Abstract
DNA barcoding has been an effective tool for species identification in several animal groups. Here, we used DNA barcoding to discriminate between 47 morphologically distinct species of Brazilian sand flies. DNA barcodes correctly identified approximately 90% of the sampled taxa (42 morphologically distinct species) using clustering based on neighbor-joining distance, of which four species showed comparatively higher maximum values of divergence (range 4.23–19.04%), indicating cryptic diversity. The DNA barcodes also corroborated the resurrection of two species within the shannoni complex and provided an efficient tool to differentiate between morphologically indistinguishable females of closely related species. Taken together, our results validate the effectiveness of DNA barcoding for species identification and the discovery of cryptic diversity in sand flies from Brazil.
Introduction
Sand flies (Diptera, Psychodidae, Phlebotominae) are small insects that are the main vectors of Leishmania Ross parasites, the etiologic agents of the leishmaniases. These insects are also vectors of other pathogens, including Bartonella Strong et al. and Phleboviruses [1]. Currently, there are over 520 species of sand flies recorded for the Neotropical region, with higher species richness in Brazil [2,3]. This high species richness is associated with the diversity of biomes in Brazil, which range from rainforests such as the Amazon and the Atlantic forests, to dry areas of cerrado and caatinga. Each biome has a different biogeographic history, created by events that shaped the landscape and molded the geographical distribution of animals, including sand flies and their amphibian, reptilian, avian, and mammalian hosts [4,5,6,7,8]. Vicariance, a historical event characterized by the geographical separation and isolation of a subpopulation, for example, can lead to speciation due reduced gene flow between subpopulations of the same species [9,10]. As a result of these geographical barriers, subpopulations begin to evolve independently and to accumulate genetic differences that can lead to speciation. In the event of speciation, a subpopulation loses the ability to mate successfully with other subpopulations, even if the geographical barrier is later removed. Over time, distinct morphological characters can also evolve; however, morphological differentiation tends to take longer because changes in morphological traits require changes in multiple genes [10,11]. Morphological differences among species have been the basis of taxonomy and classification for several groups of animals, including phlebotomine sand flies [2,12,13,14,15].
Currently, there are two classification systems for American sand flies. The first classification divided the New World sand fly species into three genera: Warileya Hertig, Brumptomyia França & Parrot, and Lutzomyia França. Lutzomyia was subdivided further into several subgenera and species groups [12,13]. The second classification also recognized the genera Warileya, Brumptomyia and Lutzomyia, but elevated several subgenera and species groups within the Lutzomyia genus sensu [12,13] to the generic rank [2,16]. This latter classification is preferred among Brazilian taxonomists because it provides a well-supported hypothesis about the evolutionary relationships among the species and genera within Phlebotominae [17,18,19,20]. Despite the additional generic ranking for some species, both sand fly classifications are based only on morphological characters. This method of morphology-based identification is limited by the inability to recognize different species that may be components of a cryptic series complex, that is, subpopulations in the early stages of speciation that have not yet evolved morphological differences. For example, the morphology of females from closely related species of sand flies belonging to Brumptomyia, Evandromyia Mangabeira, Pressatia Mangabeira, and Trichophoromyia Barretto are too similar to allow species discrimination based on morphological characteristics [21,22,23,24,25,26]. Additionally, morphology-based identifications may lead to the inappropriate grouping of subpopulations into different species because of phenotypic variation. There are several examples of intraspecific phenotypic variation among sand flies [27,28,29,30], which may prevent accurate identification of different sand fly species [31,32] Nevertheless, correct species identification is critical, particularly for species of epidemiological importance [33,34]. Therefore, the use of reliable tools for species identification to support morphology-based identification systems must be encouraged. One promising approach to the diagnosis of biological diversity is DNA barcoding, which uses a standardized fragment of DNA that is compared to a database of known sequences [35,36]. By using a 658 bp fragment from the mitochondrial gene, cytochrome c oxidase subunit I (COI), this method has facilitated the identification and discovery of new species among many taxa of insects, including butterflies, black flies and mosquitoes [33,37,38]. Previous studies have successfully utilized DNA barcoding to identify cryptic species of sand flies [39] and to validate species that were previously identified based on morphology [34,40]; however, DNA barcoding has never been applied to the identification of Brazilian sand flies. Because of high sand fly species richness in the Neotropical region, and lack of distinguishing morphological characters among females of some closely related species, we sought to test the utility of the DNA barcode approach in species identification of phlebotomine sand flies in Brazil.
Materials and Methods
Sand fly Collection and Morphological Identification
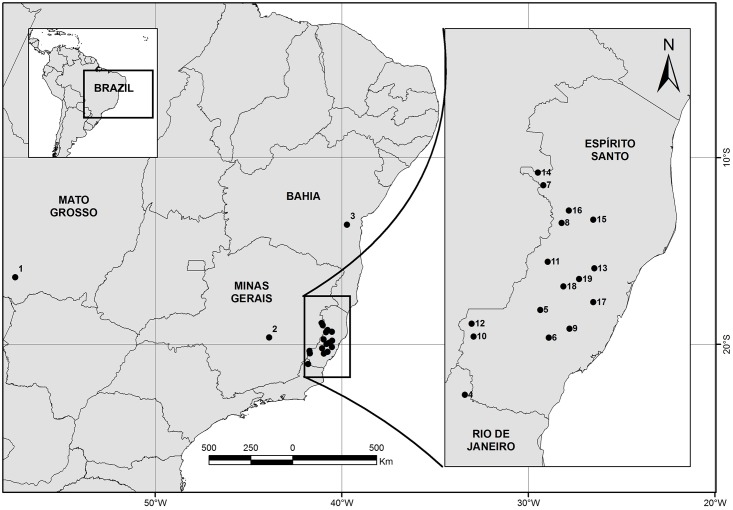
All the sand flies collections were performed using a permanent license to collect zoological material (process number: 32102) provide by the Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA). The collections performed in private areas also received a verbal permission to conduct the study on the site by the land owner. Sand flies were collected between 2011 and 2013 from 19 municipalities distributed across five Brazilian states: State of Mato Grosso: 1) Cáceres (16°24’08” S; 57°29’55” W; 286 meters at the sea level—a.s.l); State of Minas Gerais: 2) Lagoa Santa (10°45’48” S; 48°06’32” W; 740 m a.s.l.); State of Bahia: 3) Wenceslau Guimarães (13°35’04” S; 39°42’32” W; 455 m a.s.l); State of Rio de Janeiro: 4) Bom Jesus do Itabapoana (21°03’25” S; 41°47’31” W; 500 m a.s.l.); State of Espírito Santo: 5) Afonso Cláudio (20°12’53” S; 41°02’31” W; 1030 m a.s.l.), 6) Alfredo Chaves (20°29’25” S; 40°57’28” W; 1069 m a.s.l.), 7) Alto Rio Novo (18°58’35” S; 41°00’43” W; 762 m a.s.l.), 8) Baixo Guandu (19°21’04” S; 40°49’48” W; 719 m a.s.l.), 9) Domingos Martins (20°24’01” S; 40°45’11” W; 673 m a.s.l.), 10) Ibitirama (20°28’41” S; 40°42’19” W; 842 m a.s.l.), 11) Itaguaçu (19°44’13” S; 40°58’09” W; 871 m a.s.l.), 12) Iúna (20°21’02” S; 41°43’27 W; 851 m a.s.l.), 13) João Neiva (19°48’07” S; 40°30’23” W; 632 m a.s.l.), 14) Mantenópolis (18°51’09” S; 41°03’59” W; 661 m a.s.l.), 15) Marilândia (19°19’04” S; 40°31’01” W; 581 m a.s.l.), 16) Pancas (19°13’44” S; 40°45’31” W; 133 m a.s.l.), 17) Santa Leopoldina (20°08’16” S; 40°30’57 W; 51 m a.s.l.), 18) Santa Maria de Jetibá (19°58’54” S; 40°48’46” W; 844 m a.s.l.), 19) Santa Teresa (19°54’30” S; 40°39’25” W; 754 m a.s.l.). A map with these localities is shown in Fig 1.
Fig 1. Map showing the sampling sites across the Brazilian territory.
(1) Cáceres. (2) Lagoa Santa. (3) Wenceslau Guimarães. (4) Bom Jesus do Itabapoana. (5) Afonso Cláudio. (6) Alfredo Chaves. (7) Alto Rio Novo. (8) Baixo Guandu. (9) Domingos Martins. (10) Ibitirama. (11) Itaguaçu. (12) Iúna. (13) João Neiva. (14) Manenópolis. (15) Marilândia. (16) Pancas. (17) Santa Leopoldina. (18) Santa Maria de Jetibá. (19) Santa Teresa.
