Abstract
Damage and degeneration of the skeletal elements due to disease, trauma, and aging lead to a significant health and economical burden. To reduce this burden, skeletal tissue engineering strategies aim to regenerate functional bone and cartilage in the adult body. However, challenges still exist. Such challenges involve the identification of the external cues that determine differentiation, how to control chondrocyte hypertrophy, and how to achieve specific tissue patterns and boundaries. To address these issues, it could be insightful to look at skeletal development, a robust morphogenetic process that takes place during embryonic development and is commonly modeled in vitro by the micromass assay. In this review, we investigate what the tissue engineering field can learn from this assay. By comparing embryonic skeletal precursor cells from different anatomic locations and developmental stages in micromass, the external cues that guide lineage commitment can be identified. The signaling pathways regulating chondrocyte hypertrophy, and the cues required for tissue patterning, can be elucidated by combining the micromass assay with genetic, molecular, and engineering tools. The lessons from the micromass assay are limited by two major differences between developmental and regenerative skeletogenesis: cell type and scale. We highlight an important difference between embryonic and adult skeletal progenitor cells, in that adult progenitors are not able to form mesenchymal condensations spontaneously. Also, the mechanisms of tissue patterning need to be adjusted to the larger tissue engineering constructs. In conclusion, mechanistic insights of skeletal tissue generation gained from the micromass model could lead to improved tissue engineering strategies and constructs.
Introduction
The skeletal system consists of rigid bony elements and flexible cartilaginous structures, which provide both stability and flexibility to the vertebrate body. Disease, injury, and aging may cause defects and degeneration of these structures, resulting in severe health problems including loss of mobility, chronic pain, and other complications.1 Cartilage has limited intrinsic regenerative potential, however, mostly believed to be due to its nonvascularized nature.2 Bone, in contrast, is vascularized and has regenerative capacity, but the tissue becomes unable to heal when a defect extends a critical size.3 Thus, there is a high demand for tissue engineering strategies that aim to regenerate bone, cartilage, and their interface after loss, damage, or degeneration.
Over the last several decades, major advancements have been made in the field of skeletal tissue engineering, which have led to various clinical trials4 and several products that are currently being used in clinics to treat skeletal defects, such as the INFUSE® Bone Graft that locally delivers BMP2 to heal bone defects and the cell-based product ChondoCelect® for autologous chondrocyte transplantation to repair cartilage.5 However, several challenges still exist, one being the lineage capacity of the commonly used cell sources. Adult mesenchymal stem cells (MSCs) present a desirable cell source for skeletal tissue engineering applications, as they can be readily isolated in large numbers and have a broad lineage potential, including the capacity to differentiate down the osteogenic and chondrogenic lineage.6 However, the results of cartilage tissue engineering strategies using adult MSCs have been suboptimal when compared to the use of mature chondrocytes.7–9
In many cases chondrocytes derived from MSCs eventually displayed a hypertrophic phenotype,10–12 which appears to indicate that the cells are primed toward endochondral ossification and the chondrogenic state is thus only transiently present. This raises questions about the potential of adult MSCs: are they developmentally restricted, or could they be guided to generate the desired, stable type of cartilage if they were exposed to the right environmental cues?
Another challenge is that tissue-engineered cartilage constructs often lack the structural complexity distinctive of the different types of cartilage, such as the zonal organization in articular cartilage, or the desired mechanical properties.13,14 Also, it has been challenging to establish and maintain distinct tissue boundaries, especially when diffusible morphogens are utilized.15 These issues reflect a need for improved spatial and temporal control of the cues that guide cellular differentiation, and a more profound understanding of the mechanisms that yield tissue patterning and structural organization.
To improve tissue engineering strategies, it could be helpful to look at embryonic development.16 Skeletal development has been extensively studied, and these investigations have benefitted from various in vitro models that enable the study of isolated components. This review focuses on one model of developmental skeletogenesis in particular that is still to be explored by the tissue engineering field: the micromass assay. This relatively simple in vitro assay is commonly used by developmental biologists and facilitates the study of the early stages of skeletal development in a highly controlled and adaptable manner.17,18 By culturing embryonic skeletal progenitor cells in a high-density drop, this model recapitulates developmental stages of progenitor proliferation, mesenchymal condensation, and chondrogenic differentiation.
Here, we review how insights from the micromass assay can be of value to the skeletal tissue engineering field. We describe how it can be used to address the aforementioned challenges, and the implications and limitations of the assay. The studies that we cite along the way, both from the micromass and tissue engineering field, represent illustrative examples, but do not form an exhaustive list of the literature in these areas. Comprehensive comparisons of models of regenerative skeletogenesis and tissue engineering strategies can be found in other recent reviews.19–21
Skeletal Development and In Vitro Modeling
During embryonic development, the skeletal tissues develop through an intricate morphogenetic process that involves pattern formation, differentiation, and growth. In the following sections we will describe the process of in vivo skeletal development and how the micromass assay models this process in vitro.
Skeletal development
The skeletal elements originate from the mesoderm during early embryonic development.22 Bone formation can occur through two distinct mechanisms, intramembranous ossification or endochondral ossification. The former takes place mostly in the cranial region, while the majority of other bones, such as the long bones and the vertebrae, develop through endochondral ossification.23 For both processes, the first step involves proliferation and condensation of skeletal progenitor cells, alternatively called MSCs.24 During intramembranous ossification, the cells in the condensations differentiate directly into osteoblasts, and this process is therefore referred to as direct bone formation. In contrast, in endochondral ossification bone is formed via a cartilaginous intermediate.23 After the mesenchymal cells are differentiated into chondrocytes, a cartilaginous matrix is deposited, and the cells in the center undergo hypertrophy. The cartilage then starts to calcify and subsequently becomes vascularized, which enables osteoblasts to enter and replace the calcifying cartilage anlage by mineralized bone.23,24
Mesenchymal condensation is a critical step in the onset of skeletal development.25,26 Failure to form proper condensations leads to the development of abnormally sized or shaped skeletons, or prevents bone from forming at all.23,27 Since the location and number of elements is determined at this stage, it is also a determinant of skeletal patterning.27,28 Condensations are typically characterized by an increased cell density, positive staining with peanut agglutinin lectin,29 and specific markers like NCAM, Pax-1, and Pax-9.30,31 They are thought to form through local extracellular matrix (ECM)-driven cell rearrangements rather than active cell migration or localized proliferation.32–34 The predominant matrix components at this stage are fibronectin (FN) and hyaluronic acid.35–38 The increased cell density in a condensation is associated with increased cell–cell contacts, which is thought to induce the chondrogenic commitment of the MSCs.31,39,40
Micromass assay
Several methods have been developed to study skeletal development in vitro.41–43 For example, whole limb buds can be cultured in vitro and such organ cultures are used to test the effect of externally added morphogens.43 Densely packed three-dimensional (3D) aggregate cultures of adult MSCs are used to mimic the developmental condensation stage42 and knowledge of the spatiotemporal presence of certain morphogens during development is employed to prime MSC cultures via developmental pathways.41 However, organ cultures are not very adaptable with regard to experimental parameters, and models using adult MSC require preexisting knowledge about the developmental process. The micromass assay uses embryonic skeletal progenitor cells and provides a widely used, adaptable, and relatively simple in vitro model of the early stages of skeletal development.17,18
The main premise of the micromass model is to ensure a high cell density from the start of the culture, which mimics the high cell density that is found in the precondensation phase in vivo, and is required for the onset of skeletal development.18,44 Typically, progenitor cells isolated from chicken or mouse embryonic limb buds are suspended in a high-density cell suspension of which a small drop—typically around 1×105 cells in 10 μL—is placed in the center of a culture dish (Fig. 1). The cells are allowed to attach to the substrate, after which the dish is flooded with medium.45
FIG. 1.
