Abstract
Background: Selenomethionine, which is the principal dietary form of selenium, is metabolized by the liver to selenide, which is the form of the element required for the synthesis of selenoproteins. The liver synthesizes selenium-rich selenoprotein P (SEPP1) and secretes it into the plasma to supply extrahepatic tissues with selenium.
Objectives: We conducted a randomized controlled trial to determine whether cirrhosis is associated with functional selenium deficiency (the lack of selenium for the process of selenoprotein synthesis even though selenium intake is not limited) and, if it is, whether the deficiency is associated with impairment of selenomethionine metabolism.
Design: Patients with Child-Pugh (C-P) classes A, B, and C (mild, moderate, and severe, respectively) cirrhosis were supplemented with a placebo or supranutritional amounts of selenium as selenate (200 or 400 μg/d) or as selenomethionine (200 μg/d) for 4 wk. Plasma SEPP1 concentration and glutathione peroxidase (GPX) activity, the latter due largely to the selenoprotein GPX3 secreted by the kidneys, were measured before and after supplementation.
Results: GPX activity was increased more by both doses of selenate than by the placebo in C-P class B patients. The activity was not increased more by selenomethionine supplementation than by the placebo in C-P class B patients. Plasma selenium was increased more by 400 μg Se as selenate than by the placebo in C-P class C patients. Within the groups who responded to selenate, there was a considerable variation in responses.
Conclusion: These results indicate that severe cirrhosis causes mild functional selenium deficiency in some patients that is associated with impaired metabolism of selenomethionine. This trial was registered at clinicaltrials.gov as NCT00271245.
Keywords: inorganic selenium metabolism, selenium biomarkers, selenium metabolism by the liver, selenium requirement, functional selenium deficiency
INTRODUCTION
Selenium is an essential micronutrient that functions through selenoproteins. The human genome encodes 25 selenoproteins, which have a variety of biochemical functions. When the selenium supply is inadequate, selenoprotein amounts decline with biochemical and, potentially, clinical consequences (1, 2).
A number of investigators have reported that plasma selenium concentrations are depressed in patients with cirrhosis (3–9), thereby raising the possibility that such patients are selenium deficient. Years ago, our group analyzed the plasma forms of selenium in patients with cirrhosis (5). Selenoprotein P (SEPP1),6 which accounts for approximately one-half of the plasma selenium in healthy US residents and is produced predominantly by the liver for the transport of selenium to extrahepatic tissues, was depressed in direct relation to the severity of illness [Child-Pugh (C-P) classes]. In contrast, glutathione peroxidase (GPX) activity in plasma, which is a measure of GPX3 that accounts for ∼17% of plasma selenium in healthy subjects and is produced predominantly by the kidneys, was increased in proportion to the severity of the cirrhosis. Less than 3% of plasma selenium is in the form of small molecules, and the remaining large and variable fraction is in selenomethionine, most of which is in the primary structure of albumin at methionine positions (10). We tentatively concluded that the patients with cirrhosis were probably not selenium deficient largely on the basis of the finding that plasma GPX activity increased with worsening illness. However, we noted that a definitive determination of the selenium status of patients with cirrhosis would require a selenium supplementation trial.
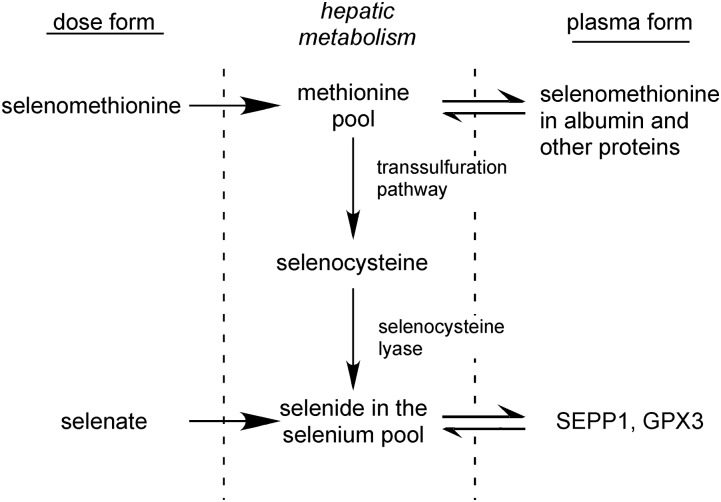
The Recommended Dietary Allowance for selenium is 55 μg (11), and daily intakes of healthy residents of the United States have generally been reported to be>80 μg. Selenomethionine is the major form of selenium in the human diet. The origin of selenomethionine is plants that incorporate selenium into the methionine carbon skeleton in place of sulfur. In animals, selenomethionine is metabolized nonspecifically in the methionine pool (Figure 1). Selenomethionine is incorporated into the primary structure of proteins at methionine positions and is also converted to selenocysteine by the transsulfuration pathway (12, 13). Free selenocysteine is catabolized by selenocysteine lyase, which produces selenide, an intermediate required for the synthesis of selenoproteins. Transsulfuration occurs predominantly in the liver, and cirrhosis impairs transsulfuration as evidenced by the increased plasma methionine concentrations observed in patients with cirrhosis (14). Thus, cirrhosis might be expected to impair the conversion of the selenium in selenomethionine to selenide, thereby causing patients to be functionally selenium deficient (i.e., lacking selenium for the process of selenoprotein synthesis even though selenium intake is not limited). The regulation of selenium metabolism has recently been reviewed (12).
FIGURE 1.
Outline of hepatic conversion of ingested forms of selenium to protein forms in plasma. The plasma forms are used as selenium biomarkers. GPX3, glutathione peroxidase-3; SEPP1, selenoprotein P.
