Abstract
Importance
Prior neuroimaging studies have suggested that alterations in brain structure may be a consequence of cannabis use. Siblings discordant for cannabis use offer an opportunity to use cross-sectional data to disentangle such causal hypotheses from shared effects of genetics and familial environment on brain structure and cannabis use.
Objective
To determine whether cannabis use is associated with differences in brain structure in a large sample of twins/siblings and to examine sibling pairs discordant for cannabis use to separate potential causal and predispositional factors linking lifetime cannabis exposure to volumetric alterations.
Design
Cross-sectional diagnostic interview, behavioral, and neuroimaging data.
Setting
Community sampling and established family registries.
Participants
Data from 483 participants (22-35 years old), enrolled in the on-going Human Connectome Project; 262 participants reported cannabis exposure, i.e. ever using cannabis in their lifetime.
Main Outcome Measures
Whole brain, hippocampus, amygdala, ventral striatum, and orbitofrontal cortex volumes were related to lifetime cannabis use (ever use, age of onset, and frequency of use) using linear regressions. Genetic (ρg) and environmental (ρe) correlations between cannabis use and brain volumes were estimated. Linear mixed-models were used to examine volume differences in sex-matched, concordant unexposed (Npairs=71), exposed (Npairs=81), or exposure discordant (Npairs=89) sibling pairs.
Results
Cannabis exposure was related to smaller left amygdala (~2.3%) and right ventral striatum volumes (~3.5%). These volumetric differences were within the range of normal variation. The relationship between left amygdala volume and cannabis use was largely due to shared genetic factors (ρg=−0.43, p=0.004), while the origin of the association with right ventral striatum volumes was unclear. Importantly, brain volumes did not differ between sex-matched siblings discordant for use. Both the exposed and unexposed siblings in pairs discordant for cannabis exposure showed reduced amygdala volumes relative to members of concordant unexposed pairs.
Conclusions and Relevance
Differences in amygdala volume in cannabis users are attributable to common predispositional factors, genetic or environmental in origin, with little support for causal influences. Causal influences, in isolation or in conjunction with predispositional factors, may exist for other brain regions (e.g. ventral striatum) or at more severe levels of cannabis involvement and deserve further study.
Introduction
Cannabis is the most widely used recreational drug in developed nations1. Its legal status has been a source of enduring controversy2, gaining particular momentum recently in the United States3. Yet, concerns about putative influences of cannabis on brain structure/function remain salient4. Neuroimaging studies have found inconsistent evidence linking cannabis to brain structure where recent meta-analyses noted possible associations between cannabis and hippocampus (and potentially amygdala) structure5,6.
Small sample sizes in prior studies (generally N<100) and varied definitions of cannabis exposure may have contributed to these inconsistent findings. Additionally, studies have implied that cannabis causes volumetric alterations, despite typically not controlling for potential confounding effects of shared predispositional factors (e.g. genes/rearing environment that contribute to both volumetric differences and cannabis use). For instance, Gilman et al. (2014) compared recreational cannabis users (N=20) with matched non-users (N=20) and posited, based on observed dose-dependent relationships, that larger left ventral striatal (VS) grey matter density was likely a consequence of cannabis use7. However, such cross-sectional case-control designs cannot account for the possibility that volumetric variations might predate cannabis use and/or might relate to cannabis use via predispositional factors, even in a dose-dependent manner.
Longitudinal studies, particularly with assessments preceding onset of cannabis use, are ideally suited to disentangle effects of cannabis on the developing brain from pre-existing differences. Such study designs have shown that smaller orbitofrontal cortex (OFC) volumes predict later cannabis initiation in adolescents8 yet also find emerging deficits in white matter as a consequence of heavy alcohol and cannabis use9. However, even in cross-sectional studies, twins/siblings discordant for cannabis exposure provide a unique opportunity for differentiating predispositional/familial factors from causal effects of cannabis on the brain. As monozygotic (MZ) twins share all genetic material identical-by-descent, neural differences between MZ twins discordant for cannabis exposure can be potentially attributed to causal effects of cannabis10,11. In contrast, if no differences are found, then large causal effects of cannabis on the brain are unlikely, and instead, the association might be attributed to genetic factors/familial environment. For instance, Gilbertson and colleagues found that PTSD severity in combat-exposed veterans negatively correlated with their hippocampal volume as well as volumes of their non-combat-exposed MZ co-twins, implicating predispositional rather than causal mechanisms12. In contrast, Lessov-Schlaggar et al. found functional differences in VS response to reward and punishment in regular tobacco smokers when compared to their non-regular-smoking MZ co-twin, implying potential causal mechanisms13. Importantly, although data from MZ twins are essential to demonstrate causality in this manner, absence of neural differences within discordant dizygotic (DZ) twin or non-twin sibling pairs is still compelling evidence against a causal hypothesis. If volume differences are not observed among discordant pairs who share only 50% of their genes, then finding an association with further genetic matching (i.e. MZ pairs) would be unlikely10.
