Highlight
Short photoperiod and apical dominance trigger a shared developmental bud programme at terminal and axillary positions, while the capacity to establish photoperiod-induced dormancy is lost in maturing para-dormant axillary buds.
Key words: Apical dominance, axillary bud, branching, BRC1-like, CENL1, dormancy, MAX1-like, PINL1-like, terminal bud.
Abstract
Tree architecture develops over time through the collective activity of apical and axillary meristems. Although the capacity of both meristems to form buds is crucial for perennial life, a comparative analysis is lacking. As shown here for hybrid aspen, axillary meristems engage in an elaborate process of axillary bud (AXB) formation, while apical dominance prevents outgrowth of branches. Development ceased when AXBs had formed an embryonic shoot (ES) with a predictable number of embryonic leaves at the bud maturation point (BMP). Under short days, terminal buds (TBs) formed an ES similar to that of AXBs, and both the TB and young AXBs above the BMP established dormancy. Quantitative PCR and in situ hybridizations showed that this shared ability and structural similarity was reflected at the molecular level. TBs and AXBs similarly regulated expression of meristem-specific and bud/branching-related genes, including CENTRORADIALIS-LIKE1 (CENL1), BRANCHED1 (BRC1), BRC2, and the strigolactone biosynthesis gene MORE AXILLARY BRANCHES1 (MAX1). Below the BMP, AXBs maintained high CENL1 expression at the rib meristem, suggesting that it serves to maintain poise for growth. In support of this, decapitation initiated outgrowth of CENL1-expressing AXBs, but not of dormant AXBs that had switched CENL1 off. This singles out CENL1 as a rib meristem marker for para-dormancy. BRC1 and MAX1 genes, which may counterbalance CENL1, were down-regulated in decapitation-activated AXBs. The results showed that removal of apical dominance shifted AXB gene expression toward that of apices, while developing TBs adopted the expression pattern of para-dormant AXBs. Bud development thus follows a shared developmental pattern at terminal and axillary positions, despite being triggered by short days and apical dominance, respectively.
Introduction
The distinctive architecture of a tree is derived from the collective activity of shoot meristems, seated at the apex and formed laterally in the axils of leaves. While the shoot apical meristem (SAM) extends the main axis, branches arise from axillary meristems (AXMs). Each species has a genetic ground plan, referred to as its ‘architectural model’ (Hallé et al., 1978; Tomlinson, 1983; Millet et al., 1999). However, the intricate and detailed form of the crown that emerges over time is the result of internal developmental competition between branches, and interaction with the external environment (Tomlinson, 1983; Barthélémy and Caraglio, 2007; Pfennig et al., 2010; Donnelly et al., 2012). The overall plasticity of development is vividly illustrated in the practice of bonsai, in which seedlings (‘sai’) of potentially huge trees are forced to grow in a miniaturized form in a ‘bon’, a tray-like pot. For temperate perennials, seasonal change is a major force that constrains and modulates the architectural process. This is particularly evident in deciduous woody perennials, where new shoots arise in spring from buds that overwintered on existing structures, which themselves were affected by past weather conditions.
The SAM, the basic organization of which is shared by all angiosperms (Sussex, 1989; Sachs, 1991; van der Schoot and Rinne, 1999; Jürgens, 2003), is the ultimate origin of the shoot system and its architectural layout (Sussex, 1989; Sachs, 1991). Nonetheless, different branching strategies have evolved. For example, in Arabidopsis, the formation of AXMs is delayed. Although in this species the identity of a leaf axil is initially secured by expression of LATERAL SUPPRESSOR (Greb et al., 2003) and REGULATOR OF AXILLARY MERISTEM1 (Keller et al., 2006), the meristem identity gene SHOOT MERISTEMLESS (STM) is expressed first in the axils of older rosette leaves (Grbic and Bleecker, 2000; Long and Barton, 2000; Greb et al., 2003). In contrast, in many angiosperms, including tree species, AXMs emerge in initial continuity with the SAM to give rise to axillary buds (AXBs) (Garrison, 1955; Esau, 1977).
The architectural possibilities of a tree crown are phyllotactically pre-determined, but realized only through differential activation of AXBs and outgrowth into branches. Some AXBs may remain inhibited for decades (Rinne et al., 1993; Meier et al., 2012). Adventitious branches, which do not follow the phyllotactic pattern, develop only under special circumstances, for example when all buds are removed (Rinne et al., 1987). Despite their significance, AXB formation and branching in woody perennials are scantily investigated. Woody perennials employ two branching strategies based on the timing of AXB outgrowth. In sylleptic branching, AXBs give rise to branches in the same season, while in ‘proleptic’ (Hallé et al., 1978) or ‘delayed’ (Barthélémy and Caraglio, 2007) branching the AXBs can only grow out after they have passed through a dormancy period (Hallé et al., 1978). Syllepsis is common in tropical species (Hallé et al., 1978; Cline and Dong-Il, 2002), but it also occurs in some temperate species (Ceulemans et al., 1990; Wu and Hinckley, 2001) in which prolepsis prevails. Syllepsis is strongly modulated by environmental factors (Wu and Settler, 1998; Ceulemans et al., 1990; Wu and Hinckley, 2001), thereby contributing to crown plasticity, while the relatively stable proleptic branching style is thought to be under strong apical dominance (Cline, 1997).
Conventionally, apical dominance denotes the phenomenon whereby AXBs are held captive in an inactive state by a proliferating apex. In this view, the inactive state, referred to as para-dormancy (Lang et al., 1987), is enforced by auxin that is produced by the apex (Thimann and Skoog, 1934; Phillips, 1975; Cline, 1991, 1997). Indeed, simply removing the sources of auxin by decapitation abolishes apical dominance and removes AXB inhibition (Rinne et al., 1993; Cline, 1997). In recent years, significant progress has been made with herbaceous species in uncovering the mechanisms that regulate branching, and the emerging concepts may serve as a heuristic paradigm for branching in proleptic hybrid aspen. In annuals, branching involves genetic controls, auxin transport, as well as long-distance signalling (Wang and Li, 2006; Hamiaux et al., 2012; Brewer et al., 2013; Wang et al., 2014a; Wang et al., 2014b). In one model, branching requires the production and export of auxin from the activated AXB to the polar auxin transport stream (PATS) in the xylem parenchyma of the stem (Li and Bangerth, 1999). In a situation in which the apex monopolizes the PATS by ‘saturating’ its transport capacity, this would be difficult to achieve (Domagalska and Leyser, 2011). A prerequisite for branching is the production and positioning of PINFORMED1 (PIN1) auxin efflux carriers in the plasma membrane of cells between the bud and the stem (Balla et al., 2011; Domagalska and Leyser, 2011). Simply decapitating the plant will remove the dominant auxin source, and allow some activated AXBs to compete for access to the stem PATS. On the other hand, vigorously proliferating apices are not incompatible with branching, suggesting that important additional mechanisms are involved (Morris et al., 2005; Dun et al., 2006; Kitazawa et al., 2008). A downstream target of apically produced auxin is strigolactone, the biosynthesis of which may require MORE AXILLARY BRANCHES1 (MAX1) (Booker et al., 2005). Auxin and phosphate starvation may promote MAX1-mediated strigolactone biosynthesis in roots (Gomez-Roldan et al., 2008; Umehara et al., 2008). From there, strigolactone is translocated to the AXBs, where it inhibits branching (Ruyter-Spira et al., 2013). The inhibitory effect of strigolactone is counteracted by the promoting effect of root-produced cytokinins (Gomez-Roldan et al., 2008; Ferguson and Beveridge, 2009). As strigolactone diminishes PIN1-mediated PATS, and enhances competition between activated AXBs, this process remains a nexus of the mechanisms that control branching (Domagalska and Leyser, 2011).
The genes that regulate branching in annuals may also play a role in deciduous trees because the basic control mechanisms are conserved among angiosperms (Wang and Li, 2006). On the other hand, emergent layers of regulation must be in place to account for the existence of juvenile and adult tree stages, and the unique presence of a seasonal dormancy cycle. This complexity was recognized by Brown et al. (1967) who referred to the mechanism that supervises the overall shape and form of the crown via various branching processes as ‘apical control’. Even though apical dominance might not be sufficient to explain branching in woody shoot systems (Wareing, 1970), it is important in preventing branching in current-year shoots of older trees (Cline, 1997). Nonetheless, even in current-year shoots, the situation is more complex than in annuals due to their overwintering capacity.
In hybrid aspen, vegetative current-year AXBs can be in more than one state. They can be quiescent, an inactive state which sensu lato includes both para-dormancy and eco-dormancy, as well as dormant, a state in which the AXM is arrested by an intrinsic mechanism that is triggered by short days. Para-dormancy, due to apical dominance, can be abolished by decapitation, but dormancy is insensitive to decapitation and requires prolonged chilling. Although these different states can be established and defined experimentally (Lang et al., 1987; van der Schoot and Rinne, 2011), the similarities and differences at the molecular level are not well understood. In addition, AXBs and terminal buds (TBs) are formed at different phases of the seasonal cycle. AXBs develop under long days from AXMs that arise as daughter meristems from the SAM, while TBs develop under short days from a transitioning SAM (Wareing, 1956; Romberger, 1963; Rinne and van der Schoot, 1998; Rohde et al., 2002).
Dormancy research has mostly focused on TBs, which are initiated under short days after the complete down-regulation of FLOWERING LOCUS T (FT) in the leaves (Böhlenius et al., 2006; Hsu et al., 2006, 2011; Ruonala et al., 2008), and the early and gradual closing of plasmodesmata (PD) in the SAM (Rinne and van der Schoot, 1998; Ruonala et al., 2008). Extensive shifts were observed in the transcriptome of developing TBs, particularly in relation to ethylene and abscisic acid (ABA) signalling (Ruttink et al., 2007), while expression of dormancy-associated MADS box genes has been reported for some species (Horvath et al., 2010; Jiménez et al., 2010). Although AXBs can also establish dormancy under short days, it is uncertain if all AXBs have this capacity and which molecular mechanisms are involved.
An intriguing problem is how the AXBs and TBs safeguard the integrity of their SAM during dormancy. In annuals, meristem maintenance and functioning require the co-ordinated action of WUSCHEL (WUS) and CLAVATA3 (CLV3), which balances cell proliferation in the SAM, while the knotted-like homeobox (KNOX) gene knotted1 (KN1) prevents premature differentiation (Jackson et al., 1994; Laux et al., 1996; Schoof et al., 2000). WUS travels though PD to overlying stem cells to regulate CLV3 (Daum et al., 2014). In hybrid aspen, such movement must be absent in dormancy as PD are blocked (Rinne and van der Schoot, 1998; Rinne et al., 2001; Ruonala et al., 2008). In Arabidopsis, experimentally induced blockage of PD distorts or terminates the SAM (Daum et al., 2014). In woody perennials, where the cellular uncoupling mechanism is part of their natural survival strategy, this does not happen. It remained unknown how WUS/CLV/KN1 are regulated during dormancy establishment in both TB and AXBs.
A gene that might be important in AXB activation is CENTRORADIALIS-LIKE1 (CENL1), the Populus orthologue of the Arabidopsis meristem-identity gene TERMINAL FLOWER1 (TFL1). CENL1 is expressed and up-regulated in the rib meristem of the apex during TB development, but switched off during dormancy (Ruonala et al., 2008). CENL1 as well as the tomato orthologue SELF-PRUNING (SP) are also expressed in para-dormant AXBs of Populus and tomato, respectively (Pnueli et al., 1998; Mohamed et al., 2010; Lifschitz et al., 2014). The fact that TFL1/SP/CENL1 are expressed in vegetative AXBs, and that in Arabidopsis TFL1 influences branching (Ratcliffe et al., 1998), suggests that they promote branching. If so, inhibitory forces must keep AXBs in check, as in hybrid aspen branching does not occur in the first year. In non-woody species, branch inhibition genes include members of the TCP family (TEOSINTE BRANCHED1, CYCLOIDEA, and PCF), which encode transcription factors that operate exclusively within AXBs (Hubbard et al., 2002). In Arabidopsis, two orthologues have been identified, BRANCHED1 (BRC1) and BRC2 (Aguilar-Martínez et al., 2007; Finlayson, 2007; Niwa et al., 2013), but in trees these genes have not been studied.
The genus Populus displays considerable variation in branching styles among species and genotypes within species (Wu and Hinckley, 2001). The present work addresses the structural development of AXBs and TBs in first-year saplings of hybrid aspen (Populus tremula x P. tremuloides), clone T89. This clone has a strictly proleptic branching style, a sign of strong apical dominance (Cline, 1997), and therefore is ideally suited to investigate the endogenous mechanisms that govern both AXB development and its outgrowth to a branch. The commonalities and differences between para-dormant and dormant states, as well as branch initiation, were investigated through molecular analyses. The data showed that TBs and AXBs both produce an identically regulated dwarfed shoot system, despite the fact that their development is under control of distinct triggers, apical dominance and short photoperiod, and in different seasons. Both require up-regulation of CENL1, but whereas in para-dormancy CENL1 expression is maintained, in dormancy it is completely down-regulated. The results suggest a working model in which two distinct signalling pathways, short days and apical dominance, converge on a shared developmental programme for bud formation. So long as buds are in the early phase of development they remain susceptible to short days and capable of establishing dormancy.