We tested the hypotheses that selenium deficiency occurs in patients with cirrhosis and that inorganic selenium in the form of selenate would correct that deficiency but selenomethionine would not. After a pilot study was compatible with the hypotheses, a randomized controlled trial in a larger number of patient subjects was carried out with the use of plasma selenium biomarkers to assess selenium nutritional status. The results of both of these trials are presented in the current article and indicated that some patients with severe cirrhosis have mild selenium deficiency that can be corrected by the administration of selenate but not of selenomethionine.
METHODS
Both studies reported in the current article were carried out in accordance with the Helsinki Declaration of 1975 as revised in 1983.
Pilot study
Twenty-four healthy control subjects aged ≥18 y were recruited from the Vanderbilt Medical Center staff and assigned to 3 supplement groups of 8 subjects each. The assignments were carried out in a predetermined order so that the first of each 3 subjects was in the group that received a placebo, the second subject was in the group that received 200 μg Se as sodium selenate, and the third subject was in the group that received 200 μg Se as l-selenomethionine. Control subjects were compensated for their participation in the trial.
Patients with C-P class C cirrhosis (15) were recruited from the Vanderbilt liver clinics and assigned to the 3 groups in the same manner as control subjects were. Recruitment was terminated after 18 patients had been enrolled because of slow subject acquisition. A requirement for the study (control and patient subjects) was that subjects were not taking >25 μg Se supplements/d. One patient in the placebo group died of complications of liver disease before completing the study.
Control and patient subjects took their supplements daily for 28 d. Blood samples (20 mL) were taken by venipuncture on the day supplementation was begun but before ingestion of the supplement, and on the morning after the day of the last dose. Blood was treated with disodium EDTA (1 mg/mL) to prevent coagulation. Plasma was separated by centrifugation and frozen at −80°C until the analysis was carried out. Plasma was analyzed for SEPP1, GPX activity, and selenium. The Vanderbilt Institutional Review Board approved the protocol.
Randomized controlled trial
Study subjects
We recruited patients with cirrhosis from the liver clinics of Vanderbilt Medical Center. Exclusion criteria were as follows: 1) history of taking >25 μg Se supplement/d at any time within the past year, 2) a creatinine concentration ≥2 mg/dL, 3) an assessment by the patient’s hepatologist of the patient being too unstable to complete the study, 4) age <18 y, and 5) ongoing substance abuse. The permission to approach patients was obtained from their hepatologists. Ninety-nine subjects were enrolled after informed consent was obtained. Subjects lived in Nashville or ≤300 miles from Nashville.
Selenium supplements
Forms of selenium supplemented were l-selenomethionine, which is hereafter referred to as selenomethionine, and sodium selenate, which is hereafter referred to as selenate. The selenomethionine was a gift from V Badmaev of Sabinsa Corp., and the selenate was purchased from Spectrum Chemical. Formulation Technology compounded the selenium-containing tablets and the placebo tablets. The main filler used was microcrystalline cellulose with other excipients being stearic acid, croscarmellose sodium, silicon dioxide, and magnesium stearate. All tablets had the same appearance.
Tablets were analyzed for their selenium contents in our laboratory. Mean ± 1 SD results were as follows: placebo, no selenium detectable (<0.05 μg Se/tablet), n = 4; 200 μg Se as selenate tablet, 189 ± 7 μg Se/tablet, n = 5; 400 μg Se as selenate tablet, 379 ± 25 μg Se/tablet, n = 6; and 200 μg Se as selenomethionine tablet, 189 ± 10 μg Se/tablet, n = 5.
Random assignment
Our goal was to study 144 subjects with cirrhosis (48 subjects from each C-P class). Within each C-P class, 12 subjects were to be studied in each of the 4 treatment groups for the first 28 d.
To preserve the random assignment in case of an early termination, we divided the study into 3 independently randomized phases, each of which contained 16 subjects of each C-P class. Each phase was completed before the next one was begun.
Tablets for the 4 treatment groups were put into plastic medication containers (28 tablets/container) and assigned subject numbers. Subject numbers for the first phase were 101–148. Numbers for the second and third phases were 201–248 and 301–348, respectively. The 48 numbers of each phase were randomly assigned so that, within each C-P class, the 4 treatment groups each contained 4 subjects. Two persons who were not otherwise involved in the study carried out this random assignment with the use of a random number generator (www.randomizer.org/form.htm). Records that matched subject numbers with treatments remained sealed and unknown to subjects and investigators until all analyses had been completed.
As subjects entered the study, they were assigned numbers sequentially within their C-P groups. We were able to complete the first 2 phases and to enroll 3 subjects in phase 3 for a total of 99 subjects enrolled. Enrollment was terminated early because of the slow acquisition of subjects. This difficulty in acquiring subjects was in part because many patients with cirrhosis were taking multivitamins that contained amounts of selenium that excluded the patients from the study.
Overall study design
Each study subject completed the same protocol. The study was designed to last 8 wk with 2 sequential 4-wk sections. The first section studied 4 treatment groups (placebo, 200 μg Se/d as selenate, 400 μg Se/d as selenate, and 200 μg Se/d as selenomethionine), and each subject was randomly assigned to one of the groups. In the second section, all study subjects received 400 μg Se/d as selenate. Plasma obtained before the start of supplementation and on the morning after each supplementation section was analyzed for selenium biomarkers.
