 1–24 | 30–266 | – | 323–454 | + | Lacking STK subdomains VIa, VII–XI | |||
| NbS00012093g0021.1 | 1 | 688 | 1–24 | 28–277 | 291–313 | 352–623 | + | Corrected ORF | |||
| NbS00020337g0016.1 | 1 | 688 | 1–24 | 29–277 | 291–313 | 352–623 | + | ||||
| Solyc02g068300.2.1 | 1 | 688 | 1–25 | 29–278 | 292–314 | 353–625 | + | ||||
| Solyc03g031980.2.1 | 1 | 678 | 1–20 | 30–270 | 289–311 | 351–614 | + | ||||
| XVII | NbS00048421g0010.1g | 0 | 707 | 1–26 | 27–261 | 294–316 | 362–632 | + | Corrected ORF | ||
| NbS00051756g0005.1g | 0 | 707 | 1–25 | 26–263 | 293–315 | 361–630 | + | Corrected ORF | |||
| Solyc10g047810.1.1g | 0 | 702 | 1–19 | 20–256 | 288–310 | 358–627 | + | ||||
| Solyc10g047680.1.1g,h | 5f | 520 | 1–21 | 42–113 | 217–239 | 259–431 | + | Truncated lectin domain; lacking STK subdomains III, IV, X, XI | |||
| XVIII | NbS00000562g0002.1g | 0 | 707 | 1–23 | 24–257 | 301–323 | 367–636 | + | |||
| Solyc01g106160.1.1g | 0 | 720 | 1–23 | 24–256 | 321–343 | 387–656 | + | ||||
| NbC25369236g0004.1 | 1f | 362 | – | 1–162 | 214–236 | 279–362 | ‒ | Lacking STK subdomains V–XI | |||
| Solyc10g047700.1.1h | 4f | 351 | – | 62–106 | – | 176–281 | ‒ | Truncated lectin domain; lacking STK subdomains VIa–XI | |||
| NbS00037263g0008.1 | 2f | 655 | 1–21 | 22–253 | 269–291 | 332–593 | + | Corrected ORF | |||
–/KN, ORF, open reading frame; OB-NTP, oligosaccharide/oligonucleotide-binding nucleoside triphosphate; STK, serine/threonine kinase.
a Grey shading represents a clade with Solanaceous LecRKs sharing over 50% similarity at the amino acid level. The Arabidopsis clades were delineated by Bouwmeester and Govers (2009).
b Signal peptide prediction based on SignalP 3.0.
c +, present; –, absent; KN, lysine/asparagine substitution.
d, e, h Tandem duplicated LecRKs.
g LecRKs sharing over 50% similarities at the amino acid level.
f No RNA-seq data available.
Multiple sequence alignment and phylogenetic analysis
Protein sequences of full-length LecRKs or either lectin or kinase domains were aligned with ClustalW using the protein weight matrix GONNET with a penalty gap opening of 10 and a gap extension of 0.1. The obtained sequence alignments were subsequently used as input to construct neighbour-joining trees and maximum-likelihood trees with 10 000 bootstrap replicates using the Jones–Taylor–Thornton substitution model (Hall, 2013) in MEGA 5.1. Branches corresponding to partitions reproduced in <60% of bootstrap replicates were collapsed in the phylogenetic trees.
Plasmid construction
Fragments for gene silencing were chosen to contain stretches of at least 25 nt with 100% identity to the target gene, and at most 20 to off-target genes (Supplementary File S2, available at JXB online). Silencing specificity of the gene fragments was verified by BLAST analysis and the virus-induced gene silencing tool at the Sol Genomics Network website (http://vigs.solgenomics.net/; last accessed 27 July 2015). Gene fragments used to generate silencing constructs were synthesized by Eurofins Genomics and subsequently cloned into the gene-silencing vector pTRV-RNA2. pTRV-RNA2 derivatives and pTRV-RNA1 vectors were transformed into Agrobacterium tumefaciens strain GV3101.
Plant growth conditions
N. benthamiana and tomato (cultivar Moneymaker) were grown in soil in a conditioned greenhouse at 19–21 °C with a 16h photoperiod and a relative humidity of 75–78%. Supplementary light (100W m–2) was applied when the light intensity dropped below 150W m–2.
Agroinfiltration and tobacco rattle virus (TRV)-mediated silencing assays
A. tumefaciens strains carrying binary vectors were grown overnight at 28 °C in yeast extract broth with appropriate antibiotics. A. tumefaciens cells were pelleted, resuspended, and incubated in induction medium (10mM MES, 10mM MgCl2, 50 μM acetosyringone, pH 5.6) for 3–4h and thereafter for 1h in infiltration medium (10mM MES, 10mM MgCl2, 200 μM acetosyringone, pH 5.6). For gene silencing, A. tumefaciens cultures carrying pTRV-RNA2 constructs and A. tumefaciens carrying pTRV1 were mixed at a ratio of 1:1 to a final OD600 of 1.0 or 2.0 before infiltration into cotyledons of 2-week-old N. benthamiana and 10-d-old tomato seedlings, respectively. TRV:PDS and TRV:GUS were used as controls. For transient expression of the elicitor genes, A. tumefaciens suspensions with appropriate concentrations were syringe infiltrated into N. benthamiana leaves. To induce elicitor-triggered cell death, N. benthamiana leaves were infiltrated with 100nM INF1.
RNA isolation and quantitative reverse transcription PCR (qRT-PCR)
For each silencing construct, six leaves (the fifth and sixth true leaves) from three individual plants were harvested and ground in liquid nitrogen. Total RNA was isolated from 100mg of leaf material with a NucleoSpin RNA Plant kit (Macherey-Nagel), and subsequently used as template for first-strand cDNA synthesis using a Moloney murine leukemia virus reverse transcriptase kit (Promega) with an oligo(dT) primer (Supplementary Table S1). qRT-PCR was performed as described by Wang et al. (2014) using Actin as endogenous control.
Phytophthora cultivation and infection assays
Culturing of P. capsici isolate LT263 and P. infestans isolate 14-3-GFP and production of zoospores were performed as described previously (Champouret et al., 2009; Wang et al., 2013; Bouwmeester et al., 2014).
