Abstract
B lymphocytes are induced to undergo Ig class switching and a complex phenotypic differentiation by the milieu of the germinal center. Partly as a result of the lack of a suitable in vitro B cell model, the relationship between these processes in the humans has never been formally established in vitro. We have identified a human monoclonal B cell line, CL-01, that expresses surface IgM and IgD and, upon induction with CD40 ligand, IL-4, and IL-10, switches to all seven downstream isotypes, showing typical DNA switch recombination preceded by germline transcription of targeted CH regions. In CL-01 cells, switch-inducing stimuli trigger concomitant changes in expression of surface IgD, CD23, CD38, and CD77 that parallel those reported in ex vivo isolated tonsillar centroblasts, centrocytes, and memory B cells. Eventually, in the presence of IL-6, CL-01 cells express CD56 and accumulate cytoplasmic IgG and IgA, both traits of plasmacytoid differentiation. Analysis of transcription and recombination of the Ig H locus in sorted CL-01 cells suggest that Ig class switching begins in centroblasts, it extends to all isotypes in centrocytes, and it is extinct in memory B cells. Thus, we have induced coordinated Ig class switching, progression through germinal center phenotypic stages, and differentiation to memory B cells and plasma cells at the level of a single B clonotype. Our data suggest that these processes are likely regulated by a common maturation program, the activation of which may require CD40 ligand, IL-4, IL-10, and IL-6 only.
B cell development proceeds through an initial Ag-independent and a subsequent Ag-dependent stage. The former leads to the emergence of a naive surface (s)3 IgM+sIgD+ B cell from the bone marrow, while the latter leads to the differentiation of an Ag-selected B cell into a plasma cell or a memory B cell in peripheral lymphoid organs. Two major processes are central to the Ag-dependent B cell maturation and to the generation of memory B cells and plasma cells: Ig class switching, and somatic hypermutation (1). Both of these processes are fostered by the specialized microenvironment of the germinal center (GC), and both contribute to the maturation of the Ab response, although in different ways. By changing the C region of the Ig H chain with a downstream CH region, class switching changes the Ig effector features to suit them to the new functions and distribution required by a maturing Ab response. By increasing the binding strength of the surface receptor for Ag, somatic hypermutation provides the structural substrate for clonal selection by Ag to operate. Ig class switching and somatic hypermutation are associated with major phenotypic changes, including the modulation of sIgD, CD23, CD38, CD77, CD80, and CD86, that are characteristic of an Ab-producing cell progressing through the GC (2–4), but their precise relationship to such phenotypic changes remains unclear.
Most of our knowledge on human B cell Ig class switching and differentiation has been gained from the study of polyclonal naive B cell fractions isolated from the peripheral blood or tonsils of healthy subjects (5–7). However, the use of such cell fractions for B lymphocyte differentiation studies is plagued by low cell viability, heterogeneous phenotype, and possibly the presence of B cells that have already switched their Ig class before the application of any switching-inducing stimuli. Some of these limitations have been circumvented by isolating sIgM+sIgD+ B cells using a solid phase anti-δ chain Ab. Using freshly isolated sIgM+sIgD+ naive B cells, a major role of CD40 in the induction of Ig class switching and phenotypic differentiation has been suggested (8–10). Nevertheless, the stimulation requirements for and the formal relationship between these two processes at the level of a single human B cell clonotype remain to be defined.
For Ig class switching and B cell differentiation studies, a monoclonal population of dividing cells would be devoid of the limitations inherent to freshly isolated polyclonal B cells, as it would be readily available in a large amount, and would be homogeneous in genetic makeup. Ideally, such monoclonal cells should be sIgM+sIgD+, they should undergo a high rate of switching to all downstream Ig classes in response to physiologic stimuli, possibly in a cytokine-directed fashion, and finally they should display the phenotypic changes that are putatively attributed to B cells that switch and progress through the GC. No B cell line with all these properties has been reported, although certain lines display some of them. The murine I.29 μ B cell line switches to IgG2a, IgA, and IgE in response to IL-4 and LPS and has been used to elucidate crucial molecular aspects of Ig class switching (11, 12). Some Abelson murine leukemia virus-transformed murine pre-B cell lines spontaneously switch to IgG2b (13); others can be induced to switch by LPS (14). The murine CH12.LX Ly-1+ B cell lymphoma and its subclone CH12F3 switch at high frequency to IgA, but not to other isotypes in response to CD40 ligand (CD40L, CD154), IL-4, and TGF-β1 (15). Finally, few murine plasmacytoma lines that spontaneously switch in culture have also been identified (16). As for the human, SSK41 and subclone 266 of the Ramos lymphoma are the only B cell lines thus far identified with some switching potential, but only to IgG (17, 18). The ability of these B cell lines to undergo other phenotypic changes in response to switch-inducing stimuli has never been investigated.
To better understand the relationship between Ig class switching and phenotypic B cell differentiation and to define the requirements for the induction of these processes, we have sought a human monoclonal cell line that would possess the features of a naive B cell and that could undergo isotype switching and phenotypic differentiation typical of normal GC B cells. Here we describe a sIgM+sIgD+ B cell line, clone CL-01, that switches the expressed Igs to all seven downstream isotypes after engagement of CD40 by CD40L and exposure to IL-4 and IL-10. In these cells, Ig class switching occurs in the context of a phenotypic differentiation program that recapitulates the features characteristic of centroblast, centrocyte, and, eventually, memory B cell, and, in the presence of IL-6, plasma cell. Ig DNA does not undergo switch recombination before induced CL-01 cells enter the centrocytic stage, suggesting that progression through a preparatory centroblastic stage is necessary for the activation of the switching machinery and indicating that Ig class switching and GC phenotypic maturation are tightly regulated by a common differentiation program, the activation of which may require CD40L, IL-4, IL-10, and IL-6 only.
Materials and Methods
Isolation of CL-01 cells
To identify a monoclonal B cell line that could switch and differentiate in vitro, more then 25 human neoplastic sIgM+ B cell lines were tested for their ability to switch from IgM to IgG following engagement of CD40 by human CD40L-expressing human embryonic kidney 293 cells (CD40L-293 cells) and addition of IL-10 and/or IL-4. BL16 cells, a Burkitt’s lymphoma carrying the t(8;14) translocation, express sIgM and sIgD and were found to secrete IgG after exposure to CD40L and IL-4 or IL-10. BL-16 cells were thoroughly subcloned and selected for sIgM and sIgD expression and high efficiency of class switching to IgG. Of three sIgM+sIgD+ subclones generated, one, CL-01, was used in all following experiments.
Cell cultures
CL-01 cells were cultured at a ratio of 1:2 with irradiated (4000 rad) CD40L-293 cells in RPMI 1640 (Life Technologies, Inc., Gaithersburg, MD) supplemented with 5% heat-inactivated fetal bovine serum (Life Technologies, Inc.), 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2% HEPES. IL-4 and IL-10 (Schering-Plough Corp., Kenilworth, NJ) were added to cultures at concentrations of 100 U/ml and 100 ng/ml, respectively. In selected experiments, the agonistic mouse mAb 89 to human CD40 (0.5 μg/ml) (Schering-Plough Corp.) was substituted for CD40L-293 cells. IL-2, IL-6, and IFN-γ (Genzyme Co., Cambridge, MA) were used at 100, 20, and 100 U/ml, respectively. Neutralizing mouse Abs to human IL-6, IL-10, and TGF-β (Genzyme Co.) were used at 30 μg/ml.
Ig and cytokine measurements
Supernatants were assayed for IgM, IgG, IgG1, IgG2, IgG3, IgG4, IgA, IgA1, IgA2, and IgE by specific ELISAs (19). Culture fluids were also tested for IL-6 and IL-10 by specific ELISAs performed according to the manufacturer’s instruction (Biosource International, Camarillo, CA). Active TGF-β was measured in the culture fluids using a bioassay based on the inhibition of [3H]TdR uptake by CCL64 mink lung epithelial cells (American Type Culture Collection, Rockville, MD) (20).
Fluorescence flow cytometric analysis
B cells (105) were reacted for 30 min on ice with the different Abs and then washed with PBS containing 3% BSA (21). Mouse FITC- or phycoerythrin (PE)-conjugated mAbs to the following human Ags were used: CD95 (Ancell Corp., Bayport, MN); CD10, CD23, CD38, CD44, CD56, and CD80 (Becton Dickinson Immunocytometry Systems, San Jose, CA); CD24 and CD39 (PharMingen, San Diego, CA); CD71 and bcl-2 (Dako Corp., Carpinteria, CA); and CD86 (Serotec Ltd., Washington, DC). FITC- or PE-conjugated goat Abs to human IgE (Biosource International), IgM, IgD, IgG, and IgA (Southern Biotechnology Associates Inc., Birmingham, AL) were also used. The unconjugated Ab to human CD77 was an IgM from rat (Immunotech Inc., Westbrook, ME). Indirect immunofluorescence was performed using an FITC-conjugated mouse Ab to rat IgM (PharMingen). For detection of bcl-2, CL-01 cells were permeabilized (40 min of incubation) with ORTHO Permeafix (Ortho Diagnostics Systems, Inc., Raritan, NJ). For three-color experiments, cells were first reacted with biotinylated mouse mAb to human IgD (Miltenyi Biotec, Inc., Sunnyvale, CA), PE-conjugated mouse mAb to human CD38, and unconjugated rat mAb to human CD77 and then with streptavidin-RED613 and FITC-conjugated mouse mAb to rat IgM. Cells (104) were analyzed using a FACScalibur (Becton Dickinson Immunocytometry Systems), with appropriate gating and propidium iodide staining to exclude cell debris and dead cells from the acquired data. Among the cells reacted with the experimental mAb, positive cells were defined as those that display a fluorescence intensity higher than that displayed by similar cells reacted with the isotype-matched control mAb with irrelevant binding activity.
Fluorescence microscopic analysis
To analyze cytoplasmic Igs (cIgs), CL-01 cells (104) were cytocentrifuged for 5 min at 200 rpm onto a microscope slide and then fixed in freshly prepared cold methanol for 20 min. Following rinsing with TBST buffer (10 mM Tris-HCl (pH 8), 150 mM NaCl, 0.02% Tween-20, and 0.02% NaN3) and incubation with TBST containing 3% BSA, cells were stained with 4′,6-diamidine-2′-phenylindole dihydrochloride (Boehringer Mannheim Corp., Indianapolis, IN), rhodamine-conjugated goat Ab to human IgG (ICN Pharmaceuticals, Inc., Costa Mesa, CA), and FITC-conjugated mouse mAb to human IgA (Sigma Chemical Co., St. Louis, MO). Slides were analyzed and photographed using a fluorescence microscope Zeiss Axioplan 2 (Atto Instruments, Inc., Rockville, MD).
