Introduction
Mouse embryonic stem cells (ESC) are derived from the inner cell mass of the pre-implantation blastocyst; accordingly, they have the ability to form all the tissues of the embryo (Evans and Kaufman, 1981; Martin 1981). There is currently great interest in understanding the molecular mechanisms involved in maintaining pluripotency as well as in achieving controlled differentiation of ESC to facilitate cell replacement therapy for the resolution of human disease. ESC can also be used as a model of lineage choice in development, providing a systematic simplification of this extraordinarily complex and otherwise inaccessible process. Pluripotency of ESC, in the absence of a feeder layer, can be maintained by leukemia inhibitory factor (LIF) signaling through Stat-3 (Smith et al., 1988; Williams et al., 1988). Blocking or stimulating other signaling pathways including: the BMP, PI3 kinase/AKT, MAP-ERK kinases, and Wnt pathways has also been suggested to be sufficient to maintain ESC pluripotency and conversely, manipulation of these pathways has also been reported to promote lineage specific differentiation of ESC (Lee et al., 2009; Paling et al., 2006; Qi et al., 2004; Watanabe et al., 2006: Ying et al., 2003). Untangling these opposing results requires rigorous and likely reversible control of each pathway individually and in combination.
The Wnt family of secreted ligands bind to frizzled receptors, inhibiting GSK-3β phosphorylation and the destruction of β-catenin, resulting in the accumulation of nuclear β-catenin that binds to Tcf/Lef transcription factors to activate or repress gene expression (Blauwkamp et al., 2008). Wnt signaling has been implicated in many diverse and seemingly opposing processes such as self-renewal and proliferation versus differentiation of both developing as well as adult tissues (Arce et al., 2006; Chien et al., 2009; van Amerongen and Nusse, 2009). In the case of ESC, there are conflicting reports regarding the role of Wnt signaling in maintaining pluripotency versus promoting differentiation. Many authors have suggested that Wnt pathway activation is sufficient to maintain self-renewal of ESC (Miyabayashi et al., 2007; Sato et al., 2004; Singla et al., 2006; Takao et al., 2007), but may require cooperative low level LIF/Stat3 signaling to inhibit differentiation (Bone et al., 2009; Hao et al., 2005; Ogawa et al., 2006). In other contexts, Wnt pathway activation promoted rather than inhibited differentiation of ESC (Gadue et al., 2006; Lindsey et al., 2006; Nakanishi et al., 2008; Otero et al., 2004; ten Berge et al., 2008). Adding to the confusion, several other groups have demonstrated a role for the Tcf3 transcription factor in maintaining the balance between self-renewal and differentiation (Cole et al., 2008), independent of the status of Wnt signaling, via its ability to act as a transcriptional repressor (Pereira et al., 2006; Tam et al., 2008; Yi et al., 2008). Certainly, nuances in experimental design and differences between mouse strains have contributed to the variability in these results, but cannot explain the dramatically different conclusions of these studies.
Wnt signaling plays a role in the specification, proliferation, or differentiation of nearly every tissue in the embryo (Arce et al., 2006; Chien et al., 2009; van Amerogen and Nusse, 2009). During gastrulation, Wnt signaling is critically involved in establishing the primitive streak and promoting the epithelial to mesenchymal transformation (EMT) required for mesendodermal differentiation of the epiblast (Doble and Woodgett, 2007; Maretto et al., 2003; Mohamed et al., 2004; Sinner et al., 2004; Yamaguchi et al., 1999), thereby controlling tri-lineage differentiation. In addition, differentiation of neural ectoderm both in the embryo as well as during ESC differentiation has been reported to result from the inhibition of Wnt signaling (Aubert et al., 2002; Cajánek et al., 2009; Haegele et al., 2003; Kelly et al., 2004; Kemler et al, 2004; ten Berge et al., 2008; Verani et al., 2007). Based on these observations, it has been postulated that inhibition of Wnt signaling during ESC differentiation indirectly promotes neural lineage specification by inhibiting mesendodermal differentiation (Aubert et al., 2002). However, loss of the mesendoderm lineage does not guarantee default differentiation to neural ectoderm (Linker and Stern, 2004) since there is first a requirement for BMP signal inhibition to induce pan-neural differentiation (Zhang et al., 2010), followed by subsequent signaling to establish neuronal and glial lineages. In fact, there is considerable evidence suggesting that Wnt pathway activation is required not only for pattering of the nervous system but also for proliferation and differentiation at multiple steps during development (Houart et al., 2002; Kalani et al., 2008; Kuwbara et al., 2009; Lagutin et al., 2002; Muroyama et al., 2003; Vanderhaeghen, 2009; Yu et al., 2007; Zechner et al., 2003; Zechner et al., 2007).
To begin to decipher the sequential roles of Wnt signaling in lineage differentiation of ESC, we developed a tetracycline inducible (Masui et al., 2005) dominant negative Tcf4 (dnTcf4) expressing mouse embryonic stem cell line. Using these cells, it is possible to block and then relieve the repression on Wnt signaling during ESC differentiation. Blocking Wnt signaling induced differentiation of Sox3 positive neural precursors that could only progress to Tuj1 positive primitive neurons when Wnt signaling was de-repressed. These results are consistent with observations in embryos null for β-catenin (Haegel et al., 1995; Huelsken et al., 2000), LRP5/LRP6 co-receptors (Kelly et al., 2004), or Wnt ligands (Liu et al., 1999; Yoshikawa et al., 1997) in which neural ectoderm differentiates at the expense of mesendoderm. There is then a requirement for active Wnt signaling for the differentiation of proliferating progenitors to mature neurons (Gao et al., 2009; Hirabayashi et al., 2004; Israsena et al., 2004; Kuwabara et al., 2009; Lie et al., 2005; Muroyama et al., 2002). Thus, it is clear that simple blockage of Wnt signaling is sufficient to inhibit tri-lineage differentiation at gastrulation producing a default (neural) ectoderm, but further differentiation to a mature neuronal phenotype requires sequential signaling/patterning.
To examine the requirement for Wnt signaling at sequential stages of neuronal differentiation in the intact embryo, we delivered a shRNA targeting β-catenin to pregnant dams to reduce Wnt signaling and observed a significant increase in differentiation of Sox3 positive neural precursors but a decrease in their conversion to mature neurons, as well as defects of embryonic axis elongation, neurulation and neural tube closure, that phenocopy the null embryo.
Materials and methods
ES cell culture and differentiation
Undifferentiated ESCs were maintained in 0.1% gelatin coated tissue culture flasks in complete media composed of DMEM (Invitrogen), 10% fetal bovine serum (Atlanta Biologicals), 50 mM HEPES (Sigma), and 1 μM β-mercaptoethanol (Sigma) with 5 ng/ml LIF (Chemicon). Neural-permissive culture conditions were achieved by plating cells at low density (2×104 cells per cm2) in gelatin coated 12 well plates in 20% Neural basal medium (Invitrogen), 80% Ham's F12 medium (Invitrogen) with N2 and B27 salts (Invitrogen), 1 μM retinoic acid (Sigma), and 0.05% Knock-out serum replacement (Invitrogen). To form embryoid bodies (EBs), cells were plated at 1×106 cells per 6cm dish in non-adherent (Petri) dishes in DMEM (Invitrogen), 10% fetal bovine serum (Atlanta Biologicals), 50 mM HEPES (Sigma), and 1 μM β-mercaptoethanol (Sigma) with or without doxycycline for 4 days, then transferred to gelatin coated 12 well plates for an additional 2 days of culture. Cells were maintained at 37°C with 5% CO2.
Inducible dnTcf4 ESC line
We obtained the MGZRTcH2 ESC cell line and corresponding exchange vector from Dr. Shinji Masui (Masui et al., 2005). The MGZRTcH2 Tet-off cell line has a tetracycline-regulatable transactivator (tTA), a tetracycline response element followed by the minimal promoter of the human cytomegalovirous (hCMV*-1) immediate early gene, a hygromycin resistance cassette, directional loxP sites, and an IRES-Venus (yellow fluorescent protein) cassette knocked into the endogenous ROSA26 locus. The corresponding exchange vector replaces the hygromycin resistance cassette with the desired cDNA in addition to adding a puromycin resistance cassette. When the exchange vector is correctly enzymatically recombined into the ROSA26 locus, the resulting clones are no longer hygromycin resistant but become puromycin resistant, allowing both positive and negative selection and ensuring the identification of cell lines with random incorporation of the exchange vector (Figure 1).
Figure 1. Expression of the dnTcf4 protein from the ROSA26 locus can be controlled by doxycycline.
