Abstract
Background: Recent studies suggest that meat intake is associated with diabetes-related phenotypes. However, whether the associations of meat intake and glucose and insulin homeostasis are modified by genes related to glucose and insulin is unknown.
Objective: We investigated the associations of meat intake and the interaction of meat with genotype on fasting glucose and insulin concentrations in Caucasians free of diabetes mellitus.
Design: Fourteen studies that are part of the Cohorts for Heart and Aging Research in Genomic Epidemiology consortium participated in the analysis. Data were provided for up to 50,345 participants. Using linear regression within studies and a fixed-effects meta-analysis across studies, we examined 1) the associations of processed meat and unprocessed red meat intake with fasting glucose and insulin concentrations; and 2) the interactions of processed meat and unprocessed red meat with genetic risk score related to fasting glucose or insulin resistance on fasting glucose and insulin concentrations.
Results: Processed meat was associated with higher fasting glucose, and unprocessed red meat was associated with both higher fasting glucose and fasting insulin concentrations after adjustment for potential confounders [not including body mass index (BMI)]. For every additional 50-g serving of processed meat per day, fasting glucose was 0.021 mmol/L (95% CI: 0.011, 0.030 mmol/L) higher. Every additional 100-g serving of unprocessed red meat per day was associated with a 0.037-mmol/L (95% CI: 0.023, 0.051-mmol/L) higher fasting glucose concentration and a 0.049–ln-pmol/L (95% CI: 0.035, 0.063–ln-pmol/L) higher fasting insulin concentration. After additional adjustment for BMI, observed associations were attenuated and no longer statistically significant. The association of processed meat and fasting insulin did not reach statistical significance after correction for multiple comparisons. Observed associations were not modified by genetic loci known to influence fasting glucose or insulin resistance.
Conclusion: The association of higher fasting glucose and insulin concentrations with meat consumption was not modified by an index of glucose- and insulin-related single-nucleotide polymorphisms. Six of the participating studies are registered at clinicaltrials.gov as NCT0000513 (Atherosclerosis Risk in Communities), NCT00149435 (Cardiovascular Health Study), NCT00005136 (Family Heart Study), NCT00005121 (Framingham Heart Study), NCT00083369 (Genetics of Lipid Lowering Drugs and Diet Network), and NCT00005487 (Multi-Ethnic Study of Atherosclerosis).
Keywords: gene–diet interaction, glucose, insulin, meat intake, diet, meta-analysis
INTRODUCTION
Hyperglycemia and hyperinsulinemia are leading risk factors for type 2 diabetes, and the worldwide burden of these risk factors continues to rise. In 2011, the WHO estimated that age-standardized fasting plasma glucose concentrations have increased by 0.07–0.09 mmol/L per decade worldwide since 1980 (1). Likewise, between 1988 and 2002, the mean concentration of fasting insulin increased 5% among nondiabetic adults in the United States (2). The rise in fasting glucose and insulin concentrations may be attributable to recent changes in lifestyle, including obesity and the adoption of Western diets high in processed meat and red meat, as well as other lifestyle-related changes. Recent studies have consistently shown that processed meat intake is associated with a higher risk of diabetes (3–16). Although the mechanism by which processed meat intake influences diabetes-related traits is complex, nitrosamines and advanced glycation end products are present in processed meats at manufacturing or formed by interactions of amino acids and nitrates within the body, and have been shown to have a toxic effect on β cells and promote the development of impaired glucose tolerance and insulin resistance (17–22).
Genome-wide association studies (GWASs)60 have identified and replicated several loci related to fasting glucose and insulin resistance in Caucasian populations (23–29). These genes are thought to encode proteins that may predispose individuals to diabetes by altering β cell function and insulin secretion or promoting cellular insulin resistance. Nevertheless, the proportion of risk attributable to these genes remains relatively small, and it is possible that part of the missing heritability in these phenotypes may be explained in part by gene–environment interactions. To date, few studies have detected gene–diet interactions in relation to diabetes-related traits (30–33), and large studies are needed to examine the potential interaction of genes and dietary factors with fasting glucose and insulin. Because many of the known diabetes-related genetic variants might affect β cell function or insulin resistance, and intake of processed meats might also affect β cell function or insulin resistance, we hypothesized that diabetes-related genetic variants that may affect β cell function or insulin resistance sensitize carriers of these variants to the effects of processed meats on β cell function and insulin resistance, resulting in a gene–diet interaction.
Using available diet and genetic data from 14 studies that are part of the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium (34), we investigated the associations of processed meat and unprocessed red meat intake with fasting glucose and insulin concentrations in Caucasians without diabetes mellitus. Additionally, we examined potential interactions of processed meat and unprocessed red meat intake with single-nucleotide polymorphisms (SNPs) previously identified as related to fasting glucose/β cell function and insulin resistance through GWASs in relation to fasting glucose and insulin concentrations.
METHODS
Study sample
The study sample comprised up to 50,345 participants from 14 cohorts that are part of the CHARGE consortium. Contributing cohorts included the Atherosclerosis Risk in Communities study; the Cardiovascular Health Study; the Family Heart Study; the Framingham Heart Study; the Greek Health Randomized Aging Study (GHRAS); the Gene–Lifestyle Interactions and Complex Traits Involved in Elevated Disease Risk (GLACIER) study; the Genetics of Lipid Lowering Drugs and Diet Network; the Health, Aging, and Body Composition (Health ABC) study; the Helsinki Birth Cohort Study; the Malmӧ Diet and Cancer Study; the Multi-Ethnic Study of Atherosclerosis; the Rotterdam Study (RS); the Hellenic Study of Interactions between SNPs and Eating in Atherosclerosis Susceptibility; and the Young Finns Study. This analysis was restricted to Caucasian participants free of prevalent diabetes mellitus (as defined by self-reported diabetes, fasting glucose ≥7 mmol/L, or use of diabetes drugs). Details on the design of each study are described in Supplemental Table 1. All procedures followed were in accordance with the Helsinki Declaration of 1975 as revised in 1983. Each participating study had local institutional review board approval, and written informed consent was obtained from all participants.
Dietary assessment
Details of the dietary assessment method for each participating cohort are described in Supplemental Table 2. Briefly, 13 cohorts used food frequency questionnaires (FFQs) to collect dietary data and one cohort used a combination of an FFQ, dietary interview, and 7-d food record. For this report, we were most interested in processed meat (e.g., hot dogs, lunch meat, breakfast sausage) and unprocessed red meat (e.g., hamburger, steak, roast) as primary dietary exposures. The individual meat line items included on each cohort’s FFQ differed and are listed in Supplemental Table 2. To obtain measures of average daily meat intake, the total daily servings for each food line item on the FFQ (or documented food item from the food diary/interview) were summed for all relevant foods. Consistent with previous studies (5), we considered 50 g and 100 g to be one serving of processed meat and unprocessed red meat, respectively.
Genotyping, SNP selection, and creation of genetic risk scores
Details on genotyping for each participating cohort are described in Supplemental Table 3. For the purposes of this meta-analysis, we included only SNPs that have been shown to be associated with fasting glucose and with known β cell function, or that have been shown to be associated with insulin resistance in previous GWASs. We identified 36 SNPs related to fasting glucose that have known β cell function (Supplemental Table 4). These SNPs included 16 SNPs from the Meta-Analyses of Glucose and Insulin-related Traits Consortium (23), 8 SNPs from another large meta-analysis of 8 GWASs (24), 5 SNPs from a meta-analysis of GWASs from the Diabetes Genetics Replication and Meta-Analysis consortium (25), 5 SNPs from a GWAS of 2 large Finnish cohorts (29), and 2 additional SNPs from GWASs of the Diabetes Genetics Initiative and Wellcome Trust Case Control Consortium (26, 28). We also identified 9 SNPs that have been shown to be associated with insulin resistance in previous meta-analyses of GWASs (Supplemental Table 5); specifically, 2 of the fasting insulin SNPs were identified from the Meta-Analyses of Glucose and Insulin-Related Traits Consortium (23) and the 7 other insulin-resistance SNPs were identified from a meta-analysis of 52 studies (27). Of all 52 GWASs considered for glucose and insulin SNP selection, 46 were population/community-based studies.
As in previous publications from the CHARGE nutrition working group (35, 36), an allele counting method was used to generate 2 genetic risk scores (GRSs) for the present analysis. The fasting glucose/β cell liability genetic risk score (GRS-FG) was calculated by summing the number of glucose-raising alleles (0, 1, or 2) for each of the 36 identified fasting glucose/β cell SNPs, and the insulin-resistance genetic risk score (GRS-IR) was calculated by adding the number of insulin-raising alleles (0, 1, or 2) for each of the 9 insulin resistance SNPs, assuming an additive genetic model. All cohorts included the 36 fasting glucose SNPs and 9 insulin resistance SNPs in the calculation of the GRS, except the GLACIER study (missing data for 3 fasting glucose SNPs) and GHRAS (missing data for 24 fasting glucose SNPs and all insulin resistance SNPs); the GRS-FG was calculated based on 33 SNPs in the GLACIER study and 12 SNPs in the GHRAS, and the GHRAS did not contribute to the analyses of the GRS-IR. A list of the missing SNPs is provided in Supplemental Table 6.