The sand flies were captured using light traps (HP model) [41] placed within the peridomiciliary environment (represented by the domiciles and their annexes, and domestic animal shelters) and/or forest environment from 18:00 to 06:00. The insects were stored in 80% ethanol and transported to the laboratory where they were screened and separated. Sand fly legs were separated for subsequent DNA extraction, and the sand fly body was mounted on glass slides as reported by [42] for identification of the species based on morphological characters [2,43], using the classification of [14,16]. The generic names abbreviations followed [44]. Sand fly vouchers were deposited in the Coleção de Flebotomíneos (COLFLEB) of the Centro de Pesquisas René Rachou, FIOCRUZ, Belo Horizonte, state of Minas Gerais.
Species were selected based on their epidemiological importance, including Lutzomyia longipalpis (Lutz & Neiva) and Lutzomyia cruzi (Mangabeira) vectors of Leishmania infantum Nicolle, Bichromomyia flaviscutellata (Mangabeira) vector of Leishmania (Leishmania) amazonensis Lainson & Shaw and Nyssomyia whitmani (Antunes & Coutinho) and Nyssomyia intermedia (Lutz & Neiva) vectors of Leishmania (Viannia) braziliensis Vianna Other species, including Migonemyia migonei (França), Pintomyia fischeri (Pinto), Psychodopygus ayrozai (Barretto & Coutinho), Psychodopygus davisi (Root) and Psychodopygus hirsutus (Mangabeira), were selected because they were previously found to be infected by Leishmania parasites in some regions of Brazil [45,46,47,48,49,50,51,52,53,54,55].
Genomic DNA Extraction and Analysis
Genomic DNA extraction was performed using legs of the sand fly specimen. Genomic DNA extraction was performed as reported by [56]. This is a rapid DNA extraction method for DNA amplification that comprises three steps: 1) the insect is macerated with 500 μl of extraction buffer (10mM Tris-HCl pH 8.2, 2mM EDTA, 0.2% Triton X-100) together with 1μl proteinase K (100μg/ml); 2) the sample is incubated at 50–56°C for 30 min in a OCR machine, and heated to 95°C for 10 min to inactivate the proteinase K and cooled down to 4°C; and 3) the sample is centrifuged at 15,000 g for 5 min at 4°C and remove the supernatant to a fresh tube. We used 20 μl of extraction buffer to macerate the legs which incubated overnight at 37°C. Polymerase chain reaction (PCR) was performed to amplify a 658 bp fragment of the COI gene, to a final volume of 50 μL reaction mixture containing 2 μL of genomic DNA template, 25 μL 2X Promega Go Taq Green™ Master Mix (Promega, Madison, WI, USA), and the primers LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HCO2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) to a final concentration of 1.0 μM [57]. The reaction cycle consisted of an initial denaturation step of 95°C for 3 min, followed by 37 cycles of 95°C for 1 min, 50°C for 1 min, 72°C for 1.5 min, and a final extension cycle 72°C for 7 min. The amplified fragments were separated by agarose (2%) gel electrophoresis and purified using Illustra GFX PCR DNA™ and Gel Band Purification Kit (GE Healthcare, Pittsburgh, PA, USA). The purified fragments were sequenced bidirectionally at Fundação Oswaldo Cruz (PDTIS/FIOCRUZ), Rio de Janeiro, Brazil with an ABI 3730 sequencer, using the same primers that were used in PCR.
Sequence Analysis
The forward and reverse sequences for each specimen were aligned using Clustal W [58] and edited to generate a consensus sequence using BioEdit 7.0 [59]. The “Sequence Composition” tool in BOLD (Barcode of Life Database–www.boldsystems.org) was used to evaluate nucleotide content of the sequences.
Pairwise nucleotide sequence divergence was estimated between all sequences using the Kimura 2-parameter (K2P) model [60] implemented in MEGA 6.0 [61]. We used the K2P model for two reasons: (1) it makes fewer assumptions about the nature of sequence changes than more heavily parameterized models [35,62] and (2) it provides a conservative estimate of long branches than more complex models, by underestimating the number of multiple hits [63]. Levels of genetic divergence within genera and species were calculated in BOLD using the “Distance Summary” tool. Calculations of intraspecific divergence were limited to those species that were represented by at least three specimens and whose DNA sequences showed nucleotide substitutions. Female specimens that were identified only at genus level due to morphological similarities among congeners were not used in the analyses at the species level.
The K2P distances were used in MEGA 6.0 to conduct a neighbor-joining (NJ) analysis and to build a dendrogram showing the similarity among taxa, including bootstrap analysis (1000 replications). One black fly species (Diptera, Simuliidae), Simulium metallicum s. l. (GenBank number: KC015102.1), and two mosquitoes species (Diptera, Culicidae), Ochlerotatus canadensis (GenBank number: JX259544.1), and Culex pipiens pallens (GenBank number: JQ350727.1) were used as outgroups. MEGA 6.0 was also used to inspect the parsimony informative sites among closest species with little to no morphological differentiation and to highlight the fixed differences (defined as sites at which all of the sequences in one sample are different from all of the sequences in a second sample) among its sequences.
Molecular Identification of Species
The ABGD software (Automatic Barcode Gap Discovery) [64] was used to find potential barcode gaps and for primary species delimitation. ABGD sorts sequences into hypothetical species based on the barcode gap, which can be observed whenever the intraspecific divergence is smaller than the interspecific divergence. ABGD uses a range of prior intraspecific divergences to infer a confidence limit for intraspecific divergence from within the data and then identifies and uses the barcode gap to partition the data. The inferred confidence limit and barcode gap are then recursively applied until the data is maximally partitioned. We limited the default range of intraspecific divergence between 0.001 and 0.1. Species were assigned the status of provisional species, indicated by the addition of the suffix PS to the species name, if they split into two or more distinct groups with sequence divergences between them that greater than value of the barcode gap found by ABGD. We used NJ bootstrap support values (≥80%) for the groups to corroborate the data and morphological identification to solve problems regarding species groups with low congener divergence.
Statistical Analysis
Regression analysis was performed using STATISTICA [65] to test: (1) the relationships between sample size and intraspecific sequence divergence (mean and maximum), and (2) the relationships between sequence divergence and geographical distance within each species. The geographical distances between the collections sites were estimated using the Geographic Distance Matrix Generator version 1.2.3 [66].
Results
A total of 576 specimens of Brazilian sand flies, belonging to 47 morphologically classified species and 14 genera, were collected and subjected to barcode analysis (S1 Table). A 658 bp length of the COI amplicon was recovered from all 576 specimens. Sequences and original trace files are available in the “AFBR-DNA barcoding for identification of sand flies (Diptera, Psychodidae, Phlebotominae) from Brazil” project in BOLD and in GenBank (accessions numbers: KP112487—KP113062) (S2 Table). The COI sequences from the sampled species showed an A + T bias (mean = 0.671) relative to the C + G content (mean = 0.329), as has been found previously for arthropods [33,67]. Individual mean nucleotide content was as follows: A = 0.290, G = 0.159, C = 0.170 and T = 0.381. The mean K2P sequence distance within nominal species was 1.41%, while the mean divergence between congeners was approximately 10-fold higher (13.34%). Frequency histograms of mean COI sequence divergences (K2P) at both the species and genus levels of the taxonomic hierarchy are shown in Fig 2. The NJ tree, built using the K2P distances between the specimens, is shown in Fig 3 and S1 Fig.
Fig 2. Frequency histograms of mean COI sequence divergences (K2P) among sand flies from Brazil.
Gray bars represent mean COI sequence divergences (K2P) for species levels and black bars for genus levels of the taxonomic hierarchy.
Fig 3. Neighbor-joining tree of COI sequence divergences (K2P) obtained from 567 specimens of sand flies analyzed.
Numbers next to the branches indicate Bootstrap percentages ≥80%. One black dash indicates groups partitioned by ABGD using the value of 2.15%. One white dash indicates the groups partitioned by ABGD using the cut-off of 1.29%. Two thin black dashes indicated groups that were not recognized by the ABGD partitions. The number of specimens is indicated behind each species name. PS1, PS2, and PS3 represent distinct groups that are hypothesized to represent provisional species.