Electron microscopic analysis
After culture with CD40L-293 cells, IL-4, IL-6, and IL-10, CL-01 cells were harvested, pelleted, and fixed in 2% glutaraldehyde-sodium cacodylate, 0.1 M HCl, pH 7.4 (15 min at 4°C). After three washes in sodium cacodylate, 0.1 M HCl, 0.2 M sucrose, pH 7.4 (15 min at 4°C), the cells were further fixed in OsO4, 1% sodium cacodylate, 0.15 M HCl, pH 7.4 (15 min at 4°C); dehydrated in a graded series of ethanols; and embedded in Epon. Ultrathin sections were prepared with an ultramicrotome and were then contrasted with methanolic uranyl acetate and lead acetate before examination with a transmission electron microscope (JEOL 100CX, JEOL USA, Inc., Peabody, MA).
Isolation of sIgD+ and sIgD− CL-01 cells after culture with CD40L, IL-4, and IL-10
At day 5 of culture, induced CL-01 cells were sequentially labeled with unconjugated mouse mAb to human IgD (Southern Biotechnology Associates, Inc.), and goat anti-mouse IgG Microbeads (Miltenyi Biotec, Inc.). Highly purified sIgD+ and sIgD− fractions were segregated using a magnetic sorter MACS (Miltenyi Biotec, Inc.). Similarly, CD77+ and CD77− cells were segregated by magnetic sorting from the sIgD− B cell population, and CD38+ and CD38− cells were segregated from the sIgD−CD77− B cell population. Unconjugated rat mAb to human CD77, mouse mAb to rat IgM, and mouse mAb to human CD38 (Becton Dickinson Immunocytometry Systems) were used in these sorting procedures.
Analysis of the human CL-01 cell CH chain gene locus configuration
Genomic DNA extracted from placenta and unstimulated CL-01 cells using the MicroTurboGen genomic DNA Purification Kit (Invitrogen Corp., San Diego, CA) was digested with BglII, HindIII, and SphI restriction enzymes; fractionated by electrophoresis on a 0.8% agarose gel; and transferred to nylon filters. Membranes were incubated overnight at 37°C in hybridization solution containing a specific radiolabeled probe. The probes for chromosomal Ig switch (S) regions, including 5′σμ, 5′σδ, 5′Sμ, 3′Sμ, 5′Sγ, 3′Sγ, 5′Sα, 3′Sα, 5′Sε, and 3′Sε, were prepared by amplification of placental DNA using appropriate oligonucleotide primer pairs (22). Blots were then washed and autoradiographed overnight at −70°C.
PCR amplification of germline and productive Ig transcripts
RNA was isolated from CL-01 cells (3 × 106) using the RNeasy Total RNA Kit (Qiagen, Inc., Chatsworth, CA) and then reverse transcribed using Moloney murine leukemia virus reverse transcriptase and an oligo(dT)12–18 primer (SuperScript Preamplification System for first strand cDNA synthesis, Life Technologies, Inc.). For the amplification of germline (IH-CH) Ig transcripts, productive (VHDJH-CH) Ig transcripts, and β-actin DNA, PCRs were performed in 50 μl using the GeneAmp PCR Reagent Kit (Perkin-Elmer/Cetus, Branchburg, NJ), and cDNA reverse-transcribed from 1 μg of RNA as template, for 30 cycles, each consisting of 1 min of denaturation at 94°C, 1 min of annealing at 68°C (52°C for Iμ-Cμ transcripts), and 1 min of extension at 72°C. To amplify IH-CH transcripts, a sense primer recognizing a given intervening (I) region was paired with an antisense primer recognizing the correspondent CH region (Table I). Due to their high degree of sequence identity, the Iγ1 and Iγ2 as well as the Iα1 and Iα2 region DNA sequences were amplified using common Iγ1/2 and Iα1/2 sense primers, respectively (8, 23). The expected sizes of IH-CH transcripts were as follows: Iμ-Cμ, 537 bp; Iγ-Cγ1, 603 bp; Iγ-Cγ2, 597 bp; Iγ3-Cγ3, 670 bp; Iγ4-Cγ4, 411 bp; Iα-Cα1, 1194 bp; Iα-Cα2, 1181 bp; and Iε-Cε, 125 bp. To amplify the VHDJH-Cμ, -Cγ1, -Cγ2, -Cγ3, -Cγ4, -Cα1, -Cα2, and -Cε productive transcripts (expected sizes, 152, 416, 416, 416, 414, 904, 891, and 179 bp, respectively) a sense primer encompassing the Ig framework (FR) 3 sequence of CL-01 cells was used in combination with one of the antisense CH primers (Table I). The expected size of β-actin cDNA was 593 bp (Table I).
Table I.
Sequences of the oligonucleotide primers used to amplify sterile Ig transcripts, productive Ig transcripts, and reciprocal DNA recombination product
| Primer Name | Sequence | GenBank Accession Number of the Related Gene Sequence |
|---|---|---|
| Sterile IH-CH transcripts and productive VHDJH-CH transcripts | ||
| Sense Iμ | 5′ GTGATTAAGGAGAAACACTTTGAT 3′ | X58529 |
| Sense Iγ1/2 | 5′ GGGCTTCCAAGCCAACAGGGCAGGACA 3′ | X17676 |
| Sense Iγ3 | 5′ AGGTGGGCAGGCTTCAGGCACCGAT 3′ | D78345 |
| Sense Iγ4 | 5′ TTGTCCAGGCCGGCAGCATCACCAGA 3′ | X56796 |
| Sense Iα1/2 | 5′ CAGCAGCCCTCTTGGCAGGCAGCCAG 3′ | L04541 |
| Sense Iε | 5′ GACGGGCCACACCATCC 3′ | L00022 |
| Sense FR3 | 5′ GACACGGCTGTGTATTACTGTGCG 3′ | M36090 |
| Antisense Cμ | 5′ CCGAATTCAGACGAGGGGGAAAAGGGTT 3′ | L23562 |
| Antisense Cγ1 | 5′ GTTTTGTCACAAGATTTGGGCTC 3′ | S76144 |
| Antisense Cγ2 | 5′ GTGGGCACTCGACACAACATTTGCG 3′ | Z49802 |
| Antisense Cγ3 | 5′ TTGTGTCACCAAGTGGGGTTTTGAGC 3′ | X99549 |
| Antisense Cγ4 | 5′ ATGGGCATGGGGGACCATTTGGA 3′ | KO1316 |
| Antisense Cα1 | 5′ GGGTGGCGGTTAGCGGGGTCTTGG 3′ | J00220 |
| Antisense Cα2 | 5′ TGTTGGCGGTTAGTGGGGTCTTGCA 3′ | S71043 |
| Antisense Cε | 5′ CGGAGGTGGCATTGGAGG 3′ | L00022 |
| Reciprocal recombination DNA productsa | ||
| Sense 5′Sγ | 5′ AAGAGTCCAGGGAGGCCCAGAAAGGCCCAG 3′ | X39737 |
| Sense 5′Iα1/2i | 5′ GCCAGCTGATACACGTCCATG 3′ | L04541 |
| Sense 5′Sε | 5′ GCTGATCTTGGCAAGTCCGAGCTGGGCGACTG 3′ | X56797 |
| Antisense 3′Sμ | 5′ TGAGTGCCCTCACTACTTGCGTCCCG 3′ | X56795 |
| Antisense 3′Sμi | 5′ CAGACTGTCATGGCTATCAGGGGTGGCGGGG 3′ | X56795 |
| Antisense 3′Sγ | 5′ CCTGCCTCCCAGTGTCCTGCATTACTTCTG 3′ | U39737 |
| Antisense 3′Sγi | 5′ CCCTGGAGTCCCACTGCAGGTG 3′ | U39737 |
| β-Actin | ||
| Sense | 5′ GTACCACTGGCATCGTGATGGACT 3′ | X00351 |
| Antisense | 5′ ATCCACACGGAGTACTTGCGCTCA 3′ | X00351 |
The external sense oligonucleotide primers specific for the Iγ, Iα, and Iε regions are reported above.
Identification of the extrachromosomal reciprocal DNA recombination products (switch circles)
A nested primer PCR strategy was devised to amplify Sγ1-Sμ, Sγ2-Sμ, Sγ3-Sμ, Sγ4-Sμ, Sα-Sμ, Sα-Sγ, Sε-Sγ, and Sε-Sμ recombination products from CL-01 cells (Fig. 1). A first round of PCR was performed in 50 μl reaction containing genomic DNA (500 ng), XL rTh DNA polymerase (1 U) (Perkin-Elmer/Cetus), 25 mM magnesium acetate, and 100 nM concentrations of the sense primer Iγ1/2, Iγ3, Iγ4, Iα1/2, or Iε coupled with an equal amount of the antisense primer 3′Sμ or 3′Sγ (Table I). The PCR conditions used were: denaturation, 1 min at 94°C; annealing, 2 min at 68°C; and extension, 6 min at 72°C for 30 cycles. A second PCR was performed on the DNA product of the first PCR under similar conditions, but using the internal sense 5′Sγ, 5′Iα1/2i, or 5′Sε and antisense 3′Sμi or 3′Sγi primer pairs (Table I). The PCR-amplified switch circles were identified using probes that were generated by PCR with specific artificial plasmids as templates and that were specific for the 5′Sγ, 5′Iα, 5′Sε, 3′Sμ, and 3′Sγ regions. The Sγ1/2-Sμ, Sγ3-Sμ, Sγ4-Sμ, Sα1/2-Sμ, Sα1/2-Sγ, Sε-Sμ, and Sε-Sγ PCR products were applied to a 0.8% agarose gel, fractionated, and transferred overnight onto nylon membranes. Amplified DNAs were first hybridized with a 5′Sγ, 5′Iα, or 5′Sε probe and then autoradiographed for 4 h. The blots were then stripped, rehybridized with the 3′Sμ or 3′Sγ probes, and reautoradiographed for 4 h.
FIGURE 1.
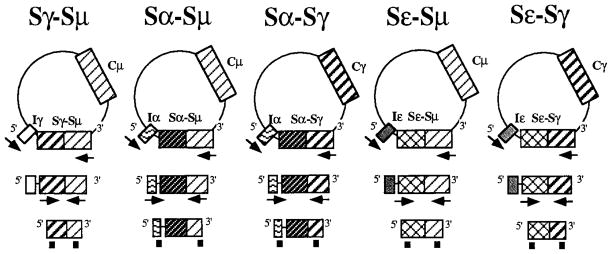
Schematic diagram depicting the strategy used to detect reciprocal DNA recombination products. The arrows indicate the relative position and the direction of the nested PCR primers used to amplify the switch junctions in deleted DNA circles. The DNA probe pairs used for the positive identification of the switch circle are depicted as black boxes below each nested PCR product. S, switch region; I, I exon; C, constant region.