Materials and methods
Plant material and designs for experiments
Hybrid aspen (Populus tremula×P. tremuloides) clone T89, and lines overexpressing oat (Avena sativa) phytochrome A (PHYA) (line 22; Ruonala et al., 2008) were micro-propagated in vitro, planted in soil, and grown in a greenhouse under long days (18h light) at ~18 °C and 75–80% relative humidity (RH), and watered twice a day. Natural light was supplemented to 200 μmol m–2 s–1 at 400–750nm (Osram). After 6 weeks, when the plants were 70–80cm tall and elongation and leaf production rates were constant, the plants were subdivided into three groups. Group one was kept in long days as a control. Group two was moved to short days (10h) for minimally 5 weeks to induce dormancy. Group three was decapitated just above a node at pre-determined distances from the apex (see below) to remove apical dominance. Following decapitation, the kinetics of AXB activation were measured for a number of consecutive days, initially with a digital micrometer (World Precision Instruments, USA) and subsequently with a ruler. Dormancy establishment after 7 weeks of short days was monitored in TBs and AXBs at different stem positions, using bud-internode cuttings under growth-promoting conditions [18h of long day, photosynthetic photon flux density (PPFD) 200 μmol m–2 s–1, 18 ○C, and 85% RH] at weekly intervals for 3 weeks (Rinne et al., 2011). This bud-internode system was also used to assess whether a xylem-fed synthetic strigolactone analogue (GR24) (Chiralix BV, The Netherlands) at concentrations of 0.5–5 μM inhibits AXB burst in hybrid aspen (n=3).
AXB and embryonic shoot development
AXB enlargement and embryonic shoot (ES) ontogeny were investigated in eight proliferating plants. From each plant the 30 uppermost AXBs were collected and their size measured under a dissection microscope. Subsequently, the AXBs were fixed in 70% alcohol, and prepared under the dissection microscope to record number, type, and position of scales, leaves, stipules, and primordia. Representative examples were documented in photographs. The information obtained was used to determine three nodal positions for stem decapitation: (i) an early stage of AXB development; (ii) a more advanced stage of development; and (iii) a mature AXB stage.
AXB anatomy
For light microscopy, AXBs were fixed overnight at 4 °C in 2% (v/v) glutaraldehyde and 3% (v/v) paraformaldehyde in 100mM phosphate citrate buffer (Rinne et al., 2001). Briefly, samples were infiltrated gradually with LR White Resin (LRW) of increasing concentration (30–70%), and kept for 4 d in 100% LRW. Polymerization was done at 55 °C for 24h. Median longitudinal sections, 1–3 μm thick, were stained with 1% aqueous Toluidine blue.
In situ hybridization of CENL1
The expression domain of CENL1 in apices and AXBs of wild-type hybrid aspen (clone T89) and transgenic lines ectopically overexpressing the oat PHYA gene (Ruonala et al., 2008) was visualized by a standard in situ hybridization technique, using digoxigenin and a Dig RNA Labeling kit (Roche) with modifications (Ruonala et al., 2008). Antisense and sense RNA probes were prepared from the CENL1 gene (Potri.004G203900, AY383600). To clone partial cDNA of PtCENL1, RNA was isolated from apices after plants were exposed to short days for 2 weeks, a time point at which CENL1 is up-regulated (Ruonala et al., 2008). cDNA was amplified using Phusion (F-530, Thermo Fisher Scientific). Forward and reverse primers for amplification were 5′-TCATGGCAAAGATGTCAGAGC and 5′-CTTTGGGCATTGAAGAAGACA, respectively. The PCR product was cloned into the EcoRV site (blunt-end cloning) of the pZErO-2 vector, which has T7 and SP6 sites flanking the multiple cloning site. The colour reaction to visualize the hybridized probe was carried out at room temperature for up to 20h. Sections were examined and photographed with a Zeiss Axioplan2, and an Olympus AX70 equipped with an Olympus DP70 digital camera.
RNA extraction and quantitative real-time PCR analysis
The apex and every second AXB between nodes 2 and 30 were collected from long-day plants. In parallel, apices and developing (growing) AXBs at nodal positions 2–14 were similarly collected from plants at short day week 2, 3, and 5. Two types of decapitation experiments were carried out. In the first experiment, the five AXBs immediately under the cut were analysed at 8 d and 14 d (not shown) post-decapitation. Based on these data, a second experiment was carried out in which the proximal AXB was collected after 1, 2, 3, 5, and 7 d. RNA was extracted from six plants and divided into two biological replicates, each containing material from three plants. Some of the investigated genes, such as PINL1, CLV1, and WUS, are potentially under diurnal regulation (http://diurnal.mocklerlab.org/). To make sure that the measured changes in gene expression were due to decapitation, and not to circadian variation, sampling was carried out at the same time of the day (Ruonala et al., 2008; Rinne et al., 2011). Specifically, all buds were harvested within the last hour of the light period under short days, and during the same hour in experiments with intact and decapitated long-day plants.
RNA was extracted from 0.2g of frozen tissue, and ground in a mortar with 750 μl of extraction buffer (Qiagen RTL buffer, containing 1% PVP-40). After addition of a 0.4 volume of KoAC at pH 6.5 and further grinding, the solution was transferred to a 2ml tube, incubated on ice for 15min, and centrifuged at 12 000rpm at 4 °C for 15min. The supernatant was transferred to a 1.5ml tube, and a 0.5 volume of 100% ethanol was added. The mix was transferred to two RNeasy-spin columns and further processed in accordance with instructions of the Qiagen Plant RNA isolation kit. RNA was DNase (Ambion) treated, cleaned using the total RNA purification system ‘Purelink RNA mini kit’ (Invitrogen), and reverse transcribed using SuperScriptIII reverse transcriptase (Invitrogen). Quantitative real-time PCR (qPCR) analyses were performed with the ABA Prism 7500Fast sequence detection system using SYBR Green PCR master mix (Applied Biosystems). Transcript levels were normalized using an actin gene. Gene-specific primer sequences for the analyses were designed using Primer3 (http://frodo.wi.mit.edu/primer3) (Supplementary Table S1 available at JXB online).
Bioinformatics
Phylogenetic analyses of Arabidopsis thaliana MAX1, BRC1, and BRC2 were carried out to identify orthologous proteins in perennial species with protein–protein BLAST searches in GenBank and the Populus trichocarpa genome v2.0 (Tuskan et al., 2006) databases (http://www.ncbi.nlm.nih.gov/BLAST; http://www.phytozome.net). ClustalW (http://www.ebi.ac.uk/Tools/msa/clustalw2) was used to perform multiple sequence alignments. A phylogenetic tree was created using the MEGA5 program (www.megasoftware.net) with the Neighbor–Joining method. Bootstrap support values are based on 1000 replicates.
Accession numbers
The P. trichocarpa gene model identifiers (Tuskan et al., 2006) and/or sequence accessions used for qPCR analysis are listed in Supplementary Table S1 at JXB online.
Results
Young AXBs contain a morphogenetically active AXM
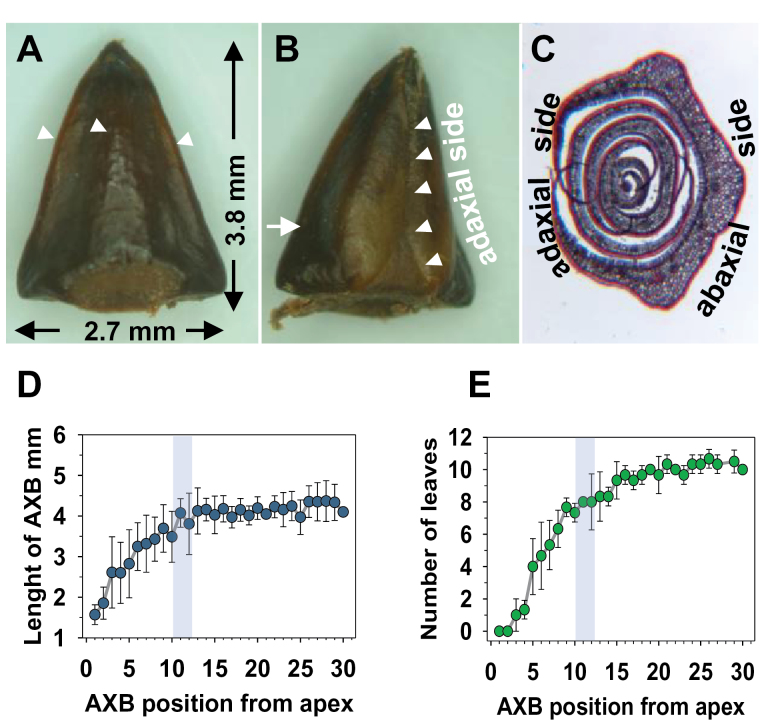
To map the developmental context in which para-dormancy, dormancy, and branching occur in hybrid aspen, the structural development and maturation of AXBs was investigated and compared with that of short-day-induced TBs. Morphometric analyses showed that the size of AXBs increased in the basipetal direction along the juvenile stem, but only up to a certain point, which was dubbed the ‘bud maturation point’ (BMP) (Fig. 1A–D). The increase in AXB size could not be due to a gradual activation of para-dormant AXBs because the hybrid aspen clone T89 delays branching to the next growing season. Surgical investigations of ~200 buds showed that the increase was due to the internal development of a dwarfed ES system (Romberger, 1963). Once the BMP was reached, the enclosed AXMs had commonly produced five scales and 10 ‘embryonic leaves’ (Fig. 1E).
Fig. 1.
Axillary bud development and structural details. (A) Abaxial face of the outer scale of a mature axillary bud (AXB), armoured with three ridges (arrowheads). (B) Adaxial side of the outer scale of a mature AXB. Overlapping scale edge (arrowheads). The arrow indicates the position of the cross-section through the AXB, as in C. (C) Cross-section of an AXB; the position of the axillary meristem is below the section plane. All scales are simple and lack conductive tissue. (D) AXB enlargement and (E) number of embryonic leaves the AXBs contain at different stages of their development. Shadowed areas indicate the approximate bud maturation point (BMP). Values represent means ±SD (n=8 plants). Toluidine blue staining (C).
