Abstract
Background
The liver plays a key role in fuel metabolism. It is well established that liver disease is associated with an increased risk for diabetes mellitus. Hepatitis C virus infection has been known to increase the risk of diabetes. However, much less is known about the role of hepatitis B virus (HBV) infection in diabetes. We examined the association of diabetes based on the vaccination status for HBV.
Methods
In this cross-sectional study, we included adult subjects (≥20 y/o) with HBV serology available from the National Health and Nutrition Examination Survey 2005–2010. Diabetes was defined as established diabetes or fasting plasma glucose concentration ≥7.0 mmol/L, 2-hour plasma glucose concentration ≥11.1 mmol/L, or HbA1c ≥ 47.5 mmol/mol (6.5%). Vaccination was based on the reported history and immunization was determined by HBV serology. The odds ratio (OR) with 95% confidence intervals (95% CI) were calculated with consideration of the following covariates: age, gender, BMI, ethnic/racial group, current smoker, current alcohol consumption, family history of diabetes, poverty index, and education.
Results
This study included 15,316 subjects. Among them, 2,320 subjects was immunized based the HBV serology. Among 4,063 subjects who received HBV vaccination, successful vaccination was only noted in 39% of subjects. The HBV vaccination was not associated with diabetes (OR: 1.08, 95%CI: 0.96–1.23). Serology evidence of HBV immunization was associated with a reduced OR of diabetes (0.75, 95%CI: 0.62–0.90). Successful HBV vaccination was also associated with a reduced OR of diabetes (0.67, 95%CI: 0.52–0.84).
Conclusions
Although our study shows the association of HBV vaccination with the reduced odds of diabetes by 33%, a prospective study is warranted to confirm and examine the impact of HBV vaccination in prevention of diabetes.
Introduction
Diabetes affects 9.3% of the US population with 21 million diagnosed and 8.1 million undiagnosed [1]. The prevalence of diabetes has increased substantially [2] and consequently so has its financial impact on society with the estimated cost of 245 billion in the US [1]. It is projected that by 2030, the global burden of diabetes in 2030 is 366 million (about 4.4% of the world’s population) with the greatest impact on developing countries and about 50% of diabetes in Asian and Pacific regions [3]. As diabetes is a highly prevalent disease with a significant and potentially life-threatening co-morbidity and financial burden, the identification of causative factors could potentially lead to better treatment and prevention strategies.
Diabetes is a syndrome complex with a common manifestation of hyperglycemia of diverse genetic and non-genetic etiologies. Infection has been implied to play a role in the pathogenesis of diabetes. Among them, the role of hepatitis C virus infection in diabetes is well recognized [4–6]. In contrast, there is much less information available about the role of hepatitis B virus (HBV) in diabetes. The rate of new HBV infections has declined significantly by about 75% in the United States since 1991, when a HBV vaccination program was implemented [7]. Despite this success, HBV infection remains a significant public health issue with up to 1.4 million carriers in the United States [8]. Furthermore, HBV infection is a major global health threat with about 350 million chronic HBV infection worldwide, especially in Asian and Pacific regions [8] where the prevalence of diabetes is recently noted to increase drastically [3].
The role that HBV plays in diabetes is less clear. Although the association with gestation diabetes has been reported [9], chronic HBV infection is not found to increase the risk of diabetes [10]. Another study demonstrated the association of HBV infection with diabetes in Asian American, but not in Pacific Islanders [11]. To further elucidate the relationship of HBV and diabetes, this study examined the data derived from the National Health and Nutrition Examination Survey (NHANES) 2005–2010. To our knowledge, this is the very first study examining the role of HBV vaccination and immunization in diabetes in large sample with multiple ethnic/racial groups. Although diabetes is also preventable with dietary change, physical activity, behavior modification, and various pharmacological interventions, the results of the present study may provide a new method of prevention via vaccination.
Materials and Methods
Ethnics statement
The National Health and Nutrition Examination Survey (NHANES) is a program of studies designed to assess the health and nutritional status of adults and children in the United States. The survey is unique in that it combines interviews and physical examinations. NHANES is a major program of the National Center for Health Statistics (NCHS). NCHS is part of the Centers for Disease Control and Prevention (CDC) and has the responsibility for producing vital and health statistics for the Nation. In 1999, the survey became a continuous program that has a changing focus on a variety of health and nutrition measurements to meet emerging needs. Data collection for (NHANES 2005–2010) was approved by the NCHS Research Ethics Review Board (http://www.cdc.gov/nchs/nhanes/irba98.htm). Informed consent was obtained from participants. The records/information was anonymized and de-identified prior to release in the NHANES website (http://www.cdc.gov/nchs/nhanes/about_nhanes.htm). Analysis of de-identified data from the survey is exempt from the federal regulations for the protection of human research participants. Only de-identified data from the survey was used in this study.