The goal of the current study was to test previously observed relationships between cannabis and brain volumes in a large normative sample of twins/siblings from the Human Connectome Project (HCP; N=483). First, we examined whether cannabis exposure, age of onset of use, and lifetime frequency of use predicted whole brain volume (WBV), amygdala, hippocampus, VS, or OFC volumes. Second, we quantified the degree to which shared genetic and individual-specific environmental factors contributed to these associations. Finally, to test whether any significant volumetric differences could be attributed to predispositional/familial or causal factors, we compared volumes across sex-matched twin/sibling pairs (henceforth referred to as sibling pairs) discordant for cannabis exposure (Npairs=89), concordant for exposure (Npairs=81), or concordantly unexposed (Npairs=71).
Methods
Participants
Participants were drawn from the June 2014 public data release from the HCP (N=527), which aims to recruit 1,200 individuals (3-4 siblings per family, most including a twin pair)14. All participants were 22-35 years old; for all inclusion/exclusion criteria, see14. Participants were not excluded for recreational drug use (unless they reported being hospitalized for ≥2 days for substance abuse or being treated by a medical specialist for ≥12 months for substance abuse [or any psychiatric or neurological condition]).
Participants were excluded from the current analyses if they lacked good-quality structural MRI data available (N=17), were missing relevant interview/questionnaire data (N=10), or screened positive for tetrahydrocannabinol on a urine screen but reported not using cannabis within the last 12 months (N=16). This resulted in a final sample size of 483 individuals (262 ever using cannabis). In analyses involving sibling pairs (eMethods S1), 36 individuals who did not have a full sibling or twin in the present data release were excluded. As sex is significantly related to both cannabis use and brain volumes, the inclusion of discordant opposite-sex non-twin sibling pairs (all twin pairs were same-sex), can result in statistical confounds. Therefore, we excluded 145 opposite-sex pairs, resulting in 241 sibling pairs (50 MZ, 45 DZ, and 146 non-twin siblings [mean sibling age difference=3.69 years]), including 89 same-sex pairs discordant for cannabis exposure, 81 concordant for cannabis exposure, and 71 concordantly unexposed pairs (eTable 1).
Brain Volume Data
High-resolution (0.7mm isotropic voxels) anatomical images were acquired using a customized Siemens Skyra 3T scanner with a 32-channel head coil. For details on data acquisition and preprocessing, see15 (see eMethods S2 for relevant pre-processing steps). Volume estimates for the regions of interest were extracted using FreeSurfer v5.3.016,17 (http://surfer.nmr.mgh.harvard.edu/). This included WBV (total gray + cortical white matter volume) and left and right amygdala, hippocampus, and VS volumes from subcortical segmentation and OFC volumes (lateral + medial) from cortical parcellation using the Desikan atlas18.
Questionnaire, Interview, and Behavioral Data
Cannabis use
Cannabis-related measures were assessed using the Semi-Structured Assessment for the Genetics of Alcoholism19 (SSAGA). Cannabis exposure was a dichotomous variable representing whether an individual reported ever using cannabis during their lifetime. Age of onset of cannabis use (reported age of first cannabis use) and lifetime frequency of use (reported number of times using cannabis across one’s lifetime) were also examined (both coded ordinally by the HCP, eMethods S3).
Covariates
The main analyses controlled for demographic factors of sex (female>male), ethnicity (White>not; African-American>not), age, zygosity (MZ>not; DZ>not), total household income (eMethods S3), and age-adjusted picture vocabulary scores as a proxy for crystallized intelligence20 (eMethods S4) – henceforth, these are referred to as primary covariates. Follow-up regression analyses controlled for a variety of other potential confounds, including personality, impulsivity, other substance use, and comorbid psychopathology (eTable 10). See eMethods S5 for rationale and assessment details.
Statistical Analysis
Analyses were performed using IBM SPSS Statistics v20 (Armonk, NY: IBM Corp.) and Rv3.121. One outlier (>3x the interquartile range away from the 25th/75th percentile) for right amygdala and right hippocampal volume was Winsorized to the next most extreme value. Independent samples t-tests and χ2 tests were used to test for differences in the covariates between cannabis-exposed and unexposed individuals.
Regression Analyses
Linear regressions were used to test whether cannabis exposure (in the full sample, N=483) and age of onset or frequency of use (among exposed individuals, N=262) predicted WBV, subcortical (left and right amygdala, hippocampus, and VS), or OFC volumes, controlling for the primary covariates and WBV (when predicting regional volumes). Bootstrapped 95% confidence intervals were calculated to account for familial clustering (R boot package22). False-discovery rate (FDR) was used to control for the nine regression analyses with each cannabis measure. Only brain regions with q<0.05 were examined in the sibling analysis.
Sources of Variance and Covariance
Sequential Oligogenic Linkage Analysis Routines23 (SOLAR) was used to attribute phenotypic correlations (ρp) between cannabis exposure and brain volumes to overlapping genetic (ρg) or individual-specific environmental (ρe) factors24. All models controlled for the primary covariates and WBV (when examining regional volumes).
