Abstract
Bee products have been used since ancient times to treat many diseases, including respiratory ailments. The present study aimed to examine the modulatory effect of honey, royal jelly, and propolis extract on peripheral blood leukocytes and lung inflammation in a mouse conalbumin-induced asthma model. The mice in group I were not sensitised or treated; they were kept as controls. The mice in group II were sensitised and challenged with conalbumin. Twenty-four hours after the first challenge with antigen, the mice in group III received 0.5 mg/kg of dexamethasone intraperitoneally per day for 18 consecutive days and kept as positive controls. The mice in groups IV, V, and VI received 650, 1000, and 30 mg/kg of honey, royal jelly, and propolis (aqueous and ethanolic extract), respectively, once per day for 18 consecutive days. Blood was collected from all of the mice for white blood cell differentiation, and the lungs were removed for histopathological studies. The groups treated with propolis extract exhibited considerable ameliorative effects against asthma, which might be explained by the flavonoids and phenolics found in propolis, which might have antioxidative effects. Otherwise, the sensitised and honey- or royal jelly-treated groups exhibited an increased incidence of asthma cascade events due to increased inflammatory cells. These results might be due to the immunostimulatory and vasodilatory effects of royal jelly and honey, which are antagonistic to bronchial asthma cases. Histopathological examination revealed that the sensitised treated propolis extract groups had significant decreases in inflammatory scores compared with other treatments and the sensitised untreated group. These results confirmed the previous data of peripheral blood cells.
Keywords: Conalbumin, Honey, Propolis, Royal jelly, Peripheral blood leukocytes, Mice
1. Introduction
Honey bees are well known as superior chemists and engineers. They biochemically synthesised beeswax, venom, and royal jelly (Bogdanovt, 2014, Viuda-Martos et al., 2008, Mahmoud, 2012), and the successful utilisation of honey bee products has been described (Schmidt, 1996). The economic importance and usefulness of honey bees and the therapeutic effects of their products have motivated scientists to conduct numerous studies.
Honey and their products have been used since ancient times as food and medicine to cure many diseases; this practice is called apitherapy (Manyi-Loh et al., 2011). Honey, propolis, wax, and other honey bee products were used in combination with herbs to treat wounds and other diseases by ancient Egyptians, Assyrians, Chinese, Greek, Romans, and Arabians (Molan, 1999, El Denshary et al., 2012). The use of honey to cure diarrhoea was recommended by the Muslim Prophet Muhammad (PBUH) (Molan, 1999), and there is a whole Surah in the Quran named “Al-Nahl,” describing the life and benefit of bees. Humans and honey bee use honey as a source of energy and as a stable sweetener (Manyi-Loh et al., 2011). The original resins are produced by various types of plants, collected by honey bees, and converted further into propolis (Wagh, 2013). Propolis has very good pharmacological activities, and it is used as an antimicrobial against microbes such as viruses, bacteria, fungi, and moulds. It is used extensively by honey bees to seal the nest cavity and to mummify the cadavers of intruders into the hive that have been killed (Wagh, 2013). Royal jelly is a complete balanced food source for bee larvae. It has various properties and is used by humans not only for moisturising, emulsifying, and stabilising, but also for antitumour and anti-inflammatory properties, antifatigue and hypotensive activity, (Nagai and Inoue, 2004, Inoue et al., 2003), antioxidant activities (Silici et al., 2009), antibacterial effects (Fujiwara et al., 1990), and enhancement of immune activity (Sver et al., 1996). With these unique properties, honey bees have become very important for human beings (Schmidt, 1996).
Bronchial asthma is considered one of the most chronic diseases affecting children; it is the main cause of children’s absence from school and for hospital admissions of patients under 15 years of age (Hockenberry and Wilson, 2007). Asthma is an inflammatory condition characterised by hypersensitivity of the airways, producing symptoms of cough, especially in the early morning or at night, dyspnoea or expiratory wheezing, and chest tightness. The prevalence of asthma, its complications, and related morbidity and mortality are increasing worldwide. This increase is attributed to air pollution, poor access to health care services, misdiagnosis, and mistreatment (Global Strategy for Asthma Management and Prevention, 2014). According to the World Health Organisation, the number of asthmatic patients will increase to 100 million by the end of 2025. Currently, allergic asthma is treated with a standard combined therapy that includes inhaled corticosteroids and leukotriene receptor antagonists (Li and Brown, 2009), which lack a consistent cure and produce side effects (Tinkelman et al., 1993). In recent years, a new trend of using complementary and alternative medicines (CAM) has increased worldwide (Barnes et al., 2008). The CAM includes herbs, acupuncture, Chinese medicine, and apitherapy. Primary health care for 80% of the world population is dependent on alternative medicine (Barnes et al., 2008), and using herbs to treat asthma is a popular practice (Ernst, 1998, Eisenberg et al., 1998). The traditional Chinese remedy ‘ma huang’, a derivative of herbs and tea leaves, is used to develop theophylline and ephedrine for asthma drugs (Ziment, 2000). Lung function has been shown to improve up to four hours using caffeine, related to theophylline, which has been used for centuries to treat asthma (Bara and Barley, 2001).