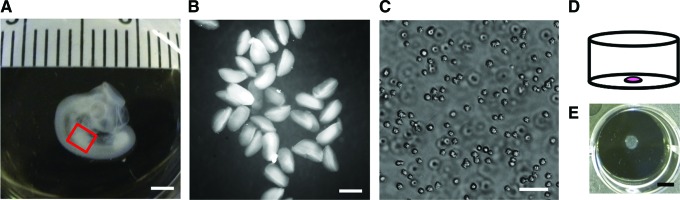
Embryonic skeletal progenitor cells are freshly isolated and cultured in micromass. (A) Photograph of representative stage 23 chicken embryo as used for cell isolations. The red box indicates the location of the limb bud used for cell isolation. Scale bar is 2 mm. (B) Collection of limb buds in phosphate-buffered saline right after dissection. Scale bar is 500 μm. (C) Micrograph of a single cell suspension of freshly isolated chicken limb bud cells, after dissociation. Scale bar is 50 μm. (D) Schematic representation of a micromass; a high cell density drop plated in the center of a culture dish. (E) Bird's eye view of a representative micromass culture after 3 days in culture in a 12-well plate after removal of the culture medium. Scale bar is 5 mm. Color images available online at www.liebertpub.com/teb
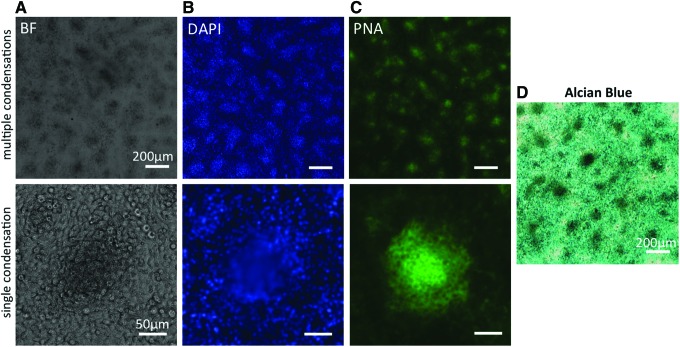
At this point, the model is considered two-dimensional. Once in culture, the cells will first undergo abundant proliferation.46,47 In combination with abundant FN deposition,37,48 the culture now contains multiple layers of cells embedded in ECM and can thus be considered 3D. Within 2–3 days, mesenchymal condensations will spontaneously form, indicated by a local increase in cell density and a local elevation in the culture (Fig. 2). Mesenchymal condensations, both in vivo and in vitro, can be detected by peanut agglutinin lectin staining29,49 (Fig. 2) and the cells in the condensations typically display a more rounded cell morphology.17,50 The increased cell density in the condensations then induces the onset of chondrogenic differentiation. This is apparent from the increased expression of typical chondrogenic genes such as Sox951 and aggrecan,52 and from the deposition of chondrogenic matrix components such as collagen type II and glycosaminoglycans (Fig. 2).53 While most micromass studies focus on the events during the first few days of culture, some studies have described how long-term cultures undergo further stages toward endochondral ossification. After 14 days in culture cellular hypertrophy could be observed in the core of the chondrogenic nodules, accompanied by increased levels of alkaline phosphatase and decreased levels of proliferation, followed by matrix mineralization.50,51
FIG. 2.
Mesenchymal condensations in micromass. Bright field (BF) and fluorescent micrographs of a representative micromass culture after 3 days in culture. Top row shows lower magnification images of multiple condensations; scale bar is 200 μm. Bottom row shows higher magnification images of a single condensation; scale bar is 50 μm. (A) In BF images, mesenchymal condensations are recognizable as dark spots. Condensations are further characterized by an increased cell density, visible as bright spots in cultures stained with DAPI (blue) (B), and increased peanut agglutinin lectin (PNA) staining (green) (C). (D) Color micrograph of a representative micromass, cultured for 3 days, stained with Alcian Blue to identify deposition of glycosaminoglycans. Mesenchymal condensations show increased staining intensity. Scale bar is 200 μm. Color images available online at www.liebertpub.com/teb
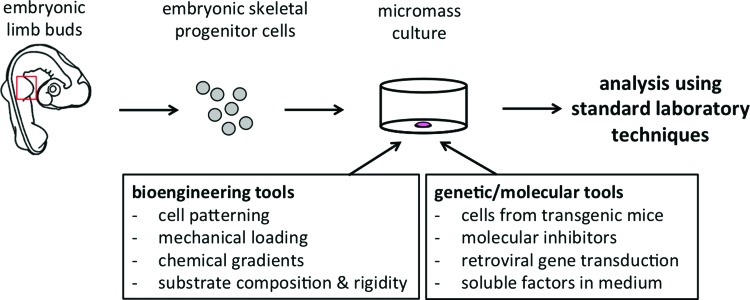
The simplicity of the micromass assay allows one to study the early stages of skeletal development using standard laboratory techniques (Fig. 3). For example, cells from transgenic mice can be used. Genetic knockout of the gene encoding for the chondroitin sulfate proteoglycan versican, for instance, has enabled the investigation of its role in chondrogenesis.54 Also, molecular inhibitors can be added to the culture medium to interfere with signaling pathways. In this manner, among many studies, it was found that the small GTPase RhoA, which is involved in regulating cytoskeletal tension, inhibits the process of early chondrogenesis, while Rac1 promotes mesenchymal condensation and chondrogenic differentiation.55 Furthermore, by simply adding the growth factor to the micromass culture medium it was shown that GDF-5, for instance, increases chondrogenesis in a dose-dependent manner.56 Taken together, the micromass assay provides a simple and highly adaptable model that allows testing of a variety of aspects of early skeletal development.
FIG. 3.
Micromass is an adaptable culture method. Skeletal progenitor cells are typically isolated from the embryonic limb buds (indicated by the red box), cultured in micromass, and analyzed by various standard laboratory techniques. The classic micromass can be supplemented with a range of genetic, molecular, and bioengineering tools to create an interdisciplinary model system to study early skeletal development in vitro. Color images available online at www.liebertpub.com/teb
Lessons and Implications from the Micromass Model
The subset of challenges in tissue engineering that are described in the Introduction section can be sorted into three categories: the early lineage commitment of skeletal progenitor cells, undesired chondrocyte hypertrophy, and the generation of tissue patterns and distinct boundaries. In this section we will first discuss how these issues can be addressed by micromass cultures using embryonic skeletal progenitor cells. Second, we will evaluate what the implications are for the tissue engineering field, considering two main differences between developmental and regenerative skeletogenesis: cell type and scale.
Lessons from the micromass model
The micromass assay is typically performed with prechondrogenic cells isolated from embryonic limb buds. The cells are freshly isolated and directly used in an experiment (Fig. 1), as they cannot be passaged without losing their phenotype. In the following section, we will discuss how this model can be used—and potentially adapted—to gain insight into the processes of lineage commitment, chondrocyte hypertrophy, and tissue patterning.