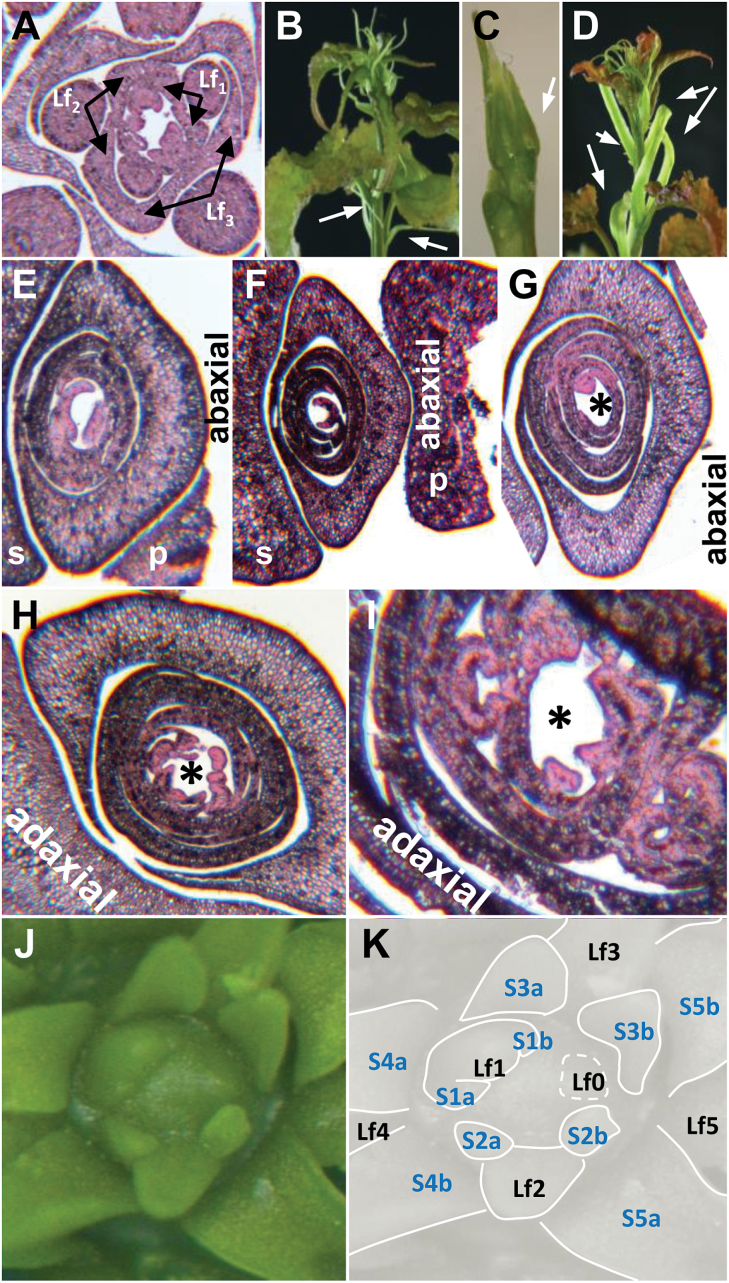
The scales of AXBs were distinct from those of TBs induced by short days. Whereas the paired scales of TBs developed from the stipules of transformed leaves (Fig. 2A–D), the scales of AXBs were ‘perfect scales’ that arose directly from the AXM without metamorphosis, and prior to leaf production. The perfect scale primordia were produced in an alternate pattern, with a 180° divergence angle, which resulted in a flattened overall shape (Figs 2E–K, 3A), whereas scales of TBs were positioned in a radial pattern (Fig. 3B). In AXBs, from leaf primordium six and onward, there was an abrupt change in fate, and primordia developed into miniaturized, embryonic leaves that arose in a spiral pattern. The ES of AXBs was highly similar to that of TBs, although in AXBs the divergence angle was larger, 2/5 (144°) compared with 3/8 (137°) in TBs (Fig. 3). The angle between the first two embryonic leaves in AXBs was deviant, enforced by the flattened shape of the AXB (Fig. 3A). Below the BMP, the AXMs ceased primordia production, although the youngest primordia would still become more pronounced and leaf like (Fig. 1D, E), tightly packing the bud space. It took ~4 weeks for a newly initiated AXB to reach the BMP, and during this period the proliferating SAM of the main stem had produced ~10 younger phytomers. The virtual absence of cell elongation in the rib meristem of the ES ensured that the AXB remained closed even after the BMP was reached. Briefly, the present data show that up to the BMP an AXB contains a growing and developing ES, and that AXBs become morphogenetically inactive only below this point.
Fig. 2.
Bud development at apical and axillary positions. (A–D) Short photoperiod-induced development of a terminal bud (TB) at the shoot apex. (E–K) Default axillary bud (AXB) development under long photoperiod. (A) Stipules, paired leaf base extensions, are initiated early during leaf development. Arrows indicate stipules of three leaf primordia (Lf1–Lf3). Three younger leaf primordia are visible in the centre. The meristem is below the section plane. (B) Stipules are paired thread-like structures that form under long days (arrows), but which under short days metamorphose into scales while the leaf lamina fails to develop. (C) An emerging TB, after a 2-week exposure to short days. Overarching leaves were surgically removed (note brownish colour on the developing scale, arrow). (D) Reversion of the apex to a regular growth pattern under long days, after a restricted 2–3 week short day exposure. Note the reverted scale-like stipules (arrows). (E) Immature AXBs with the first three scales, (F) all five scales, and subsequently one (G), three (H) and seven (I) embryonic leaves. The asterisk in (G–I) points to the axillary meristem position, just below the section plane. (J) Immature fixed AXB, opened under the microscope (digitally coloured, Photoshop). (K) Diagram of (J), depicting the arrangement of embryonic leaves. The youngest leaf buttress is Lf0. Each embryonic leaf has two stipules (e.g. S1a and S1b, stipules of Lf1). (A, E–I) Transverse sections, toluidine blue staining. s, stem; p, petiole.
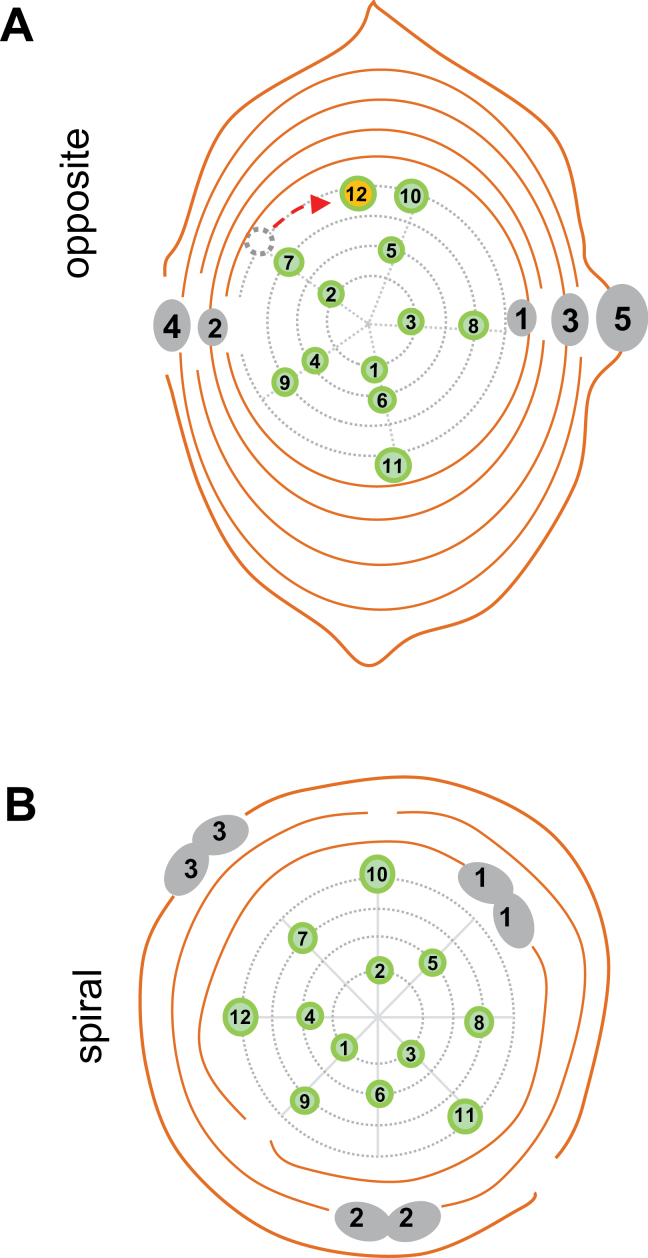
Fig. 3.
Schematic diagrams depicting primordia development and patterning in the apex (terminal bud) and axillary bud. (A) The first five primordia in axillary buds develop with opposite (decussate) arrangement (180°) and become scales. Number 5 is the abaxial outer scale. Subsequent 12 primordia develop into dwarfed embryonic leaves in a spiral pattern 2/5 (144°). The first embryonic leaf has a 90° divergence angle due to space constrains (red stippled arrow). (B) In the shoot apex the stipules of the first three (to five) primordia develop into scales (dual numbered brown lines) and the subsequent 12 primordia develop into dwarfed embryonic leaves. All primordia arise with 3/8 spiral pattern (135°) similar to proliferating apex.
AXBs are poised to burst around the BMP
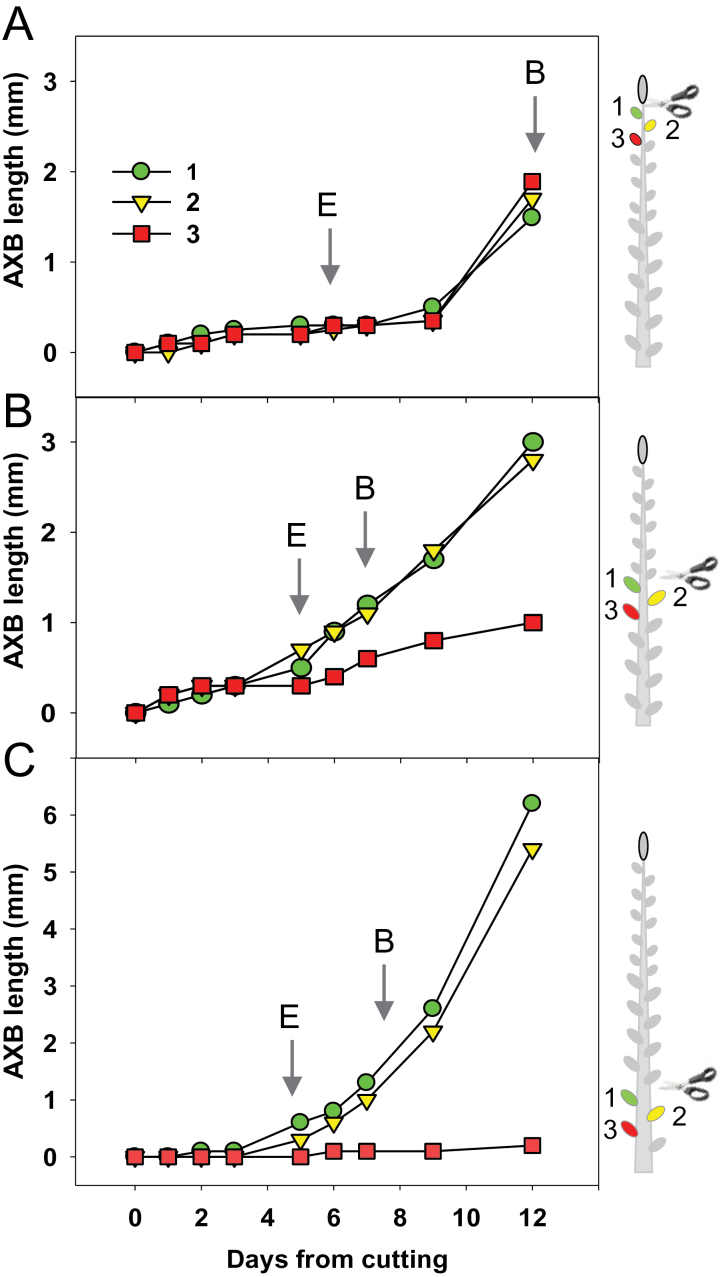
In the proleptic hybrid aspen clone T89, all branching from AXBs is absent during the first growing season, regardless of their maturation level, and even in the following season branching is restricted to a subset of AXBs. Nonetheless, first-season para-dormant AXBs can be activated by decapitation. To assess if AXB activation depends on the developmental status of the enclosed ES, plants were decapitated at various heights of the stem. This showed that, eventually, all AXBs are able to elongate and burst, but that the time required for activation is different (Fig. 4). The youngest AXBs needed an extended period of up to 2 weeks before bud burst was detectable (Fig. 4A), probably reflecting the time required to advance internal development. This suggests that a certain minimum stage of maturity (i.e. ES development) is required before bud elongation can take place. In contrast, AXBs at or below the BMP burst within a single week (Fig. 4B, C). Additional ageing of AXBs did not further advance the timing of bud burst, although older AXBs commonly produced faster growing branches (Fig. 4; Supplementary Fig. S1 at JXB online).
Fig. 4.
Decapitation-induced axillary bud (AXB) activation. Activation was assessed by measuring AXB elongation and burst. Stems were cut immediately under the apex (A), at the bud maturation point (BMP) (B), and at a lower position among mature AXBs (C) as illustrated in the schemes on the right (leaves are not depicted). The arrow marks the day when AXBs had significantly enlarged (E) (n=5; P<0.01; Student’s t-test), and the arrow ‘B’ when AXBs burst.
AXBs above the BMP are responsive to short days
Photoperiodic signals that are generated in the leaves are involved in triggering developmental transitions at the apex. Growing apices are strong sinks in which cellular proliferation is fuelled by sugars and nitrogenous compounds imported via the phloem. Simultaneously, phloem sap delivers mobile signals that redirect development, among others the signal peptide FLOWERING LOCUS T (FT) and a host of small RNAs. The AXBs above the BMP are also sinks, because they are actively producing cells and tissues for the developing ES, albeit that cell stretching is absent. This raised the question of whether, and to what degree, AXBs are susceptible to phloem-delivered signals, and if this would influence their ability to establish dormancy under short days. To address this experimentally, plants were exposed to a dormancy-inducing short-day regime, and subsequently the burst capacity of AXBs was tested at various stages of development and ageing. In the long-day conditions used here, the apex harbours ~10 leaf primordia, each with an AXM. During exposure to a short photoperiod, they produced AXBs that occupied positions 1–10 (Supplementary Fig. S2 at JXB online). For bud burst testing, this cluster was divided into two groups containing AXB 1–5 and AXB 6–10 (Table 1). The ~10 visible AXBs between the apex and the BMP were subdivided into group 1 and 2. After a short photoperiod, the AXBs of these groups occupied positions 11–15 and 16–20, respectively. The 10 AXBs directly below the BMP represented a mature and an ageing group (group 3 and 4, respectively). After a short photoperiod, the AXBs of these groups occupied positions 21–25 and 26–30, respectively (Table 1; Supplementary Fig. S2 at JXB online). For each category, the dormancy status of AXBs was assessed in single-node cutting tests under growth-promoting conditions (Table 1; Rinne et al., 2011). A pronounced negative correlation was consistently found between AXB maturity and the capacity to establish a dormant state (Table 1). The AXBs in the axils of the 10 youngest leaves (position 1–10) were dormant as they did not show any signs of burst, whereas the large majority of AXBs that in long days were clearly below the BMP (position 21–25) or in the ageing phase (position 26–30) did not establish dormancy. Above the BMP, most of the developing AXBs (position 11–15) established dormancy, but this capacity rapidly diminished during AXB completion toward the BMP (position 16–20). Together this showed that as a rule AXBs could establish dormancy during the phase of early development. Being a sink might thus be a major determinant of the capacity to establish dormancy.