Studied subjects
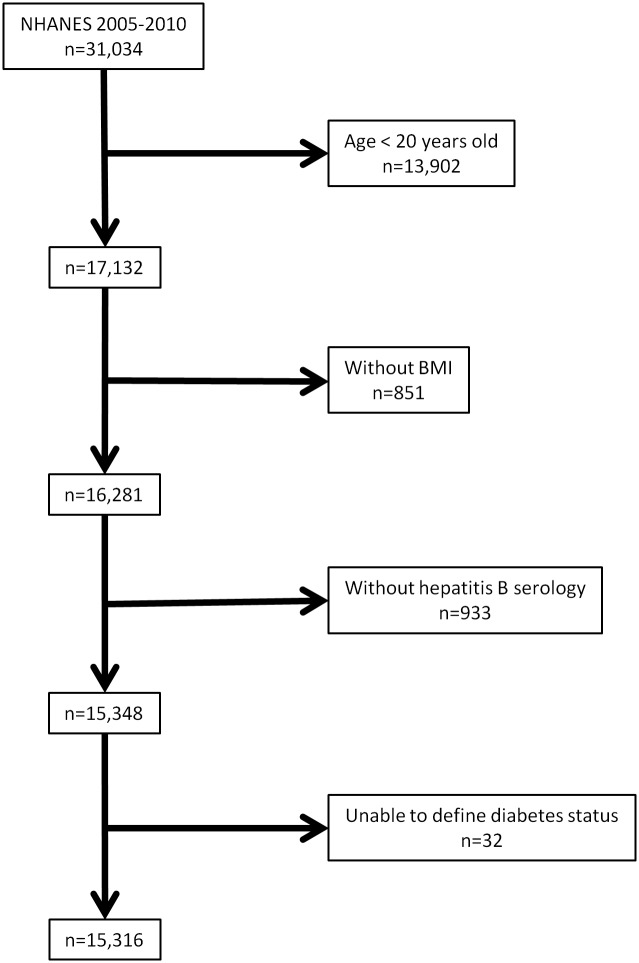
Data from the NHANES from 2005 through 2010 were evaluated for the study (n = 31,034). As shown in Fig 1, we excluded 13,902 subjects who were younger than 20 years of age at the time of the screening interview and 851 subjects without body mass index (BMI) information. Then, we excluded 933 subjects whom hepatitis B serology was not available and 32 subjects whose diabetes status could not be determined by either history or laboratory measurement as described below.
Fig 1. Sampling scheme.
Definition of diabetes
Established diabetes (n = 1,807) was based on the history of self-reported diabetes or by the use of insulin and/or oral anti-diabetic agents. Diabetes was defined in accordance with the guidelines set forth by the American Diabetes Association [12]. As treatment affected HbA1c, fasting plasma glucose concentration and 2-hour plasma glucose concentration, 658 subjects with established diabetes were noted to have HbA1c <47.5 mmol/mol, 330 subjects with established diabetes were noted to have fasting plasma glucose concentration <7.0 mmol/L, and 31 subjects with established diabetes were noted to have 2-hour plasma glucose concentration <11.1 mmol/L. Diabetes was defined as established diabetes or fasting plasma glucose concentration ≥7.0 mmol/L, 2-hour plasma glucose concentration ≥11.1 mmol/L, or HbA1c ≥47.5 mmol/mol (6.5%). In this sample set, we were able to define diabetes in 2,626 subjects by above criteria. Since sampling weights were only available for subsampling for plasma fasting glucose and 2-hour plasma glucose concentrations, multiple criteria, in addition to plasma fasting glucose and 2-hour plasma glucose concentrations, were used defined diabetes in the present study. Thus, sampling weights were not used in this study.
Hepatitis B vaccination history
The information on the hepatitis B vaccination was categorized as receiving all 3 doses or less than 3 doses. Among them, 91.07% of vaccinated subjects received all 3 doses of hepatitis B vaccination. As the analysis was mainly based on the serological results, we did not separate the subjects received all 3 doses or less than 3 doses.
Laboratory methods
Plasma glucose
Fasting plasma glucose concentrations were obtained from subjects examined in the morning after a 9 hour fast. After fasting samples were obtained, subjects were asked to drink a calibrated dose (75 grams) of glucose with a second venipuncture 2 hours (± 15 minutes) later for 2-hour plasma glucose concentrations. Plasma glucose concentrations were determined by a hexokinase method. Due to different laboratory instruments were used in 2005–2006 and 2007–2010, we applied regression equations to align fasting plasma glucose and 2-hour plasma glucose concentrations obtained in 2005–2006 as recommended by the NHANES.
HbA1c
HbA1c was measured using high performance liquid chromatography based assays. Although different HbA1c laboratory instruments and laboratories were used between 2005 and 2010, both laboratories were standardized by participating in the National Glycohemoglobin Standardization Program (NGSP). The NGSP reviewed and concluded that both NHANES laboratories met NGSP criteria for bias and precision from 1999–2010. As recommended by the NGSP, no cross-over regression was made in the present study. The re-released HbA1c data for 2007–2008 (GHB_E) and 2009–2010 (GHB_F) in March 2012 were used in this study.
Hepatitis B surface antibody (HBsAb)
The AUSAB Enzyme immunoassay for HBsAb used the “sandwich principle” a solid phase enzyme-linked immunoassay technique to detect HBsAb levels in serum or plasma.
Hepatitis B core antibody (HBcAb)
The Ortho HBc ELISA Test System was a qualitative enzyme-linked immunosorbent assay (ELISA) for the detection of total antibody to HBcAb in human serum or plasma. HBcAb appeared in virtually all individuals infected with HBV and was an accurate serological marker of current and past infection.