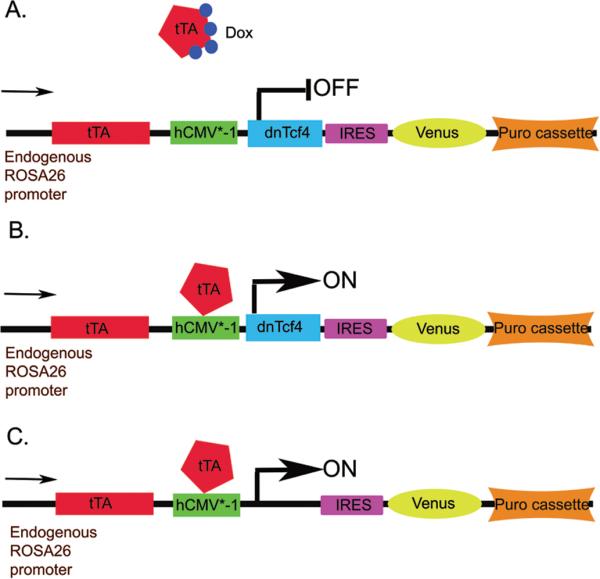
A. In the presence of Doxycycline (Dox), the tetracycline-regulated transactivator (tTA) is unable to bind to the hCMV*-1 promoter (Tetracycline response element followed by the minimal promoter of the human cytomegalovirus immediate early gene) and initiate downstream gene expression.
B. Once the drug is removed from the tissue culture medium, the tTA binds the promoter and the dnTcf4 and Venus proteins are transcribed.
C. Control cells inducibly express Venus, but lack the dnTcf4 cDNA.
The dnTcf4 cDNA was a gift from Dr. Eric Fearon at the University of Michigan (Kolligs et al., 1999). We added a Kozak's translation initiation sequence to the 5’ end of the cDNA and subcloned it into the pPthC exchange vector (Masui et al., 2005). We created a second exchange vector with no insert to generate control cell lines. The dnTcf4 and control exchange vectors were co-transfected with pCAGGS-CRE into MGZRTcH2ES cells using Lipofectamine Plus, following the manufacturer's recommendations. Selection of inducible dnTcf4 and control cells was carried out using 2μg/ml puromycin with negative selection of improperly targeted colonies using 100μg/ml hygromycin. Correctly targeted colonies were expanded in complete media with 1μg/ml doxycycline (Sigma) to inhibit expression of the transgene and 2μg/ml puromycin to maintain selection. Inducible expression of dnTcf4 was verified by western blot, semi-quantitative RT-PCR, and TOPflash assay.
Immunohistochemistry
For immunohistochemical (IHC) localization of cell type-restricted proteins, ES cells were fixed with 2% PFA for 15 minutes at room temperature and washed twice with PBS. Fixed cells were incubated with 10% donkey serum and 0.5% TritonX-100 in PBS for 30 minutes followed by overnight incubation at 4°C with primary antibody (mouse anti-βIII tubulin/Tuj1, Chemicon 1:500; rabbit anti-Sox3, Mike Klymkowski, University of Colorado 1:1000; goat anti-Oct3/4, Santa Cruz 1:500; rabbit anti-Foxa2, Upstate, 1:500; goat anti-brachyury, Santa Cruz, 1:500; rabbit anti-phosphohistone 3, Millipore, 1:500). Cells were washed in PBS and incubated with secondary antibodies (1:400) conjugated to CY3 or FITC (Jackson Immunoresearch) for 2 hours at room temperature. Nuclei were visualized with Hoechst 33258 (Sigma). Images were obtained using a Leica DM inverted fluorescence microscope and Olympus DP70 camera with associated software. Quantitative analysis of neuronal differentiation was performed with NIH Image J software (Version 1.4). Results are expressed as mean number of Tuj1 positive pixels divided by Hoechst positive pixels. Ten microscopic fields from triplicate cultures in four biological replicates (12 wells; 120 fields) were analyzed for each cell line. Data were averaged and analyzed using Student's t test.
Fluorescence activated cell sorting (FACS)
Cells were harvested with trypsin, triturated to a single cell suspension, washed with FACS buffer (PBS with 1% donkey serum and 0.02% sodium azide), and fixed with 2% paraformaldehyde (PFA) for 10 minutes at 4°C. Cells were permeabilized with 0.2% saponin in FACS buffer for 10 minutes at 4°C, incubated with rabbit antibodies against Sox3 (1:1000) for 30 minutes at 4°C followed by 3 washes with FACS buffer with saponin. Anti-rabbit CY5 secondary (Jackson Immunoresearch, 1:200) was used for detection followed by 3 washes with FACS buffer with saponin. Analysis was performed on a Becton Dickinson FACSCalibur.
Western blot
ES cells were lysed in Laemmli or RIPA buffer, cell debris pelleted by centrifugation, protein loaded onto polyacrylamide SDS PAGE gels, then transferred to a PVDF membrane. The membranes were blocked with 5% milk powder/TBST (Tris buffered saline and 0.1% Tween 20) and incubated with goat anti-Tcf4 (1:500, Santa Cruz), or mouse anti-β-catenin (1:1000, Millipore) and mouse anti-β-actin (1:10,000, Sigma) primary antibodies in 5% milk powder/TBST overnight at 4°C. Membranes were washed in TBST and incubated for 1 hour at room temperature in donkey anti-goat (1:1000) and donkey anti-mouse (1:10,000) horseradish peroxidase conjugated secondary antibodies (Jackson Immunoresearch) in 5% milk powder/TBST. Membranes were developed with Pierce Supersignal West Pico chemiluminescent substrate. Quantification of band intensity was carried out using ImageJ software (NIH).
Quantitative and semi-quantitative RT PCR
RNA was harvested with Trizol (Invitrogen) following the manufacturer's protocol and genomic DNA was removed by DNAse (Sigma) digestion. Complete DNA digestion was confirmed by semi-quantitative PCR using primers for β-actin before reverse transcription. A 0.5 to 1.0 μg RNA sample was used for reverse transcription with Verso RT (Thermo Scientific) using random nonamers (Invitrogen) following the manufacturer's protocols. For quantitative PCR, cDNAs were diluted 1:2 and 1μl was used per reaction with Abgene SYBR green master mix (Thermo-Fisher). All primer pairs were rigorously screened to eliminate primer dimer and reaction conditions were optimized to result in reaction efficiencies between 90% and 110%. Quantitative PCR results were calculated and statistical analysis was performed with REST2008 software (Pfaffl et al., 2002) and displayed as box and whisker plots. Semi-quantitative PCR was performed as described for the genomic DNA test above. Primer sequences and detailed reaction conditions are available upon request.
Top/Fopflash assay
Control and dnTcf4 cells were grown in DMEM (Invitrogen), 10% fetal bovine serum (Atlanta Biologicals), 50 mM HEPES (Sigma), and 1 μM β-mercaptoethanol (Sigma) without doxycycline for 48 hours to induce transgene expression. Cells were transfected with TOPFlash or FOPFlash plasmids (Millipore) using Lipofectamine Plus (Invitrogen) according to the manufacturer's instructions. A Renilla plasmid (pRL-CMV, Promega) was used as a transfection control. After 24 hours, cells were lysed with passive lysis buffer and subjected to the dual-luciferase assay (Promega) following the manufacturer's instructions. Samples were quantified with a Veritas microplate luminometer (Turner Biosystems). Data are expressed as the ratio of TOPFlash relative to Renilla over FOPFlash relative to Renilla.
Post-implantation mouse embryos
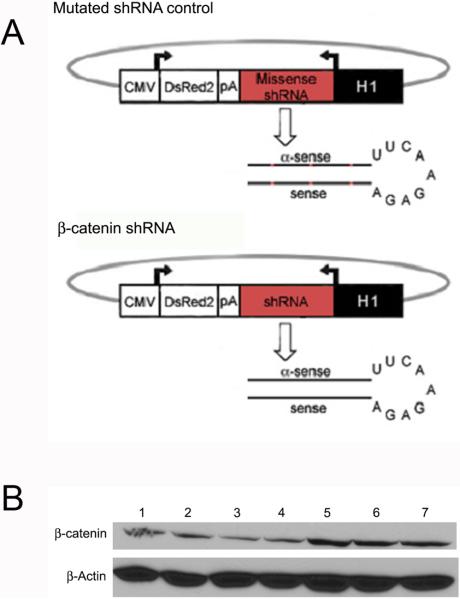
Wnt reporter mice (Mohamaed et al., 2004) were obtained (from Dr. Philip Gage, University of Michigan) and bred to generate time pregnant females. On day 5.5 post-coitum, pregnant females were injected via the tail vein (Gratsch et al., 2003) with 200 μl Ringer's saline containing 10 μg of two separate plasmids containing a short hairpin RNA against β-catenin or containing a mutated short hairpin RNA that does not target any sequence in the mouse genome (Figure 6A). Prior to use in pregnant dams, we verified that each construct efficiently decreased β-catenin protein in ESC by western blot (Figure 6B). Transfection with shRNAs produced a 40-64% reduction in β-catenin protein compared with the mutated control as quantified by ImageJ software. To harvest embryos, dams were killed by cervical dislocation and embryos dissected from uteri and decidua. Membranes were removed and embryos photographed using a Wild stereomicroscope, then allocated for analysis by scanning electron microscopy, X-Gal staining, O-Nitrophenyl -β-D-Galctopyranosidase (ONPG) analysis (quantification of β-Galactosidase activity), flow cytometry, or immunohistochemistry as described in detail below.