Measurement of fasting glucose and fasting insulin
Cohort-specific methods for assessing fasting glucose and fasting insulin are described in Supplemental Table 3. For the purposes of this analysis, fasting insulin values were log-transformed because of their skewed distribution.
Measurement of covariates
Cohort-specific definitions for other measurements of interest, including smoking, alcohol intake, BMI, education, physical activity, and dietary factors, are described in Supplemental Table 7.
Cohort-specific analyses
An analysis request that outlined the statistical analysis plan was sent to each cohort. For each cohort, linear regression was used to examine the associations of processed meat and unprocessed red meat with fasting glucose or fasting insulin. Analyses for most cohorts were cross-sectional, with the exception of the Health ABC study (dietary assessment in 1998–1990 and fasting glucose and insulin assessments in 1997–1998) and the RS (dietary assessment in 1990–1993 and fasting glucose and insulin assessments in 1997–1999). Each cohort reported β coefficients and robust SEs for 3 models (specified a priori) for each analysis, and sent these summary statistics to the project lead. Model 1 (a minimally adjusted model) adjusted for age, sex, energy intake (kilocalories per day), and field center/population substructure (if relevant). A second model was additionally adjusted for a priori confounders, including education, smoking, alcohol use, physical activity, and other dietary factors. Because we were most interested in examining the associations of processed meat and unprocessed red meat intake with fasting glucose and insulin concentrations above and beyond the effect that other foods and nutrients—including saturated fat, a component of many meats—may have on fasting glucose and insulin concentrations, we included daily servings of fish, fruits, vegetables, whole grains, sugar-sweetened beverages, nuts, and other meats, and saturated fat (grams per day) as covariates in model 2. In model 3, we additionally adjusted for BMI to better determine whether obesity might confound or mediate the relation of meat intake and fasting glucose or insulin. In secondary analyses, all cohorts (1) assessed the relation of GRS-FG and GRS-IR with fasting glucose and insulin concentrations in a model adjusted for age, sex, energy intake, and field center/population substructure, and (2) repeated all analyses with the use of total meat (i.e., total servings of both processed meat and unprocessed red meat) as the exposure of interest.
Each cohort examined the potential interactions of processed meat and unprocessed red meat intake with GRS-FG on fasting glucose to investigate whether SNPs related to fasting glucose/β cell function modify the association of meat intake and fasting glucose. Similarly, the cohorts assessed the potential interaction of processed meat and unprocessed red meat with the GRS-IR on fasting insulin to better understand whether SNPs related to insulin resistance modify the association of meat intake and fasting insulin. For each of these analyses, participating cohorts provided β coefficients and robust SEs [for a model that also adjusted for age, sex, energy intake, and field center/population substructure (if relevant)] to the project lead for meta-analyses.
Meta-analyses
The project lead used the summary statistics provided by each cohort to perform meta-analyses to examine the associations of 1) processed meat and unprocessed red meat with fasting glucose or insulin (main effects), and 2) interactions of processed meat and unprocessed red meat with GRS-FG or GRS-IR on fasting glucose or insulin (interaction effects). In secondary analyses, we also assessed the associations of the GRS-FG and GRS-FI on fasting glucose and insulin. Meta-analyses were performed with the use of inverse-variance–weighted fixed-effects models in STATA 10.0 (Stata Corporation). We chose to use a fixed-effects model rather than a random-effects model because we were most interested in understanding the relation of meat, GRS, and fasting glucose and insulin among existing studies (37). Heterogeneity between studies was assessed with the use of the I2 index derived from the Cochran Q statistic (38). A Bonferroni correction was used to adjust for multiple comparisons; the significance threshold used for the current analysis was P = 0.006 (based on 8 comparisons for primary analyses). In sensitivity analyses, we repeated each meta-analysis, omitting one cohort at a time to confirm that individual cohorts were not driving the observed associations. We also performed additional exploratory sensitivity analyses stratified by mean age (<60 y or ≥60 y), region (Europe or United States), mean daily processed meat intake (<0.50 servings/d or ≥0.50 servings/d), and mean unprocessed red meat intake (<0.50 servings/d or ≥0.50 servings/d). Because it is possible that the chemical composition of processed meats may have changed over time (i.e., temporal changes in additives or curing methods over time), we also performed sensitivity analyses among cohorts for which FFQs were completed before 1990 or after 2000. We chose 1990 and 2000 as cutoffs based on the distribution of the years for dietary ascertainment for each cohort. We also repeated all analyses while excluding cohorts with incomplete genetic data (i.e., the GLACIER study and the GHRAS) or cohorts in which the diet and fasting glucose or insulin measures were not collected at the same study visit (i.e., the Health ABC study and the RS). To be consistent with the serving sizes used in previously published studies (5), for all analyses, one serving of unprocessed red meat is twice as large as a serving of processed meat. To compare similar portions of unprocessed red meat and processed meat, the β coefficient for processed meat must be doubled. In secondary analyses, all analyses were repeated for total meat.
RESULTS
Demographic, metabolic, and dietary characteristics for each of the 14 participating cohorts are described in Table 1. The mean age across the cohorts ranged from 37.7 y to 73.7 y, and ∼50–70% of participants from each cohort were female. Mean fasting glucose concentrations of each cohort ranged from 5.1 to 5.7 mmol/L. Mean fasting insulin concentrations ranged from 50.2 to 97.7 pmol/L. Reported mean intake of both processed meat and unprocessed red meat also varied across the cohorts, ranging from 0.2 to 1.8 servings/d for processed meat and 0.4 to 2.0 servings/d for unprocessed red meat. No differences in meat intake were evident based on region (Europe or United States), mean age of cohort, or year of dietary assessment (data not shown).
TABLE 1.
Participant characteristics in 14 participating cohorts1
| Cohort (country) (ref) | n2 | Age, y | F | Fasting glucose, mmol/L | Fasting insulin, pmol/L | Processed meat intake, servings/d | Unprocessed red meat intake, servings/d | Energy intake, kcal/d | Saturated fat intake, % calories | BMI, kg/m2 |
| ARIC (USA) (39) | 8591 | 54.2 ± 5.7 | 53.7 | 5.5 ± 0.5 | 72.2 ± 52.6 | 0.4 ± 0.5 | 0.6 ± 0.4 | 1642.0 ± 604.0 | 12.2 ± 3.1 | 26.7 ± 4.6 |
| CHS (USA) (40) | 2468 | 72.2 ± 5.3 | 61.4 | 5.5 ± 0.5 | 91.5 ± 45.7 | 0.2 ± 0.3 | 0.5 ± 0.4 | 2019.6 ± 645.7 | 10.3 ± 2.2 | 25.9 ± 4.3 |
| Family HS (USA) (41) | 3187 | 51.4 ± 13.6 | 53.6 | 5.2 ± 0.5 | 71.0 ± 49.1 | 1.8 ± 1.0 | 0.7 ± 0.5 | 1748.7 ± 614.6 | 11.2 ± 3.2 | 27.4 ± 5.3 |
| FHS (USA) (42–44) | 5325 | 48.7 ± 13.6 | 55.0 | 5.3 ± 0.5 | 85.9 ± 37.5 | 0.3 ± 0.4 | 0.5 ± 0.4 | 1965.7 ± 654.2 | 11.1 ± 10.9 | 27.0 ± 5.2 |
| GHRAS (Greece) (45) | 774 | 71.8 ± 7.4 | 72.0 | 5.7 ± 1.3 | 54.4 ± 40.8 | 0.3 ± 0.6 | 2.0 ± 0.9 | 2146.0 ± 657.0 | N/A | 25.6 ± 3.9 |
| GLACIER (Sweden) (46) | 15,204 | 52.0 ± 8.8 | 60.7 | 5.4 ± 0.6 | 50.2 ± 35.3 | 0.2 ± 0.1 | 0.4 ± 0.2 | 1723.7 ± 599.0 | 14.0 ± 3.3 | 25.8 ± 4.0 |
| GOLDN (USA) (47) | 821 | 48.3 ± 15.9 | 50.5 | 5.7 ± 1.1 | 97.7 ± 83.3 | 0.4 ± 0.5 | 0.6 ± 0.5 | 2139.3 ± 1258.1 | 11.8 ± 2.7 | 28.5 ± 5.5 |
| HBCS (Finland) (48) | 1447 | 61.5 ± 2.9 | 59.3 | 5.5 ± 0.6 | 68.9 ± 55.5 | 0.6 ± 0.8 | 0.7 ± 0.6 | 2231.5 ± 801.4 | 12.2 ± 2.6 | 27.1 ± 4.3 |
| Health ABC (USA) (49) | 1254 | 73.7 ± 2.8 | 50.6 | 5.1 ± 0.5 | 52.2 ± 35.8 | 0.4 ± 0.3 | 0.4 ± 0.4 | 1807.0 ± 599.3 | 9.4 ± 2.5 | 26.2 ± 4.0 |
| Malmӧ (Sweden) (50) | 4746 | 57.5 ± 6.0 | 60.0 | 5.6 ± 0.8 | 53.9 ± 54.6 | 0.7 ± 0.6 | 0.6 ± 0.5 | 2330 ± 670.0 | 16.2 ± 3.9 | 25.6 ± 3.9 |
| MESA (USA) (51) | 2317 | 62.8 ± 10.3 | 52.3 | 5.1 ± 1.2 | 63.1 ± 38.0 | 0.2 ± 0.2 | 0.4 ± 0.3 | 1535.8 ± 656.7 | 10.7 ± 3.4 | 27.7 ± 5.0 |
| RS (Netherlands) (52) | 2305 | 71.9 ± 6.6 | 58.7 | 5.5 ± 0.5 | 73.0 ± 41.0 | 1.5 ± 1.2 | 0.7 ± 0.5 | 1991.0 ± 504.0 | 14.1 ± 3.1 | 26.6 ± 3.8 |
| THISEAS (Greece) (53) | 366 | 59.0 ± 13.1 | 47.0 | 5.3 ± 0.6 | 73.0 ± 46.8 | 0.2 ± 0.4 | 0.7 ± 0.5 | 2261 ± 947.8 | 11.6 ± 3.4 | 29.9 ± 4.9 |
| YFS (Finland) (54) | 1728 | 37.7 ± 5.0 | 56.0 | 5.3 ± 0.5 | 50.7 ± 39.8 | 1.1 ± 0.9 | 0.9 ± 0.7 | 2382.5 ± 765.3 | 11.8 ± 2.4 | 25.8 ± 4.5 |
Values are means ± SDs or percentages. ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; Family HS, Family Heart Study; FHS, Framingham Heart Study; GHRAS, Greek Health Randomized Aging Study; GLACIER, Gene–Lifestyle Interactions and Complex Traits Involved in Elevated Disease Risk; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network; HBCS, Helsinki Birth Cohort Study; Health ABC, Health, Aging, and Body Composition; Malmӧ, Malmӧ Diet and Cancer Study; MESA, Multi-Ethnic Study of Atherosclerosis; N/A, not available; ref, reference(s); RS, Rotterdam Study; THISEAS, Hellenic Study of Interactions between SNPs and Eating in Atherosclerosis Susceptibility; YFS, Young Finns Study.
