Abstract
Monoclonal antibodies remain a primary product option for novel cancer treatment. The properties of an antibody are a function of the antigen specificity and constant region incorporated. The rapid advance in molecular understanding of cancer biology and the host–tumor interaction has defined a new range of targets for antibody development. The clinical success of the checkpoint inhibitors has validated immune modulation and mobilization as a therapeutic approach. Solid cancers are distinguished from hematologic malignancies because the solid tumor stroma contains significant tumor promoting and immune dampening elements less prominent in hematologic cancer. This review highlights how engineered monoclonal antibody products are emerging as potential cornerstones of new more personalized cancer treatment paradigms that target both tumor and the stromal environment.
Keywords: check point, IgE, IgG, ipilimumab, macrophage, monocyte, myeloid, nivolumab, oregovomab, PD-1, second signal, T cell, tumor stroma
The 2010 approval of Yervoy® (ipilimumab, Bristol-Meyers Squibb, NY, USA), the first monoclonal antibody targeting a checkpoint pathway, was shown to induce regressions and prolong survival in advanced melanoma. This has triggered an emerging revolution in the treatment of solid malignancies focused on immune regulation and activation now gathering momentum at multiple levels of both basic and clinical understanding [1]. Monoclonal antibody technology is central not only as a tool for the scientific characterization of the tumor–host interaction, but as an expedient strategy to generate therapeutics precisely targeting these cellular pathways and processes. Antibodies are the B-cell product of adaptive immunity. Individual antibodies recognize distinct antigen structures through their variable region (Fab) while their constant regions (Fc) engage specific cellular and plasma components to mediate immune effects according to subclass and isotype. A carefully selected or engineered antibody can be a powerful tool to target and modify immune and growth dynamics in tissue and serve as a payload to bring drugs to defined locations. Because antibodies are produced naturally in living cells, toxicity is not inherent in the molecule, but rather derivative of biological response affected by antibody exposure.
Herceptin® (trastuzumab, Genentech, CA, USA), the first commercially available monoclonal antibody for the treatment of cancer, was approved almost 25 years after the original report by Kohler and Milstein describing monoclonal antibody technology [2,3]. Efficacy of the product was established by identifying a subset of breast cancer patients with over-expression of the HER2/neu growth regulating receptor who responded to this antagonistic antibody [4]. The expectation has remained that novel products would individually revolutionize treatment; however the advances during this initial period have only been incremental. The complexity of biological systems, redundancy in pathways and plasticity in cellular dynamics have limited progress especially in the treatment of solid malignancies. It is impossible in the scope of a brief review to comprehensively summarize all the monoclonal antibody products in development as cancer treatments. Inspection of pipelines for the major global pharmaceutical firms describes hundreds of programs with a myriad of individual product candidates. New insights into the dynamics of cancer biology and immune response provide a framework to consider and prioritize among these emerging programs. The safeguards and systems established to foster rationale and diligent drug development of monotherapies do create barriers for the development of products best suited to succeed as a component of combination treatment with other novel entities. This review argues that the combinational approach is where cancer immunotherapy should focus in order to overcome the redundancy of growth supporting and immune suppressive elements that are prevalent in a growing solid tumor.
Monoclonal antibodies can be used to precisely impact host tumor biology. Considering individual antibody product candidates with this perspective provides context for consideration of novel antibody applications and therapeutic strategies. Homeostatic forces that limit immune activation in tissue are dominant in solid tumor stroma and contribute to the difficulty in treating advanced solid cancer. This review will focus on the interactions of growing tumor cells and the tissue matrix that supports them. A multifocal strategy using scheduled combinations to manipulate the immune environment to the disadvantage of the tumor holds great promise, whereas individual products in isolation may only be able to accomplish limited benefit for the majority of treated patients. Advances in molecular conjugation and protein engineering to construct synthetic large molecule treatments are not considered here, however, the lesson and approach proposed in this review can be applied equally to evaluate such products and other compounds expected to influence the host–tumor cell interaction.
Antibodies as therapeutic tools
Antibody classes
Monoclonal antibodies can be engineered according to species of origin as well as isotype and subclass as a product of rational design. The variable antigen binding region which is the target of a given antibody consists of the variable regions of heavy and light chains which are linked together by disulfide bonds in the constant regions of each. The antigen specificity is of unique affinity for each antibody and can be stimulatory or antagonistic if targeting a signal transducing receptor. The core of all antibody isotypes is four chain complexes consisting of two heavy and two light chains. IgG of all subclasses and IgE, both circulate as individual molecules in solution or bound to specific cell surface receptors. In contrast, IgA isotypes exist as a secretory dimers and IgM as pentamers. Antibody structure and function is well reviewed in ‘The Immunoglubulin Facts Book’ [5]. IgA is the predominant secretory antibody of mucosal immunity while IgM is an acute phase antibody and the first class of immunoglobulin produced prior to recombinant class switching evolution of the B-cell clone. Both IgA and IgM form multimeric complexes less practical for development as monoclonal antibody cancer products. IgG and IgE are the two antibody classes actively being pursued for use as parenteral cancer therapies. For human clinical use, most antibodies are human or humanized chimeric, although the use of xenotypic IgG antibodies can be useful for inducing antigen-specific immunity [5,6].
Antibody subclasses & Fc receptors
Human IgGs are classified into four subclasses (IgG1–IgG4) and the functional biology of these four constant region γ heavy chain (Fcγ) variants differs. Murine antibodies have an analogous classification, however the subclasses between mouse and human do not correspond directly. For example, classic antibody-dependent cellular cytotoxicity is mediated by human NK cells with human IgG1 and IgG3, but murine IgG1 does not mediate ADCC whereas murine IgG2a does. The affinity of the human IgG Fc for the Fc receptor (FcR) binding varies across the subclasses. There are three Fc γ chain receptors CD64 (FcγRI), CD32 (FcγRII) and CD16 (FcγRIII) expressed on antigen processing and effector cells. CD64 is the high-affinity receptor classically associated with antigen processing, whereas CD32 a lower affinity receptor expressed notably on B cells mediates inhibition of antibody production. CD16 is also a lower affinity receptor and is expressed on NK and a range of myeloid cells. Fcγ affinity for carbohydrate moieties and ability to fix complement is subclass specific, with IgG1 and IgG3 being stronger complement fixers. IgG4 does not fix complement or mediate ADCC. In addition to the classic Fc receptors, binding of antibody to antigen processing cells is also mediated by various carbohydrate binding elements. For example, mannosylated IgG can be bound and internalized by antigen presenting cells via the mannose receptor CD206 [7]. The pharmacologic effects of an individual monoclonal antibody are thus dictated by its specific Fab and Fc elements. Human IgG persists in human circulation for multiple weeks and thus can achieve high-steady state serum levels and be used as a pharmacologic ligand targeting receptor function. IgG1 has been a popular subclass for use in receptor targeting. Trastuzumab (inhibitor of Her2/ neu), cetuximab (Erbitux®, Eli Lily and Company, IN, USA) (inhibitor of EGFR) and also the hematologic antibody rituximab (Rituxan®, Genentech, CA, USA) which targets the B-cell marker CD20, highly expressed in B-cell lymphoma, are all IgG1 molecules. This subclass is also optimal for inducing NK cell mediated ADCC [8]. The importance of the biological interaction with multiple cell types is illustrated by the finding that the best responding patients to rituximab treatment carry a germ line variant with a conserved single amino substitution variant in their antibody binding receptor-FcγRIII gene that mediates enhanced ADCC activity [9,10]. Appreciation of the variation in Fc constant regions across alternative antibody candidates is critical to understanding that despite similar target antigen binding affinities, variations in Fc structure, class or subclass will also alter the pharmacology associated with a given antibody therapeutic.