Figure 6. Transfection of ESC with a plasmid containing a β-catenin shRNA decreases the level of β-catenin protein.
A. Diagrams of plasmids containing mutated shRNA control or β-catenin shRNA that were expressed in ESC and pregnant dams.
B. Western blot of protein from ESC transfected with shRNA constructs for 24 hours targeting β-catenin singly (lanes 2,3) or in combination (lane 4) versus mutated control shRNA constructs singly (lanes 5,6) or in combination (lane 7) demonstrating 40-64% knock-down. Top blot is β-catenin and bottom blot is a loading control blotted for β-actin. Lane 1 is protein from untransfected ESC.
Scanning electron microscopy
Embryos were fixed for 30 minutes in 1% gluteraldehyde, washed, and stored in PBS at 4°C. They were dehydrated through graded alcohols followed by hexamethyldisilazane. Embryos were oriented on stubs, sputter coated with gold palladium, viewed, and photographed in an Amray scanning electron microscope.
X-Gal staining
Embryos were harvested and photographed as described above. To genotype individual embryos, a portion of the amnion/chorion was removed and placed into 25 μl alkaline lysis buffer (25 mM NaOH and 0.2mM EDTA) then incubated for 30 minutes at 95°C to release genomic DNA (Truett et al., 2000). The lysis buffer was neutralized with 25 μl of 40 mM Tris-HCL and 1 μl of each sample was used for genotyping PCR performed as described in Mohamed et al., 2004. Embryos were fixed for 15 to 30 minutes at room temperature in 0.1 M phosphate buffer (3.74 g monobasic sodium phosphate and 10.35 g dibasic sodium phosphate in 1 L water pH 7.3), 5 mM EGTA pH 7.3, 2 mM MgCl, and 0.2% glutaraldehyde then washed 3 times for 5 minutes in wash buffer (0.1 M phosphate buffer pH7.3, 2 mM MgCl, 0.01% deoxcholate, and 0.02% NP-40). Embryos were stained for 15 minutes to 1 hour at 37°C in stain buffer (0.1 M phosphate buffer pH7.3, 2 mM MgCl, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 0.01% deoxcholate, 0.02% NP-40, and 1 mg/ml X-gal).
ONPG assay
Embryos were harvested, photographed, and embryonic membranes genotyped as described above. Embryos were lysed in 25 μl of PM-2 buffer (20mM NaH2PO4, 80mM Na2HPO4, 0.1mM MnCl2, 2mM MgSO4, 40mM β-mercaptoethanol, pH7.3) by 5 cycles of freeze thaw then passed through a 12 gauge needle 5 times. Cellular debris was pelleted by centrifugation at 14,000 × g for 15 minutes at 4°C. Protein concentration was determined by the Bradford protein assay (BioRad). Total embryo protein was added to 400 μl of PM-2 buffer and samples were incubated at 37°C for 15 minutes. Next, 100ml of prewarmed ONPG solution (4mg/ml O-Nitrophenyl -β-D-Galctopyranosidase, Sigma, dissolved in PM-2 buffer made fresh for each assay) was added to each tube, assay start time was noted, and protein incubated at 37°C until a yellow color was visible. Finally, 250μl Na2CO3 was added to stop the reaction, stop time recorded, and the absorbance was read at 420nm in a spectrophotometer (BioRad SmartSpec 3000). β-galactosidase activity was calculated with the equation: Units = (380 × A420 / time)/ total protein. BSA protein dissolved in PM-2 buffer was used as a negative control and PM-2 buffer was used as the blank in the spectrophotometer.
FACS analysis of neural precursors and neurons
On E11, shRNA and mutated hairpin control embryos were harvested as described above then embryonic membranes and central nervous system dissected free of heart, liver, gut and branchial arch tissues. Embryos were digested with trypsin for 15-20 minutes at 37°C then triturated to a single cell suspension before fixation and analysis as described above for ESC. Each embryo sample was divided in half and incubated with either rabbit anti-Sox3 (1:1000) or mouse anti-NeuN antibody (Chemicon 1:500) prior to FACS analysis.
Embryo immunohistochemistry
Embryos were harvested and photographed as described above. Selected embryos were embedded in OCT (EMS), frozen in isopentane and sectioned at 8μm using a Microm cryostat. Sections were fixed with 2% paraformaldehyde for 15 minutes at room temperature then washed in PBS. They were permeabilized with 0.2% TritonX-100 in PBS then blocked with 10% donkey serum, 0.2% TritonX-100, and 0.05% sodium azide. Sections were incubated overnight at 4°C in rabbit anti-Sox3 (1:1000) and mouse anti-NeuN (1:500). They were washed in PBS and incubated with secondary antibodies conjugated to CY3 or FITC (1:200, Jackson Immunoresearch) for 2 hours at room temperature. Nuclei were visualized with Hoechst 33258 (Sigma). Sections were examined and photographed in a Leitz DM microscope and Olympus camera.
For whole mount immunohistochemistry, selected embryos were fixed in 2% PFA for 30 minutes at room temperature then washed in PBS. Embryos were dehydrated in 30%, 50%, and 80% methanol: DMSO (1:4) then incubated in methanol/DMSO/30% hydrogen peroxide (4:1:1) for 4 hours at room temperature to block endogenous peroxidase activity. Embryos were rehydrated in 50% and 15% methanol/PBS and cut in half before processing to increase antibody penetration. Embryos were permeabilized further and non-specific binding blocked by incubation in PBSMTX (2% milk powder and 0.1% TritonX-100 in PBS) for 2 hours at room temperature. Embryos were incubated overnight at 4°C in rabbit anti-Sox3 (1:2000) and mouse anti-NeuN (Chemicon, 1:1000) then washed 2 hours at 4°C followed by 3 hours at room temperature in PBSMTX. Embryos were incubated overnight at 4°C in secondary antibodies (1:200) conjugated to horseradish peroxidase (Jackson Immunoresearch) then washed 2 hours at 4°C followed by 3 hours at room temperature in PBSMTX. Embryos were washed in PBT (0.2% BSA, 0.1% TritonX-100 in PBS) for 20 minutes followed by incubation in 0.3mg/ml diaminobenzidine (Sigma) with 0.03% hydrogen peroxide in PBT for 40 minutes at room temperature. Embryos were washed in PBT and photographed using a Wild dissecting scope and a Nikon camera.
Results
Inducible expression of the dnTcf-4 protein blocks Wnt signaling in ESC
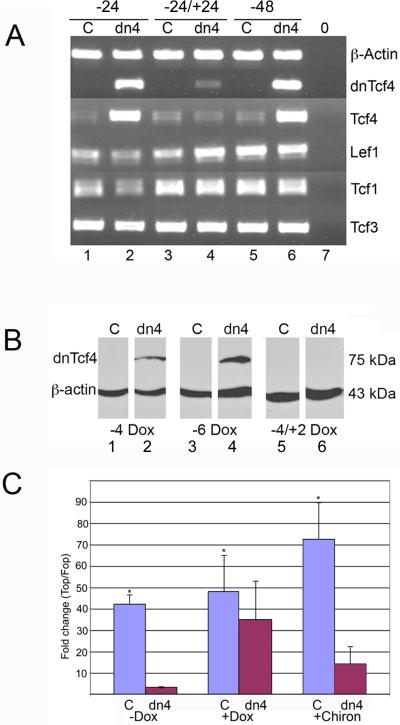
To explore the role of Wnt signaling in multi-lineage differentiation of ESC, a cell line was created that inducibly expresses a dominant negative Tcf4 (dnTcf4) protein (Tet-off) thereby inhibiting canonical Wnt signaling (Figure 2). A total of 24 dnTcf4 and 24 control lines (puromycin resistant cells that inducibly express only Venus yellow fluorescent protein) were cloned and expanded for further study. Proper targeting of all chosen lines was verified by loss of hygromycin resistance. Several lines of each were selected for further study based on doxycycline regulated dnTcf4 protein and/or Venus protein expression. In the presence of doxycycline, the dnTcf4 cell line divides and grows similarly to the control cell line (Supplemental Figure 1). Control (C) and dnTcf4 (dn4) cells express all four Tcf/Lef transcription factors (Figure 2A). The dnTcf4 mRNA is highly expressed within 24 hours of doxycycline withdrawal and is three-fold down-regulated when doxycycline is added back to the culture medium for an additional 24 hours. Neither doxycycline exposure nor expression of the dnTcf4 affected expression of either native Tcf4 or the three other Tcf/Lef transcription factors (Figure 2A). The transgenic dnTcf4 protein is detectable at four and six days of doxycycline withdrawal and disappears completely following reintroduction of doxycycline for 48 hours (Figure 2B).
Figure 2. Inducible expression of the dnTcf4 protein blocks Wnt signaling in ESC.