This study was conducted to examine the ameliorating effect of honey, royal jelly, and propolis on peripheral blood leukocytes and lung inflammation in conalbumin-sensitised mice.
2. Materials and methods
2.1. Animals
Thirty-six albino CD1 mice (male, ∼6 weeks old, weighing 18–20 g), obtained from Helwan Breeding Farm, Ministry of Health, Helwan, Cairo, Egypt, were kept in well-maintained animal facilities at Biotechnology Research Laboratory, Gastroenterology Surgery Centre, Mansoura University, with proper food and water ad libitum throughout the experiment. The room was maintained on a 12 h light–dark schedule at a temperature of 25 ± 2 °C and a relative humidity of 58.41–72.18%. The experiments were performed in accordance with the guidelines for the use of laboratory animals established by the Institute of Laboratory Animal Resources Commission of Life Sciences (1996).
2.2. Honey
One kg of pure, freshly gathered honey was purchased from Meet El-Aamel apiary, Dakahlyia.
2.3. Royal jelly
Freshly gathered honeybee or royal jelly was purchased from Agricultural Research Centre Station, Tag El-Ezz, Dakahlyia.
2.4. Propolis
Crude propolis was purchased from Agricultural Research Centre Station, Manzala, Dakahlyia.
2.4.1. Extraction of crude propolis
Propolis was extracted following the protocol developed by Boeru and Derevici (1978), with slight modifications. A small piece of propolis (approximately 50 g) was sliced and squashed with a mortar and pestle, and the extract was cultured for ten days in the dark at 45 °C with continuous shaking (100 rpm) in a flask containing ethyl alcohol (95%) at a ratio of 1:5. It was then filtered through Whatman paper, and the crude extract of propolis (66.6% concentration) was dried at 60 °C. Following the same technique, aqueous propolis extract was obtained (36.9 mg/ml) using distilled water in a separate flask; it was stored at 4 °C for further studies.
2.5. Conalbumin sensitisation and induction of lung inflammation
Intraperitoneal injections were administered to the mice for sensitisation, using 200 μg of conalbumin along with 2 mg of alum in 0.3 ml of PBS on alternating weeks. The mice were anesthetised and challenged with conalbumin intratracheally by aspiration seven days after the last sensitisation (Keane-Myers et al., 1998, Li et al., 2000). Specifically, the mice were placed on a board in a supine position, the tongue was extended using lined forceps, and conalbumin (100 μg/50 μl PBS) was applied to the back of the tongue. The intratracheal challenges were repeated after 20 and 30 days (Group II).
2.6. Treatment of allergic pulmonary inflammation
The animals were divided into six groups (six mice in each group). The mice in group I were not sensitised or treated; they were kept as controls. The mice in group II were sensitised and challenged with conalbumin as mentioned previously. Twenty-four hours after the first antigen challenge, the mice in group III received 0.5 mg/kg of dexamethasone intraperitoneally once per day for 18 consecutive days, and they were kept as a positive control. The mice in groups IV, V, and VI received 650, 1000, and 30 mg/kg honey (Ali et al., 1997), royal jelly (Fujii et al., 1990), and propolis (aqueous and ethanolic extract), respectively (Campos et al., 1998), once per day for 18 consecutive days.
Peripheral blood was collected from the caudal vein of all the animals 24 h after the final treatment dose. Blood smears were made immediately, following routine procedures, and stained with Leishman’s stain for white blood cell differentiation. The lungs were removed immediately after administering a formalin injection via the trachea to evacuate air from the alveoli, and they were preserved in 10% formalin for histopathological studies.
2.7. Peripheral white blood count
The blood smears were fixed with absolute methanol and stained with Leishman’s stain for white blood cell differentiation. Approximately 300 inflammatory cells counted by two researchers using a light microscope were subsequently classified as neutrophils, monocytes, lymphocytes, eosinophils, and basophils (Chang et al., 2004).
2.8. Histopathological examination
The formalin-infused lungs were embedded in paraffin wax blocks and sliced into 6 μm-thick sections. The slides were examined under a light microscope after staining with haematoxylin and eosin. The scoring of the airway inflammation was blindly investigated by two separate investigators. A standard scale (0–3) was used to evaluate the severity of perivascular and peribronchial inflammation (Park et al., 2001) and to assign value: 0 for no evident inflammation, 1+ for foci or infrequent cuffing with inflammatory cells, 2+ if most bronchi or vessels were surrounded by a thin layer, and 3+ for the most severe inflammation (more than five cells deep) of inflammatory cells. The average of the peribronchial and perivascular inflammation scores was used to define total lung inflammation.