The IgE class & tissue stroma
For the treatment of solid malignancies, a limitation of antibodies of the IgG class is that these antibodies are present in highest concentration in the plasma and interstitial fluids and may not penetrate into less vascular and poorly perfused tissues against a pressure gradient which is a common condition in many solid tumors. This requires large dosing requirements, with most products being dosed in the range of 150–1000 mg per infusion and demanding large capacity of costly cell culture manufacturing capacity to assure clinical supply. There is a growing body of researchers advancing tumor antigen specific IgE as a complementary alternative and addition to the IgG classes of antibody for use as cancer therapies [11,12]. IgE is similar to IgG, sharing the identical light chains. IgE is a product of class switch recombination from IgG and thus the variable region is of shared pedigree. The IgE heavy chain constant region (Fcε) lacks a hinge structure and has four constant domains rather than the three found in IgG. Additionally, there are more glycosylation sites on IgE than IgG, meaning that carbohydrate mediated binding and interactions, although not yet well characterized in the literature, are likely to be more complex than for IgG. Multiple monoclonal IgE molecules, specific for a range of tumor-associated antigens, have now been developed preclinically and reported in the literature. Details of IgE receptor interactions and consequential biology are now emerging [13]. IgE has an extremely short plasma half-life but rapidly redistributes into tissues primarily bound to cells bearing the high-affinity FcγR1. The glycosylation profile of IgE may also contribute to its rapid clearance. Its persistence in tissue and its ability to penetrate solid tissues and modulate vascular perfusion make the antibody class of potential significance for the use in immunotherapy of solid malignancy. FcγR1 exists as a tetrameric transmembrane receptor (αβγ2) highly expressed on mast cells and basophils (up to 200,000 receptors per cell). In humans, other primates and rats (but not in mice), FcεR1 is also expressed as a trimeric form (αγ2) lacking the β chain on myeloid cells including dendritic cells, monocytes and eosinophils. The trimeric receptor is generally expressed in lower copy numbers (100s per cell). The affinity of Fcε for the high-affinity receptor FcεR1 is several orders of magnitude greater than for FcεR2 (CD23). The affinity of γ receptor CD64 for IgG is similar to the affinity of IgE to its low-affinity receptor. FcεR2 is a calcium-dependent C-type lectin, and is inducible on a range of cells including B and T lymphocytes, myeloid cells including macrophages, monocytes, dendritic cells and eosinophils, but also can be found on airway epithelium and smooth muscle cells. When cleaved, soluble CD23 (sCD23) is released to the circulation. Soluble CD23 can bind the complement receptor 2 on B cells and has a role in regulation of endogenous IgE synthesis as well as other cytokine like properties [14]. Thus, monoclonal IgE represents a second emerging antibody strategy to engage tumor cells directly and influence a different array of effector cells of potential relevance to host tumor biology and cancer therapy. Figure 1 summarizes an array of possible ways antibodies can be used to mediate therapeutic effect in the treatment of cancer.
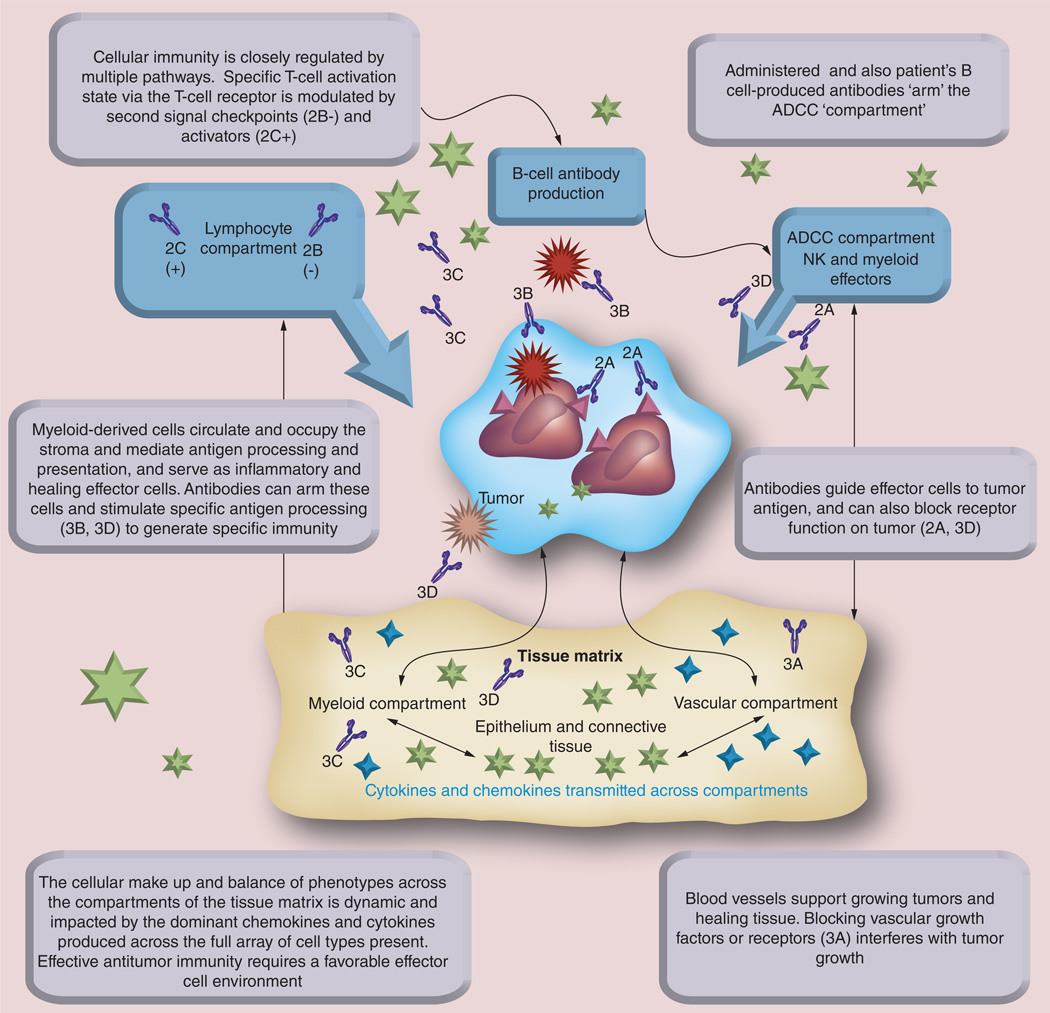
Figure 1. The balance of host–tumor interaction is defined by the tissue matrix in which a tumor is growing.
Antibodies not only arm effector cells such as NK cells and Fc receptor-bearing cells for the IgG and IgE classes to give them specificity in function, but can directly interfere with receptor-mediated tumor cell growth and activation (2A). By targeting second signal interactions between lymphocytes and both antigen processing cells and the lymphocyte targets, specific antibodies can serve to enhance T-cell immunity by blocking checkpoint down regulation (2B) and by stimulating second-signal activators (2C). Vascular support of the tumor microenvironment can be reduced by antibodies to vascular growth factors and their receptors on growing microvasculature, thus limiting tumor growth and nutrition (3A). Antibodies to tumor antigen can selectively enhance antigen presentation and emergence of tumor-specific immunity (3B). Antibody neutralization of specific chemokine and cytokine activity (3C) can further modulate the balance of a tumor microenvironment to favor tumor-eliminating immunity. Monoclonal IgE targeting tumor antigens (3D) can enhance tumor antigen processing and T-cell immunity and also arm myeloid effector cells to target tumor cells in the tissue stroma. Drugs of multiple classes can also influence these interactions, such that optimal cancer therapy is multimodality and should be crafted to modulate the tumor environment to favor growth impairment, cytotoxicity and immune-mediated tumor destruction. Antibody products labelled 2A–C and 3A–D correspond with Table 2A–C and Table 3A–D, where each antibody product is described in-depth.
