Abstract
Objective
We aimed to systematically review available literature linking adipokines to gestational diabetes mellitus (GDM) for a comprehensive understanding of the roles of adipokines in the development of GDM.
Methods
We searched PubMed/MEDLINE and EMBASE databases for published studies on adipokines and GDM through October 21, 2014. We included articles if they had a prospective study design (i.e., blood samples for adipokines measurement were collected before GDM diagnosis). Random-effects models were used to pool the weighted mean differences comparing levels of adipokines between GDM cases and non-GDM controls.
Results
Of 1,523 potentially relevant articles, we included 25 prospective studies relating adipokines to incident GDM. Our meta-analysis of nine prospective studies on adiponectin and eight prospective studies on leptin indicated that adiponectin levels in the first or early second trimester of pregnancy were 2.25 μg/ml lower (95% CI: 1.75–2.75), whereas leptin levels were 7.25 ng/ml higher (95% CI 3.27–11.22), among women who later developed GDM than women who did not. Prospective data were sparse and findings were inconsistent for visfatin, retinol binding protein (RBP-4), resistin, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and vaspin. We did not identify prospective studies for several novel adipokines, including chemerin, apelin, omentin, or adipocyte fatty acid-binding protein. Moreover, no published prospective studies with longitudinal assessment of adipokines and incident GDM were identified.
Conclusion
Adiponectin levels in the first or second trimester of pregnancy are lower among pregnant women who later develop GDM than non-GDM women, whereas leptin levels are higher. Well-designed prospective studies with longitudinal assessment of adipokines during pregnancy are needed to understand the trajectories and dynamic associations of adipokines with GDM risk.
Keywords: gestational diabetes mellitus, adipokines, adiponectin, leptin
1. INTRODUCTION
Gestational diabetes mellitus (GDM), a common pregnancy complication, is defined as glucose intolerance with onset or first recognition during pregnancy [1]. Approximately 7% (ranging from 1% to 14%) of all pregnancies in the United States are complicated by GDM, resulting in more than 200,000 cases annually [1]. Women with GDM have an increased risk for prenatal morbidity and a considerably elevated risk for type 2 diabetes mellitus (T2DM) after pregnancy [1]. Furthermore, the offspring of women with GDM are more likely to be obese and have impaired glucose tolerance and T2DM in their early adulthood [2].
Adiposity is an important modifiable risk factor for the development of GDM [3], although mechanisms linking excess adiposity to elevated risk of GDM are not completely understood. Adipose tissue is not only involved in energy storage but also functions as an active endocrine organ [4]. Recent evidence points to a crucial role of specific hormones and cytokines (i.e., adipokines) secreted by the adipose tissue. A major breakthrough in understanding the link between adiposity and glucose intolerance has come from the demonstration of crosstalk between adipose tissue and other insulin target tissues such as skeletal muscles and the liver [5]. Such crosstalk is mediated by a number of molecules that are secreted by adipocytes [4]. Among those identified to date are adiponectin, leptin, resistin, retinol binding protein 4 (RBP4), and tumor necrosis factor-α (TNF-α), etc. In concert, these adipokines are believed to adapt metabolic fluxes to the amount of stored energy. Dysregulation of this network is a critical factor in the deterioration of insulin sensitivity [4, 6].
Despite the promising role of these adipokines in glucose homeostasis, their roles in the development of GDM remain to be elucidated. Although there have been a number of human studies on adipokines and GDM during the past decades, inferences have been hindered due to significant heterogeneities in these studies concerning design, population characteristics, assay methods, timing of blood sample collection, and definition/diagnosis of GDM. Moreover, the majority of previous studies had a small sample size that may lead to false positive or negative findings. We aimed to systematically review the current literature and quantitatively synthesize prospective data regarding adipokines and GDM risk, and to identify important data gaps.
2. MATERIALS AND METHODS
When conducting the study, we adhered to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [7].
2.1 Literature search and study selection
We conducted a comprehensive electronic search of PubMed and EMBASE databases for literature on adipokines and GDM through October 21, 2014. To maximize the coverage in our literature search, we used a combination of free text (e.g., gestational diabetes) and subheadings from MeSH (e.g., “Diabetes, Gestational”[Mesh]) or EMTREE terms (e.g., ‘pregnancy diabetes mellitus’/exp). In addition to using the generic term for adipokines, we also specifically named each key adipokine (e.g., adiponectin, leptin, resistin, etc.) when conducting the literature search. Detailed search terms are listed in the Supplementary Materials. We restricted the literature search to English language. All reference lists from the main articles and relevant reviews were hand searched to identify additional studies.
