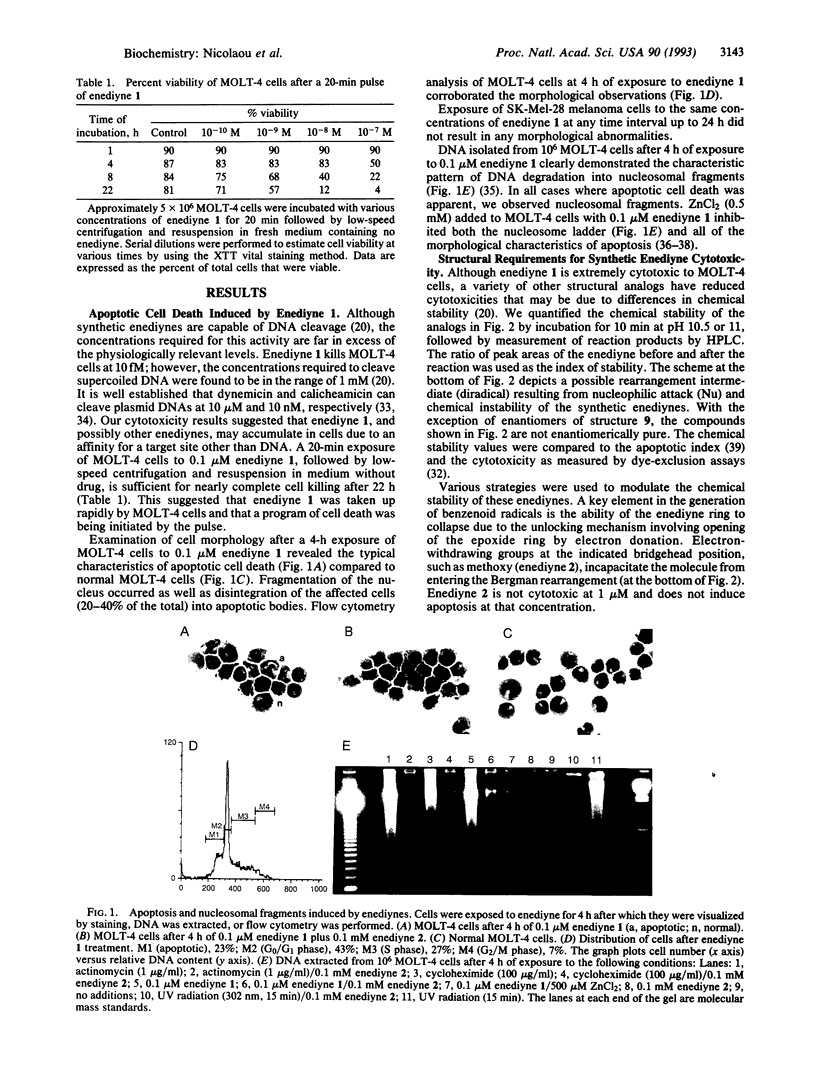
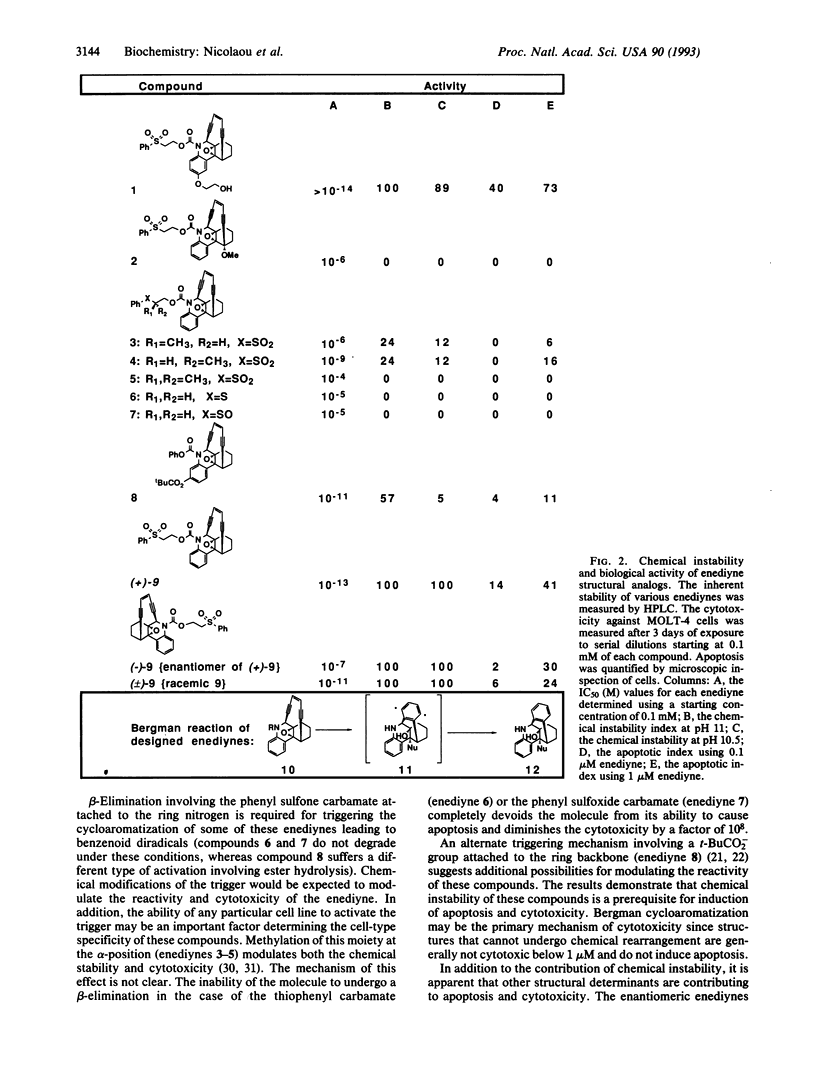
Abstract
The naturally occurring enediyne antibiotics are a unique class of antitumor drugs that combine reactive enediynes with additional structural features conferring affinity for DNA. Dynemicin A, in which an enediyne core is attached to an anthraquinone group capable of DNA intercalation, readily cleaves double-stranded DNA. This activity is thought to be the basis of its potent antitumor cytotoxicity. To investigate cell-specific mechanisms of cytotoxicity in the absence of DNA affinity, we have synthesized a variety of dynemicin-like enediynes that lack the anthraquinone moiety. We have found that the cytotoxicity of these compounds is dependent on their chemical instability and their enantiomeric form. Their selective toxicity results from a potent induction of apoptosis primarily in human leukemic cells. A group of synthetic enediynes were designed to be highly stable. These compounds were found to inhibit apoptotic cell death. This inhibition was observed in competition with the chemically unstable enediynes, including dynemicin and calicheamicin. The stable synthetic enediynes could also block the apoptotic morphology induced by unrelated cytotoxic agents such as cycloheximide, actinomycin D, and ultraviolet radiation. The results suggest that the cellular target(s) of synthetic enediynes may play a central role in regulating programmed cell death; a specific receptor-ligand interaction is proposed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bansal N., Houle A., Melnykovych G. Apoptosis: mode of cell death induced in T cell leukemia lines by dexamethasone and other agents. FASEB J. 1991 Feb;5(2):211–216. doi: 10.1096/fasebj.5.2.2004665. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Dedon P. C., Goldberg I. H. Influence of thiol structure on neocarzinostatin activation and expression of DNA damage. Biochemistry. 1992 Feb 25;31(7):1909–1917. doi: 10.1021/bi00122a003. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall E., Wyllie A. H., Morris R. G. Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunology. 