The purpose of the 4–8-wk supplementation of all patients with 400 μg Se/d was to allow us to inform subjects, before the study was concluded, whether their selenoprotein biomarkers suggested selenium deficiency. Preliminary SEPP1 and GPX assays of the initial and 8-wk samples were carried out ≤6 mo after the completion of each supplementation. We judged that selenium deficiency was possible if both selenoprotein biomarkers increased by 20% between the initial value and the 8-wk value. Five of the subjects were notified of their suggested selenium deficiency.
Study protocol
The study was conducted from 13 March 2006 through 9 December 2011. Patients were interviewed when they attended a liver clinic, and after informed consent was obtained, a 20-mL blood sample was taken by venipuncture in conjunction with the clinic blood sample or separately in the Vanderbilt General Clinical Research Center. The blood sample was treated with 1 mg disodium EDTA/mL to prevent coagulation, and plasma was separated by centrifugation. Each subject was given a medication container with 28 tablets that was identified only by a subject number. The subject was instructed to take one tablet each morning. On the day after the last dose had been taken another blood sample was obtained and the subject began taking 400 μg Se/d as selenate for 28 d. The final blood sample was taken on the day after the last 400-μg dose. The second and third blood samples were taken either at the Vanderbilt General Clinical Research Center or at clinics in towns that were convenient to the subjects.
Assays
Plasma samples were stored in aliquots at −80°C. Aliquots of initial and final plasma samples were assayed within 6 mo of their acquisition for purposes of notifying patients of the results. To generate the data in this report, all plasma samples from the randomized controlled trial were assayed in one run in our laboratory.
The SEPP1 concentration was determined with the use of an ELISA (16), and GPX activity was determined by the coupled assay in the presence of 0.25 mmol H2O2/L as a substrate (17). Selenium was determined by the fluorometric assay of Koh and Benson (18) as modified (19). The analytic services core of the Vanderbilt Diabetes Center determined the methionine in the initial plasma sample with the use of the Waters Acutag method (Waters Corp.), and the Vanderbilt Hospital clinical laboratory performed the liver tests. Hybridomas that produced the 2 monoclonal antibodies that were used in the ELISA were given to us by Takeshi Naruse (Kaketsukenn) and were deposited in the Developmental Studies Hybridoma Bank at the University of Iowa (http://dshb.biology.uiowa.edu).
Outcomes
The primary outcome measure was to determine whether selenate supplementation would increase the 2 plasma selenoproteins. If so, these increases would indicate that the subject had been selenium deficient. The secondary outcome measure was to determine whether selenium as selenomethionine would increase the selenoproteins. If selenate increased the selenoproteins and selenomethionine did not, these results would indicate that the selenium deficiency had been accompanied by an impairment of selenomethionine metabolism. Because selenomethionine is largely metabolized in the liver, the finding would have implicated cirrhosis as the cause of impairment. These outcome measures were assessed after the study had been concluded and all analyses had been completed.
Compliance and withdrawals
Of the 12 intervention groups, only one group lost 3 randomly assigned subjects from the analysis (Supplemental Figure 1). Five groups lost 2 subjects each, and 4 groups lost one subject each. In 2 groups, all randomly assigned subjects completed the study, and their results were analyzed. Seven of 17 subjects who were excluded from analysis were not compliant or gave no reason for their withdrawal. Five subjects withdrew because of complications that were attributable to their liver disease, and one subject had a liver transplant. Four subjects withdrew for personal reasons. Joseph A Awad served as safety monitor and reviewed the study activity each year.
Statistical analysis
Because of our finding that one-third of C-P class C subjects in the pilot study had responded to selenate, we estimated that a study of 12 patients/arm would have 80% statistical power to detect changes of ≥1.2 SDs. This estimate was based on a 2-sided α level of 0.05 with the use of Student’s t test (20). A Spearman correlation was used to assess bivariate continuous relations. Categorical variables were assessed with the use of a chi-square test in the 4 treatment groups except for variables with small cells, such as race, for which the likelihood ratio test was used. Continuous variables were assessed with the use of a nonparametric Kruskal-Wallis H test across the 4 treatment groups. For changes in continuous values, the nonparametric Wilcoxon’s signed-rank test was used. Finally, a general linear model was used for the multivariate analyses of changes over time. All analyses were performed with the software program R (version 3.1.0; R Core Team) or IBM SPSS (version 22).
RESULTS
Pilot study
Responses of plasma selenium biomarkers to selenium supplements were assessed in healthy control subjects and in subjects with C-P class C cirrhosis. The original intent was to supplement 8 members of each group with the placebo, 200 μg Se as selenate, or 200 μg Se as selenomethionine daily for 4 wk. All controls were studied, but only 17 subjects with cirrhosis completed the study. Five of the subjects with cirrhosis received the placebo, and 6 subjects each received selenate and selenomethionine.
Supplemental Table 1 shows that none of the treatments significantly increased the mean of either selenoprotein biomarker in control or patient subjects. However, on a percentage basis, selenate had an apparently greater effect on both selenoproteins in patient subjects than did selenomethionine or the placebo. Supplemental Figure 2 shows that in 2 of 6 patient subjects administered selenate, both selenoproteins increased ≥30%. The increase in selenoproteins was reflected in increases of plasma selenium in the same subjects. No such increases in all 3 biomarkers occurred in other patient or control subjects (results not shown). These results indicate that control subjects in Nashville were selenium replete but suggest that some patients with C-P class C cirrhosis were selenium deficient. The findings are compatible with the hypotheses that some patients with C-P class C cirrhosis have functional selenium deficiency associated with impaired selenomethionine metabolism. Those results justified a larger trial.