Using ABGD, we identified nine values for potential barcode gaps (S2 Fig). We only considered barcode gaps with values of prior intraspecific divergence between 1% and 2.5% because values below 1% or above 2.5% can overestimate or underestimate, respectively, the number of species. We found two values for barcode gaps within this range: 1.29% and 2.15%. Using the value of 2.15%, the species were partitioned into 48 groups, as indicated by one black dash (Fig 3). Using the value of 1.29%, the species were partitioned into 49 groups because one of the original 48 groups [Psychodopygus matosi (Barretto & Zago)] was split in two groups, as indicated using one white dash (Fig 3). Seven species were not recognized by the ABGD partitioning: Nyssomyia intermedia was grouped with Ny. whitmani; Ev. carmelinoi (Ryan et al.) was grouped with Evandromyia lenti (Mangabeira) as indicated with two thin black dashes in the NJ tree (Fig 3); and Lu. longipalpis, which if analyzed separately, showed deep intraspecific variation that suggested it should form two groups. One of the two groups, comprising Lu. longipalpis specimens from the central-western region of Brazil, clustered with Lu. cruzi specimens, while the other group, comprising Lu. longipalpis from southeastern of Brazil, clustered with Lutzomyia alencari Martins, Souza & Falcão (Fig 3). Four nominal species, Psathyromyia bigeniculata (Floch & Abonnenc), Evandromyia edwardsi (Mangabeira), Pintomyia monticola (Costa Lima), and Sciopemyia microps (Mangabeira), showed deep intraspecific variation, forming two or more intraspecific barcode groups with mean divergence greater than 2.15% (Fig 3). Psychodopygus matosi showed moderate intraspecific variation and only formed two intraspecific barcode groups with mean divergence greater than 1.29% (Fig 3).
We found fixed differences within Pa. bigeniculata, Ev. edwardsi, Pi. monticola, and Brumptomyia genus and between Evandromyia tupynambai (Mangabeira) and Evandromyia spp. (S3 Fig). Because of the morphological similarities between the species, we also compared Pa. bigeniculata (PS1 and PS2) sequences to the sequences from Pa. limai (Fonseca). Psathyromyia limai showed 41 fixed differences in relation to both provisional species of Pa. bigeniculata (PS1 and PS2), while Pa. bigeniculata PS1 showed nine fixed differences in relation to both Pa. bigeniculata PS2 and Pa. limai. Likewise, Pa. bigeniculata PS2 showed 13 fixed differences in relation to both Pa. bigeniculata PS1 and Pa. limai. These three species still showed one fixed difference among each other that can be used as diagnostic site (421st position of the COI sequence) (S3A Fig). The three species within the Ev. edwardsi nominal species lacked a diagnostic site among them. However, Ev. edwardsi PS1 showed eight fixed differences in relation to Ev. edwardsi PS2 and PS3, while Ev. edwardsi PS2 showed ten fixed differences in relation to Ev. edwardsi PS1 and PS3, and Ev. edwardsi PS3 showed 16 fixed differences in relation to Ev. edwardsi PS1 and PS2 (S3B Fig). The two species of Pi. monticola (PS1 and PS2) showed 36 fixed (S3C Fig). The three species of the Brumptomyia genus showed seven fixed differences that can be used as diagnostic sites (S3D Fig). Evandromyia tupynambai showed 27 fixed differences in relation to Evandromyia spp. (S3E Fig).
The mean intraspecific sequence divergence values (adjusted R2 = -0.0265, p = 0.587) (S4A Fig) and maximum intraspecific sequence divergence values (adjusted R2 = -0.0372, p = 0.861) (S4B Fig) were not significantly correlated to sample size. After a Bonferroni correction sequences divergences were significantly correlated to geographical distance within ten species (S4C, S4D, S4E, S4F, S4G, S4H, S4I, S4J, S4L, and S4M Fig).
Discussion
The molecular identification of species based COI DNA barcoding has been useful for species recognition and the discovery of different taxa of insect vectors of parasites [33,34,39]. Here, we show that COI DNA barcoding is an effective tool for the discovery and recognition of sand fly species from Brazil.
First, there existed a possibility that we would not accurately detect amplification of the COI gene when using the sand fly genomic DNA. This concerned was based on the amplification of COI nuclear pseudogenes of mitochondrial origin (NUMTs) in a small number of previous DNA barcode studies using animal DNAs [68,69]. The presence of NUMT contamination is often indicated by the presence of PCR ghost bands, extra bands in restriction profiles, sequence ambiguities in polymorphic sites, or when both template strands are sequenced, by the presence of frameshift mutations, stop codons, and unexpected phylogenetic placements [68]. Additionally, in the DNA barcode studies, the amplicons generated from NUMT sequences often were shorter than the full-length barcode sequences of COI [69]. As shown above, all of the sequence reads from this study recovered full-length barcodes sequence, with no evidence of insertions, deletions, or stop codons, indicating the absence of NUMTs in the sequences analyzed. This finding is similar to a previous barcoding study that did not find NUMTs in Colombian sand fly species [34]. From our results, we concluded that COI sequences could reliably be used for barcode analysis of sand flies collected from Brazil.
The DNA barcode analyses using the automatic partitioning performed by ABGD allowed us to correctly discriminate approximately 85% (40 species) of all previously morphologically identified species (Fig 3). This percentage becomes closer to 90% when we consider that the COI gene can be used to discriminate between Ny. intermedia and Ny. whitmani. Although the genetic divergence between these species was too low to allow partitioning by ABGD, Ny. intermedia and Ny. whitmani both are composed of monophyletic groups with high bootstrap support values (Fig 3). Even at 90%, this identification percentage was somewhat lower than the percentages found in DNA barcode studies of other sand fly fauna. The identification percentages from studies using sand flies from Colombia and India, for example, were close to 100% [34,39]. Nevertheless, a number of DNA barcode studies in which specimen for each species were collected from several localities showed discrimination percentages similar to those reported here [33,70]. Differences between the species discrimination percentages of sand flies from Brazil and others regions may be related to sampling effects. In the study of sand flies from Colombia, 36 species were collected from different geographic localities and analyzed, 31 of which included the analysis of more than one specimen; however, only ten of these 31 species were sampled from more than one locality. Two of these ten species, Lutzomyia gomezi (Nitzulescu) and Psychodopygus panamensis Shannon, showed the maximum intraspecific COI sequence divergence within one species [34]. This result suggests that the increase in sampling localities for each species could influence their discrimination percentages. Our data supports this possibility, since ABGD accurately delimited between species collected from the same locality. In the study of sand flies from India, although the species were all collected from multiple localities, the high percentage of discrimination between the species may be explained by the small number of species analyzed [39]. The genetic distances between species also can be larger if some species are missing from the assemblage and, consequently, the delimitation becomes easier [71]. This raised concerns regarding the effect of sample size and geographical scale of sampling in the COI sequence divergence (intraspecific genetic variation and interspecific genetic divergence to congeners) for sand flies from Brazil.
A global study using diving beetles showed that both intraspecific genetic variation and interspecific genetic divergence to congenerics are correlated to the geographical scale of sampling [71]. This correlation is supported by a number of theories and concepts, including those of isolation by distance and distance decay [72,73]. Therefore, it is expected that a species sampled from a wide geographical coverage will show greater genetic variation than if the variation was estimated in a species sampled from a single smaller region [71]. As predicted, we found that intraspecific sequence divergence (K2P) in COI was significantly correlated to geographical distance (S4 Fig) within several, but not all, of the species analyzed. Only Pa. bigeniculata (PS1 from Santa Leopoldina, state of Espírito Santo; and PS2 from Cáceres, state of Mato Grosso) and Sc. microps (PS1 from Iúna, state of Espírito Santo; and PS2 from Pancas, state of Espírito Santo) were clustered according to geographical distance. From these samples, we were able to infer that the barcoding gene reliably discriminates between the nominal species, even with correlation between the intraspecific sequence divergence (K2P) at COI and the geographical distance within some species.