Maximum available observations; sample sizes varied in some cohorts depending on availability of data on covariates.
Associations between processed meat intake and fasting glucose and fasting insulin
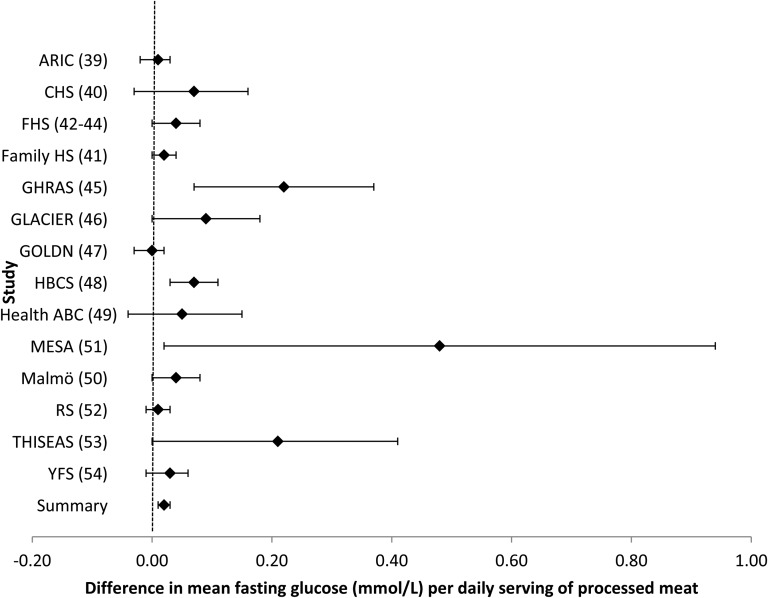
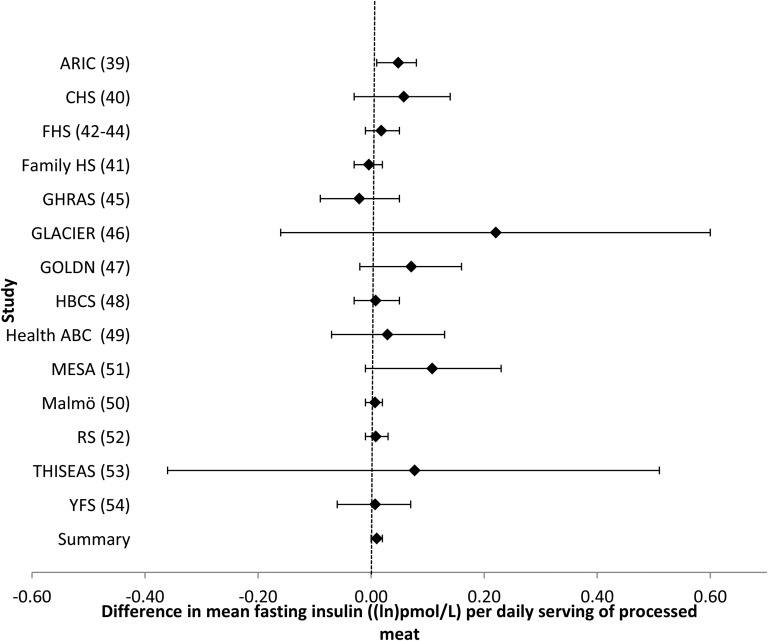
Intake of processed meat was associated with higher fasting glucose. For every additional serving of processed meat per day (i.e., 50 g), fasting glucose was 0.021 mmol/L (95% CI: 0.011, 0.030 mmol/L) higher after adjustment for potential confounders (model 2) (Table 2, Figure 1). Additional adjustment for BMI (model 3) largely attenuated the association (Table 2). After a Bonferroni correction was applied, there was no association of processed meat and fasting insulin (Table 2, Figure 2). Omitting one cohort at a time and restricting analyses to younger or older cohorts, American or European cohorts, cohorts whose dietary assessment was completed before 1990 or after 2000, or cohorts with diet and glucose and insulin measures collected at the same time did not materially alter risk estimates (data not shown).
TABLE 2.
Meta-analysis of associations of meat intake with fasting glucose or fasting insulin1
| Values |
||||
| n | β (95% CI) | P | I2 | |
| Change in fasting glucose for every additional daily serving of processed meat | ||||
| Model 1 | 50,345 | 0.032 (0.023, 0.040) | <0.0001 | 79.4% |
| Model 2 | 48,590 | 0.021 (0.011, 0.030) | <0.0001 | 57.8% |
| Model 3 | 48,538 | 0.010 (0.001, 0.019) | 0.03 | 50.6% |
| Change in fasting insulin for every additional daily serving of processed meat | ||||
| Model 1 | 35,182 | 0.024 (0.015, 0.032) | <0.0001 | 77.4% |
| Model 2 | 34,321 | 0.011 (0.002, 0.019) | 0.016 | 12.2% |
| Model 3 | 34,267 | −0.006 (−0.013, 0.002) | 0.146 | 0% |
| Change in fasting glucose for every additional daily serving of unprocessed red meat | ||||
| Model 1 | 50,471 | 0.061 (0.049, 0.074) | <0.0001 | 54.9% |
| Model 2 | 48,590 | 0.037 (0.023, 0.051) | <0.0001 | 24.0% |
| Model 3 | 48,532 | 0.021 (0.007, 0.035) | 0.004 | 0% |
| Change in fasting insulin for every additional daily serving of unprocessed red meat | ||||
| Model 1 | 35,306 | 0.073 (0.060, 0.086) | <0.0001 | 65.8% |
| Model 2 | 34,321 | 0.049 (0.035, 0.063) | <0.0001 | 44.9% |
| Model 3 | 34,267 | 0.017 (0.004, 0.029) | 0.008 | 19.9% |
Model 1 was adjusted for age, sex, energy intake (kilocalories per day), and field center/population substructure. Model 2 was additionally adjusted for education, smoking, alcohol use, physical activity, saturated fat (grams per day), and daily servings of other meat (i.e., unprocessed red meat for analyses of processed meat and fasting glucose or insulin, and processed meat for analyses of unprocessed red meat and fasting glucose or insulin), fish, fruit, vegetables, whole grains, sugar-sweetened beverages, and nuts. Model 3 was additionally adjusted for BMI. Fasting glucose was measured as millimoles per liter; fasting insulin was measured as natural log-picomoles per liter. The GOLDN did not adjust for sugar-sweetened beverage intake because these data were not available. The GHRAS did not adjust for saturated fat intake because these data were not available. Meta-analyses were performed with the use of inverse-variance–weighted fixed-effects models. GHRAS, Greek Health Randomized Aging Study; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network.
FIGURE 1.