1985 Oct;56(2):351–358. [PMC free article] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- HAMBURGER J., RICHET G. Enseignements tirés de la pratique du rein artificiel pour l'interprétation des désordres électrolytiques de l'urémie aiguë. Rev Fr Etud Clin Biol. 1956 Jan;1(1):39–55. [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Davidson N. E., Isaacs J. T. Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res. 1991 Jan 1;51(1):162–166. [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Isaacs J. T. Programmed cell death during regression of PC-82 human prostate cancer following androgen ablation. Cancer Res. 1990 Jun 15;50(12):3748–3753. [PubMed] [Google Scholar]
- Lesser B., Bruchovsky N. The effects of testosterone, 5 -dihydrotestosterone and adenosine 3',5'-monophosphate on cell proliferation and differentiation in rat prostate. Biochim Biophys Acta. 1973 May 18;308(3):426–437. doi: 10.1016/0005-2787(73)90336-5. [DOI] [PubMed] [Google Scholar]
- Meyaard L., Otto S. A., Jonker R. R., Mijnster M. J., Keet R. P., Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992 Jul 10;257(5067):217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- Nicolaou K. C., Dai W. M., Tsay S. C., Estevez V. A., Wrasidlo W. Designed enediynes: a new class of DNA-cleaving molecules with potent and selective anticancer activity. Science. 1992 May 22;256(5060):1172–1178. doi: 10.1126/science.256.5060.1172. [DOI] [PubMed] [Google Scholar]
- Nuñez G., Hockenbery D., McDonnell T. J., Sorensen C. M., Korsmeyer S. J. Bcl-2 maintains B cell memory. Nature. 1991 Sep 5;353(6339):71–73. doi: 10.1038/353071a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Savill J. S., Henson P. M., Haslett C. Phagocytosis of aged human neutrophils by macrophages is mediated by a novel "charge-sensitive" recognition mechanism. J Clin Invest. 1989 Nov;84(5):1518–1527. doi: 10.1172/JCI114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989 Mar;83(3):865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. F., Sahai B. M., Green D. R. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature. 1989 Jun 22;339(6226):625–626. doi: 10.1038/339625a0. [DOI] [PubMed] [Google Scholar]