2.9. Statistical analysis
Statistical Package for the Social Sciences (©SPSS Inc. 1989–1999, Chicago, IL, USA) was used for statistical analyses. The test of normal distribution by the Kolmogorov–Smirnov test was used to determine parametric and nonparametric data. The parametric data were analysed by a one-way ANOVA test used for analysis of variance within and between the groups. The least significance difference test was used as a post hoc test for multiple comparisons between the tested groups after the analysis of variance. For nonparametric data of categorical variables, comparisons were carried out by Chi-square (χ2) test. The inflammatory score data were expressed as means ± standard deviation. The significant statistical difference was accepted at p < 0.05, and all p-values reported are double-sided (two-tailed distributions).
3. Results
3.1. Haematological studies
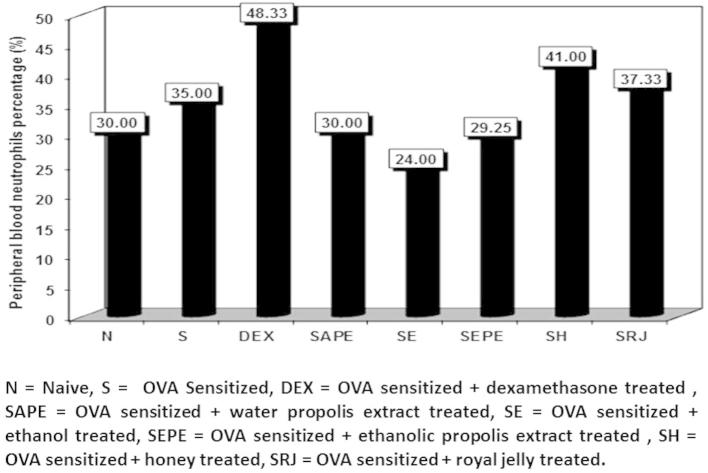
Examination of the blood smears showed significant differences in the differential peripheral blood counts of the different animal groups. As shown in Fig. 1, the percentages of peripheral blood neutrophils were significantly higher in the dexamethasone-treated group (48.33%) than in the other groups. Thus, dexamethasone had the best results as a standard corticosteroid drug. The other groups, with naïve and sensitised non-treated animals, exhibited insignificant differences in neutrophil percentages, but there were significant increases in the royal jelly treated group and significant decreases in the dexamethasone, sensitised honey, and sensitised royal jelly treated groups (Figure 2, Figure 3).
Figure 1.
The percentage of peripheral blood neutrophils in normal control, cone albumin sensitised, dexamethasone, water and ethanol propolis extract, ethanol, honey and royal jelly sensitised and treated groups.
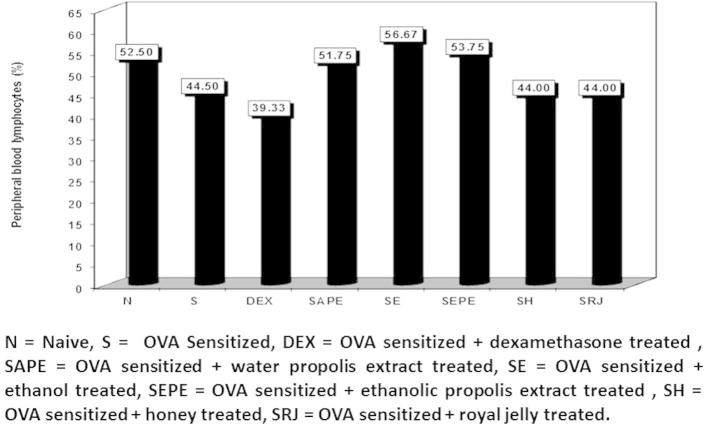
Figure 2.
The percentage of peripheral blood lymphocytes in normal control, cone albumin sensitised, dexamethasone, water and ethanol propolis extract, ethanol, honey and royal jelly sensitised and treated groups.
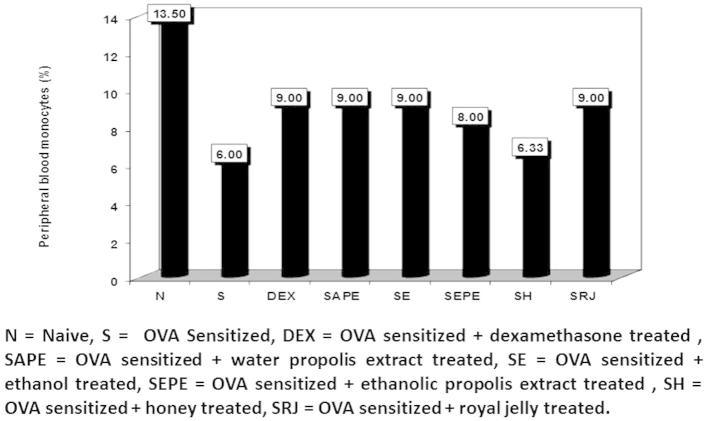
Figure 3.