Table 1.
Short day (SD)-induced AXB dormancy
The upper AXBs (position 1–10) that developed under SDs from meristems in the axils of existing primordia all established dormancy. The older AXBs (position 21–30), which were at or below the bud maturation point (BMP) when SD started, had already completed their development, and the majority did not develop dormancy. AXBs in the middle positions (11–20) represents the AXBs above the BMP in long-day plants. The youngest of these AXBs, which were still developing when SDs started, mostly established dormancy (11–15), but this capacity diminished in AXBs closer to the BMP (16–20). The morphologically determined BMP of long-day plants, at which new primordia no longer emerge, was reached around AXB position 12 (n=10 plants). SD-induced dormancy was assessed by testing the bud burst capacity under growth-promoting conditions.
| AXB position | Photoperiod during development | AXB burst % |
|---|---|---|
| 1–5 | SD | 0 |
| 6–10 | SD | 0 |
| 11–15 | LD/SD | 38 |
| 16–20 | LD/SD | 90 |
| 21–25 | LD | 90 |
| 26–30 | LD | 100 |
Abbreviations: AXB, axillary bud; BMP, bud maturation point; ES, embryonic shoot; PATS, polar auxin transport stream; PD, plasmodesmata; SAM, shoot apical meristem; TB, terminal bud.
Meristem-specific genes in AXB and TB development
The AXM, which produces an ES inside a developing AXB, is the equivalent of the SAM that produces an ES in the short-day-induced TB. The SAM and the AXM are actively engaged with primary morphogenesis so long as the ES is not complete. To map the expression of selected meristem identity genes during ES development, putative orthologues of the Arabidopsis genes WUS, CLV1, CLV3, and KN1 were identified in the P. trichocarpa genome (Tuskan et al., 2006), and their expression levels were analysed by qPCR.
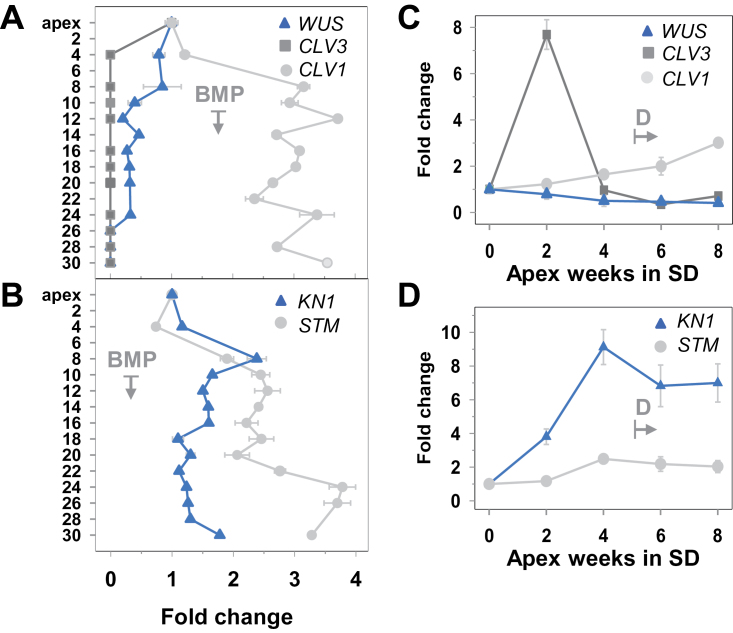
In apices, the two identified WUS-like genes were expressed at a similar level. The gene with highest similarity to the Arabidopsis WUS gene (WUSL1; Supplementary Table S1 at JXB online) was selected for further analysis. WUS expression in AXBs was lower than in the apex, and was gradually down-regulated towards the BMP. In ageing AXBs (below node 24), transcript levels fell below the detection limit (Fig. 5A). In short-day-induced TBs WUS was moderately down-regulated (Fig. 5C), but less than in AXBs.
Fig. 5.
Expression analysis of genes involved in meristem organization and maintenance during axillary bud (AXB) and terminal bud (TB) development. Expression patterns (fold changes) of hybrid aspen (A and C) WUSCHEL-like (WUS), CLAVATA 3-like (CLV3), and CLAVATA1-like (CLV1), and (B and D) KNOTTED1-like (KN1) and SHOOT MERISTEMLESS-like (STM) genes. (A and B) Expression levels in the apex and AXBs in long days (C and D), and expression in the apex under short days (SD). T-shaped arrows mark AXBs below the bud maturation point (BMP) (A and B), and the time point of dormancy induction (D) in TBs (C and D). Values represent the means of six plants ±SE, analysed in two pooled samples.
Blasting the Arabidopsis CLV1 against the P. trichocarpa genome resulted in a large number of highly significant hits, but CLV3 gave only three sequences with very low scores. A CLV3-like gene was selected on the basis of the presence of a C-terminus similar to the one in the Arabidopsis CLV3, and its presence in the shoot meristem library (http://popgenie.org/). The selected CLV1 and CLV3 genes were both expressed in the shoot apex of hybrid aspen. In developing AXBs, CLV1 was up-regulated 3-fold, corresponding to the level found in short-day-induced TBs (Fig. 5A, C). In the apex, CLV3 was expressed at a similar low level to WUS, whereas in all AXBs it was hardly expressed, or was below the detection limit (Fig. 5A). Under short days, CLV3 expression was up-regulated in TBs after 2 weeks of short days, but down-regulated at week 4, and further until dormancy was established (Fig. 5C).
The expression levels of the two selected KNOX genes, with high similarity to KN1 and STM, increased 2- and 3-fold during AXB development (Fig. 5B). While STM was further up-regulated during AXB ageing, KN1 decreased in AXBs below the BMP to apex levels (Fig. 5B). During short-day-induced TB formation, both genes were up-regulated, particularly KN1 (Fig. 5D). These patterns show that short-day-induced TBs and developing long-day AXBs share grossly similar gene expression trends, except for the transient expression peak in the putative CLV3 gene during early TB development.
Genes involved in SAM identity and branching
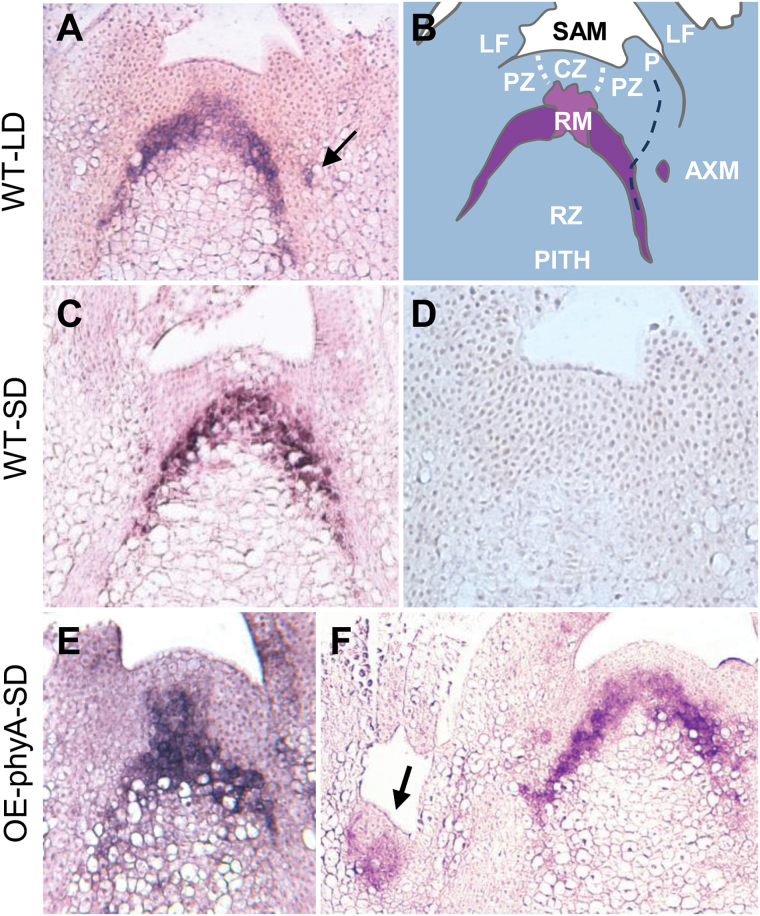
In trees, CENL1 is characteristically expressed in AXBs (Mohamed et al., 2010), as well as in the apex and short-day-induced TBs prior to dormancy establishment (Ruonala et al., 2008). As shown previously by qPCR, CENL1 is expressed in an area that corresponds approximately to the rib meristem in the apex of hybrid aspen (Ruonala et al., 2008). To establish this more precisely, the CENL1 expression domain was mapped by in situ hybridization in long-day apices and developing short-day-induced TBs at the time point when its transcript levels are known to rise (Ruonala et al., 2008). In the growing apex, CENL1 was expressed specifically in the rib meristem and the early descendant cells that encircled the radially expanding pith. In sections this was visible as a bell-shaped domain (Fig. 6A, B). In the rib meristem, where radial expansion is still absent, CENL1 was detected in a hat-shaped domain immediately subjacent to the SAM, and with the same width as the central zone of the meristem. CENL1 was also detected very early in emerging AXMs (Fig. 6A, B). In short-day-induced TBs the hat-shaped domain faded, corresponding to the arrest of cell elongation in the rib meristem (Fig. 6C). That CENL1 is involved in rib meristem activity is corroborated by observations on hybrid aspen lines that overexpress the oat PHYA gene. Whereas under long days the saplings are stunted, they accelerate internode elongation under short days, while the plastochron remains unchanged (Ruonala et al., 2008). Here it is shown that this is accompanied by a pronounced hat-shaped CENL1 expression domain (Fig. 6E). Although this was not visible in all sections (Fig. 6F), possibly relating to plastochron stage and rib meristem rhythmicity, it links enhanced rib meristem activity to expansion of the expression domain (Fig. 6E).
Fig. 6.
In situ hybridization of CENTRORADIALIS-LIKE1 (CENL1). (A–D) Expression patterns in the apex of wild-type (WT), and (E–F) transgenic hybrid aspen overexpressing oat phytochrome A (OE-phyA). (A) Long day (LD) apices show a bell-shaped expression domain, sheathing the uppermost part of the pith. Note CENL1 expression in young axillary meristem (AXM, arrow). (B) Schematic depiction of the expression domain in (A). The upper part of the CENL1 domain is the rib meristem (RM), immediately subjacent to the central zone (CZ). CENL1 expression reaches to cell layer 7 or 6, which may be upper rib meristem or lower corpus. Leaf primordia (P), and the position of pro-vascular tissue (stippled). PZ, peripheral zone; LF, leaf; RZ, rib zone, where cell division and elongation occur simultaneously. (C) CENL1 expression after 2 weeks of short days (SD). (D) Sense probe. (E) CENL1 expression after 1 week of short days. The domain reaches 1–2 cell layers higher than in the WT. (F) Expression domain after 2 weeks in short days. Note expression in AXM (arrow).