Randomized controlled trial
Design and subject characteristics
The primary goal of the randomized controlled trial was to determine whether patients with cirrhosis have suboptimal selenium status. A significant increase in plasma selenoprotein biomarkers after patients had been supplemented with selenium would have indicated suboptimal status. Furthermore, an increase in biomarkers in response to selenate without such a response to selenomethionine would have suggested that the cause of suboptimal status was the impairment of the transsulfuration pathway or selenocysteine lyase by cirrhosis (Figure 1). Only patients with cirrhosis were enrolled in the randomized controlled trial because healthy controls had been shown not to respond to selenium supplementation in the pilot study (Supplemental Table 1) and in a larger study carried out in Nashville (16).
Table 1 lists the causes of cirrhosis in the patients studied. Approximately one-third of subjects had cirrhosis of an unknown cause, and another one-third of subjects had hepatitis C. Several diseases were responsible for cirrhosis in the remaining subjects. Table 2 presents demographics of subjects by treatment group. With the exception of age, demographic characteristics were randomly distributed in the treatment groups.
TABLE 1.
Causes of cirrhosis in patients in the randomized controlled trial
| Cause of cirrhosis | n |
| Unknown (cryptogenic) | 28 |
| Hepatitis C | 27 |
| Hepatitis B | 7 |
| Alcohol | 7 |
| Autoimmune hepatitis | 6 |
| Primary biliary cirrhosis | 4 |
| Other | 3 |
| Total | 82 |
TABLE 2.
Baseline patient demographic characteristics by treatment1
| Placebo | Selenate (200 μg Se) | Selenate (400 μg Se) | Selenomethionine (200 μg Se) | P | |
| n | 22 | 21 | 18 | 21 | — |
| Age | 50.8 ± 11.12,x | 59.5 ± 6.7y | 54.9 ± 7.9x,y | 56.2 ± 10.0x,y | 0.011 |
| Sex, M, % | 64 | 57 | 61 | 62 | 0.9773 |
| BMI, kg/m2 | 29.7 ± 6.1 | 29.9 ± 3.8 | 30.2 ± 6.3 | 31.2 ± 6.8 | 0.935 |
| Race, n | 0.1674 | ||||
| Caucasian | 22 | 21 | 14 | 21 | |
| Black | 0 | 0 | 1 | 0 | |
| Hispanic | 0 | 0 | 2 | 0 | |
| Asian | 0 | 0 | 1 | 0 | |
| Taking vitamin supplements containing selenium, n | 2 | 2 | 1 | 1 | 0.9113 |
Values that do not share a common superscript letter are significantly different at P < 0.05 on the basis of the Kruskal-Wallis H test.
Mean ± 1 SD (all such values).
On the basis of Pearson’s chi-square test.
On the basis of the likelihood ratio test.
Determinants of the severity of liver disease were distributed evenly in the treatment groups (Table 3). Plasma SEPP1 and selenium concentrations were also evenly distributed in the treatment groups, but plasma GPX activity was unevenly distributed with the group that received 400 μg Se/d as selenate having higher GPX-activity values than those of the other treatment groups.
TABLE 3.
Baseline biomarkers of selenium status and liver disease severity by treatment1
| Placebo | Selenate (200 μg Se) | Selenate (400 μg Se) | Selenomethionine (200 μg Se) | P | |
| n | 22 | 21 | 18 | 21 | — |
| Child-Pugh class, n | 0.9972 | ||||
| A | 8 | 7 | 5 | 7 | |
| B | 8 | 7 | 7 | 8 | |
| C | 6 | 7 | 6 | 6 | |
| SEPP1, mg/L | 4.5 ± 1.63 | 4.4 ± 1.8 | 3.9 ± 1.2 | 4.3 ± 1.3 | 0.6544 |
| GPX, U/L | 127 ± 32x | 126 ± 44x | 151 ± 30y | 124 ± 31x | 0.0294 |
| Selenium, μg/L | 93 ± 22 | 91 ± 19 | 92 ± 15 | 93 ± 16 | 0.9404 |
| MELD | 12.0 ± 4.0 | 13.2 ± 5.0 | 12.5 ± 3.6 | 12.9 ± 4.1 | 0.9464 |
| Albumin, g/dL | 3.5 ± 0.6 | 3.4 ± 0.5 | 3.3 ± 0.6 | 3.5 ± 0.7 | 0.6524 |
| Bilirubin, mg/dL | 2.1 ± 1.8 | 2.2 ± 1.5 | 2.2 ± 1.3 | 1.8 ± 1.1 | 0.8314 |
| INR, seconds prolonged | 1.3 ± 0.3 | 1.4 ± 0.4 | 1.3 ± 0.2 | 1.4 ± 0.29 | 0.6794 |
| Creatinine, mg/dL | 0.95 ± 0.26 | 1.1 ± 0.37 | 0.93 ± 0.31 | 1.1 ± 0.30 | 0.1284 |
| Methionine, μmol/L | 37 ± 13 | 52 ± 84 | 36 ± 11 | 37 ± 18 | 0.9664 |
Values that do not share a common superscript letter are significantly different at P < 0.05 on the basis of the Kruskal-Wallis H test. GPX, glutathione peroxidase; INR, International Normalized Ratio; MELD, model for end-stage liver disease; SEPP1, selenoprotein P.
On the basis of Pearson’s chi-square test.
Mean ± 1 SD (all such values).
On the basis of the Kruskal-Wallis H test.
Cirrhosis severity affects plasma selenium biomarkers and methionine concentration
In this study, the C-P class was used to designate disease severity (15). As an additional indicator of disease severity, model for end-stage liver disease scores were calculated for the patients (21). Means ± 1 SDs were as follows: class A, 9.3 ± 2.0, n = 27; class B, 13 ± 3.9, n = 30; class C, 16 ± 3.5, n = 25.