The percentage of peripheral blood monocytes in normal control, cone albumin sensitised, dexamethasone, water and ethanol propolis extract, ethanol, honey and royal jelly sensitised and treated groups.
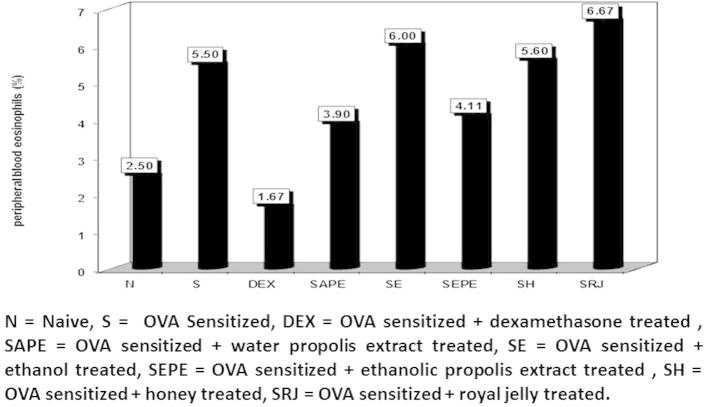
There was a significant decrease in percentage of monocyte count in the sensitised untreated group (6.0%), which is an indicator of successful asthma inducing. The same was observed for the sensitised honey treated group (6.3%). There was a significant increase in the percentage of eosinophils in the sensitised group (asthmatic mice) compared to the naïve one. On the contrary, there were significant increases in the ethanol, honey, and royal jelly -treated groups compared with the naïve group (6.0%, 5.6%, and 6.66%, respectively), and insignificant differences in the sensitised untreated group. These increases might be due to the immunostimulatory characteristics of honey, and royal jelly (Fig. 4).
Figure 4.
The percentage of peripheral blood eosinophils in normal control, cone albumin sensitised, dexamethasone, water and ethanol propolis extract, ethanol, honey and royal jelly sensitised and treated groups.
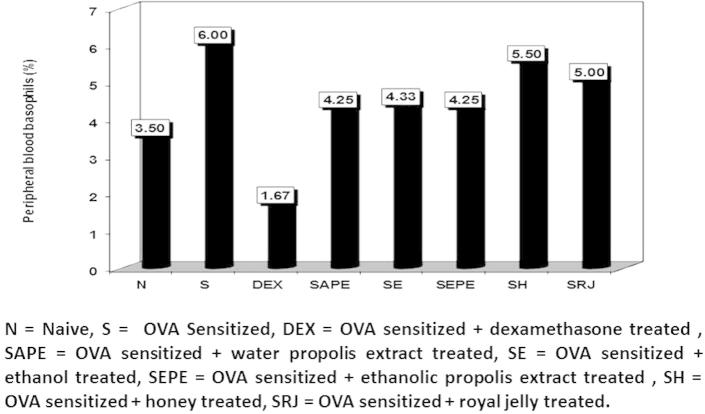
Fig. 5 illustrates the basophil percentages of the different animal groups. The highest level was recorded in the sensitised group (6.0%) compared to the naïve group (3.5%). There was a significant decrease in the dexamethasone treated group (1.67%) compared to the sensitised untreated group. The other groups showed insignificant differences compared with the naïve group, with the exception of the honey (5.5%) and royal jelly (5.0%) treated groups, which increased significantly compared with the naïve group, but which differed insignificantly from the sensitised untreated group.
Figure 5.
The percentage of peripheral blood basophils in normal control, cone albumin sensitised, dexamethasone, water and ethanol propolis extract, ethanol, honey and royal jelly sensitised and treated groups.
The groups treated with propolis extracts exhibited considerable ameliorative effects against asthma. Propolis has flavonoids and phenolics, which may have antioxidative effects, thus explaining that result. Otherwise, the sensitised and honey or royal jelly treated groups showed an increase in asthma cascade events by increasing the inflammatory cells. These results might be due to the immunostimulatory and vasodilatory effects of royal jelly and honey, which are antagonistic to bronchial asthma cases.