Lineage commitment
The embryonic limb buds offer a convenient source of skeletal progenitor cells for in vitro culture, as the buds are relatively easily accessible and protocols for isolation are readily available.45 Chicken and mouse embryos are most commonly used, but rat and rabbit limb bud cell isolations have been reported as well.57,58 Chicken embryos are typically used at Hamburger Hamilton stage 20–2417,59 and mouse embryos at embryonic day 11.5–12.5,45 comparable developmental stages at which the mesenchymal cells in the limb buds have not formed condensations yet. Apart from limb bud cells, other sources of skeletal precursor cells can be used as well. There are reports of the use of sclerotomal cells, the mesenchymal cells from the paraxial mesoderm that give rise to the vertebral column.60 Thus, embryonic skeletal progenitor cells of varying species, developmental stage, and anatomic location can be used in the micromass assay.
In the embryo, the skeletal elements develop from several sources of skeletal progenitor cells, the lateral plate mesoderm (limbs), the paraxial mesoderm (vertebral column), and the cranial neural crest (craniofacial cartilage).22,61 Each of these cell sources gives rise to bone and varying types of cartilage, indicating that they have similar lineage potential. Still, widely different types, shapes, and sizes of bone and cartilage develop at different anatomic sites.62 Comparing the behavior of progenitor cells isolated from different locations in vitro could provide insight into the mechanisms that are common to the formation of all skeletal elements, and those that distinguish the different skeletal features. Furthermore, comparing cultures using cells from different stages of development could answer the question whether and when these cells become intrinsically different.
For example, fore and hind limb skeletal progenitor cells derived from stage 21–26 chicken embryos were compared in micromass culture.63 In the early cultures, wing cells produced broad condensations that eventually merged, resulting in a continuous sheet of cartilage. The condensations in the leg cultures, on the other hand, were more nodular and remained discrete, leading to punctate chondrogenic differentiation and a higher overall amount of proteoglycan deposition. The cultures also responded differently to soluble factors such as transforming growth factor (TGF)-β and retinoic acid, which indicates that both intrinsic and induced differences exist between these two cell populations. Similarly, it would be interesting to compare the behavior of limb bud cells and sclerotomal cells in micromass culture, since they develop into such different skeletal structures in vivo.
Investigating the pattern of condensations and the overall extent of differentiation under various culture conditions could elucidate potential cellular differences and the role of the local microenvironment. In conclusion, by taking cells from different anatomic locations out of their native microenvironment and following their progression in micromass culture, one can specifically investigate whether local environmental or acquired intrinsic cellular differences are causing the distinctive shapes and patterns of skeletal elements. Moreover, comparing precursor cells isolated at different stages in development allows investigation at which point the cells obtain the intrinsic differences that lead to the various skeletal tissue types.
Chondrocyte hypertrophy
To prevent undesired hypertrophy in cartilage tissue engineering constructs, a better understanding of the mechanisms in skeletal development that determine the bifurcation between stable cartilage and the transient cartilage anlagen could be insightful.
The micromass model provides a convenient platform for such investigations, and has facilitated the identification of signaling pathways involved in hypertrophy in embryonic skeletal progenitor cells. For example, in long-term micromass cultures of mouse limb bud cells, it was shown that TGF-β treatment resulted in the delay of chondrocyte hypertrophy and inhibition of matrix calcification and its associated gene expression.51 In another study, it was found that ectopic expression of sonic hedgehog (Shh) through retroviral transduction leads to the formation of additional chondrogenic nodules with elevated levels or hypertrophic genes such as alkaline phosphatase and collagen X.64 Indian hedgehog (Ihh) and parathyroid hormone-related protein (PTHrP) are involved in a negative feedback loop that blocks maturation of prehypertrophic chondrocytes.65
A recent study suggests that actively controlling oxygen tension, following oxygen exposure patterns known from embryonic development, can steer adult MSCs in a tissue engineering construct toward either permanent hyaline cartilage or hypertrophic cartilage through metabolic programming.66,67 The micromass assay could be useful to investigate in more detail the role of spatial and temporal gradients of oxygen tension in chondrocyte hypertrophy during developmental skeletogenesis. Among many, these studies add to a fundamental understanding of the pathways governing chondrocyte hypertrophy, providing insight into the external cues that can be used to control this process and underscoring the usefulness of the micromass assay in unraveling signaling pathways by facilitating the use of genetic and molecular tools.
Controlling tissue organization and boundaries
In vivo, highly organized skeletal structures result from the tight temporal and spatial control of soluble and nonsoluble cues during early skeletogenesis.68,69 In contrast, the bulk induction of lineage commitment of skeletal progenitor cells that is often employed in tissue engineering strategies provides little control over tissue boundaries and structural organization. The micromass can be used to provide insight into the cues that guide tissue patterning and boundary formation in skeletal development.
Growth factors and other morphogens form an important group of soluble cues that are known to guide morphogenesis. Specifically, it is the spatial and temporal gradients of soluble cues that are thought to guide the generation of controlled tissue patterns in the embryo.70–72 For example, a localized source of Shh in the tip of the limb creates a gradient through the tissue, which is shown to determine the specific pattern of skeletal elements in the limbs,69 and the spatiotemporal expression patterns of Ihh and PTHrP are key in generating and maintaining the highly organized growth plate in long bones.65 To mimic this type of exposure, a range of methods could be used to establish spatial gradients of growth factors in culture. For example, gradients could be achieved in vitro by using growth factor-soaked beads positioned at specific locations with respect to the cells, a technique that is used more often in developmental biology.43,73 Microfluidics is also a powerful tool to establish highly controlled spatial and temporal gradients of soluble factors in cell culture.74,75
In addition to soluble morphogens, there is a range of physical cues that are known or hypothesized to affect progenitor cell behavior and tissue pattering.76–78 The role of these cues in skeletal patterning could be investigated in a controlled manner by combining the micromass model with tools that have emerged from the bioengineering field, such as mechanical loading devices, traction force microscopy, and cellular patterning (Fig. 3) and quantifying the numbers and spacing of condensations in the culture as a measure of patterning.79,80 For example, a developing tissue is subjected to geometric boundary conditions by neighboring tissues.
Recently, to mimic the geometric boundaries of the developing vertebral column, we have subjected embryonic skeletal progenitor cells to narrow geometric constraints by combining the micromass assay with a microchannel cell patterning technique.81 It was found that patterning of mesenchymal condensations, quantified by their spacing and density in culture, is modulated by geometric boundary conditions. Moreover, the pattern of condensations at the site of the developing vertebral column in vivo in chicken embryos was shown to match the correlation between geometric constraints and the distance between neighboring condensations found in vitro.
Differential growth rates of distinctive tissues and cellular traction forces cause tissue-level stresses and deformations.82,83 Different mechanical loading regimes have been applied to micromass cultures to address those effects. In a recent study for example, compressive loading was applied through the culture medium at days 2 and 3 of culture, which resulted in increased levels of chondrogenic differentiation and matrix deposition.84 In contrast, in a different study stepwise uniaxial strain applied daily at days 4–7 was shown to lead to the inhibition of chondrogenesis.85 These studies indicate that additional investigations are required to characterize the exact mechanical loading conditions that are relevant to skeletal progenitor cells during skeletal development, and how they affect tissue patterning. Overall, the micromass assay can facilitate a wide range of soluble and physical cues. This enables the well-controlled investigation of the role of these cues in skeletal patterning, which can guide the design of structurally complex tissue engineering constructs.