A. Control (C) and dnTcf4 (dn4) ESC were grown without Dox to induced transgene expression for 24h (-24, lanes 1,2) or 48h (-48, lanes 5,6) or for 24 hours without Dox followed by 24 hours with Dox (-24/+24, lanes 3,4). Lane 7 (0) is a no template negative control. PCR with a primer that anchors in the FLAG tag present in only the dnTcf4 mRNA shows that transgene expression is tightly controlled by Dox exposure and that reversible induction of the dnTcf4 had no effect on expression of endogenous Tcf4 or other Tcf/Lef transcription factors (Lef1, Tcf1, Tcf3).
B. Western blot demonstrating expression of the dnTcf4 protein 4 and 6 days after doxycycline withdrawal (Lanes 2,4). Expression is eliminated when doxycycline is reintroduced into the culture media for 48 hours (Lane 6). Expression is not detected in control cells (Lanes 1,3,5).
C. Wnt signaling is strikingly inhibited by dnTcf4 expression even in the presence of a GSKβ inhibitor (Chir99021) that strongly stimulates the Wnt pathway in luciferase assays. The mean fold change (TOP Flash/FOP Flash) in the control line (C) was significantly higher than the dnTcf4 (dn4) line without Dox for 4 days (-Dox), after Dox was withdrawn for 3 days and added back to cells for 1 day (+Dox), and without Dox for 4 days but with Chir99021 for 1 day (+Chiron,) * = p ≤ 0.05, Student's t test (n=3). Error bars represent SEM.
The dnTcf4 protein reduced canonical Wnt signaling approximately 40 fold in the TopFlash luciferase assay (Figure 2C), and after reintroduction of doxycycline for 24 hours, the levels of Wnt signaling were restored to nearly normal in dn4 cells. More importantly, the dnTcf4 inhibited Wnt signaling even in the presence of a Gsk3β inhibitor (Chir99021, Stemgent) previously shown to promote Wnt signaling (Finaly et al., 2004). These data indicate that expression of the dnTcf4 transgene is controlled by doxycycline, and functionally inhibits Wnt signaling.
Regulated expression of the dnTcf4 protein promotes neural differentiation of ESC in a monolayer assay
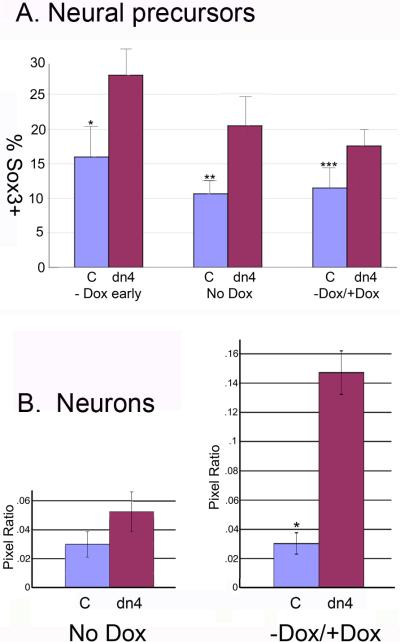
To explore the role of Wnt signaling in the neuronal differentiation of ESC, control and dnTcf4 expressing cells were plated in a serum free monolayer differentiation assay in neural permissive media with or without doxycycline. When Wnt signaling was abrogated throughout the culture period by removal of doxycycline from the medium, there was a dramatic increase in the number of Sox3 positive neural precursor cells after as little as 4 days (Figures 3,4), but there was little neuronal differentiation even if the cells were kept in culture as long as 8 days. These results are not attributable to alterations in cell density as proliferation of dn4 cells and control cells were similar as assessed by IHC localization of anti-phosphohistone H3 (Figure 5A; Supplemental Figure 1), and cell behavior. FACS analysis indicated that there was a statistically significant increase in the number of Sox3 positive cells in dnTcf4 versus control cells (Figure 4A) that was particularly robust when the transgene was induced early in differentiation. When the dnTcf4 protein was expressed early in the culture period (days 1 to 3) then down-regulated by addition of doxycycline during the second phase of differentiation (days 3-6) to permit Wnt signaling, there was a striking increase in the number of βΙΙΙ tubulin positive neurons in the cultures (Figures 3,4). Culture of the dn4 cells with doxycycline for 6 days resulted in differentiation that was very similar to control cells (Supplemental Figure 1). Because FACS sorting of Tuj1 positive cells resulted in high levels of background signal that did not accurately reflect differentiation, we used ImageJ software to determine the mean number of green (βΙΙΙ tublin positive) pixels in each condition. Data are presented as mean number of Tuj1 pixels/mean number of Hoechst pixels (nuclei). Consistent with our IHC results, ImageJ analysis indicated that there was maximal differentiation of primitive neurons when Wnt signaling was initially inhibited, then released for the final differentiation of precursors to neurons (Figure 4B).
Figure 3. Regulated expression of the dnTcf4 protein promotes neural differentiation of ESC in a monolayer assay.
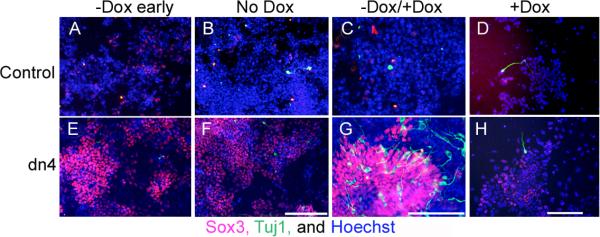
Immunohistochemical localization of cell type restricted antigens in Control (A-D) and dnTcf4 (E-H) ESC grown in monolayer. When the transgene was induced and Wnt signaling was abrogated for 4 (-Dox early; E) or 6 days (No Dox; F) of differentiation there was widespread expression of the neural precursor marker Sox3 (red, Cy3 secondary) compared with controls (A,B), but was little expression of the neuronal marker βIII tubulin (Tuj1 antibody, green, FITC secondary; A,B, E,F). Reactivation of Wnt signaling during the last 3 days of differentiation (-Dox/+Dox; G) resulted in a significant increase in the conversion of Sox3 positive precursors to immature neurons, compared with Controls (C). There was little neuronal differentiation when Control or dn4 cells were grown continuously in doxycycline for 6 days (+Dox; D,H). Nuclei were stained with Hoechst (blue). Scale bar = 200 μM.
Figure 4. Regulated expression of the dnTcf4 protein significantly increases neural and neuronal differentiation of ESC in a monolayer assay.
A. FACS analysis of neural precursors (Sox3 +cells) following induction of the dnTcf4 transgene. Cells were cultured for 3 days without Dox (-Dox early), 6 days without Dox (No Dox), or 3 days without Dox followed by 3 days with Dox (-Dox / +Dox). The number of Sox3 positive cells was significantly increased in dnTcf4 (dn4) expressing cells compared to control cells (C) at all time points. *p≤0.001, p**≤0.01, p***≤0.05, Student's t test. The graphs represent averages of 5 experiments with 3 replicates/experiment.
B. The number of neurons in control and dnTcf4 ESC grown in monolayer culture was quantified using NIH Image J. IHC localization of βIII tubulin was carried out in cultures in which Wnt signaling was abrogated during the entire culture period (No Dox) or when signaling was abrogated only during the first half of the culture period (-Dox / +Dox). The average number of βIII tubulin pixels was divided by the average number of Hoechst pixels in 10 fields per well, 3 replicates/experiment, in 4 biological experiments (n=120 fields/group). There was a significant increase in neuronal differentiation only when Sox3 positive precursors were allowed to complete differentiation in the presence of Wnt signaling during the last half of the culture period (-Dox / +Dox). *p≤0.003 Student's t test.
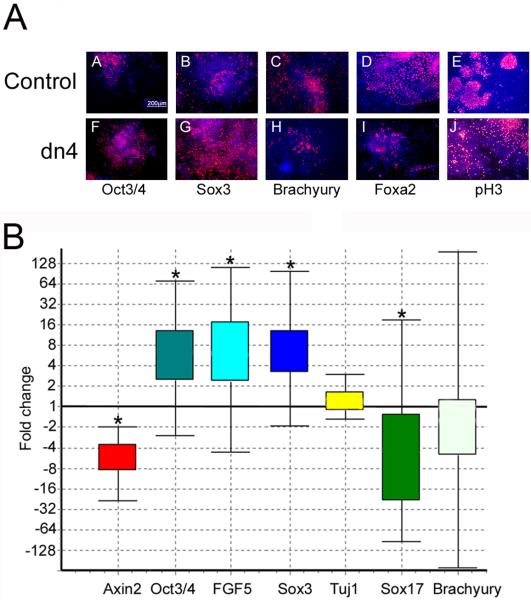
Figure 5. Wnt signaling blockade increases differentiation of neural precursors and decreases mesendoderm differentiation in an EB assay.