Using antibodies to alter immune homeostasis
Monoclonal antibody technology remains a prominent tool to modulate immune mechanisms to therapeutic advantage. In addition to tumor targeting, antibodies can be used to antagonize or stimulate hormonal, cytokine and chemokine pathways, to modify antigen recognition uptake and presentation, and to help guide specific cellular effectors of the immune response to a selected site and thus shift the balance of immune homeostasis. The skillful use of monoclonal antibodies with therapeutic intent will require carefully orchestrating approaches with other molecular modalities including adjuvants and chemotherapeutic agents in scheduled and coordinated fashion. Application of these products is dependent on a sophisticated understanding of the interaction between a growing tumor, the local tissue where it resides and the evolving host immune response [15]. The scientific approach of reducing pathology and cellular interactions into narrow models to reduce noise has influenced biomedical clinical drug development pathways. Monotherapies are typically developed first in isolation targeting a receptor or marker. It is assumed that the target contributes significantly to the clinical disease. Once commercialized, a novel treatment is subject to empiric combination in the clinic that leads to further refinement and improvement in clinical outcomes. The development of multiple component treatments which in isolation may lack clinical effect, but in coordinated and sequenced combination may achieve substantial benefit, is a bigger challenge for the modern drug development process. The institutional, legal and regulatory process established to safeguard patients, protect innovators and facilitate incremental progress hampers development of more complex treatment paradigms. The clinical development of monotherapies has led to unprecedented advancement in medical understanding and produced many effective medicines, however for disorders that are heterogeneous within a population, monotherapies result in less dramatic benefits and statistical demonstration of efficacy requires randomized studies of large size, demanding substantial clinical resources to complete. For cancer immunotherapy, highly simplified immune therapeutic strategies although effective in controlled laboratory settings have historically succeeded only by anecdote in the clinic, and most often failed to reproduce outcomes when applied more generally. For more than 100 years following Coley’s report of success with his original Coley’s toxin [16], cancer immunotherapy did not prove robust for general application. Until the successful product registration of ipilimumab in mobilizing cellular immunity to fight cancer in 2010 [1], belief that mobilization of cellular immunity was a viable therapeutic strategy to treat human cancer was at best controversial.
Second signal activation & checkpoint pathways
To trigger and control a cellular immune response following T-cell receptor engagement, second signal ligand interactions between the T cell and the antigen presenting cell are necessary. These pathways initially activate and then dampen and turn off the specific immune response over time. This biology has been thoroughly reviewed by Pardoll [17] who has inventoried the growing panel of second signal ligands as activating or inhibitory. The ‘checkpoint inhibitors’ anti-CTLA4, anti-PD-1 and anti-PDL-1 are examples of monoclonal antibodies that inhibit the natural second signal down regulatory component of a T-cell response mediated by the second signal ligands CTLA4, PD-1 and PDL-1, respectively. By inhibiting the down regulation of activated T cells, tumor-specific immune responses can be amplified and extended. T cells exposed to these antibodies can persist in active states without being ‘checked’ or turned off.
Specific immune suppression is the natural state of immunity in health. Induction of an immune response follows encounter with a pathogen-associated molecular pattern (danger signal) that triggers activation of antigen processing, immune recognition and innate and also specific adaptive humoral and cellular responses. These are rapid, orderly and self-limited. The activated pathways are then returned to a state of passive surveillance via highly redundant homeostatic mechanisms evolved to counter the breadth of potential insults which an individual organism or patient might face, neutralize, and then and recover from overtime. The pathways of tissue growth and wound healing are integral to this process and reflect the biology and interaction of the tissue matrix and the classical myeloid and lymphoid derived immune effector cells. The receptors active in immune regulation share common pedigrees, notably in the immunoglobulin superfamily of genes and the TNF receptor superfamily [18,19]. There are multiple pathways of immune amplification and inhibition, facilitating shifts in response reflective of integrated micro environmental signals [20,21]. Unfortunately, growing tumors are especially adept at changing their phenotypes in response to negative selection and finding gene expression patterns that select for tumor growth and limit neutralizing immunity. Table 1 summarizes selected examples of immune down-regulatory and up-regulatory pathways in immune homeostasis. Identified are second signal receptors which serve as both activators and checkpoints, active cellular participants that are also stimulating or suppressive, common cytokine and chemokine patterns associated with both, and selected features of the resultant biological response. These all represent potential targets for immune-modulating monoclonal antibody therapy.
Table 1.
Selected pathways for immune modulation and homeostasis.
| Pathway | Comment |
|---|---|
T-cell activation checkpoints:
|
The first element in each pair of this category except NKG2A is located on the T cell, and ligand interaction results in direct down-regulatory cell signaling in the T cell [17]. NKG2A is the ligand for HLA-E and down regulates NK-cell activity [22] |
T-cell activation stimulators:
|
The first element of the pair in this activator list is on the T cell. Ligand binding promotes T-cell activation, except for CD40L which stimulates APC leading to indirect T-cell activation [17] |
| Pathogen associated molecular patterns:
|
A primary interface between intrinsic and adaptive immunity is mediated by recognition by local cells of foreign materials. Characteristic molecular patterns including microbial DNA/RNA, microbial cell wall elements and microbial glycolipid structures bind these receptors and trigger immune stimulator cytokine activation [23]. Toll-like receptor agonists may be useful for combination with immune modulatory antibodies |
Metabolic T-cell modulators:
|
Adenosine accumulates at the site of cell death and is stimulatory to Treg cells, thus down regulating inflammatory sites [24]. Tryptophan is a needed amino acid for T-cell growth, indoleamine oxidase metabolizes tryptophan and is overexpressed in tumors facilitating immune escape [25] |
| Cell types with immune-modulatory function:
|
Many cells involved in coordinating a primary immune response and facilitating healing are capable of producing inflammation limiting cytokines, promoting angiogenesis, collagen synthesis and scar tissue formation. Tumors can find growth advantage by expressing factors that favor a ‘healing microenvironment’. These cell types thus can be tumor promoting in states of chronic inflammatory wound healing but can also mediate antitumor effects when armed with a specific antibody [26,27] |
Immune stimulatory cytokines:
|
A few select cytokines that are central to activating an immune response and favoring cell-mediated T-cell immunity are listed here; however many other cytokines influence the immune balance and may be highly relevant to effective cancer immunotherapy [28] |
Immune suppressive cytokines:
|
There are many other factors and cytokines that also influence the balance of immunity, however IL-10 and TGF-β are especially well characterized in this regard [28] |
Effector cell chemokines:
|
Chemokines facilitate local accumulation of effector cells including activated T cells, NK cells and inflammatory monocytes [21] |
Suppressor cell chemokines:
|
CCL22 is locally chemotactic to Tregs; there are other chemokines that are chemotactic to immune-regulatory cell phenotypes that may also be targets [21] |
Cellular immune phenotypes & function
Well described immune cellular phenotypes often polarized or subclassified into ‘good and bad’ actors of the immune response include T lymphocytes, dendritic cells, myeloid cells such as monocytes, eosinophils, macrophages and mast cells and the list can be further extended to include stromal components such as fibroblasts, endothelial cells, epithelial cells, adipocytes and other ectodermal and mesenchymal constituents [26,29,30]. In certain circumstances, these cell types may be terminally differentiated and appropriately categorized for a definitive cell function; however, the assumption that a given cell exists typically at the polarized extreme of differentiation may not always be the case in an evolving tumor microenvironment. A therapeutic strategy introduced with the checkpoint inhibitors modifies the tumor microenvironment to enrich for preferred phenotypes that would favor immune-effector pathways. Chemotactic migration to a site, or simple transformation in phenotype and proliferation of existing cells, both can result in a shifted microenvironment. Combinatorial strategies may be particularly useful in achieving this objective.
Manipulating the local chemokine environment
The recent report by Muthuswamy illustrates the concept. The authors assessed the biological patterns in tumor explants in response to coordinated exposure with the components of the triad of IFN-α, poly IC (a TLR3 agonist) and cyclooxygenase inhibition. Individual cell responses and whole tissue expression were measured in the tumor microenvironment of tumor explants [21,31]. The study dissects cellular sources of selected chemokines known to favor desired cellular patterns and observed variant responses in different tissue samples that was further modified by the presence or absence of the individual components of the modulating therapeutic triad. For example, stromal tissue macrophage and fibroblasts expressed CCL5 (RANTES, a T effector cell attracting chemokine),in response to the combination of IFN-α and poly IC but much less in response to either agent in isolation. Macrophages produced the Treg chemotactic chemokine CCL22 in response to poly IC which was ablated by the presence of IFN-α, whereas fibroblasts did not produce this chemokine in response to either stimulus. The important message illustrated in that report is that the biological response at the tissue level for a given tumor is modified in the presence, absence and concentration of combinatorial stimulants and that the response is cell type specific. This report teaches how the use of triple combinations of immune modifiers can modulate the tissue microenvironment to favor immune effector pathways and reduce immune suppressive elements. Furthermore, the response elicited from a given cellular element may be changed by imposing a conditioning environment. The ideal formula, if it exists, is not identified in this report, but the principle is clearly established. Monoclonal antibodies with their multiple functional elements are ideal agents to modulate the tumor microenvironment in coordination with other agents such as select cytotoxic agents, adjuvants and cyclooxygenase inhibitors. Many antibodies currently in development or in use for the treatment of autoimmune disease, may also have useful roles in this emerging treatment paradigm as well.
