Abstract
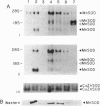
Introduction of a normal human chromosome 6 into human melanoma cell lines results in suppression of tumorigenicity. This suggests that a gene(s) on chromosome 6 controls the malignant phenotype of human melanoma. Because antioxidants can suppress the tumor-promotion phase of carcinogenesis, and because the antioxidant enzyme manganese superoxide dismutase (MnSOD) has been localized to a region of chromosome 6 frequently lost in melanomas, we have examined the effect of transfecting sense and antisense human MnSOD cDNAs into melanoma cell lines. Cell lines expressing abundant (+)-sense MnSOD-5 cDNAs significantly altered their phenotype in culture and lost their ability to form colonies in soft agar and tumors in nude mice. In contrast, the introduction of antisense MnSOD or +psv2neo had no effect on melanoma tumorigenicity. These findings indicate that stable transfection of MnSOD cDNA into melanoma cell lines exerts a biological effect that mimics that observed after introduction of an entire human chromosome 6.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. G., Balin A. K., Reimer R. J., Sohal R. S., Nations C. Superoxide dismutase induces differentiation in microplasmodia of the slime mold Physarum polycephalum. Arch Biochem Biophys. 1988 Feb 15;261(1):205–211. doi: 10.1016/0003-9861(88)90119-1. [DOI] [PubMed] [Google Scholar]
- Allen R. G. Oxygen-reactive species and antioxidant responses during development: the metabolic paradox of cellular differentiation. Proc Soc Exp Biol Med. 1991 Feb;196(2):117–129. doi: 10.3181/00379727-196-43171a. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Beckman B. S., Balin A. K., Allen R. G. Superoxide dismutase induces differentiation of Friend erythroleukemia cells. J Cell Physiol. 1989 May;139(2):370–376. doi: 10.1002/jcp.1041390220. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Borek C., Troll W. Modifiers of free radicals inhibit in vitro the oncogenic actions of x-rays, bleomycin, and the tumor promoter 12-O-tetradecanoylphorbol 13-acetate. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1304–1307. doi: 10.1073/pnas.80.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravard A., Sabatier L., Hoffschir F., Ricoul M., Luccioni C., Dutrillaux B. SOD2: a new type of tumor-suppressor gene? Int J Cancer. 1992 May 28;51(3):476–480. doi: 10.1002/ijc.2910510323. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chen L. B. Mitochondrial membrane potential in living cells. Annu Rev Cell Biol. 1988;4:155–181. doi: 10.1146/annurev.cb.04.110188.001103. [DOI] [PubMed] [Google Scholar]
- Church S. L., Farmer D. R., Nelson D. M. Induction of manganese superoxide dismutase in cultured human trophoblast during in vitro differentiation. Dev Biol. 1992 Jan;149(1):177–184. doi: 10.1016/0012-1606(92)90274-k. [DOI] [PubMed] [Google Scholar]
- Church S. L., Grant J. W., Meese E. U., Trent J. M. Sublocalization of the gene encoding manganese superoxide dismutase (MnSOD/SOD2) to 6q25 by fluorescence in situ hybridization and somatic cell hybrid mapping. Genomics. 1992 Nov;14(3):823–825. doi: 10.1016/s0888-7543(05)80202-2. [DOI] [PubMed] [Google Scholar]
- Church S. L. Manganese superoxide dismutase: nucleotide and deduced amino acid sequence of a cDNA encoding a new human transcript. Biochim Biophys Acta. 1990 Oct 23;1087(2):250–252. doi: 10.1016/0167-4781(90)90213-l. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge R. C., Fine S. M., Unanue E. R., Chaplin D. D. Expression of membrane interleukin 1 by fibroblasts transfected with murine pro-interleukin 1 alpha cDNA. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5649–5653. doi: 10.1073/pnas.85.15.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Hoak D., Gough F., McNutt M. A. Monoclonal antibodies specific for melanocytic tumors distinguish subpopulations of melanocytes. Am J Pathol. 1986 May;123(2):195–203. [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler T. W., Bush D. M., Kozumbo W. J. Inhibition of tumor promotion by a biomimetic superoxide dismutase. Science. 1983 Jul 1;221(4605):75–77. doi: 10.1126/science.6857269. [DOI] [PubMed] [Google Scholar]