A. Control and dnTCF4 ESC were grown as EB for 4 days then transferred to adherent culture for an additional 2 days. Cells were grown without Dox during the entire culture period to induce transgene expression. IHC localization of the pluripotency marker (Oct3/4; A,F), the neural precursor marker (Sox3; B,G), mesodermal marker (Brachyury; C,H), or the endodermal maker (Foxa2; D,I), or phosphohistone3 to identify dividing cells (pH3; E,J). When Wnt signaling is inhibited, cells continue to express high levels of Oct3/4 and Sox3 (F,G) with a concomitant decrease in mesendoderm differentiation (H,I), compared with controls (A-D). Panels E,J illustrate the very similar pattern of cell division identified using anti-phosphohistone3 antibody. Secondary antibodies were conjugated to Cy3 (red) and Hoechst (blue) identifies nuclei.
B. Quantitative PCR indicated that expression of the Wnt target gene Axin2 was decreased 5-fold in dnTcf4 compared to control cells after 6 days of transgene induction. Genes expressed in the epiblast: Oct3/4 and Fgf5 were increased 6 fold suggesting that Wnt signaling is required for differentiation of the epiblast. The neural precursor gene Sox3 was increased 6 fold, but Tuj1, a marker of immature neurons, was not changed. Sox17 was down-regulated 5 fold and there was a trend towards a reduction of Brachyury expression (mesoderm) when Wnt signaling was inhibited. Box and whisker plot produced with (REST software, Pfaffl et al., 2002). The top and bottom “whiskers” indicate the range while the box indicates the upper and lower quartile values. Gene expression was calculated relative to β-actin and gene expression in the control ESC line was set to 1. Asterisks indicate statistically significant differences (p≤0.05) calculated by the REST software (n=3).
Wnt signaling is required for mesendodermal differentiation in a model of gastrulation
To investigate the role of Wnt signaling in a more complex three-dimensional multi-lineage differentiation assay, we employed an embryoid body (EB) differentiation paradigm. Cells were plated in non-adherent dishes (in the absence of LIF, with and without doxycycline but with serum) promoting the formation of EBs and thereby inducing trilineage differentiation that has been widely employed as a model of lineage differentiation at gastrulation. Cells were grown as spheres for four days then plated into adherent tissue culture dishes for an additional two days before fixation for immunhistochemistry (IHC) or harvesting of RNA.
Axin2, a well-characterized canonical Wnt target gene (Hughes and Brady 2006; Jho et al., 2002; Leung et al., 2002; Yan et al., 2001), was down-regulated 5-fold after 6 days in culture (Figure 5B), confirming a functional Wnt signaling blockade. The block in canonical Wnt signaling caused a delay or stalling of differentiation with increased expression of the ESC and epiblast markers Fgf5 (6-fold) and Oct3/4 (6-fold; Figure 5 A,B). Decreased expression of the endoderm markers Foxa2 (Figure 5A) and Sox-17 (4.5-fold; Figure 5B), and a trend toward decreased expression of the mesoderm marker T (2-fold) also indicated a reduction in mesendodermal differentiation, while primitive neural ectoderm differentiation increased as identified by Sox3 expression (6-fold; Figure 5A,B), but Tuj1 did not change. Addition of doxycycline to control and dn4 cells during EB culture eliminated changes in differentiation seen when Wnt signaling was blocked (Supplemental Figure 1). As observed in dispersed cultures, the dnTcf4 expressing cells were unable to progress to βΙΙΙ-tubulin positive neurons in the continued Wnt signaling blockade. When doxycycline was reintroduced midway through differentiation, there was no increase in the number of βΙΙΙ-tubulin positive neurons, likely because of the crowded conditions within each EB (data not shown). These data indicate that in dense culture conditions, blocking Wnt signaling delays differentiation and promotes differentiation of neural precursors at the expense of mesendoderm.
β-catenin knock-down affects survival, gastrulation, and neuronal differentiation of post-implantation embryos
To examine the role of Wnt signaling in lineage differentiation in vivo, we delivered shRNAs targeting β-catenin via the tail vein of pregnant mice on E5.5 of gestation; n=26 litters exposed to scrambled control plasmid, and 27 litters to β-catenin shRNA. Knock-down avoids the peri-implantation lethality of conventional knock-out approaches and results in graded knock-down of gene expression allowing the correlation of phenotype and level of gene expression. Reduced β-catenin expression was often embryo lethal, increasing the number of resorptions observed from 5.6% in control litters to 25.9% in β-catenin shRNA embryos on E7.5. Since Wnt signaling is required first to position the primitive streak then for EMT at gastrulation, we examined the displacement of primitive endoderm (PE) by newly formed (embryonic) definitive endoderm (DE) in individual embryos and using scanning electron microscopy (SEM). On E7.5, gastrulation was fully underway in embryos exposed to the scrambled hairpin (controls) with the boundary marking PE and DE moving 75% of the way to its final position at the embryonic—extra-embryonic boundary (Figure 7A.A). Control embryos had typically expanded in the proximal-distal, but not anterior-posterior axis at this stage of development. In embryos exposed to β-catenin shRNA, gastrulation was slowed, DE typically progressing only 1/4 to 1/3 of the way along the proximal-distal axis (Figure 7A.B). Occasionally, newly formed mesendodermal cells piled up at the node in β-catenin targeted embryos, remaining as an ectopic cluster, rather than migrating anteriorly to replace PE and form new endoderm. We also measured the distance the DE had migrated in individual embryos. In control embryos (n=27) the distance the DE had migrated was 1.75 ± 0.4 vs 0.45 ± 0.1 in embryos exposed to the shRNAs (n=51 embryos), p ≤ 0.003, Student's t-test.
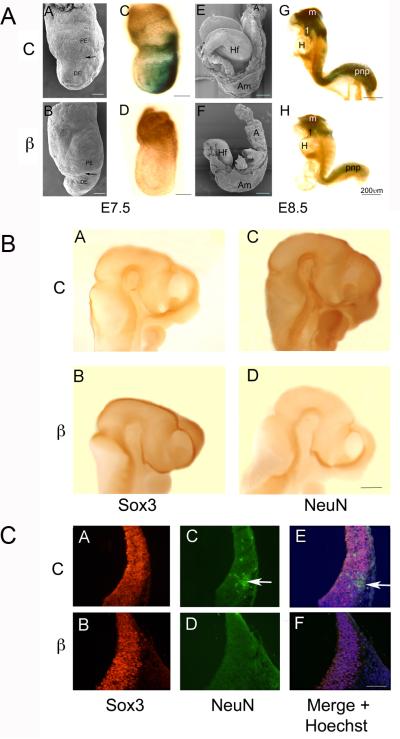
Figure 7. Wnt signaling is required in the embryo for the conversion of Sox3 neural precursors to NeuN positive neurons.
A. Compared with control (C) embryos (A,C,E,G), embryos (β) exposed to shRNA targeting β-catenin (B,D,F,H) exhibit defects in gastrulation, axis elongation, and neural differentiation. Side views of E7.5 and 8.0-8.5 embryos examined using SEM (AB, EF) or stained with Xgal to identify sites of β-galactosidase expression and Wnt signaling (CD, GH). The arrows in A and B indicate the boundary between primitive endoderm (PE) and definitive endoderm (DE) that largely replaces the PE at gastrulation. The proximal-distal displacement of the boundary is an indication of the progress of gastrulation movements. The control embryo (A) has expanded in both the proximal-distal (P-D) and anterior-posterior (A-P) axes compared to the β-catenin shRNA exposed embryo (B). In control embryos (C) Wnt signaling is strong in the posterior primitive streak (PS) compared with shRNA exposed embryos (D) where signaling is nearly abrogated. By day 8 in control embryos (E) the neural folds are elevating and later embryos (G) are beginning the process of adopting the fetal C shape. β-galactosidase is expressed at high levels in the midbrain (m), first branchial arch (1), and posterior neuropore (pnp) in control embryos (G), but is strikingly down-regulated in β-catenin shRNA treated embryos (H). Anterior is to the left in each figure, Am=amnion, A=Allantois, Hf=headfold, H=heart. SBs = 200μm
B. Side views of control (A, C) and embryos exposed to shRNA against β-catenin (B, D). Anterior is to the right in A-D. Whole mount IHC was carried out to compare Sox3 positive neural precursors (A, B) and NeuN positive neurons (C, D). By E10.5 in control embryos there were more NeuN positive neurons (C) and fewer Sox3 positive precursors (A) compared with β-catenin shRNA exposed embryos (B, D). Embryos exposed to β-catenin shRNA also exhibited increased midbrain mesenchyme and abnormal positioning of midbrain flexure.
C. When similar embryos were sectioned, there was widespread expression of Sox3 in both control and β-catenin shRNA exposed embryos (A, B) while NeuN was observed in differentiating neurons in control embryos (C, arrow) but not in embryos exposed to β-catenin shRNA (D). E and F are overlays of AC+BD with Hoechst staining of nuclei. SB=200μ
When we examined β-galactosidase expression in control “Wnt indicator” mice, the X-gal reaction product clearly marked the primitive streak at the posterior pole of the embryo (Figure 7A.C). In β-catenin shRNA targeted embryos at this stage, there was a range in β—gal expression from slight to undetectable levels (Figure 7A.D), confirming the range of knock-down, and presumably inhibition of Wnt signaling.