Discordant Sibling Analyses
All possible same-sex sibling pairs were drawn from the data (N=241 pairs from 146 families; details in eMethods S1). Linear mixed models (lmer function in R package lme425) were used, which nested individuals within sibling pairs and nested pairs within families. The mixed models included fixed effects for the primary covariates and WBV (when predicting regional volumes). The main effect of interest was membership in a sibling pair concordant or discordant for cannabis exposure. To test this, participants were divided into four groups – individuals from concordant unexposed pairs, individuals from concordant exposed pairs, exposed members of discordant pairs, and unexposed members of discordant pairs. This four-group factor was tested as a fixed effect using Helmert contrast coding (eTable 2). Contrast 1 compared exposed and unexposed siblings from discordant pairs to test a causal hypothesis, i.e. cannabis causes altered brain volumes (depicted as smaller volumes for exposed vs. unexposed individuals in Figure 1a). Siblings share 50% of their genes and much of their rearing environment. Therefore, within-pair volumetric differences would be preliminary evidence for causation, pending replication in MZ pairs. Contrast 2 and 3 both hypothesize no volumetric differences between the exposed and unexposed members of the discordant pairs and thus tested facets of the hypothesis that cannabis use and brain volumes share predispositional factors. This would suggest that differences in volume likely pre-date (or co-occur with) cannabis use and that other variables, like genetic liability or rearing environment, may lead to both neural differences and liability to cannabis use. Alternatively, these other factors could contribute to neural differences, in turn increasing liability to cannabis use. Contrast 2 compared brain volumes from concordant exposed pairs to both members of discordant pairs to test exposure-related differences by concordance/discordance (Figure 1b). A significant effect might indicate that concordantly exposed pairs are at greater liability for cannabis use and altered brain volumes (because both siblings have used cannabis) than discordant pairs, i.e. graded liability. Contrast 3 compared volumes from concordant unexposed pairs to all other groups to test whether altered brain volumes and cannabis exposure were associated with a shared predisposition (Figure 1c). A significant effect here implies that both concordant exposed and discordant pairs are at the same genetic liability regardless of whether one or both siblings use cannabis.
Figure 1. Hypothetical Causal and Predispositional Effects.
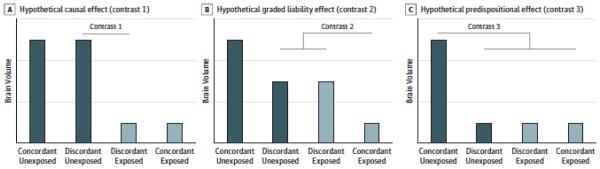
Three hypothetical patterns of results from the linear mixed model analyses are presented here. A, The hypothesis that cannabis causes alterations in brain volumes (depicted as reductions in volume) was tested with contrast 1. As denoted by the bar labeled contrast 1, this contrast tested for differences between exposed and unexposed members of pairs discordant for cannabis use. Finding smaller volumes among exposed members of these pairs compared with their siblings would support this causal hypothesis (pending replication with monozygotic twin pairs). B, contrast 2 compares volumes for concordant exposed pairs with discordant pairs to test the hypothesis that brain volumes and cannabis use share familial/predispositional factors where concordant exposed pairs are at increased liability (both siblings are exposed vs only one), ie, graded liability. C, Contrast 3 compares volumes for the concordant unexposed pairs with all other groups to test the hypothesis that brain volumes and cannabis use share familial/predispositional factors but that liability does not differ by concordance vs discordance for use.
Control Analysis
To confirm that differences in brain volumes among discordant pairs in the linear mixed models (Contrast 1) were non-significant due to familial matching rather than a reduction in sample size, we randomly paired each cannabis user from a same-sex discordant pair (N=89) with a sex-matched, unrelated unexposed individual. Paired t-tests were performed to compare volumes for these unrelated pairs, i.e. to remove the familial control while matching for sample size.
Results
Sample Characteristics
Of the 483 participants, 262 (54.2%) reported ever using cannabis. Forty-nine percent of the exposed individuals reported first using cannabis by 17-years-old and 29.4% reported using cannabis >100 times (eFigure 1). Cannabis users were significantly more likely to be male, to be non-White, to report lower income, to report greater alcohol, cigarette, and other illicit drug use, to report more childhood conduct problems, to be less agreeable and more impulsive, and to show steeper delay discounting as compared to never users (Table 1). Similar relationships were also observed with increasing frequency of use and decreasing age of onset (eTable 3). Descriptive statistics and inter-correlations among brain volumes are presented in eTable 4 and inter-correlations among all covariates are in eTable 5.
Table 1.