N. benthamiana and tomato leaves were collected 3–4 weeks after TRV treatment and placed in water-saturated floral foam in trays as described by Vleeshouwers et al. (1999). Leaves were inoculated on the abaxial sides with fresh mycelial plugs (diameter 0.5cm), or 10 µl droplets containing 1×105 ml–1 P. capsici zoospores or 5×105 ml–1 P. infestans zoospores. Inoculated leaves were kept in transparent plastic boxes to maintain high humidity and placed in a climate chamber with a 12h photoperiod and appropriate temperature settings. Boxes were kept in the dark for the first 24h. The diameters of P. capsici lesions were measured at 3 d post-inoculation (dpi) and P. infestans lesions at 4 or 6 dpi. Lesion sizes were calculated as described previously (Vleeshouwers et al., 1999).
Results and discussion
Identification of LecRKs in N. benthamiana and tomato
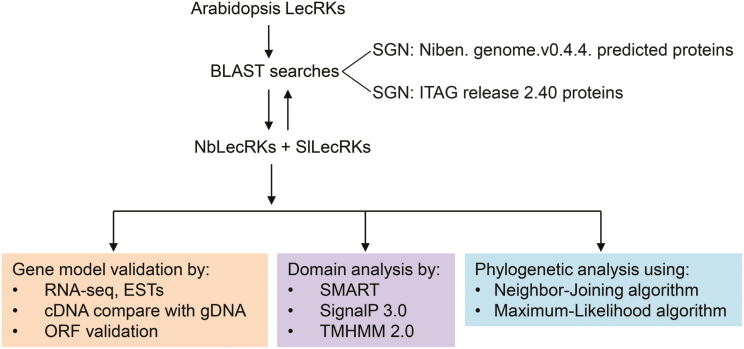
LecRKs in N. benthamiana and tomato were identified following the pipeline depicted in Fig. 1. Protein sequences of Arabidopsis LecRKs (AtLecRKs) were used as queries for BLAST searches against the predicted protein databases of N. benthamiana and tomato via the SGN website. The presence of both a lectin domain and a kinase domain was used as the criterion for the selection of putative LecRKs. Using reciprocal BLAST searches, we identified 37 (Nb)LecRKs and 22 (Sl)LecRKs in N. benthamiana and tomato, respectively (Table 1). The predicted cDNA sequences were retrieved and, in combination with EST data, RNA-seq data, and genomic DNA sequences, were used for gene model validation. Strikingly, over half of the NbLecRK gene models were found to be incorrect due to erroneous open reading frame prediction and were corrected (Table 1). An additional full-length NbLecRK, NbS00021029g0001.1, that is annotated to lack the kinase domain in the SGN database, was identified by gene model verification using RNA-seq data (Table 1). The coding sequence of the previously described NbLRK1 (deposited in GenBank as AB247455.1; Kanzaki et al., 2008) was found to contain multiple single-nucleotide polymorphisms when compared with N. benthamiana genomic DNA or RNA-seq data. Hence, we PCR amplified and sequenced the coding sequence and found that it matched the corrected gene model of NbS00026192g0010.1. For the SlLecRKs, three were found to be erroneous and these were corrected according to the RNA-seq data (Table 1). The obtained LecRK sequences, including those revised, are listed in Supplementary File S1 (available at JXB online) and have been deposited in GenBank.
Fig. 1.
Pipeline depicting the procedures used for identification and analysis of LecRKs in N. benthamiana and tomato. (This figure is available in colour at JXB online.)
In Arabidopsis, only six out of the 45 LecRKs contain introns. Five of these have one intron and the other one contains two introns. By comparing Solanaceous LecRK cDNA sequences with genomic DNA sequences, 12 NbLecRKs were found to contain introns ranging from one to four per gene (Table 1). For six of these, the presence of the intron could be confirmed based on RNA-seq data, but for the other six there was no RNA-seq data available. Three out of the 22 SlLecRKs were confirmed to contain one intron based on RNA-seq data, whereas another three SlLecRKs were predicted to contain one, four, and five introns, respectively (Table 1).
Chromosomal location of tomato LecRKs
To investigate tandem duplication events, we examined the chromosomal distribution of the SlLecRKs in tomato. For N. benthamiana, no such information is available. The 22 SlLecRKs were distributed over eight of the 12 tomato chromosomes (chromosomes 1, 2, 3, 4, 5, 7, 9, and 10), with the number of LecRKs ranging from one to six per chromosome (Table 1). Most of the SlLecRKs were located on chromosomes 9 and 10, with six SlLecRKs on each chromosome. In addition, two tandem duplicated SlLecRK gene pairs were found on chromosome 9 and one on chromosome 10 based on the criterion that tandem duplicated genes are located within 10 adjacent gene models (Shiu and Bleecker, 2003). In comparison, the LecRKs in Arabidopsis show a much higher tandem duplication rate; there are nine distinct clusters, each with two to six AtLecRKs (Bouwmeester and Govers, 2009).
Domain composition of NbLecRKs and SlLecRKs
Domain analysis, which was performed using multiple bioinformatics webtools, revealed that, of the 38 identified NbLecRKs, 32 contained the typical composition of a LecRK, i.e. a signal peptide (SP), an extracellular L-type lectin domain, a single transmembrane (TM) domain, and a cytosolic serine/threonine kinase domain. Of the remaining six, three had no clear TM domain, and two had no SP, whereas one lacked both the SP and the TM domain. Most of the 22 SlLecRKs contained all representative LecRK features; the remaining one was predicted to lack both the SP and the TM domain (Table 1). One SlLecRK (Solyc09g011060.2.1) contained an additional domain, namely an oligosaccharide/oligonucleotide-binding domain (Pfam accession: PF07717) at the C terminus adjacent to the kinase domain. Although NbS00043874g0006.1, Solyc10g047680.1.1, and Solyc10g047700.1.1 are predicted to contain a lectin signature, the lectin domain is truncated (Table 1). Kinase domains of RLKs are in general highly conserved and contain 12 subdomains that are essential for kinase activity (Shiu and Bleecker, 2001; Hanks, 2003; Afzal et al., 2008). Alignment of the predicted kinase domains revealed that six NbLecRKs and three SlLecRKs lacked one or more subdomains, which could impair kinase activity (Table 1). Protein kinases that are activated by phosphorylation in the activation loop typically carry a conserved arginine (R) that precedes the catalytic aspartate (D) in subdomain VIb (Nolen et al., 2004; Kornev et al., 2006). They are therefore known as RD kinases, whereas kinases that lack the RD motif are collectively termed non-RD kinases. It has been found that activation of non-RD kinases does not require phosphorylation of the activation loop (Dardick and Ronald, 2006). Nearly all identified Solanaceous LecRKs contained a RD motif with the exception of three NbLecRKs and two SlLecRKs. NbC25369236g0004.1 and Solyc10g047700.1.1 did not contain an RD motif due to absence of the kinase subdomain VIb, whereas in NbS00029224g0003.1, NbS00001395g0006.1, and Solyc03g080060.1.1 RD was substituted by KN (Table 1). This is also the case for some of the Arabidopsis LecRKs: AtLecRK-I.2 lacks the kinase subdomain VIb, while AtLecRK-III.1 and AtLecRK-III.2 contain GN residues instead of RD.