The effects of geographical scale on sampling in the COI sequence divergence has been proposed to occur because the distance of one species to the closest morphologically different species significantly decreases with increasing geographical scale of sampling [71]; however, our data did not corroborate this hypothesis. Though Lu. longipalpis, Lu. cruzi and Lu. alencari are closely related species [2,19,74], for example, specimens from these three species of the same geographical region showed less sequence divergence than the closest morphologically different species from others regions (Fig 3). Lutzomyia longipalpis specimens from Pancas, state of Espírito Santo, were closely related to Lu. alencari specimens from the same region, and Lu. longipalpis specimens from Cáceres, state of Mato Grosso clustered with Lu. cruzi specimens from the same region (Fig 3). Despite the morphological differences between these species, this low level of sequence divergence, suggests that Lu. alencari also belongs to the Lu. longipalpis complex. This possibility is further supported by the presence of a spot in the abdominal tergite of both species, which appears to be a synapomorphy of the Lu. longipalpis complex [19]. The low sequence divergence found between these sympatric species can be the result of introgression [75,76], a common phenomenon among closely species that tends to occur much more readily in the mitochondrial DNA than in the nuclear DNA [77,78].
Introgression can also explain the low divergence found among Ny. intermedia and Ny. whitmani (Fig 3). The evidence of introgression among these species is corroborated by studies using mitochondrial DNA (mtDNA) and using nuclear DNA [79,80,81]. Introgression of mtDNA was detected in studies analyzing cytochrome b gene from females that morphologically were identified as Ny. intermedia specimens from Viana, state of Espírito Santo, but showed mtDNA haplotypes from Ny. whitmani [79]. Likewise, introgression of nuclear DNA was detected by studies analyzing the period gene in order to differentiate species of the Ny. intermedia complex. This latter analysis indicated a low level of divergence, high similarities among sympatric sequences, and the presence of shared haplotypes between Nyssomyia species specimens from the locality of Posse, state of the Rio de Janeiro [80,81]. It is hard to distinguish introgression from the persistence of ancestral polymorphisms, but the introgression hypothesis has been the preferred model based on data analysis which suggested that Ny. intermedia and Ny. whitmani might be exchanging alleles [80,81].
Unlike the results for Ny. intermedia and Ny. whitmani, the bootstrap values did not support the clades of Ev. carmelinoi and Ev. lenti, and ABGD was not able to discriminate between them (Fig 3), indicating very low sequence divergence between these species. These two species are very similar morphologically, but can be differentiated by the male genital filamental tips (genital filament tips arrow-like and elongate 1/8th the length of the genital filaments for Ev. lenti; and genital filaments scrolled less than 1/20th the length of genital filaments for Ev. carmelinoi) and differences in the spermathecae of females (ratio of the widths of common/individual ducts is 1.5-fold higher in Ev. lenti than in Ev. carmelinoi) (See Figures 4 (1–2) and 5 (1–2) from [82]). Unfortunately, we were only able to evaluate a small number of specimens from these two species, and the sequence divergence among them (0.1% approximately) was lower than the mean of intraspecific COI sequence divergence within almost of all the species (S1 Table). Nonetheless, we expected that collecting the species from well separated sites (Ev. carmelinoi from Cáceres, state of Mato Grosso and Ev. lenti from Pancas, state of Espírito Santo), would favor reduction of the gene flow, thereby increasing sequence divergence. Furthermore, this similarity of sequences cannot be explained by the phenomenon of decreasing interspecific divergence with increasing geographical scale of sampling, because the geographical distance among the areas (S3 Table) is not enough to achieve such similarity (See Figure 4 from [71]). If these two nominal species really represent two different taxa, the only likely explanation is that strong introgressive hybridization occurs between Ev carmelinoi and Ev. lenti in Cáceres, where these two species are sympatric [83]. It should be noted, however, that only Ev. lenti has been reported in Pancas. Molecular and morphological revision of the species group using a larger number of specimens from several localities throughout its distribution range is required to resolve this discrepancy.
While our study was in progress, the shannoni group of the genus Psathyromyia was revised [43], including species previously reported for the state of Espírito Santo as Psathyromyia shannoni (Dyar) and Psathyromyia pestanai (Barretto & Coutinho) [84,85,86]. From the revision, two species of the shannoni complex, Pa. bigeniculata and Pa. limai, were resurrected from the synonymy of Pa. shannoni, while a third species, Pa. pestanai, was proposed to be a junior synonym of Pa. limai on the basis of morphological characters. Therefore, we reexamined all slides of the specimens morphologically identified as Pa. shannoni from states of Espírito Santo state and Mato Grosso and found them to be Pa. bigeniculata from Cáceres, Mato Grosso and from Santa Leopoldina, state of Espírito Santo. We also found Pa. limai from the remaining areas of the state of Espírito Santo. Corroborating the results of the morphological revision, the DNA barcode discriminated between Pa. limai and Pa. bigeniculata, showing a high mean sequence divergence (10%) between them (Fig 3). It is noteworthy that the DNA barcode analysis from our study split the specimens of Pa. bigeniculata into two groups (PS1 from state of Espírito Santo and PS2 from Cáceres from state of Mato Grosso) that represent putative species within the Pa. bigeniculata complex (Fig 3). These groups had a high number of fixed differences among them, including a diagnostic site (421st position of the 658 bp fragment of COI gene) that enabled identification of Pa. bigeniculata (PS1 and PS2) and Pa. limai (S3A Fig). Based on the distribution of the analyzed specimens, Pa. bigeniculata PS1 represented a coastal species (Atlantic Forest) and Pa. bigeniculata PS2 represented an interior species (cerrado). Besides supporting the resurrection of two species within the shannoni group that are morphologically similar, the DNA barcode also provided a reliable method to discriminate among them, corroborating the utility of the DNA barcode for sand fly identification.
Our analyses also suggested that three other nominal species belongs to cryptic species complex: Ev. edwardsi, Pi. monticola, and Sc. microps. Evandromyia edwardsi was split into three species (PS1, PS2, and PS3) (Fig 3), each with fixed differences in relation to the other species (S3B Fig), but without a diagnostic site. The fixed differences set can be used to differentiate between these three species within the Ev. edwardsi complex. Evandromyia edwardsi does not morphologically resemble other sand fly species, and therefore, can be easily identified based on the male paramere and shape of the spermathecae of the female. This morphological distinction and the low number of specimens collected throughout the distribution range of Ev. edwardsi might have contributed to the failure to recognize slight morphological differences among them. We also failed to find morphological differences within the Pi. monticola complex; however, fixed differences in the COI sequences resulted in diagnostic sites useful for distinguishing the two species (PS1 and PS2), which were split with substantial mean sequence divergence among them (8.1%) (Fig 3, S3C Fig). We found slight morphological differences between the females of the two species (PS1 and PS2) within Sc. microps, which also showed high mean sequence divergence among them (11.9%). Sciopemyia microps PS1 was composed of one female specimen, while Sc. microps PS2 was composed of two male and two female specimens, identified as Sciopemyia sp. The females grouped as Sc. microps PS2 were morphologically identified as Sciopemyia sp. because, due to slide preparation, it was not possible to exam the spermathecae, and because the cibarium (horizontal teeth less-development) was very different from the morphological description of the females of Sc. microps (horizontal teeth well-developed). Unlike the Sc. microps PS2 females, the female of Sc. microps PS1 was morphologically similar to the description of Sc. microps [87], with well-developed horizontal teeth in the cibarium. Two other female specimens (Sc_sp255f and Sc_sp299f, S1 Fig) were also identified as Sciopemyia sp. and were partitioned into a third species based on our DNA barcode analyses (Fig 3). This result supports a previous sand fly survey of the area, which identified these specimens as belonging to Sc. aff. microps rather than Sc. microps [24] because of differences in the diameter of the female spermathecae ducts (AL Ferreira, personal communication). These slight morphological differences are difficult to detect and require considerable technical skills. Therefore, DNA barcode analysis is a useful alternative for discriminating among specimens with small morphological differences.