Table 4 presents presupplement (baseline) selenium biomarkers and methionine concentrations. As disease severity increased, plasma SEPP1 and selenium concentrations fell as had been reported in an earlier publication (5). The GPX activity trended upward with increasing disease severity as reported previously, but the trend was not as statistically significant as it was in the previous study. Thus, presupplement plasma selenium biomarkers of subjects in the current study were consistent with results that we reported earlier, which showed that cirrhosis affected all 3 selenium biomarkers (5).
TABLE 4.
Baseline plasma selenium biomarkers and methionine concentration in Child-Pugh classes1
| Child-Pugh class A (n = 27) | Child-Pugh class B (n = 30) | Child-Pugh class C (n = 25) | P | |
| SEPP1, mg/L | 5.6 ± 1.6x | 4.0 ± 0.9y | 3.3 ± 0.8y | <0.001 |
| GPX, U/L | 123 ± 26 | 124 ± 30 | 148 ± 46 | 0.062 |
| Selenium, μg/L | 107 ± 18x | 88 ± 13y | 82 ± 12y | <0.001 |
| Methionine, μmol/L | 29 ± 10x | 36 ± 14y | 58 ± 76z | <0.001 |
All values are means ± 1 SDs. P values were based on the Kruskal-Wallis H test. Values that do not share a common superscript letter are significantly different at P < 0.05. GPX, glutathione peroxidase; SEPP1, selenoprotein P.
Plasma methionine concentration doubled between C-P classes A and C (Table 4). This finding was compatible with earlier reports (14) and indicates that cirrhosis impaired methionine metabolism in the current study subjects.
Adverse effects in subjects
Subjects in the current study were questioned at least twice about changes in nails and loss of hair. One subject who was taking 400 μg Se as selenate reported brittle nails toward the end of the study. No other subjects reported potential adverse effects.
Response of selenium biomarkers to selenium supplementation of subjects with cirrhosis
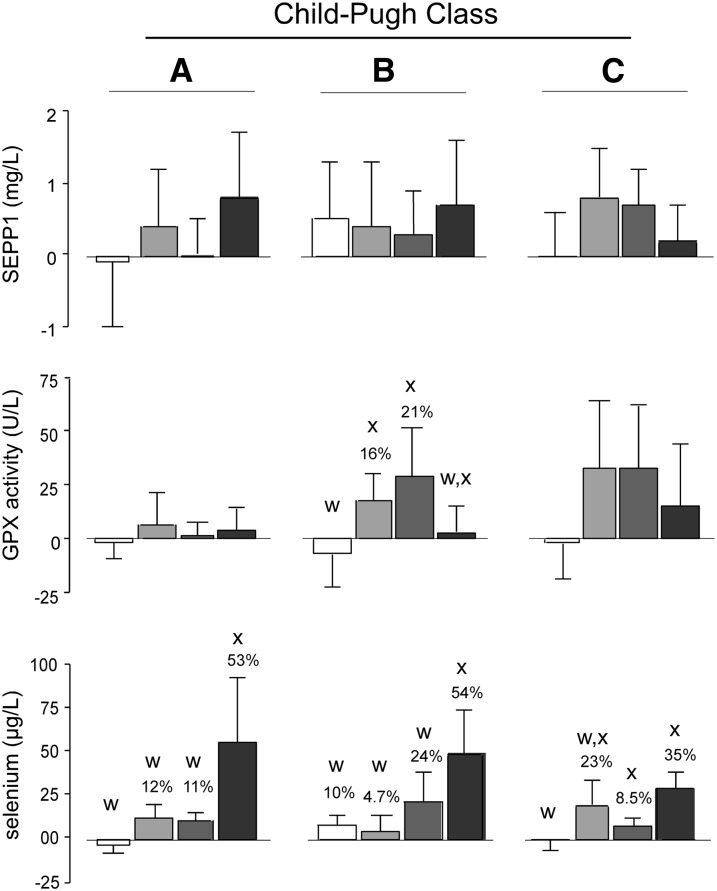
Figure 2 presents changes in selenium biomarkers of the 4 supplement groups after 4 wk of supplementation. The statistical analysis was carried out by 2 tests. The Kruskal-Wallis H test was used to compare the changes of a given biomarker between groups within a C-P class that were caused by the 4 different supplements. This test assessed the efficacy of the supplements relative to one another. The Wilcoxon’s signed-rank test was used to determine whether biomarkers were increased from their initial (baseline) values by the specific treatments. This test was applied to treatment groups within C-P classes that were identified by the Kruskal-Wallis H test and allowed us to determine the magnitude of treatment group increases caused by supplementation.
FIGURE 2.
Mean ± 1 SD effects of selenium supplementation on selenium biomarkers in patients with cirrhosis. Values represented by bars are means of differences between initial (baseline) and 4-wk values with 1 SDs indicated. Colors of bars designate supplement groups as follows: placebo (white); 200 μg Se as selenate (light gray); 400 μg Se as selenate (dark gray); and 200 μg Se as selenomethionine (black). The number of patient values included in each group calculation was 5–8; the exact numbers in each group are shown in Table 3. When significant differences between treatment values were detected within C-P classes with the use of the Kruskal-Wallis H test, letters are present above bars. Bars that do not share a common letter were significantly different at P < 0.05. Percentage values over bars within C-P classes containing significant differences between treatment values as determined with the use of the Kruskal-Wallis H test indicate how much the values were significantly increased (P < 0.05) by the supplement as determined with the use of Wilcoxon’s signed-rank test. GPX, glutathione peroxidase; SEPP1, selenoprotein P.