The specificity of our method was verified using artificial reciprocal recombination DNA products as positive controls. The Sγ-Sμ, Sε-Sμ, and Sε-Sγ control plasmids were prepared as described (9). The Sα-Sμ and Sα-Sγ control plasmids were constructs containing a 1-kb PstI-StuI fragment from the Iα region ligated either to a 0.9-kb SstI-EcoRI fragment from the 3′ portion of Sμ or to a 1.3-kb PvuII-HindIII fragment from the 3′ portion of Sγ (9, 24).
Ig H chain region cloning and sequencing
Purified PCR products were ligated into the pCR II plasmid vector (Invitrogen Corp.). The inserts were excised from the cloning vectors by EcoRI digestion. Sequencing was performed by the dideoxynucleotide chain termination method using the TaqTrack Sequencing System (Promega Corp., Madison, WI).
Results
CL-01 cells are sIgM+sIgD+ and switch to IgG, IgA, or IgE in response to CD40L and IL-4
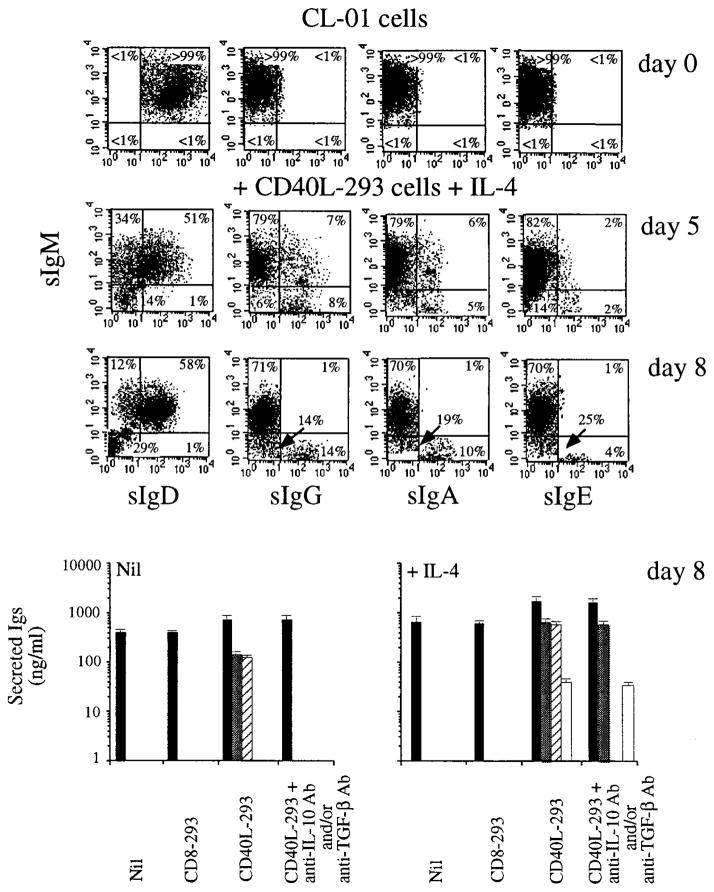
Flow cytometry analysis showed that unstimulated CL-01 B cells expressed sIgM and sIgD but lacked sIgG, sIgA, and sIgE (Fig. 2, day 0). These cells are phenotypically stable, as suggested by multiple time point flow cytometric analysis performed throughout a period of >1 year (not shown). To analyze the phenotypic changes induced by the application of physiologic differentiative stimuli, CL-01 cells were incubated alone or in the presence of CD40L-293 cells or control 293 cells expressing human CD8 (CD8-293 cells), in the presence or the absence of IL-4 or in IL-4 only. After 5 days in culture with IL-4 plus CD40L-293 cells, the fraction of sIgM+sIgD+ cells dropped from 100% to 51%, with compensating increases in cells positive for IgM only (34%) and double negative (14%); a negligible proportion of cells expressed sIgD only (Fig. 2, day 5). These changes were not observed in cells cultured with IL-4 only or IL-4 and CD8-293 cells (not shown). Approximately 15% of the total cells at day 5 expressed sIgG, 11% sIgA, and 4% sIgE. About one-half of these switched cells also expressed sIgM, likely due to the persistence of residual μ mRNA and/or protein, as suggested by the complete disappearance of such double sIg+ B cells by day 8, at which time sIgM−sIgD− cells accounted for almost one-third of all cells (Fig. 2, day 8). Virtually all these sIgM−sIgD−, but virtually none of their sIgM+sIgD− counterparts, bore sIgG, sIgA, or sIgE.
FIGURE 2.
Fluorescence flow cytometric analysis of the expression of sIgM, sIgG, sIgA, and sIgE by CL-01 cells and analysis of secreted IgM, IgG, IgA, and IgE. Dot plots depict the expression of sIgM, sIgD, sIgG, and sIgA by CL-01 cells (day 0) and by CL-01 cells cocultured with CD40L-293 cells and IL-4 (100 U/ml) for 5 or 8 days. Numbers inside quadrants are percentages of the total cells. Results are representative of three independent experiments. Histograms show the concentration of IgM (■), IgG (
 ), IgA (▨), and IgE (□) accumulated in the fluids of CL-01 cells incubated for 8 days with or without IL-4, and cultured alone, with CD8-293 cells, with CD40L-293 cells, and with CD40L-293 cells in the presence of neutralizing anti-IL-10 and/or anti-TGF-β Abs (the concentrations of IgM and IgE were from cultures with both anti-IL-10 and anti-TGF-β Abs, whereas the concentrations of IgG and IgA were from cultures with anti-IL-10 and anti-TGF-β Abs, respectively). Values are mean ± SD of four determinations from three independent experiments.
), IgA (▨), and IgE (□) accumulated in the fluids of CL-01 cells incubated for 8 days with or without IL-4, and cultured alone, with CD8-293 cells, with CD40L-293 cells, and with CD40L-293 cells in the presence of neutralizing anti-IL-10 and/or anti-TGF-β Abs (the concentrations of IgM and IgE were from cultures with both anti-IL-10 and anti-TGF-β Abs, whereas the concentrations of IgG and IgA were from cultures with anti-IL-10 and anti-TGF-β Abs, respectively). Values are mean ± SD of four determinations from three independent experiments.
To verify that the expression of sIgG, sIgA, and sIgE by induced CL-01 cells was associated with the secretion of these isotypes, fluids from day 8 cultures were analyzed for Ig content (Fig. 2, bottom histograms). The CL-01 cells produced similar amounts of IgM, regardless of the presence of CD8-293 cells or IL-4. None of these CL-01 cell cultures produced detectable amounts of IgG, IgA, or IgE. In contrast, and consistent with the results of the sIg studies, CL-01 cells cultured in the presence of CD40L-293 cells and IL-4 produced relatively large amounts of IgG, IgA, and IgE; the IgG were IgG1, IgG2, IgG3, and IgG4, and the IgA were both IgA1 and IgA2 (not shown). Significantly smaller but consistent amounts of IgG (IgG1) and IgA (IgA1), but not IgE, were produced by the CL-01 cells cultured with CD40L-293 cells in the absence of IL-4 or cultured with the agonistic mouse mAb 89 to human CD40 in the absence of IL-4 (not shown). CD40L-induced IgG and IgA production was preceded by expression of sIgG and sIgA (8% and 5% of sIgG+ and sIgA+ cells, respectively; not shown) and was ablated by the neutralization of endogenous IL-10 or TGF-β, as effected by specific mouse Abs to human IL-10 and TGF-β (30 μg/ml), respectively, but not irrelevant Abs (not shown). CL-01 cells incubated for 2 days with CD8-293 cells secreted significant amounts of IL-10 (0.2 ng/ml) and TGF-β (0.3 ng/ml), which increased in the presence of CD40L-293 cells (0.8 and 0.6 ng/ml, respectively). Thus, CD40L-induced production of IgG and IgA is partly mediated by IL-10 and TGF-βendogenously secreted by CD40-activated CL-01 cells. IL-4 dramatically enhances IgG and IgA expression and secretion, and, concomitant with CD40 engagement, it induces the expression of membrane and secreted IgE.
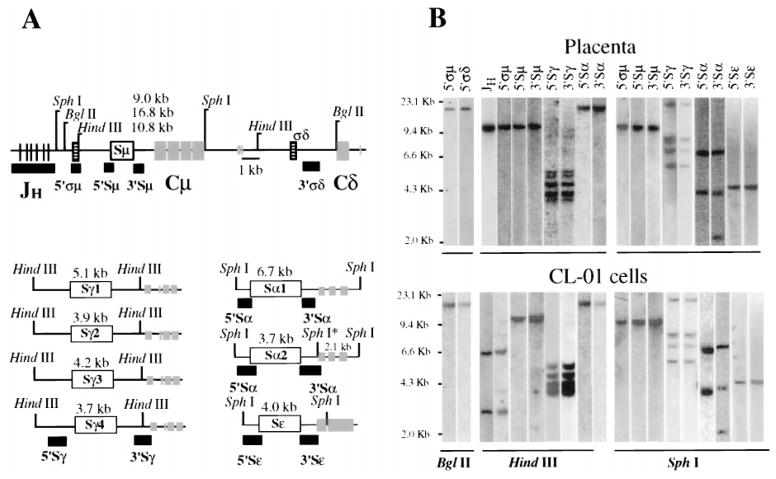
CL-01 cells are monoclonal and bear the Sμ, Sγ, Sα, and Sε DNA regions in germline configuration
The configuration of the Ig H chain locus was analyzed in unstimulated CL-01 cells using probes specific for each Ig S region (Fig. 3). Both the 5′ σμ and the JH probes identified the same HindIII fragments of rearranged DNA in CL-01, but a single germline fragment in placenta cell DNA, suggesting that CL-01 cells are monoclonal and have rearranged the Ig JH genes on both chromosomes. The VHDJH gene sequence of the productive chromosome has been fully characterized and is reported elsewhere (W. Ikematsu, P. Riboldi, R. Dalla-Favera, and P. Casali, manuscript in preparation). All remaining probes, 5′σδ, 5′ and 3′Sμ, Sγ, Sα, and Sε, cohybridized with identical restriction fragment(s) in DNA from CL-01 cells and placenta. Thus, unstimulated CL-01 cells are monoclonal and bear Ig S regions in germline configuration on both chromosomes.
FIGURE 3.