Finally, the DNA barcode analysis was able to associate morphologically indistinguishable females with males of the genera Brumptomyia, Evandromyia, and Pressatia. Based on the male morphology, three species of Brumptomyia [Brumptomyia cunhai Mangabeira, Brumptomyia ortizi Martins, Silva & Falcão, and Brumptomyia nitzulescui (Costa Lima)] were identified and all the females were identified as Brumptomyia sp. The specimens of Br. cunhai were collected from a sand fly survey from Pancas, Espírito Santo and females from this locality were not analyzed. Unfortunately, this species was not included in the sand fly survey from that area because, at that time, Br. cunhai specimens were misidentified as Br. nitzulescui [24]. Since the initial survey, we performed a morphological revision of the specimens based on a preliminary barcode analyses which indicated two groups of Br. nitzulescui (one from Pancas and others from others areas). Indeed, we find that the group from Pancas was composed of specimens of Br. cunhai. This result highlights the importance of integrative approaches based on morphology and molecular biology to reach accurate results with sand fly identification. In our study, Br. cunhai showed 46 fixed differences in relation to Br. ortizi and Br. nitzulescui, including seven fixed differences that can be used as diagnostic sites among these three species. Besides the seven diagnostic sites, Br. ortizi showed 26 fixed differences in relation to Br. cunhai and Br. nitzulescui (S3 Fig). Five of the ten females identified as Brumptomyia sp. were grouped with the Br. ortizi males, sharing its fixed differences in relation to others Brumptomyia species. The automatic partitioning by ABGD also indicated that these females belong to Br. ortizi taxon. In addition to the similarities between the COI gene sequences, the specimens were collected at the same locality, which further supports that they belong to the same taxon. This finding is very interesting and underscores the suitability of DNA barcoding for describing Br. ortizi females, since females of Br. ortizi have not been previously described [2] because it is morphologically similar to other females of the Brumptomyia genus. Females of Br. nitzulescui can be morphologically differentiated from five others Brumptomyia species, but when sympatric with others species [88], the differentiation is difficult to achieve. Our DNA barcode analysis clustered five Brumptomyia females specimens within the Br. nitzulescui clade, which showed a high number of fixed differences and, consequently, high sequence divergence in relation to others Brumptomyia species. As noted for Br. ortizi, this result highlights the utility of the DNA barcode tool for sand fly species identification and its potential for female and male species association, even for species without formal morphological description of both sexes. Our DNA barcode analysis also indicated an association between the males and the females of the Evandromyia genus. Evandromyia tupynambai was described for both sexes, but when found to be sympatric with Evandromyia callipyga (Martins & Silva) or Evandromyia costalimai (Mangabeira) the females of these three species cannot be distinguished. We did not analyze the males of Ev. callipyga or Ev. costalimai; however our data suggest that the barcode gene can discriminate among these species since the females identified as Evandromyia sp. were clustered in two different groups with high sequence divergence and fixed differences among them (Fig 3). The first group belonged to Ev. tupynambai and was composed of females. The second group of three females belonged to either to Ev. callipyga or Ev. costalimai, but could not be distinguished further because of morphological similarities with the females of Ev. tupynambai of the first group and because we did not analyze DNA barcode from their males. The high genetic difference among the females further underscores the usefulness of the DNA barcode method for associating males with females of a species. Finally, we provided a DNA barcode profile for the females of the Pressatia genus, which indicated that these females belong to Pressatia choti (Floch & Abonnenc) (Fig 3). This result is supported by the absence of other Pressatia species in the region where our specimens were collected [24].
In conclusion, except in cases of species that have undergone introgressive hybridization, DNA barcoding allowed discrimination of sand fly species from Brazil and the discovery of species within putative sand fly species complex. This method also reliably detected sand fly species misidentification and allowed the association between males and females among species that are morphologically similar. Finally, this study highlights the importance of utilizing integrative approaches for sand fly species identification in order to achieve accurate and reliable results.
Supporting Information
Each tip label in the tree contains the sand fly species name abbreviation (five words), the sample ID (number) and sex of the specimen (f = female or m = male). The sand fly species names were abbreviated as follows: Bi_fla = Bichromomyia flaviscutellata; Br_nit = Brumptomyia nitzulescui; Br_cun = Brumptomyia cunhai; Br_ort = Brumptomyia ortizi; Br_sp = Brumptomyia spp.; Ev_car = Evandromyia carmelinoi; Ev_edw = Evandromyia edwardsi; Ev_len = Evandromyia lenti; Ev_sp = Evandromyia spp.; Ev_ter = Evandromyia termitophila; Ev_tup = Evandromyia tupynambai; Ex_fir = Expapillata firmatoi; Lu_ale = Lutzomyia alencari; Lu_cru = Lutzomyia cruzi; Lu_dis = Lutzomyia dispar; Lu_ren = Lutzomyia renei; Lu_sp = Lutzomyia sp.; Mi_cap = Micropygomyia capixaba; Mi_ech = Micropygomyia echinatopharynx; Mi_fer = Micropygomyia ferreirana; Mi_per = Micropygomyia peresi; Mi_qui = Micropygomyia quinquefer; Mi_sch = Micropygomyia schreiberi; Mg_mig = Migonemyia migonei; Ny_int = Nyssomyia intermedia; Ny_whi = Nyssomyia whitmani; Ny_yui = Nyssomyia yuilli yuilli; Pi_bia = Pintomyia bianchigalatiae; Pi_fis = Pintomyia fischeri; Pi_mis = Pintomyia misionensis; Pi_mon = Pintomyia monticola; Pr_cho = Pressatia choti; Pr_sp = Pressatia spp.; Pa_big = Psathyromyia bigeniculata; Pa_lim = Psathyromyia limai; Pa_lut = Psathyromyia lutziana; Pa_pas = Psathyromyia pascalei; Pa_pel = Psathyromyia pelloni; Ps_ayr = Psychodopygus ayrozai; Ps_dav = Psychodopygus davisi; Ps_hir = Psychodopygus hirsutus; Ps_mat = Psychodopygus matosi; Sc_mic = Sciopemyia microps; Sc_sor = Sciopemyia sordellii; Sc_sp = Sciopemyia spp.; Th_via = Trichophoromyia viannamartinsi.
(PDF)
(PDF)
A) Psathyromyia bigeniculata (PS1 and PS2); B) Evandromyia edwardsi (PS1, PS2, and PS3); C) Pintomyia monticola (PS1 and PS2); D) Brumtomyia genus (Brumptomyia cunhai, Brumptomyia ortizi, and Brumptomyia nitzulescui); and E) Evandromyia tupynambai and Evandromyia spp. (yellow = fixed differences; red = diagnostic sites).
(PDF)
(PDF)
Maximum and mean intraspecific values of genetic divergence (Kimura 2-parameter pairwise distances) are shown. As implemented by the Barcode of Life Database (BOLD), values are given only to species represented by three or more individuals and showing at least one nucleotide substitution. Nominal species marked with one asterisk showed distinct intraspecific lineages suggesting cryptic species complexes. Classification follows [14,16] and the generic abbreviations follow [44].
(PDF)
(PDF)
All the distances were estimated in kilometers. (BA = state of Bahia; ES = state of Espírito Santo; MG = state of Minas Gerais; MT = Mato Grosso; RJ = state of Rio de Janeiro).
(PDF)
Acknowledgments
We thank Agenor Barbosa, Antonio Teva, Daniel Kiefer, Felipe Vigoder, Gabriela Thomazim, João F. R. Tonini, Luiz G. S. R. Bauzer and Mateus Borges for the help in the fieldwork. We also thank Gustavo R. Leite for the preparation of the map, Elisa Cupolillo for comments on an earlier version of the manuscript, and Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) for provide the collecting permissions. This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and FIOCRUZ. We are grateful to the Plataforma Genomica-Sequenciamento de DNA- RPT01A- PDTIS/FIOCRUZ.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the CNPq and FIOCRUZ.
References
- 1. Ready P. Biology of Phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol. 2013; 58: 227–250. 10.1146/annurev-ento-120811-153557 [DOI] [PubMed] [Google Scholar]
- 2. Galati EAB. Morfologia, terminologia de adultos e identificação dos táxons da América In: Rangel EF, Lainson R, editors. Flebotomíneos do Brasil. Rio de Janeiro: Fiocruz; 2003. pp. 53–175. [Google Scholar]
- 3.Galati EAB. Phlebotominae (Diptera, Psychodidae). Classificação, morfologia, terminologia e identificação de Adultos. Apostila da Disciplina HEP 5752—Bioecologia e Identificação de Phlebotominae 2014, Programa de Pós-Graduação em Saúde Pública, Faculdade de Saúde Pública, Universidade de São Paulo. 22 august 2014. Available: <www.fsp.usp.br/~egalati>.