When analyzed with the use of the Kruskal-Wallis H test, significant differences in the GPX activity between each of the selenate groups and the placebo group were detected in C-P class B (Figure 2). No other significant differences were detected by this test in either selenoprotein biomarker between treatment groups. There were differences in selenium amounts between placebo treatment groups and selenomethionine treatment groups in all 3 C-P classes. Also, the group that was taking 400 μg Se as selenate in C-P class C was significantly different from the placebo group.
The Wilcoxon’s signed-rank test was applied to treatment values in C-P classes in which values were significantly different from one another according to the Kruskal-Wallis H test. GPX values were increased significantly (16% and 21%) by both doses of selenate in C-P class B (Figure 2) but not by the placebo or selenomethionine. No significant differences between selenoprotein responses of men and women to supplementation were detected (not shown) although patient numbers were too small to reach firm conclusions.
Selenium concentration increased significantly in all C-P classes supplemented with selenomethionine. The selenium concentration was increased significantly by both selenate doses in all C-P classes. Both selenoproteins measured as biomarkers contributed to the selenium concentration, and thus, this result was consistent with increases in the selenoprotein biomarkers in these groups even though individual selenoprotein biomarker values did not always increase significantly.
Supplemental Figure 2 shows that 2 of 6 C-P class C subjects supplemented with selenate in the pilot study responded with increases of all biomarkers, which suggested that the selenium status of patients with cirrhosis of a given C-P severity varies. Percentages of subjects in the randomized controlled trial who responded with a ≥20% increase in all 3 biomarkers are presented in Supplemental Figure 3. Of 39 subjects supplemented with the 2 dose amounts of selenate, 8 subjects (21%) responded with a ≥20% increase in all 3 selenium biomarkers, whereas one of 21 subjects (5%) supplemented with selenomethionine responded in this manner. These observations are consistent with selenium status varying in patients with similar clinical assessments and also with an impairment of selenomethionine metabolism that causes functional selenium deficiency in some, but not all, patients with cirrhosis.
DISCUSSION
In the current randomized controlled trial, the Kruskal-Wallis H test indicated that, in C-P class B, both doses of selenate supplementation increased the plasma GPX activity significantly more than did the placebo, whereas selenomethionine supplementation did not cause such an increase. These results are consistent with some patients with cirrhosis having selenium deficiency. The failure of GPX activity to respond to selenomethionine supports the hypothesis that the selenium deficiency in cirrhosis is functional (i.e., caused by impairment of selenomethionine metabolism). Plasma selenoprotein biomarkers did not increase in healthy subjects supplemented with selenomethionine (16) (Supplemental Table 1).
What do the responses to selenate reveal about the frequency and severity of selenium deficiency in patients with cirrhosis? Twenty-one percent of the 39 subjects supplemented with selenate had ≥20% increases in all 3 selenium biomarkers (Supplemental Figure 3). Broken down by C-P class, the percentages were 8% in class A, 21% in class B, and 31% in class C. Thus, it appears that selenium deficiency is more likely to occur as cirrhosis worsens and that its incidence approaches one-third in C-P class C patients.
The maximum group average response of a selenoprotein biomarker to selenate in the current study was 24% (with the SEPP1 concentration responding to 200 μg Se as selenate in C-P class C), which implied that the group average of the SEPP1 concentration had been 76% of the optimal concentration (defined as not limited by selenium availability) before supplementation. The cause of selenium deficiency in humans is usually inadequate dietary intake. Selenium intakes in US residents are usually >80 μg/d, and selenium deficiency has not been reported in these individuals (16). Other regions of the world have lower selenium intakes that are directly related to low soil-selenium bioavailability in the locales where their foods are produced. Many people in Europe have mild selenium deficiency with plasma SEPP1 concentrations ∼73% of the optimal concentration in one British study (22). In 1999, residents of the South Island of New Zealand were reported to be moderately selenium deficient with SEPP1 concentrations ∼55% of the optimal concentration (23). In 1987, a study of residents in a low-selenium area of China revealed that residents were more severely selenium deficient with dietary intakes ∼10 μg Se/d and plasma SEPP1 concentrations that were ∼14% of the optimal concentration (24). The principal causes of this severe selenium deficiency were the local production of food on soil with low selenium bioavailability and limited access to food from other areas.
Although no morbidity associated with the selenium deficiencies in Europe or New Zealand has been consistently identified, the more-severe deficiency that occurred in China until ∼1990 was a prerequisite for the occurrence of a childhood cardiomyopathy named Keshan disease (25). A similar cardiac injury has been produced in selenium-deficient mice by infection with the Coxsackie B3 virus (26), which suggested that a viral infection might have triggered the cardiomyopathy that occurred in some of the severely selenium-deficient children.
Keshan disease disappeared in the late 20th century when improving economic conditions in China resulted in food being distributed from selenium-adequate areas to the selenium-deficient areas. In a 2007 study of one of the selenium-deficient areas in China where Keshan disease had been endemic before 1980, healthy adults had plasma SEPP1 concentrations that were 36% of the values attained after selenium supplementation (27). We did not study patients with cirrhosis in China but, on the basis of the results presented in the current study, patients with cirrhosis living in low-selenium areas might be expected to have more-severe selenium deficiency than healthy people do in the same areas.