By E8.0 of development, control embryos had well-developed headfolds, were expanded in the proximal-distal plane; AP axis expansion was underway. The neural folds were elevating and invagination of the optic vesicles was just beginning in the cephalic region (Figure 7A.E). Gene targeted embryos were characterized by extremely shortened PD axes, often with an expanded AP axis and shortened posterior region giving them a “rocking horse” appearance (Figure 7A.F). The anterior neural folds were slightly elevated, but were characteristically flattened in the midbrain region; the border between neural ectoderm and epidermal ectoderm was wavy rather than smooth. The allantois appeared elongated, the first branchial arch reduced and abnormally displaced posteriorly (ventrally). Neural tube closure defects were common in the midbrain, where there was a characteristic eversion of neural tissue. However, in extremely shortened embryos, the entire neural tube had often failed to close.
Wnt signaling activity increased significantly by E8.5, with strong β-gal expression throughout the cranial neural ectoderm, especially high in midbrain and hindbrain regions in control embryos. X-gal staining was also high in the posterior neuropore and regressing primitive streak, as well as in the closed neural tube. Signaling was also strong in the first branchial arch, in neural crest forming the extra-ocular muscles, surrounding the otic vesicles and in the cardiac neural crest (Figure 7A.G). In targeted embryos, there was a consistent reduction in the expression of β-gal—particularly in the first branchial arch, midbrain and heart (Figure 7A.H).
To quantify Wnt signaling activity in control versus β-catenin shRNA embryos, we used the O-nitrophenyl-beta-D- galactopyranoside (ONPG) β-galactosidase assay in Wnt indicator mice. In control embryos (6 litters, 40 embryos) β-galactosidase activity was significantly higher 0.29 ± 0.22 versus 0.13 ± 0.04 (p ≤ 0.03, Student's t test) than in β-Catenin shRNA treated embryos (8 litters, 59 embryos) demonstrating significant inhibition of Wnt signaling.
Immunohistochemisty and FACS analysis
Since Wnt signaling is required for neuronal differentiation of proliferating precursors, we examined the conversion of neural precursor cells to neurons using immunohistochemistry and FACS analysis in individual control versus β-catenin shRNA exposed embryos. There was a slight increase in Sox3 positive cells in whole mount as well as in sections through the neural tube (Figure 7B,C) in β-catenin shRNA exposed embryos compared with control embryos exposed to a mutated shRNA. Immunohistochemical localization of NeuN demonstrated a clear decrease in differentiation of immature neurons that were beginning to stratify within the neural ectoderm in conditions of reduced Wnt signaling (Figure 7C). To quantify these changes, we dissected the CNS from E10.5 embryos, dissociated each to a single cell suspension, divided each embryo into two equal samples, and then carried out IHC for Sox3 or NeuN. Cell number from the central nervous system of individual embryos was quantified in FACS analysis; 40 scrambled and 43 β-catenin shRNA exposed embryos from 12 litters. There was a significant increase in Sox3 positive cells in embryos exposed to the β-catenin shRNA compared to control embryos (20.7 ± 6.3 versus 26.5 ± 7.2, p≤ 0.004) and a corresponding decrease in NeuN positive cells (7.8 ± 1.6 versus 6.2 ± 1.3, p≤ 0.004), confirming that in vivo as in vitro, Wnt signaling is required at multiple stages of neuronal differentiation, in this case in the conversion of neural precursors to primitive neurons.
Discussion
To probe the role of Wnt pathway activation in lineage differentiation, we derived a tetracycline regulated dnTcf4 expressing ES cell line in which Wnt signaling could be tightly controlled. There are four Tcf/Lef factors in the mouse genome that act as the downstream transcriptional effectors of the canonical Wnt signaling pathway. All four transcription factors bind highly similar promoter sequences (Arce et al., 2006; Kelly et al., 2011) and can act as repressors in the absence of Wnt ligand activity (by binding Groucho/TLE family of co-repressor proteins) but can also switch to transcriptional activation when β-catenin enters the nucleus and displaces Groucho/TLE proteins (Brantjes et al., 2001; Daniels and Weiss, 2005). However, in ESC Tcf3 retains its repressor function even in the presence of nuclear β-catenin (Pereira et al., 2006) and acts to regulate levels of genes involved in pluripotency. Rather than using extracellular inhibitors to abrogate Wnt signaling, we chose to employ a dnTcf4 protein, which lacks the β-catenin binding domain, to block all transcription downstream of the Wnt pathway.
Mouse ESC have been widely employed to model both neural induction and tri-lineage differentiation at gastrulation. Simply removing cells from a source of BMPs or adding BMP inhibitors to the culture medium of ESC that promotes neuronal differentiation has been cited as support for the default model (Gaulden and Reiter, 2008). However, it is clear that additional variables including cell density and other unidentified factors in supplements and serum also play a role in controlling differentiation. Further, since FGFs and Nodal are required to maintain BMP4 expression (Ben-Haim et al., 2006) it is likely that as in the intact embryo, the situation is more complex. Inhibition of Wnt signaling in ESC has previously been reported to be sufficient to promote neuronal differentiation (Aubert et al., 2002; Cajánek et al., 2009; Haegele et al., 2003; Nordin et al., 2008; Verani et al., 2007), despite observations that constitutive over-expression of a dnTcf4 and dnLef1 abrogated neuronal differentiation in vitro as well as in teratomas (Kelly et al., 2011). However, monolayer culture of the dnTCF4 cells in neural permissive media absent doxycycline (to induce transgene expression) promoted differentiation of Sox3 positive neural precursors, but these cells were unable to differentiate further into Tuj1 positive neurons, unless Wnt signaing was restored.
In the developing nervous system, Wnt signaling is required for both proliferation of neural precursors (Chesnutt et al., 2004; Israsena et al., 2004; Kalani et al., 2008; Shimizu et al., 2008; Zechner et al., 2003) and later for neuronal differentiation (Hirabayashi et al., 2004: Muroyama et al., 2004), where its source is likely astrocytes. Subsequent patterning of the nervous system requires Wnt signaling (Ciani and Salinas, 2005; Nordstrom et al., 2002), and the key bHLH transcription factors that drive neuronal specification, including Neurogenin1 and NeuroD1, are transcriptional targets of the canonical Wnt pathway (Hirabayashi et al., 2004; Israsena et al., 2004; Kuwabara et al., 2009). Therefore, while Wnt signal inhibition may initially be required to increase neuronal precursor differentiation by inhibiting mesendodermal lineage differentiation; active signaling appears to be required for precursor proliferation and neuronal differentiation. When dnTcf4 expression was abrogated midway through the culture period there was a dramatic conversion of Sox3 positive precursors to immature neurons. Previous conclusions that a simple block in Wnt signaling was sufficient to promote neuronal differentiation (Aubert et al., 2002; Cajánek et al., 2009; Kong and Zhang 2009; Verani et al., 2006) likely resulted from a reduction (rather than a complete block) in signaling, since a single Wnt gene or co-receptor (Wnt 1 or LRP6) was deleted. Similarly, over-expression of extracellular inhibitors (DKK1 or SFRP2) likely did not reach a sufficiently high concentration to eliminate Wnt signaling. However, the dnTcf4 employed in the current investigation should inhibit all canonical Wnt signaling at the promoter of target genes preventing all downstream signaling.
To examine the role of Wnt signaling in tri-lineage differentiation at gastrulation, we employed an embryoid body differentiation paradigm. When ESC are grown in the absence of LIF in suspension culture or in hanging drops of medium without substrate contact, they form cell aggregates termed embryoid bodies (EBs) due to the loose organization of cells into stratified aggregates with some resemblance to the early post-implantation mouse embryo (Desbaillets et al., 2000). However, the EB is significantly different from the embryo since there are no organized axes, primitive streak, or signaling centers. Lacking organization, differentiation within the EB has been characterized as “chaotic” or random. Despite the disorganization and lack of organized signaling centers, EB differentiation has been widely employed to model early lineage differentiation; particularly, as a model of gastrulation and the role of Wnt signaling in this process (ten Berge et al., 2008; Nakanishi et al., 2008; Gaude et al., 2006). Using this model, induction of the dnTcf4 protein resulted in sustained expression of the pluripotency gene Oct3/4 and the epiblast marker Fgf5, likely identifying cells that retain an epiblast phenotype and are unable to further differentiate due to a requirement for Wnt signaling in formation of the primitive streak and mesendoderm differentiation. Consistent with this result, expression of markers of endoderm (Sox17 and Foxa2) and mesoderm (Brachyury) were significantly reduced. Only one lineage, primitive neural ectoderm marked by Sox3 expression, increased in the absence of Wnt signaling. Surprisingly, in this EB model these cells failed to differentiate into Tuj1 positive neurons when Wnt signaling was de-repressed. Since neuronal differentiation is exquisitely sensitive to cell density (Otero et al., 2004), the high-density microenvironment of the EB likely inhibited neuronal differentiation possibly via Notch signaling.