Monoclonal antibodies for the treatment of solid malignancies
Specific monoclonal antibodies can be rapidly generated against pathways of interest. Cell surface markers and secreted molecules can be targeted. Receptor activity typically is inhibited, however if appropriate screening assays are available, agonist activity can be identified and selected. Table 2 illustrates selected antibodies in use or in clinical development for the treatment of solid malignancy that target tumors to interfere with receptor function and modulate cellular and antibody-dependent effector cell activity. Table 3 illustrates selected antibodies that target the myeloid and stromal compartments as well as cytokines and chemokines to impact tumor access, tumor growth and immune activation and regulation.
Table 2.
Selected cancer antibodies in development and clinical use: effector cell modulation.
| Target | Site | Products | Comments |
|---|---|---|---|
| (A) Surface receptors | |||
| HER2 | Tumor | Trastuzumab [32], pertuzumab [33] |
Pertuzumab interferes with HER2 dimerization |
| EGFR1 | Tumor | Cetuximab [35], panitumumab [36] |
EGFR1 is expressed especially in gastrointestinal and head and neck tumors and is modulated by heterodimerization with EGFR3 |
| EGFR3 (HER3) | Tumor | Patritumumab [37,38] | – |
| Glypican 3 | Tumor | GC-33 [39] | Glypican3 is highly expressed in hepatocellular cancer |
| Ganglioside GD2 | Tumor | Dinutuximab [40] | GD2 is over expressed in peripheral neuroblastoma [34] |
| (B) Checkpoint targets | |||
| CTLA4 | T cell | Ipilimumab [1], tremelimumab [41] |
Ipilumumab was the first approved check point agent |
| PD-1 | T cell | Nivolumab [42], pembrolizumab [43] pidilizumab [44] |
Nivolumab is approved in melanoma and lung cancer; pembrolizumab is approved for advanced melanoma refractory to ipilumumab, other agents remain in development |
| PDL-1 | APC/tumor | BMS936559 [45], MPDL3280A[46], MEDI- 4736 [47], avelumab [48] |
Blocking PDL1 has theoretical advantage of interfering with PD-1 stimulation and also targeting tumor cells for ADCC |
| KIR | T cell | Lirilumab [49] | – |
| LAG-3 | T cell | BMS 986016 [50] | – |
| (C) Second signal targets | |||
| CD28 | T cell | TGN1412 [51,52] | TGN 1412 was the CD-28 agonist that induced severe cytokine storm in Phase I |
| CD27 | T cell | CDX-1127 (varlilumab) [53] | CDX 1127 is being developed for solid and hematologic indications in early-phase studies |
| CD137(41BB) | T cell | Urelumab [54], PF05082566 [55] | The 41BB agonist urelumab and PF05082566 are lgG4 and lgG2 antibodies, respectively |
| CD40 [56] | APC | Dacetuzumab, CP870893 [57,58] | CD40 agonists directly stimulate APC and B cells. Their development has been slowed by toxicity issues |
| CD134 (OX40) | T cell | MEDI-6383 [59] | The Ox40 agonist MEDI-6383 is a T-cell activator in Phase I trials |
| GITR [60] | T, B, and NK cells |
TRX518 [61], MK4166 [62] | GITR agonists deplete Treg and activates T effectors |
Table 3.
Selected cancer antibodies in development and clinical use: matrix modifiers.
| Target | Site | Products | Comments |
|---|---|---|---|
| (A) Angiogenesis inhibitors | |||
| VEGF | Circulation | Bevacizumab [63] | Bevacizumab was the first approved antiangiogenesis antibody, now has multiple indications |
| Angiopoietin 2 | Circulation | Nesvacumab [64] | Ang 2 is a tumor growth factor and angiogenesis inducer, inhibitory antibodies are being studied |
| VEGFR2 | Vessel | Ramucirumab [65] | Ramucirumab is approved for gastric and lung cancers |
| (B) Circulating tumor-associated antigens | |||
| MUC16 | Tumor and circulation | Oregovomab [66] | Oregovomab binds CA125 (MUC16) in circulation and is studied in combination with chemotherapy and adjuvants in ovarian cancer and pancreatic cancer |
| MUC1 | AR20.5 [67] | The anti-MUC1 antibody AR20.5 completed a Phase I trial MUC1-expressing solid cancers |
|
| (C) Chemokines/cytokines | |||
| TGF-β | Fresolimumab [68] | Several monoclonal antibodies are being developed/marketed as anti-inflammatory antibody in autoimmune disease. Fresolimumab inhibits TGF-β and is in development for fibrosis and cancer |
|
| IL-10 [69–71] | Circulation | IL-10 has paradoxical effects and has been studied as a cytokine drug and also as a target of antibody neutralization |
|
| IL-6 | Tocilizumab [72] | Tocilizumab is approved to treat rheumatoid arthritis, but may prove useful in modulating stromal environments and controlling cytokine storm |
|
| (D) IgE-targeted tumor-associated antigens | |||
| MUC1 | Tumor and circulation | Anti-MUC1 IgE [73,74] |
Preclinical reports published for all of these stroma targeting agents. Preclinical development continuing. The therapeutic strategy combines induction of T-cell immunity, arming of myeloid effector cells to bring antitumor effects and may improve penetration of solid tumors by chemotherapeutics |
| PSA | Anti-PSA-lgE [75] | ||
| HER2 | Anti-HER2 IgE [76,77] |
||
| FBP | Anti-FBP IgE [78] | ||
Antibodies to surface receptors
Tumor antigen-targeting antibodies that target specific tumor-associated antigens and also interfere with receptor function have been the first commercially successful monoclonal antibodies developed for solid tumors (Table 2A). Problems of tissue penetration, hypoxic microenvironment, and a stromal environment that shares many features with a healing wound limit effectiveness of current tumor targeting therapies. Herceptin (trastuzumab) is the prototypic monoclonal antibody for treatment of solid cancer. Approved in 1998 for the treatment of HER2 amplified breast cancer, the antibody inhibits the EGFR (HER2/neu, EGFR2). Its activity is greatest in patients with amplified HER2 receptors which reflects a more dominant growth factor activity for interference and an increased copy number for NK cell-mediated ADCC. Both mechanisms likely contribute to the clinical effect [4]. Cetuximab developed to target EGFR1 was the second antibody commercialized to treat solid cancers, in this case initially for colorectal cancer [79]. Treatment effects are real and beneficial but generally not dramatic. In both cases, the antibody is dosed to maintain a high blood level that pharmacologically saturates the target receptor over time. Second generation products have provided some incremental improvements, expanded indications, provided carrier scaffolds for radioisotopes and drug conjugates and established a multibillion dollar market.
Additional monoclonal antibodies to surface markers are of emerging importance. Chugai Pharmaceuticals (Tokyo, Japan) is developing a monoclonal antibody to the oncofetal proteoglycan known as glypican-3. This receptor is overexpressed on hepatocellular carcinoma and mediates biological activities that are still being elucidated. A similar high-pharmacologic dose of antibody to trastuzumab and cetuximab to saturate the antigen and facilitate the NK-mediated immune eradication of the host tumor is being studied [39]. A second novel receptor, this one in the EGFR family that targets HER3 being codeveloped by Daiichi Sankyo (Tokyo, Japan) and Amgen (CA, USA) is also in early clinical development for multiple solid cancers [35]. Both programs show promise, and their potential may be expanded by integration with the novel immunotherapeutic strategies described below. In March 2015, the ganglioside GD2 targeting monoclonal ch14.18 with generic name dinutuximab (Unituxin™ , United Therapeutics, MD, USA) was approved for use in combination with IL-2, GMCSF and 13-cis-retinoic acid following at least partial response to chemotherapy for treatment of neuroblastoma [34,40].