Implications for tissue engineering
When interpreting insights from the approaches outlined above, one has to consider two important differences between developmental and regenerative skeletogenesis: cell type and scale. In this section, we will discuss the implications for the tissue engineering field.
Lineage commitment
To investigate the difference between embryonic and adult MSCs with respect to their lineage commitment, we look into studies that have performed micromass assays with adult or immortalized mesenchymal cells, instead of the commonly used primary embryonic mesenchymal cells.86–89 Although the micromass provides an environment that favors chondrogenic differentiation in these cultures,86,87 adult or immortalized cells typically do not form condensations. Mesenchymal condensations, indicated by foci of increased cell density—within the high density culture—appear spontaneously within 2–3 days in micromass cultures using primary embryonic cells (Fig. 2).17,45,46 In contrast, the cell density in cultures using adult or immortalized cells is equally high but remains homogeneous (Fig. 4).87,89 Consequently, the chondrogenic commitment, typically shown by Alcian Blue staining, in these micromass cultures is fairly homogeneous86,88 instead of nodulated.45,88
FIG. 4.
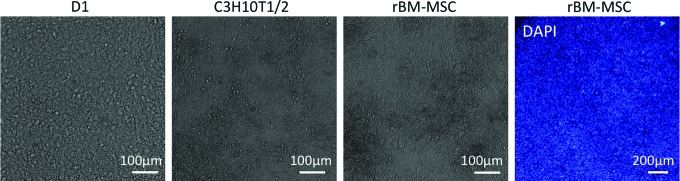
Various adult and/or immortalized skeletal progenitor cell types in micromass. BF micrographs of immortalized mouse bone marrow-derived mesenchymal stem cells (MSCs) (D1), immortalized mouse embryonic fibroblasts (C3H10T1/2), and primary rat bone marrow-derived MSCs (rBM-MSC) cultured in micromass for 3 days. Mesenchymal condensations, indicated by local increases in cell density (Fig. 1), have not formed in any culture. Although the rat MSC micromass culture appears to show inhomogeneities in cell density in the BF image, a micromass stained with DAPI (right) shows homogeneous cell density. For all BF images, scale bar is 100 μm. For fluorescent DAPI image, scale bar is 200 μm. Color images available online at www.liebertpub.com/teb
It has been shown for both embryonic and adult MSCs that a high cell density, which allows for cell–cell contacts and thus facilitates cell–cell communication, is required for the induction of chondrogenic differentiation.28,90 We can conclude that in embryonic cells this high cell density is established in specific locations, through an intrinsic mechanism of mesenchymal condensation, while adult MSCs do not go through this process spontaneously, potentially due to differences in the expression of adhesion molecules and/or their interaction with the available ECM.
Adult skeletal progenitor cells thus lack the capacity of mesenchymal condensation, yet it is possible to induce chondrogenic commitment by enhancing the cell density to secure the required cell–cell interactions. In cartilage tissue engineering, such high cell density can be achieved by the use of pellet or high-density 3D cultures.91,92 However, as oxygen and nutrient supplies do not penetrate far into the densely packed culture, the size of such constructs is limited. Recent studies describe a possible solution by maintaining small high-density spheres using chondrocytes or adult human MSC in culture for a certain amount of time while inducing cartilage tissue phenotype, before merging several spheres into a larger construct.93,94 Alternatively, 3D printing could be exploited to place small cellular aggregates into a large porous structure.95,96 Such an approach would facilitate sufficient diffusion and provide structural support while maintaining a high cell density, and thus meet both the microscopic and macroscopic requirements of a cartilage tissue-engineering construct. These and various other approaches could be used to overcome the lack of spontaneous condensation in adult skeletal progenitor cells.
Chondrocyte hypertrophy
Among various signaling pathways identified through micromass and in vivo studies, it was shown that Ihh is expressed in prehypertrophic chondrocytes and is associated with PTHrP in a negative feedback loop that modulates the rate of chondrocyte hypertrophy and maturation.65 Among many approaches, (over)exposure of Ihh has been proposed and investigated as a potential way to prevent hypertrophy in cartilage tissue engineering.97,98 While chondrogenic commitment was enhanced in these studies, continuous exposure resulted in limited prevention of chondrocyte hypertrophy. Intermittent exposure to PTHrP, however, was shown to lead to reduced hypertrophy in human MSCs.97 In another study, it was shown that BMP2, a growth factor that is commonly used in skeletal tissue engineering strategies, increases hypertrophy in adult chondrocytes, while BMP7 acts as a suppressor. Both effects are mediated through the transcription factor Bapx1/Nkx3.2.99 Altogether, the signaling pathways that are identified through micromass and in vivo studies in embryonic skeletal progenitor cells form a valuable basis for studies that aim to prevent hypertrophy in adult progenitor cells.
Patterning and tissue boundaries
During skeletal development, patterning occurs at the early stage of mesenchymal condensation, a stage that is not recapitulated by adult MSCs in regenerative skeletogenesis. Potential methods to overcome the lack of spontaneous condensation are discussed above. With respect to the difference in size, cues that are involved in guiding tissue patterning could be adjusted to account for the larger dimensions. In terms of growth factor presentation, for instance, engineering strategies are being used to gain spatial and temporal control of growth factor release in constructs, which allows for the controlled onset and spatially regulated differentiation.
For example, well-defined spatial and temporal control of the release of osteogenic and chondrogenic growth factors, in combination with inhibiting antibodies, is being exploited to establish multilineage differentiation of MSCs in sharply defined zones. Such an approach could be useful in the regeneration of the bone-cartilage interface.100 Even matrix properties can be controlled in time and space, for example, by hydrogels of which the mechanical properties can be changed over time to modulate mechanoresponsive effects in the encapsulated cells101 or spatial gradients of adhesion ligands to control cell–matrix interactions.102
In addition to these controlled spatiotemporal inhomogeneities, patterned differentiation has also been induced spontaneously in soft hydrogels in vitro through cell-mediated contraction. For example, organized cell contraction of immortalized embryonic fibroblasts in nanofiber scaffolds was shown to lead to inhomogeneous gene expression and spatially regulated chondrogenic differentiation.103 Also, cell-mediated contraction of adult MSCs in collagen gels under time-varying boundary conditions led to spatially regulated osteogenic differentiation.104 Taken together, fine spatiotemporal control of both soluble and physical cues could be achieved through the advanced engineering of scaffolds and their boundary conditions. Among many others, this is one of the strategies being explored to establish tissue patterns and boundaries in large tissue engineering constructs.
Conclusions and Future Directions
Skeletal tissue engineering aims to regenerate bone and cartilage tissues in the adult body. These strategies have not reached their full potential, partly due to an incomplete understanding of the molecular and mechanobiological mechanisms of skeletal regeneration. The micromass assay provides a convenient platform to study the process of early skeletal development in vitro and can thus provide relevant insight into the tissue engineering field. For example, the assay can be performed with embryonic skeletal precursor cells from different anatomic locations and different developmental stages. By taking them out of their native microenvironment, one could learn whether and when the cells become intrinsically different and what factors determine their lineage commitment.