Hepatitis B surface antigen (HBsAg)
The AUSZYME Monoclonal test was a solid-phase “sandwich” enzyme immunoassay used to detect the presence of HBsAg, which indicated current infection with HBV.
Other measures
We considered gender, age, BMI, ethnic/race group, current smoker, current alcohol consumption, family history of diabetes, education, and poverty index as covariates. Age was recorded in years at the time of the screening interview. Gender was based on self-reported categories from the participants, as well as race/ethnicity which were categorized as Mexican Americans, other Hispanics, non-Hispanics whites, non-Hispanic blacks, and other ethnic/racial groups. BMI (kg/m2) was calculated from measured weight (kg) divided by the square of standing height (meter). Current smoker was defined as using tobacco/nicotine last 5 days or not. Current alcohol consumption was defined as at least 12 alcohol drinks per years or not in the past year. Family history of diabetes was defined as any of blood relatives including father, mother, sisters or brothers, ever told by a health professional that they had diabetes or not. Education was categorized based on the self-reported highest education as follows: less than 9th grade education, 9-11th grade education (includes 12th grade and no diploma), high school graduate, some college or associates degree, and college graduate or higher. Poverty index was an index for the ratio of family income to poverty based on the US Department of Health and Human Services’ poverty guidelines [13]. A poverty index of 1.0 represents the level of family income that is at the federal poverty level, and a poverty index of 2.0 represents a family income that is 200% of the federal poverty level.
Statistical analysis
Continuous data were expressed in mean ± standard deviation. Continuous differences were examined using a two-tailed Student’s t-test. Categorical differences were given in proportions and examined using Chi-square test. Logistic regression analysis was used to calculate the odds ratio with 95% confidence intervals of diabetes based on vaccination history, HBV serology markers and successful HBV vaccination with the consideration of covariates as described above. In model 1, no covariates were considered; in model 2, gender, age, and BMI were considered; in model 3, ethnic/racial groups were entered in addition to gender, age, and BMI; in model 4, all covariates were entered. A nominal P value of < 0.05 was considered to be significant. SYSTAT 13.0 for windows package from SPSS, INC. (Chicago, IL, USA) was used for statistical analysis.
Results
Hepatitis B status
The clinical features of studied subjects were shown in Table 1. In this representative US population, the prevalence of HBV infection was 6.35% based on positive HBcAb, in contrast the prevalence of positive HBsAg was 0.40%. The prevalence of positive HBsAb was 19.66%. Among those with positive HBcAb, 6.28% were positive for HBsAg, while only 0.13% of HBsAb positive subjects were positive for HBsAg. Based on positive HBsAb and negative for both HBcAb and HBsAg, 2,320 subjects (15.15%) were immunized. As 4,063 subjects (26.53%) reported to receive HBV vaccination, successful HBV vaccination rate was 39.01% (Table 2).
Table 1. Clinical and demographic characteristics.
| n | mean (n) STD (%) | ||
|---|---|---|---|
| Gender | female | 15,316 | 7,905 (51.61%) |
| Age | year | 15,316 | 49 ± 18 |
| BMI | kg/m2 | 15,316 | 28.99 ± 6.73 |
| Current alcohol consumption | yes | 15,316 | 10,028 (65.47%) |
| Current smoking | yes | 15,316 | 3,685 (24.06%) |
| Family history of diabetes | yes | 15,316 | 6,127 (40.00%) |
| Poverty index* | 15,316 | 2.82 ± 1.81 | |
| Education** | 15,316 | 10,859 (70.90%) | |
| Ethnic/racial group | 15,316 | ||
| Mexican American | 2,876 (17.57%) | ||
| Other Hispanic | 1,305 (7.97%) | ||
| Non-Hispanic white | 7,484 (45.71%) | ||
| Non-Hispanic black | 2,945 (17.99%) | ||
| Others | 706 (4.31%) | ||
| Fasting plasma glucose | mmol/L | 7,421 | 6.0 ± 2.0 |
| 2-hour plasma gluccose | mmol/L | 5,394 | 6.8 ± 3.0 |
| HbA1c | |||
| NGSP | % | 15,307 | 5.7 ± 1.0 |
| IFCC | mmol/mol | 15,307 | 38.8 ± 10.9 |
Mean ± standard deviation, or n (percent).
*In reference to the level of family income that is at the federal poverty level as 1.00.
**High school graduate or higher.
Table 2. Distribution of hepatitis B vaccination by serological evidence of immunization for hepatitis B.
| Hepatitis B vaccination | |||||||
|---|---|---|---|---|---|---|---|
| Yes | No | Subtotal | |||||
| Imminzation for hepatitis B | Yes | 1,587 | (39.06%) | 733 | (6.51%) | 2,320 | (15.15%) |
| Imminzation for hepatitis B | No | 2,476 | (60.94%) | 10,520 | (93.49%) | 12,996 | (84.85%) |
| Subtotal | 4,063 | (26.53%) | 11,253 | (73.47%) | 15,316 | (100.00%) | |
Pearson Chi-Square = 2,460.1284, degree of freedom = 1.000, P<0.001
Hepatitis B vaccination: Yes, vaccination per self-report; No, no vaccination per self-report
Immunization for hepatitis B: Yes, positive for hepatitis B surface antibody and negative for both hepatitis B core antibody and surface antigen; No, others.