Forest plot of association of processed meat intake and fasting glucose. For each cohort, linear regression was used to examine the association of processed meat and fasting glucose. Meta-analyses were performed with the use of inverse-variance–weighted fixed-effects models. Regression coefficients and 95% CIs are represented by a filled diamond and horizontal line for each cohort and overall (summary). Regression coefficients and 95% CIs represent the difference in mean fasting glucose per one daily serving of processed meat in a model adjusted for model 2 covariates, including age, sex, energy intake (kilocalories per day), field center/population substructure, education, smoking, alcohol use, physical activity, and unprocessed red meat, fish, fruit, vegetable, whole grain, sugar-sweetened beverage, nut, and saturated fat (grams per day) intake. Summary regression coefficient (95% CI): 0.021 (0.011, 0.030). The GOLDN did not adjust for sugar-sweetened beverage intake because these data were not available. The GHRAS did not adjust for saturated fat intake because these data were not available. ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; Family HS, Family Heart Study; FHS, Framingham Heart Study; GHRAS, Greek Health Randomized Aging Study; GLACIER, Gene–Lifestyle Interactions and Complex Traits Involved in Elevated Disease Risk; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network; HBCS, Helsinki Birth Cohort Study; Health ABC, Health, Aging, and Body Composition; Malmӧ, Malmӧ Diet and Cancer Study; MESA, Multi-Ethnic Study of Atherosclerosis; RS, Rotterdam Study; THISEAS, Hellenic Study of Interactions between SNPs and Eating in Atherosclerosis Susceptibility; YFS, Young Finns Study.
FIGURE 2.
Forest plot of association of processed meat intake with fasting insulin. For each cohort, linear regression was used to examine the associations of processed meat with fasting insulin. Meta-analyses were performed with the use of inverse-variance–weighted fixed-effects models. Regression coefficients and 95% CIs are represented by a filled diamond and horizontal line for each cohort and overall (summary). Regression coefficients and 95% CIs represent the difference in mean fasting insulin per one daily serving of processed meat in a model adjusted for model 2 covariates, including age, sex, energy intake (kilocalories per day), field center/population substructure, education, smoking, alcohol use, physical activity, and unprocessed red meat, fish, fruit, vegetable, whole grain, sugar-sweetened beverage, nut, and saturated fat (grams per day) intake. Summary regression coefficient (95% CI): 0.011 (0.002, 0.019). The GOLDN did not adjust for sugar-sweetened beverage intake because these data were not available. The GHRAS did not adjust for saturated fat intake because these data were not available. ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; Family HS, Family Heart Study; FHS, Framingham Heart Study; GHRAS, Greek Health Randomized Aging Study; GLACIER, Gene–Lifestyle Interactions and Complex Traits Involved in Elevated Disease Risk; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network; HBCS, Helsinki Birth Cohort Study; Health ABC, Health, Aging, and Body Composition; Malmӧ, Malmӧ Diet and Cancer Study; MESA, Multi-Ethnic Study of Atherosclerosis; RS, Rotterdam Study; THISEAS, Hellenic Study of Interactions between SNPs and Eating in Atherosclerosis Susceptibility; YFS, Young Finns Study.
Associations between unprocessed red meat intake and fasting glucose and fasting insulin
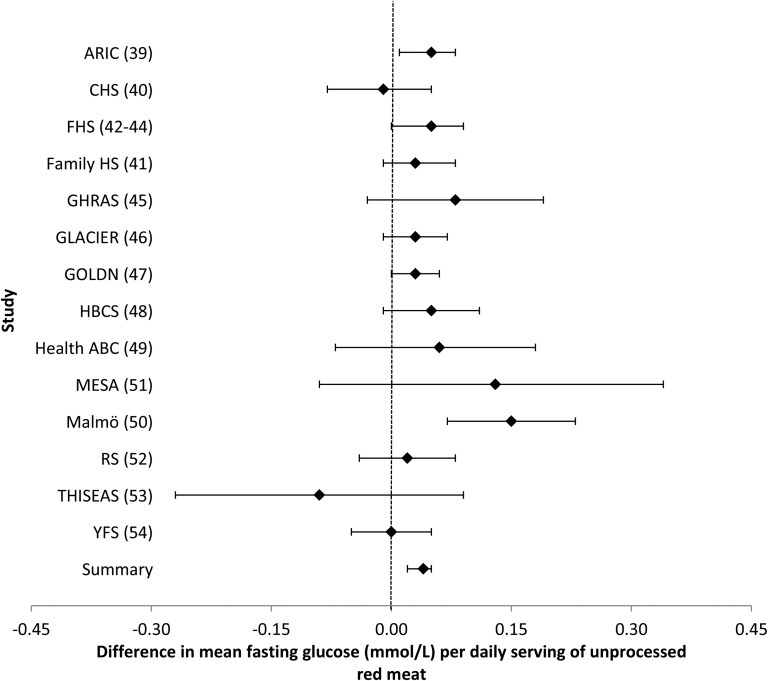
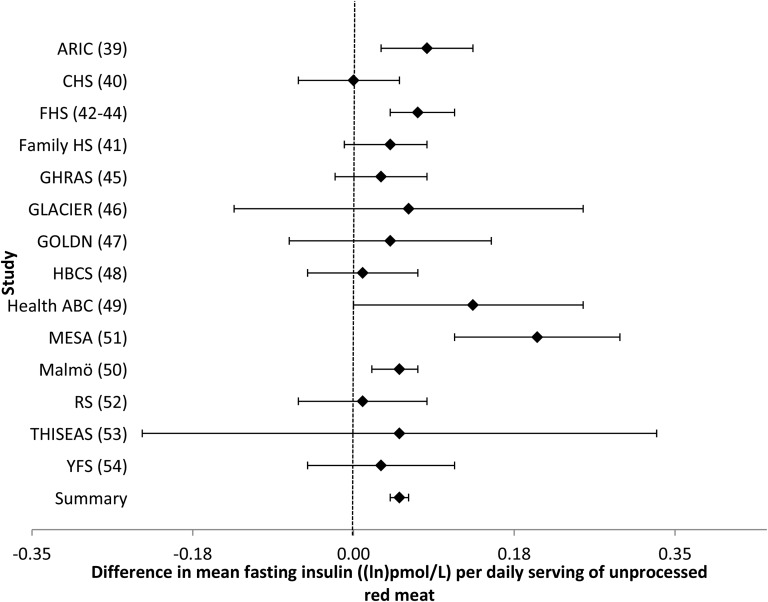
Intake of unprocessed red meat was associated with higher concentrations of fasting glucose and insulin. In a model that adjusted for potential confounders (model 2), every additional serving of unprocessed red meat per day (i.e., 100 g) was associated with a 0.037-mmol/L (95% CI: 0.023, 0.051-mmol/L) higher fasting glucose concentration (Table 2, Figure 3), whereas every additional serving of unprocessed red meat was associated with a 0.049–ln-pmol/L (95% CI: 0.035, 0.063–ln-pmol/L) higher fasting insulin concentration (Table 2, Figure 4). Similar to the analyses for processed meat, adjustment for BMI (model 3) largely attenuated these associations. Likewise, omitting one cohort at a time and restricting analyses to younger or older cohorts, American or European cohorts, cohorts whose dietary assessment was completed before 1990 or after 2000, or cohorts with diet and glucose and insulin measures collected at the same time yielded similar findings (data not shown).
FIGURE 3.
Forest plot of association of unprocessed red meat intake and fasting glucose. For each cohort, linear regression was used to examine the associations of unprocessed red meat with fasting glucose. Meta-analyses were performed with the use of inverse-variance–weighted fixed-effects models. Regression coefficients and 95% CIs are represented by a filled diamond and horizontal line for each cohort and overall (summary). Regression coefficients and 95% CIs represent the difference in mean fasting glucose per one daily serving of unprocessed red meat in a model adjusted for model 2 covariates, including age, sex, energy intake (kilocalories per day), field center/population substructure, education, smoking, alcohol use, physical activity, and processed meat, fish, fruit, vegetable, whole grain, sugar-sweetened beverage, nut, and saturated fat (grams per day) intake. Summary regression coefficient (95% CI): 0.037 (0.023, 0.051). The GOLDN did not adjust for sugar-sweetened beverage intake because these data were not available. The GHRAS did not adjust for saturated fat intake because these data were not available. ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; Family HS, Family Heart Study; FHS, Framingham Heart Study; GHRAS, Greek Health Randomized Aging Study; GLACIER, Gene–Lifestyle Interactions and Complex Traits Involved in Elevated Disease Risk; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network; HBCS, Helsinki Birth Cohort Study; Health ABC, Health, Aging, and Body Composition; Malmӧ, Malmӧ Diet and Cancer Study; MESA, Multi-Ethnic Study of Atherosclerosis; RS, Rotterdam Study; THISEAS, Hellenic Study of Interactions between SNPs and Eating in Atherosclerosis Susceptibility; YFS, Young Finns Study.
FIGURE 4.