The assay can further be used to gain insight into the signaling pathways that are involved in chondrocyte hypertrophy, facilitated by the possibility to use cells from transgenic mice, apply retroviral gene transduction, and use molecular inhibitors in the culture medium. The micromass model can also be combined with a range of engineering tools to allow for the spatiotemporal control of soluble and physical cues, to investigate their effect on skeletal tissue patterning. To translate the results from micromass studies to tissue engineering applications, however, it is important to consider the two main differences between developmental and regenerative skeletogenesis: cell type and scale. For example, it was observed that adult MSCs differ from embryonic skeletal progenitor cells in that they do not spontaneously form mesenchymal condensations. Also, with regard to determining tissue patterning and boundaries, gradients of soluble and physical cues thus need to be adjusted to account for the different scale.
In future studies, the micromass assay could be further improved and exploited to explore the mechanisms of skeletal development from an interdisciplinary perspective. The classic micromass assay models the biological process of early skeletal development in vitro, but it does not capture the full complexity of the in vivo microenvironment. Various coculture methods, 3D culture approaches, and bioengineering tools, such as the cell patterning methods and mechanical loading devices described in this review, could complement the existing micromass assay to create a model that incorporates the biological, chemical, and mechanical components of the developing skeletal elements (Fig. 3).
Cross-disciplinary laboratory techniques such as traction force microscopy and advanced imaging methods could generate more detailed and quantitative information from these cell cultures, which will provide a more profound understanding of the mechanisms involved in skeletal development. When cells are cultured on elastic substrates with embedded fluorescent beads, traction force microscopy uses the displacements of the beads during culture to quantify the forces that cells exert on their substrate, and the forces at cell–cell interactions in multicellular aggregates.105,106 The balance between cell–cell and cell–matrix interactions and the forces applied at these adhesions are considered in the process of mesenchymal condensation,107,108 but this hypothesis is hardly explored in embryonic cultures. To this end, a combination of the micromass model with traction force microscopy, adhesion-blocking antibodies, and chemical interference with matrix deposition and degradation could be used.
To better understand what regenerative medicine can learn from studying skeletal development, further studies are required to identify the specific differences and similarities between embryonic and adult skeletal progenitor cells. To overcome the possible limitations of adult MSCs, the use of induced pluripotent stem cells (iPSCs) for regenerative strategies is being explored. Several methods have already been developed to generate MSCs from iPSCs.109,110 It would be useful to know whether these induced MSCs are more similar to embryonic or adult skeletal progenitor cells in their ability to generate bone and cartilage, for example, by studying whether they have the ability to spontaneously form mesenchymal condensations. Alternatively, skeletal tissue-specific cells can be generated directly without the need of a pluripotent intermediate. For example, chondrocytes were transdifferentiated directly from somatic cells using a combination of reprogramming and chondrogenic factors.111–113 Such approaches have great potential but should be well characterized and regulated to diminish the risk of teratoma formation and immune rejection.
In tissue engineering constructs it is key that the cellular microenvironment is designed to guide proper stem cell differentiation. At the same time, the bulk properties of the construct need to meet the tissue-level requirements such as size, as discussed in the review, diffusion-permitting porosity, and mechanical properties that can bear tissue level loads. Moreover, it might be desirable for the conditions to change over time to facilitate different stages of tissue generation or to obtain proper structural organization. Further investigations into this direction might build upon current efforts to design constructs of which the properties can be changed on demand through for example thermally induced shrinking, cleavable peptides, or on-demand delivery of growth factors.114–116 Alternatively, constructs could be engineered with the ability to adapt and/or grow autonomously in vivo.117,118 Investigations along these lines, combining mechanistic biological insight with advanced bioengineering tools, hold great potential for the advancement of skeletal tissue engineering.
In conclusion, micromass studies using embryonic skeletal precursor cells provide fundamental insight into the mechanisms of lineage commitment, the regulation of chondrocyte hypertrophy, and the cues that determine tissue patterning. Various bioengineering tools, such as 3D printing and traction force microscopy, can be used to further exploit this model. To fully understand what the tissue engineering field can learn from the micromass model, it is important to supplement these studies with investigations that address the main differences between developmental and regenerative skeletogenesis: cell type and size.
Acknowledgments
The authors thank Dr. Tim J.M. Welting for critically reading the article. Funding from the National Institutes of Health (R37 DE013033), ZonMW-VICI grant 918.11.635 (The Netherlands), and Research Institute MOVE at the VU University, Amsterdam is acknowledged. T.H.S. was supported by a visiting scholarship from the Wyss Institute for Biologically Inspired Engineering at Harvard University.
Disclosure Statement
No competing financial interests exist.
References
- 1.Woolf A.D., and Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 81, 646, 2003 [PMC free article] [PubMed] [Google Scholar]
- 2.Tew S.R., Kwan A.P., Hann A., Thomson B.M., and Archer C.W. The reactions of articular cartilage to experimental wounding: role of apoptosis. Arthritis Rheum 43, 215, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Greenbaum M.A., and Kanat I.O. Current concepts in bone healing. Review of the literature. J Am Podiatr Med Assoc 83, 123, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Mastbergen S.C., Saris D.B., and Lafeber F.P. Functional articular cartilage repair: here, near, or is the best approach not yet clear? Nat Rev Rheumatol 9, 277, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Lee Buckler R., Margolin R., and Haecker S.A. 15 State of the global regenerative medicine industry. In: Prescott C.D., and Polak J., eds. The Delivery of Regenerative Medicines and Their Impact on Healthcare. Boca Raton, FL: CRC Press, Taylor & Francis Group, 2010, pp. 213–237 [Google Scholar]