Sample Characteristics of 483 Participants Included From the September 2014 Data Release of the Human Connectome Projecta
| Cannabis, Mean (SD) |
||
|---|---|---|
| Characteristic | Unexposed (n = 221) |
Exposed (n = 262) |
| Age, y | 29.30 (3.46) | 29.32 (3.43) |
|
| ||
| Total household Incomeb | 5.24 (2.10) | 4.82 (2.21) |
|
| ||
| Age-adjusted picture vocabulary | 108.42 (14.57) | 106.35 (19.99) |
|
| ||
| NEO-FFI | ||
|
| ||
| Contentiousness | 34.92 (5.55) | 34.60 (5.66) |
|
| ||
| Extraversion | 30.18 (6.21) | 30.81 (6.22) |
|
| ||
| Neuroticism | 16.66 (7.32) | 16.26 (6.99) |
|
| ||
| Openness | 27.37 (5.78) | 28.42 (6.29) |
|
| ||
| Agreeablenessb | 32.59 (4.66) | 31.60 (4.72) |
|
| ||
| Delay discounting (AUC)c | 0.28 (0.22) | 0.21 (0.16) |
|
| ||
| ASR Impulsivityb | 1.14 (1.18) | 1.40 (1.30) |
|
| ||
| Female, %c | 69.68 | 52.29 |
|
| ||
| White,%c,d | 79.64 | 70.23 |
|
| ||
| Twins, % | 53.85 | 46.56 |
|
| ||
| Monozygotic twins, % | 50.42 | 43.44 |
|
| ||
| Lifetime depression history, % | 8.60 | 7.25 |
|
| ||
| Any childhood conduct problems,%b | 33.94 | 42.75 |
|
| ||
| Times ever used illicit drugs, %c | 2.71 | 34.35 |
|
| ||
| ≥2 Alcoholic drinks per day (heaviest), %c,e |
63.35 | 88.17 |
|
| ||
| Never smoked cigarettes (heaviest), %c,e | 90.95 | 57.63 |
|
| ||
| Cannabls use | ||
|
| ||
| Age at onset <17 y, % | NA | 48.85 |
|
| ||
| Lifetime quantity of use > 11 times, % | NA | 51.15 |
|
| ||
| Current abuse/dependence diagnosis, % |
NA | 17.56 |
|
| ||
| Use in the past 12 mo, % | NA | 36.26 |
Abbreviations: AUC, area under the curve; ASR, Achenbach Adult Self-Report; NA, not applicable; NEO-FFI, NEO Five-Factor Inventory.
Independent-samples t tests were used to compare means for the exposed and unexposed groups. χ2 Tests were used to compare ordinal/binary variables across groups. eAppendix 5 in the Supplement provides further details.
P < .05.
P < .001.
Race/ethnicity was coded as white, black or African American, or other race/ethnicity (including Asian, Pacific Islander, Native Hawaiian, multiracial, other, or not reported).
Alcoholic drinks per day and heaviness of cigarette smoking variables refer to use during the 12-month period of heaviest use.
Regression Analyses
Table 2 summarizes the linear regressions results, controlling for the primary covariates (full results in eTables 6-8). Relative to unexposed individuals, cannabis users had smaller left amygdala (~2.3%) and right VS volumes (~3.5%). Post-hoc comparisons (eTable 9) showed similar volumetric differences in those who used <100 or ≥100 times, with no statistical difference between the regression coefficients across these levels of use. Among cannabis users, heavier use predicted smaller left hippocampus volumes (though this effect did not pass FDR correction). There were no significant associations with age of onset of use (eTable 8). These results remained significant when also controlling for a variety of personality factors, impulsivity, other substance use, and psychiatric history (eTable 10).
Table 2.
Regression Results for the Association Between Cannabis-Related Measures and Brain Volumesa
| Cannabis Exposed vs Unexposed (n = 483) |
Lifetime Amount of Use Among Exposed Individuals (n = 262) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Regression Coefficients |
Regression Coefficients |
|||||||
| Volume | Unstandardized (b) | Standardized (β) | P Value | Bootstrapped 95%CI |
Unstandardized (b) | Standardized (β) | P Value | Bootstrapped 95%CI |
| Whole brain | −6684.22 | −0.06 | .38 | −21717.0 to 8106.0 |
−6568.31 | −0.06 | .06 | −13893.0 to 501.0 |
|
| ||||||||
| Amygdala | ||||||||
|
| ||||||||
| Left | −34.68b,c | −0.18b,c | .007b,c | −60.65 to −11.01b,c |
−4.72 | −0.03 | .43 | −14.97 to 5.20 |
|
| ||||||||
| Right | −20.64 | −0.10 | .13 | −45.94 to 6.65 |
−10.50 | −0.05 | .09 | −23.11 to 0.75 |
|
| ||||||||
| Hippocampus | ||||||||
|
| ||||||||
| Left | −29.51 | −0.06 | .30 | −91.44 to 33.27 |
−31.83c | −0.07c | .05c | −61.96 to −3.03c |
|
| ||||||||
| Right | 14.90 | 0.03 | .61 | −43.83 to 73.13 |
−15.58 | −0.04 | .26 | −43.46 to 11.25 |
|
| ||||||||
| Ventral striatum | ||||||||
|
| ||||||||
| Left | −0.59 | −0.01 | .94 | −15.12 to 13.15 |
0.26 | 0.00 | .94 | −6.09 to 7.1 |
|
| ||||||||
| Right | −20.87b,c | −0.22b,c | .005b,c | −35.69 to −6.44b,c |
−2.83 | −0.03 | .43 | −10.01 to 4.03 |
|
| ||||||||
| Orbitofrontal cortex |
||||||||
|
| ||||||||
| Left | −88.72 | −0.06 | .24 | −232.34 to 50.1 |
−26.00 | −0.02 | .48 | −87.24 to 42.66 |
|
| ||||||||
| Right | −21.51 | −0.02 | .76 | −156.19 to 112.22 |
−39.73 | −0.03 | .24 | −106.98 to 23.96 |
Unstandardized (b) and standardized (β) regression coefficients and their associated P values are presented for the effects of cannabis exposure and lifetime quantity of use (among cannabis-exposed individuals) from separate linear regression models predicting whole-brain or regional volume. Bootstrapped confidence intervals are also presented for each estimate (5000 bootstrap iterations; bias-corrected accelerated percentile intervals). Regressions controlled for sex, age (years), race/ethnicity (white vs not; African American vs not), zygosity (monozygotic vs not; dizygotic vs not), total household income, picture vocabulary, and whole-brain volume (when predicting regional volumes). Negative regression coefficients indicate smaller volumes for exposed vs unexposed individuals or greater lifetime quantity of use.