- 4. Costa LP, Leite YLR, Fonseca GAB, Fonseca MT. Biogeography of South American forest mammals: endemism and diversity in the Atlantic Forest. Biotropica. 2000; 32: 872–881. [Google Scholar]
- 5. Costa LP. The historical bridge between the Amazon and the Atlantic Forest of Brazil: a study of molecular phylogeography with small mammals. J Biogeogr. 2003; 30: 71–86. [Google Scholar]
- 6. Watts PC, Hamilton JG, Ward RD, Noyes HA, Souza NA, Kemp SJ, et al. Male sex pheromones and the phylogeographic structure of the Lutzomyia longipalpis species complex (Diptera: Psychodidae) from Brazil and Venezuela. Am J Trop Med Hyg. 2005; 73: 734–743. [PubMed] [Google Scholar]
- 7. Santos AMM, Cavalcanti DR, Silva JMC, Tabarelli M. Biogeographical relationships among tropical forests in north-eastern Brazil. J Biogeogr. 2007; 34: 437–446. [Google Scholar]
- 8. Carnaval AC, Moritz C. Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic Forest. J Biogeogr. 2008; 35: 1187–1201. [Google Scholar]
- 9. Avise JC. Phylogeography: the history and formation of species. Harvard University Press Cambridge, MA: Harvard University Press; 2000. [Google Scholar]
- 10. Ridley M. Evolution. 3rd edition Malden: Blackwell Publishing; 2004. [Google Scholar]
- 11. Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer Associates, Inc; 2004. [Google Scholar]
- 12. Lewis DJ, Young DG, Minter DM, Fairchild GB. Proposals for a stable classification of the Phlebotomine sandflies (Diptera: Psychodidae). Syst Entomol. 1977; 2: 235–262. [Google Scholar]
- 13. Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Gainesville, FL: Associated Publishers; (1994. [Google Scholar]
- 14. Galati EAB. Classificação de Phlebotominae In: Rangel EF, Lainson R, editors. Flebotomíneos do Brasil. Rio de Janeiro: Fiocruz; 2003. pp. 23–51. [Google Scholar]
- 15. Sinclair BJ, Borkent A, Wood DM. The male genital tract and aedeagal components of the Diptera with a discussion of their phylogenetic significance. Zool J Linn Soc-Lond. 2007; 150: 711–742. [Google Scholar]
- 16. Galati EAB. Phylogenetic systematics of the Phlebotominae (Diptera, Psychodidae) with emphasis on American groups. Bol Dir Malariol San Amb. 1995; 35: 133–142. [Google Scholar]
- 17. Beati L, Cáceres AG, Lee JA, Munstermann LE. Systematic relationships among Lutzomyia sand flies (Diptera: Psychodidae) of Peru and Colombia based on the analysis of 12S and 28S ribosomal DNA sequences. Int J Parasitol. 2004; 34: 225–234. [DOI] [PubMed] [Google Scholar]
- 18. Andrade-Filho JD, Galati EAB, Brazil RP. Review of American fossil Phlebotominae (Diptera: Psychodidae) with a description of two new species. J Med Entomol. 2009; 46: 969–979. [DOI] [PubMed] [Google Scholar]
- 19. Pinto IS, Filho JD, Santos CB, Falqueto A, Leite YL. Phylogenetic relationships among species of Lutzomyia, subgenus Lutzomyia (Diptera: Psychodidae). J Med Entomol. 2010; 47: 16–21. [DOI] [PubMed] [Google Scholar]
- 20. Rodrigues AAF, Barbosa VA, Andrade-Filho JD, Brazil RP. The sandfly fauna (Diptera: Psychodidae: Phlebotominae) of the Parque Estadual da Serra da Tiririca, Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 2013; 108: 943–946. 10.1590/0074-0276130688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Virgens TM, Santos CB, Pinto IS, Silva KS, Leal FC, Falqueto A. Phlebotomine sand flies (Diptera, Psychodidae) in an American tegumentary leishmaniasis transmission area in northern Espírito Santo state, Brazil. Cad Saúde Pública. 2008; 24: 2969–2978. [DOI] [PubMed] [Google Scholar]
- 22. Virgens TM, Rezende HR, Pinto IS, Falqueto A. Sand fly fauna (Diptera: Psychodidae) from the Goytacazes National Forest and surrounding areas of southeastern Brazil. J Vector Ecol. 2015; 40: 28–35. 10.1111/jvec.12129 [DOI] [PubMed] [Google Scholar]
- 23. Oliveira AG, Galati EAB, Fernandes CE, Dorval MEC, Brazil RP. Ecological aspects of phlebotomines (Diptera: Psychodidae) in endemic area of Visceral leishmaniasis, Campo Grande, state of Mato Grosso do Sul, Brazil. J Med Entomol. 2012; 49: 43–50. [DOI] [PubMed] [Google Scholar]
- 24. Ferreira AL, Falqueto A, Grimaldi G, Peixoto AA, Pinto IS. Ecological and epidemiological aspects of the sand fly (Diptera, Psychodidae) fauna of the National Monument of Pontões Capixabas, state of Espírito Santo, southeastern Brazil. J Med Entomol. 2013; 50: 1215–1223. [DOI] [PubMed] [Google Scholar]
- 25. Figueira EAG, Silva G, Chagas ECS, Shimabukuro PHF. Phlebotomine sandflies (Diptera: Psychodidae) from Lábrea, state of Amazonas, Brazil, with a description of Evandromyia (Aldamyia) apurinan Shimabukuro, Figueira & Silva, sp. nov. Mem Inst Oswaldo Cruz. 2013; 108: 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ladeia-Andrade S, Fé NF, Sanguinette CC, Andrade-Filho JD. Description of Trichophoromyia uniniensis, a new phlebotomine species (Diptera: Psychodidae: Phlebotominae) of Amazonas State, Brazil. Parasit Vectors. 2014; 7: 400 10.1186/1756-3305-7-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andrade-Filho JD, Carvalho GML, Saraiva L, Falcão AL. Bilateral anomaly in the style of Micropygomyia schreiberi (Martins, Falcão & Silva) (Diptera, Psychodidae). Rev Bras Entomol. 2004; 48: 583–585. [Google Scholar]
- 28. Galati EAB, Marassá AM, Fonseca MB, Gonçalves-Andrade RM, Consales CA, Bueno EFM. Phlebotomines (Diptera, Psychodidae) in the Speleological Province of the Ribeira Valley: 3. Serra district—area of hostels for tourists who visit the Parque Estadual do Alto Ribeira (PETAR), state of São Paulo, Brazil. Rev Bras Entomol. 2010; 54: 665–676. [Google Scholar]
- 29. Pinto IS, Santos CB, Ferreira AL, Falqueto A. Variations in the gonostyle of Nyssomyia intermedia (Lutz & Neiva) (Diptera: Psychodidae). Neotrop Entomol. 2010; 39: 732–735. [DOI] [PubMed] [Google Scholar]
- 30. Sanguinette CC, Faustino JX, Meira PCS, Botelho HA, Carvalho GM, Gontijo CM, et al. Anomalies in the sand fly Lutzomyia longipalpis (Diptera: Psychodidae) in Brazil. J Am Mosq Control Assoc. 2013; 29: 54–58. [DOI] [PubMed] [Google Scholar]
- 31. Shannon RC, Del Ponte E. Cuatro notas sobre especies nuevas de dipteros nematóceros, hematófagos o no, de la República Argentina. Rev Inst Bacteriol Dep Nac Hig. 1927; 4: 724–736. [Google Scholar]
- 32. Coutinho JO, Barretto MP. Contribuição para o conhecimento dos flebótomos do estado de São Paulo. III. Descrição do macho de Phlebotomus alphabeticus Fonseca, 1936 e de Phlebotomus pascalei n. sp. (Diptera: Psychodidae). An Fac Med Univ São Paulo. 1940; 16: 193–206. [Google Scholar]
- 33. Rivera J, Currie DC. Identification of Nearctic black flies using DNA barcodes (Diptera: Simuliidae). Mol Ecol Resour. 2009; 9: 224–236. 10.1111/j.1755-0998.2009.02648.x [DOI] [PubMed] [Google Scholar]