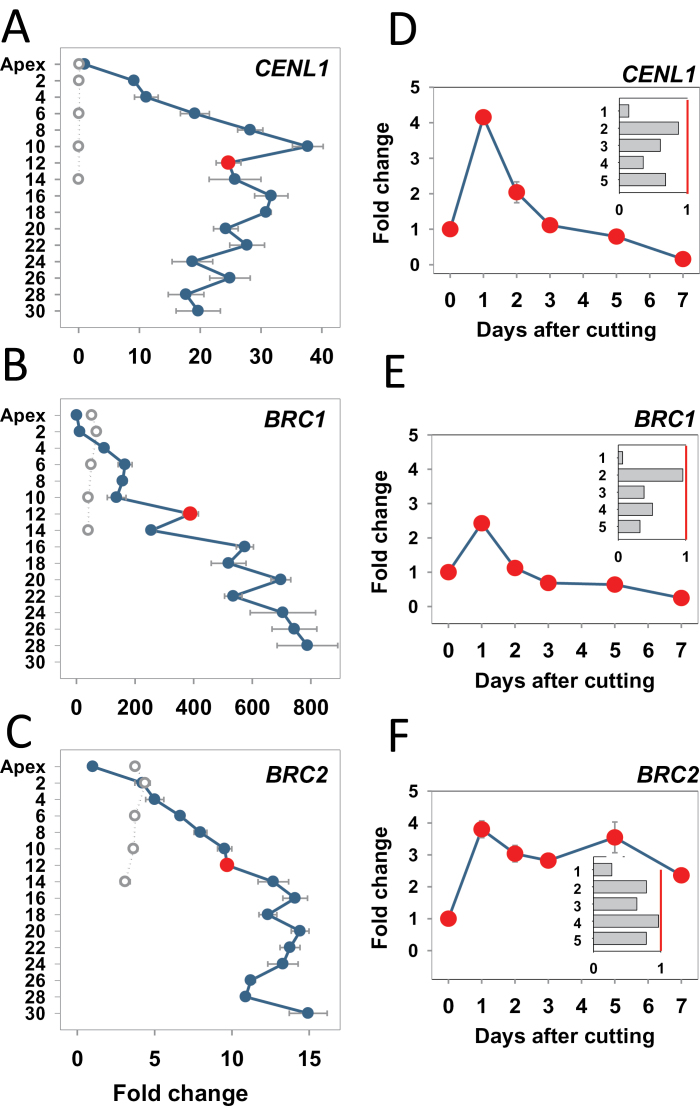
CENL1 expression was also established by qPCR in apices and AXBs. In developing AXBs, transcript levels gradually rose to ~35-fold at the BMP, relative to the apex (Fig. 7A). Below the BMP, CENL1 expression in AXBs was maintained at high levels. In strong contrast, in all AXBs that established dormancy, CENL1 was completely down-regulated (Fig. 7A). Thus, maintenance of CENL1 expression after bud completion characterizes para-dormancy and corresponds to a state that is poised for vegetative growth, whereas its complete down-regulation reflects dormancy establishment. Other phosphatidylethanolamine-binding protein family genes, CENL2, FT1, and FT2 (Supplementary Table S1 at JXB online), were hardly expressed in buds (not shown). In contrast, the P. trichocarpa homologue of BROTHER OF FT (BFT) showed a gradual 8- to 10-fold up-regulation (Supplementary Fig. S3A at JXB online), a trend similar to that of CENL1. Expression levels of BFT in the dormant TB were elevated 4-fold, relative to the long-day apex. Under short days, AXBs increased BFT expression approximately to the same level as they would have done under long days, but at 5 weeks BFT expression was equalized between TBs and AXBs that established dormancy.
Fig. 7.
Expression analysis of genes involved in meristem identity and branching in plants grown under long and short photoperiods and after stem decapitation. (A and D) CENTRORADIALIS-LIKE1 (CENL1); (B and E) BRANCHED1-like (BRC1); (C and F) BRANCHED2-like (BRC2). (A–C) Intact plants grown under long days (LD; blue dots and lines) and after 5 weeks under short days (SD; open circles, stippled lines). Long-day samples include the apex and axillary buds (AXBs) until node 30, and short-day samples include terminal buds and AXBs up to node 14. The red dot indicates the expression level (x-axis fold change) in AXB at node 12 of intact plants, the bud maturation point (BMP). (D–F) Stems were cut just above the BMP, and gene expression was followed for 7 d in AXB 12. Inserts indicate relative expression in five successive AXBs (position 1–5) proximal to the cut, 8 d after decapitation (y-axis fold change). Values in A–C are calculated relative to the apex (set at 1). Values in D–F are relative to AXB 12 of the intact plant (set at 1, red dot in A–C). Values in the insets of D–F are relative to each individual AXB position in the intact plant (set at 1, red line). Values represent the means of six plants ±SE, analysed in two pooled samples.
Two TCP-like genes, BRC1 and BRC2, which are similar to the Arabidopsis branching-inhibiting genes, were identified in the P. trichocarpa genome (Supplementary Fig. S4 at JXB online). In long days, the expression levels of these genes increased considerably in developing AXBs, and even below the BMP in ageing AXBs (Fig. 7B, C). A 5-week short day exposure gradually induced significant increases in BRC1 and BRC2 levels also in TBs, ~50- and 4-fold, respectively. However, the values were lower than those of long-day AXBs at the BMP. Under short days, AXBs increased BRC1 and BRC2 levels to approximately the same level as they would have done under long days, but at 5 weeks the expression was equalized to the same level between TBs and AXBs.
Hormone biosynthesis and signalling genes in TBs and AXBs
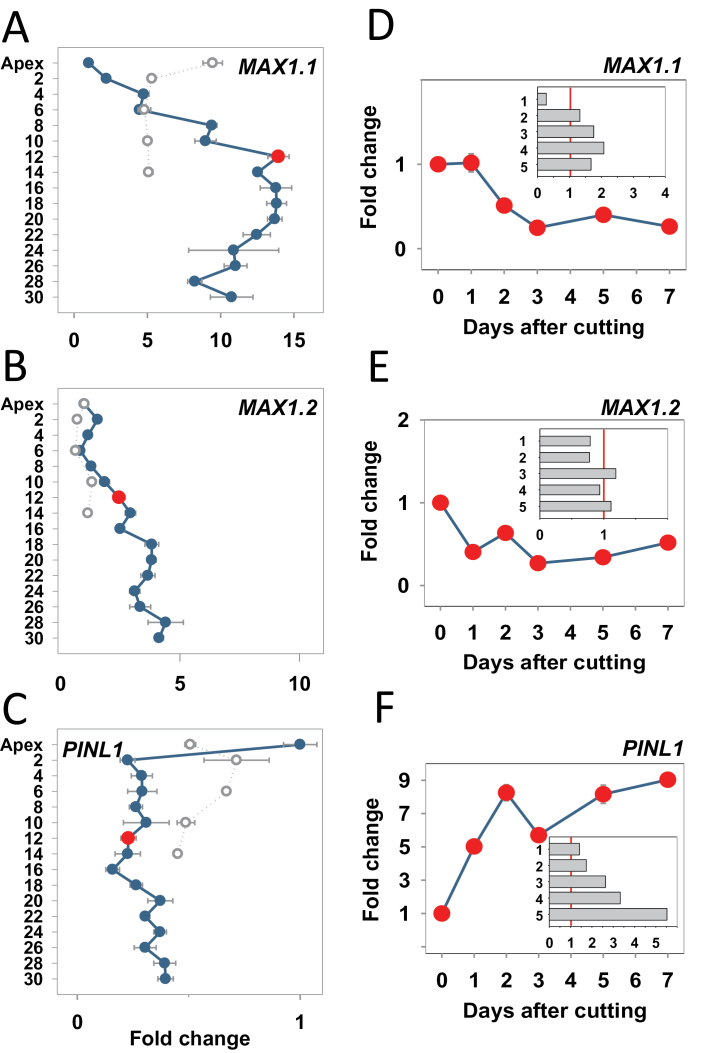
Hormones play a key role in AXB activation in herbaceous plants, but their role in perennial AXBs is less clear. The xylem-fed strigolactone analogue GR24 could inhibit the AXB burst, starting at a concentration of 0.5 μM (not shown), suggesting that endogenous strigolactone could be involved in inhibiting access of AXBs to the stem PATS. To assess the potential involvement of strigolactone and auxin in para-dormancy in hybrid aspen, the expression of a few P. trichocarpa genes, putatively belonging to these hormone pathways, were analysed. Two Populus genes were identified as homologues to the Arabidopsis putative strigolactone biosynthesis gene MAX1 (Supplementary Fig. S5 at JXB online). These paralogues, named MAX1.1 and MAX1.2, were both expressed in apices of hybrid aspen. In AXBs, the expression level of MAX1.1 steadily increased towards the BMP, after which it remained relatively unchanged at a 10- to 12-fold level (Fig. 8A). MAX1.2 expression was only slightly up-regulated when AXBs approached the BMP, but more strongly in the ageing AXBs below the BMP (Fig. 8B). They were also differentially expressed during dormancy. In dormant TBs, MAX1.1 was strongly up-regulated, ~10-fold, approaching the expression levels of mature long-day AXBs (i.e. AXBs at the BMP and below) (Fig. 8A). The AXBs that matured under short days initially up-regulated MAX1.1 to the long day level (not shown), but at 5 weeks in dormant AXBs, the level was lower than in TB (Fig. 8A). MAX1.2, in contrast, was not up-regulated in TBs. Although in AXBs developing under short days the MAX1.2 expression was initially up-regulated (not shown), at dormancy both TBs and AXBs showed equal low expression levels (Fig. 8B).
Fig. 8.
Expression analysis of genes of the strigolactone and auxin pathways in plants grown under long and short photoperiods and after stem decapitation. (A and D) MORE AXILLARY BRANCHES1 (MAX1.1); (B and E) MAX1.2; (C and F) PINFORMED1 (PINL1). (A–C) Intact plants grown under long days (LD; blue dots and lines) and for 5 weeks under short days (SD; open circles, stippled lines). Long-day samples include the apex and axillary buds (AXBs) until node 30 and short-day samples include the terminal bud and AXBs up to node 14. The red dot indicates the expression level (fold change on the x-axis) in AXB 12 of intact plants, the bud maturation point (BMP). (D–F) Stems were cut above the BMP and gene expression was followed for 7 d in AXB 12. Inserts indicate relative expression in five successive AXBs (1–5) proximal to the cut 8 d after decapitation (y-axis fold change). Values in A–C are calculated relative to the apex (set at 1). Values in D–F are relative to AXB 12 of intact plants (set at 1, red dot in A–C). Values in the insets of D–F are relative to each individual AXB position in the intact plants (set at 1, red line). Values represent the means of six plants ±SE, analysed in two pooled samples.
To assess the possible involvement of auxin, two Populus genes with 73% and 65% identity to Arabidopsis PIN1 auxin efflux carriers, named PINL1 and PINL2, were selected. These genes, belonging to subclass-type 1 (Mravec et al., 2009; Liu et al., 2014), were both expressed in apices of hybrid aspen. Under long days, expression of PINL1 and PINL2 was about four times lower in developing para-dormant AXBs than in the growing apex (Fig. 8C; Supplementary Fig. S3B at JXB online). The low expression patterns of the PINL1 and PINL2 genes correspond to a lack of internode elongation in the ES, and may serve to inhibit branching. In ageing AXBs, below the BMP, their expression slightly increased. In short-day-exposed apices, expression of PINL1 and PINL2 was reduced within 2 weeks (not shown), whereas after 5 weeks they were further down-regulated in dormant TBs (Fig. 8C; Supplementary Fig. S3B at JXB online). In AXBs that developed under short days, the expression levels were up-regulated to a similar level to that in the TBs. The similarity in expression levels suggests that equalized expression levels may promote competition for access to the stem PATS, once dormancy is released and activation becomes possible.
Decapitation changes gene expression in AXBs
As decapitation results in burst of AXBs and growth of the enclosed ES, it was investigated if this was preceded by changes in the expression of genes that are involved in branching, including CENL1, MAX1.1, MAX1.2, BRC1, BRC2, PINL1, and PINL2. To avoid measuring gene expression that is related to the developmental completion of the ES, stems were decapitated just below the BMP, at nodal position 12. Gene expression was first monitored over a 14 d period (not shown) and an 8 d period in five successive AXBs proximal to the cut (insets in Figs 7 and 8). This showed that only a few AXBs proximal to the cut substantially changed their gene expression pattern. This is congruent with the observation that, as a rule, decapitation only activated a few of the uppermost AXBs, giving rise to branches. Another experiment, with daily analyses of the proximal AXB only, showed that significant changes in gene expression took place already within the first day (e.g. Figs 7D–F, 8D–F). Enlargement of these already mature AXBs, a pre-stage of bud burst, became visible only later (Fig. 4).
CENL1 expression, which in AXBs of intact plants increased toward the BMP, was up-regulated 4-fold 1 d after decapitation (Fig. 7D), but subsequently down-regulated to the level of growing apices (Fig. 7D, inset). This boost of CENL1 expression prior to any visible enlargement of the AXBs may serve to activate the rib meristem. The subsequent lower CENL1 expression levels, typical of the growing apex, may indicate that the former AXM had assumed the role of the SAM for the side shoot. The BFT gene, in contrast, was up-regulated during AXB development, maintained during para-dormancy, and down-regulated after decapitation in the proximal AXB (Supplementary Fig. S3B at JXB online). In four AXBs under the proximal AXB, expression levels of BFT were up-regulated, possibly reflecting the inhibition of multiple branch outgrowth.