Configuration of the Ig H chain locus in CL-01 cells. A, Schematic map of the human Ig H locus with the positions of the S regions (□), σ sequences (▤), JH and CH region exons (
 and lines), JH, 5′S, 3′S, and σ probes (■), and restriction sites. The expected sizes of restriction fragments detected by each switch probe are also indicated. The asterisk indicates a SphI site that is present in the 3′ end of the Sα2 locus, but not in the Sα1 locus. B, Genomic DNA from placenta (upper gel) and unstimulated CL-01 cells (bottom gel) was digested with BglII, HindIII, and SphI restriction enzymes; subjected to electrophoresis on a 0.7% agarose gel; blotted; and probed sequentially with the various σμ, Sμ, σδ, Sγ, Sα, and Sε region probes (shown above each lane).
and lines), JH, 5′S, 3′S, and σ probes (■), and restriction sites. The expected sizes of restriction fragments detected by each switch probe are also indicated. The asterisk indicates a SphI site that is present in the 3′ end of the Sα2 locus, but not in the Sα1 locus. B, Genomic DNA from placenta (upper gel) and unstimulated CL-01 cells (bottom gel) was digested with BglII, HindIII, and SphI restriction enzymes; subjected to electrophoresis on a 0.7% agarose gel; blotted; and probed sequentially with the various σμ, Sμ, σδ, Sγ, Sα, and Sε region probes (shown above each lane).
CL-01 cells express germline IH-CH and productive VHDJH-CH γ, α, and ε transcripts in response to CD40L and IL-4
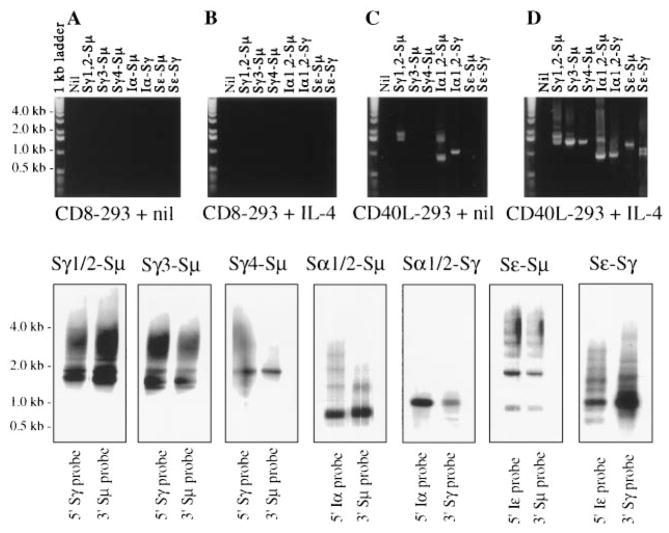
Switch recombination from μ to a downstream CH gene is preceded by the transcriptional activation of that specific CH gene in the form of an mRNA containing a noncoding exon, called I region, that lies 5′ to each CH gene (25, 26). Switching brings the assembled variable VHDJH DNA near one of the seven CH genes downstream of Cμ and Cδ and gives rise to a VHDJH-CH productive transcript. cDNAs from CL-01 cells cultured for 5 days were utilized as templates to amplify Iγ-Cγ1, Iγ-Cγ2, Iγ3-Cγ3, Iγ4-Cγ4, Iα-Cα1, Iα-Cα2, and Iε-Cε transcripts using appropriate sense and antisense primer pairs. In each case, the identity of the amplified germline Ig product was confirmed by the cDNA sequence (not shown). CL-01 cells cultured with CD8-293 cells with or without IL-4 failed to express germline transcripts (Fig. 4, A and B). A similar result was obtained when CL-01 cells were cultured alone or with IL-4 only (not shown). The engagement of CD40 by CD40L or mAb 89 (not shown) was sufficient to initiate germline Iγ-Cγ1, Iγ-Cγ2, Iγ3-Cγ3, and Iα-Cα1 transcription (Fig. 4C). The addition of IL-4 to CD40L-293 cells extended germline transcription to all isotypes to include Iγ4-Cγ4, Iα-Cα2, and Iε-Cε (Fig. 4D). Under these culture conditions, germline transcripts were detectable as early as 24 to 36 h of culture (not shown).
FIGURE 4.
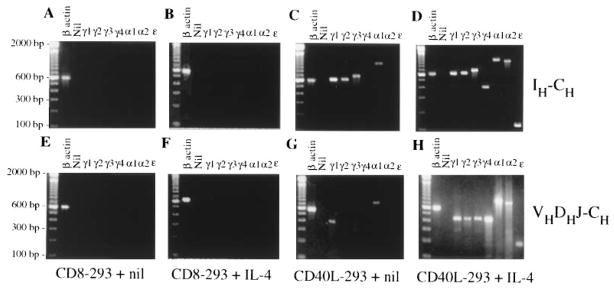
The germline IH-CH and productive VHDJH-CH Ig transcripts in induced CL-01 cells. Fractionation of the germline and mature Ig transcript cDNA PCR amplified from CL-01 cells cocultured with CD8-293 cells only (A and E), CD8-293 cells and IL-4 (B and F), CD40L-293 cells only (C and G), or CD40L-293 cells and IL-4 (D and H) for 5 days (2% ethidium bromide-stained agarose gel). Gels were obtained from one of three independent experiments yielding similar results.
As in the case of germline transcripts, CL-01 cells cultured for 5 days with CD8-293 cells in the presence or the absence of IL-4 failed to express VHDJH-CH gene products (Fig. 4, E and F). A similar result was obtained when CL-01 cells were cultured alone or with IL-4 only (not shown). Engagement of CD40 by CD40L or mAb 89 (not shown) yielded the productive VHDJH-Cγ1 and -Cα1 transcripts (Fig. 4G), but not VHDJH-Cγ2, -Cγ3, -Cγ4, -Cα2, or -Cε transcripts. VHDJH-Cγ1 and -Cα1 transcription was completely inhibited by anti-TGF-β Ab (30 μg/ml), suggesting that CD40L-induced switching to Cγ1 and Cα1 was related to endogenous TGF-β (H. Zan, A. Cerutti, A. Schaffer, and P. Casali, manuscript in preparation). Addition of IL-4 to CD40L-293 cells induced all remaining downstream productive transcripts in CL-01 cells, including VHDJH-Cγ2, -Cγ3, -Cγ4, -Cα2, and -Cε (Fig. 4H), that were all positively identified by sequencing (see below). Under these culture conditions, productive transcripts were detectable as early as day 5 of culture.
Ig class switching in CL-01 cells is associated with DNA recombination
Ig class switching occurs through a deletional DNA recombination between tandemly repeated S regions located 5′ to each CH gene (25, 26). To formally demonstrate that CL-01 cells undergo switch recombination, a nested primer PCR was used to detect the reciprocal switch junctions Sγ1-Sμ, Sγ2-Sμ, Sγ3-Sμ, Sγ4- Sμ, Sα1-Sμ, Sα2-Sμ, Sα1-Sγ, Sα2-Sγ, Sε-Sμ, and Sε-Sγ expected in circular DNAs (Fig. 1). These reciprocal switch junctions were analyzed in CL-01 cells from the same cultures (day 5) used for the identification of switch IH-CH and VHDJH-CH transcripts. Genomic DNA from CL-01 cells cultured with CD8-293 cells in the presence or the absence of IL-4 failed to yield any detectable bands in gel electrophoresis (Fig. 5, A and B), and so did genomic DNA from cells cultured in the presence or the absence of IL-4 only (not shown). Engagement of CD40 by CD40L gave rise to reciprocal Sγ1 and/or Sγ2-Sμ, Sα1 and/or Sα2-Sμ, and Sα1 and/or Sα2-Sγ recombination products of different sizes (Fig. 5C). Addition of IL-4 to CD40L gave rise also to Sγ3-Sμ, Sγ4-Sμ, Sε-Sμ, and Sε-Sγ recombination fragments (Fig. 5D). The sequential hybridization with DNA probes specific for the 5′ (I or S) and 3′ ends of each S region (Fig. 5, lower panels), and the subsequent sequencing of five independent positive DNA clones (not shown) confirmed the specificity of the amplified DNA recombination products. In contrast to the heterogeneous hybridization pattern observed in polyclonal normal B cells (8, 9, 27–29) and, perhaps, reflecting the monoclonality of our switch model, one major band or few dominant bands were obtained from each of the different switch circles.
FIGURE 5.
Identification of extrachromosomal reciprocal DNA recombination products in induced CL-01 cells. Fractionation of the Sγ1,2-Sμ, Sγ3-Sμ, Sγ4-Sμ, Sα-Sμ, Sα-Sγ, Sε-Sμ, and Sε-Sγ extrachromosomal reciprocal DNA recombination products PCR amplified from the genomic DNA of CL-01 cells cocultured with CD8-293 cells only (A), with CD8- 293 cells and IL-4 (B), with CD40L-293 cells only (C), or with CD40L-293 cells and IL-4 (D) for 5 days (0.8% ethidium bromide-stained agarose gel). Bottom panels, Southern blot analysis of the Sγ1/2-Sμ, Sγ3- Sμ, Sγ4-Sμ, Sα1/2-Sμ, Sα1/2-Sγ, Sε-Sμ, and Sε-Sγ extrachromosomal reciprocal recombination DNAs specifically amplified from CL-01 cells induced by CD40L-293 cells and IL-4, and hybridized with [γ-32P]dCTP probes as listed at the top of each panel.