- Kho C. J., Zarbl H. Fte-1, a v-fos transformation effector gene, encodes the mammalian homologue of a yeast gene involved in protein import into mitochondria. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2200–2204. doi: 10.1073/pnas.89.6.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marshall C. J. Tumor suppressor genes. Cell. 1991 Jan 25;64(2):313–326. doi: 10.1016/0092-8674(91)90641-b. [DOI] [PubMed] [Google Scholar]
- Millikin D., Meese E., Vogelstein B., Witkowski C., Trent J. Loss of heterozygosity for loci on the long arm of chromosome 6 in human malignant melanoma. Cancer Res. 1991 Oct 15;51(20):5449–5453. [PubMed] [Google Scholar]
- Muehlematter D., Larsson R., Cerutti P. Active oxygen induced DNA strand breakage and poly ADP-ribosylation in promotable and non-promotable JB6 mouse epidermal cells. Carcinogenesis. 1988 Feb;9(2):239–245. doi: 10.1093/carcin/9.2.239. [DOI] [PubMed] [Google Scholar]
- Oberley L. W., Oberley T. D., Buettner G. R. Cell differentiation, aging and cancer: the possible roles of superoxide and superoxide dismutases. Med Hypotheses. 1980 Mar;6(3):249–268. doi: 10.1016/0306-9877(80)90123-1. [DOI] [PubMed] [Google Scholar]
- Oberley L. W., Oberley T. D. The role of superoxide dismutase and gene amplification in carcinogenesis. J Theor Biol. 1984 Feb 7;106(3):403–422. doi: 10.1016/0022-5193(84)90038-9. [DOI] [PubMed] [Google Scholar]
- Oberley T. D., Oberley L. W., Slattery A. F., Lauchner L. J., Elwell J. H. Immunohistochemical localization of antioxidant enzymes in adult Syrian hamster tissues and during kidney development. Am J Pathol. 1990 Jul;137(1):199–214. [PMC free article] [PubMed] [Google Scholar]
- Pedersen P. L. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J. Human tumor suppressor genes. Annu Rev Genet. 1990;24:615–657. doi: 10.1146/annurev.ge.24.120190.003151. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Strutton G. M., Parsons P. G. Determination of proliferating fractions in malignant melanomas by anti-PCNA/cyclin monoclonal antibody. Histopathology. 1991 Mar;18(3):221–227. doi: 10.1111/j.1365-2559.1991.tb00829.x. [DOI] [PubMed] [Google Scholar]
- Torroni A., Stepien G., Hodge J. A., Wallace D. C. Neoplastic transformation is associated with coordinate induction of nuclear and cytoplasmic oxidative phosphorylation genes. J Biol Chem. 1990 Nov 25;265(33):20589–20593. [PubMed] [Google Scholar]
- Trent J. M., Stanbridge E. J., McBride H. L., Meese E. U., Casey G., Araujo D. E., Witkowski C. M., Nagle R. B. Tumorigenicity in human melanoma cell lines controlled by introduction of human chromosome 6. Science. 1990 Feb 2;247(4942):568–571. doi: 10.1126/science.2300817. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. The retinoblastoma gene and cell growth control. Trends Biochem Sci. 1990 May;15(5):199–202. doi: 10.1016/0968-0004(90)90162-5. [DOI] [PubMed] [Google Scholar]
- Weissman B. E., Saxon P. J., Pasquale S. R., Jones G. R., Geiser A. G., Stanbridge E. J. Introduction of a normal human chromosome 11 into a Wilms' tumor cell line controls its tumorigenic expression. Science. 1987 Apr 10;236(4798):175–180. doi: 10.1126/science.3031816. [DOI] [PubMed] [Google Scholar]
- Westerhausen D. R., Jr, Hopkins W. E., Billadello J. J. Multiple transforming growth factor-beta-inducible elements regulate expression of the plasminogen activator inhibitor type-1 gene in Hep G2 cells. J Biol Chem. 1991 Jan 15;266(2):1092–1100. [PubMed] [Google Scholar]
- Wispé J. R., Clark J. C., Burhans M. S., Kropp K. E., Korfhagen T. R., Whitsett J. A. Synthesis and processing of the precursor for human mangano-superoxide dismutase. Biochim Biophys Acta. 1989 Jan 19;994(1):30–36. doi: 10.1016/0167-4838(89)90058-7. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988 Nov 11;242(4880):941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- Woods A. L., Hall P. A., Shepherd N. A., Hanby A. M., Waseem N. H., Lane D. P., Levison D. A. The assessment of proliferating cell nuclear antigen (PCNA) immunostaining in primary gastrointestinal lymphomas and its relationship to histological grade, S+G2+M phase fraction (flow cytometric analysis) and prognosis. Histopathology. 1991 Jul;19(1):21–27. doi: 10.1111/j.1365-2559.1991.tb00890.x. [DOI] [PubMed] [Google Scholar]