Forest plot of association between unprocessed red meat intake and fasting insulin. For each cohort, linear regression was used to examine the associations between unprocessed red meat and fasting insulin. Meta-analyses were performed with the use of inverse-variance–weighted fixed-effects models. Regression coefficients and 95% CIs are represented by a filled diamond and horizontal line for each cohort and overall (summary). Regression coefficients and 95% CIs represent the difference in mean fasting insulin per one daily serving of unprocessed red meat in a model adjusted for model 2 covariates, including age, sex, energy intake (kilocalories per day), field center/population substructure, education, smoking, alcohol use, physical activity, and processed meat, fish, fruit, vegetable, whole grain, sugar-sweetened beverage, nut, and saturated fat (grams per day) intake. Summary regression coefficient (95% CI): 0.049 (0.035, 0.063). The GOLDN did not adjust for sugar-sweetened beverage intake because these data were not available. The GHRAS did not adjust for saturated fat intake because these data were not available. ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; Family HS, Family Heart Study; FHS, Framingham Heart Study; GHRAS, Greek Health Randomized Aging Study; GLACIER, Gene–Lifestyle Interactions and Complex Traits Involved in Elevated Disease Risk; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network; HBCS, Helsinki Birth Cohort Study; Health ABC, Health, Aging, and Body Composition; Malmӧ, Malmӧ Diet and Cancer Study; MESA, Multi-Ethnic Study of Atherosclerosis; RS, Rotterdam Study; THISEAS, Hellenic Study of Interactions between SNPs and Eating in Atherosclerosis Susceptibility; YFS, Young Finns Study.
Associations between GRS-FG and GRS-IR and fasting glucose and fasting insulin
The GRS-FG was associated with higher fasting glucose, and the GRS-IR was associated with higher fasting insulin. For every additional copy of a GRS-FG risk allele, fasting glucose concentrations were 0.020 mmol/L (95% CI, 0.19. 0.21 mmol/L) higher. Similarly, for every additional copy of a GRS-IR risk allele, fasting insulin concentrations were 0.013 ln-pmol/L (95% CI, 0.011, 0.016 ln-pmol/L) higher (Supplemental Table 8, Supplemental Figures 1 and 2).
Interactions of GRS-FG and GRS-IR with intake of processed meat and unprocessed red meat on fasting glucose and fasting insulin
Results of meta-analyses that examined the relation of the interactions of processed meat and unprocessed red meat with the GRS-FG or GRS-IR on fasting glucose and insulin concentrations are shown in Table 3 and Figures 5–8. There was no evidence of GRS–meat interactions on either fasting glucose or insulin. Sensitivity analyses (omitting one cohort at a time or restricting analyses by age, region, or year of dietary assessment, as described above) did not materially alter the results (data not shown). Additionally, restricting analyses to cohorts with no missing genetic data (i.e., omitting the GLACIER study and GHRAS from analyses) produced similar risk estimates.
TABLE 3.
Meta-analysis of interactions of meat intake with GRS-FG or GRS-IR on fasting glucose or insulin1
| Values | ||||
| n | β (95% CI) | P | I2 | |
| Processed meat × GRS-FG interaction on fasting glucose | 48,756 | 0.001 (−0.001, 0.003) | 0.38 | 40.5% |
| Processed meat × GRS-FI interaction on fasting insulin | 33,426 | −0.003 (−0.007, 0.002) | 0.23 | 24.2% |
| Unprocessed red meat × GRS-FG interaction on fasting glucose | 48,882 | 0.000 (−0.002, 0.003) | 0.87 | 40.1% |
| Unprocessed red meat × GRS-FI interaction on fasting insulin | 33,550 | −0.004 (−0.010, 0.003) | 0.26 | 24.2% |
β was adjusted for age, sex, energy intake (kilocalories per day), and field center/population substructure. Meta-analyses were performed with the use of inverse-variance–weighted fixed-effects models. GRS-FG, fasting glucose/β cell liability genetic risk score; GRS-IR, insulin-resistance genetic risk score.
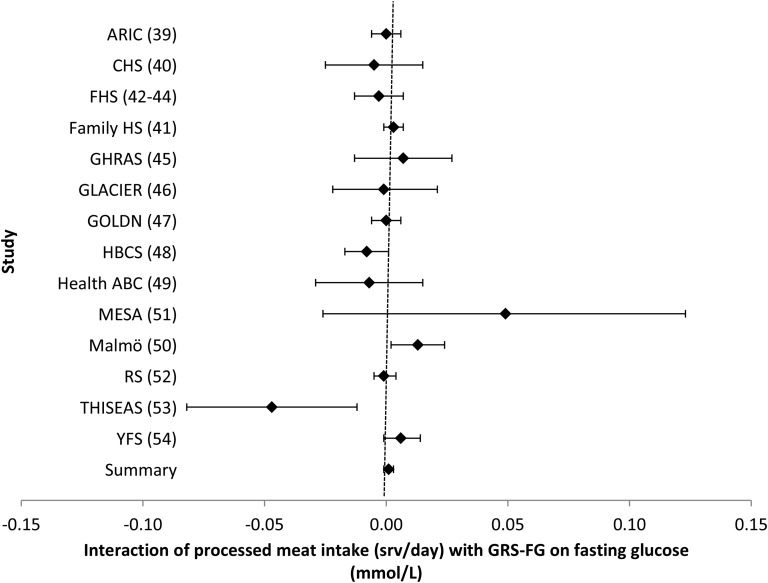
FIGURE 5.
Forest plot of interaction of processed meat intake with the GRS-FG on fasting glucose. For each cohort, linear regression was used to examine the interaction of processed meat intake with the GRS-FG on fasting glucose. Meta-analyses were performed with the use of inverse-variance–weighted fixed-effects models. Regression coefficients and 95% CIs are represented by a filled diamond and horizontal line for each cohort and overall (summary). Regression coefficients (95% CIs) are adjusted for age, sex, energy intake, and field center/population substructure. Summary regression coefficient (95% CI): 0.001 (−0.001, 0.003). ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; Family HS, Family Heart Study; FHS, Framingham Heart Study; GHRAS, Greek Health Randomized Aging Study; GLACIER, Gene–Lifestyle Interactions and Complex Traits Involved in Elevated Disease Risk; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network; GRS-FG, β cell liability genetic risk score; HBCS, Helsinki Birth Cohort Study; Health ABC, Health, Aging, and Body Composition; Malmӧ, Malmӧ Diet and Cancer Study; MESA, Multi-Ethnic Study of Atherosclerosis; RS, Rotterdam Study; srv, servings; THISEAS, Hellenic Study of Interactions between SNPs and Eating in Atherosclerosis Susceptibility; YFS, Young Finns Study.
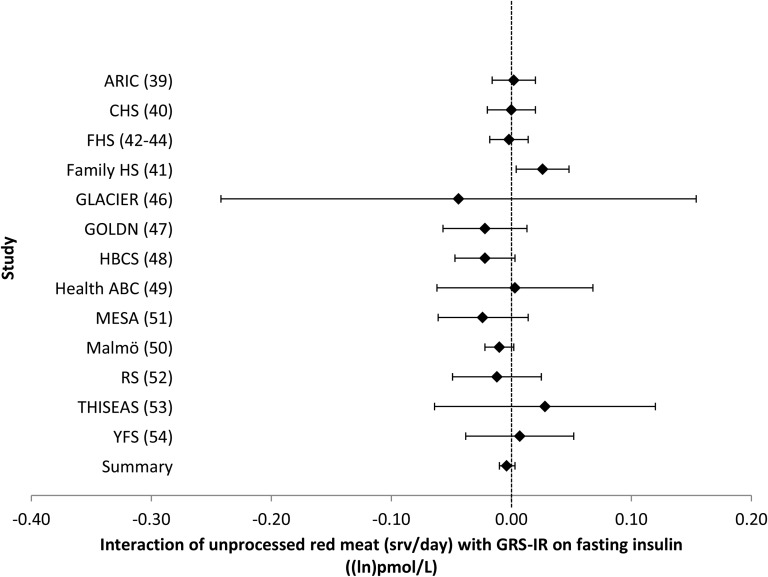
FIGURE 8.
Forest plot of interaction of unprocessed red meat intake with the GRS-IR on fasting insulin. For each cohort, linear regression was used to examine the interaction of unprocessed red meat intake with the GRS-IR on fasting insulin. Meta-analyses were performed with the use of inverse-variance–weighted fixed-effects models. Regression coefficients and 95% CIs are represented by a filled diamond and horizontal line for each cohort and overall (summary). Regression coefficients (95% CIs) are adjusted for age, sex, energy intake, and field center/population substructure. Summary regression coefficient (95% CI): −0.004 (−0.010, 0.003). ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; Family HS, Family Heart Study; FHS, Framingham Heart Study; GLACIER, Gene–Lifestyle Interactions and Complex Traits Involved in Elevated Disease Risk; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network; GRS-IR, insulin-resistance genetic risk score; HBCS, Helsinki Birth Cohort Study; Health ABC, Health, Aging, and Body Composition; Malmӧ, Malmӧ Diet and Cancer Study; MESA, Multi-Ethnic Study of Atherosclerosis; RS, Rotterdam Study; srv, servings; THISEAS, Hellenic Study of Interactions between SNPs and Eating in Atherosclerosis Susceptibility; YFS, Young Finns Study.