Hepatitis B vaccination history and diabetes
Among 4,063 subjects with a history of HBV vaccination, diabetes was noted in 11.62%, in compared to 19.14% in those without a history of HBV vaccination (P<0.001, Table 3). The subjects with a history of vaccination were more female (P<0.001), younger (P<0.001), more current alcohol consumption (P = 0.001), more current smokers (P = 0.02), and higher education (P<0.001) with better glucose profiles (P<0.001) when compared to those without a history of HBV vaccination (Table 4). Analysis with covariates revealed age, gender, BMI, education, current alcohol consumption, and family history of diabetes had significant impacts (P<0.05) on the results while ethnic/racial groups, poverty index, and current smoking had not. However, the logistic regression analyses did not confirm its protective effect for diabetes (Table 3).
Table 3. Odds ratio and 95% confidence intervals for the risk of diabetes by vaccination and immunization of hepatitis B.
| Diabetes | Model 1 | Model 2 | Model 3 | Model 4 | |||
|---|---|---|---|---|---|---|---|
| n | % | OR (95% CI) | OR(95% CI) | OR (95% CI) | OR (95% CI) | ||
| Vaccination | Yes | 472 | (11.62%) | 0.56 | 1.01 | 1.03 | 1.08 |
| Vaccination | No | 2,154 | (19.14%) | (0.50–0.62) | (0.89–1.14) | (0.91–1.16) | (0.96–1.23) |
| Immunization | Yes | 156 | (6.72%) | 0.31 | 0.68 | 0.69 | 0.75 |
| Immunization | No | 2,470 | (19.01%) | (0.26–0.36) | (0.56–0.95) | (0.58–0.83) | (0.62–0.90) |
| Successfu vaccination | Yes | 85 | (5.36%) | 0.25 | 0.57 | 0.59 | 0.67 |
| Successful vaccination | No | 2,541 | (18.51%) | (0.20–0.31) | (0.45–0.72) | (0.46–0.75) | (0.52–0.84) |
Hepatitis B vaccination: Yes, vaccination per self-report; No, no vaccination per self-report
Hepatitis B immunization: Yes, positive for hepatitis B surface antibody and negative for both hepatitis B core antibody and surface antigen; No, others
OR, odds ratio; 95% CI, 95% confidence intervals
Model 1, unadjusted; Model 2, adjusted for gender, age, and BMI; Model 3, adjusted for gender, age, BMI, and ethnic/racial group; Model 4, adjusted for gender, age, BMI, ethnic/race group, active smoker, active alcohol consumption, family history of diabetes, poverty index, and education.
Table 4. Comparison of clinical features by history of hepatitis B vaccination.
| Hepatitis B vaccination | P | |||
|---|---|---|---|---|
| Yes | No | |||
| n | 4,063 | 11,253 | ||
| Gender | female | 2,392 (58.87%) | 5,513 (48.99%) | <0.001 |
| Age | year | 40 ± 16 | 53 ± 18 | <0.001 |
| Body mass index | kg/m2 | 29.06 ± 7.14 | 28.96 ± 6.57 | 0.41 |
| Current alcohol consumption | yes | 2,722 (66.99%) | 7,306 (64.92%) | 0.001 |
| Current smoker | yes | 1,032 (25.40%) | 2,653 (23.58%) | 0.02 |
| Family history of diabetes | yes | 1,598 (39.33%) | 4,529 (40.24%) | 0.31 |
| Poverty index* | 2.80 ± 1.79 | 2.82 ± 1.81 | 0.52 | |
| Education** | 3,218 (79.20%) | 7,661 (68.08%) | <0.001 | |
| Ethnic/racial group | <0.001 | |||
| Mexican American | 671 (16.51%) | 2,686 (19.56%) | ||
| Other Hispanic | 389 (9.58%) | 1,189 (8.66%) | ||
| Non-Hispanic white | 1,826 (44.94%) | 6,639 (48.36%) | ||
| Non-Hispanic black | 935 (23.01%) | 2,620 (19.08%) | ||
| Others | 242 (5.96%) | 595 (4.33%) | ||
| Fasting plasma glucose*** | mmol/L | 5.7 ± 1.6 | 6.1 ± 2.1 | <0.001 |
| 2-hour plasma glucose*** | mmol/L | 6.1 ± 2.4 | 7.0 ± 3.1 | <0.001 |
| HbA1c*** | ||||
| NSPG | % | 5.5 ± 0.9 | 5.8 ± 1.1 | <0.001 |
| IFCC | mmol/mol | 36.6 ± 9.8 | 39.9 ± 12.0 | <0.001 |
Mean ± standard deviation, or n (percent).
Hepatitis B vaccination: Yes, vaccination per self-report; No, no vaccination per self-report
*In reference to the level of family income that is at the federal poverty level as 1.00.