BRC1 and BRC2 expression, which in AXBs of intact plants was up-regulated toward the BMP (Fig. 7B, C), was transiently further up-regulated after decapitation (Fig. 7E, F). BRC1 was down-regulated 2 d after decapitation, before activation was detectable. In contrast, BRC2 expression remained elevated longer until AXBs were visibly elongating at day 8 (Fig. 7F, inset), suggesting that it does not function early in the branching process.
Expression of MAX1-like genes was under complex regulation in AXBs after decapitation. Expression of MAX1.1, which increased during AXB development and was maintained at maturity (Fig. 8A), decreased significantly in the proximal AXB 1 d after decapitation, and before AXB elongation was detectable (Fig. 8D). While the low expression level was maintained after 8 d, the AXBs at a lower position had slightly increased expression levels (Fig. 8D, insert), possibly reflecting the inhibition of multiple branch outgrowth, as in the case of BFT (Supplementary Fig. S3 at JXB online). Decapitation resulted in a rapid reduction of MAX1.2 expression in the AXB closest to the cut, while the two uppermost buds maintained slightly reduced levels even at 8 d post-decapitation (Fig. 8E, inset).
Although in para-dormant AXBs of intact plants PINL1 and PINL2 were little expressed (Fig. 8C), they were significantly up-regulated 1 d after decapitation in the AXBs proximal to the cut, and further during subsequent days (Fig. 8F; Supplementary Fig. S3B at JXB online), reflecting the branching process. In independent sets of experiments expression levels in the upper AXB returned to moderate values 8 d post-decapitation (Fig. 8F, inset; Supplementary Fig. S3, inset at JXB online).
Discussion
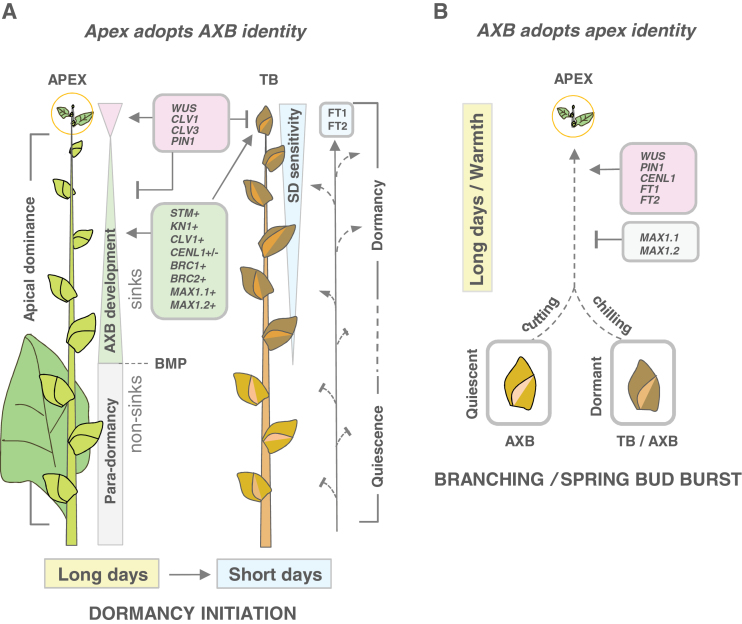
The genetic and physiological parameters that govern plant architecture are conserved among angiosperms (Wang and Li, 2006). Nevertheless, growth habits are so variable that one might expect that branching mechanisms have diversified, reflecting variable implementation of conserved branching processes. For example, the rosette growth habit of Arabidopsis is very different from the caulescent growth form of pea. On another level, woody perennials are vastly different from both annuals, and unique regulatory principles must operate to account for the presence of juvenile and adult stages (Brunner et al., 2014) as well as a seasonal dormancy cycle (van der Schoot and Rinne, 2011). To explore this unknown territory, AXB and TB development was mapped in hybrid aspen, Populus orthologues of relevant Arabidopsis genes were identified, and their expression patterns were analysed in AXBs during their development and during decapitation-induced activation and outgrowth. For the sake of experimental simplicity, the investigations were carried out with first-year saplings that were grown under controlled conditions. Moreover, because in first-year saplings the AXBs are repressed by apical dominance (Cline et al., 1997), a comparison with branching in annuals is a realistic possibility. Nonetheless, distinct differences were found related to AXM initiation, and AXB development, structure, and composition. The findings are summarized in a model (Fig. 9).
Fig. 9.
Conceptual model of the interactive environmental and molecular regulation of identity swapping of apices and axillary meristem in hybrid aspen. (A) In long days (LD), the shoot apical meristem (SAM), seated at the apex (encircled), produces internodes, leaves, and para-dormant AXBs. Under short days (SD), the SAM adopts axillary bud (AXB) identity and produces a terminal bud (TB). SAM-specific gene expression is down-regulated in developing AXBs as well as in developing TBs (pink triangle and pink gene box). During development, AXBs and TBs produce an embryonic shoot, while up-regulating bud-specific genes (green triangle and green gene box). Development is complete at the bud maturation point (BMP; stippled line), after which gene expression remains relatively stable (grey square). Key genes for SAM function and AXB development are mutually exclusive (opposing triangle summits; pink and green gene boxes; + indicates up-regulation, +/– up-/down-regulation). AXBs keep the same basic programme in both photoperiods, but after 5 weeks of SDs in both AXBs and TBs CENL1 is completely down-regulated during dormancy establishment. AXBs gradually lose their responsiveness to SDs (inverted blue triangle) towards the BMP, where sink activity and accessibility to phloem-delivered photoperiodic signals such as FT1 and FT2 have ceased (blue peptide box, arrows). These AXBs overwinter in a suppressed, quiescent state. AXB number is arbitrary. (B) During bud burst and branching, AXBs adopt apex identity. This requires release from dormancy by chilling and LDs/warmth, or removal of para-dormancy by cutting. Genes that promote development of second-generation apices (pink gene box) are shared with the apex, and genes that oppose this (grey gene box, T-shape) are shared with AXBs.
AXBs and TBs of hybrid aspen are perennating structures
In hybrid aspen, AXMs arise in the axils of all emerging leaves, and immediately start forming bud scales and an ES (Figs 1, 2). As a result, a range of different developmental stages is present along the elongating parent stem (Fig. 9). Because as a rule AXBs remain inhibited, changes in gene expression reflect exclusively AXB development, and not the sudden activation of AXBs. Although in the rosette plant Arabidopsis the initiation of AXMs starts in the axils of lower rosette leaves (Grbic and Bleecker, 2000; Long and Barton, 2000; Greb et al., 2003), the developmental gradient of AXBs has a similar orientation. In both cases, the more developed AXBs are the lowest on the stem. Nonetheless, the AXBs in hybrid aspen are very different from those in the annual Arabidopsis. In Arabidopsis, the term ‘AXB’ denotes an AXM that has produced a couple of small leaves but no scales, possibly resembling the incomplete AXBs that give rise to the sylleptic branches of woody perennials (Wu and Hinckley, 2001). The perennating AXBs of the proleptic hybrid aspen clone T89 are sturdy structures with hardening scales that form irrespective of photoperiod to serve as protective devices for the ES in winter (Figs 1, 2, 9) (van der Schoot et al., 2014).
Unique to woody perennials is that the SAM transforms under short days into a perennating structure that is similar to an AXB (Figs 1, 2, 9). Interestingly, the SAM adopts the developmental programme of its daughter meristems, not only structurally, but also in terms of how they regulate BRC1, BRC2, and MAX1.1 (Figs 7, 8). Although scales of TBs and AXBs are formed differently, corresponding to their different origin (Figs 2, 3), they are identical in function. In both cases, bud formation requires switches in the identity of meristem primordia: in TBs from leaf-to-scale-to-embryonic leaf identity, and in AXBs from scale-to-embryonic leaf identity. These tightly regulated processes are crucial underpinnings of the perennial lifestyle.
Redefining para-dormancy in hybrid aspen
In the dormancy literature, absence of branching is often referred to as para-dormancy, to distinguish it from dormancy. More specifically, it may be defined as the suspension of axial development and growth imposed by other plant parts (reviewed in Atwell et al., 1999; van der Schoot and Rinne, 2011). The term correlative inhibition may refer to the same phenomenon, denoting suppression of AXB outgrowth, usually by an actively proliferating apex. The literature is ambivalent about their precise meaning, and both terms may refer to absence of branch formation, or to AXBs that either grow slowly or not at all. The present data suggest that for hybrid aspen the term para-dormancy can be defined in a specific way. Although none of the AXBs bursts, only those below the BMP suspend axial growth and development. Up to that point, AXBs show considerable developmental activity, even if the shoot is compressed and shielded from vision. They produce a tightly packed rosette of ~10 embryonic leaves (Figs 1, 2, 9) in a period as short as 4 weeks. In the same period, the SAM had produced ~10 younger phytomers. The developmental activity of the SAM/apex and the AXM/AXB thus appears quite comparable. In conclusion, the ES (Romberger, 1963; van der Schoot et al., 2014), or ‘pre-formed shoot’ (Brunner et al., 2014), is a genuine and complete shoot, albeit with unextended internodes and unexpanded leaves. It is in some ways reminiscent of a rosette growth form (Romberger, 1963). While elongation of this ES is prevented by apical dominance, its development continues up to the BMP. Shoot elongation at the apex is similarly prevented under short days. Thus, there appears to be a direct parallel between AXB and TB formation. In TBs, ES completion is followed by dormancy establishment, whereas in AXBs it is followed by para-dormancy under long days and, when completed under short days, by dormancy (Fig. 9).
Developing AXBs (above the BMP) and para-dormant AXBs (at and below the BMP) were also functionally distinct. They differed in the ease with which they produced a branch upon decapitation (Fig. 3), and in their capacity to establish dormancy (Table 1). The AXBs above the BMP, emerging TBs, and apices, are all supplied by the phloem. They import not only sugars and nitrogenous compounds, but also photoperiodic signals (Fig. 9; Sachs, 1991). To some degree this is even true in Arabidopsis, where FT imported in AXBs is neutralized by BRC1 (Niwa et al., 2013). The strongly diminished capacity to establish dormancy in AXBs around and below the BMP (Table 1) might therefore reflect cessation of sink activity. In brief, AXBs above the BMP resemble developing TBs more than the para-dormant AXBs below the BMP.
CENL1 is a rib meristem-identity gene
In hybrid aspen, CENL1 is expressed in growing apices (Ruonala et al., 2008) as well as in AXBs (Fig. 7A). Expression in AXBs was reported previously for species as distinct as tomato and Populus (Pnueli et al., 1998; Mohamed et al., 2010). The current data show that CENL1 is considerably up-regulated during AXB development, and that the highest expression levels are reached in para-dormant AXBs at and below the BMP (Fig. 7A). Up-regulation characterizes bud formation as such, as CENL1 is also transiently up-regulated in short-day-induced TBs, prior to dormancy establishment (Ruonala et al., 2008). The in situ hybridizations show that the CENL1 expression domain is located at the rib meristem (Fig. 6), confirming earlier qPCR-based estimates for apices (Ruonala et al., 2008).
That CENL1 expression is required for rib meristem activity is concluded from the following facts. Hybrid aspen saplings that overexpress the oat PHYA gene, which reduces stem elongation (Jordan et al., 1995), are stunted. However, under short days these transgenic plants up-regulate CENL1 and increase elongation (Ruonala et al., 2008), while concomitantly expanding the CENL1 expression domain at the rib meristem (Fig. 6E). It is proposed, therefore, that in hybrid aspen CENL1 functions as a rib meristem-identity gene. The rib meristem may not only support stem elongation by supplying cells (Rinne et al., 2005), but it may also function as a putative signal relay station between the stem and SAM (Ruonala et al., 2008; Paul et al., 2014). During AXB and TB formation, cell divisions in the rib meristem continue, but cell stretching is virtually absent, enforcing the dwarfed stature of the ES. This is accompanied by a steady increase in the level of CENL1 expression (Fig. 7A), suggesting that up-regulation is needed to support the completion of the ES before the inhibiting forces that promote para-dormancy take the upper hand.
Does CENL1 keep AXBs poised for vegetative growth?
In AXBs below the BMP, the expression levels of CENL1 are kept high (Fig. 7A), possibly keeping these para-dormant AXBs poised for growth. This conjecture is supported by correlative evidence. First, preparedness for growth is lost when AXBs and TBs down-regulate CENL1 during dormancy establishment. Secondly, decapitation triggers AXB activation and branching in CENL1-expressing cells, but not in dormant AXB/TBs that have shut off CENL1. Thirdly, during the first 2–3 weeks of short-day-induced TB formation, when CENL1 expression is up-regulated, reversal to normal growth is possible, but not at later stages, when the gene is switched off (Ruonala et al., 2008). Lastly, CENL1 expression is boosted immediately after decapitation, to return subsequently to levels representative of the growing apex (Fig. 7A). The proposed CENL1 function is congruent with the functions of its orthologue TFL1 in Arabidopsis. TFL1 overexpressors have highly branched inflorescences and delayed flowering (Ratcliffe et al., 1998). In contrast, tfl mutants are deficient in secondary inflorescences and accelerate flowering (Bradley et al., 1997).