Sequence divergence of Arabidopsis, N. benthamiana, and tomato LecRKs
Sequence alignment of LecRKs of the three plant species revealed high levels of sequence divergence. At the protein level, the identity of the most similar AtLecRK homologues in N. benthamiana and tomato was only 66 and 58%, respectively. At the nucleotide level, the identities between AtLecRKs and SolLecRKs were even lower, at the most 56%. Between LecRK homologues of the two Solanaceous species, the identity was much higher, reaching up to 87% at the protein level and 73% at the nucleotide level.
In Arabidopsis, LecRKs are divided into nine clades based on the definition that a clade contains at least two homologues with a minimum of 50% similarity at the nucleotide level (Bouwmeester and Govers, 2009). However, due to high sequence divergence, this criterion is not applicable for the Solanaceous LecRKs (data not shown). Hence, Solanaceous LecRKs were grouped in one clade if they share over 50% similarity at the amino acid level. In this way, 17 clades were found by means of pairwise alignment (Table 1), with three to 10 members per clade.
Phylogeny of LecRKs in Arabidopsis, N. benthamiana, and tomato
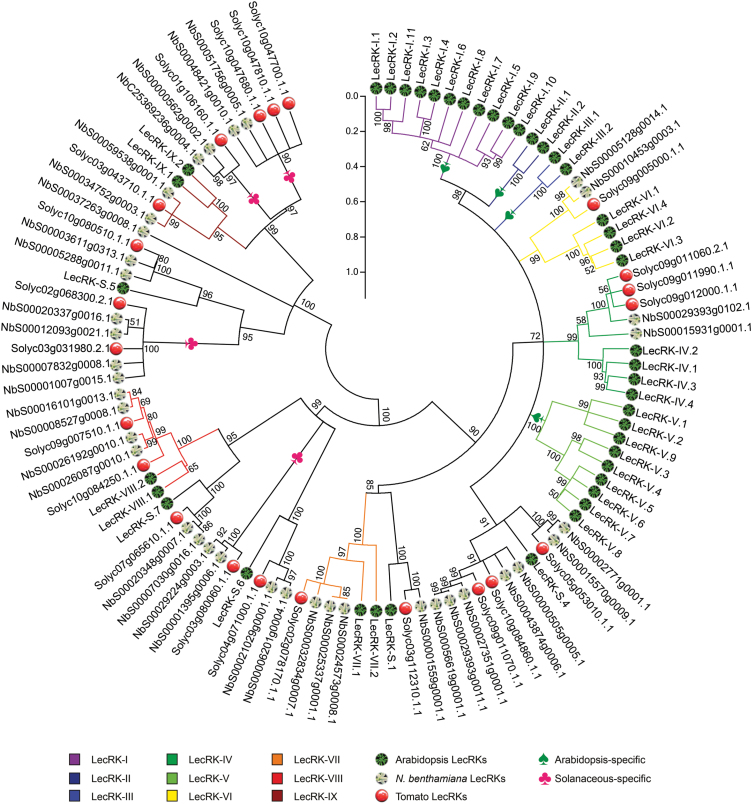
To evaluate the evolutionary relationship among LecRKs from different plant species, an unrooted phylogenetic tree containing 43 full-length AtLecRKs, 38 NbLecRKs and 22 SlLecRKs was generated using the neighbour-joining algorithm (Fig. 2). The reliability of the phylogram was determined by bootstrap analysis with 10 000 replicates. A comparable consensus tree was obtained using the maximum-likelihood algorithm with the same bootstrap replicates (data not shown). Similar phylogenetic analyses were also performed using solely the extracellular lectin domains or intracellular kinase domains, and this resulted in tree topologies that were in overall agreement with the phylogenetic tree obtained with the full-length LecRK protein sequences (Fig. 2 and Supplementary Fig. S1, available at JXB online). It thus seems that LecRKs that cluster in one clade based on the full-length phylogeny also have similar extracellular domains and intracellular kinase domains, and this is in line with the finding that plant RLKs containing similar extracellular domains are prone to have similar kinase domains (Shiu and Bleecker, 2003).
Fig. 2.
Phylogenetic tree of LecRKs from Arabidopsis, N. benthamiana, and tomato. A neighbour-joining tree based on predicted full-length amino acid sequences, comprising 43 AtLecRKs, 38 NbLecRKs, and 22 SlLecRKs. Branches are coloured according to the clades delineated by Bouwmeester and Govers (2009). Numbers above the branches represent the level of clade support inferred by 10 000 bootstrap replicates. The vertical branch-length scale bar represents 0.2 amino acid substitutions per site. (This figure is available in colour at JXB online.)