3.2. Histopathological examination
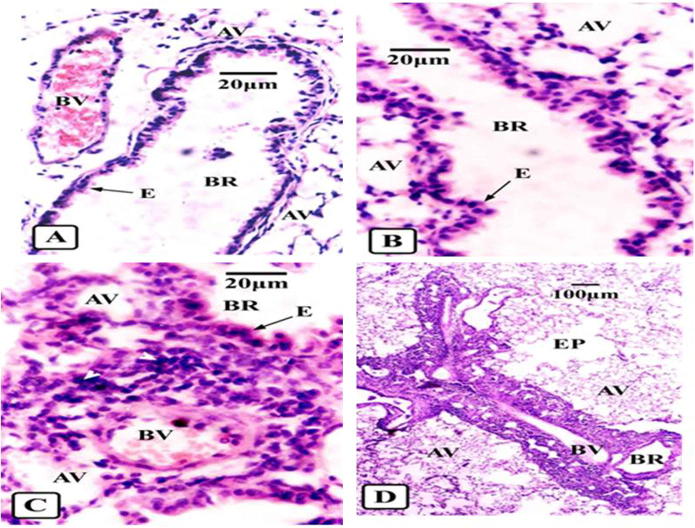
The normal lungs of the naïve mice revealed an intact bronchiolar structure of a pseudostratified epithelial layer lining the bronchi and bronchioles, normal alveoli, and normal blood vessels (Fig. 6a). Histopathological examination of the pulmonary tissue included the definition of peribronchial and perivascular inflammatory cell scores. The inflammatory cells, including eosinophils, monocytes, neutrophils, and lymphocytes are one of the allergic asthma indicators. The score of inflammation was considered 1+ if the inflammatory cells were occasional (Fig. 6b), 2+ if they were all-around peribronchial or perivascular and less than five layers deep (Fig. 6c), and 3+ if all-around inflammation with more than five layers (Fig. 6d).
Figure 6.
Light photomicrographs of sections in lungs of different animal groups showing control for different degrees of asthma (H & E). (A) Normal small bronchus (BR) without detectable inflammatory reaction showing intact epithelium (E), blood vessel (BV) and alveoli (AV). (B) Focal perivascular inflammatory cells (arrow head) around small bronchus (BR). Slight oedema appears in epithelial layer (E) and inflation of alveoli (AV) in mild asthma. (C) All-around peribronchial (BR) and perivascular (BV) inflammation (arrow heads) forming less than 5 cell-deep layers with more oedematous epithelium (E) and inflation of alveoli (AV) in mild persistent asthmatic mice. (D) All-around peribronchial (BR) and perivascular (BV) inflammation (arrow heads) forming more than 5 cells-deep layers with inflated alveoli (AV) and burst fused others forming emphysema (EP) in severe asthmatic cases.
Some of the asthma histopathological findings were observed as shedding or oedema of some epithelial cells in focal cases (Fig. 6b). The dilation of alveoli in focal or mild cases is one of the common findings in asthmatics, as the alveoli become filled with air as a result of airways constriction (Fig. 6c). In severe cases, there is emphysema, in which the alveoli burst and are confused with each other due to their hyperinflation with air, which is an irreversible complication of airways constriction (Fig. 6d). The inflammatory cell scores of the studied groups are summarised in Table 1. The sensitised treated propolis extract groups showed greater significant decreases in inflammatory scores than the other treatment groups compared with the sensitised untreated group. These results are in agreement with the previous data of peripheral blood cells.
Table 1.
Histopathological examination of lung tissues in normal control, cone albumin sensitised, dexamethasone, water and ethanol propolis extract, honey and Royal Jelly sensitised and treated groups and their statistical differences using Chi-square (χ2) test.
| N | S | DEX | SAPE | SE | SEPE | SH | SRJ | |
|---|---|---|---|---|---|---|---|---|
| 1+ | 2 | 1 | 2 | 2 | 1 | 1 | 0 | 0 |
| 2+ | 1 | 0 | 1 | 1 | 2 | 2 | 0 | 2 |
| 3+ | 0 | 2 | 0 | 0 | 0 | 0 | 3 | 1 |
| P | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |||
| S | a | b | b | b | b | a | a |
N = Naive, S = Sensitised, DEX = Dexamethasone, SAPE = Sensitised water propolis extract, SE = Sensitised ethanol, SEPE = Sensitised ethanolic propolis extract, SH = Sensitised honey, SRJ = Sensitized royal jelly.
1+ = focal inflammation.
2+ = all around inflammation (<5 layers cells deep).
3+ = all around inflammation (>5 layers cells deep).
P = probability.
S = significance.
a = significant increase in inflammatory cells comparable to the naive group.
b = significant decrease in inflammatory cells comparable to the sensitised group.