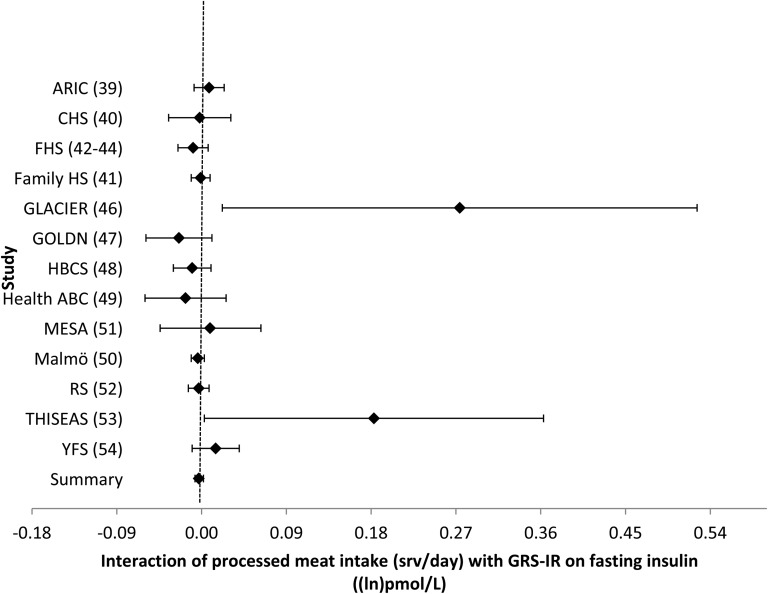
FIGURE 6.
Forest plot of interaction of processed meat intake with the GRS-IR on fasting insulin. For each cohort, linear regression was used to examine the interaction of processed meat intake with the GRS-IR on fasting insulin. Meta-analyses were performed with the use of inverse-variance–weighted fixed-effects models. Regression coefficients and 95% CIs are represented by a filled diamond and horizontal line for each cohort and overall (summary). Regression coefficients (95% CIs) are adjusted for age, sex, energy intake, and field center/population substructure. Summary regression coefficient (95% CI): −0.003 (−0.007, 0.002). ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; Family HS, Family Heart Study; FHS, Framingham Heart Study; GLACIER, Gene–Lifestyle Interactions and Complex Traits Involved in Elevated Disease Risk; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network; HBCS, Helsinki Birth Cohort Study; GRS-IR, insulin-resistance genetic risk score; Health ABC, Health, Aging, and Body Composition; Malmӧ, Malmӧ Diet and Cancer Study; MESA, Multi-Ethnic Study of Atherosclerosis; RS, Rotterdam Study; srv, servings; THISEAS, Hellenic Study of Interactions between SNPs and Eating in Atherosclerosis Susceptibility; YFS, Young Finns Study.
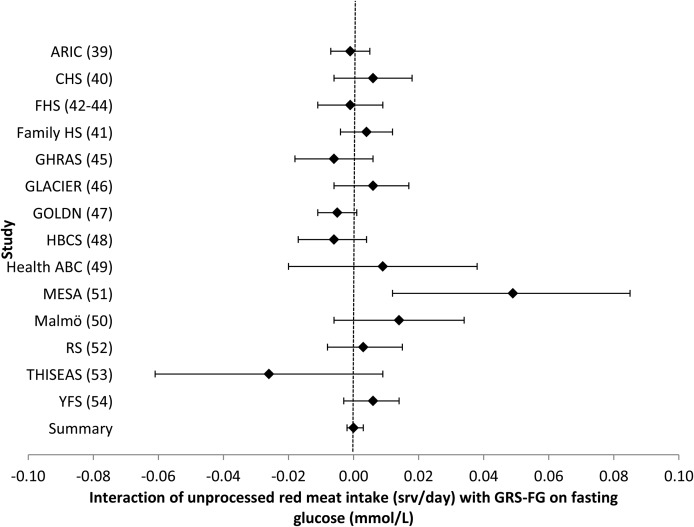
FIGURE 7.
Forest plot of interaction of unprocessed red meat intake with the GRS-FG on fasting glucose. For each cohort, linear regression was used to examine the interaction of unprocessed red meat intake with the GRS-FG on fasting glucose. Meta-analyses were performed with the use of inverse-variance–weighted fixed-effects models. Regression coefficients and 95% CIs are represented by a filled diamond and horizontal line for each cohort and overall (summary). Regression coefficients (95% CIs) are adjusted for age, sex, energy intake, and field center/population substructure. Summary regression coefficient (95% CI): 0.000 (−0.002, 0.003). ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; Family HS, Family Heart Study; FHS, Framingham Heart Study; GHRAS, Greek Health Randomized Aging Study; GLACIER, Gene–Lifestyle Interactions and Complex Traits Involved in Elevated Disease Risk; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network; GRS-FG, β cell liability genetic risk score; HBCS, Helsinki Birth Cohort Study; Health ABC, Health, Aging, and Body Composition; Malmӧ, Malmӧ Diet and Cancer Study; MESA, Multi-Ethnic Study of Atherosclerosis; RS, Rotterdam Study; srv, servings; THISEAS, Hellenic Study of Interactions between SNPs and Eating in Atherosclerosis Susceptibility; YFS, Young Finns Study.
Results of analyses that examined the associations of total meat intake (and the interaction of the GRS-FG and GRS-IR with total meat intake) with fasting glucose and fasting insulin are shown in Supplemental Tables 9 and 10 and Supplemental Figures 3–6. All analyses were repeated with the use of random-effects models, and risk estimates were not meaningfully changed.
DISCUSSION
The results from this large meta-analysis of 14 epidemiologic studies indicate that intake of processed meat is associated with higher fasting glucose, and intake of unprocessed red meat is associated with higher fasting glucose and insulin concentrations in Caucasians without diabetes mellitus. Observed associations were not modified by β cell function/fasting glucose or insulin resistance GRSs.
Results were largely attenuated after adjustment for BMI, possibly because of the independent effects of BMI on both meat intake and fasting glucose and insulin concentrations. However, obesity may be in the causal pathway of processed meat or unprocessed red meat intake and fasting glucose or insulin concentrations—that is, consuming a diet high in meat may cause weight gain and obesity, and obesity is a risk factor for impaired fasting glucose and insulin resistance. As such, adjustment for BMI may underestimate the associations of meat intake with fasting glucose or insulin concentrations.
The mechanism by which the consumption of meat may influence fasting glucose and insulin concentrations is complex. Nitrosamines are present in processed meats at manufacturing or are formed by interactions of amino acids and nitrates within the body, and have been shown to have a toxic effect on β cells and promote the development of diabetes mellitus in rodents and humans (19, 20, 22). Additionally, the intake of meat has been associated with markers of inflammation, such as C-reactive protein (13, 55). Consumption of foods high in saturated fat, such as processed meat and unprocessed red meat, may promote obesity, a leading risk factor for glucose intolerance, insulin resistance, and incident diabetes (56, 57). Red meats are also rich in heme iron, advanced glycation end products, and amino acids (e.g., leucine), which may influence β cell function, insulin secretion, and the pathogenesis of diabetes mellitus (17, 18, 58, 59).
Several previously published studies have examined the relation of meat intake with metabolic outcomes. Most of these studies have focused on incident diabetes or weight gain as outcomes of interest, and, to our knowledge, only one study has examined the associations of meat intake with fasting glucose and insulin concentrations in individuals without diabetes mellitus (60). In that study, each additional serving of red meat per week was associated with 0.42 ± 0.17-mg/dL higher fasting glucose concentration and 0.32 ± 0.15-μU/mL higher fasting insulin concentration. These findings support our results and suggest a positive association of meat intake with fasting glucose and insulin.
Several prospective studies have assessed the associations of meat with development of diabetes. These studies have consistently demonstrated that processed meat intake is associated with a higher risk of incident diabetes (3–10, 13–16), whereas the association of unprocessed red meat with diabetes risk is less clear, with much smaller (8, 13, 15, 16) or even no associations in several studies (3–5, 14). However, previous studies have consistently shown that the magnitude of the effect of meat intake on long-term weight gain is similar for processed meat and unprocessed red meat (61–63). In our analysis, the magnitude of the association of unprocessed red meat with fasting glucose was double that of processed meat. However, a standard serving of unprocessed red meat was twice as large as a standard serving of processed meat (100 g vs. 50 g, respectively); thus, per gram of intake, the magnitude of the association of unprocessed red meat and processed meat with fasting glucose was similar. Interestingly, the magnitude of the association of unprocessed red meat intake with fasting insulin was higher than the magnitude of the association of processed meat intake with fasting insulin. This was an unexpected finding and it is difficult to explain, because, on average, unprocessed red meats have fewer calories, lower concentrations of total fat, less sodium, fewer nitrates, and similar concentrations of saturated fat than processed meats have (5, 64). However, unprocessed red meats contain more heme iron than processed meats (65). If the association of meat intake with fasting glucose and insulin concentrations is primarily driven through the effects of heme iron on fasting glucose and insulin concentrations, this may at least partly explain our findings.