Our results are consistent with previous reports demonstrating that canonical Wnt signaling is crucial for formation of the primitive streak, gastrulation, and mesendoderm differentiation during embryogenesis. Deletion of core components of the Wnt pathway such as β-catenin (Haegel et al., 1995; Huelsken et al., 2000), Wnt3a (Yoshiakai et al., 1997), Wnt3 (Liu et al., 1999), frizzled co-receptors Lrp5 and Lrp6 (Kelly et al., 2004), or Lef1 and Tcf1 (Galceran et al., 1999) produced embryos lacking the primitive streak or paraxial mesoderm, with an expansion of neural ectoderm in surviving embryos. Conversely, mouse embryos in which canonical Wnt signaling was activated via deletion of inhibitory proteins including: Axin2 (Zeng et al., 1997), Tcf3 (Merill et al., 2003) or APC (Ishikawa et al., 2003), or transgenic mis-expression of Wnt8c (Popperl et al., 1997), formed multiple axes (multiple primitive streaks), and were characterized by increased differentiation of mesendoderm at the expense of neural ectoderm. It is clear both in ESC and the early embryo that Wnt signaling is required for mesendodermal differentiation and in its absence more of the embryonic epiblast can be converted to neural ectoderm.
To examine the role of Wnt signaling neural differentiation in the intact embryo, shRNA plasmids directed against β-catenin were delivered to pregnant dams, thereby eliminating Wnt signaling at the nuclear level in embryos during early post-implantation development. Using knock-down in lieu of knock-out, many embryos were able to escape the lethality associated with early β-catenin loss. In vivo, β-catenin shRNA phenocopied previous results, with Wnt signaling knock-down embryos exhibiting gastrulation abnormalities and resulting axis elongation defects. When embryos survived gastrulation lethality, it was possible to examine the requirement for active Wnt signaling in neuronal differentiation. Consistent with our observations in ESC, when Wnt signaling was down-regulated, neural ectoderm precursors failed to differentiate to NeuN positive neurons. It is appears that β-catenin function is required both in the embryo and in ESC to maintain epithelial characteristics of the cells (e.g., Li et al., 2010).
As in the CNS, lineage differentiation of most cells and tissues likely involves multiple rounds of Wnt signaling, signal inhibition, and resumption of signaling rather than acting as an on-off switch. The inducible dnTcf4 cell line should be extremely useful in uncovering previously unidentified sequential requirements for Wnt signaling in many additional tissues that were unappreciated in transgenic experiments.
Supplementary Material
Supplemental Figure 1.
To control for possible effects of doxycycline exposure, control (A-E) and dnTCF4 (dn4; F-J) cells were grown in doxycycline during the entire 6 day culture period (4 days as EB, followed by an additional 2 days in adherent culture). In these conditions, there was little difference in gene expression between control cells and dn4 cells. These data are strikingly similar to the results of culture of control cells without doxycycline (Figure 5A), suggesting that doxycycline exposure itself did not affect lineage differentiation.
Acknowledgements
The authors gratefully acknowledge Mike Klymkowsky (University of Colorado) for the Sox3 antibody, Daniel Dufort and Phil Gage (University of Michigan) for Wnt indicator mice, Eric Feron (University of Michigan) for the dnTcf4 cDNA, the University of Michigan Flow Cytometry Core, and Maria Morell and Yao Chang Tsan for many helpful discussions. This work was supported by NIH grants NS-04187 and RR- 023187.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25:7492–504. doi: 10.1038/sj.onc.1210056. [DOI] [PubMed] [Google Scholar]
- Aubert J, Dunstan H, Chambers I, Smith AG. Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat. Biotechnol. 2002;20:1240–5. doi: 10.1038/nbt763. [DOI] [PubMed] [Google Scholar]
- Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell. 2006;11:313–23. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Blauwkamp TA, Chang MV, Cadigan KM. Novel TCF-binding sites specify transcriptional repression by Wnt signalling. EMBO J. 2008;27:1436–46. doi: 10.1038/emboj.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone HK, Damiano T, Bartlett S, Perry A, Letchford J, Ripoll YS, Nelson AS, Welham MJ. Involvement of GSK-3 in regulation of murine embryonic stem cell self-renewal revealed by a series of bisindolylmaleimides. Chem Biol. 2009;16:15–27. doi: 10.1016/j.chembiol.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Brantjes H, Roose J, van De Wetering M, Clevers H. All Tcf HMG box transcription factors interact with Groucho-related co-repressors. Nucleic Acids Res. 2001;29:1410–9. doi: 10.1093/nar/29.7.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajanek L, Ribeiro D, Liste I, Parish CL, Bryja V, Arenas E. Wnt/beta-catenin signaling blockade promotes neuronal induction and dopaminergic differentiation in embryonic stem cells. Stem Cells. 2009;27:2917–27. doi: 10.1002/stem.210. [DOI] [PubMed] [Google Scholar]
- Chesnutt C, Burrus LW, Brown AM, Niswander L. Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev Biol. 2004;274:334–47. doi: 10.1016/j.ydbio.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129:1614–27. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6:351–62. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008;22:746–55. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DL, Weis WI. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol. 2005;12:364–71. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]
- Desbaillets I, Ziegler U, Groscurth P, Gassmann M. Embryoid bodies: an in vitro model of mouse embryogenesis. Exp Physiol. 2000;85:645–51. [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions. Cells Tissues Organs. 2007;185:73–84. doi: 10.1159/000101306. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Finlay D, Patel S, Dickson LM, Shpiro N, Marquez R, Rhodes CJ, Sutherland C. Glycogen synthase kinase-3 regulates IGFBP-1 gene transcription through the thymine-rich insulin response element. BMC Mol Biol. 2004;5:15. doi: 10.1186/1471-2199-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:16806–11. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a-/--like phenotype and limb deficiency in Lef1(-/-)Tcf1(-/-) mice. Genes Dev. 1999;13:709–17. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–2. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulden J, Reiter JF. Neurons and neur-offs: regulators of neural induction in vertebrate embryos and embryonic stem cells. Hum Mol Genet. 2008;17:R60–6. doi: 10.1093/hmg/ddn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratsch TE, DeBoer LS, O'Shea KS. inhibition of BMP-4 gene expression in postimplantation mouse embryos. RNA. genesis. 2003;37:12–7. doi: 10.1002/gene.10221. [DOI] [PubMed] [Google Scholar]
- Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–37. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- Hao J, Li TG, Qi X, Zhao DF, Zhao GQ. WNT/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- Horn Z, Papachristou P, Shariatmadari M, Peyronnet J, Eriksson B, Ringstedt T. Wnt7a overexpression delays beta-tubulin III expression in transgenic mouse embryos. Brain Res. 2007;1130:67–72. doi: 10.1016/j.brainres.2006.10.090. [DOI] [PubMed] [Google Scholar]
- Houart C, Caneparo L, Heisenberg C, Barth K, Take-Uchi M, Wilson S. Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron. 2002;35:255–65. doi: 10.1016/s0896-6273(02)00751-1. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–78. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TA, Brady HJ. Regulation of axin2 expression at the levels of transcription, translation and protein stability in lung and colon cancer. Cancer Lett. 2006;233:338–47. doi: 10.1016/j.canlet.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Ishikawa TO, Tamai Y, Li Q, Oshima M, Taketo MM. Requirement for tumor suppressor Apc in the morphogenesis of anterior and ventral mouse embryo. Dev Biol. 2003;253:230–46. doi: 10.1016/s0012-1606(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Israsena N, Hu M, Fu W, Kan L, Kessler JA. The presence of FGF2 signaling determines whether beta-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev Biol. 2004;268:220–31. doi: 10.1016/j.ydbio.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani MY, Cheshier SH, Cord BJ, Bababeygy SR, Vogel H, Weissman IL, Palmer TD, Nusse R. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci U S A. 2008;105:16970–5. doi: 10.1073/pnas.0808616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KF, Ng DY, Jayakumaran G, Wood GA, Koide H, Doble BW. beta-Catenin Enhances Oct-4 Activity and Reinforces Pluripotency through a TCF-Independent Mechanism. Cell Stem Cell. 2011;8:214–27. doi: 10.1016/j.stem.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly OG, Pinson KI, Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–15. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- Kemler R, Hierholzer A, Kanzler B, Kuppig S, Hansen K, Taketo MM, de Vries WN, Knowles BB, Solter D. Stabilization of beta-catenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development. 2004;131:5817–24. doi: 10.1242/dev.01458. [DOI] [PubMed] [Google Scholar]