4. Discussion
Lung function can potentially be affected in many patients by chronic inflammatory disorders, finally resulting in asthma. Due to interaction of inflammatory cells with resident cells, chronic inflammation and clinical manifestations occur and asthmatic inflammation develops. The clinical manifestations include bronchial epithelial damage, airway smooth muscle spasm, airway mucus secretion, further inflammation, and airway oedema and narrowing (Lemanske, 2000). Animal models, including mice, are used extensively in the study of allergic disease mechanisms because of their silent features of allergic airway inflammation in humans and other properties such as low cost and a well-known immune system. Conalbumin is a known aeroallergen that is used as a successful asthma inducer. The sensitised non-treated group of mice in this study exhibited significantly increased peripheral blood eosinophils and basophils. However, lymphocytes, monocytes, and neutrophils decreased in the sensitised group compared with the naïve (non-sensitised) group. This result indicates successful sensitisation and development of an allergic model. Peripheral blood eosinophilia has been documented in bronchial asthma in both human (Simon, 2013) and murine (Nakashima et al., 2014) models.
In this work, a significant increase in the number of inflammatory cells invading the lungs in the sensitised non-treated group, compared to the control group, was observed. This finding is in agreement with some previous studies that revealed invasion of the lung tissues by eosinophils, lymphocytes, neutrophils, and monocytes after sensitisation in murine models of atopic asthma (Blyth et al., 1996, Kumar et al., 2003). That work showed that the dexamethasone-treated group exhibited a significant reduction in percentage of circulating eosinophils compared to the sensitised non-treated group, and an insignificant increase compared to the control group. Dexamethasone is a golden drug in bronchial asthma treatment. It suppresses Th2, which leads to a decline in the percentage of eosinophils and basophils, hence releasing the ability of Th1 (which was suppressed by the dominance of Th2 due to asthma) to express their cytokines. In turn, these cytokines elevate the percentage of neutrophils, monocytes, and lymphocytes (autocrine). The reduction of inflammatory cells in the circulation system and in tissues by systemically administered corticosteroids has been reported in allergic murine asthma models (De Bie et al., 1996, Trifilieff et al., 2000). In that work, sensitised aqueous propolis extract and sensitised ethanolic propolis extract treated groups exhibited significant increases in the percentage of peripheral blood neutrophils, lymphocytes, and monocytes.
The effect of propolis extract was evaluated previously and found to be a better therapeutic and immunomodulatory agent for asthma (Araujo et al., 2012). In that work, the sensitised and honey treated group showed a significant decrease in Th1-mediated cells (neutrophils, lymphocytes, and monocytes) and a significant increase in Th2-mediated cells (eosinophiles and basophiles) in peripheral blood and lung tissues than in the naïve group. Honey is known as a wound-healing and tissue-repairing agent that acts by stimulating the inflammatory cytokines from monocytic cells (Tonks et al., 2003). These characteristics may stimulate inflammatory cell proliferation, which might explain the increasing cascade of events of bronchial asthma disease. In this study, administration of royal jelly at a dose of 1 g/kg/day resulted in a significant increase in peripheral blood eosinophilia and lung inflammatory cell accumulation compared with the control (naïve) group. At the same time, there were insignificant differences between this group and the sensitised untreated group. Similarly, increased atopic manifestations were observed in a study on royal jelly consumption in Hong Kong (Leung et al., 1997). The lipid peroxidation model was used to evaluate the antioxidative effects of honeys, royal jelly, and propolis (Nagai et al., 2001). Their superoxide-scavenging activities, arranged in a decreasing manner, are propolis > royal jelly > honey; these findings greatly support the results of the current study.
5. Conclusion
The percentage of circulating and lung-infiltrated eosinophils and basophils decreased significantly in the sensitised and propolis extract treated groups. In addition, the percentages were still insignificantly higher than those of the naïve and dexamethasone-treated groups. These findings support the hypothesis that propolis extracts may have an anti-inflammatory role, and hence, ameliorative effects in allergic asthma by free radical scavenging. Indeed, there is a need for new or alternative approaches to the control of this disease.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgements
The authors would like to thank the staff members of Biotechnology Research Laboratories, Gastroenterology Surgery Centre, Mansoura University, Mansoura, Egypt, for their assistance.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ali A.T., Al-Swayeh O.A., Al-Humayed M.S. Natural honey prevents ischemia-reperfusion-induced gastric mucosal lesions and increased vascular permeability in rats. Eur. J. Gastroenterol. Hepatol. 1997;9:1101–1105. doi: 10.1097/00042737-199711000-00014. [DOI] [PubMed] [Google Scholar]
- Araujo M.A.R., Libério S.A., Guerra R.N.M., Ribeiro M.N.S., Nascimento F.R.F. Mechanisms of action underlying the anti-inflammatory and immunomodulatory effects of propolis: a brief review. Rev. Bras. Farm. 2012;22:208–219. [Google Scholar]
- Bara A.I., Barley E.A. Caffeine for asthma. Cochrane Database. Cochrane Database Syst. Rev. 2001;4:CD001112. doi: 10.1002/14651858.CD001112. [DOI] [PubMed] [Google Scholar]
- Barnes P.M., Bloom B., Nahin R.L. Complementary and alternative medicine use among adults and children. United States Natl. Health Stat. Rep. 2008;12:1–23. [PubMed] [Google Scholar]
- Blyth D.I., Pedrick M.S., Savage T.J. Lung inflammation and epithelial changes in a murine model of atopic asthma. Am. J. Respir. Cell Mol. Biol. 1996;14:425–438. doi: 10.1165/ajrcmb.14.5.8624247. [DOI] [PubMed] [Google Scholar]
- Boeru, V., Derevici, A., 1978. Some chemical and physical data on Romanian propolis. Apimondia “propolis”, Bucharest. 19–26.