Antibodies causing checkpoint inhibition or second signal activation
Table 2B & C highlight progress in the field of second signal and checkpoint immunology. Candidates for check point inhibition are the ligands or receptors that naturally down regulate activated T-cell immunity. Drew Pardoll’s review in Nature Medicine elegantly lays out the biology of the ‘second signal’ which includes second signal activators and inhibitors [17]. These direct T-cell pathways are a first line of regulation to either perpetuate or evolve into memory and shut off a specific cellular immune response. NK-cell activity mediating ADCC has also been found to have inhibitory down regulator (checkpoint) mediated by the receptors NKG2A and NKG2B interacting with HLA-E, a specialized MHC class I molecule. The checkpoint products target pathways that normally dampen or turn off a specific activated T cell (and for NKG2A NK cell)-mediated immune effector activity. By blocking these inhibitory pathways, an ongoing specific immune response can be perpetuated and amplified. Monoclonal antibodies are ideal agents to mediate this specific blockade. CTLA4 is an inhibitory second signal that arises with delay after a specific T-cell receptor has been engaged and activated. CTLA4 competes with the T cell-activating second signal CD28 and thus dampens specific activated immunity. Two lead anti-CTLA4 antibodies have been advanced through clinical development. Ipilimumab (Yervoy® Bristol-Myers Squibb, NY, USA) was the first therapy of any kind demonstrated to improve survival outcomes in advanced melanoma [1] and is now a standard treatment. A second anti-CTLA4 candidate, tremelimumab which was developed by Pfizer (NY, USA) but did not show a survival benefit in its first Phase III melanoma trial [80] and was deprioritized for less aggressive continuing development in collaboration with AstraZeneca (London, UK). The different outcomes of clinical development are instructive, as both products have similar activity and similar toxicities and both can induce durable responses in what had traditionally been considered untreatable melanoma. The use of CTLA4 is associated with significant autoimmune toxicity including colitis, vitiligo and hypophysitis. Preventive clinical management of these toxicities was better accomplished in the ipilimumab program using corticosteroids and anti-TNF agents and dosing was kept lower, but more frequent and of shorter duration 10 mgs/kg four doses over 3 months versus 15 mgs/kg per quarter on a continuing basis. Responses with ipilimumab have translated into durable survival benefit over time, suggesting a persistent effective antitumor immune response in the absence of continuing CTLA4 blockade [17].
The lessons of CTLA4 clinical development were immediately applied to a second check point axis PD-1 and PDL1 and in 2014, two PD-1 antagonists have achieved initial approval in advanced melanoma. PD-1 is an inhibitory second signal receptor that arises following T-cell activation and its ligand PDL-1 is expressed on antigen presenting cells but also frequently on tumors. PD-1 functions by inhibiting T-cell receptor (TCR) signaling utilizing the SHP2 phosphatase that is a fundamental component of the regulation of acute T-cell responses to infection [81–83]. Many companies are advancing antibodies to both PD-1 and PD1-1 in melanoma and multiple other solid cancers and lymphoma [84]. Autoimmune toxicities, notably colitis, dermatitis and occasionally endocrinopathy have been encountered, but in general, the PD pathway products have been better tolerated and more easily managed than the CTLA4 antagonists. Merck (NJ, USA) has achieved accelerated approval of its anti-PD-1, pembrolizumab (Keytruda®), by establishing efficacy in advanced melanoma patients with a BRAF mutation which had previously failed therapy with ipilimumab treatment alone or ipilimumab and a BRAF inhibitor. The objective response rate was 24% and the responses were durable lasting beyond 1.4–8.5 months in most patients [85], while Bristol-Myers Squibb (NY, USA) established the superiority of nivolumab (Opdivo®, Bristol-Myers Squibb, NY, USA) to standard dacarbazine treatment in patients with previously untreated advanced unresectable melanoma. The objective response rate was 32% in the nivolumab study. In that series, a third of patients responding had a durable response greater than 6 months [86]. Importantly, the principle of combinatorial therapy is illustrated by the demonstration that anti-CTLA-4 and anti-PD-1 effects are additive and complementary.
Confirmatory Phase III studies are ongoing in support of both of these accelerated approvals. With at least six additional products in development in this category, there is much ongoing clinical research in multiple indications. A definitive biomarker for clinical response has not yet been identified, and although activity in indications previously refractory to any treatment is being reported, the majority of patients still fail to respond to the treatment implying that additional steps are needed to further improve outcomes. To that end, combination studies with many alternative agents have been initiated across many cancer indications. Several reports presented at ASCO in June 2014 provide important clues for how immunity may be better mobilized, based on genomic and proteomic profiling of patients who have responded to check point therapy. Snyder, et al. found that in a series of patients with melanoma, the patients who responded to ipilimumab had high likelihood to carry mutations on whole-exome sequencing of tumor explants coding for neoantigens known to be associated with the generation of tumor-specific T-cell immunity [87]. At the same session, Kefford et al. associated better clinical outcomes in melanoma patients treated with prembrolizumab with expression of PDL1 on the patient’s tumors. The investigators did note, however that clinical response was sometimes also seen in patients lacking tumor expression of PDL-1 [88]. In a third report, Adaniel and colleagues using a gene set enrichment analysis reported that the presence of germ line mutations in the gene locus 3.p21.31 which includes the genes for three immune response related chemokine receptors (CCR2, CCRL2 and CCR5), was associated with a failure to respond to ipilimumab. This suggests subtle germ line mutations negatively altering the chemokine-mediated trafficking of inflammatory cells in the tumor microenvironment may impact the effectiveness of checkpoint blockade therapy [89]. There is an implication from these observations that a strategy that increases the immunogenicity of tumors; enhances the function and trafficking of inflammatory cells; and stimulates expression of tumor PDL-1 may be useful to improve the activity of check point treatments. Additional antagonists of inhibitory pathways in the immune response are being advanced through clinical development as a way to further build on this progress. Lirilumab is an antagonist to the KIR receptor [49] and BMS 986016 is an antagonist of LAG3 [50]. A third inhibitory checkpoint pathway is the TIM-3-Galectin-9 pathway that is also a promising target for checkpoint inhibition [90]. Finally, an NKG2A inhibitory antibody that limits down regulation of activated NK cells, IPH2201 is being developed by Innate Pharma (Marseille, France) and will be subject to combinatorial studies in association with the PD1-1 antagonist Medi4736 [22].
The alternative to checkpoint inhibition is to stimulate the immune activating second signal receptors using an agonist antibody. Table 2C highlights five such pathways that are being targeted by antibodies in early clinical development, including CD137, CD27, Ox40, GITR and CD-40. Toxicity seen with checkpoint antagonists has been less acute and more manageable than the severe cytokine storms encountered in a Phase I study of a CD-28 agonist TGN1412 [51,52], but the B7-CD28 second signal modulatory pathway is a potent modulator of T-cell activity for which overactive T-cell response is a patient management risk. The experience derived from the development efforts with the CD28 pathway and inconsistencies in toxic effects between animals and humans have slowed development of agonists of stimulatory immune pathways. Modulating inhibitory pathways has been more easily managed clinically, however early progress in this area is promising.
Antibodies inhibiting tumor vascularization
Antiangiogenesis is another well-established strategy now to treat cancer with antibodies (Table 3A). Bevacizumab was the first treatment approved in this therapeutic category [63]. This monoclonal antibody binds VEGF in the circulation and thus prevents it from binding VEGFR and promoting vascular growth and a nurturing tumor microenvironment. Initially approved as a treatment for colon cancer, it is being studied in many other cancer indications and has approval for other tumor types in some geographies. In addition to bevacizumab, Table 3D highlights anti-Ang-2 targeting the angiopoietin pathway [64] and ramucirumab which is a monoclonal antibody that directly blocks VEGFR [65] that are both promising targets for cancer therapy.
Antibodies to induce tumor-specific immunity
Table 3B describes monoclonal antibodies in development intended to induce immunity to targeted tumor antigens. Two antibodies being developed by Quest PharmaTech (Alberta, Canada) are oregovomab and AR20.5 which target MUC16 (CA125) and MUC1, respectively. These antibodies have been studied in tumors associated with overexpression of these tumor markers and are dosed at much lower doses than other monoclonal antibodies with the specific intention of modifying antigen processing and inducing antigen-specific cellular immunity. The pharmacologic activity has been documented in both preclinical and clinical studies and Braly established a schedule-dependent effect of carboplatin paclitaxel on oregovomab-induced immunity [66,67]. The recent progress with checkpoint inhibition suggests that a strategy to induce specific T-cell immunity using these antibodies could improve the therapeutic outcomes associated with checkpoint inhibition in malignancies associated with their targeted antigens.