**High school graduate or higher.
***For fasting plasma glucose, n = 1,989 for those with hepatitis B vaccination and n = 5,432 for those without hepatitis B vaccination; for 2-hour plasma glucose, n = 1,476 and n = 3,918, respectively; for HbA1c, n = 4,062 and n = 11,245, respectively.
Hepatitis B immunization and diabetes
In this population, 15.15% of the subjects showed serological evidence of HBV immunization. Among them, 6.72% were diabetic while diabetes was noted in 19.01% of the subjects without serological evidence of HBV immunization (P<0.001). Again, there were significant differences in the clinical features between the two groups (Table 5). Although the logistic regression analyses confirmed the influence of covariates and ORs increased, the protective effect of HBV immunization remained persistent after adjustment of all covariates with an OR of 0.75 (95%CI: 0.62–0.90, Table 3). The impact of each covariate on the results was highly similar to the analysis of HBV vaccination history and diabetes with minor differences in P values.
Table 5. Comparison of clinical features by states of hepatitis B immunization per hepatitis B serology.
| Hepatitis B immunization | P | |||
|---|---|---|---|---|
| Yes | No | |||
| n | 2,320 | 12,996 | ||
| Gender | female | 1,438(61.98%) | 6,467(49.76%) | <0.001 |
| Age | year | 37±16 | 52±18 | <0.001 |
| Body mass index | kg/m2 | 27.60±6.19 | 29.24±6.79 | <0.001 |
| Current alcohol consumption | yes | 1,572(67.76%) | 8,456(65.07%) | 0.01 |
| Current smoker | yes | 563(24.27%) | 3,122(24.02%) | 0.80 |
| Family history of diabetes | yes | 829(35.73%) | 5,298(40.77%) | <0.001 |
| Poverty index* | 2.93±1.81 | 2.80±1.80 | <0.001 | |
| Education** | 1,937(83.49%) | 8,943(68.81%) | <0.001 | |
| Ethnic/racial group | <0.001 | |||
| Mexican American | 329(14.18%) | 2,547(19.60%) | ||
| Other Hispanic | 182(7.84%) | 1,123(8.64%) | ||
| Non-Hispanic white | 1,134(48.88%) | 6,350(48.86%) | ||
| Non-Hispanic black | 492(21.21%) | 2,453(18.88%) | ||
| Others | 183(7.89%) | 523(4.02%) | ||
| Fasting plasma glucose*** | mmol/L | 5.5±1.4 | 6.1±2.1 | <0.001 |
| 2-hour plasma glucose*** | mmol/L | 5.9±2.1 | 6.9±3.1 | <0.001 |
| HbA1c*** | ||||
| NSPG | % | 5.4±0.8 | 5.7±1.1 | <0.001 |
| IFCC | mmol/mol | 35.5±8.7 | 38.8±12.0 | <0.001 |
Mean ± standard deviation, or n (percent).
Hepatitis B immunization: Yes, positive for hepatitis B surface antibody and negative for both hepatitis B core antibody and surface antigen; No, others
*In reference to the level of family income that is at the federal poverty level as 1.00.
**High school graduate or higher.
***For fasting plasma glucose, n = 1,123 for those with immunization to hepatitis B and n = 6,298 for those without immunization to hepatitis B; for 2-hour plasma glucose, n = 885 and n = 4,509, respectively; for HbA1c, n = 2,319 and n = 12,988, respectively.
Successful hepatitis B vaccination and diabetes
Among those who were tested positive for HBsAb and negative for both HBcAb and HBsAg with a history of HBV vaccination, 1,587 (10.36%) subjects were deemed successfully vaccinated for HBV. The prevalence of diabetes was significantly lower in subjects with successful HBV vaccination than those without it (5.36% vs. 18.51%, P<0.001, Table 3), suggesting the protective effect for diabetes by successful HBV vaccination. Again two groups differed significantly in clinical features except for current smoker (Table 6). The logistic regression analyses confirmed the protective effects for diabetes (Table 3). Successful HBV vaccination was associated with a reduced odds of diabetes (OR: 0.67, 95%CI: 0.52–0.84). Again age, gender, BMI, education, current alcohol consumption, and family history of diabetes had significant impacts (P<0.05) on the results while ethnic/racial groups, poverty index, and current smoking had not.
Table 6. Comparison of clinical features by states of successful vaccination confirmed by hepatitis B serology.
| Successful vaccination for hepatitis B | P | |||
|---|---|---|---|---|
| Yes | No | |||
| n | 1,587 | 13,729 | ||
| Gender | female | 1,038(65.41%) | 6,867(50.02%) | <0.001 |
| Age | year | 36±14 | 51±18 | <0.001 |
| Body mass index | kg/m2 | 27.74±6.18 | 29.13±6.77 | <0.001 |
| Current alcohol consumption | yes | 1,097(69.12%) | 8,931(65.05%) | 0.001 |
| Current smoker | yes | 359(22.62%) | 3,326(24.23%) | 0.16 |
| Family history of diabetes | yes | 565(35.60%) | 5,562(40.51%) | <0.001 |
| Poverty index* | 3.02±1.76 | 2.79±1.81 | <0.001 | |
| Education** | 1,404(88.47%) | 9,455(68.87%) | <0.001 | |
| Ethnic/racial group | <0.001 | |||
| Mexican American | 190(11.97%) | 2,686(19.56%) | ||
| Other Hispanic | 116(7.31%) | 1,189(8.66%) | ||
| Non-Hispanic white | 845(53.25%) | 6,639(48.36%) | ||
| Non-Hispanic black | 325(20.48%) | 2,620(19.08%) | ||
| Others | 111(6.99%) | 595(4.33%) | ||
| Fasting glucose*** | mmol/L | 5.4±1.0 | 6.1±2.1 | <0.001 |
| 2h glucose*** | mmol/L | 5.8±1.9 | 6.9±3.1 | <0.001 |
| HbA1c*** | ||||
| NGSP | % | 5.3±0.6 | 5.7±1.1 | <0.001 |
| IFCC | mmol/mol | 34.4±6.6 | 38.8±12.0 | <0.001 |
Mean ± standard deviation, or n (percent).
