Abstract
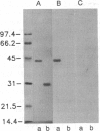
Complex formation between the human erythrocyte transglutaminase (protein-glutamine:amine gamma-glutamyltransferase, EC 2.3.2.13) and fibronectin or its fragments was examined by immunoanalytical procedures and by fluorescence polarization. A 42-kDa gelatin-binding structure, obtained from human plasma fibronectin by thermolytic digestion, showed as high an affinity for the cytosolic enzyme as the parent fibronectin chains themselves. A 21-kDa fragment comprising type I modules 8 and 9, the last two modules in the 42-kDa fragment, bound with an affinity 100-fold less than the 42-kDa fragment. Binding was remarkably specific and could be exploited for the affinity purification of transglutaminase directly from the hemoglobin-depleted erythrocyte lysate. In spite of the high affinity, it was possible to elute active enzyme from the 42-kDa fragment column with 0.25% monochloroacetic acid. This solvent might have general applicability in other systems involving separation of tightly bound ligands.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borsi L., Castellani P., Balza E., Siri A., Pellecchia C., De Scalzi F., Zardi L. Large-scale procedure for the purification of fibronectin domains. Anal Biochem. 1986 Jun;155(2):335–345. doi: 10.1016/0003-2697(86)90443-4. [DOI] [PubMed] [Google Scholar]
- Brenner S. C., Wold F. Human erythrocyte transglutaminase. Purification and properties. Biochim Biophys Acta. 1978 Jan 12;522(1):74–83. doi: 10.1016/0005-2744(78)90323-6. [DOI] [PubMed] [Google Scholar]
- Churchich J. E. The rotational relaxation time of aspartate aminotransferase. Biochim Biophys Acta. 1967 Dec 12;147(3):511–517. doi: 10.1016/0005-2795(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Freyssinet J. M., Lewis B. A., Holbrook J. J., Shore J. D. Protein-protein interactions in blood clotting. The use of polarization of fluorescence to measure the dissociation of plasma factor XIIIa. Biochem J. 1978 Feb 1;169(2):403–410. doi: 10.1042/bj1690403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham K. C., Brew S. A., Migliorini M. M. Further localization of the gelatin-binding determinants within fibronectin. Active fragments devoid of type II homologous repeat modules. J Biol Chem. 1989 Oct 15;264(29):16977–16980. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeMosy E. K., Erickson H. P., Beyer W. F., Jr, Radek J. T., Jeong J. M., Murthy S. N., Lorand L. Visualization of purified fibronectin-transglutaminase complexes. J Biol Chem. 1992 Apr 15;267(11):7880–7885. [PubMed] [Google Scholar]
- Litvinovich S. V., Strickland D. K., Medved L. V., Ingham K. C. Domain structure and interactions of the type I and type II modules in the gelatin-binding region of fibronectin. All six modules are independently folded. J Mol Biol. 1991 Feb 5;217(3):563–575. doi: 10.1016/0022-2836(91)90758-x. [DOI] [PubMed] [Google Scholar]
- Lorand L., Campbell-Wilkes L. K., Cooperstein L. A filter paper assay for transamidating enzymes using radioactive amine substrates. Anal Biochem. 1972 Dec;50(2):623–631. doi: 10.1016/0003-2697(72)90074-7. [DOI] [PubMed] [Google Scholar]
- Lorand L., Credo R. B., Janus T. J. Factor XIII (fibrin-stabilizing factor). Methods Enzymol. 1981;80(Pt 100):333–341. doi: 10.1016/s0076-6879(81)80029-8. [DOI] [PubMed] [Google Scholar]
- Lorand L., Dailey J. E., Turner P. M. Fibronectin as a carrier for the transglutaminase from human erythrocytes. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1057–1059. doi: 10.1073/pnas.85.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Siefring G. E., Jr, Tong Y. S., Bruner-Lorand J., Gray A. J., Jr Dansylcadaverine specific staining for transamidating enzymes. Anal Biochem. 1979 Mar;93(2):453–458. doi: 10.1016/s0003-2697(79)80178-5. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Umfleet R. A. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J Biol Chem. 1970 Nov 10;245(21):5728–5736. [PubMed] [Google Scholar]
- Murthy S. N., Lorand L. Cross-linked A alpha.gamma chain hybrids serve as unique markers for fibrinogen polymerized by tissue transglutaminase. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9679–9682. doi: 10.1073/pnas.87.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S. N., Wilson J., Guy S. L., Lorand L. Intramolecular crosslinking of monomeric fibrinogen by tissue transglutaminase. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10601–10604. doi: 10.1073/pnas.88.23.10601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Shainoff J. R., Urbanic D. A., DiBello P. M. Immunoelectrophoretic characterizations of the cross-linking of fibrinogen and fibrin by factor XIIIa and tissue transglutaminase. Identification of a rapid mode of hybrid alpha-/gamma-chain cross-linking that is promoted by the gamma-chain cross-linking. J Biol Chem. 1991 Apr 5;266(10):6429–6437. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. M., Lorand L. Complexation of fibronectin with tissue transglutaminase. Biochemistry. 1989 Jan 24;28(2):628–635. doi: 10.1021/bi00428a032. [DOI] [PubMed] [Google Scholar]