Antibodies to chemokines & cytokines
The redundancy of immune homeostatic pathways and the capacity of tumors to express cytokines, chemokines and receptors to select for these pathways implies that narrow strategies to stimulate specific immunity may likely work only in select patients in whom a tumor-specific immune response is dominantly gated by the select pathway of the chosen treatment. Additional tools to address cell trafficking, the phenotypic character of the myeloid compartment at a tumor site and the stroma tissues themselves should greatly facilitate mobilization of effective tumor-specific immunity in a broader range of patients. Standard cytotoxic therapies themselves have unique immune modulatory properties, which if better appreciated, may lead to improved combinatorial strategies. There are monoclonal antibodies approved or in development for the treatment of inflammatory states, fibrosis and autoimmune disease which target chemokines/cytokines and may be highly useful for modulating stromal environments (Table 3C). TGF-β is a primary cytokine associated with wound healing and downregulation of inflammatory states. Fresolimumab (Sanofi-Aventis, Paris, France) is being developed for the treatment of pulmonary fibrosis and other fibrotic conditions but its value to modulate antitumor immunity by limiting TGF-β is also under investigation [68]. The use of IL-6 to limit cytokine storm has been reported in early studies of chimeric antigen receptor autologous T-cell treatments [72]. Although not yet reported, this suggests that combination treatments with an anti-IL-6 antibody such as tocilizumab (Actemra®, Genentech, CA, USA) may permit more aggressive use of agonists to immune stimulators [72]. Finally, IL-10 is another cytokine with anti-inflammatory properties that has been explored to control fibrotic conditions [69]. Using an antibody to modulate this pathway in conjunction with other antitumor immune stimulatory treatments deserves further exploration.
IgE antibodies to modulate tumor stroma
The IgE class of monoclonal antibody is generating interest in some groups as an anticancer therapeutic strategy [11,12]. Antibodies specific for FBP, MUC1, HER2, PSA, have been advanced to late preclinical development (Table 3D) and all have been demonstrated to have antitumor activity in animal models. With emerging appreciation of the significance of the stromal compartment and the ability to modify the behavior of cells otherwise classified as immune suppressive, IgE is an interesting area for development. The risk of uncontrolled hypersensitivity should be easily managed pre-emptively. The ability of monoclonal IgE to induce cross presentation and potent T-cell reactivity to soluble self-antigen has been demonstrated for HER2 [76] and PSA [75]. The arming of myeloid cells with specific IgE including mast cells and basophils, but also monocytes, eosinophils and others appears to modulate their activity and permit antibody-dependent cytotoxicity and antibody-dependent phagocytosis by these effector cells [77] and also to interfere with tumor metastasis [73]. Preliminary data suggests specific IgE may also facilitate perfusion of tumor stroma facilitating penetration of cytotoxic therapy but confirmatory studies are ongoing. Whether cytotoxic therapy and other immune modulators will have additive effects in the presence of tumor antigen-specific IgE should be determined in the near future.
Conclusion
The potential to substantially advance the current state of success with treating solid cancers in most cases will rely on sophisticated combinatorial strategies that not only target the cancer itself, but also modulate the surrounding tissues to reduce homeostatic immune suppression and foster tumor focused attack. The success of tumor-targeting antibodies achieved with first generation products such as trastuzumab and cetuximab will be expanded and improved by incorporation of these approaches with combinations of check point inhibitors, second signal agonists and other small molecule stromal modifiers and scheduled chemotherapy. Antibodies can also be used to enrich for antigen-specific immunity and to target and modulate the inflammatory cells of myeloid lineage that often are immune suppressive in tumor dominated microenvironments. The suppressive phenotypes are strongly characteristic of tissue repair and wound healing states that are a physiologic part of a tissue injury and repair cycle. Antibodies to select chemokines and cytokines or their receptors may influence this biology directly and products of the IgE isotype have the potential to mobilize and activate as effectors, myeloid cells which otherwise might be immune suppressive. It is of interest that the current success with chimeric antigen receptor autologous cell therapy technology has been most evident in pediatric hematology, but much less so in the management of solid tumors and in adult patients [91]. This suggests that similar elements of the tumor stroma that are limiting the success of solid tumor targeting antibodies relative to hematologic targeting antibodies may also be limiting autologous cell therapy. New treatment paradigms incorporating antibody driven strategies to modulate the solid tissue stroma will be important for the advancement of that technology as well. The preclinical models which predict clinical activity are not adequately reflective of human cancer biology to definitively predict which emerging strategies will be most useful clinically, but the antibodies in development and also in current use for noncancer indications in the modulation of autoimmune states, all have the potential to be important components of future therapy. In the emerging ‘post genomics’ era, the majority of relevant pathways are now most likely defined but some are likely not yet fully appreciated. The objective of finding ‘cure all’ monotherapy products should give way to a new more methodical era of systematic study of combinations. Importantly, conclusions drawn from negative clinical studies of bioactive compounds as monotherapy are highly instructive in revising combinatorial strategies but should not be considered conclusive evidence for elimination of products. Clinical utilization of such products may depend on more sophisticated clinical strategies. If precisely selected combinations can induce more dramatic clinical effect in the control of typically refractory late-stage solid tumors, definitive registration studies for these combinations should be achievable in small patient cohorts reducing development costs. Furthermore, if the precisely selected use of highly effective combinations replaces the general use of often ineffective or marginally effective monotherapies, overall costs of medical treatment may be better controlled. Improvement in diagnostic profiling will likely be required. This will be the subject of intense review in coming years as the current trends in cancer treatment costs are non-sustainable.
Future perspective
There is a growing appreciation that a revised approach should be taken to advance cancer drug development, however institutional, regulatory and procedural barriers established in the name of safety, quality, good science and also to foster product innovation, do not favor combinatorial studies with experimental investigational new drug application drug candidates. This may provide a competitive incentive for global regions with less mature biomedical science and regulatory infrastructures and with underserved populations with unmet medical need to facilitate such systematic combinatorial research. Institutional barriers and costs are becoming increasingly limiting in the regions where modern biomedical clinical science first blossomed.
The numbers of products seeking to enter clinical study, the costs, the deficiency of clinical resources and also a paucity of available study patients in some geographies suggest a shift in development paradigms and biomedical research dominance may emerge. Just as the AIDS crisis revolutionized the rapid combinatorial screening of antiretroviral agents to advance the fight against epidemic HIV; the continuing marginal performance of current treatment paradigms in the management of advanced stage solid tumors suggests a new dynamic approach to developmental research should be considered. Methods to better appreciate the nature of immune suppression operative in individual patients may be as important as the pathologic diagnosis of the malignancy itself in dictating what an optimal individual treatment plan should be. This is a model of personalized medicine that will likely emerge over time. Monoclonal antibodies specific for relevant immune modulatory elements, able to induce and foster tumor-specific cellular immunity, able to inhibit downregulation and enhance penetration of combinatorial cytotoxics, will all have potential roles in future patient treatment plans. The coordinated use of adjuvants such as selective TLR agonists that are able to enrich responses [92] and appreciation of the immune modulatory impact and combinatorial limitations of standard chemotherapeutics will be additional factors to consider in establishing future treatment regimens. The challenge is multidimensional and includes a strong temporal component that makes schedule as important as dose. Many of the needed tools have been created and the targets defined. How rapidly this advance can progress is hard to predict, but the road map forward is now defined.
Executive summary.
The success of checkpoint inhibitors has established the potential of immunotherapy to control even advanced treatment refractory cancer.
Monoclonal antibody products have had greater impact to date in hematologic malignancy rather than in solid tumors.
Recent insights into cell biology have established redundancies in immune homeostatic mechanisms that are especially prevalent in the tissue matrix and solid tumors.
Monoclonal antibody technology is ideal to manipulate the tumor-host interaction to therapeutic advantage.
Antibodies as therapeutic tools
Antibodies interact with target antigen through the Fab antigen-binding domain and immune effector pathways via the Fc region.
The function of the Fc component is determined by the antibody class and subclass.
IgG subclasses lgG1, lgG2 and lgG4 are commonly used as therapeutic antibodies and the IgE is an emerging class due to Fc extensive interaction with the myeloid compartment critical to solid tumor biology.