- Sugiura Y., Shiraki T., Konishi M., Oki T. DNA intercalation and cleavage of an antitumor antibiotic dynemicin that contains anthracycline and enediyne cores. Proc Natl Acad Sci U S A. 1990 May;87(10):3831–3835. doi: 10.1073/pnas.87.10.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szende B., Srkalovic G., Groot K., Lapis K., Schally A. V. Regression of nitrosamine-induced pancreatic cancers in hamsters treated with luteinizing hormone-releasing hormone antagonists or agonists. Cancer Res. 1990 Jun 15;50(12):3716–3721. [PubMed] [Google Scholar]
- Teh H. S., Kisielow P., Scott B., Kishi H., Uematsu Y., Blüthmann H., von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988 Sep 15;335(6187):229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- Telford W. G., King L. E., Fraker P. J. Evaluation of glucocorticoid-induced DNA fragmentation in mouse thymocytes by flow cytometry. Cell Prolif. 1991 Sep;24(5):447–459. doi: 10.1111/j.1365-2184.1991.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Terai C., Kornbluth R. S., Pauza C. D., Richman D. D., Carson D. A. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J Clin Invest. 1991 May;87(5):1710–1715. doi: 10.1172/JCI115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Uesawa Y., Sugiura Y. Heat-induced DNA cleavage by esperamicin antitumor antibiotics. Biochemistry. 1991 Sep 24;30(38):9242–9246. doi: 10.1021/bi00102a016. [DOI] [PubMed] [Google Scholar]
- Walker S., Landovitz R., Ding W. D., Ellestad G. A., Kahne D. Cleavage behavior of calicheamicin gamma 1 and calicheamicin T. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4608–4612. doi: 10.1073/pnas.89.10.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring P. DNA fragmentation induced in macrophages by gliotoxin does not require protein synthesis and is preceded by raised inositol triphosphate levels. J Biol Chem. 1990 Aug 25;265(24):14476–14480. [PubMed] [Google Scholar]
- Weislow O. S., Kiser R., Fine D. L., Bader J., Shoemaker R. H., Boyd M. R. New soluble-formazan assay for HIV-1 cytopathic effects: application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J Natl Cancer Inst. 1989 Apr 19;81(8):577–586. doi: 10.1093/jnci/81.8.577. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. The biology of cell death in tumours. Anticancer Res. 1985 Jan-Feb;5(1):131–136. [PubMed] [Google Scholar]
- Zychlinsky A., Prevost M. C., Sansonetti P. J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992 Jul 9;358(6382):167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]