In the phylogenetic tree, 38 of the 43 AtLecRKs fall into nine distinct clades (I–IX) and the other five are singletons, which is in agreement with the tree constructed by Bouwmeester and Govers (2009), which included only the AtLecRKs. Moreover, the degree of similarity between LecRKs was found to be indicative of the phylogenetic relationship between the three plant species (Table 1, Fig. 2). Most of the NbLecRKs and SlLecRKs grouped together with AtLecRKs in five of the nine clades, i.e. IV, VI, VII, VIII, and IX, and with the five AtLecRK singletons. In these cases, often one AtLecRK clade member or an AtLecRK singleton grouped with one SlLecRK and two NbLecRKs in one subbranch. In such a subbranch, the NbLecRKs and SlLecRK were often closer to each other than to their Arabidopsis counterpart, as expected. The duplicated number of NbLecRKs is most probably due to the allotetraploid nature of N. benthamiana. Next to the subbranch that contained LecRK-S.4 homologues from all three species, there was a subbranch that comprised only Solanaceous LecRKs, two from tomato and five from N. benthamiana. In contrast to these expansions of certain clades and on certain branches of the tree, there were four clades that were expanded. None of the Solanaceous LecRKs fell into clades I, II, III, and V and similarly none of the LecRKs identified in cucumber by Wu et al. (2014) belonged to any of these four clades. It should be noted that the AtLecRKs in clades I, II, III, and V exhibit a high frequency of tandem duplication (Bouwmeester and Govers, 2009). Moreover, these four LecRK clades are well conserved among various plant species of the Brassicaceae family (Hofberger et al., 2015), pointing to a lineage-specific expansion of these LecRKs after the split of the Brassicales. Several LecRKs in Arabidopsis belonging to clades I, II, III, and V were found to be involved in resistance to Phytophthora, Alternaria brassicicola, and bacterial pathogens (Bouwmeester et al., 2011; Wang et al., 2014), suggesting that this lineage-specific expansion of LecRKs is important for Arabidopsis adaptation. Next to the Arabidopsis-specific clades, the phylogenetic analysis revealed four Solanaceous-specific clades and one NbLecRK that ended up as a singleton (Fig. 2). The three Solanaceous LecRKs that carry a substitution in the RD motif formed a well-supported clade separated from other LecRKs.
N. benthamiana homologues of clade IX AtLecRKs function in Phytophthora resistance
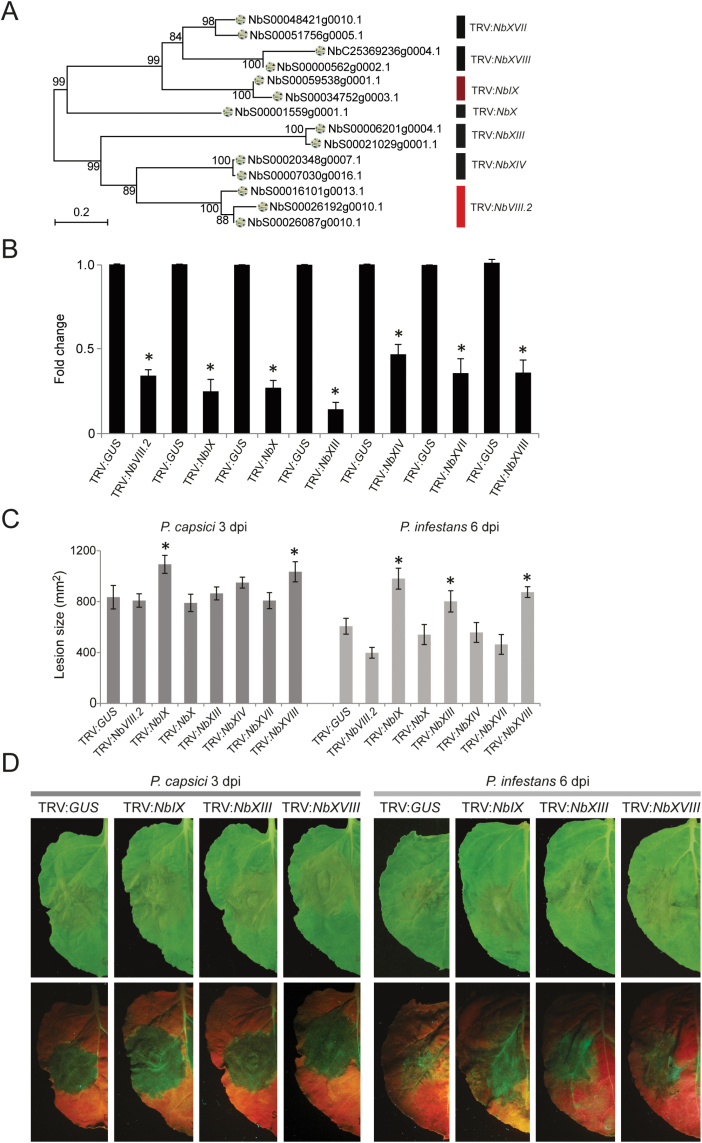
To determine the role of NbLecRKs in Phytophthora resistance we selected homologues of AtLecRKs that, in a previous study, were identified as putative resistance components in Arabidopsis (Wang et al., 2014). That study revealed 14 AtLecRKs implicated in Phytophthora resistance. TRV-based constructs were designed to silence NbLecRKs that are homologous to six of these AtLecRKs (i.e. AtLecRK-VIII.2, AtLecRK-IX.1, AtLecRK-IX.2, AtLecRK-S.1, AtLecRK-S.6 and AtLecRK-S.7) and NbLecRKs, belonging to two Solanaceous-specific clades, clades XVII and XVIII. Loss of function of a single gene might fail to cause any phenotypic change as its function can be either completely or partially complemented by the presence of homologous genes. To avoid this problem, we made TRV-based constructs to simultaneously silence several LecRKs with high sequence similarity. As shown in Fig. 3A, seven TRV constructs were generated, each of which was designed to target only LecRKs belonging to an assigned clade. Expression levels were determined using clade-specific primers (Supplementary Table S1). In all cases, at least a 50% reduction in transcript levels of the targeted NbLecRKs was observed in the NbLecRK-silenced N. benthamiana plants when compared with TRV:GUS-treated plants (Fig. 3B). NbLecRK silencing did not affect plant growth and development, as none of the NbLecRK-silenced plants showed consistent phenotypic changes in terms of plant size, leaf colour, leaf morphology, or shoot growth (Supplementary Fig. S2, available at JXB online). Upon inoculation with P. capsici LT263 or P. infestans 14-3-GFP, TRV:NbIX- and TRV:NbXVIII-treated N. benthamiana leaves showed significantly larger lesions compared with those observed on TRV:GUS-treated plants (Fig. 3C, D). The increased susceptibility of TRV:NbIX- and TRV:NbXVIII-treated N. benthamiana plants indicated that the corresponding silenced LecRKs are involved in Phytophthora resistance. In contrast, none of the plants treated by TRV:NbVIII.2, TRV:NbX, TRV:NbXIV, or TRV:NbXVII showed significant differences in susceptibility to either P. capsici or P. infestans when compared with TRV:GUS-treated plants (Fig. 3C). TRV:NbXIII-treated plant, however, showed increased susceptibility upon infection by P. infestans 14-3-GFP but not when inoculated with P. capsici LT263 (Fig. 3C). It is possible that the activity of some of these NbLecRKs was simply too weak to prevent Phytophthora infection. This is exemplified by the fact that TRV:NbXIII-treated plants allowed P. infestans isolate 14-3-GFP to expand but not P. capsici LT263, an isolate with relatively higher virulence (Fig. 3D). Also, the sequence divergence between the AtLecRKs and their N. benthamiana homologues may contribute to the functional divergence that we observed.