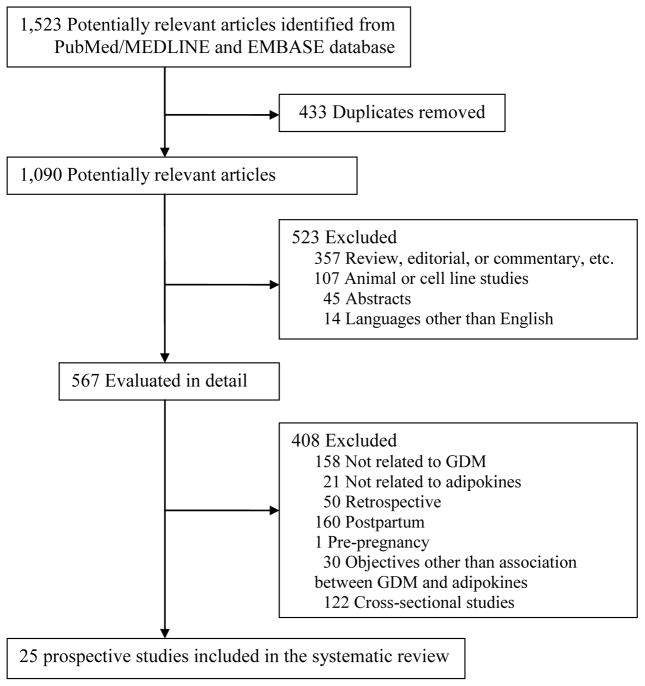
Figure 1 summarizes the process of literature search and study selection. Articles were eligible for inclusion if they had a prospective study design (i.e., blood samples for adipokines measurement were collected before the diagnosis of GDM, typically before 24 weeks of gestational age), and they compared adipokine levels measured in pregnant women who later developed GDM with women with normoglycemic pregnancies. We excluded the following types of articles: review articles or editorials, non-human studies (i.e., cell culture or animal studies), studies that did not include GDM as the primary concern, and studies that did not evaluate adipokine levels. We also excluded articles that combined GDM with impaired glucose tolerance or previous cases of type 1 diabetes or T2DM. Disagreement about eligibility was settled by consensus between all authors.
Figure 1.
Flow chart for literature search and study selection
2.2 Data extraction
The following data were extracted from each eligible article: the first author’s name, year of publication, sample size, number of GDM cases, ethnicity, study design, time for blood samples collected for adipokine measurement, method of adipokine measurement, time and criteria for GDM diagnosis, and mean and standard deviation (SD) of adipokine levels among GDM cases and the comparison group. When necessary, we contacted the corresponding authors of the original articles by email to request relevant data or information. We also extracted odds ratios, risk ratios, and 95% confidence intervals if they were available. If multiple articles were published using data from the same cohort, we extracted the report with the information most relevant to the analysis.
2.3 Data synthesis and statistical analysis
To quantitatively summarize the available data, we conducted meta-analyses for adipokines with more than five independent studies. We calculated weighted mean differences (WMDs) in adipokine levels for each of the included studies, and pooled them in the meta-analysis using random-effects model [8]. We also calculated standardized mean differences (SMDs) when different units across studies were used for a certain adipokine. When means and SDs were not reported in the full-text article, we approximated them using the median and interquartile range. When applicable, the standard error of the mean was transformed into SD. Forest plots and funnel plots were used for visualizing the overall effect size and evaluating publication bias, respectively. The probability of publication bias was also statistically assessed using Egger regression asymmetry test [9].
We assessed between-study heterogeneity using the χ2-based Cochran’s Q statistic and the I2 metric (I2 value of 25, 50, and 75% were considered as low, medium, and high heterogeneity, respectively) [10]. Potential sources of between-study heterogeneity were also investigated by a priori-defined stratification analyses. Specifically, we stratified the included studies by geographical location, sample size, time for determination of exposure (i.e., adipokines) and outcome (i.e., GDM), assay methods for adipokines, and diagnostic criteria for GDM. A formal meta-regression was also performed by the aforementioned factors, but the potential for robust conclusions from meta-regression analyses may be very limited [11], because the number of included studies was small for some adipokines. Sensitivity analyses were performed by omitting one study at a time and computing the pooled the effect size of the remaining studies to evaluate whether the results were affected markedly by a single study. All statistical analyses were performed using Stata software version 11.0 (Stata Corp, College Station, TX, USA).
3. RESULTS
3.1 Characteristics of the included studies
Our initial literature search identified 1,523 articles from PubMed/MEDLINE and EMBASE databases. After applying the inclusion and exclusion criteria, 25 prospective studies [12–36] on eight adipokines were ultimately included in the systematic review (Figure 1). These adipokines were adiponectin, leptin, visfatin, RBP-4, resistin, TNF-α, IL-6, and vaspin. Supplementary Table 1 summarizes the potential functions of these adipokines that may link them to the pathogenesis of GDM. In general, the number of prospective studies on GDM risk and these adipokines, except adiponectin and leptin, was sparse. We did not identify prospective studies relating several novel adipokines, such as chemerin, apelin, omentin, or adipocyte fatty acid-binding protein, to GDM risk.