Ig class switching in CL-01 cells is associated with GC-like phenotypic differentiation
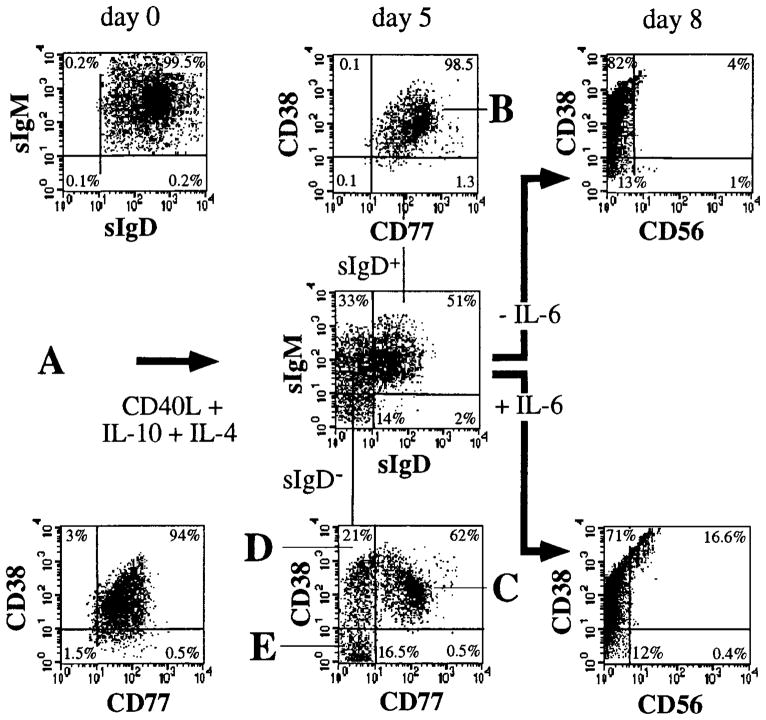
Together with CD40L, IL-10 seems to be an absolute requirement for the in vitro-induced differentiation of GC B cells to memory B cells and to plasma cells (30). To test whether Ig class switching was accompanied by differentiative changes typical of GC B cells, sIgM+sIgD+ CL-01 cells were cultured with CD40L-293 cells, IL-4, and IL-10. Tonsillar naive B cells and founder centroblasts express sIgD, which is absent in centroblasts, centrocytes, and memory B cells. Founder centroblasts and centroblasts express CD77 (globotriaosyl-ceramide), which is absent in centrocytes and memory B cells. CD38 (nicotinamide adenine dinucleotide glycohydrolase) is expressed by tonsillar founder centroblasts, centroblasts, and centrocytes; is further up-regulated in plasma cells; but is absent in memory B cells (4, 7, 31). Flow cytometric analysis of the expression of sIgM, sIgD, CD77, and CD38 on CL-01 cells allowed us to define five tentative differentiation stages (Fig. 6). Unstimulated CL-01 cells expressed sIgM, sIgD, CD77, and CD38 (sIgM+sIgD+CD77+CD38+) and were considered to be at the initial differentiation stage A, possibly equivalent to founder centroblast. After 5 days of culture, about one-third of the cells did not express sIgD, and almost one-half of these cells also did not display sIgM. The remaining B cells still expressed sIgM and sIgD, but at a density lower (sIgMlowsIgDlow) than that of most unstimulated B cells. These sIgMlowsIgDlow B cells expressed CD77 and CD38 at a density comparable with and higher than that of the stage A cells; they were assumed to have just entered the differentiation pathway and were referred to as stage B cells (sIgMlowsIgDlowCD77+CD38+). sIgD− cells, whether sIgM+ or sIgM−, were assumed to have progressed further in differentiation and were tentatively interpreted to include three sequential maturation stages corresponding to successive loss of sIgD, CD77, and CD38. Cells at stage C still expressed CD77 and CD38 (sIgMlow/−sIgD− CD77+CD38+), evocative of centroblasts; cells at stage D completely lost CD77 but were still CD38+ (sIgMlow/−sIgD− CD77− CD38+), resembling centrocytes; and finally, cells at stage E lost both CD77 and CD38 (sIgMlow/−sIgD− CD77−CD38−), resembling memory B cells. When IL-10 was substituted by IL-2 or formalinized particles of Staphylococcus aureus strain Cowan I, two potent polyclonal B cell activators, CD40-induced CL-01 cells underwent only partial GC-like phenotypic changes and did not differentiate into CD38− memory-like B cells (not shown).
FIGURE 6.
Phenotypic GC and plasmacytoid differentiation in induced CL-01 cells. The expression of sIgM, sIgD, CD38, and CD77 was analyzed by double fluorescence in unstimulated CL-01 cells, which were referred to as at stage A (sIgM+sIgD+CD77+CD38+). After 5 days of culture with CD40L-293 cells, IL-4, and IL-10, almost one-half of the CL-01 cells (center panel, cells in the upper and left quadrants; see also Fig. 1, day 5, left panel) down-regulated or lost sIgM and completely lost sIgD, a hallmark of the transition from naive to GC B cells. Similar cells at day 5 were submitted to triple fluorescence to analyze the expression of CD38 and CD77 by the sIgD+ and sIgD− CL-01 fractions, as defined by appropriate electronic gating, and were segregated in stages B (sIgMlowsIgDlowCD77+CD38+), C (sIgMlow/−sIgD−CD77+CD38+), D (sIgMlow/−sIgD−CD77−CD38+), and E (sIgMlow/−sIgD−CD77−CD38−). To verify the ability of CL-01 cells to undergo plasmacytoid differentiation, CL-01 cells stimulated with CD40L-293 cells, IL-4, and IL-10 for 5 days were cultured for additional 3 days with or without IL-6 and were analyzed for their expression of CD56 and CD38. The results depicted here were obtained from one of four experiments yielding similar results.
IgG-switched CL-01 cells differentiate to CD56+ plasmacytoid cells in the presence of IL-6
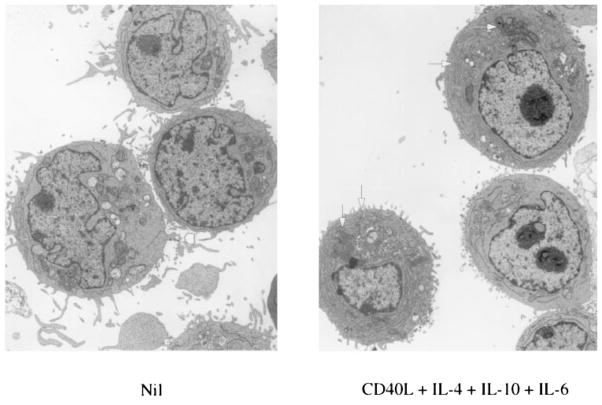
B cell plasmacytoid differentiation is promoted by IL-6 and in humans has been associated with the expression of high levels of CD38 and low levels of CD56, an adhesion molecule characteristically expressed by multiple myeloma cells and involved in homotypic cell contact (32). Because some of the CL-01 cells cultured for 5 days in the presence of CD40L-293 cells, IL-4, and IL-10 resembled normal centrocytes (stage D) and memory B cells (stage E), these cultures were tested for their potential to promote plasmacytoid differentiation in response to IL-6. More then 20% of the cells that received IL-6 at day 5, compared with 4% of those that did not, acquired CD56 and expressed CD38 at high density at day 8 of culture (Fig. 6). The induction of CD56 by IL-6 required the preincubation of CL-01 cells with CD40L-293 cells and IL-10 for at least 5 days. CD56 was not expressed by unstimulated CL-01 cells or by CL-01 cells cultured in the presence of S. aureus strain Cowan I, IL-2, IL-4, or IFN-γ (not shown). The plasmacytoid phenotype of CL-01 cells induced by CD40L, IL-4, IL-6, and IL-10 was further emphasized by significant accumulation of cIgG and cIgA. More than 20% of the CL-01 cells cultured with CD40L, IL-4, and IL-10 for 5 days and with exogenous IL-6 for additional 3 days contained a high load of IgG or IgA in their cytoplasm; <8% of their counterparts cultured in the absence of exogenous IL-6 displayed significant amounts of cIgG or cIgA (Fig. 7). Such minimal plasmacytoid differentiation was likely due to the secretion of endogenous IL-6 by the stimulated CL-01 cells, which, after 5 days, secreted as much as 0.7 ng/ml of IL-6. The biologic activity of this CL-01-derived IL-6 was completely abrogated by neutralizing anti-IL-6 Abs, which also induced a significant decrease of Ig secretion (not shown). Finally, electron microscopic analysis showed that a significant proportion of CL-01 cells stimulated by CD40L-293 cells, IL-4, IL-10, and IL-6, but virtually none of CL-01 cells cultured in medium alone, possessed ultra-structural features of plasmacytoid cells, including parallel arrays of rough endoplasmic reticulum and aggregates of mitochondrias (Fig. 8). Thus, after application of appropriate physiologic stimuli, CL-01 cells undergo GC-like phenotypic differentiation and, in the presence of IL-6, acquire plasmacytoid features.
FIGURE 7.
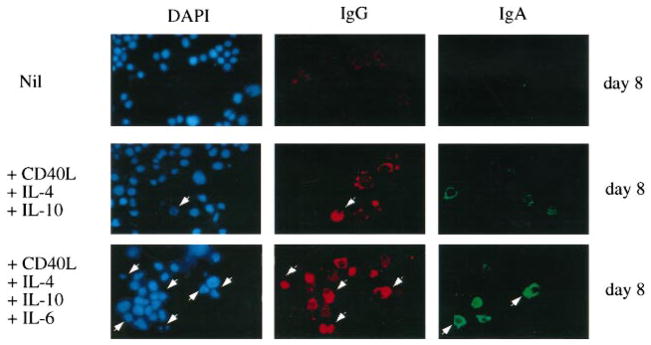
IL-6 induces plasmacytoid differentiation in IgG-switched CL-01 cells. CL-01 cells were incubated with medium only, with CD40L-293 cells, IL-4, and IL-10, and with CD40L-293 cells, IL-4, IL-10, and IL-6 for 8 days. IL-6 was added at day 5 of culture. 4′,6-Diamidine-2′-phenylindole dihydrochloride (blue), rhodamine-conjugated mouse Abs to human IgG (red), and FITC-conjugated mouse mAb to human IgA were used to visualize cellular nuclei, IgG, and IgA, respectively. Arrows indicate CL-01 cells that show accumulation of cIgG and cIgA as well as plasmacytoid morphology (×1000). Data were obtained from one of four experiments yielding similar results.
FIGURE 8.
Electron microscopic analysis of CL-01 cells cultured for 8 days in the presence or in the absence of CD40L-293 cells, IL-4, IL-10, and IL-6. Arrows indicate the presence of ultrastructural plasmacytoid features, including parallel arrays of rough endoplasmic reticulum, and aggregates of mitochondrias (×11,800).
In CL-01 cells, different Ig H chain locus transcription and recombination stages are associated with discrete phenotypic changes
To investigate the relationship of the above phenotypic differentiation stages to the transcriptional and recombinatorial status of the Ig H chain locus, germline and productive μ, γ1, γ2, γ3, γ4, α1, α2, and ε transcripts were amplified from stage A, B, C, D, and E cells that had been sorted on the basis of their surface expression of sIgD, CD77, and CD38 (Fig. 9). No germline IH-CH transcripts were detected in CL-01 cells at stages A and B. Consistent with the expression of sIgM and the lack of sIgG, sIgA, and sIgE, only VHDJH-Cμ productive transcripts could be detected in these cells. At stage C, Iμ-Cμ, Iγ-Cγ1, and Iα-Cα1 germline transcripts were detected. Extension of germline transcripts to include all isotypes occurred only at stage D, at which stage all productive transcripts were also expressed. Completion of the switch process was associated with transition to stage E, at which B cells expressed productive VHDJH-Cμ, -Cγ1, -Cγ2, -Cγ3, -Cγ4, -Cα1, -Cα2, and -Cε, but not germline transcripts. These productive transcripts were found to be identical in the FR3-complementarity-determining region (CDR) 3-FR4 area (Fig. 10), and identical with the corresponding sequence of the Ig VHDJH gene expressed by unstimulated CL-01 cells (W. Ikematsu, P. Riboldi, R. Dalla-Favera, and P. Casali, manuscript in preparation).