Fig. 3.
Response of NbLecRK-silenced N. benthamiana to Phytophthora infection. (A) NbLecRKs targeted by different TRV constructs. Colours refer to clades as represented in Table 1. (B) Relative NbLecRK transcript levels in TRV:GUS- and TRV:NbLecRK-treated N. benthamiana leaves. Transcript levels were normalized with NbActin and expressed as mean fold changes across four biological replicates (±SD) relative to the transcript level in TRV:GUS-treated leaves, which was arbitrarily set as 1. * indicates significant difference in expression levels (P<0.05, two-tailed t-test) between TRV:GUS- and TRV:NbLecRK-treated plants. (C) Lesion sizes on TRV:GUS- and TRV:NbLecRK-treated N. benthamiana leaves upon inoculation with P. capsici LT263 (1×105 zoospores ml–1) and P. infestans 14-3-GFP (5×105 zoospores ml–1) at 3 and 6 dpi, respectively. Bars represent mean lesion sizes (±SE) of over 20 inoculation sites from six independent plants. * indicates significant difference in lesion sizes (P<0.05, two-tailed t-test) between TRV:GUS- and TRV:NbLecRK-treated plants. This experiment was repeated four times with similar results. (D) Disease symptoms on TRV:GUS-, TRV:NbIX- TRV:NbXIII-, and TRV:NbXVIII-treated N. benthamiana leaves after inoculation with P. capsici at 3 dpi and P. infestans at 6 dpi. The pictures in the bottom row were taken under UV light. (This figure is available in colour at JXB online.)
NbLecRK silencing does not alter elicitor-induced cell death in N. benthamiana
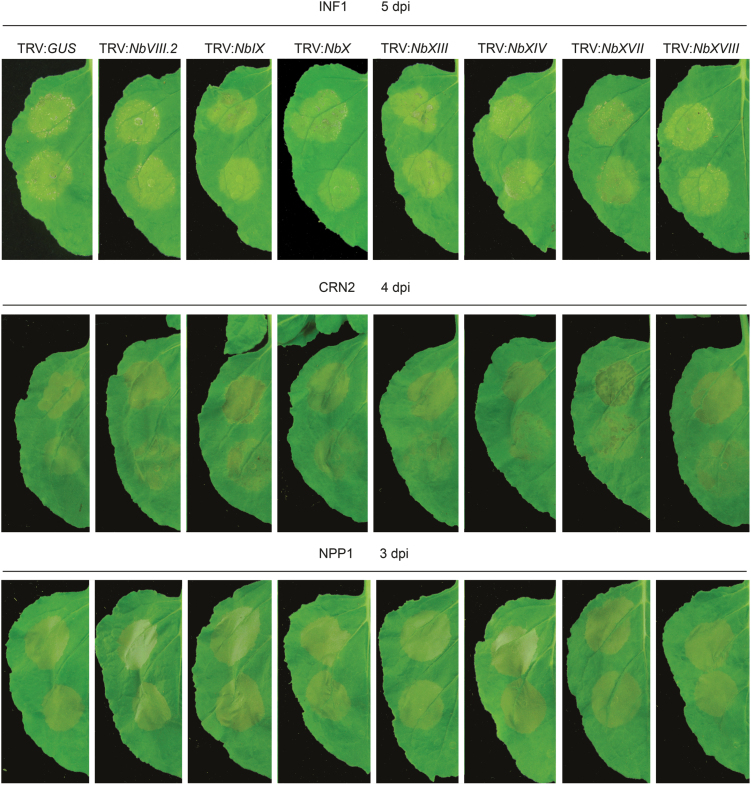
NbLRK1 was found previously to interact with the P. infestans elicitor INF1 via its kinase domain (Kanzaki et al., 2008). In that study, silencing of NbLRK1 in N. benthamiana compromised INF1-induced cell death and was proposed to be an important component of a host receptor complex that recognizes INF1 (Kanzaki et al., 2008). We found four LecRKs in N. benthamiana that were homologous to NbLRK1 (Table 1). Sequence alignment of the cDNA sequences revealed that three of these four were probably silenced by the fragment used by Kanzaki et al. (2008) (termed NbVIII.2-2 in Supplementary Fig. S3, available at JXB online), raising the question of which of the three genes is responsible for the compromised cell death that they observed. In this study, we used another fragment, named TRV:NbVIII.2, to simultaneously silence these three NbLecRKs (Fig. 3A and Supplementary Fig. S3) and confirmed by qRT-PCR using clade-specific primers that the overall NbVIII.2 transcript level was reduced (Fig. 3B). In contrast to the results of Kanzaki et al. (2008), we found no difference in the appearance of cell death upon transient expression of inf1 between N. benthamiana plants treated with TRV:NbVIII.2 and TRV:GUS (Fig. 4). This discrepancy could be due to the fact that Kanzaki et al. (2008) infiltrated the leaves with INF1 protein, whereas we expressed the inf1 gene in planta by Agrobacterium-mediated transient expression. To exclude this, we also performed the assay in a similar way as described by Kanzaki et al. (2008), namely by infiltrating INF1 protein into silenced N. benthamiana leaves, but again no differences in hypersensitive response were observed between N. benthamiana plants treated with TRV:NbVIII.2 and TRV:GUS at 3 or 6 dpi (Supplementary Fig. S4, available at JXB online). In the natural situation, INF1 is secreted by P. infestans into the plant apoplast and is assumed to remain in the apoplastic space (Kamoun, 2006). Hence, it is puzzling how INF1 can interact with the cytoplasmic kinase domain of NbLRK1 to mediate cell death induction. Kanzaki et al. (2008) hypothesized that INF1 could be translocated into plant cells via receptor-mediated endocytosis, but they were not able to show an interaction between INF1 and NbLRK1 in planta. More recently, it was shown that INF1 recognition in Solanum microdontum is mediated by the receptor-like protein ELR (Du et al. 2015).