- 34. Gutiérrez MAC, Vivero RJ, Vélez ID, Porter CH, Uribe S. DNA Barcoding for the identification of sand fly species (Diptera, Psychodidae, Phlebotominae) in Colombia. Plos One. 2014; 9: e85496 10.1371/journal.pone.0085496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hebert PDN, Cywinska A, Ball SL, deWaard JR. Biological identifications through DNA barcodes. Proc R Soc Lond B Biol Sci. 2003; 270: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hebert PDN, Ratnasingham S, deWaard JR. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc R Soc Lond B Biol Sci. 2003; 270: S96–S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the Neotropical skipper butterfly Astraptes fulgerator . Proc Natl Acad Sci USA. 2004; 101: 14812–14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruiz-Lopez F, Wilkerson RC, Conn JE, McKeon SN, Levin DM, Quiñones ML, et al. DNA barcoding reveals both known and novel taxa in the Albitarsis Group (Anopheles: Nyssorhynchus) of Neotropical malaria vectors. Parasit Vectors. (2012; 5: 44 10.1186/1756-3305-5-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kumar NP, Srinivasan R, Jambulingam P. DNA barcoding for identification of sand flies (Diptera: Psychodidae) in Índia. Mol Ecol Resour. 2012; 12: 414–420. 10.1111/j.1755-0998.2012.03117.x [DOI] [PubMed] [Google Scholar]
- 40. Azpurua J, De La Cruz D, Valderama A, Windsor D. Lutzomyia sand fly diversity and rates of infection by Wolbachia and an exotic Leishmania species on Barro Colorado Island, Panama. PLoS Negl Trop Dis. 2010; 4: e627 10.1371/journal.pntd.0000627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pugedo H, Barata RA, França-Silva JC, Silva JC, Dias ES. HP: um modelo aprimorado de armadilha luminosa de sucção para a captura de pequenos insetos. Rev Soc Bras Med Trop. 2005; 38: 70–72. [DOI] [PubMed] [Google Scholar]
- 42. Barretto MP, Coutinho JO. Contribuicão ao conhecimento dos Flebotomus de São Paulo. II—Descrição do macho de Phlebotomus limai Fonseca, 1935 e de duas novas espécies: Phlebotomus ayrozai e P. amarali (Diptera, Psychodidae). Arq Fac Hig Saude Publica Univ São Paulo. 1940; 6: 34–35. [Google Scholar]
- 43. Sábio PB, Andrade AJ, Galati EAB Assessment of the taxonomic status of some species included in the Shannoni complex, with the description of a new Species of Psathyromyia (Diptera: Psychodidae: Phlebotominae). J Med Entomol. 2014; 51: 331–341. [DOI] [PubMed] [Google Scholar]
- 44. Marcondes CB. A proposal of generic and subgeneric abbreviations for Phlebotomine sandflies (Diptera: Psychodidae: Phlebotominae) of the world. Entomol News. 2007; 118: 351–356. [Google Scholar]
- 45. Lainson R, Shaw JJ, Ward RD, Fraiha H. Leishmaniasis in Brazil, IX. Considerations on the Leishmania braziliensis complex, importance of sandflies of the genus Psychodopygus (Mangabeira) in the transmission of L. braziliensis braziliensis in north Brazil. Trans R Soc Trop Med Hyg. 1973; 67: 184–196. [DOI] [PubMed] [Google Scholar]
- 46. Rangel EF, Ryan L, Lainson R, Shaw JJ. Observations on the sandfly (Diptera: Psychodidae) fauna of Além Paraíba, state of Minas Gerais, Brazil, and the isolation of a parasite of the Leishmania braziliensis complex from Psychodopygus hirsuta hirsuta. Mem Inst Oswaldo Cruz. 1985; 80: 373–374. [DOI] [PubMed] [Google Scholar]
- 47. Arias JR, Miles MA, Naiff RD, Povoa MM, Freitas RA, Biancardi CB, et al. Flagellate infections of Brazilian sand flies (Diptera: Psychodidae): isolation in vitro and biochemical identification of Endotrypanum and Leishmania. Am J Trop Med Hyg. 1985; 34; 1098–1108. [DOI] [PubMed] [Google Scholar]
- 48. Gil LHS, Basano SA, Souza AA, Silva MGS, Barata I, Ishikawa EA, et al. Recent observations on the sand fly (Diptera: Psychodidae) fauna of the State of Rondônia, Western Amazônia, Brazil: the importance of Psychdopygus davisi as a vector of zoonotic cutaneous leishmaniasis. Mem Inst Oswaldo Cruz. 2003; 98: 751–755. [DOI] [PubMed] [Google Scholar]
- 49. Rangel EF, Lainson R. Flebotomíneos do Brasil. Rio de Janeiro: Editora Fiocruz; 2003. [Google Scholar]
- 50. Pita-Pereira D, Alves CR, Souza MB, Brazil RP, Bertho AL, Barbosa AF, et al. Identification of naturally infected Lutzomyia intermedia and Lutzomyia migonei with Leishmania (Viannia) braziliensis in Rio de Janeiro (Brazil) revealed by a PCR multiplex non-isotopic hybridization assay. Trans R Soc Trop Med Hyg. 2005; 99: 905–913. [DOI] [PubMed] [Google Scholar]
- 51. Pita-Pereira D, Cardoso MAB, Alves CR, Brazil RP, Britto C. Detection of natural infection in Lutzomyia cruzi and Lutzomyia forattinii (Diptera: Psychodidae: Phlebotominae) by Leishmania infantum in an endemic area of visceral leishmaniasis in Brazil using PCR multiplex assay. Acta Trop. 2008; 107: 66–69. 10.1016/j.actatropica.2008.04.015 [DOI] [PubMed] [Google Scholar]
- 52. Carvalho MR, Valença HF, Silva FJ, Pita-Pereira D, Pereira TA, Britto C, et al. Natural Leishmania infantum infection in Migonemyia migonei (França, 1920) (Diptera: Psychodidae: Phlebotominae) the putative vector of visceral leishmaniasis in Pernambuco State, Brazil. Acta Trop. 2010; 116: 108–110. 10.1016/j.actatropica.2010.03.009 [DOI] [PubMed] [Google Scholar]
- 53. Rocha LS, Falqueto A, Santos CB, Ferreira AL, Graça GC, Grimaldi G Jr, et al. Survey of natural infection by Leishmania in sand fly species collected in southeastern Brazil. Trans R Soc Trop Med Hyg. 2010; 104: 461–466. 10.1016/j.trstmh.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 54. Missawa NA, Veloso MA, Maciel GB, Michalsky EM, Dias ES. Evidence of transmission of visceral leishmaniasis by Lutzomyia cruzi in the municipality of Jaciara, State of Mato Grosso, Brazil. Rev Soc Bras Med Trop. 2011; 44: 76–78. [DOI] [PubMed] [Google Scholar]
- 55. Falqueto A, Ferreira AL. Reservatórios extra-humanos do complexo Leishmânia e dinâmica de transmissão da infecção ao homem In Coura JR, editor. Dinâmica das doenças infecciosas e parasitárias. 2ed Rio de Janeiro: Guanabara Koogan; 2013. pp. 786–797. [Google Scholar]
- 56. Jowett T. Preparation of nucleic acids In: Roberts DB, ed. Drosophila: A practical approach. Oxford: IRL Press; 1998. pp. 347–371. [Google Scholar]
- 57. Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994; 3: 294–299. [PubMed] [Google Scholar]
- 58. Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994; 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999; 41: 95–98. [Google Scholar]
- 60. Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980; 16: 111–120. [DOI] [PubMed] [Google Scholar]
- 61. Tamura K, Stecher G, Peterson D, Filipski A, and Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013; 30; 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Barrett RDH, Hebert PDN. Identifying spiders through DNA barcodes. Can J Zool. 2005; 83: 481–491. [Google Scholar]
- 63. Nei M, Kumar S. Molecular Evolution and Phylogenetics. New York: Oxford University Press; 2000. [Google Scholar]
- 64. Puillandre N, Lambert A, Brouillet S, Achaz G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol. 2012; 21: 1864–1877. 10.1111/j.1365-294X.2011.05239.x [DOI] [PubMed] [Google Scholar]
- 65.StatSoft, Inc. (2011). STATISTICA (data analysis software system), version 10. www.statsoft.com.
- 66.Ersts PJ. Geographic Distance Matrix Generator (version 1.2.3). American Museum of Natural History, Center for Biodiversity and Conservation. 22 august 2014. Available: http://biodiversityinformatics.amnh.org/open_source/gdmg.