FIGURE 9.
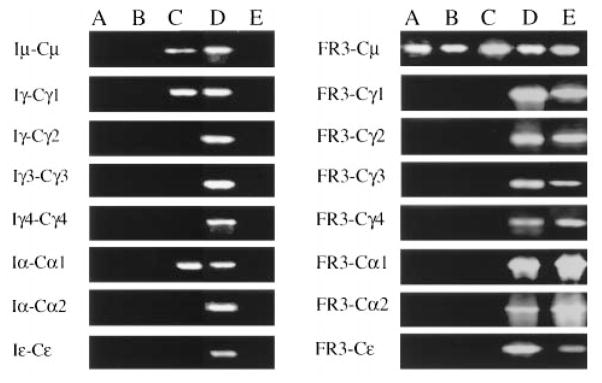
Transcriptional and recombinational status of the IgH chain locus in CL-01 cells at different stages of phenotypic differentiation. CL-01 cells were cultured with CD40L-293 cells, IL-4, and IL-10 for 5 days. Germline IH-CH and productive VHDJH-CH μ, γ1, γ2, γ3, γ4, α1, α2, and ε transcripts were amplified by PCR from cells at stages A (sIgM+sIgD+CD77+CD38+), B (sIgMlow/−sIgDlowCD77+CD38+), C (sIgMlow/−sIgD−CD77+CD38+), D (sIgMlow/−sIgD+CD77+CD38+), and E (sIgMlow/−sIgD−CD77−CD38−), as defined in Figure 6. The different cell fractions were segregated on the basis of their surface expression of sIgD, CD38, and CD77. The results depicted here were obtained from one of two independent experiments yielding similar results.
FIGURE 10.

FR3-CDR3-FR4 sequences of the Ig VHDJH-Cμ, Cγ1, -Cγ2, -Cγ3, -Cγ4, -Cα1, -Cα2, and -Cε productive transcripts from CL-01 cells cultured with CD40L-293 cells, IL-4, and IL-10 for 5 days. The top sequence of each cluster is used as the term of comparison. Dashes indicate identities. The solid line above the first cluster depicts CDR3. The full dot indicates the beginning of the CH1 exon (residue −1). The sequences of the sense FR3 and antisense Cμ and Cε primers are underlined. The antisense Cγ1, Cγ2, Cγ3, Cγ4, Cα1, and Cα2 primers encompassed the sequences spanning nucleotides −285 to −308, −283 to −308, −282 to −308, −283 to −306, −772 to −796, and −758 to −783, respectively, and are not shown.
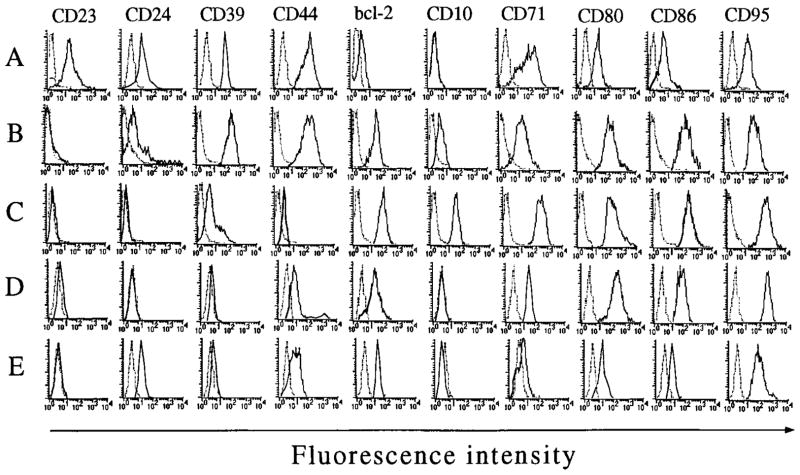
The differential Ig H transcription and switch recombination of the five CL-01 stages defined by expression of sIgM, sIgD, CD77, and CD38 also correlates with changes in expression of other surface markers. As shown in Figure 11, the “naive” cell markers CD23 (FcεRII), CD24 (heat-stable Ag), CD39 (ATP diphosphohydrolase), and CD44 (addressin) were all expressed in fraction A and dramatically down-regulated through fraction C (although CD24 and CD44 were re-expressed by fraction E). In contrast, the naive marker bcl-2 (survival molecule) and the “GC” markers CD10 (neutral endopeptidase 24.11), CD71 (transferrin R), CD80 (CD28 and CD152/CTLA-4 counter-receptor), CD86 (CD28 and CD152/CTLA-4 counter-receptor), and CD95 (Fas/APO-1) all showed their peak expression in fraction C. These changes are similar to those inferred from immunohistochemical studies on tonsillar specimens and from phenotypic studies on sorted tonsillar B cells (2–4, 7).
FIGURE 11.
Expression of naive and GC markers by CL-01 cells at stages A, B, C, D, and E. Naive (CD23, CD24, CD39, CD44, and bcl-2), and GC (CD10, CD71, CD80, CD86, and CD95) B cell markers were analyzed on cells from fractions A (sIgM+sIgD+CD77+CD38+), B (sIgMlow/−sIgDlowCD77+CD38+), C (sIgMlow/−sIgD−CD77+CD38+), D (sIgMlow/−sIgD−CD77−CD38+), and E (sIgMlow/−sIgD−CD77−CD38−), that were isolated as described in Materials and Methods. The solid histograms were obtained using the mAb under study, and the dotted histograms were obtained using the respective isotype-matched control mAb with indifferent binding activity. Histograms were obtained from one of two independent experiments yielding similar results.
Discussion
When induced by CD40L, IL-4, and IL-10, human sIgM+sIgD+ CL-01 B cells undergo a differentiation program that recapitulates, within a single clonotype, several features of normal GC B cell maturation. Induced CL-01 cells switch to all downstream isotypes via DNA recombination, as formally demonstrated by the detection of reciprocal DNA recombination products. This switching is associated with the expression of typical germline transcripts and the acquisition of a centrocytic-like phenotype. Eventually, in the presence of IL-6, switched CL-01 cells effectively differentiate to plasma cells. Thus, these studies allow a single human sIgM+sIgD+ B clonotype to be followed through sequential GC-like differentiation stages and allow these stages to be formally associated with the transcriptional and recombinatorial status of the Ig H chain locus.
Consistent with their naive sIgM+sIgD+ phenotype, CL-01 cells have recombined their JH genes on both chromosomes but retain downstream CH genes in germline configuration. After stimulation with CD40L and IL-4 for 8 days, almost one-third of CL-01 cells are negative for sIgM and sIgD and express sIgG, sIgA, or sIgE. This extent of switching is comparable with that achieved in many experiments with polyclonal cells (murine splenic B cells or human tonsillar B cells), in which failure to achieve higher switching efficiency even under optimal culture conditions could be attributed to the heterogeneity of the B cell population, such that only a certain fraction of cells were “switch competent.” In a monoclonal cell line like CL-01, in which heterogeneity is minimized, the explanation for the incomplete switching response is particularly puzzling. We have observed that CL-01 cells that remain unswitched after culture with optimal conditions of IL-4 plus CD40L are not permanently switch resistant, since if they are selected and recultured with IL-4 and anti-CD40L, a similar fraction undergoes isotype switching, as was observed in the original culture (unpublished results). We are currently exploring whether the switch-resistant cells correspond to a subpopulation that is temporarily less actively proliferating, consistent with the relatively low expression of germline Iγ1-Cγ1 transcripts observed in G0 cells vs those in G1 and S (33). By showing that CD40L and IL-4 can trigger switching to all Cγ subclasses, including Cγ2, and that these stimuli can induce not only direct μ-α but also sequential μ-γ-α switching, our findings extend those in freshly isolated human B cells (8, 28). Interestingly, in CL-01 cells, the application of switch-promoting stimuli induces not only Iγ-Cγ, Iα-Cα, or Iε-Cε transcripts but also Iμ-Cμ transcripts. Iμ-Cμ transcripts were reported to be constitutively expressed in murine B cell lines that spontaneously switch to IgG2b (34) and may be necessary for switch recombination.
In the present human B cell differentiation model, IL-4 was not sufficient to trigger Ig class switching, as suggested by the failure of this cytokine to induce any germline Ig H chain gene transcripts in the absence of CD40L. In an earlier study, IL-4 induced germline Iγ-Cγ transcripts in tonsillar sIgD+ B cells (8), but these were observed at higher IL-4 dose (200 U/ml) than we used and, in agreement with our data, were not followed by the appearance of mature VHDJH-Cγ transcripts or Sγ-Sμ switch circles. Our findings further support the notion that Ig class switching is a highly CD40-dependent process. They show that both IL-4 and CD40 engagement are necessary to trigger switching to IgG2, IgG3, IgG4, IgA2, and IgE. CD40 engagement can induce CL-01 cells to switch to Cγ1 and Cα1, but this switching is dependent on endogenously secreted IL-10 and TGF-β1, as suggested by the detection of these cytokines in the culture fluids of CD40-activated CL-01 cells and by the ablation of IgG1 and IgA1 secretion in the presence of neutralizing anti-IL-10 or anti-TGF-β Abs, respectively (Fig. 2). These results extend earlier work showing that CD40-mediated Iα-Cα germline transcription could be abolished by neutralizing endogenous TGF-β (23) and that IL-10 and TGF-β are produced by normal activated B lymphocytes (35, 36). It is unlikely that CD40L-293 cells expressed cytokines or membrane-bound molecules that are critical for the responses that we observed in CL-01 cells, since similar responses were induced by the agonistic anti-CD40 mAb 89. While the levels of endogenous IL-10 and TGF-β induced by CD40-activated CL-01 cells are sufficient to induce class switching to IgG1 and IgA1, respectively (Fig. 4G), higher levels of these cytokines may be required to induce switching to other isotypes, including IgG3 and IgA2 (23, 37). Higher IL-10 and/or TGF-β levels may be achievable only through further activation of B cells by T cell-dependent stimuli, such as IL-4 (Figs. 2 and 4, D and H). In addition, this cytokine could be directly responsible for inducing switching to certain IgG subclasses, as suggested by the failure of anti-IL-10 and anti-TGF-β Abs to inhibit the switching to IgG of CD40-activated CL-01 cells cultured in the presence of CD40L and IL-4 (Fig. 2). IL-4 also constitutes an absolute requirement in the CD40L-triggered switching to IgG4 and IgE (Figs. 2 and 4, D and H).