Successful vaccination for hepatitis B: Yes, positive for hepatitis B surface antibody and negative for both hepatitis B core antibody and surface antigen with a history of HBV vaccination; No, others
*In reference to the level of family income that is at the federal poverty level as 1.00.
**High school graduate or higher.
***For fasting glucose, n = 802 for those with successful vaccination and n = 6,619 for those without successful vaccination; for 2-hour plasma glucose, n = 650 and n = 4,744, respectively; for HbA1c, n = 1,586 and n = 13,721, respectively.
Discussion
To investigate the role of HBV in diabetes, we examined the association of HBV vaccination history and HBV serology with diabetes in a representative US population. Our results suggest that serological evidence of HBV immunization is associated with reduced odds of diabetes (33%), and successful HBV vaccination, defined by a history of hepatitis B vaccination with positive HBsAb, negative HBcAb, and negative HBsAg, was a protective factor for diabetes. Since only a very small subset of subjects in this population were chronic HBV carriers (positive HBsAg, 0.40%), we were not able to conduct a meaning analysis of the odds of diabetes in the group with chronic HBV infection.
In this study, we had no information on the age of HBV vaccination. As the titer of HBV declines with time, the high vaccination failure rate in this sample set could be a time related issue. However, we could not exclude other causes from the limited information available. As shown in Table 6, subjects who were successful vaccination for HBV were more female, younger, leaner by BMI, of better financial status by poverty index, and completed higher education. HBV vaccination could be a selective process for a lower diabetes risk. If the observed association is from a self-selection of health behavior, we should observe the association of HBV vaccination alone with diabetes, regardless of HBV serological status. However, no association of HBV vaccination with diabetes was found in this population after adjustment for covariates (Table 3). In contrast, the protective effect against diabetes was only observed with serological evidence of HBV immunization and successful HBV vaccination. Thus, the observed association is not a result of self-selective process of heath behavior.
Furthermore, logistic regression analysis provided the means to examine the differences in covariates between the subjects with and without successful HBV vaccination. It revealed that ethnic/racial groups, poverty index, and active smoker had no effect on the reduced odds of diabetes (P>0.05), while significant effects (P<0.001) were noted for gender, age, BMI, current alcohol consumption, family history of diabetes, and education. Although a much lower risk (OR: 0.25) for those with successful HBV vaccination was noted in Model 1 (unadjusted analysis), the ORs increased drastically after adjustment for differences in gender, age, and BMI (Model 2 in Table 3). Additional adjustment with other covariates only provided a fairly modest change in the OR. The results confirmed that after adjustment for covariates, successful HBV vaccination was independently associated with reduced odds of diabetes: 0.67 (95% CI: 0.52–0.84).
Among 2,626 diabetic subjects included in this study, 819 subjects were undiagnosed prior to taking the survey. As the concern of undiagnosed may represent a different group, we also examined the association based on 1,807 subjects with established diabetes only. The OR for HBV vaccination within the subjects with established diabetes was 1.12 (95% CI: 0.97–1.29), 0.74 (95% CI: 0.60–0.93) for those with HBV immunization by serological evidence, and 0.63 (95%CI: 0.47–0.84) for those with successful hepatitis B vaccination by both HBV vaccination history and serological evidence of immunization after adjustment for all covariates. The results were very consistent with the analysis based on all 2,626 diabetic subjects.
The major strength of the current study is derived from a fairly large representative US population. Furthermore, the results of HBV vaccination and successful HBV vaccination are complimentary to each other. The major limitation of the current study is that it is a retrospective association study. The causal relationship of diabetes and HBV is unknown as well as underlying molecular mechanisms. Furthermore, the low rate of successful immunization in diabetic subjects after HBV vaccination (16.77% vs. 41.02% in non-diabetic subjects), as noted in other studies [14,15], could also play some role in this association. Diabetes is associated with an increased risk for acute HBV infection [16]. Although the role of HBV infection in the pathogenesis of diabetes is unknown, the underlying mechanistic insights could stem from several lines of evidence. The liver plays a key role in maintaining glucose homeostasis, including insulin-mediated processes and the clearance of insulin itself [17]. Various liver diseases are associated with diabetes [18,19]. Furthermore, the association of hepatitis C virus infection and diabetes is well recognized [4]. Insulin resistance is associated with hepatitis C virus infection and has been inferred to play a role in the pathogenesis of diabetes [20]. The presence of hepatitis C virus in human pancreatic beta cells is associated with morphological cell changes and beta cell dysfunction [21]. Additionally, inflammation with the production of pro-inflammatory cytokines has also been demonstrated in diabetic patients with hepatitis C virus infection [22]. Thus, HBV infection might increase the risk of diabetes through similar mechanisms and, accordingly, immunization with HBV might reduce the risk of diabetes.