Fig. 4.
Silencing of NbLecRKs does not affect cell death induced by Phytophthora elicitors. Cell death in TRV:GUS- and TRV:NbLecRK-treated N. benthamiana leaves expressing inf1, crn2, and npp1. Each experiment included at least six leaves from three independent plants per construct. This experiment was repeated three times with similar results. (This figure is available in colour at JXB online.)
We also investigated whether other NbLecRKs affected cell death triggered by INF1 and whether NbLecRKs play a role in cell death triggered by two other Phytophthora elicitors, CRN2 and NPP1. Transient expression of inf1, crn2, and npp1 in NbLecRK-silenced plants resulted in cell death in all cases, and no visible differences in the appearance of cell death were observed when compared with TRV:GUS-treated leaves (Fig. 4). These results suggested that none of the tested NbLecRKs plays an essential role in cell death induction trigged by these three elicitors.
Silencing of the tomato LecRK homologue of AtLecRK-IX.1/LecRK-IX.2 reduces Phytophthora resistance
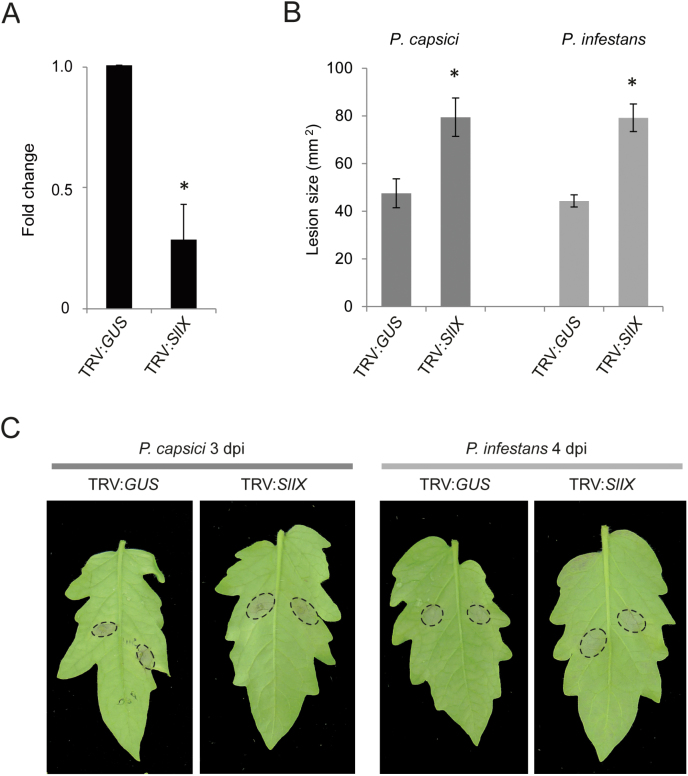
To assess whether the homologue of AtLecRK-IX.1/LecRK-IX.2 in tomato also plays a role in Phytophthora resistance, the TRV construct TRV:SlIX was generated to silence Solyc03g043710.1.1, the homologue closest to LecRK-IX.1 and LecRK-IX.2 (Fig. 2). Quantitative analysis of transcript levels in TRV:SlIX-treated plants revealed around a 60% reduction in expression in comparison with TRV:GUS-treated plants (Fig. 5A). Inoculation with zoospores of P. capsici LT263 or P. infestans 14-3-GFP on TRV:SlIX-treated plants resulted in lesions that were significantly larger than the lesions on TRV:GUS-treated plants (Fig. 5B, C). Larger lesions were also observed on TRV:SlIX-treated tomato leaves upon inoculation with mycelial plugs of P. capsici LT263 (Supplementary Fig. S5, available at JXB online). Taken together, these results indicated that the closely related tomato homologue of AtLecRK-IX.1 and AtLecRK-IX.2 plays a role in Phytophthora resistance similar to NbLecRK-IX.1/2, the homologue in N. benthamiana.
Fig. 5.
Silencing of SlLecRK-IX.1/LecRK-IX.2 compromises Phytophthora resistance in tomato. (A) Relative SlLecRK transcript levels in TRV:GUS- and TRV:SlIX-treated tomato leaves. Transcript levels were normalized with SlActin and expressed as mean fold changes across four biological replicates (±SD) relative to the transcript level in TRV:GUS-treated leaves, which was arbitrarily set as 1. * indicates significant difference in expression levels (P<0.05, two-tailed t-test) between TRV:GUS- and TRV:SlIX-treated plants. (B, C) Disease symptoms (B) and quantified lesion sizes (C) on TRV:GUS- and TRV:SlIX-treated tomato leaves inoculated with P. capsici LT263 (1×105 zoospores ml–1) and P. infestans 14-3-GFP (5×105 zoospores ml–1). Each experiment included at least 20 leaves from four independent plants treated with each construct. Bars represent the mean lesion sizes (±SE). * indicates significant difference (P<0.05) in lesion sizes between TRV:GUS- and TRV:SlIX-treated plants according to a two-tailed t-test. The infection assay with P. capsici was performed twice, whereas infection assays with P. infestans were repeated four times with similar results. (This figure is available in colour at JXB online.)
Conclusions
In total, 38 and 22 LecRKs were identified in the genomes of N. benthamiana and tomato, respectively. Multiple Solanaceous LecRK gene models deposited in the SGN database were found to be erroneous due to mis-prediction of the open reading frame and were corrected based on transcriptome data. Domain composition analysis indicated that most of the identified LecRKs have a typical LecRK structure, but there are several LecRKs, especially those from N. benthamiana, that lack an SP domain, a TM domain, or both. Phylogenetic analysis revealed that most of the Solanaceous LecRKs group together with Arabidopsis LecRKs, whereas four clades seem to be Solanaceous specific. For TRV-mediated gene silencing, we designed constructs that targeted all genes within a specific clade. Functional analysis using these constructs demonstrated that homologues of AtLecRK-IX.1/LecRK-IX.2 in both N. benthamiana and tomato function in resistance to different Phytophthora pathogens, and apparently the Phytophthora resistance function of clade IX LecRKs is conserved across different plant species. Although computational sequence analysis confirmed that the constructs were clade specific, we cannot fully exclude off-target silencing, nor can we predict to what extent the expression level of an individual gene within a clade is affected. Future research focused at unravelling the role of Solanaceous LecRKs in Phytophthora resistance requires more precise methods that allow functional analyses of each individual gene either within clade IX or within the Solanaceous-specific LecRK clades.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Table S1. Primers used in this study.