Effects passing false-discovery rate (q < 0.05) correction.
Effects significant at P < .05.
Sources of Variance and Covariance
The heritability of cannabis exposure (ever vs. never used) was estimated at 67.2% (standard error=13.6%, p<0.001; Table 3). All brain volumes of interest were also significantly heritable. There was no evidence for contributions of shared rearing environment. As above, only the left amygdala (ρp=−0.175,p=0.005) and right VS (ρp=−0.154,p=0.015) showed significant phenotypic correlations with cannabis use. Decomposing these correlations, we found a significant genetic correlation between left amygdala volume and cannabis use (ρg=−0.433,p=0.004), but not a significant environmental correlation (ρe=0.280,p=0.189). Neither ρg nor ρe was significant for right VS volume.
Table 3.
Results of SOLAR Analysesa
| Variable | Whole-Brain Volume |
Amygdala |
Hippocampus |
Ventral Striatum |
Orbitofrontal Cortex |
||||
|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | ||
| Heritability/familiality (SE), % |
95.3 (1.0)b | 52.9 (7.8)b | 53.6 (10.4)b | 22.3 (9.8)b | 77.7 (6.8)b | 49.3 (8.2)b | 62.4 (7.6)b | 45.8 (8.9)b | 60.2 (7.2)b |
|
| |||||||||
| P value | <.001b | <.001b | <.001b | .01b | <.001b | <.001b | <.001b | <.001b | <.001b |
|
| |||||||||
| Phenotypic correlation | −0.064 | −0.175b | −0.091 | −0.050 | 0.042 | 0.005 | −0.154b | −0.062 | −0.004 |
|
| |||||||||
| P value | >.99 | .005b | .15 | .41 | .53 | .94 | .02b | .33 | .95 |
|
| |||||||||
| Environmental correlation (SE) |
0.062 (0.277) |
0.280 (0.215) |
0.059 (0.201) |
0.072 (0.172) |
−0.080 (0.254) |
−0.026 (0.170) |
−0.015 (0.197) |
0.055 (0.188) |
−0.275 (0.194) |
|
| |||||||||
| P value | .82 | .19 | .78 | .68 | .76 | .89 | .94 | .77 | .17 |
|
| |||||||||
| Genetic/familial correlation (SE) |
−0.090 (0.124) |
−0.433 (0.139)b |
−0.192 (0.180) |
−0.220 (0.248) |
0.090 (0.167) |
0.027 (0.167) |
−0.231 (0.148) |
−0.159 (0.186) |
0.145 (0.150) |
|
| |||||||||
| P value | >.99 | .004b | .27 | .36 | .55 | .87 | .12 | .38 | .32 |
Abbreviation: SOLAR, sequential oligogenic linkage analysis routines.
All models controlled for age, sex, race/ethnicity, income, picture vocabulary, and whole-brain volume (when predicting regional volumes). Heritability estimates were derived from a standard polygenic model (not including cannabis use as a covariate) Household effects (living with the same biological mother) were used to test for shared/rearing environment and found to be nonsignificant at P > .05. Phenotypic, environmental, and genetic/familial correlations were derived from bivariate models including each regional volume and cannabis use (ever vs never). Standard errors are presented for the estimates at heritability and environmental and genetic correlations.
Effects significant at P < .05.