It has been well recognized that the primary purpose of vaccination is to reduce infectious disease and its sequelae. In addition to reduction of HBV infection, it has been demonstrated that vaccination with HBV also reduces the incidence of hepatocellular carcinoma [23]. This study further demonstrates an additional potential benefit of HBV vaccination by reducing the risk of diabetes, which is an unforeseen bonus of HBV vaccination, as if confirmed by an interventional study.
Diabetes can be delayed or prevented by various ways. The Diabetes Prevention Program demonstrates that lifestyle intervention reduces the incidence of diabetes by 58% and metformin by 31%, as compared to placebo after an average of 2.8 years [24]. The similar effect of behavior modification on the reduction of diabetes was also noted from other studies [25–27]. In addition to metformin, various pharmacological interventions are also very effective in the prevention of diabetes [28–30]. However, it requires significant cost and commitment to achieve the target goal of diabetes prevention [31]. Furthermore, cost-effectiveness of diabetes prevention through the above mentioned behavior modification has been challenged [32]. Once an intervention study is conducted and confirms the observation of our study, HBV vaccination may potentially act as a relatively cheap and cost-effective method of reducing the risk of diabetes in addition to the current recommendations of behavior and lifestyle modifications.
In this representative US population, the HBV vaccination rate was relatively low with only 26.53% of subjects having been vaccinated and almost three-fourths of subjects aged 20 years or older were not vaccinated for HBV. The first vaccine for HBV was approved by the FDA in 1981 and the CDC recommended HBV vaccination for all newborns in the United States in 1991. In this study, we included subjects 20 years or older in the NHANES from 2005 to 2010. Thus, it is very unlikely the subjects vaccinated in this population were the results of the national newborn vaccination program. With a relatively low HBV vaccination rate in the population aged 20 years or older, it provides a unique opportunity to intervene the epidemic of diabetes through HBV vaccination.
Conclusion
The results of this study suggest that successful HBV vaccination (positive HBsAb, negative HBcAb, and negative HBsAg with HBV vaccination) is associated with reduced odds of diabetes by 33%. As the HBV vaccination rate is relatively low (26.53%) in the current US population aged 20 years or older, prevention of diabetes through HBV vaccination is a relatively low cost and can be easily achieved, as compared to known measures that require long-term commitment of behavior modification and/or costly pharmacological intervention. Furthermore, about 50% of projected increases in the prevalence of diabetes by 2030 will occur in developing countries, especially Asian and Pacific regions [3], where hepatitis B has become an epidemic [8]. Thus, HBV vaccination could have a significant impact on the epidemic of diabetes in these countries. An intervention trial is warranted before promoting a large scale application of HBV vaccination for prevention of diabetes.
Acknowledgments
We are in debt to Ding-Shinn Chen, M.D. for his critical discussion and guidance in the revision of the manuscript, Henry Lin and Jemily Juan for medical writing support and revision, and Karen Ramos for excellent logistic and administrative support of this research endeavor and assistance in the preparation of the manuscript.
The preliminary results which were analyzed based on the serology markers only without consideration of vaccination history was presented as a guided audio poster at 2014 74th American Diabetes Association Scientific Sessions, San Francisco, California, USA (http://app.core-apps.com/tristar-ada14/abstract/e3048dedfd3d5fbcc516bc86384167f7). The poster presentation was covered by the news media (http://www.medscape.com/viewarticle/826772 and http://www.physiciansweekly.com/ada-2014-hepatitis-b-vaccination-diabetes/").
Data Availability
Data are available from: http://www.cdc.gov/nchs/nhanes.htm.
Funding Statement
The authors have no support or funding to report.