- Kolligs FT, Hu G, Dang CV, Fearon ER. Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol Cell Biol. 1999;19:5696–706. doi: 10.1128/mcb.19.8.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong XB, Zhang C. Dickkopf (Dkk) 1 promotes the differentiation of mouse embryonic stem cells toward neuroectoderm. In Vitro Cell Dev Biol Anim. 2009;45:185–93. doi: 10.1007/s11626-008-9157-2. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagutin OV, Zhu CC, Kobayashi D, Topczewski J, Shimamura K, Puelles L, Russell HR, McKinnon PJ, Solnica-Krezel L, Oliver G. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 2003;17:368–79. doi: 10.1101/gad.1059403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Lim HW, Lee SH, Han HJ. Smad, PI3K/Akt, and Wnt-dependent signaling pathways are involved in BMP-4-induced ESC self-renewal. Stem Cells. 2009;27:1858–68. doi: 10.1002/stem.124. [DOI] [PubMed] [Google Scholar]
- Leimeister C, Bach A, Gessler M. Developmental expression patterns of mouse sFRP genes encoding members of the secreted frizzled related protein family. Mech Dev. 1998;75:29–42. doi: 10.1016/s0925-4773(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657–65. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- Li R, Liang J, Ni S, Zhou T, Qing X, Li H, He W, Chen J, Li F, Zhuang Q, Qin B, Xu J, Li W, Yang J, Gan Y, Qin D, Feng S, Song H, Yang D, Zhang B, Zeng L, Lai L, Miguel Esteban MA, Pei D. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–5. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–96. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- Linker C, Stern CD. Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development. 2004;131:5671–81. doi: 10.1242/dev.01445. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–5. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui S, Shimosato D, Toyooka Y, Yagi R, Takahashi K, Niwa H. An efficient system to establish multiple embryonic stem cell lines carrying an inducible expression unit. Nucleic Acids Res. 2005;33:e43. doi: 10.1093/nar/gni043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill BJ, Pasolli HA, Polak L, Rendl M, Garcia-Garcia MJ, Anderson KV, Fuchs E. Tcf3: a transcriptional regulator of axis induction in the early embryo. Development. 2004;131:263–74. doi: 10.1242/dev.00935. [DOI] [PubMed] [Google Scholar]
- Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M. Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 2007;104:5668–73. doi: 10.1073/pnas.0701331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed OA, Clarke HJ, Dufort D. Beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev Dyn. 2004;231:416–24. doi: 10.1002/dvdy.20135. [DOI] [PubMed] [Google Scholar]
- Muroyama Y, Fujihara M, Ikeya M, Kondoh H, Takada S. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 2002;16:548–53. doi: 10.1101/gad.937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi M, Kurisaki A, Hayashi Y, Warashina M, Ishiura S, Kusuda-Furue M, Asashima M. Directed induction of anterior and posterior primitive streak by Wnt from embryonic stem cells cultured in a chemically defined serum-free medium. FASEB J. 2009;23:114–22. doi: 10.1096/fj.08-111203. [DOI] [PubMed] [Google Scholar]
- Nordin N, Li M, Mason JO. Expression profiles of Wnt genes during neural differentiation of mouse embryonic stem cells. Cloning Stem Cells. 2008;10:37–48. doi: 10.1089/clo.2007.0060. [DOI] [PubMed] [Google Scholar]
- Nordstrom U, Jessell TM, Edlund T. Progressive induction of caudal neural character by graded Wnt signaling. Nat Neurosci. 2002;5:525–32. doi: 10.1038/nn0602-854. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun. 2006;343:159–66. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- Otero JJ, Fu W, Kan L, Cuadra AE, Kessler JA. Beta-catenin signaling is required for neural differentiation of embryonic stem cells. Development. 2004;131:3545–57. doi: 10.1242/dev.01218. [DOI] [PubMed] [Google Scholar]
- Paling NR, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem. 2004;279:48063–70. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- Pereira L, Yi F, Merrill BJ. Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Mol Cell Biol. 2006;26:7479–91. doi: 10.1128/MCB.00368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popperl H, Schmidt C, Wilson V, Hume CR, Dodd J, Krumlauf R, Beddington RS. Misexpression of Cwnt8C in the mouse induces an ectopic embryonic axis and causes a truncation of the anterior neuroectoderm. Development. 1997;124:2997–3005. doi: 10.1242/dev.124.15.2997. [DOI] [PubMed] [Google Scholar]
- Qi X, Li TG, Hao J, Hu J, Wang J, Simmons H, Miura S, Mishina Y, Zhao GQ. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A. 2004;101:6027–32. doi: 10.1073/pnas.0401367101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Kagawa T, Inoue T, Nonaka A, Takada S, Aburatani H, Taga T. Stabilized beta-catenin functions through TCF/LEF proteins and the Notch/RBP-Jkappa complex to promote proliferation and suppress differentiation of neural precursor cells. Mol Cell Biol. 2008;28:7427–41. doi: 10.1128/MCB.01962-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla DK, Schneider DJ, LeWinter MM, Sobel BE. wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem Biophys Res Commun. 2006;345:789–95. doi: 10.1016/j.bbrc.2006.04.125. [DOI] [PubMed] [Google Scholar]
- Sinner D, Rankin S, Lee M, Zorn AM. Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development. 2004;131:3069–80. doi: 10.1242/dev.01176. [DOI] [PubMed] [Google Scholar]
- Smith SK, Charnock-Jones DS, Sharkey AM. The role of leukemia inhibitory factor and interleukin-6 in human reproduction. Hum Reprod. 13 Suppl. 1998;3:237–43. doi: 10.1093/humrep/13.suppl_3.237. discussion 244-6. [DOI] [PubMed] [Google Scholar]
- Takao Y, Yokota T, Koide H. Beta-catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochem Biophys Res Commun. 2007;353:699–705. doi: 10.1016/j.bbrc.2006.12.072. [DOI] [PubMed] [Google Scholar]
- Tam WL, Lim CY, Han J, Zhang J, Ang YS, Ng HH, Yang H, Lim B. T-cell factor 3 regulates embryonic stem cell pluripotency and self-renewal by the transcriptional control of multiple lineage pathways. Stem Cells. 2008;26:2019–31. doi: 10.1634/stemcells.2007-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Berge D, Koole W, Fuerer C, Fish M, Eroglu E, Nusse R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–18. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques. 2000;29:52–54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–14. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen P. Wnts blow on NeuroD1 to promote adult neuron production and diversity. Nat Neurosci. 2009;12:1079–81. doi: 10.1038/nn0909-1079. [DOI] [PubMed] [Google Scholar]
- Verani R, Cappuccio I, Spinsanti P, Gradini R, Caruso A, Magnotti MC, Motolese M, Nicoletti F, Melchiorri D. Expression of the Wnt inhibitor Dickkopf-1 is required for the induction of neural markers in mouse embryonic stem cells differentiating in response to retinoic acid. J Neurochem. 2007;100:242–50. doi: 10.1111/j.1471-4159.2006.04207.x. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006;25:2697–707. doi: 10.1038/sj.onc.1209307. [DOI] [PubMed] [Google Scholar]
- Wexler EM, Paucer A, Kornblum HI, Palmer TD, Geschwind DH. Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells. 2009;27:1130–41. doi: 10.1002/stem.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–7. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999;13:3185–90. doi: 10.1101/gad.13.24.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, Sakamoto D, Caothien RH, Fuller JH, Reinhard C, Garcia PD, Randazzo FM, Escobedo J, Fantl WJ, Williams LT. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc Natl Acad Sci U S A. 2001;98:14973–8. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Pereira L, Merrill BJ. Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells. 2008;26:1951–60. doi: 10.1634/stemcells.2008-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–6. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Fujimori T, McMahon AP, Takada S. Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol. 1997;183:234–42. doi: 10.1006/dbio.1997.8502. [DOI] [PubMed] [Google Scholar]
- Yu HM, Liu B, Costantini F, Hsu W. Impaired neural development caused by inducible expression of Axin in transgenic mice. Mech Dev. 2007;124:146–56. doi: 10.1016/j.mod.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, 3rd, Birchmeier W, Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–18. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Zechner D, Muller T, Wende H, Walther I, Taketo MM, Crenshaw EB, 3rd, Treier M, Birchmeier W, Birchmeier C. Bmp and Wnt/beta-catenin signals control expression of the transcription factor Olig3 and the specification of spinal cord neurons. Dev Biol. 2007;303:181–90. doi: 10.1016/j.ydbio.2006.10.045. [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–92. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Zhang K, Li L, Huang C, Shen C, Tan F, Xia C, Liu P, Rossant J, Jing N. Distinct functions of BMP4 during different stages of mouse ES cell neural commitment. Develop. 2010;137:2095–2105. doi: 10.1242/dev.049494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1.
To control for possible effects of doxycycline exposure, control (A-E) and dnTCF4 (dn4; F-J) cells were grown in doxycycline during the entire 6 day culture period (4 days as EB, followed by an additional 2 days in adherent culture). In these conditions, there was little difference in gene expression between control cells and dn4 cells. These data are strikingly similar to the results of culture of control cells without doxycycline (Figure 5A), suggesting that doxycycline exposure itself did not affect lineage differentiation.