The consequences of very-severe dietary selenium deficiency with a plasma SEPP1 concentration <5% of selenium-replete values are not known in humans but have been studied in experimental animals. In the livers of severely selenium-deficient mice, the nuclear factor (erythroid-derived 2)-like 2–antioxidant responsive element pathway is activated with the induction of glutathione synthesis and the induction of a number of protective enzymes including heme oxygenase-1 and glutathione transferases (1). Some toxicities in animals are lessened by selenium deficiency [e.g., acetaminophen and aflatoxin toxicities) (28), but other toxicities are worsened. The redox cycling compound diquat causes massive liver necrosis in selenium-deficient rats (29) as does vitamin E deficiency (30). It is not known whether the relatively mild selenium deficiency associated with cirrhosis predisposes patients to injuries caused by other agents or clinical conditions.
Studies to determine human selenium requirements have all been carried out in healthy subjects. In 2000, the US Recommended Dietary Allowance for selenium was set at 55 μg/d on the basis of plasma GPX activity optimization (reaching a plateau during progressively increasing selenium supplementation) (11). A subsequent study that used the optimization of the plasma SEPP1 concentration to estimate the selenium requirement arrived at a value of ∼75 μg/d (27). The doses of 200 and 400 μg used in this study were chosen to ensure that adequate selenium would be provided for selenoprotein synthesis. Such doses cannot be recommended for general use because epidemiologic studies have linked high selenium amounts in plasma and toenails with diabetes and other pathologic conditions (31). Moreover, as shown in Figure 2, the 200-μg dose was as effective as the 400-μg dose, which suggested that the dose needed to optimize the biomarkers in patients with cirrhosis is ≤200 μg. Thus, any selenium supplementation of patients with cirrhosis should be undertaken with caution.
What are some limitations of the current study? The study was designed to enroll 144 patients but enrolled only 99 subjects. Of the 99 enrolled patients, 82 subjects completed the study. These factors lessened the statistical power.
Another potential problem was the use of the plasma selenoprotein biomarkers in patients with cirrhosis. Previous studies have validated these biomarkers as indexes of selenium status in healthy subjects (27), but it is possible that cirrhosis might alter the biomarker response to supplemental selenium and, thereby, impair its use in the assessment of selenium status (12). Plasma SEPP1 originates mostly in the liver (32), and cirrhosis causes it to be depressed even in patients who have been supplemented with selenate (Figure 2). Plasma GPX3 originates mostly in the kidney (33), and cirrhosis causes plasma GPX activity to increase. It is not known whether GPX3 or another GPX is responsible for this increased GPX activity or whether the incremental plasma GPX activity originates in the kidney or elsewhere. Despite these potential problems, GPX activity responded to supplementation with selenate in subjects with C-P class B cirrhosis, suggesting that it could be used to detect selenium deficiency.
In conclusion, we interpret the results of the current study as supporting the hypothesis that cirrhosis causes functional selenium deficiency in some patients by impairing selenomethionine metabolism, and the incidence of selenium deficiency increases as cirrhosis becomes more severe. These results raise the issue of whether patients with cirrhosis should be supplemented with inorganic selenium. Because the selenium deficiency we detected was mild, and selenium supplementation might have adverse effects, no recommendations for supplementation can be made at the current time. Additional studies will be needed to determine the dose of inorganic selenium needed to correct the functional selenium deficiency and, then, to determine whether correcting the selenium deficiency diminishes morbidity in patients with cirrhosis. In addition, assessments of selenium status in patients with cirrhosis residing in low-selenium areas of the world are needed.
Acknowledgments
We thank Joseph A Awad for serving as the safety monitor for the randomized controlled trial.
The authors’ responsibilities were as follows—RFB and KEH: were responsible for the design of the concept and plans of the projects, wrote the manuscript, and had primary responsibility for the final content of the manuscript; RFB, KEH, and DWB: analyzed the data or performed the statistical analysis; KEH, AKM, and BKN: conducted the research; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: C-P, Child-Pugh; GPX, glutathione peroxidase; SEPP1, selenoprotein P.
REFERENCES
- 1.Burk RF, Hill KE, Nakayama A, Mostert V, Levander XA, Motley AK, Johnson DA, Johnson JA, Freeman ML, Austin LM. Selenium deficiency activates mouse liver Nrf2-ARE but vitamin E deficiency does not. Free Radic Biol Med 2008;44:1617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J. An original discovery: selenium deficiency and Keshan disease (an endemic heart disease). Asia Pac J Clin Nutr 2012;21:320–6. [PubMed] [Google Scholar]
- 3.Aaseth J, Alexander J, Thomassen Y, Blomhoff JP, Skrede S. Serum selenium levels in liver diseases. Clin Biochem 1982;15:281–3. [DOI] [PubMed] [Google Scholar]
- 4.Bettinger D, Schultheiss M, Hennecke N, Panther E, Knuppel E, Blum HE, Thimme R, Spangenberg HC. Selenium levels in patients with hepatitis C virus-related chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma: a pilot study. Hepatology 2013;57:2543–4. [DOI] [PubMed] [Google Scholar]
- 5.Burk RF, Early DS, Hill KE, Palmer IS, Boeglin ME. Plasma selenium in patients with cirrhosis. Hepatology 1998;27:794–8. [DOI] [PubMed] [Google Scholar]
- 6.Chin SE, Shepherd RW, Thomas BJ, Cleghorn GH, Patrick MK, Wilcox JA, Ong TH, Lynch SV, Strong R. The nature of malnutrition in children with end-stage liver disease awaiting orthotopic liver transplantation. Am J Clin Nutr 1992;56:164–8. [DOI] [PubMed] [Google Scholar]
- 7.Dworkin B, Rosenthal WS, Jankowski RH. Low blood selenium levels in alcoholics with and without advanced liver disease. Dig Dis Sci 1985;30:838–44. [DOI] [PubMed] [Google Scholar]
- 8.Johansson U, Johnsson F, Joelsson B, Berglund M, Åkesson B. Selenium status in patients with liver cirrhosis and alcoholism. Br J Nutr 1986;55:227–33. [DOI] [PubMed] [Google Scholar]
- 9.Thuluvath PJ, Triger DR. Selenium in chronic liver disease. J Hepatol 1992;14:176–82. [DOI] [PubMed] [Google Scholar]
- 10.Burk RF, Hill KE, Motley AK. Plasma selenium in specific and non-specific forms. Biofactors 2001;14:107–14. [DOI] [PubMed] [Google Scholar]
- 11.Food and Nutrition Board, Institute of Medicine. Selenium. In: Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington (DC): National Academy Press; 2000. p. 284–324.