References
- 1.National Diabetes Statistics Report, 2014. Available: http://www.cdc.gov/diabetes/pubs/statsreport14.htm. Accessed 2015 March 20
- 2. Selvin E, Parrinello CM, Sacks DB, Coresh J (2014) Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med 160: 517–525. 10.7326/M13-2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 4. Antonelli A, Ferri C, Ferrari SM, Colaci M, Sansonno D, Fallahi P (2009) Endocrine manifestations of hepatitis C virus infection. Nat Clin Pract Endocrinol Metab 5: 26–34. 10.1038/ncpendmet1027 [DOI] [PubMed] [Google Scholar]
- 5. Negro F, Alaei M (2009) Hepatitis C virus and type 2 diabetes. World J Gastroenterol 15: 1537–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. White DL, Ratziu V, El-Serag HB (2008) Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol 49: 831–844. 10.1016/j.jhep.2008.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. (2005) A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 54: 1–31. [PubMed] [Google Scholar]
- 8. Te HS, Jensen DM (2010) Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis 14: 1–21, vii 10.1016/j.cld.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 9. Lao TT, Chan BC, Leung WC, Ho LF, Tse KY (2007) Maternal hepatitis B infection and gestational diabetes mellitus. J Hepatol 47: 46–50. [DOI] [PubMed] [Google Scholar]
- 10. Huang ZS, Huang TS, Wu TH, Chen MF, Hsu CS, Kao JH (2010) Asymptomatic chronic hepatitis B virus infection does not increase the risk of diabetes mellitus: a ten-year observation. J Gastroenterol Hepatol 25: 1420–1425. 10.1111/j.1440-1746.2010.06268.x [DOI] [PubMed] [Google Scholar]
- 11. Li-Ng M, Tropp S, Danoff A, Bini EJ (2007) Association between chronic hepatitis B virus infection and diabetes among Asian Americans and Pacific Islanders. Dig Liver Dis 39: 549–556. [DOI] [PubMed] [Google Scholar]
- 12.(2014) Diagnosis and classification of diabetes mellitus. Diabetes Care 37 Suppl 1:S81–90. 10.2337/dc14-S081: S81–S90. [DOI] [PubMed] [Google Scholar]
- 13. Malamug LR, Karnchanasorn R, Samoa R, Chiu KC (2014) The Role of Helicobacter pylori Seropositivity in Insulin Sensitivity, Beta Cell Function, and Abnormal Glucose Tolerance. Scientifica (Cairo) 2014:870165 10.1155/2014/870165 Epub@2014 Mar 25.: 870165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Douvin C, Simon D, Charles MA, Deforges L, Bierling P, Lehner V, et al. (1997) Hepatitis B vaccination in diabetic patients. Randomized trial comparing recombinant vaccines containing and not containing pre-S2 antigen. Diabetes Care 20: 148–151. [DOI] [PubMed] [Google Scholar]
- 15. Bouter KP, Diepersloot RJ, Wismans PJ, Gmelig Meyling FH, Hoekstra JB, Heijtink RA, et al. (1992) Humoral immune response to a yeast-derived hepatitis B vaccine in patients with type 1 diabetes mellitus. Diabet Med 9: 66–69. [DOI] [PubMed] [Google Scholar]
- 16. Reilly ML, Schillie SF, Smith E, Poissant T, Vonderwahl CW, Gerard K, et al. (2012) Increased risk of acute hepatitis B among adults with diagnosed diabetes mellitus. J Diabetes Sci Technol 6: 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeFronzo RA (2009) Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58: 773–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moscatiello S, Manini R, Marchesini G (2007) Diabetes and liver disease: an ominous association. Nutr Metab Cardiovasc Dis 17: 63–70. [DOI] [PubMed] [Google Scholar]
- 19. Picardi A, D'Avola D, Gentilucci UV, Galati G, Fiori E, Spataro S, et al. (2006) Diabetes in chronic liver disease: from old concepts to new evidence. Diabetes Metab Res Rev 22: 274–283. [DOI] [PubMed] [Google Scholar]
- 20. Eslam M, Khattab MA, Harrison SA (2011) Insulin resistance and hepatitis C: an evolving story. Gut 60: 1139–1151. 10.1136/gut.2010.228262 [DOI] [PubMed] [Google Scholar]
- 21. Masini M, Campani D, Boggi U, Menicagli M, Funel N, Pollera M, et al. (2005) Hepatitis C virus infection and human pancreatic beta-cell dysfunction. Diabetes Care 28: 940–941. [DOI] [PubMed] [Google Scholar]
- 22. Lecube A, Hernandez C, Genesca J, Simo R (2006) Proinflammatory cytokines, insulin resistance, and insulin secretion in chronic hepatitis C patients: A case-control study. Diabetes Care 29: 1096–1101. [DOI] [PubMed] [Google Scholar]
- 23. Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, et al. (1997) Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med 336: 1855–1859. [DOI] [PubMed] [Google Scholar]
- 24. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. (2008) The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 371: 1783–1789. 10.1016/S0140-6736(08)60766-7 [DOI] [PubMed] [Google Scholar]
- 26. Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, et al. (2006) Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 368: 1673–1679. [DOI] [PubMed] [Google Scholar]
- 27. Kosaka K, Noda M, Kuzuya T (2005) Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract 67: 152–162. [DOI] [PubMed] [Google Scholar]
- 28. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M (2004) Acarbose for the prevention of Type 2 diabetes, hypertension and cardiovascular disease in subjects with impaired glucose tolerance: facts and interpretations concerning the critical analysis of the STOP-NIDDM Trial data. Diabetologia 47: 969–975. [DOI] [PubMed] [Google Scholar]
- 29. DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, et al. (2011) Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 364: 1104–1115. 10.1056/NEJMoa1010949 [DOI] [PubMed] [Google Scholar]
- 30. Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, et al. (2006) Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 368: 1096–1105. [DOI] [PubMed] [Google Scholar]
- 31. Hernan WH, Brandle M, Zhang P, Williamson DF, Matulik MJ, Ratner RE, et al. (2003) Costs associated with the primary prevention of type 2 diabetes mellitus in the diabetes prevention program. Diabetes Care 26: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kahn R, Davidson MB (2014) The reality of type 2 diabetes prevention. Diabetes Care 37: 943–949. 10.2337/dc13-1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from: http://www.cdc.gov/nchs/nhanes.htm.