- 67. Crease TJ. The complete sequence for the mitochondrial genome of Daphnia pulex (Cladocera: Crustacea). Gene. 1999; 233: 89–99. [DOI] [PubMed] [Google Scholar]
- 68. Bensasson D, Zhang D, Hartl DL, Hewitt GM. Mitochondrial pseudogenes: evolution’s misplaced witnesses. Trends Ecol Evol. 2001; 16; 314–321. [DOI] [PubMed] [Google Scholar]
- 69. Kerr KCR, Stoeckle MY, Dove CJ, Weigt LA, Francis CM, Hebert PDN. Comprehensive DNA barcode coverage of North American birds. Mol Ecol Notes. 2007; 7: 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Clare EL, Lim BK, Engstrom MD, Eger JL, Hebert PDN. DNA barcoding of Neotropical bats: species identification and discovery within Guyana. Mol Ecol Notes. 2007; 7: 184–190. [Google Scholar]
- 71. Bergsten J, Bilton DT, Fujisawa T, Elliott M, Monaghan M, Balke M, et al. The effect of geographical scale of sampling on DNA barcoding. Syst Biol. 2012; 61: 851–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wright S. (1943) Isolation by distance. Genetics. 1943; 31: 114–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nekola JC, White PS. The distance decay of similarity in biogeography and ecology. J Biogeogr. 1999; 26: 867–878. [Google Scholar]
- 74. Vigoder FM, Araki AS, Bauzer LG, Souza NA, Brazil RP, Peixoto AA. Lovesongs and period gene polymorphisms indicate Lutzomyia cruzi (Mangabeira, 1938) as a sibling species of the Lutzomyia longipalpis (Lutz and Neiva, 1912) complex. Infect Genet Evol. 2010; 10: 734–739. 10.1016/j.meegid.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 75. Lins RMMA, Souza NA Brazil RP Maingon RDC Peixoto AA. Fixed differences in the paralytic gene define two lineages within the Lutzomyia longipalpis complex producing different types of courtship songs. PLoS ONE. 2012; 7: e44323 10.1371/journal.pone.0044323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Araki AS, Ferreira GEM, Mazzoni CJ, Souza NA, Machado RC, Bruno RV, et al. Multilocus analysis of divergence and introgression in sympatric and allopatric sibling species of the Lutzomyia longipalpis complex in Brazil. PLoS Negl Trop Dis. 2013; 7: e2495 10.1371/journal.pntd.0002495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Powell J. Interspecific cytoplasmic gene flow in the absence of nuclear gene flow: evidence from Drosophila . Proc Natl Acad Sci USA. 1983; 80: 492–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bachtrog D, Thornton K, Clark A, Andolfatto P. Extensive introgression of mitochondrial DNA relative to nuclear genes in the Drosophila yakuba species group. Evolution. 2006; 60: 292–302. [PubMed] [Google Scholar]
- 79. Marcondes CB, Day JC, Ready PD. Introgression between Lutzomyia intermedia and both Lu. neivai and Lu. whitmani, and their roles as vectors of Leishmania braziliensis . Trans R Soc Trop Med Hyg. 1997; 91: 725–726. [DOI] [PubMed] [Google Scholar]
- 80. Mazzoni CJ, Souza NA, Andrade-Coelho C, Kyriacou CP, Peixoto AA. Molecular polymorphism, differentiation and introgression in the period gene between Lutzomyia intermedia and Lutzomyia whitmani . BMC Evol Biol. 2006; 6: 85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mazzoni CJ, Araki AS, Ferreira GEM, Azevedo RVDM, Barbujani G, Peixoto AA. Multilocus analysis of introgression between two sand fly vectors of leishmaniasis. BMC Evol Biol. 2008; 8: 141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ryan L, Fraiha H, Lainson R, Shaw JJ. New phlebotomine sandflies of the Walkeri group (Diptera: Psychodidae) from Pará state, Brazil, with a pictorial key 1. Mem Inst Oswaldo Cruz. 1986; 81: 323–331. [Google Scholar]
- 83. Alves GB, Oshiro ET, Leite MC, Melão AV, Ribeiro LM, Mateus NLF, et al. Phlebotomine sandflies fauna (Diptera: Brazil) (Diptera: Psychodidae) at rural settlements in the municipality of Cáceres, State of Mato Grosso, Brazil. Rev Soc Bras Med Trop. 2012; 45: 437–443. [DOI] [PubMed] [Google Scholar]
- 84. Pinto IS, Santos CB, Ferreira AL, Falqueto A. American visceral leishmaniasis dissociated from Lutzomyia longipalpis (Diptera, Psychodidae) in the State of Espírito Santo, Brazil. Cad Saúde Pública. 2010; 26: 365–372. [DOI] [PubMed] [Google Scholar]
- 85. Pinto IS, Santos CB, Ferreira AL, Falqueto A. Richness and diversity of sand flies (Diptera, Psychodidae) in an Atlantic rainforest reserve in southeastern Brazil. J Vect Ecol. 2010; 35: 325–332. [DOI] [PubMed] [Google Scholar]
- 86. Pinto IS, Tonini JFR, Ferreira AL, Falqueto A. A brief inventory of sand flies (Diptera, Psychodidae) from the National Forest of the Rio Preto, state of the Espírito Santo, southeastern Brazil. Biota Neotrop. 2012; 12: 323–326. [Google Scholar]
- 87. Martins AV, Falcão AL, Silva JE. Estudos sobre os flebótomos do estado de Minas Gerais—X—Descrição das fêmeas de Lutzomyia microps (Mangabeira, 1942) e Lutzomyia firmatoi (Barretto, Martins & Pellegrino, 1956) (Diptera, Psychodidae, Phlebotominae). Rev Bras Biol. 1975; 35: 259–263. [Google Scholar]
- 88. Shimabukuro PHF, Tolezano JE, Galati EAB. Chave de identificação ilustrada dos Phlebotominae (Diptera, Psychodidae) do estado de São Paulo, Brasil. Pap Avulsos Zool. 2011; 51: 399–441. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each tip label in the tree contains the sand fly species name abbreviation (five words), the sample ID (number) and sex of the specimen (f = female or m = male). The sand fly species names were abbreviated as follows: Bi_fla = Bichromomyia flaviscutellata; Br_nit = Brumptomyia nitzulescui; Br_cun = Brumptomyia cunhai; Br_ort = Brumptomyia ortizi; Br_sp = Brumptomyia spp.; Ev_car = Evandromyia carmelinoi; Ev_edw = Evandromyia edwardsi; Ev_len = Evandromyia lenti; Ev_sp = Evandromyia spp.; Ev_ter = Evandromyia termitophila; Ev_tup = Evandromyia tupynambai; Ex_fir = Expapillata firmatoi; Lu_ale = Lutzomyia alencari; Lu_cru = Lutzomyia cruzi; Lu_dis = Lutzomyia dispar; Lu_ren = Lutzomyia renei; Lu_sp = Lutzomyia sp.; Mi_cap = Micropygomyia capixaba; Mi_ech = Micropygomyia echinatopharynx; Mi_fer = Micropygomyia ferreirana; Mi_per = Micropygomyia peresi; Mi_qui = Micropygomyia quinquefer; Mi_sch = Micropygomyia schreiberi; Mg_mig = Migonemyia migonei; Ny_int = Nyssomyia intermedia; Ny_whi = Nyssomyia whitmani; Ny_yui = Nyssomyia yuilli yuilli; Pi_bia = Pintomyia bianchigalatiae; Pi_fis = Pintomyia fischeri; Pi_mis = Pintomyia misionensis; Pi_mon = Pintomyia monticola; Pr_cho = Pressatia choti; Pr_sp = Pressatia spp.; Pa_big = Psathyromyia bigeniculata; Pa_lim = Psathyromyia limai; Pa_lut = Psathyromyia lutziana; Pa_pas = Psathyromyia pascalei; Pa_pel = Psathyromyia pelloni; Ps_ayr = Psychodopygus ayrozai; Ps_dav = Psychodopygus davisi; Ps_hir = Psychodopygus hirsutus; Ps_mat = Psychodopygus matosi; Sc_mic = Sciopemyia microps; Sc_sor = Sciopemyia sordellii; Sc_sp = Sciopemyia spp.; Th_via = Trichophoromyia viannamartinsi.
(PDF)
(PDF)
A) Psathyromyia bigeniculata (PS1 and PS2); B) Evandromyia edwardsi (PS1, PS2, and PS3); C) Pintomyia monticola (PS1 and PS2); D) Brumtomyia genus (Brumptomyia cunhai, Brumptomyia ortizi, and Brumptomyia nitzulescui); and E) Evandromyia tupynambai and Evandromyia spp. (yellow = fixed differences; red = diagnostic sites).
(PDF)
(PDF)
Maximum and mean intraspecific values of genetic divergence (Kimura 2-parameter pairwise distances) are shown. As implemented by the Barcode of Life Database (BOLD), values are given only to species represented by three or more individuals and showing at least one nucleotide substitution. Nominal species marked with one asterisk showed distinct intraspecific lineages suggesting cryptic species complexes. Classification follows [14,16] and the generic abbreviations follow [44].
(PDF)
(PDF)
All the distances were estimated in kilometers. (BA = state of Bahia; ES = state of Espírito Santo; MG = state of Minas Gerais; MT = Mato Grosso; RJ = state of Rio de Janeiro).
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.