- 6.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., and Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., and Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331, 889, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Lima E.G., Bian L., Ng K.W., Mauck R.L., Byers B.A., Tuan R.S., Ateshian G.A., and Hung C.T. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage 15, 1025, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauck R.L., Yuan X., and Tuan R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage 14, 179, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Ichinose S., Yamagata K., Sekiya I., Muneta T., and Tagami M. Detailed examination of cartilage formation and endochondral ossification using human mesenchymal stem cells. Clin Exp Pharmacol Physiol 32, 561, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Mueller M.B., and Tuan R.S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum 58, 1377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelttari K., Winter A., Steck E., Goetzke K., Hennig T., Ochs B.G., Aigner T., and Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum 54, 3254, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Sharma B., Williams C.G., Kim T.K., Sun D., Malik A., Khan M., Leong K., and Elisseeff J.H. Designing zonal organization into tissue-engineered cartilage. Tissue Eng 13, 405, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Sophia Fox A.J., Bedi A., and Rodeo S.A. The basic science of articular cartilage: structure, composition, and function. Sports Health 1, 461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel V.V., Zhao L., Wong P., Pradhan B.B., Bae H.W., Kanim L., and Delamarter R.B. An in vitro and in vivo analysis of fibrin glue use to control bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth. Spine J 6, 397, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Ingber D.E., and Levin M. What lies at the interface of regenerative medicine and developmental biology? Development 134, 2541, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Ahrens P.B., Solursh M., and Reiter R.S. Stage-related capacity for limb chondrogenesis in cell culture. Dev Biol 60, 69, 1977 [DOI] [PubMed] [Google Scholar]
- 18.Umansky R. The effect of cell population density on the developmental fate of reaggregating mouse limb bud mesenchyme. Dev Biol 13, 31, 1966 [DOI] [PubMed] [Google Scholar]
- 19.Dennis S.C., Berkland C.J., Bonewald L.F., and Detamore M.S. Endochondral ossification for enhancing bone regeneration: converging native extracellular matrix biomaterials and developmental engineering in vivo. Tissue Eng Part B Rev 21, 247, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mravic M., Peault B., and James A.W. Current trends in bone tissue engineering. Biomed Res Int 2014, 865270, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee E.J., Kasper F.K., and Mikos A.G. Biomaterials for tissue engineering. Ann Biomed Eng 42, 323, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray H., and Collins P. Embryology and Development. Gray's Anatomy. New York: Churchill Livingstone, 1995 [Google Scholar]
- 23.Hall B.K. Earliest evidence of cartilage and bone development in embryonic life. Clin Orthop Relat Res 255, 1987 [PubMed] [Google Scholar]
- 24.Karsenty G., and Wagner E.F. Reaching a genetic and molecular understanding of skeletal development. Dev Cell 2, 389, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Hall B.K., and Miyake T. The membranous skeleton: the role of cell condensations in vertebrate skeletogenesis. Anat Embryol (Berl) 186, 107, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Hall B.K., and Miyake T. Divide, accumulate, differentiate: cell condensation in skeletal development revisited. Int J Dev Biol 39, 881, 1995 [PubMed] [Google Scholar]
- 27.Grüneberg H. The Athology of Development: A Study of Inhereted Skeletal Disorders in Animals. Blackwells Scientific Publications; Oxford, UK, 1963 [Google Scholar]
- 28.Hall B.K., and Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays 22, 138, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Zimmermann B., and Thies M. Alterations of lectin binding during chondrogenesis of mouse limb buds. Histochemistry 81, 353, 1984 [DOI] [PubMed] [Google Scholar]
- 30.LeClair E.E., Bonfiglio L., and Tuan R.S. Expression of the paired-box genes Pax-1 and Pax-9 in limb skeleton development. Dev Dyn 214, 101, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Tavella S., Raffo P., Tacchetti C., Cancedda R., and Castagnola P. N-CAM and N-cadherin expression during in vitro chondrogenesis. Exp Cell Res 215, 354, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Janners M.Y., and Searls R.L. Changes in rate of cellular proliferation during the differentiation of cartilage and muscle in the mesenchyme of the embryonic chick wing. Dev Biol 23, 136, 1970 [DOI] [PubMed] [Google Scholar]
- 33.Newman S.A., Frenz D.A., Tomasek J.J., and Rabuzzi D.D. Matrix-driven translocation of cells and nonliving particles. Science 228, 885, 1985 [DOI] [PubMed] [Google Scholar]
- 34.Summerbell D., and Wolpert L. Cell density and cell division in the early morphogenesis of the chick wing. Nat New Biol 239, 24, 1972 [DOI] [PubMed] [Google Scholar]
- 35.Dessau W., von der Mark H., von der Mark K., and Fischer S. Changes in the patterns of collagens and fibronectin during limb-bud chondrogenesis. J Embryol Exp Morphol 57, 51, 1980 [PubMed] [Google Scholar]
- 36.Knudson C.B., and Toole B.P. Changes in the pericellular matrix during differentiation of limb bud mesoderm. Dev Biol 112, 308, 1985 [DOI] [PubMed] [Google Scholar]
- 37.Kulyk W.M., Upholt W.B., and Kosher R.A. Fibronectin gene expression during limb cartilage differentiation. Development 106, 449, 1989 [DOI] [PubMed] [Google Scholar]
- 38.Stern C.D. Mini-review: hyaluronidases in early embryonic development. Cell Biol Int Rep 8, 703, 1984 [DOI] [PubMed] [Google Scholar]
- 39.Oberlender S.A., and Tuan R.S. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development 120, 177, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Widelitz R.B., Jiang T.X., Murray B.A., and Chuong C.M. Adhesion molecules in skeletogenesis: II. Neural cell adhesion molecules mediate precartilaginous mesenchymal condensations and enhance chondrogenesis. J Cell Physiol 156, 399, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Centola M., Tonnarelli B., Scharen S., Glaser N., Barbero A., and Martin I. Priming 3D cultures of human mesenchymal stromal cells toward cartilage formation via developmental pathways. Stem Cells Dev 22, 2849, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sart S., Tsai A.C., Li Y., and Ma T. Three-dimensional aggregates of mesenchymal stem cells: cellular mechanisms, biological properties, and applications. Tissue Eng Part B Rev 20, 365, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ten Berge D., Brugmann S.A., Helms J.A., and Nusse R. Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development 135, 3247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tacchetti C., Tavella S., Dozin B., Quarto R., Robino G., and Cancedda R. Cell condensation in chondrogenic differentiation. Exp Cell Res 200, 26, 1992 [DOI] [PubMed] [Google Scholar]
- 45.DeLise A.M., Stringa E., Woodward W.A., Mello M.A., and Tuan R.S. Embryonic limb mesenchyme micromass culture as an in vitro model for chondrogenesis and cartilage maturation. Methods Mol Biol 137, 359, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Edwall-Arvidsson C., and Wroblewski J. Characterization of chondrogenesis in cells isolated from limb buds in mouse. Anat Embryol (Berl) 193, 453, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Kaplowitz P.B., D'Ercole A.J., and Underwood L.E. Stimulation of embryonic mouse limb bud mesenchymal cell growth by peptide growth factors. J Cell Physiol 112, 353, 1982 [DOI] [PubMed] [Google Scholar]
- 48.Glant T.T., Hadhazy C., Mikecz K., and Sipos A. Appearance and persistence of fibronectin in cartilage. Specific interaction of fibronectin with collagen type II. Histochemistry 82, 149, 1985 [DOI] [PubMed] [Google Scholar]
- 49.Aulthouse A.L., and Solursh M. The detection of a precartilage, blastema-specific marker. Dev Biol 120, 377, 1987 [DOI] [PubMed] [Google Scholar]