Current insights into immune homeostasis
Immune quiescence is the physiologic state in health and redundant pathways exist to dampen acute immune response to an insult and to promote healing.
Specific T-cell immunity is regulated by second signal activators and inhibitors.
The solid tumor tissue matrix and stromal environment includes many cells associated with tissue repair, which have a predominant anti-inflammatory bias.
Tumors selectively favor growth promoting environments and colonies in a tumor clones will grow to advantage when expressing immune suppressive factors and receptors.
Monoclonal antibodies for the treatment of solid malignancies
Tumor-targeting antibodies inhibiting receptors such as HER2 and EGFR1 have established the commercial success of therapeutic anti-cancer antibodies.
The clinical response to checkpoint inhibition has been dramatic and gratifying, however the majority of patients are not yet cured of their advanced cancers.
Strategies to improve outcomes should focus on strategies to induce specific immunity, modulate the biology of the myeloid compartment, evaluate combinations and elucidate the effect of schedule.
The IgE class of antibody and a variety of immune modulating antibodies currently in use in management of autoimmune disease as well as selective novel adjuvants are likely be important for future success.
Conclusion
The potential of monoclonal antibody therapy to revolutionize outcomes in the management of solid malignancy is near.
Institutional and regulatory conventions to foster development of new chemical entities does not favor the combinatorial research approaches most likely needed to establish the most effective future treatment strategies.
Regions with large unmet medical need and emerging institutions may be more facile at bringing cancer therapy to this next level of success.
Acknowledgments
C Nicodemus provides strategic and scientific guidance to academic and industrial clients in the biomedical field. The author receives compensation and owns shares of Quest PharmaTech, a research and development stage company innovating novel antibody products as cancer immunotherapeutics to which the author has assigned issued and pending patents. This work was supported in part by NIH/NCI R41A137881.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest.
- 1. Hodi FS, O’Day SJ, Mcdermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466.• Original report of checkpoint blockade with CTLA4 extending survival in melanoma.
- 2.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 3.Genentech. www.gene.com/media/product-information/herceptin.
- 4.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Lefranc MP, Lefranc G. The Immunoglobulin FactsBook. MA, USA: Academic Press; 2001. [Google Scholar]
- 6.Nicodemus CF, Smith LM, Schultes BC. Role of monoclonal antibodies in tumor-specific immunity. Expert Opin. Biol. Ther. 2007;7(3):331–343. doi: 10.1517/14712598.7.3.331. [DOI] [PubMed] [Google Scholar]
- 7.Gorovits B, Krinos-Fiorotti C. Proposed mechanism of off-target toxicity for antibody-drug conjugates driven by mannose receptor uptake. Cancer Immunol. Immunother. 2013;62(2):217–223. doi: 10.1007/s00262-012-1369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiner GJ. Rituximab: mechanism of action. Semin. Hematol. 2010;47(2):115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefebvre ML, Krause SW, Salcedo M, Nardin A. Ex vivo-activated human macrophages kill chronic lymphocytic leukemia cells in the presence of rituximab: mechanism of antibody-dependent cellular cytotoxicity and impact of human serum. J.Immunother. 2006;29(4):388–397. doi: 10.1097/01.cji.0000203081.43235.d7. [DOI] [PubMed] [Google Scholar]
- 10.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99(3):754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 11.Josephs DH, Spicer JF, Karagiannis P, Gould HJ, Karagiannis SN. IgE immunotherapy: a novel concept with promise for the treatment of cancer. MAbs. 2014;6(1):54–72. doi: 10.4161/mabs.27029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nigro E, Siccardi A, Vangelista L. Role and redirection of IgE against cancer. Antibodies. 2013;2(2):371–391.•• Comprehensive review of the monoclonal IgE field including discussion of possible mechanisms.
- 13.Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat. Rev. Immunol. 2007;7(5):365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 14.Edkins AL, Borland G, Acharya M, Cogdell RJ, Ozanne BW, Cushley W. Differential regulation of monocyte cytokine release by αV and β(2) integrins that bind CD23. Immunology. 2012;136(2):241–251. doi: 10.1111/j.1365-2567.2012.03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul WE. Fundamental Immunology. 7th Edition. PA, USA: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 16.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin. Orthop. Relat. Res. 1991;(262):3–11. [PubMed] [Google Scholar]
- 17. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239.•• Comprehensive review of immune second signals and checkpoints.
- 18.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 2003;3(9):745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 19.Peggs KS, Allison JP. Co-stimulatory pathways in lymphocyte regulation: the immunoglobulin superfamily. Br. J. Haematol. 2005;130(6):809–824. doi: 10.1111/j.1365-2141.2005.05627.x. [DOI] [PubMed] [Google Scholar]
- 20.Guilliams M, Ginhoux F, Jakubzick C, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014;14(8):571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muthuswamy R, Berk E, Junecko BF, et al. NF-κB hyperactivation in tumor tissues allows tumor-selective reprogramming of the chemokine microenvironment to enhance the recruitment of cytolytic T effector cells. Cancer Res. 2012;72(15):3735–3743. doi: 10.1158/0008-5472.CAN-11-4136.• Original report describing the variable responses to specific stimulate based on cell type and presence of cofactors.
- 22.Innate Pharma. www.innate-pharma.com/en/product-pipeline.
- 23.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 24.Cekic C, Day YJ, Sag D, Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014;74(24):7250–7259. doi: 10.1158/0008-5472.CAN-13-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platten M, Von Knebel Doeberitz N, Oezen I, Wick W, Ochs K. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol. 2014;5:673. doi: 10.3389/fimmu.2014.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khazaie K, Blatner NR, Khan MW, et al. The significant role of mast cells in cancer. Cancer Metastasis Rev. 2011;30(1):45–60. doi: 10.1007/s10555-011-9286-z. [DOI] [PubMed] [Google Scholar]
- 27.Movahedi K, Laoui D, Gysemans C, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 28.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14(6):e218–e228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 29.Butt AQ, Mills KH. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene. 2014;33(38):4623–4631. doi: 10.1038/onc.2013.432. [DOI] [PubMed] [Google Scholar]
- 30.Tan W, Zhang W, Strasner A, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470(7335):548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118(20):5498–5505. doi: 10.1182/blood-2011-07-365825.• Original report demonstrating impact of Cox-2 inhibition on response to stimulation of immune microenvironment.
- 32.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl. J. Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 33.Harbeck N, Beckmann MW, Rody A, et al. HER2 dimerization inhibitor pertuzumab - mode of action and clinical data in breast cancer. Breast Care (Basel) 2013;8(1):49–55. doi: 10.1159/000346837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010;363(14):1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawakami H, Okamoto I, Yonesaka K, et al. The anti-HER3 antibody patritumab abrogates cetuximab resistance mediated by heregulin in colorectal cancer cells. Oncotarget. 2014;5(23):11847–11856. doi: 10.18632/oncotarget.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price TJ, Peeters M, Kim TW, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority Phase 3 study. Lancet Oncol. 2014;15(6):569–579. doi: 10.1016/S1470-2045(14)70118-4. [DOI] [PubMed] [Google Scholar]
- 37.Wakui H, Yamamoto N, Nakamichi S, et al. Phase 1 and dose-finding study of patrkumab (U3-1287), a human monoclonal antibody targeting HER3, in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2014;73(3):511–516. doi: 10.1007/s00280-014-2375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorusso P, Janne PA, Oliveira M, et al. Phase I study of U3-1287, a fully human anti-HER3 monoclonal antibody, in patients with advanced solid tumors. Clin. Cancer. Res. 2013;19(11):3078–3087. doi: 10.1158/1078-0432.CCR-12-3051. [DOI] [PubMed] [Google Scholar]
- 39.Zhu AX, Gold PJ, El-Khoueiry AB, et al. First-in-man Phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2013;19(4):920–928. doi: 10.1158/1078-0432.CCR-12-2616. [DOI] [PubMed] [Google Scholar]
- 40.US FDA. www.fda.gov/newsevents/newsroom/pressannouncements.
- 41.Ascierto PA. Is there still a role for tremelimumab in the treatment of cancer? Transl. Cancer Res. 2013;2(1):48–50. [Google Scholar]
- 42.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 43.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a Phase 1 trial. Lancet. 2014;384(9948):1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 44.Atkins MB, Kudchadkar RR, Sznol M, et al. Phase 2, multicenter, safety and efficacy study of pidilizumab in patients with metastatic melanoma. ASCO Meeting Abstracts. 2014;32(15 Suppl) 9001. [Google Scholar]
- 45.NIH. Study NCT00729664. www.clinicaltrials.gov.