- 12.Burk RF, Hill KE. Regulation of selenium metabolism and transport. Annu Rev Nutr 2015;35:109–34. [DOI] [PubMed] [Google Scholar]
- 13.Esaki N, Nakamura T, Tanaka H, Soda K. Selenocysteine lyase, a novel enzyme that specifically acts on selenocysteine. Mammalian distribution and purification and properties of pig liver enzyme. J Biol Chem 1982;257:4386–91. [PubMed] [Google Scholar]
- 14.Bosy-Westphal A, Ruschmeyer M, Czech N, Oehler G, Hinrichsen H, Plauth M, Lotterer E, Fleig W, Muller MJ. Determinants of hyperhomocysteinemia in patients with chronic liver disease and after orthotopic liver transplantation. Am J Clin Nutr 2003;77:1269–77. [DOI] [PubMed] [Google Scholar]
- 15.Pugh RNH, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–9. [DOI] [PubMed] [Google Scholar]
- 16.Burk RF, Norsworthy BK, Hill KE, Motley AK, Byrne DW. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol Biomarkers Prev 2006;15:804–10. [DOI] [PubMed] [Google Scholar]
- 17.Xia YM, Hill KE, Burk RF. Biochemical studies of a selenium-deficient population in China: Measurement of selenium, glutathione peroxidase and oxidant defenses in blood. J Nutr 1989;119:1318–26. [DOI] [PubMed] [Google Scholar]
- 18.Koh TS, Benson TH. Critical re-appraisal of fluorometric method for determination of selenium in biological materials. J Assoc Off Anal Chem 1983;66:918–26. [PubMed] [Google Scholar]
- 19.Sheehan TMT, Gao M. Simplified fluorometric assay of total selenium in plasma and urine. Clin Chem 1990;36:2124–6. [PubMed] [Google Scholar]
- 20.Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trials 1990;11:116–28. [DOI] [PubMed] [Google Scholar]
- 21.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464–70. [DOI] [PubMed] [Google Scholar]
- 22.Méplan C, Crosley LK, Nicol F, Beckett GJ, Howie AF, Hill KE, Horgan G, Mathers JC, Arthur JR, Hesketh JE. Genetic polymorphisms in the human selenoprotein P gene determine the response of selenoprotein markers to selenium supplementation in a gender-specific manner (the SELGEN study). FASEB J 2007;21:3063–74. [DOI] [PubMed] [Google Scholar]
- 23.Duffield AJ, Thomson CD, Hill KE, Williams S. An estimation of selenium requirements for New Zealanders. Am J Clin Nutr 1999;70:896–903. [DOI] [PubMed] [Google Scholar]
- 24.Hill KE, Xia Y, Åkesson B, Boeglin ME, Burk RF. Selenoprotein P concentration in plasma is an index of selenium status in selenium-deficient and selenium-supplemented Chinese subjects. J Nutr 1996;126:138–45. [DOI] [PubMed] [Google Scholar]
- 25.Observations on effect of sodium selenite in prevention of Keshan disease. Chin Med J (Engl) 1979;92:471–6. [PubMed] [Google Scholar]
- 26.Beck MA, Shi Q, Morris VC, Levander OA. Rapid genomic evolution of a non-virulent coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat Med 1995;1:433–6. [DOI] [PubMed] [Google Scholar]
- 27.Xia Y, Hill KE, Li P, Xu J, Zhou D, Motley AK, Wang L, Byrne DW, Burk RF. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: a placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am J Clin Nutr 2010;92:525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burk RF, Lane JM. Modification of chemical toxicity by selenium deficiency. Fundam Appl Toxicol 1983;3:218–21. [DOI] [PubMed] [Google Scholar]
- 29.Burk RF, Lawrence RA, Lane JM. Liver necrosis and lipid peroxidation in the rat due to paraquat and diquat administration. J Clin Invest 1980;65:1024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz K, Foltz CM. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J Am Chem Soc 1957;79:3292–3. [PubMed] [Google Scholar]
- 31.Stranges S, Sieri S, Vinceti M, Grioni S, Guallar E, Laclaustra M, Muti P, Berrino F, Krogh V. A prospective study of dietary selenium intake and risk of type 2 diabetes. BMC Public Health 2010;10:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill KE, Wu S, Motley AK, Stevenson TD, Winfrey VP, Capecchi MR, Atkins JF, Burk RF. Production of selenoprotein P (Sepp1) by hepatocytes is central to selenium homeostasis. J Biol Chem 2012;287:40414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avissar N, Ornt DB, Yagil Y, Horowitz S, Watkins RH, Kerl EA, Takahashi K, Palmer IS, Cohen HJ. Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am J Physiol 1994;266:C367–75. [DOI] [PubMed] [Google Scholar]