To date, to our knowledge, no published studies have examined the interaction of meat intake with genes related to fasting glucose/β cell function or insulin resistance on fasting glucose and insulin concentrations in non-diabetic patients, and only one published study has examined the interaction of meat intake with genes related to diabetes on risk of incident diabetes (30). In that candidate–gene study, both processed meat and unprocessed red meat showed modest, albeit significant, interactions with the GRS in relation to diabetes risk. However, we found no evidence that processed or red meat interacts with fasting glucose or insulin loci to influence fasting glucose or insulin concentrations in non-diabetic patients.
Our analysis has several strengths. This analysis comprised data from 14 epidemiologic studies, and, to our knowledge, this is the largest analysis to date to examine the associations of meat intake with fasting glucose and insulin. Additionally, we were able to employ a standardized analysis plan because of the richness of the available data from the cohorts.
This study also has limitations. First, some participants might not have accurately recalled dietary information, thereby limiting our ability to obtain accurate estimates of meat intake. Although serving sizes and line items for meats were harmonized across the participating studies, some studies had more detailed questions on meat intake than other studies, and misclassification of intake is possible. Such misclassification is likely nondifferential, biasing risk estimates toward the null. Although analyses are adjusted for several factors related to meat intake and fasting glucose/insulin concentrations, residual confounding by unmeasured factors is possible. Moreover, although more than 30,000 participants composed the study population for the interaction meta-analyses, we may have had insufficient power to detect an interaction if the gene–diet interaction effect size is small. This is a cross-sectional analysis, and it is not possible to determine whether meat intake influences fasting glucose or insulin concentrations, or, alternatively, if participants with higher fasting glucose or insulin concentrations are more likely to consume meat than participants with lower fasting glucose or insulin concentrations. For the purposes of this analysis, we chose to use GRSs to examine the interaction of SNPs related to β cell function/fasting glucose and insulin resistance with meat intake on fasting glucose and insulin concentrations. Although this may have maximized the power to find a gene–diet interaction, we did not assess the interaction of individual SNPs and meat intake, and the use of a GRS might have concealed potentially strong interactions for individual SNPs. Moreover, the SNPs used in the GRSs were selected a priori based on SNPs with known β cell function or related to insulin resistance identified from GWASs from a literature search performed in 2011. Finally, this analysis comprised Caucasians without diabetes mellitus, and results may not be generalizable to other populations.
In conclusion, the results of this study suggest that meat intake is associated with fasting glucose and insulin concentrations in Caucasians without diabetes mellitus. This association is not dependent on genetic variation of loci previously shown to be associated with a fasting glucose/β cell function or insulin resistance from GRSs. This study adds to the growing body of evidence that suggests that meat intake is associated with higher glucose and insulin concentrations.
Acknowledgments
The authors’ responsibilities were as follows—AMF, JAN, DSS, and JBM: designed the research; AMF, JLF, JAN, MKW, JSN, IPK, TVV, FR, ACF-W, JL, MAN, DKH, UE, EHvdH, and VM: analyzed the data; AMF, GVD, JCK-dJ, DM, and RNL: wrote the manuscript; JLF, JAN, KEN, JSP, MG, LD, JIR, KM, BMP, KR, DSS, MKW, MFF, MAP, IBB, JSN, NMM, LAC, IPK, SK, TVV, FR, FBH, IJ, PWF, ACF-W, CES, C-QL, JMO, DKA, JL, A-MT, M-MP, SM, JGE, MAN, DKH, MEG, MFK, YL, UE, ES, MO-M, AM, EHvdH, MD, PD, VM, JBM, AH, OR, MK, AGU, IS, OHF, TL, and FJAvR: reviewed and revised the manuscript; KR: provided statistical expertise; AMF, DSS, GVD, JCK-dJ, and DM: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. DM has received ad hoc honoraria from Bunge, Pollock Institute, and Quaker Oats; has conducted ad hoc consulting for Foodminds, Nutrition Impact, Amarin, Astra Zeneca, Winston and Strawn, and Life Sciences Research Organization; is a member of the Unilever North America Scientific Advisory Board; and has received chapter royalties from UpToDate. No other authors reported a conflict of interest related to this study. Data for this project were obtained from each participating study, and are not publically available. Access to study-specific data is at the discretion of the participating studies. Representing authors from each cohort and study-specific acknowledgments may be found in Supplemental Table 11.
Footnotes
Abbreviations used: CHARGE, Cohorts for Heart and Aging Research in Genomic Epidemiology; FFQ, food frequency questionnaire; GHRAS, Greek Health Randomized Aging Study; GLACIER, Gene–Lifestyle Interactions and Complex Traits Involved in Elevated Disease Risk; GRS, genetic risk score; GRS-FG, fasting glucose/β cell liability genetic risk score; GRS-IR, insulin-resistance genetic risk score; GWAS, genome-wide association study; Health ABC, Health, Aging, and Body Composition; RS, Rotterdam Study; SNP, single-nucleotide polymorphism.
REFERENCES
- 1.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, et al. . National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011;378:31–40. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Ford ES, McGuire LC, Mokdad AH, Little RR, Reaven GM. Trends in hyperinsulinemia among nondiabetic adults in the US. Diabetes Care 2006;29:2396–402. [DOI] [PubMed] [Google Scholar]
- 3.Van Dam RM, Willett W, Rimm E, Stampfer M, Hu F. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002;25:417–24. [DOI] [PubMed] [Google Scholar]
- 4.Song Y, Manson JE, Buring JE, Liu S. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: the women's health study. Diabetes Care 2004;27(9):2108–15. [DOI] [PubMed]
- 5.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010;121(21):2271–83. [DOI] [PMC free article] [PubMed]
- 6.Schulze MB, Manson JE, Willett WZ, Hu FB. Processed meat intake and incidence of type 2 diabetes in younger and middle-aged women. Diabetologia 2003;46:1465–73. [DOI] [PubMed] [Google Scholar]
- 7.Fung TT, Schulze M, Manson J, Willett W, Hu F. Dietary patterns, meat intake and the risk of type 2 diabetes in women. Arch Intern Med 2004;164:2235–40. [DOI] [PubMed] [Google Scholar]
- 8.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, Hu FB. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr 2011;94:1088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannistö S, Kontto J, Kataja-Tuomola M, Albanes D, Virtamo J. High processed meat consumption is a risk factor of type 2 diabetes in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention study. Br J Nutr 2010;103:1817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinbrecher A, Erber E, Grandinetti A, Kolonel LN, Maskarinec G. Meat consumption and risk of type 2 diabetes: the Multiethnic Cohort. Public Health Nutr 2011;14:568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendinelli B, Consortium I. Association between dietary meat consumption and incident type 2 diabetes: the EPIC-InterAct study. Diabetologia 2013;56:47–59. [DOI] [PubMed] [Google Scholar]
- 12.Pan A, Sun Q, Bernstein AM, Manson JE, Willett WC, Hu FB. Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus three cohorts of US men and women. JAMA Intern Med 2013;173:1328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Woudenbergh GJ, Kuijsten A, Tigcheler B, Sijbrands EJ, van Rooij FJ, Hofman A, Witteman JC, Feskens EJ. Meat consumption and its association with C-reactive protein and incident type 2 diabetes: the Rotterdam Study. Diabetes Care 2012;35:1499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lajous M, Tondeur L, Fagherazzi G, de Lauzon-Guillain B, Boutron-Ruaualt MC, Clavel-Chapelon F. Processed and unprocessed red meat consumption and incident type 2 diabetes among French women. Diabetes Care 2012;35:128–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aune D, Ursin G, Veierod MB. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia 2009;52:2277–87. [DOI] [PubMed] [Google Scholar]
- 16.Feskens EJM, Sluik D, van Woudenbergh GJ. Meat consumption, diabetes, and its complications. Curr Diab Rep 2013;13:298–306. [DOI] [PubMed] [Google Scholar]
- 17.Peppa M, Goldberg T, Cai W, Rayfield E, Vlassara H. Glycotoxins: a missing link in the “relationship of dietary fat and meat intake in relation to risk of type 2 diabetes in men”. Diabetes Care 2002;25:1898–9. [DOI] [PubMed] [Google Scholar]
- 18.Piercy V, Toseland CD, Turner NC. Potential benefit of inhibitors of advanced glycation end products in the progression of type II diabetes: a study with aminoguanidine in C57/BLKsJ diabetic mice. Metabolism 1998;47:1477–80. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann SM, Dong HJ, Li Z, Cai WJ, Altomonte J, Thung SN, Zeng F, Fisher EA, Vlassara H. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes 2002;51:2082–9. [DOI] [PubMed] [Google Scholar]