- 50.Mello M.A., and Tuan R.S. High density micromass cultures of embryonic limb bud mesenchymal cells: an in vitro model of endochondral skeletal development. In Vitro Cell Dev Biol Anim 35, 262, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Zhang X., Ziran N., Goater J.J., Schwarz E.M., Puzas J.E., Rosier R.N., Zuscik M., Drissi H., and O'Keefe R.J. Primary murine limb bud mesenchymal cells in long-term culture complete chondrocyte differentiation: TGF-beta delays hypertrophy and PGE2 inhibits terminal differentiation. Bone 34, 809, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Mallein-Gerin F., Kosher R.A., Upholt W.B., and Tanzer M.L. Temporal and spatial analysis of cartilage proteoglycan core protein gene expression during limb development by in situ hybridization. Dev Biol 126, 337, 1988 [DOI] [PubMed] [Google Scholar]
- 53.Hascall V.C., Oegema T.R., and Brown M. Isolation and characterization of proteoglycans from chick limb bud chondrocytes grown in vitro. J Biol Chem 251, 3511, 1976 [PubMed] [Google Scholar]
- 54.Gillotte D.M., Fox P.L., Mjaatvedt C.H., Hoffman S., and Capehart A.A. An in vitro method for analysis of chondrogenesis in limb mesenchyme from individual transgenic (hdf) embryos. Methods Cell Sci 25, 97, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Woods A., Wang G., Dupuis H., Shao Z., and Beier F. Rac1 signaling stimulates N-cadherin expression, mesenchymal condensation, and chondrogenesis. J Biol Chem 282, 23500, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Francis-West P.H., Abdelfattah A., Chen P., Allen C., Parish J., Ladher R., Allen S., MacPherson S., Luyten F.P., and Archer C.W. Mechanisms of GDF-5 action during skeletal development. Development 126, 1305, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Brown N.A., and Wiger R. Comparison of rat and chick limb bud micromass cultures for developmental toxicity screening. Toxicol In Vitro 6, 101, 1992 [DOI] [PubMed] [Google Scholar]
- 58.Hansen J.M., Carney E.W., and Harris C. Altered differentiation in rat and rabbit limb bud micromass cultures by glutathione modulating agents. Free Radic Biol Med 31, 1582, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Hamburger V., and Hamilton H. A series of normal stages in the development of the chick embryo. J Morphol 88, 49, 1951 [PubMed] [Google Scholar]
- 60.Sohn P., Cox M., Chen D., and Serra R. Molecular profiling of the developing mouse axial skeleton: a role for Tgfbr2 in the development of the intervertebral disc. BMC Dev Biol 10, 29, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Somoza R.A., Welter J.F., Correa D., and Caplan A.I. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev 20, 596, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naumann A., Dennis J.E., Awadallah A., Carrino D.A., Mansour J.M., Kastenbauer E., and Caplan A.I. Immunochemical and mechanical characterization of cartilage subtypes in rabbit. J Histochem Cytochem 50, 1049, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Downie S.A., and Newman S.A. Morphogenetic differences between fore and hind limb precartilage mesenchyme: relation to mechanisms of skeletal pattern formation. Dev Biol 162, 195, 1994 [DOI] [PubMed] [Google Scholar]
- 64.Stott N.S., and Chuong C.M. Dual action of sonic hedgehog on chondrocyte hypertrophy: retrovirus mediated ectopic sonic hedgehog expression in limb bud micromass culture induces novel cartilage nodules that are positive for alkaline phosphatase and type X collagen. J Cell Sci 110 (Pt 21), 2691, 1997 [DOI] [PubMed] [Google Scholar]
- 65.Vortkamp A., Lee K., Lanske B., Segre G.V., Kronenberg H.M., and Tabin C.J. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science 273, 613, 1996 [DOI] [PubMed] [Google Scholar]
- 66.Leijten J., Georgi N., Moreira Teixeira L., van Blitterswijk C.A., Post J.N., and Karperien M. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc Natl Acad Sci U S A 111, 13954, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leijten J.C., Moreira Teixeira L.S., Landman E.B., van Blitterswijk C.A., and Karperien M. Hypoxia inhibits hypertrophic differentiation and endochondral ossification in explanted tibiae. PLoS One 7, e49896, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burke A.C., Nelson C.E., Morgan B.A., and Tabin C. Hox genes and the evolution of vertebrate axial morphology. Development 121, 333, 1995 [DOI] [PubMed] [Google Scholar]
- 69.Riddle R.D., Johnson R.L., Laufer E., and Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75, 1401, 1993 [DOI] [PubMed] [Google Scholar]
- 70.Pourquie O. The segmentation clock: converting embryonic time into spatial pattern. Science 301, 328, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Tabata T., and Takei Y. Morphogens, their identification and regulation. Development 131, 703, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol 25, 1, 1969 [DOI] [PubMed] [Google Scholar]
- 73.Cohn M.J., Izpisua-Belmonte J.C., Abud H., Heath J.K., and Tickle C. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell 80, 739, 1995 [DOI] [PubMed] [Google Scholar]
- 74.Chung B.G., Flanagan L.A., Rhee S.W., Schwartz P.H., Lee A.P., Monuki E.S., and Jeon N.L. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip 5, 401, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Dertinger S.K.W., Chiu D.T., Jeon N.L., and Whitesides G.M. Generation of gradients having complex shapes using microfluidic networks. Anal Chem 73, 1240, 2001 [Google Scholar]
- 76.Beloussov L.V., and Grabovsky V.I. Morphomechanics: goals, basic experiments and models. Int J Dev Biol 50, 81, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Gjorevski N., and Nelson C.M. The mechanics of development: models and methods for tissue morphogenesis. Birth Defects Res C Embryo Today 90, 193, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ingber D.E. Mechanical control of tissue morphogenesis during embryological development. Int J Dev Biol 50, 255, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Bhat R., Lerea K.M., Peng H., Kaltner H., Gabius H.J., and Newman S.A. A regulatory network of two galectins mediates the earliest steps of avian limb skeletal morphogenesis. BMC Dev Biol 11, 6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Christley S., Alber M.S., and Newman S.A. Patterns of mesenchymal condensation in a multiscale, discrete stochastic model. PLoS Comput Biol 3, e76, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klumpers D.D., Mao A.S., Smit T.H., and Mooney D.J. Linear patterning of mesenchymal condensations is modulated by geometric constraints. J R Soc Interface 11, 20140215, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henderson J.H., and Carter D.R. Mechanical induction in limb morphogenesis: the role of growth-generated strains and pressures. Bone 31, 645, 2002 [DOI] [PubMed] [Google Scholar]
- 83.Wozniak M.A., and Chen C.S. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol 10, 34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Juhasz T., Matta C., Somogyi C., Katona E., Takacs R., Soha R.F., Szabo I.A., Cserhati C., Szody R., Karacsonyi Z., Bako E., Gergely P., and Zakany R. Mechanical loading stimulates chondrogenesis via the PKA/CREB-Sox9 and PP2A pathways in chicken micromass cultures. Cell Signal 26, 468, 2014 [DOI] [PubMed] [Google Scholar]
- 85.Onodera K., Takahashi I., Sasano Y., Bae J.W., Mitani H., Kagayama M., and Mitani H. Stepwise mechanical stretching inhibits chondrogenesis through cell-matrix adhesion mediated by integrins in embryonic rat limb-bud mesenchymal cells. Eur J Cell Biol 84, 45, 2005 [DOI] [PubMed] [Google Scholar]