Discordant Sibling Analyses
Given the above results, we focused on the left amygdala and right VS for the sibling analyses. Contrast 3 significantly predicted left amygdala volumes (β=12.56, t(302.80)=2.97, p=0.003), supporting the predispositional hypothesis. We did not find evidence for either the causal (Contrast 1) or graded liability (Contrast 2) hypotheses. Concordant unexposed pairs had larger amygdala volumes than all other groups, even unexposed members of discordant pairs (Figure 2). Despite not using cannabis, these unexposed individuals from discordant pairs had amygdala volumes resembling cannabis-exposed individuals, including their exposed siblings. None of the three contrasts significantly predicted right VS volumes (fixed effects predicting all volumes are presented in eTable 11).
Figure 2. Left Amygdala Volume by Cannabis Exposure Group.
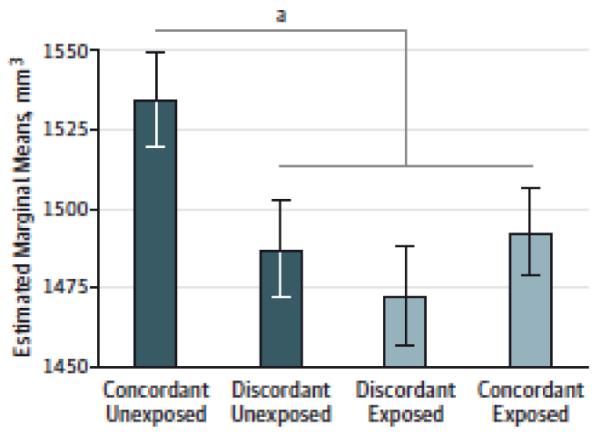
Estimated marginal means (error bars Indicate standard errors) for left amygdala volumes by cannabis exposure group are presented. The significant fixed effect of contrast 3 is denoted: concordant unexposed pairs showed larger volumes than all other groups.
a P < .05.
Results including opposite-sex sibling pairs are presented in eTable 12. We observed significant effects of contrast 3 (predisposition), contrast 1 (causal), and an interaction between contrast 1 and pair sex-concordance underscoring the confounding effect of sex, within-pairs, with respect to cannabis use and brain volume.
Control Analyses
Finally, the exposed members of the same-sex discordant pairs (N=89) were compared with unrelated but sex-matched unexposed individuals. Significantly lower volumes for the left amygdala (~5.1%) were observed for the exposed vs. unexposed members of these unrelated pairs (eTable 13). This confirmed that the lack of volumetric differences between exposed and unexposed members of discordant pairs (Contrast 1) was attributable to familial matching and not a reduced sample size.
Discussion
To our knowledge, this is the largest study to date examining relationships between cannabis exposure (ever vs. never used) and brain volumes. Cannabis exposure was associated with smaller left amygdala and right VS volumes; these findings persisted even after controlling for a host of covariates. While other studies have noted reductions in amygdala volumes26-28, the right VS finding is somewhat unique to this study. It contradicts the increased VS volume in occasional cannabis users reported by Gilman and colleagues7. It remains to be explored whether this is due to the cannabis measures explored (e.g. cannabis-exposed vs. joints/week), sample size (483 vs. 40), or other sample characteristics (e.g. ethnicity; 74.5% White in our sample but unreported by Gilman et al.).
Importantly, by leveraging the familial design of the HCP, we demonstrated that amygdala volumetric reductions among cannabis users are primarily attributed to familial factors shared by twins/siblings. Overlapping genetic factors (ρg) were the only significant source of covariance; a significant correlation between individual-specific environmental factors (ρe) would be expected if the causal hypothesis were supported29. The discordant sibling analyses further confirmed this; even in the absence of cannabis exposure, smaller amygdala volumes were observed among individuals with heightened familial liability, given their sibling’s cannabis use. However, interpretations from our sibling analyses should be tempered by our limited sample size to examine discordant MZ pairs only. This predisposition to smaller brain volumes, even in the absence of manifest cannabis use, casts considerable doubt on hypotheses that cannabis use, at least at the levels noted in this sample, causes reductions in amygdala volumes. Instead, both the exposed and unexposed siblings in discordant pairs and concordant exposed pairs tended to have smaller amygdala volumes than concordant unexposed individuals.