Supplementary Fig. S1. Neighbour-joining trees constructed based on the lectin domains (A) and kinase domains (B) of 43 AtLecRKs, 38 NbLecRKs and 22 SlLecRKs.
Supplementary Fig. S2. Morphology of N. benthamiana plants treated by TRV:NbLecRKs, TRV:PDS and TRV:GUS.
Supplementary Fig. S3. Sequence alignment of NbS00016101g0013.1, NbS00026192g0010.1, NbS00026 087g0010.1 and the fragments used for silencing.
Supplementary Fig. S4. Cell death induced by INF1 on TRV:GUS- and TRV:NbVIII.2-treated plants. Pictures were taken at three and six days after syringe-infiltration. Each experiment consisted of at least six infiltration sites. This experiment was repeated twice with similar results.
Supplementary Fig. S5. Quantified lesion sizes on TRV:GUS- and TRV:SlIX-treated tomato leaves 3 d after inoculation with P. capsici plugs (diameter 0.5cm). This experiment included 16 leaves from four independent plants treated with each construct. * indicates significant difference (P < 0.05) in lesion sizes between TRV:GUS- and TRV:SlIX-treated plants according to a two-tailed t test. This experiment was repeated twice with similar results.
Supplementary File S1. LecRK sequences of N. benthamiana and tomato. Highlighted protein sequences where shown to be inconsistent with RNA-seq data. Revised sequences are labelled ‘Corrected’. Introns within genomic sequences are underlined.
Supplementary File S2. DNA sequences of the fragments used for TRV-mediated silencing in N. benthamiana and tomato.
Acknowledgements
We thank Harold Meijer, Michael Seidl and Luigi Faino for discussion, Huchen Li and David Nsibo for assistance, Patrick Smit and Daniela Sueldo for providing the pSol2095 vector and GFP-HA construct, and Henk Smid and Bert Essenstam at Unifarm for excellent plant care. This research was supported by a Wageningen University sandwich-PhD fellowship and a Huygens scholarship (YW), a VENI grant (KB) from The Netherlands Organization for Scientific Research (Technology Foundation NWO-STW) and the Food-for-Thought campaign of the Wageningen University Fund.
Glossary
Abbreviations:
- DAMP
damage-associated molecular pattern
- dpi
days post-inoculation
- EST
expressed sequence tag
- ETI
effector-triggered immunity
- LecRK
L-type lectin receptor kinase
- MAMP
microbe-associated molecular pattern
- NLR
nucleotide-binding leucine-rich repeat receptor
- PRR
pattern recognition receptor
- qRT-PCR
quantitative reverse transcription PCR
- RLK
receptor-like kinase
- RNA-seq
RNA sequencing
- SP
signal peptide
- TM
transmembrane
- TRV
tobacco rattle virus.
References
- Afzal AJ, Wood AJ, Lightfoot DA. 2008. Plant receptor-like serine threonine kinases: roles in signaling and plant defense. Molecular Plant–Microbe Interactions 21, 507–517. [DOI] [PubMed] [Google Scholar]
- Boller T, Felix G. 2009. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annual Review of Plant Biology 60, 379–406. [DOI] [PubMed] [Google Scholar]
- Bouwmeester K, de Sain M, Weide R, Gouget A, Klamer S, Canut H, Govers F. 2011. The lectin receptor kinase LecRK-I.9 is a novel Phytophthora resistance component and a potential host target for a RXLR effector. PLoS Pathogens 7, e1001327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester K, Govers F. 2009. Arabidopsis L-type lectin receptor kinases: phylogeny, classification, and expression profiles. Journal of Experimental Botany 60, 4383–4396. [DOI] [PubMed] [Google Scholar]
- Bouwmeester K, Han M, Blanco-Portales R, Song W, Weide R, Guo L, van der Vossen EAG, Govers F. 2014. The Arabidopsis lectin receptor kinase LecRK-I.9 enhances resistance to Phytophthora infestans in Solanaceous plants. Plant Biotechnology Journal 12, 10–16. [DOI] [PubMed] [Google Scholar]
- Bouwmeester K, van Poppel PMJA, Govers F. 2009. Genome biology cracks enigmas of oomycete plant pathogens. In: Parker J, ed. Molecular aspects of plant disease resistance. Oxford, UK: Wiley-Blackwell, 102–133. [Google Scholar]
- Champouret N, Bouwmeester K, Rietman H, et al. 2009. Phytophthora infestans isolates lacking class I ipiO variants are virulent on Rpi-blb1 potato. Molecular Plant–Microbe Interactions 22, 1535–1545. [DOI] [PubMed] [Google Scholar]
- Choi J, Tanaka K, Cao Y, Qi Y, Qiu J, Liang Y, Lee SY, Stacey G. 2014. Identification of a plant receptor for extracellular ATP. Science 343, 290–294. [DOI] [PubMed] [Google Scholar]
- Dardick C, Ronald P. 2006. Plant and animal pathogen recognition receptors signal through non-RD kinases. PLoS Pathogens 2, 14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclos-Theveniau M, Arnaud D, Huang TY, Lin GJC, Chen WY, Lin YC, Zimmerli L. 2012. The Arabidopsis lectin receptor kinase LecRK-V.5 represses stomatal immunity induced by Pseudomonas syringae pv. tomato DC3000. PLoS Pathogens 8, e1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Verzaux E, Chaparro-Garcia A, et al. 2015. Elicitin recognition confers enhanced resistance to Phytophthora infestans in potato. Nature Plants 1, doi:10.1038/nplants.2015.34. [DOI] [PubMed] [Google Scholar]
- Fry W. 2008. Phytophthora infestans: the plant (and R gene) destroyer. Molecular Plant Pathology 9, 385–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gomez L, Boller T. 2000. FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis . Molecular Cell 5, 1003–1011. [DOI] [PubMed] [Google Scholar]
- Gouget A, Senchou V, Govers F, Sanson A, Barre A, Rouge P, Pont-Lezica RP, Canut H. 2006. Lectin receptor kinases participate in protein-protein interactions to mediate plasma membrane-cell wall adhesions in Arabidopsis . Plant Physiology 140, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouhier-Darimont C, Schmiesing A, Bonnet C, Lassueur S, Reymond P. 2013. Signalling of Arabidopsis thaliana response to Pieris brassicae eggs shares similarities with PAMP-triggered immunity. Journal of Experimental Botany 64, 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BG. 2013. Building phylogenetic trees from molecular data with MEGA. Molecular Biology and Evolution 30, 1229–1235. [DOI] [PubMed] [Google Scholar]