Unlike the sylleptic branching style, which is affected by growth vigour and environmental conditions (Ceulemans et al., 1990; Wu and Settler, 1998), prolepsis is a stable branching style. Why then would a proleptic genotype, in which branching is delayed to the next growing season, keep AXBs poised for growth? It seems likely that the readiness of AXBs to grow out may be a strategy to deal with incidental damage to the apex, or adverse weather conditions during the growing season. Because in sylleptic genotypes branching from current-year AXBs can proceed without decapitation, its regulation might involve partly different mechanisms, although this remains to be investigated. Briefly, the up-regulation of CENL1 at the rib meristem during ES formation supports the hypothesis that it is required to sustain cell divisions, and counteract increasingly strong inhibitory forces to maintain the preparedness of AXBs for growth.
Are BRC1 and BRC2 counteracting CENL1?
Factors that could potentially counterbalance CENL1 include BRC genes, which repress branching in Arabidopsis (Aguilar-Martínez et al., 2007; Finlayson, 2007; Niwa et al., 2013). This is supported by the fact that the Populus orthologues BRC1 and BRC2 were up-regulated during AXB formation and short day induction of TBs (Fig. 7B, C). Below the BMP, the expression of BRC1, possibly the main inhibitor of branching, continues to increase (Fig. 7B, C), whereas CENL1 expression levels off (Fig. 7A). This could suppress further cell division activity at the rib meristem and halt ES development. Other inhibitory factors positively correlate with AXB inhibition, including hormones such as ABA, ethylene (Ruttink et al., 2007), and strigolactone (Booker et al., 2005; Brewer et al., 2009, 2013). Indeed, the data support that MAX1.1-mediated strigolactone production might counterbalance CENL1 during AXB formation and para-dormancy (Figs 7A, 8A).
An additional role for BRC1 and BRC2 could parallel a function of CENL1, which is to safeguard the vegetative status of the AXBs. TFL1 can interact with FD in nuclei (Hanano and Goto, 2011) and compete with FT for binding to an FD–14-3-3 receptor complex (Conti and Bradley, 2007; Jaeger et al., 2013). Up-regulation of the TFL1 orthologue CENL1 in para-dormant AXBs of hybrid aspen might thus help to diminish the change of floral induction. BRC1 and BRC2 might have a similar role in suppressing flowering in AXBs. In Arabidopsis, BRC1 selectively binds and neutralizes FT that is imported into the AXB, thereby suppressing floral transition (Niwa et al., 2013). Although in adult Populus trees FT1 induces flowering in a subset of AXBs (Böhlenius et al., 2006; Hsu et al., 2011; Brunner et al., 2014), saplings of hybrid aspen do not flower despite the fact that chilling-induced release from dormancy hyper-induces FT1 (Rinne et al., 2011; Paul et al., 2014). Although it is not known if chilling up-regulates BRC1 and BRC2 in hybrid aspen, the genes are up-regulated in the apex and AXBs during dormancy establishment (Fig. 7B, C). In spring, the TB and most AXBs burst, suggesting that equalization of BRC1 and BRC2 expression may safeguard indeterminacy and competitiveness in the race to become a leading shoot.
AXB behaviour and PIN1 and MAX1 genes
Auxin has an important role in the initiation of leaf primordia at the SAM (Reinhardt et al., 2003), and it is likely also to be involved in ES formation in AXBs and TBs. However, its polar transport toward the stem PATS might be prevented as PIN1 levels are low in both AXBs and TBs, and branching and apical expansion are inhibited. When released from apical dominance by decapitation, the para-dormant AXBs just above the BMP increased the expression of PINL1 and PINL2 (Fig. 8C; Supplementary Fig. S3B at JXB online), the Populus orthologues of class I PIN1 in Arabidopsis (Liu et al., 2014). For example, PINL1 expression increased to 9-fold, temporarily exceeding apex levels by >2-fold (Fig. 8C). Such an increase is congruent with data of Liu et al. (2014) showing that PINL1 and PINL2 (referred to as PIN1a and PIN1c) were up-regulated >5-fold during shoot regeneration in Populus. The low expression levels of PINL1 and PINL2 in almost all AXBs reflects apical dominance, as decapitation resulted in up-regulation in the AXBs proximal to the cut (Fig 8C; Supplementary Fig. S3B). Interestingly, short-day exposure enhanced PINL1 and PINL2 expression in AXBs, while somewhat lowering it in TBs (Fig 8C; Supplementary Fig. S3B). This equalization makes sense in a strategy in which all buds, once released from dormancy, have similar changes to initiate growth and access the stem supply routes.
As the genes for strigolactone biosynthesis are conserved across many species, including willow and Populus (Challis et al., 2013; Ward et al., 2013; Czarnecki et al., 2014), their function might be widely shared. The strong up-regulation of MAX1.1 during AXB development in hybrid aspen (Fig. 8A) may therefore be an important factor in inhibition of branching at current-year AXBs. That MAX1.2 expression reached the highest levels in AXBs below the BMP may help to counteract the greater likelihood of burst in older AXBs where the ES is completed (Fig. 8B). Application of strigolactone causes rapid depletion of PIN1 proteins from the plasma membrane in Arabidopsis and pea, resulting in a loss of PATS (Balla et al., 2011; Shinohara et al., 2013; Waldie et al., 2014). Given the conserved nature of PIN1 and MAX1 genes, and their role in annuals, the combination of low PINL1/PINL2 expression (Fig. 8C; Supplementary Fig. S3B at JXB online) and elevated MAX1.1/MAX1.2 expression (Fig. 8A, B) is likely to contribute to the inhibition of branching in hybrid aspen.
The change in expression of MAX1.1 and MAX1.2 in apex/TB and AXBs, during the switch from long days to short days, was opposite compared with that of the PIN1 genes, but their expression was similarly equalized. This provides further support for the hypothesis that equalized gene expression facilitates the synchronized bud burst in spring, prior to the establishment of apical dominance by a leader shoot. This strategy could serve to spread risk among different shoots, allowing initial competition between the apex and apical branches.
In hybrid aspen and other woody perennials, MAX1-regulated strigolactone production might have an unexpected additional role, absent in herbaceous species. MAX1.1, but not MAX1.2, was highly expressed during short-day-induced dormancy in TBs as well as the uppermost AXBs (Fig. 8A, B). Since strigolactone typically controls AXB behaviour, the surprising finding that short days induced expression of the MAX1.1 gene in the TB supports the hypothesis that under short days the apex adopts an AXB-like identity. Notably, both MAX genes were expressed by AXBs, implying that they produce strigolactone themselves, instead of relying on import (Ruyter-Spira et al., 2013; Cavar et al., 2014). Possibly, the production of strigolactone by AXBs is an adaptation enforced by a continuously expanding shoot system.
Conclusions
Structural analyses and gene expression data suggest a conceptual model in which TBs and AXBs, despite being formed under different conditions in subsequent seasons, share overall development, structure, activation, and marker gene expression (Fig. 9). Under short days, the apex adopts the developmental trajectory of AXBs. TBs and developing AXBs are photoperiod-responsive sink organs, which establish dormancy after having completed their ES under short days (Fig. 9A). Under long days, branching is prevented by apical dominance, and the AXBs enter para-dormancy once they are complete and cease to be sinks. Quiescent AXBs and the dormant TB/AXBs that are activated by decapitation and chilling, respectively, adopt the developmental programme of the apex (Fig. 9B). CENL1 is a rib meristem-identity gene, whose activity is required for growth at the apex, developing buds, and branching. High CENL1 expression levels keep AXBs poised for growth, but are counterbalanced by BRC1 and BRC2. Branching is further impeded by local strigolactone biosynthesis and repressed access to the stem PATS, as suggested by the contrasting and decapitation-reversible expression patterns of the studied MAX1 and PIN1-like genes.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Decapitation effects on AXB branching.
Figure S2. A priori defined categories of AXM/AXB.
Figure S3. BFT and PINL2 expression.
Figure S4. Phylogenetic analysis of TB/BRC-like proteins.
Figure S5. Phylogenetic analysis of MAX1-like proteins.
Table S1. P. trichocarpa genes, identifiers, and primer pairs.
Acknowledgements
We are grateful for the excellent technical help of Linda Ripel with qPCR analysis, Sheetal Babu Paul for microscopy sample preparation, and Marit Siira for taking care of the plants. We thank the reviewers for helpful comments. We acknowledge the support of the Norwegian Research Council to CvdS and PLHR (FRIPRO project no. 192013).
References
- Atwell PE, Kriedemann PE, Turnbull CGN. 1999. Plants in action: adaptation in nature, performance in cultivation. Australia: MacMillan Publishers PTY LTD. [Google Scholar]
- Aguilar-Martínez JA, Poza-Carrión C, Cubas P. 2007. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. The Plant Cell 19, 458–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla J, Kalousek P, Reinöhl V, Friml J, Procházka S. 2011. Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. The Plant Journal 65, 571–577. [DOI] [PubMed] [Google Scholar]
- Barthélémy D, Caraglio Y. 2007. Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Annals of Botany 99, 375–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O. 2006. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312, 1040–1043. [DOI] [PubMed] [Google Scholar]
- Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, Turnbull C, Srinivasan M, Goddard P, Leyser O. 2005. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Developmental Cell 8, 443–449. [DOI] [PubMed] [Google Scholar]
- Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E. 1997. Inflorescence commitment and architecture in Arabidopsis . Science 275, 80–83. [DOI] [PubMed] [Google Scholar]
- Brewer P, Dun E, Ferguson B, Rameau C, Beveridge C. 2009. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis . Plant Physiology 150, 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PB, Koltai H, Beveridge CA. 2013. Diverse roles of strigolactones in plant development. Molecular Plant 6, 18–28. [DOI] [PubMed] [Google Scholar]
- Brunner AM, Evans LM, Hsu C-Y, Sheng X. 2014. Vernalization and the chilling requirement to exit bud dormancy: shared or separate regulation? Frontiers in Plant Science 5, 732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CL, McAlpine RG, Kormanik PP. 1967. Apical dominance and form in woody plants: a reappraisal. American Journal of Botany 54, 153–162. [Google Scholar]
- Cavar S, Zwanenburg B, Tarkowski P. 2014. Strigolactones: occurrence, structure, and biological activity in the rhizosphere. Phytochemisty Reviews (in press). [Google Scholar]
- Ceulemans R, Steller RF, Hinckly TM, Isebrands JG, Heilman PE. 1990. Crown architecture of Populus clones as determined by branch orientation and branch characteristics. Tree Physiology 7, 157–167. [DOI] [PubMed] [Google Scholar]
- Challis RJ, Hepworth J, Mouchel C, Waites R, Leyser O. 2013. A role for MORE AXILLARY GROWTH1 (MAX1) in evolutionary diversity in strigolactone signaling upstream of MAX2. Plant Physiology 161, 1885–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline MG. 1991. Apical dominance. Botanical Review 57, 318–358. [Google Scholar]
- Cline MG. 1997. Concepts and terminology of apical dominance. American Journal of Botany 84, 1064–1069. [PubMed] [Google Scholar]
- Cline MG, Dong-Il K. 2002. A preliminary investigation of the role of auxin and cytokinin in sylleptic branching of three hybrid poplar clones exhibiting contrasting degrees of sylleptic branching. Annals of Botany 90, 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti L, Bradley D. 2007. TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture. The Plant Cell 19, 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki O, Yang J, Wang X, Wang S, Muchero W, Tuskan GA, Chen JG. 2014. Characterization of MORE AXILLARY GROWTH genes in Populus . PLoS One 9, e102757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G, Medzihradszky A, Suzaki T, Lohmann JU. 2014. A mechanistic framework for noncell autonomous stem cell induction in Arabidopsis . Proceedings of the National Academy of Sciences, USA 111, 14619–14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O. 2011. Signal integration in the control of shoot branching. Nature Reviews Molecular Cell Biology 12, 211–221. [DOI] [PubMed] [Google Scholar]
- Donnelly A, Caffarra A, Kelleher CT, et al. 2012. Surviving in a warmer world: environmental and genetic responses. Climate Research 53, 245–262. [Google Scholar]
- Dun EA, Ferguson BJ, Beveridge CA. 2006. Apical dominance and shoot branching. Divergent opinions or divergent mechanisms? Plant Physiology 142, 812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K. 1977. Anatomy of seed plants. New York: John Wiley & Sons. [Google Scholar]