- Bogdanovt, S., 2014. Propolis: Composition, Health, Medicine: A Review, Bee Product Science, www.bee-hexagon.net February.
- Chang Y.S., Kim Y.K., Kim T.B., Kang H.R., Kim S.S., Bahn J.W., Min K.U., Kim Y.Y., Cho S.H. Airway inflammation and allergen specific IgE production may persist longer than airway hyperresponsiveness in mice. J. Korean Med. Sci. 2004;19:69–73. doi: 10.3346/jkms.2004.19.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bie J.J., Hessel E.M., Van Ark I., Van Esch B., Hofman G., Nijkamp F.P., Van Oosterhout A.J. Effect of dexamethasone and endogenous corticosterone on airway hyperresponsiveness and eosinophilia in the mouse. Br. J. Pharmacol. 1996;119:1484–1490. doi: 10.1111/j.1476-5381.1996.tb16062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCampos R.O., Paulino N., da Silva C.H., Scremin A., Calixto J.B. Anti-hyperalgesic effect of an ethanolic extract of propolis in mice and rats. J. Pharm. Pharmacol. 1998;50:1187–1193. doi: 10.1111/j.2042-7158.1998.tb03333.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg D.M., Davis R.B., Ettner S.L., Appel S., Wilkey S., Van Rompay M., Kessler R.C. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- El Denshary E.S., Al-Gahazali M.A., Mannaa F.A., Salem H.A., Hassan N.S., Abdel-Wahhab M.A. Dietary honey and ginseng protect against carbon tetrachloride-induced hepatonephrotoxicity in rats. Exp. Toxicol. Pathol. 2012;64:753–760. doi: 10.1016/j.etp.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Ernst E. Complementary therapies for asthma: what patients use. J. Asthma. 1998;35:667–671. doi: 10.3109/02770909809048969. [DOI] [PubMed] [Google Scholar]
- Fujii A., Kobayashi S., Kuboyama N., Furukawa Y., Kaneko Y., Ishihama S., Yamamoto H., Tamura T. Augmentation of wound healing and royal jelly (RJ) in streptozotocin-diabetic rats. Jpn. J. Pharmacol. 1990;53:331–337. doi: 10.1254/jjp.53.331. [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Imai J., Fujiwara M., Yaeshima T., Kawashima T., Kobayashi K.A. Potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J. Biol. Chem. 1990;15 11333–113. [PubMed] [Google Scholar]
- Global Strategy for Asthma Management and Prevention; Updated December 2011.
- Hockenberry M.J., Wilson D. 8th ed. Mosby Elsevier; St. Louis: 2007. Wong’s nursing care of infants and children. [Google Scholar]
- Inoue S., Koya-Miyata S., Ushio S., Iwaki K., Ikeda M., Kurimoto M. Royal jelly prolongs the life span of C3H/HeJ mice: correlation with reduced DNA damage. Exp. Gerontol. 2003;38:965–969. doi: 10.1016/s0531-5565(03)00165-7. [DOI] [PubMed] [Google Scholar]
- Keane-Myers A.M., Gause W.C., Finkelman F.D., Xhou X.D., Wills-Karp M. Development of murine allergic asthma is dependent upon B7–2 co-stimulation. J. Immunol. 1998;160:1036–1043. [PubMed] [Google Scholar]
- Kumar R.K., Herbert C., Thomas P.S. Inhibition of inflammation and remodeling by roflumilast and dexamethasone in murine chronic asthma. J. Pharmacol. Exp. Ther. 2003;307:349–355. doi: 10.1124/jpet.103.053819. [DOI] [PubMed] [Google Scholar]
- Lemanske R.F. Inflammatory events in asthma: An expanding equation. J. Allergy Clin. Immunol. 2000;105:633–636. doi: 10.1067/mai.2000.106155. [DOI] [PubMed] [Google Scholar]
- Leung R.Ho.A., Chan J., Choy D., Lai C.K.W. Royal jelly consumption and hypersensitivity in the community. Clin. Exp. Allergy. 1997;27:333–336. [PubMed] [Google Scholar]
- Li X.M., Brown L. Efficacy and mechanisms of action of traditional Chinese medicines for treating asthma and allergy. J. Allergy Clin. Immunol. 2009;123:297–306. doi: 10.1016/j.jaci.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.M., Huang C.K., Zhang T.F., Teper A.A., Srivastava K., Schofield B.H., Sampson H.A. The Chinese herbal medicine formula MSSM-002 suppresses allergic airway hyperreactivity and modulates TH1/TH2 responses in a murine model of allergic asthma. J. Allergy Clin. Immunol. 2000;106:660–668. doi: 10.1067/mai.2000.110102. [DOI] [PubMed] [Google Scholar]