Limitations and Future Directions
First, while the normative sampling of the HCP is a strength, we were limited by sample size from examining heavy/problematic cannabis use, which has been inconsistently associated with volumetric alterations27,30. Furthermore, as heavy/problem cannabis use is often associated with psychiatric problems31, severity and chronicity of use may have been limited by the HCP’s exclusion of individuals receiving extended psychiatric treatment or hospitalization. Also, data was not available on recent duration/frequency of use, which has been linked to structural changes32. Thus, even though we noted similar evidence of association with lighter (<100 times) and heavier (≥100 times) lifetime cannabis exposure, we were underpowered to test this in our sibling analyses and cannot exclude the role of causal factors at higher levels of cannabis exposure. Second, though right VS volume and cannabis use were significantly related, we were unable to disentangle the etiology of this relationship. Neither genetic nor environmental correlations were significant and within-pair differences could be equated across all groups. Thus, causal and/or predispositional influences may link cannabis exposure and VS volume. Third, while the family structure in the HCP data is powerful, longitudinal data that are collected from prior to cannabis onset through later development is critical for substantiating causal claims (e.g. recent NIH efforts33). Fourth, the small sample size of discordant MZ twin pairs (Npairs=9) is a limitation. Fifth, the role of additional covariates cannot be excluded (e.g. childhood trauma is linked to both amygdala volumes34 and cannabis use35). Sixth, while we selected a priori regions of interest based on prior studies, other regions should be examined in future work (e.g. using whole brain voxel-wise analysis). Seventh, exploring other brain-related measures, like white matter integrity and task-related activity, might reveal different findings. Eighth, evidence for potential causal effects in opposite-sex sibling pairs was driven by a small number (N=14) of discordant pairs where the female sibling was the exposed member. A thorough examination of phenotypic data failed to distinguish these discordant exposed females from others in the sample. However, based on evidence for sex differences in the endocannabinoid system36,37, such differences in related individuals may reflect qualitatively different pathways and warrants further study. Finally, future work should further explore the graded liability hypothesis.
Summary
Despite speculation regarding neurotoxic effects of tetrahydrocannabinol based on preclinical research (e.g.38,39), the observed cannabis-related volumetric differences were well within the range of normal variation. When using a simple index of exposure (i.e. ever vs. never use), we found no evidence for the causal influence of cannabis exposure on amygdala volume. Future work characterizing the roles of causal and predispositional factors underpinning neural changes at various degrees of cannabis involvement may provide targets for substance abuse policy and prevention programs.
Supplementary Material
Acknowledgements
Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. A.A.’s work was supported by a grant from the National Institute of Drug Abuse (No. 5K02DA32573). D.P.’s work was supported by a grant from the National Institute of General Medical Sciences (No. 5T32GM081739). RB receives support from the Klingenstein Third Generation Foundation. D.P. had full access to all study data and takes responsibility for the integrity of the data and accuracy of the data analysis. We thank all participants and their families who provided time and effort to making this study possible.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Degenhardt L, Whiteford HA, Ferrari AJ, et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1564–1574. doi: 10.1016/S0140-6736(13)61530-5. doi:10.1016/S0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- 2.Hall W, Lynskey M. The challenges in developing a rational cannabis policy. Curr Opin Psychiatry. 2009;22(3):258–262. doi: 10.1097/YCO.0b013e3283298f36. doi:10.1097/YCO.0b013e3283298f36. [DOI] [PubMed] [Google Scholar]
- 3.Room R. Cannabis legalization and public health: legal niceties, commercialization and countercultures. Addiction. 2014;109(3):358–359. doi: 10.1111/add.12481. doi:10.1111/add.12481. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse Health Effects of Marijuana Use. N Engl J Med. 2014;370(23):2219–2227. doi: 10.1056/NEJMra1402309. doi:10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorenzetti V, Lubman DI, Whittle S, Solowij N, Yücel M. Structural MRI findings in long-term cannabis users: what do we know? Subst Use Misuse. 2010;45(11):1787–1808. doi: 10.3109/10826084.2010.482443. doi:10.3109/10826084.2010.482443. [DOI] [PubMed] [Google Scholar]
- 6.Rocchetti M, Crescini A, Borgwardt S, et al. Is cannabis neurotoxic for the healthy brain? A meta-analytical review of structural brain alterations in nonpsychotic users. Psychiatry Clin Neurosci. 2013;67(7):483–492. doi: 10.1111/pcn.12085. doi:10.1111/pcn.12085. [DOI] [PubMed] [Google Scholar]
- 7.Gilman JM, Kuster JK, Lee S, et al. Cannabis Use Is Quantitatively Associated with Nucleus Accumbens and Amygdala Abnormalities in Young Adult Recreational Users. Journal of Neuroscience. 2014;34(16):5529–5538. doi: 10.1523/JNEUROSCI.4745-13.2014. doi:10.1523/JNEUROSCI.4745-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheetham A, Allen NB, Whittle S, Simmons JG, Yücel M, Lubman DI. Orbitofrontal Volumes in Early Adolescence PredictInitiation of Cannabis Use: A 4-Year Longitudinal andProspective Study. BPS. 2012;71(8):684–692. doi: 10.1016/j.biopsych.2011.10.029. doi:10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Jacobus J, Squeglia LM, Infante MA, Bava S, Tapert SF. White matter integrity pre- and post marijuana and alcohol initiation in adolescence. Brain Sci. 2013;3(1):396–414. doi: 10.3390/brainsci3010396. doi:10.3390/brainsci3010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression. A causal analysis. Archives of general psychiatry. 1993;50(1):36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- 11.Lynskey MT, Heath AC, Bucholz KK, et al. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA. 2003;289(4):427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- 12.Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242–1247. doi: 10.1038/nn958. doi:10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lessov-Schlaggar CN, Lepore RL, Kristjansson SD, et al. Functional neuroimaging study in identical twin pairs discordant for regular cigarette smoking. Addict Biol. 2013;18(1):98–108. doi: 10.1111/j.1369-1600.2012.00435.x. doi:10.1111/j.1369-1600.2012.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Essen DC, Ugurbil K, Auerbach E, et al. The Human Connectome Project: A data acquisition perspective. NeuroImage. 2012;62(4):2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. doi:10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasser MF, Sotiropoulos SN, Wilson JA, et al. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage. 2013;80(C):105–124. doi: 10.1016/j.neuroimage.2013.04.127. doi:10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dale A. Cortical Surface-Based Analysis I. Segmentation and Surface Reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. doi:10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 17.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically Parcellating the Human Cerebral Cortex. 2004. [DOI] [PubMed]
- 18.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. doi:10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Bucholz KK, Cadoret R, Cloninger CR, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 20.Gershon RC, Slotkin J, Manly JJ, et al. IV. NIH Toolbox Cognition Battery (CB): measuring language (vocabulary comprehension and reading decoding) Monographs of the Society for Research in Child Development. 2013;78(4):49–69. doi: 10.1111/mono.12034. doi:10.1111/mono.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Team RC. R: A language and environment for statistical computing. 2012.