- 46.NIH. Study NCT02174172. www.clinicaltrials.gov.
- 47.Lutzky J, Antonia SJ, Blake-Haskins A, et al. A Phase 1 study of MEDI4736, an anti-PD-L1 antibody, in patients with advanced solid tumors. ASCO Meeting Abstracts. 2014;32(15 Suppl):3001. [Google Scholar]
- 48.NIH. Study NCT01772004. www.clinicaltrials.gov.
- 49.Segal NH, Hodi FS, Sanborn RE, et al. A Phase I dose escalation and cohort expansion study of lirilumab (anti-KIR; BMS-986015) in combination with nivolumab (anti-PD-1; BMS-936558, ONO-4538) in advanced solid tumors. ASCO Meeting Abstracts. 2014;32(15 Suppl) TPS3115. [Google Scholar]
- 50.Goding SR, Wilson KA, Xie Y, et al. Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma. J. Immunol. 2013;190(9):4899–4909. doi: 10.4049/jimmunol.1300271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a Phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355(10):1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 52.Yiu HH, Graham AL, Stengel RF. Dynamics of a cytokine storm. PLoS ONE. 2012;7(10):e45027. doi: 10.1371/journal.pone.0045027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vitale LA, He LZ, Thomas LJ, et al. Development of a human monoclonal antibody for potential therapy of CD27-expressing lymphoma and leukemia. Clin. Cancer Res. 2012;18(14):3812–3821. doi: 10.1158/1078-0432.CCR-11-3308. [DOI] [PubMed] [Google Scholar]
- 54.Kohrt HE, Godwin JE, Lossos IS, et al. A Phase Ib, open-label, multicenter study of urelumab (BMS-663513) in combination with rituximab in subjects with relapsed/ refractory B-cell malignancies. A SCO Meeting Abstracts. 2013;31(15 Suppl) TPS3108. [Google Scholar]
- 55.Segal NH, Gopal AK, Bhatia S, et al. A Phase 1 study of PF-05082566 (anti-4–1BB) in patients with advanced cancer. ASCO Meeting Abstracts. 2014;32(15 Suppl):3007. [Google Scholar]
- 56.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin. Cancer Res. 2013;19(5):1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beatty GL, Torigian DA, Chiorean EG, et al. A Phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2013;19(22):6286–6295. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.NIH. Study NCT02221960. www.clinicaltrials.gov.
- 60.Lu L, Xu X, Zhang B, Zhang R, Ji H, Wang X. Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs. J.Transl. Med. 2014;12:36. doi: 10.1186/1479-5876-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.NIH. Study NCT01239134. www.clinicaltrials.gov.
- 62.NIH. Study NCT02132754. www.clinicaltrials.gov.
- 63.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 64.Thomas M, Kienast Y, Scheuer W, et al. A novel angiopoietin-2 selective fully human antibody with potent anti-tumoral and anti-angiogenic efficacy and superior side effect profile compared with Pan-Angiopoietin-1/-2 inhibitors. PLoS ONE. 2013;8(2):e54923. doi: 10.1371/journal.pone.0054923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, Phase 3 trial. Lancet. 2014;383(9911):31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 66.Braly P, Nicodemus CF, Chu C, et al. The Immune adjuvant properties of front-line carboplatin-paclitaxel: a randomized Phase 2 study of alternative schedules of intravenous oregovomab chemoimmunotherapy in advanced ovarian cancer. J.Immunother. 2009;32(1):54–65. doi: 10.1097/CJI.0b013e31818b3dad. [DOI] [PubMed] [Google Scholar]
- 67.De Bono JS, Rha SY, Stephenson J, et al. Phase I trial of a murine antibody to MUCl in patients with metastatic cancer: evidence for the activation of humoral and cellular antitumor immunity. Ann. Oncol. 2004;15(12):1825–1833. doi: 10.1093/annonc/mdh472. [DOI] [PubMed] [Google Scholar]
- 68.Morris JC, Tan AR, Olencki TE, et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFbeta) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS ONE. 2014;9(3):e90353. doi: 10.1371/journal.pone.0090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bijjiga E, Martino A. Interleukin 10 (IL-10) regulatory cytokine and its clinical consequences. J. Clin. Cell. Immunol. 2013 (Epub ahead of print). [Google Scholar]
- 70.Oft M. IL-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol. Res. 2014;2(3):194–199. doi: 10.1158/2326-6066.CIR-13-0214. [DOI] [PubMed] [Google Scholar]
- 71.Llorente L, Richaud-Patin Y, Garcia-Padilla C, et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000;43(8):1790–1800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 72.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mollick J, Madiyalakan M, Nicodemus C. Muci specific IGE to modify myeloid derived cells of the tumor microenvironment. J. Immunother. Cancer. 2013;1(Suppl. 1):P245. [Google Scholar]
- 74.Teo PZ, Utz PJ, Mollick JA. Using the allergic immune system to target cancer: activity of IgE antibodies specific for human CD20 and MUCl. Cancer Immunol. Immunother. 2012;61(12):2295–2309. doi: 10.1007/s00262-012-1299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Daniels-Wells TR, Helguera G, Leuchter RK, et al. A novel IgE antibody targeting the prostate-specific antigen as a potential prostate cancer therapy. BMC Cancer. 2013;13:195. doi: 10.1186/1471-2407-13-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Daniels TR, Leuchter RK, Quintero R, et al. Targeting HER2/neu with a fully human IgE to harness the allergic reaction against cancer cells. Cancer Immunol. Immunother. 2012;61(7):991–1003. doi: 10.1007/s00262-011-1150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karagiannis P, Singer J, Hunt J, et al. Characterisation of an engineered trastuzumab IgE antibody and effector cell mechanisms targeting HER2/neu-positive tumour cells. Cancer Immunol. Immunother. 2009;58(6):915–930. doi: 10.1007/s00262-008-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gould HJ, Mackay GA, Karagiannis SN, et al. Comparison of IgE and IgG antibody-dependent cytotoxicity in vitro and in a SCID mouse xenograft model of ovarian carcinoma. Eur. J. Immunol. 1999;29(11):3527–3537. doi: 10.1002/(SICI)1521-4141(199911)29:11<3527::AID-IMMU3527>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 79.Jonker DJ, O’callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N. Engl. J. Med. 2007;357(20):2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 80.Ribas A. Clinical development of the anti-CTLA-4 antibody tremelimumab. Semin. Oncol. 2010;37(5):450–454. doi: 10.1053/j.seminoncol.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 81.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibkory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 83.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J.Immunol. 2004;173(2):945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 84.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl., J. Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.US FDA. www.fda.gov/newsevents/newsroom/pressannouncements.
- 86.US FDA. www.fda.gov/newsevents.
- 87.Snyder Charen A, Makarov V, Merghoub T, et al. The neoantigen landscape underlying clinical response to ipilimumab. ASCO Meeting Abstracts. 2014;32(15 Suppl):3003. [Google Scholar]
- 88.Kefford R, Ribas A, Hamid O, et al. Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. ASCO Meeting Abstracts. 2014;32(15 Suppl):3005. [Google Scholar]
- 89.Adaniel C, Rendleman J, Polsky D, et al. Germline genetic determinants of immunotherapy response in metastatic melanoma. ASCO Meeting Abstracts. 2014;32(15 Suppl.):3004. [Google Scholar]
- 90.Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol. Res. 2014;2(5):393–398. doi: 10.1158/2326-6066.CIR-14-0039. [DOI] [PubMed] [Google Scholar]
- 91. Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123(17):2625–2635. doi: 10.1182/blood-2013-11-492231.• Review of chimeric antigen receptor technology that may well benefit from cancer therapy in combination with select monoclonal antibodies.
- 92.Nagato T, Lee YR, Harabuchi Y, Celis E. Combinatorial immunotherapy of polyinosinic-polycytidylic acid and blockade of programmed death-ligand 1 induce effective CD8 T-cell responses against established tumors. Clin. Cancer Res. 2014;20(5):1223–1234. doi: 10.1158/1078-0432.CCR-13-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]