- Mahmoud A.M. Studies on bee venom and its medical uses. Int. J. Adv. Res. Technol. 2012;1(2) [Google Scholar]
- Manyi-Loh C.E., Clarke A.M., Ndip R.N. An overview of honey: Therapeutic properties and contribution in nutrition and human health. Afr. J. Microbiol. Res. 2011;5:844–852. [Google Scholar]
- Molan C. Why honey is effective as a medicine. I. Its use in modern medicine. Bee World. 1999;80:80–92. [Google Scholar]
- Nagai T., Inoue R. Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004;84:181–186. [Google Scholar]
- Nagai T., Sakai M., Inoue R., Inoue H., Suzuki N. Antioxidative activities of some commercially honeys, royal jelly, and propolis. Food Chem. 2001;75:237–240. [Google Scholar]
- Nakashima T., Hayashi T., Mizuno T. Regulation of the Development of Asthmatic Inflammation by In Situ CD4(+)Foxp3 (+) T Cells in a Mouse Model of Late Allergic Asthma. Inflammation. 2014;37:1642–1653. doi: 10.1007/s10753-014-9892-3. [DOI] [PubMed] [Google Scholar]
- Park Y.J., Chang Y.S., Lee S.W., Cho S.Y., Kim Y.K., Min K.U., Kim Y.Y., Cho S.H., Sung Y.C. The enhanced effect of a hexameric deoxyriboguanosine run conjugation to CpG oligoeoxynucleotides on the protection against allergic asthma. J. Allergy Clin. Immunol. 2001;108:570–576. doi: 10.1067/mai.2001.118517. [DOI] [PubMed] [Google Scholar]
- Schmidt J.O. Chemical composition and application. In: Mizrahi, Lensky, editors. Bee Products. Plenum Press; New York: 1996. pp. 15–20. [Google Scholar]
- Silici S., Ekmekcioglu O., Eraslan G., Demirtas A. Antioxidative effect of royal jelly in cisplatin-induced testes damage. Urology. 2009;15:545–551. doi: 10.1016/j.urology.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Simon H.U. Allergic inflammation: focus on eosinophils. Allergy. 2013;68:823–824. doi: 10.1111/all.12231. [DOI] [PubMed] [Google Scholar]
- Sver L., Orsolic N., Tadic Z., Njari B., Valpotic I., Basic I. A royal jelly as a new potential immunomodulator in rats and mice. Comp. Immunol. Microbiol. Infect. Dis. 1996;15:31–38. doi: 10.1016/0147-9571(95)00020-8. [DOI] [PubMed] [Google Scholar]
- Tinkelman D.G., Reed C.E., Nelson H.S., Offord K.P. Aerosol beclomethasone dipropionate compared with theophylline as primary treatment of chronic, mild to moderately severe asthma in children. Pediatrics. 1993;92:64–77. [PubMed] [Google Scholar]
- Tonks A.J., Cooper R.A., Jones K.P., Blair S., Parton J., Tonks A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine. 2003;21:242–247. doi: 10.1016/s1043-4666(03)00092-9. [DOI] [PubMed] [Google Scholar]
- Trifilieff A., El-Hashim A., Bertrand C. Time course of inflammatory and remodeling events in a murine model of asthma: effect of steroid treatment. Am. J. Physiol. Lung Cell Physiol. 2000;279:1120–1128. doi: 10.1152/ajplung.2000.279.6.L1120. [DOI] [PubMed] [Google Scholar]
- Viuda-Martos M., Ruiz-Navajas Y., Fernández-López J., Pérez-Alvarez J.A. Functional properties of honey, propolis, and royaljelly. J. Food Sci. 2008;73:117–124. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- Wagh V.D. Propolis: A Wonder Bees Product and Its Pharmacological Potentials. Adv. Pharmacol. Sci. 2013;2013:1–11. doi: 10.1155/2013/308249. Article ID: 308249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziment I. Recent advances in alternative therapies. Curr. Opin. Pulm. Med. 2000;6:71–78. doi: 10.1097/00063198-200001000-00013. [DOI] [PubMed] [Google Scholar]