Since Ig class switching is part of the differentiative program of B cells in the GC, we investigated whether the stimuli that induce switch recombination in CL-01 cells can simultaneously trigger those phenotypic changes that are characteristic of tonsillar GC B cells. Unstimulated CL-01 cells (stage A) express not only sIgM and sIgD but also a cohort of other naive B cell markers, including CD23, CD24, CD39, CD44, and bcl-2, as well as GC markers, including CD38, CD71, CD77, CD80, CD86, and CD95, suggesting that these cells represent the equivalent of a founder centroblast (31, 38). The combination of CD40L, IL-4, and IL-10 induces CL-01 cells to progress throughout stages B, C, D, and E of a differentiation pathway that would approximate the stages of early centroblast, centroblast, centrocyte, and memory B cell, respectively. The differentiation of CL-01 cells is associated with phenotypic changes in addition to those of sIgM, sIgD, CD77, and CD38 that were used to define the above maturation stages. The transition of CL-01 cells from founder centroblast (stage A) to early centroblast (stage B) is characterized not only by the downregulation of sIgM and sIgD but also by the loss of CD23 and the up-regulation of CD10, CD80, CD86, and CD95. The acquisition of a full centroblastic phenotype (stage C) is distinguished by the complete loss of sIgD, partial or complete loss of sIgM, down-regulation of CD24, CD39, and CD44, and up-regulation of bcl-2, CD10, CD71, CD77, and CD95. These phenotypic changes overlap with those that are thought to be associated with the progression of a B cell through a full centroblastic stage, as suggested by the analysis of tonsillar B cells (7, 10, 39). bcl-2 up-regulation of CL-01 cells at stages B and C is at variance with the scarcity of this marker in histochemical analysis of tonsillar GC B cells (40) but is consistent with the findings of other in vitro studies (41, 42). The maturation of CL-01 cells from centroblast to centrocyte (stage D) is primarly characterized by CD77 loss, which has been hypothesized to be a hallmark of the centroblastic to centrocytic transition in tonsillar B cells (2) and by the complete loss of CD10. Finally, the putative differentiation of centrocytic-like CL-01 cells (stage D) to memory B cells (stage E) is associated with the loss of CD38. Complete lack of CD38 in tonsillar sIgD−CD77− lymphocytes has been suggested to be characteristic of memory B cells (7). In conclusion, when triggered by switch-inducing stimuli, sIgM+sIgD+ CL-01 cells progress through centroblastic, centrocytic, and memory- like B cell differentiation stages.
That CL-01 cells differentiate effectively in response to physiologic stimuli is further emphasized by their ability to acquire a plasmacytoid phenotype in the presence of IL-6 after switching to IgG. A recent report pointed out the crucial role of IL-6 in inducing terminal plasmacytoid differentiation of sIgG+ B cells (43). Our findings on the progression of a single sIgD+ B clonotype through plasma cell in the presence of switch-inducing stimuli strengthen previous studies on heterogeneous sIgD+ GC B cells (30). A small proportion of CL-01 cells (~4%) expressed high amounts of cIgG in response to CD40L, IL-4, and IL-10, possibly as a result of plasmacytoid differentiation induced by endogenous IL-6 secretion. This proportion significantly increases after exposure to exogenous IL-6, which induces switched CL-01 cells to further up-regulate CD38 and to express CD56, an adhesion molecule involved in homotypic cell-to-cell contacts. The expression of CD38 at high density is a phenotypic hallmark of normal plasma cells (4). The expression of CD56 has never been investigated in human B cells induced to differentiate in vitro but is frequent in normal bone marrow plasma cells and in myeloma cells (32). Our model suggests that CD56 is expressed by switched B cells after exposure to IL-6. Whether CD56:CD56-mediated B-to-B cell contacts occur and play a role in the late phases of plasmacytoid differentiation remains to be established.
In normal B cells, knowledge of the relation of the phenotypic changes to the Ig H chain locus transcriptional and recombinational status has been limited by their polyclonality, their phenotypic heterogeneity, their modest viability, and the uncertain status with respect to their in vivo “pre-priming” by CD40L and cytokines. In spite of these limitations, Liu et al. (3) selectively amplified germline Ig transcripts in sorted tonsillar centrocytes (Bm4). Nevertheless, it is unknown whether in vitro-induced Ig class switching selectively occurs at a specific B cell differentiation stage. In CL-01 cells, PCR amplification showed that germline transcription started at the centroblastic stage C, with Iμ-Cμ, Iγ-Cγ1, and Iα-Cα1; continued through the centrocytic stage D, with the addition of Iγ-Cγ2, Iγ3-Cγ3, Iγ4-Cγ4, Iα-Cα2, and Iε-Cε; and extinguished at the memory-like stage E. The presence of VHDJH-Cμ transcripts at stages A throughout E was consistent with the presence of a significant proportion of unswitched IgM+ B cells in all the corresponding CL-01 fractions. At stage D, both germline and productive downstream transcripts were observed. At stage E, germline transcription was absent, but mature transcription of all isotypes still continued, strongly suggesting that stage E CL-01 cells represent the equivalent of memory B lymphocytes. Thus, our studies suggest that in vitro-induced switch recombination begins in centroblasts (stage C), it extends to all isotypes in centrocytes (stage D), and it is extinct in memory-like B cells (stage E), as indicated by the absence of germline transcripts in sIgD−CD77−CD38− CL-01 cells. These data indicate that in human B cells, Ig class switching and GC phenotypic differentiation likely obey a tightly regulated program that is activated by CD40L, IL-4, and IL-10.
In conclusion, following engagement of CD40 by CD40L and exposure to appropriate cytokines, human monoclonal sIgM+sIgD+ CL-01 cells undertake an extensive maturation program that includes Ig class switching to all seven downstream isotypes, progression through GC stages, and differentiation to memory-like B cells and plasma cells. Our studies allow also formal association of specific GC phenotypic stages with the transcriptional and recombinatorial status of the Ig H chain locus and suggest that founder centroblasts are competent to undergo complete GC differentiation with no more stimuli than CD40L, IL-4, IL-6, and IL-10. The more advanced differentiation stage of CL-01 cells compared with naive B cells may explain their ability to undergo both GC and plasmacytoid phenotypic differentiation in the absence of sIg engagement. sIg engagement, however, is necessary for the induction of Ig gene hypermutation in CL-01 cells, as suggested by our unpublished experiments, and consistent with that shown in mouse B cells (44). Engagement of sIg induces CL-01 cells to undergo somatic hypermutation of the expressed VHDJH gene sequences in both primary and switched isotypes. Because of their monoclonality and their ability to undergo a complete differentiation program in response to physiologic stimuli, CL-01 cells should be useful for studying the mechanisms and regulatory elements that underlie the last stages of human B cell maturation.
Acknowledgments
We are grateful to Dr. R. Dalla-Favera (College of Physicians and Surgeons, Columbia University, New York, NY) for providing us with more than a dozen human neoplastic B cell lines, including the progenitors of CL-01 cells; to Dr. S. Lederman (College of Physicians and Surgeons, Columbia University, New York, NY) for providing us with CD8- and CD40L-transfected human embryonic kidney 293 cells; to Dr. P. Crow (Hospital for Special Surgery, New York Hospital, New York, NY) and Dr. T. A. McCaffrey (Department of Internal Medicine, Cornell University Medical College, New York, NY) for helping us with the measurements of IL-6, IL-10, and TGF-β; and to Ms. L. Friedman (Department of Pathology, Cornell University Medical College, New York, NY) for her expert help with electon microscopy. We thank Dr. S. Narula (Schering-Plough Research Institute, Kenilworth, NJ) and Dr. J. Banchereau (Schering-Plough Laboratory for Immunologic Research) for providing us with recombinant IL-4, recombinant IL-10, and mouse mAb 89 to human CD40. We are also grateful to Dr. F. W. Alt (Howard Hughes Medical Institute, Children’s Hospital, Boston, MA) and to Dr. J. Stavnezer (University of Massachusetts Medical School, Worchester, MA) for helpful discussions on Ig class switching. Finally, we thank Mr. A. Francis, Ms. N. Pacheco, and Ms. S. Shah for their skillful technical assistance.
Footnotes
This work was supported by United States Public Health Service Grants AR 40908 and CA 68541.
Abbreviations used in this paper: s, surface as in sIg; GC, germinal center; CD40L, CD40 ligand; CD40L-293 cells, CD40L-transfected human embryonic kidney 293 cells; PE, phycoerythrin; c, cytoplasmic as in cIg; S, switch region; IH-CH, germline Ig transcripts; VHDJH-CH, productive Ig transcripts; I, intervening region; FR, framework region; CD8-293 cells, CD8-transfected human embryonic kidney 293 cells; CDR, complementarity-determining region.