- 86.Denker A.E., Haas A.R., Nicoll S.B., and Tuan R.S. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: I. Stimulation by bone morphogenetic protein-2 in high-density micromass cultures. Differentiation 64, 67, 1999 [DOI] [PubMed] [Google Scholar]
- 87.Denker A.E., Nicoll S.B., and Tuan R.S. Formation of cartilage-like spheroids by micromass cultures of murine C3H10T1/2 cells upon treatment with transforming growth factor-beta 1. Differentiation 59, 25, 1995 [DOI] [PubMed] [Google Scholar]
- 88.Hatakeyama Y., Tuan R.S., and Shum L. Distinct functions of BMP4 and GDF5 in the regulation of chondrogenesis. J Cell Biochem 91, 1204, 2004 [DOI] [PubMed] [Google Scholar]
- 89.Song J.J., Aswad R., Kanaan R.A., Rico M.C., Owen T.A., Barbe M.F., Safadi F.F., and Popoff S.N. Connective tissue growth factor (CTGF) acts as a downstream mediator of TGF-beta1 to induce mesenchymal cell condensation. J Cell Physiol 210, 398, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Mauck R.L., Seyhan S.L., Ateshian G.A., and Hung C.T. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng 30, 1046, 2002 [DOI] [PubMed] [Google Scholar]
- 91.Rosowski M., Falb M., Tschirschmann M., and Lauster R. Initiation of mesenchymal condensation in alginate hollow spheres—a useful model for understanding cartilage repair? Artif Organs 30, 775, 2006 [DOI] [PubMed] [Google Scholar]
- 92.Zhang L., Su P., Xu C., Yang J., Yu W., and Huang D. Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol Lett 32, 1339, 2010 [DOI] [PubMed] [Google Scholar]
- 93.Bhumiratana S., Eton R.E., Oungoulian S.R., Wan L.Q., Ateshian G.A., and Vunjak-Novakovic G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc Natl Acad Sci U S A 111, 6940, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moreira Teixeira L.S., Leijten J.C., Sobral J., Jin R., van Apeldoorn A.A., Feijen J., van Blitterswijk C., Dijkstra P.J., and Karperien M. High throughput generated micro-aggregates of chondrocytes stimulate cartilage formation in vitro and in vivo. Eur Cell Mater 23, 387, 2012 [DOI] [PubMed] [Google Scholar]
- 95.Boland T., Xu T., Damon B., and Cui X. Application of inkjet printing to tissue engineering. Biotechnol J 1, 910, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Kolesky D.B., Truby R.L., Gladman A.S., Busbee T.A., Homan K.A., and Lewis J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 26, 3124, 2014 [DOI] [PubMed] [Google Scholar]
- 97.Fischer J., Aulmann A., Dexheimer V., Grossner T., and Richter W. Intermittent PTHrP(1-34) exposure augments chondrogenesis and reduces hypertrophy of mesenchymal stromal cells. Stem Cells Dev 23, 2513, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Steinert A.F., Weissenberger M., Kunz M., Gilbert F., Ghivizzani S.C., Gobel S., Jakob F., Noth U., and Rudert M. Indian hedgehog gene transfer is a chondrogenic inducer of human mesenchymal stem cells. Arthritis Res Ther 14, R168, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Caron M.M., Emans P.J., Cremers A., Surtel D.A., Coolsen M.M., van Rhijn L.W., and Welting T.J. Hypertrophic differentiation during chondrogenic differentiation of progenitor cells is stimulated by BMP-2 but suppressed by BMP-7. Osteoarthritis Cartilage 21, 604, 2013 [DOI] [PubMed] [Google Scholar]
- 100.Arany P.R., Huang G.X., Gadish O., Feliz J., Weaver J.C., Kim J., Yuen W.W., and Mooney D.J. Multi-lineage MSC differentiation via engineered morphogen fields. J Dent Res 93, 1250, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guvendiren M., and Burdick J.A. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat Commun 3, 792, 2012 [DOI] [PubMed] [Google Scholar]
- 102.Burdick J.A., Khademhosseini A., and Langer R. Fabrication of gradient hydrogels using a microfluidics/photopolymerization process. Langmuir 20, 5153, 2004 [DOI] [PubMed] [Google Scholar]
- 103.Quintana L., Muinos T.F., Genove E., Del Mar Olmos M., Borros S., and Semino C.E. Early tissue patterning recreated by mouse embryonic fibroblasts in a three-dimensional environment. Tissue Eng Part A 15, 45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klumpers D.D., Zhao X., Mooney D.J., and Smit T.H. Cell mediated contraction in 3D cell-matrix constructs leads to spatially regulated osteogenic differentiation. Integr Biol (Camb) 5, 1174, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Butler J.P., Tolic-Norrelykke I.M., Fabry B., and Fredberg J.J. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol 282, C595, 2002 [DOI] [PubMed] [Google Scholar]
- 106.Tambe D.T., Hardin C.C., Angelini T.E., Rajendran K., Park C.Y., Serra-Picamal X., Zhou E.H., Zaman M.H., Butler J.P., Weitz D.A., Fredberg J.J., and Trepat X. Collective cell guidance by cooperative intercellular forces. Nat Mater 10, 469, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murray J.D., and Oster G.F. Cell traction models for generating pattern and form in morphogenesis. J Math Biol 19, 265, 1984 [DOI] [PubMed] [Google Scholar]
- 108.Oster G.F., Murray J.D., and Harris A.K. Mechanical aspects of mesenchymal morphogenesis. J Embryol Exp Morphol 78, 83, 1983 [PubMed] [Google Scholar]
- 109.Koyama N., Miura M., Nakao K., Kondo E., Fujii T., Taura D., Kanamoto N., Sone M., Yasoda A., Arai H., Bessho K., and Nakao K. Human induced pluripotent stem cells differentiated into chondrogenic lineage via generation of mesenchymal progenitor cells. Stem Cells Dev 22, 102, 2013 [DOI] [PubMed] [Google Scholar]
- 110.Liu Y., Goldberg A.J., Dennis J.E., Gronowicz G.A., and Kuhn L.T. One-step derivation of mesenchymal stem cell (MSC)-like cells from human pluripotent stem cells on a fibrillar collagen coating. PLoS One 7, e33225, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meng X., Su R.J., Baylink D.J., Neises A., Kiroyan J.B., Lee W.Y., Payne K.J., Gridley D.S., Wang J., Lau K.H., Li G., and Zhang X.B. Rapid and efficient reprogramming of human fetal and adult blood CD34+ cells into mesenchymal stem cells with a single factor. Cell Res 23, 658, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hiramatsu K., Sasagawa S., Outani H., Nakagawa K., Yoshikawa H., and Tsumaki N. Generation of hyaline cartilaginous tissue from mouse adult dermal fibroblast culture by defined factors. J Clin Invest 121, 640, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Outani H., Okada M., Yamashita A., Nakagawa K., Yoshikawa H., and Tsumaki N. Direct induction of chondrogenic cells from human dermal fibroblast culture by defined factors. PLoS One 8, e77365, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hashmi B., Zarzar L.D., Mammoto T., Mammoto A., Jiang A., Aizenberg J., and Ingber D.E. Developmentally-inspired shrink-wrap polymers for mechanical induction of tissue differentiation. Adv Mater 26, 3253, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mehta M., Schmidt-Bleek K., Duda G.N., and Mooney D.J. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv Drug Deliv Rev 64, 1257, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fonseca K.B., Gomes D.B., Lee K., Santos S.G., Sousa A., Silva E.A., Mooney D.J., Granja P.L., and Barrias C.C. Injectable MMP-sensitive alginate hydrogels as hMSC delivery systems. Biomacromolecules 15, 380, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alsberg E., Anderson K.W., Albeiruti A., Rowley J.A., and Mooney D.J. Engineering growing tissues. Proc Natl Acad Sci U S A 99, 12025, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Emans P.J., van Rhijn L.W., Welting T.J., Cremers A., Wijnands N., Spaapen F., Voncken J.W., and Shastri V.P. Autologous engineering of cartilage. Proc Natl Acad Sci U S A 107, 3418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]