- 22.Canty A, Ripley B. boot: Bootstrap R (S-Plus) functions. R package version. 2012;1(7) [Google Scholar]
- 23.Blangero J, Almasy L. Solar: sequential oligogenic linkage analysis routines. Population Genetics Laboratory Technical Report. 1996;6 [Google Scholar]
- 24.Evans DM, Gillespie NA, Martin NG. Biometrical genetics. Biol Psychol. 2002;61(1-2):33–51. doi: 10.1016/s0301-0511(02)00051-0. doi:10.1016/S0301-0511(02)00051-0. [DOI] [PubMed] [Google Scholar]
- 25.Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. 2012.
- 26.Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: Results of a VBM study in heavy cannabis users and healthy controls. NeuroImage. 2012;59(4):3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. doi:10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 27.Schacht JP, Hutchison KE, Filbey FM. Associations between Cannabinoid Receptor-1 (CNR1) Variation and Hippocampus and Amygdala Volumes in Heavy Cannabis Users. Neuropsychopharmacology. 2012;37(11):2368–2376. doi: 10.1038/npp.2012.92. doi:10.1038/npp.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yücel M, Solowij N, Respondek C, et al. Regional brain abnormalities associated with long-term heavy cannabis use. Archives of general psychiatry. 2008;65(6):694–701. doi: 10.1001/archpsyc.65.6.694. doi:10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal A, NEALE MC, Prescott CA, Kendler KS. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychol Med. 2004;34(7):1227–1237. doi: 10.1017/s0033291704002545. [DOI] [PubMed] [Google Scholar]
- 30.Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, Hutchison KE. Daily Marijuana Use Is Not Associated with Brain Morphometric Measures in Adolescents or Adults. The journal of Neuroscience. 2015;35(4):1505–1512. doi: 10.1523/JNEUROSCI.2946-14.2015. doi:10.1523/JNEUROSCI.2946-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2014;110(1):19–35. doi: 10.1111/add.12703. doi:10.1111/add.12703. [DOI] [PubMed] [Google Scholar]
- 32.Battistella G, Fornari E, Annoni J-M, et al. Long-term effects of cannabis on brain structure. Neuropsychopharmacology. 2014;39(9):2041–2048. doi: 10.1038/npp.2014.67. doi:10.1038/npp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adolescent Brain Cognitive Development Study [Accessed March 14, 2015];addictionresearchnihgov. Available at: http://addictionresearch.nih.gov/adolescent-brain-cognitive-development-study.
- 34.Hanson JL, Nacewicz BM, Sutterer MJ, et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biological Psychiatry. 2015;77(4):314–323. doi: 10.1016/j.biopsych.2014.04.020. doi:10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sartor CE, Waldron M, Duncan AE, et al. Childhood sexual abuse and early substance use in adolescent girls: the role of familial influences. Addiction. 2013;108(5):993–1000. doi: 10.1111/add.12115. doi:10.1111/add.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burston JJ, Wiley JL, Craig AA, et al. Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Δ9-tetrahydrocannabinol exposure. British journal of pharmacology. 2010;161(1):103–112. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubino T, Realini N, Guidali C, et al. Chronic Δ9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33(11):2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- 38.Scallet AC. Neurotoxicology of cannabis and THC: A review of chronic exposure studies in animals. Pharmacol Biochem Behav. 1991;40(3):671–676. doi: 10.1016/0091-3057(91)90380-k. doi:10.1016/0091-3057(91)90380-K. [DOI] [PubMed] [Google Scholar]
- 39.Landfield PW, Cadwallader LB, Vinsant S. Quantitative changes in hippocampal structure following long-term exposure to delta 9-tetrahydrocannabinol: possible mediation by glucocorticoid systems. Brain Research. 1988;443(1-2):47–62. doi: 10.1016/0006-8993(88)91597-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