References
- 1.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 2.Pascual V, Liu YJ, Magalski A, de Boutellier O, Banchereau J, Capra JD. Analysis of somatic mutation in five B cell subsets of human tonsils. J Exp Med. 1994;180:329. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu YL, Malisan F, de Bouteiller O, Guret C, Lebecque S, Banchereau J, Mills FC, Max EE, Martinez-Valdez H. Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity. 1996;4:241. doi: 10.1016/s1074-7613(00)80432-x. [DOI] [PubMed] [Google Scholar]
- 4.Merville P, Déchanet J, Desmoulière A, Durand I, de Boutellier O, Garrone P, Banchereau J, Liu YJ. Bcl-2+ tonsillar plasma cells are rescued from apoptosis by bone marrow fibroblasts. J Exp Med. 1996;183:227. doi: 10.1084/jem.183.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Punnonen J, Aversa G, Cocks BG, McKenzie AN, Menon S, Zurawski G, de Waal Malefyt R, de Vries JE. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jumper MD, Splawski JB, Lipsky PE, Meek K. Ligation of CD40 induces germline transcripts of multiple Ig H chain isotypes in human B cells. J Immunol. 1994;152:438. [PubMed] [Google Scholar]
- 7.Liu YJ, Barthélémy C, de Boutellier O, Arpin C, Durand I, Banchereau J. Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid up-regulation of B7-1 and B7-2. Immunity. 1995;2:239. doi: 10.1016/1074-7613(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 8.Fujieda S, Zhang K, Saxon A. IL-4 plus CD40 monoclonal antibody induces human B cells gamma subclass-specific isotype switch: switching to γ1, γ3 and γ4, but not γ2. J Immunol. 1995;155:2318. [PubMed] [Google Scholar]
- 9.Malisan F, Brière F, Bridon JM, Harindranath N, Mills FC, Max EE, Banchereau J, Martinez-Valdez H. Interleukin-10 induces immunoglobulin G isotype switch recombination in human CD40-activated naive B lymphocytes. J Exp Med. 1996;183:937. doi: 10.1084/jem.183.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galibert L, Burdin N, de Saint-Vis B, Garrone P, Van Kooten C, Banchereau J, Rousset F. CD40 and B cell antigen receptor dual triggering of resting B lymphocytes turns on a partial germinal center phenotype. J Exp Med. 1996;183:77. doi: 10.1084/jem.183.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stavnezer J, Sirlin S, Abbott J. Induction of immunoglobulin isotype switching in cultured I.29 B lymphoma cells: characterization of the accompanying rearrangements of heavy chain genes. J Exp Med. 1985;161:577. doi: 10.1084/jem.161.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delphin S, Stavnezer J. Characterization of an interleukin 4 (IL-4) responsive region in the immunoglobulin heavy chain germline ε promoter: regulation by NF-IL-4, a C/EBP family member and NF-κB/p50. J Exp Med. 1995;181:181. doi: 10.1084/jem.181.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burrows PD, Beck-Engeser GB, Wabl MR. Immunoglobulin heavy-chain class switching in a pre-B cell line is accompanied by DNA rearrangement. Nature. 1983;306:243. doi: 10.1038/306243a0. [DOI] [PubMed] [Google Scholar]
- 14.Lutzker S, Rothman P, Pollock R, Coffman R, Alt FW. Mitogen- and IL-4-regulated expression of germ-line Ig γ2b transcripts: evidence for directed heavy chain class switching. Cell. 1988;53:177. doi: 10.1016/0092-8674(88)90379-0. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura M, Kondo S, Sugai M, Nazarea M, Imamura S, Honjo T. High frequency class switching of an IgM+ B lymphoma clone CH12F3 to IgA+ cells. Int Immunol. 1996;8:193. doi: 10.1093/intimm/8.2.193. [DOI] [PubMed] [Google Scholar]
- 16.Spira G, Gregor P, Aguila HL, Scharff MD. Clonal variants of hybridoma cells that switch isotype at a high frequency. Proc Natl Acad Sci USA. 1994;91:3423. doi: 10.1073/pnas.91.8.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sideras P, Mizuta TR, Kanamori H, Suzuki N, Okamoto M, Kuze K, Ohno H, Doi S, Fukuhara S, Hassan MS, Hammarstrom L, Smith E, Shimuzu A, Honjo T. Production of germline transcripts of Cγ genes in an IgM-producing human neoplastic B cell line that switches to IgG-producing cells. Int Immunol. 1989;1:631. doi: 10.1093/intimm/1.6.631. [DOI] [PubMed] [Google Scholar]
- 18.Lederman S, Yellin MJ, Cleary AM, Pernis A, Inghirami G, Cohn LE, Covey LR, Lee JJ, Rothman P, Chess L. T-BAM/CD40-L on helper T lymphocytes augments lymphokine-induced B cell Ig isotype switch recombination and rescues B cells from programmed cell death. J Immunol. 1994;152:2163. [PubMed] [Google Scholar]
- 19.Ichiyoshi Y, Casali P. Analysis of the structural correlates for antibody polyreactivity by multiple reassortments of chimeric human immunoglobulin heavy and light chain variable segments. J Exp Med. 1994;180:885. doi: 10.1084/jem.180.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCaffrey TI, Consigli S, Du B, Falcone DJ, Sanborn TA, Spokojny AM, Bush HL., Jr Decreased type II/type I TGF-β receptor ratio in cells derived from human atherosclerotic lesions: conversion from an antiproliferative to profibrotic response to TGF-β1. J Clin Invest. 1995;96:2667. doi: 10.1172/JCI118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasaian MT, Ikematsu H, Casali P. Identification and analysis of a novel human surface CD5− B lymphocyte subset producing natural antibodies. J Immunol. 1992;148:2690. [PMC free article] [PubMed] [Google Scholar]
- 22.Bergsagel PL, Chesi M, Nardini E, Brents LA, Kirby SL, Kuehl WM. Promiscuous translocations into immunoglobulin heavy chain switch regions in multiple myeloma. Proc Natl Acad Sci USA. 1996;93:13931. doi: 10.1073/pnas.93.24.13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitani A, Strober W. Differential regulation of Cα1 and Cα2 germ-line and mature mRNA transcripts in human peripheral blood B cells. J Immunol. 1994;153:1466. [PubMed] [Google Scholar]
- 24.Nilsson L, Islam KB, Olafsson O, Zalcberg I, Samakovlis C, Hammarstrom L, Smith CIE, Sideras P. Structure of TGF-β1- induced human immunoglobulin Cα1 and Cα2 germ-line transcripts. Int Immunol. 1991;5:1107. doi: 10.1093/intimm/3.11.1107. [DOI] [PubMed] [Google Scholar]
- 25.Stavnezer J. Antibody class switching. Adv Immunol. 1996;61:79. doi: 10.1016/s0065-2776(08)60866-4. [DOI] [PubMed] [Google Scholar]
- 26.Snapper CM, Marcu KB, Zelazowski P. The immunoglobulin class switch: beyond “accessibility”. Immunity. 1997;6:217. doi: 10.1016/s1074-7613(00)80324-6. [DOI] [PubMed] [Google Scholar]
- 27.Mills FC, Thyphronytis F, Finkelman F, Max EE. Ig μ-ε isotype switch in IL-4-treated human B lymphoblastoid cells: evidence for a sequential switching. J Immunol. 1992;149:1075. [PubMed] [Google Scholar]
- 28.Mills FC, Mitchell MP, Harindranath N, Max EE. Human Ig Sγ regions and their participation in sequential switching to IgE. J Immunol. 1995;155:3021. [PubMed] [Google Scholar]
- 29.Fujieda S, Waschek JA, Zhang K, Saxon A. Vasoactive intestinal peptide induces Sα/Sμ switch circular DNA in human B cells. J Clin Invest. 1996;98:1527. doi: 10.1172/JCI118944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arpin C, Déchanet J, Van Kooten C, Merville P, Grouard G, Brière F, Banchereau J, Liu YJ. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- 31.Billian G, Bella C, Mondière C, Defrance T. Identification of a tonsil IgD+ B cell subset with phenotypical and functional characteristics of germinal center B cells. Eur J Immunol. 1996;26:1712. doi: 10.1002/eji.1830260808. [DOI] [PubMed] [Google Scholar]
- 32.Pellat-Deceunynck C, Bataille R, Robillard N, Harousseau JL, Rapp MJ, Juge-Morinau N, Wjidenes J, Amiot M. Expression of CD28 and CD40 in human myeloma cells: a comparative study with normal plasma cells. Blood. 1994;84:2597. [PubMed] [Google Scholar]
- 33.Lundgren M, Strom L, Bergqvist LO, Skog S, Heiden T, Stavnezer T, Severinson E. Cell cycle regulation of germline immunoglobulin transcription: potential role of Ets family members. Eur J Immunol. 1995;25:2042. doi: 10.1002/eji.1830250736. [DOI] [PubMed] [Google Scholar]
- 34.Li SC, Rothman PB, Zhang J, Chan C, Hirsh D, Alt FW. Expression of Iμ-Cγ hybrid germline transcripts subsequent to immunoglobulin heavy chain class switching. Int Immunol. 1994;6:491. doi: 10.1093/intimm/6.4.491. [DOI] [PubMed] [Google Scholar]
- 35.Matthes T, Werner-Favre C, Tang H, Zhang X, Kindler V, Zubler RH. Cytokine mRNA expression during an in vitro response of human B lymphocytes: kinetics of B cell tumor necrosis factor alpha, interleukin (IL)-6, IL-10, and transforming growth factor beta 1 mRNAs. J Exp Med. 1993;178:521. doi: 10.1084/jem.178.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burdin N, Van Kooten C, Galibert L, Abrams JS, Wijdenes J, Banchereau J, Rousset F. Endogenous IL-6 and IL-10 contribute to the differentiation of CD40-activated human B lymphocytes. J Immunol. 1995;154:2533. [PubMed] [Google Scholar]
- 37.Brière F, Servet-Delprat C, Bridon JM, Saint-Remy JM, Banchereau J. Human IL-10 induces naive surface IgD+ B cells to secrete IgG1 and IgG3. J Exp Med. 1994;179:757. doi: 10.1084/jem.179.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu JL, Arpin C, de Bouteiller O, Guret C, Banchereau J, Martinez-Valdez H, Lebecque S. Sequential triggering of apoptosis, somatic mutation, and isotype switching during germinal center development. Semin Immunol. 1996;8:169. doi: 10.1006/smim.1996.0021. [DOI] [PubMed] [Google Scholar]
- 39.Kremmidiotis G, Zola H. Changes in CD44 expression during B cell differentiation in the human tonsil. Cell Immunol. 1995;161:147. doi: 10.1006/cimm.1995.1021. [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Valdez H, Guret C, de Bouteiller O, Fugier I, Banchereau J, Liu YJ. Human germinal center B cells express the apoptosis-inducing genes Fas, c-myc, P53, and Bax but not the survival gene bcl-2. J Exp Med. 1996;183:971. doi: 10.1084/jem.183.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lagresle C, Bella C, Daniel PT, Krammer PH, Defrance T. Regulation of germinal center B cell differentiation: role of the human APO-1/Fas (CD95) molecule. J Immunol. 1995;154:5746. [PubMed] [Google Scholar]
- 42.Choe J, Kim HS, Zhang X, Armitage RJ, Choi YS. Cellular and molecular factors that regulate the differentiation and apoptosis of germinal center B cells. Anti-Ig down-regulates Fas expression on CD40 ligand-stimulated germinal center B cells and inhibits Fas-mediated apoptosis. J Immunol. 1996;157:1006. [PubMed] [Google Scholar]
- 43.Morse L, Chen D, Gray D, Franklin D, Xiong Y, Chen-Kiang S. Induction of cell cycle arrest and B cell terminal differentiation by CDK inhibitor p18INK4c and IL-6. Immunity. 1997;6:47. doi: 10.1016/s1074-7613(00)80241-1. [DOI] [PubMed] [Google Scholar]
- 44.Kälberg E, Jainandunsing S, Gray D, Leanderson T. Somatic mutation of immunoglobulin V genes in vitro. Science. 1996;271:1285. doi: 10.1126/science.271.5253.1285. [DOI] [PubMed] [Google Scholar]