- Ferguson BJ, Beveridge CA. 2009. Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiology 149, 1929–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson SA. 2007. Arabidopsis TEOSINTE BRANCHED-LIKE 1 regulates axillary bud outgrowth and is homologous to monocot TEOSINTE BRANCHED1. Plant and Cell Physiology 48, 667–677. [DOI] [PubMed] [Google Scholar]
- Garrison R. 1955. Studies in the development of axillary buds. American Journal of Botany 42, 257–266. [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, et al. 2008. Strigolactone inhibition of shoot branching. Nature 455, 189–194. [DOI] [PubMed] [Google Scholar]
- Grbic V, Bleecker AB. 2000. Axillary meristem development in Arabidopsis thaliana . The Plant Journal 21, 215–223. [DOI] [PubMed] [Google Scholar]
- Greb T, Clarenz O, Schäfer E, Müller D, Herrero R, Schmitz G, Theres K. 2003. Molecular analysis of the LATERAL SUPPRESSOR gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Genes and Development 17, 1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallé F, Oldeman RAA, Tomlinson PB. 1978. Tropical trees and forests. Berlin: Springer-Verlag. [Google Scholar]
- Hamiaux C, Drummond RSM, Janssen BJ, Ledger SE, Cooney JM, Newcomb RD, Snowden KC. 2012. DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Current Biology 22, 2032–2036. [DOI] [PubMed] [Google Scholar]
- Hanano S, Goto K. 2011. Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. The Plant Cell 23, 3172–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath DP, Sung S, Kim D, Chao W, Anderson J. 2010. Characterization, expression and function of DORMANCY ASSOCIATED MADS-BOX genes from leafy spurge. Plant Molecular Biology 73, 169–179. [DOI] [PubMed] [Google Scholar]
- Hsu C-Y, Adams JP, Kim H, et al. 2011. FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proceedings of the National Academy of Sciences, USA 108, 10756–10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C-Y, Liu Y, Luthe DS, Yuceer C. 2006. Poplar FT2 shortens the juvenile phase and promotes seasonal flowering. The Plant Cell 18, 1846–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard L, McSteen P, Doebley J, Hake S. 2002. Expression patterns and mutant phenotype of teosinte branched1 correlate with growth suppression in maize and teosinte. Genetics 162, 1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D, Veit B, Hake S. 1994. Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120, 405–413. [Google Scholar]
- Jaeger KE, Pullen N, Lamzin S, Morris RJ, Wigge PA. 2013. Interlocking feedback loops govern the dynamic behavior of the floral transition in Arabidopsis . The Plant Cell 25, 820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez S, Reighard GL, Bielenberg DG. 2010. Gene expression of DAM5 and DAM6 is suppressed by chilling temperatures and inversely correlated with bud break rate. Plant Molecular Biology 73, 157–167. [DOI] [PubMed] [Google Scholar]
- Jordan ET, Hatfield PM, Hondred D, Talon M, Zeevaart JAD, Vierstra RD. 1995. Phytochrome A overexpression in transgenic tobacco. Plant Physiology 107, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens G. 2003. Growing up green: cellular basis of plant development. Mechanisms of Development 120, 1395–1406. [DOI] [PubMed] [Google Scholar]
- Keller T, Abbott J, Moritz T, Doerner P. 2006. Arabidopsis REGULATOR OF AXILLARY MERISTEMS1 controls a leaf axil stem cell niche and modulates vegetative development. The Plant Cell 18, 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa D, Miyazawa Y, Fujii N, Hoshino A, Iida S, Nitasaka E, Takahashi H. 2008. The gravity-regulated growth of axillary buds is mediated by a mechanism different from decapitation-induced release. Plant and Cell Physiology 49, 891–900. [DOI] [PubMed] [Google Scholar]
- Lang GA, Early JD, Martin GC, Darnell RL. 1987. Endodormancy, paradormancy, and ecodormancy—physiological terminology and classification for dormancy research. HortScience 22, 371–377. [Google Scholar]
- Laux T, Mayer KFX, Berger J, Jürgens G. 1996. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis . Development 122, 87–96. [DOI] [PubMed] [Google Scholar]
- Li CJ, Bangerth F. 1999. Autoinhibition of indoleacetic acid transport in the shoots of two-branched pea (Pisum sativum) plants and its relationship to correlative dominance. Physiologia Plantarum 106, 415–420. [Google Scholar]
- Lifschitz E, Ayre BG, Eshed Y. 2014. Florigen and anti-florigen—a systemic mechanism for coordinating growth and termination in flowering plants. Frontiers in Plant Science 5, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Zhang J, Wang L, Li J, Zheng H, Chen J, Lu M. 2014. A survey of Populus PIN-FORMED family genes reveals their diversified expression patterns. Journal of Experimental Botany 65, 2437–2448. [DOI] [PubMed] [Google Scholar]
- Long J, Barton MK. 2000. Initiation of axillary and floral meristems in Arabidopsis . Developmental Biology 218, 341–353. [DOI] [PubMed] [Google Scholar]
- Meier AR, Saunders MR, Michler CH. 2012. Epicormic buds in trees: a review of bud establishment, development and dormancy release. Tree Physiology 32, 565–584. [DOI] [PubMed] [Google Scholar]
- Millet P, Bouchard A, Edelin C. 1999. Architecture and successional status of trees in a temperate deciduous forest. Ecoscience 6, 187–203. [Google Scholar]
- Mohamed R, Wang CT, Ma C, et al. 2010. Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identity and dormancy release in Populus . The Plant Journal 62, 674–688. [DOI] [PubMed] [Google Scholar]
- Morris SE, Cox MCH, Ross JJ, Krisantini S, Beveridge CA. 2005. Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiology 138, 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec J, Skupa P, Baily A, et al. 2009. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 459, 1136–1140. [DOI] [PubMed] [Google Scholar]
- Niwa M, Daimon Y, Kurotani K, et al. 2013. BRANCHED1 interacts with FLOWERING LOCUS T to repress the floral transition of the axillary meristems in Arabidopsis . The Plant Cell 25, 1228–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LK, Rinne PLH, van der Schoot C. 2014. Shoot meristems of deciduous woody perennials: self-organization and morphogenetic transitions. Current Opinion in Plant Biology 17, 86–95. [DOI] [PubMed] [Google Scholar]
- Pfennig DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP. 2010. Phenotypic plasticity’s impacts on diversification and speciation. Trends in Ecology and Evolution 25, 459–467. [DOI] [PubMed] [Google Scholar]
- Phillips IDJ. 1975. Apical dominance. Annual Review of Plant Physiology 26, 341–367. [Google Scholar]
- Pnueli L, Carmel-Goren L, Hareven D, Gutfinger T, Alvarez J, Ganal M, Zamir D, Lifschitz E. 1998. The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1 . Development 125, 1979–1989. [DOI] [PubMed] [Google Scholar]
- Ratcliffe OJ, Amaya I, Vincent CA, Rothstein S, Carpenter R, Coen ES, Bradley DJ. 1998. A common mechanism controls the life cycle and architecture of plants. Development 125, 1609–1615. [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Pesce E-R, Stieger P, Mandel T, Baltensperger K, Bennet M, Traas J, Friml J, Kuhlemeier C. 2003. Regulation of phyllotaxis by polar auxin transport. Nature 426, 255–260. [DOI] [PubMed] [Google Scholar]
- Rinne P, Kauppi A, Ferm A. 1987. Induction of adventitious buds and sprouts on birch seedlings (Betula pendula Roth and Betula pubescens Ehrh). Canadian Journal of Forest Research 17, 545–555. [Google Scholar]
- Rinne P, Tuominen H, Sundberg B. 1993. Growth patterns and endogenous indole-3-acetic-acid concentrations in current-year coppice shoots and seedlings of two Betula species. Physiologia Plantarum 88, 403–412. [Google Scholar]
- Rinne PLH, van der Schoot C. 1998. Symplasmic fields in the tunica of the shoot apical meristem coordinate morphogenetic events. Development 125, 1477–1485. [DOI] [PubMed] [Google Scholar]
- Rinne PLH, Kaikuranta PM, van der Schoot C. 2001. The shoot apical meristem restores its symplasmic organization during chilling-induced release from dormancy. The Plant Journal 26, 249–264. [DOI] [PubMed] [Google Scholar]
- Rinne PLH, van den Boogaard R, Mensink MGJ, Kopperud C, Kormelink R, van der Schoot C. 2005. Tobacco plants respond to the constitutive expression of the tospovirus movement protein NSM with a heat-reversible sealing of plasmodesmata that impairs development. The Plant Journal 43, 688–707. [DOI] [PubMed] [Google Scholar]
- Rinne PLH, Welling A, Vahala J, Ripel L, Ruonala R, Kangasjärvi J, van der Schoot C. 2011. Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-β-glucanases to reopen signal conduits and release dormancy in Populus . The Plant Cell 23, 130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde A, Prinsen E, De Rycke R, Engler G, Van Montagu M, Boerjan W. 2002. PtABI3 impinges on the growth and differentiation of embryonic leaves during bud set in poplar. The Plant Cell 14, 1885–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberger JA. 1963. Meristems, growth and development in woody plants. USDA Technical Bulletin no. 1293. Washington, DC: US Department of Agriculture. [Google Scholar]
- Ruonala R, Rinne PLH, Kangasjärvi J, van der Schoot C. 2008. CENL1 expression in the rib meristem affects stem elongation and the transition to dormancy in Populus . The Plant Cell 20, 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttink T, Arend M, Morreel K, Storme V, Rombauts S, Fromm J, Bhalerao RP, Boerjan W, Rohde A. 2007. A molecular timetable for apical bud formation and dormancy induction in poplar. The Plant Cell 19, 2370–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyter-Spira C, Al-Babili S, van der Krol S, Bouwmeester H. 2013. The biology of strigolactones. Trends in Plant Science 18, 72–83. [DOI] [PubMed] [Google Scholar]
- Sachs T. 1991. Pattern formation in plant tissues. Cambridge: Cambridge University Press. [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KFX, Jürgens G, Laux T. 2000. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100, 635–644. [DOI] [PubMed] [Google Scholar]
- Shinohara N, Taylor C, Leyser O. 2013. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biology 11, e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussex IM. 1989. Developmental programming of the shoot apical meristem. Cell 56, 225–229. [DOI] [PubMed] [Google Scholar]
- Thimann KV, Skoog F. 1934. On the inhibition of bud development and other functions of growth substances in Vicia faba . Proceedings of the Royal Society B: Biological Sciences 114, 317–339. [Google Scholar]
- Tomlinson PB. 1983. Tree architecture. American Scientist 71, 141–149. [PubMed] [Google Scholar]
- Tuskan GA, Difazio S, Jansson S, et al. 2006. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313, 1596–1604. [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, et al. 2008. Inhibition of shoot branching by new terpenoid plant hormones. Nature 455, 195–200. [DOI] [PubMed] [Google Scholar]
- van der Schoot C, Paul LK, Rinne PLH. 2014. The embryonic shoot: a lifeline through winter. Journal of Experimental Botany 65, 1699–1712. [DOI] [PubMed] [Google Scholar]
- van der Schoot C, Rinne P. 1999. Networks for shoot design. Trends in Plant Science 4, 31–37. [DOI] [PubMed] [Google Scholar]
- van der Schoot C, Rinne PLH. 2011. Dormancy cycling at the shoot apical meristem: transitioning between self-organization and self-arrest. Plant Science 180, 120–131. [DOI] [PubMed] [Google Scholar]
- Waldie T, McCulloch H, Leyser O. 2014. Strigolactones and the control of plant development: lessons from shoot branching. The Plant Journal 79, 607–622. [DOI] [PubMed] [Google Scholar]
- Wang Q, Kohlen W, Rossmann S, Vernoux T, Theres K. 2014a. Auxin depletion from the leaf axil conditions competence for axillary meristem formation in Arabidopsis and tomato. The Plant Cell 26, 2068–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang J, Shi B, Yu T, Qi J, Meyerowitz EM, Jiao Y. 2014b. The stem cell niche in leaf axils is established by auxin and cytokinin in Arabidopsis . The Plant Cell 26, 2055–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Li JY. 2006. Genes controlling plant architecture. Current Opinion in Biotechnology 17, 123–129. [DOI] [PubMed] [Google Scholar]
- Ward SP, Salmon J, Hanley SJ, Karp A, Leyser O. 2013. Using Arabidopsis to study shoot branching in biomass willow. Plant Physiology 162, 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareing PF. 1956. Photoperiodism in woody plants. Annual Review of Plant Physiology 7, 191–214. [Google Scholar]
- Wareing PF. 1970. Growth and its co-ordination in trees. In: Luckwill L, Curttings C, eds. Physiology of crop trees. New York: Academic Press, 1–21. [Google Scholar]
- Wu R, Hinckley TM. 2001. Phenotypic plasticity of sylleptic branching: genetic design of tree architecture. Critical Reviews in Plant Science 20, 467–485. [Google Scholar]
- Wu R, Steller RF. 1998. Quantitative genetics of growth and development in Populus. III. Phenotypic plasticity of crown structure and function. Heredity 81, 299–310. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.