- 20.Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, Rayfield EJ. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA 2002;99:15596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storlien LH, Baur LA, Kriketos AD, Pan DA, Cooney GJ, Jenkins AB, Calvert GD, Campbell LV. Dietary fats and insulin action. Diabetologia 1996;39:621–31. [DOI] [PubMed] [Google Scholar]
- 22.Lijinsky W. N-Nitroso compounds in the diet. Mutat Res 1999;443:129–38. [DOI] [PubMed] [Google Scholar]
- 23.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, et al. . New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010;42:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, et al. . Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 2010;42:579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, de Bakker PI, Abecasis GR, Almgren P, Andersen G, et al. . Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 2008;40:638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florez JC. Newly identified loci highlight beta cell dysfunction as a key cause of type 2 diabetes: where are the insulin resistance genes? Diabetologia 2008;51:1100–10. [DOI] [PubMed] [Google Scholar]
- 27.Manning AK, Hivert MF, Scott RA, Grimsby JL, Bouatia-Naji N, Chen H, Rybin D, Liu CT, Bielak LF, Prokopenko I, et al. . A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet 2012;44:659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parikh H, Lyssenko V, Groop LC. Prioritizing genes for follow-up from genome wide association studies using information on gene expression in tissues relevant for type 2 diabetes mellitus. Bmc Med Genomics 2009;2. [DOI] [PMC free article] [PubMed]
- 29.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, et al. . A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007;316:1341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi L, Cornelis MC, Zhang C, van Dam RM, Hu FB. Genetic predisposition, Western dietary pattern, and the risk of type 2 diabetes in men. Am J Clin Nutr 2009;89:1453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanoni S, Nettleton JA, Hivert MF, Ye Z, van Rooij FJ, Shungin D, Sonestedt E, Ngwa JS, Wojczynski MK, Lemaitre RN, et al. . Total zinc intake may modify the glucose-raising effect of a zinc transporter (SLC30A8) variant: a 14-cohort meta-analysis. Diabetes 2011;60:2407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornelis MC, Qi L, Kraft P, Hu FB. TCF7L2, dietary carbohydrate, and risk of type 2 diabetes in US women. Am J Clin Nutr 2009;89:1256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi L, Liang J. Interactions between genetic factors that predict diabetes and dietary factors that ultimately impact on risk of diabetes. Curr Opin Lipidol 2010;21:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Psaty BM, O’Donnell CJ, Gudnason V, Lunetta KL, Folsom AR, Rotter JI, Uitterlinden AG, Harris TB, Witteman JC, Boerwinkle E. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium. Design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet 2009;2:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nettleton JA, McKeown NM, Kanoni S, Lemaitre RN, Hivert MF, Ngwa J, van Rooij FJA, Sonestedt E, Wojczynski MK, Ye Z, et al. . Interactions of Dietary Whole-Grain Intake With Fasting Glucose- and Insulin-Related Genetic Loci in Individuals of European Descent A meta-analysis of 14 cohort studies. Diabetes Care 2010;33:2684–91. Corrected and republished from: Diabetes Care 2011; 34:785–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hruby A, Ngwa JS, Renstrom F, Wojczynski MK, Ganna A, Hallmans G, Houston DK, Jacques PF, Kanoni S, Lehtimaki T, et al. . Higher magnesium intake is associated with lower fasting glucose and insulin, with no evidence of interaction with select genetic loci, in a meta-analysis of 15 CHARGE Consortium studies. J Nutr 2013;143:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper H, Hedges L, Valentine J. The handbook of research synthesis and meta-analysis. New York: Russell Sage Foundation Publications; 2009. [Google Scholar]
- 38.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 40.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. . The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–76. [DOI] [PubMed] [Google Scholar]
- 41.Higgins M, Province M, Heiss G, Eckfeldt J, Ellison RC, Folsom AR, Rao DC, Sprafka JM, Williams R. NHLBI Family Heart Study: objectives and design. Am J Epidemiol 1996;143:1219–28.8651220 [Google Scholar]
- 42.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979;110:281–90. [DOI] [PubMed] [Google Scholar]
- 43.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci 1963;107:539–56. [DOI] [PubMed] [Google Scholar]
- 44.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB Sr, Fox CS, Larson MG, Murabito JM, et al. . The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 2007;165:1328–35. [DOI] [PubMed] [Google Scholar]
- 45.Kanoni S, Dedoussis GV. Design and descriptive characteristics of the GHRAS: the Greek Health Randomized Aging Study. Med Sci Monit 2008;14:CR204–12. [PubMed] [Google Scholar]
- 46.Kurbasic A, Poveda A, Chen Y, Agren A, Engberg E, Hu FB, Johansson I, Barroso I, Brandstrom A, Hallmans G, et al. . Gene-lifestyle interactions in complex diseases: Design and description of the GLACIER and VIKING Studies. Curr Nutr Rep 2014;3:400–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aslibekyan S, Kabagambe EK, Irvin MR, Straka RJ, Borecki IB, Tiwari HK, Tsai MY, Hopkins PN, Shen J, Lai CQ, et al. . A genome-wide association study of inflammatory biomarker changes in response to fenofibrate treatment in the Genetics of Lipid Lowering Drug and Diet Network. Pharmacogenet Genomics 2012;22:191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eriksson JG. Early growth and adult health outcomes–lessons learned from the Helsinki Birth Cohort Study. Matern Child Nutr 2005;1:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM, Harris T. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci 2001;56:M644–9. [DOI] [PubMed] [Google Scholar]
- 50.Berglund G, Elmstahl S, Janzon L, Larsson SA. The Malmo Diet and Cancer Study. Design and feasibility. J Intern Med 1993;233:45–51. [DOI] [PubMed] [Google Scholar]
- 51.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, et al. . Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 52.Hofman A, Darwish Murad S, van Duijn CM, Franco OH, Goedegebure A, Ikram MA, Klaver CC, Nijsten TE, Peeters RP, Stricker BH, et al. . The Rotterdam Study: 2014 objectives and design update. Eur J Epidemiol 2013;28:889–926. [DOI] [PubMed] [Google Scholar]
- 53.Kalandidi A, Tzonou A, Toupadaki N, Lan SJ, Koutis C, Drogari P, Notara V, Hsieh CC, Toutouzas P, Trichopoulos D. A case-control study of coronary heart disease in Athens, Greece. Int J Epidemiol 1992;21:1074–80. [DOI] [PubMed] [Google Scholar]
- 54.Raitakari OT, Juonala M, Ronnemaa T, Keltikangas-Jarvinen L, Rasanen L, Pietikainen M, Hutri-Kahonen N, Taittonen L, Jokinen E, Marniemi J, et al. . Cohort profile: the cardiovascular risk in Young Finns Study. Int J Epidemiol 2008;37:1220–6. [DOI] [PubMed] [Google Scholar]
- 55.Lee CC, Adler AI, Sandhu MS, Sharp SJ, Forouhi NG, Erqou S, Luben R, Bingham S, Khaw KT, Wareham NJ. Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis. Diabetologia 2009;52:1040–7. [DOI] [PubMed] [Google Scholar]
- 56.Hu G, Lindstrom J, Valle TT, Eriksson JG, Jousilahti P, Silventoinen K, Qiao Q, Tuomilehto J. Physical activity, body mass index, and risk of type 2 diabetes in patients with normal or impaired glucose regulation. Arch Intern Med 2004;164:892–6. [DOI] [PubMed] [Google Scholar]
- 57.Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, Speizer FE, Manson JE. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol 1997;145:614–9. [DOI] [PubMed] [Google Scholar]
- 58.Rajpathak SN, Crandall JP, Wylie-Rosett J, Kabat GC, Rohan TE, Hu FB. The role of iron in type 2 diabetes in humans. Biochim Biophys Acta 2009;1790:671–81. [DOI] [PubMed] [Google Scholar]
- 59.Newsholme P, Bender K, Kiely A, Brennan L. Amino acid metabolism, insulin secretion and diabetes. Biochem Soc Trans 2007;35(Pt 5):1180–6. [DOI] [PubMed]
- 60.Panagiotakos DB, Tzima N, Pitsavos C, Chrysohoou C, Papakonstantinou E, Zampelas A, Stefanadis C. The relationship between dietary habits, blood glucose and insulin levels among people without cardiovascular disease and type 2 diabetes; the ATTICA study. Rev Diabet Stud 2005;2:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vergnaud AC, Norat T, Romaguera D, Mouw T, May AM, Travier N, Luan J, Wareham N, Slimani N, Rinaldi S, et al. . Meat consumption and prospective weight change in participants of the EPIC-PANACEA study. Am J Clin Nutr 2010;92:398–407. [DOI] [PubMed] [Google Scholar]
- 62.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gilsing AMJ, Weijenberg MP, Hughes LAE, Ambergen T, Dagnelie PC, Goldbohm RA, van den Brandt PA, Schouten LJ. Longitudinal changes in BMI in older adults are associated with meat consumption differentially, by type of meat consumed. J Nutr 2012;142:340–9. [DOI] [PubMed] [Google Scholar]
- 64.Micha R, Michas G, Lajous M, Mozaffarian D. Processing of meats and cardiovascular risk: time to focus on preservatives. Bmc Med 2013;11. [DOI] [PMC free article] [PubMed]
- 65.Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes—An updated review of the evidence. Curr Atheroscler Rep 2012;14:515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]