- Hanks SK. 2003. Genomic analysis of the eukaryotic protein kinase superfamily: a perspective. Genome Biology 4, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann DR, Rathjen JP. 2007. Early events in the pathogenicity of Pseudomonas syringae on Nicotiana benthamiana . The Plant Journal 49, 607–618. [DOI] [PubMed] [Google Scholar]
- Hofberger JA, Nsibo DL, Govers F, Bouwmeester K, Schranz ME. 2015. A complex interplay of tandem- and whole genome duplication drives expansion of the L-type lectin receptor kinase gene family in the Brassicaceae. Genome Biology and Evolution 7, 720–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PY, Zimmerli L. 2014. Enhancing crop innate immunity: new promising trends. Frontiers in Plant Science 5, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun S. 2006. A catalogue of the effector secretome of plant pathogenic oomycetes. Annual Review of Phytopathology 44, 41–60. [DOI] [PubMed] [Google Scholar]
- Kanzaki H, Saitoh H, Takahashi Y, Berberich T, Ito A, Kamoun S, Terauchi R. 2008. NbLRK1, a lectin-like receptor kinase protein of Nicotiana benthamiana, interacts with Phytophthora infestans INF1 elicitin and mediates INF1-induced cell death. Planta 228, 977–987. [DOI] [PubMed] [Google Scholar]
- Kasuga T, Gijzen M. 2013. Epigenetics and the evolution of virulence. Trends in Microbiology 21, 575–582. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Meinke D. 2010. The development of Arabidopsis as a model plant. The Plant Journal 61, 909–921. [DOI] [PubMed] [Google Scholar]
- Kornev AP, Haste NM, Taylor SS, Ten Eyck LF. 2006. Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proceedings of the National Academy of Sciences, USA 103, 17783–17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon LPNM, Brouwer H, de Cock AWAM, Govers F. 2012. The genus Phytophthora anno 2012. Phytopathology 102, 348–364. [DOI] [PubMed] [Google Scholar]
- Lamour KH, Stam R, Jupe J, Huitema E. 2012. The oomycete broad-host-range pathogen Phytophthora capsici . Molecular Plant Pathology 13, 329–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim F, Nakasugi K, Crowhurst RN, Hilario E, Zwart AB, Hellens RP, Taylor JM, Waterhouse PM, Wood CC. 2012. Advanced engineering of lipid metabolism in Nicotiana benthamiana using a draft genome and the V2 viral silencing-suppressor protein. PLoS One 7, e52717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasugi K, Crowhurst RN, Bally J, Wood CC, Hellens RP, Waterhouse PM. 2013. De novo transcriptome sequence assembly and analysis of RNA silencing genes of Nicotiana benthamiana . PLoS One 8, e59534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen B, Taylor S, Ghosh G. 2004. Regulation of protein kinases: controlling activity through activation segment conformation. Molecular Cell 15, 661–675. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Dubery IA, van Heerden H. 2013. Identification and molecular characterisation of a lectin receptor-like kinase (GhLecRK-2) from cotton. Plant Molecular Biology Reporter 31, 9–20. [Google Scholar]
- Robatzek S, Bittel P, Chinchilla D, Köchner P, Felix G, Shiu SH, Boller T. 2007. Molecular identification and characterization of the tomato flagellin receptor LeFLS2, an orthologue of Arabidopsis FLS2 exhibiting characteristically different perception specificities. Plant Molecular Biology 64, 539–547. [DOI] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB. 2001. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proceedings of the National Academy of Sciences, USA 98, 10763–10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB. 2003. Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis . Plant Physiology 132, 530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Chien CC, Mishra S, Tsai CH, Zimmerli L. 2013. The Arabidopsis LECTIN RECEPTOR KINASE-VI.2 is a functional protein kinase and is dispensable for basal resistance to Botrytis cinerea . Plant Signaling & Behavior 8, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Kuo YC, Mishra S, et al. 2012. The lectin receptor kinase-VI.2 is required for priming and positively regulates Arabidopsis pattern-triggered immunity. The Plant Cell 24, 1256–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai R, Isogai A, Takayama S, Che FS. 2008. Analysis of flagellin perception mediated by flg22 receptor OsFLS2 in rice. Molecular Plant–Microbe Interactions 21, 1635–1642. [DOI] [PubMed] [Google Scholar]
- Vleeshouwers VGAA, van Dooijeweert W, Keizer LCP, Sijpkes L, Govers F, Colon LT. 1999. A laboratory assay for Phytophthora infestans resistance in various Solanum species reflects the field situation. European Journal of Plant Pathology 105, 241–250. [Google Scholar]
- Vleeshouwers VGAA, Raffaele S, Vossen JH, et al. 2011. Understanding and exploiting late blight resistance in the age of effectors. Annual Review of Phytopathology 49, 507–531. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bouwmeester K, Beseh P, Shan W, Govers F. 2014. Phenotypic analyses of Arabidopsis T-DNA insertion lines and expression profiling reveal that multiple L-type lectin receptor kinases are involved in plant immunity. Molecular Plant–Microbe Interactions 27, 1390–1402. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bouwmeester K, van de Mortel JE, Shan W, Govers F. 2013. A novel Arabidopsis-oomycete pathosystem: differential interactions with Phytophthora capsici reveal a role for camalexin, indole glucosinolates and salicylic acid in defense. Plant, Cell & Environment 36, 1192–1203. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang M, Ke K, Lu Y. 2005. Cellular localization and biochemical characterization of a novel calcium-dependent protein kinase from tobacco. Cell Research 15, 604–612. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang R, Xu X, He X, Sun B, Zhong Y, Liang Z, Luo S, Lin Y. 2014. Cucumis sativus L-type lectin receptor kinase (CsLecRK) gene family respond to Phytophthora melonis, Phytophthora capsici and water immersion in disease resistant and susceptible cucumber cultivars. Gene 549, 214–222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.