Table 1 and Table 2 show characteristics of the 13 prospective studies about adiponectin and nine prospective studies about leptin, respectively. Overall, the reporting of the included studies was generally well described with sufficient details concerning key parameters such as the number of cases and controls, adipokine measurements (time for blood collection and assay method), and GDM diagnosis (time and criteria).
Table 1.
Characteristics of available prospective studies relating adiponectin levels to GDM risk
| Author, year | Location | Participants (Cases/Controls) | Adiponectin measurement | GDM diagnosis | ||
|---|---|---|---|---|---|---|
| Time for blood collection | Assay method | Time for diagnosis | Diagnostic criteria | |||
| Williams et al., 2004 [15] | USA | 111 (41/70) | 13 weeks | EIA | 24–28 weeks | ADA 2002 |
| Gao et al., 2008 [18] | China | 42 (22/20) | 14–20 weeks | EIA | 24–32 weeks | Yang, 2005; Li, 2006 |
| Georgiou et al., 2008 [19] | Australia | 28 (14/14) | 11 weeks | EIA | 28 weeks | ADIPS |
| Lain et al., 2008 [20] | USA | 59 (30/29) | <16 weeks | RIA | 24–28 weeks | Carpenter and Coustan, 1982 |
| Low et al., 2010 [24] | Malaysia | 79 (26/53) | <18 weeks | EIA | 24–28 weeks | WHO |
| Paradisi et al., 2010 [21] | Italy | 50 (12/38) | 8–11 weeks | EIA | 23–25 weeks | Carpenter and Coustan, 1982 |
| Savvidou et al., 2010 [22] | UK | 372 (124/248) | 11–19 weeks | EIA | 24–28 weeks | WHO |
| Ferreira et al., 2011 [23] | UK | 400 (100/300) | 11–13 weeks | EIA | 24–28 weeks | WHO |
| Ianniello et al., 2013 [33] | Italy | 32 (16/16) | 8–11 weeks | EIA | 24–28 weeks | Carpenter and Coustan, 1982 |
| LaCroix et al., 2013 [31] | Canada | 445 (38/407) | 6–13 weeks | RIA | 24–28 weeks | IADPSG |
| Rasanen et al., 2013 [34] | Finland | 182 (92/90) | 5–13 weeks | EIA | 2nd trimester | ADA 2008 |
| Guillemette et al., 2014 [35] | Canada | 749 (61/688) | 5–16 weeks | RIA | 24–28 weeks | IADPSG |
| Maitland et al., 2014 [36] | UK | 106 (29/77) | 16–18 weeks | EIA | 27–28 wks | IADPSG |
Abbreviations: ADA, American Diabetes Association; ADIPS, Australasian Diabetes in Pregnancy Society; EIA, Enzyme Immunoassay; IADPSG, International Association of Diabetes and Pregnancy Study Groups; N/A, not available or not reported; RIA, Radioimmunoassay; WHO, World Health Organization.
Table 2.
Characteristics of available prospective studies relating leptin levels to GDM risk
| Author, year | Location | Participants (Cases/Controls) | Leptin measurement | GDM diagnosis | ||
|---|---|---|---|---|---|---|
| Time for blood collection | Assay method | Time for diagnosis | Diagnostic criteria | |||
| Kirwan et al., 2002 [12] | USA | 15 (5/10) | 12–14 weeks | RIA | 3rd trimester | Carpenter and Coustan (1982) |
| Atawi et al., 2004 [13] | Saudi Arabia | 36 (8/28) | 1st trimester | RIA | N/A | N/A |
| Qiu et al., 2004 [14] | USA | 823 (47/776) | 13 weeks | EIA | 26–28 weeks | ADA (2003) |
| D’Anna et al., 2007 [16] | Italy | 75 (32/43) | 15–17 weeks | RIA | 24–28 weeks | Carpenter and Coustan (1982) |
| Gao et al., 2008 [18] | China | 42 (22/20) | 14–20 weeks | EIA | 24–32 weeks | Yang, 2005; Li, 2006 |
| Georgiou et al., 2008 [19] | Australia | 28 (14/14) | 11 weeks | EIA | 28 weeks | ADIPS |
| Mendieta-Zeron et al., 2012 [26] | Mexico | 35 (16/19) | 10–12 weeks | RIA | 24–28 weeks | NDDG (1979) |
| Guillemette et al., 2014 [35] | Canada | 749 (61/688) | 5–16 weeks | RIA | 24–28 weeks | IADPSG 2010 |
| Maitland et al., 2014 [36] | UK | 106 (29/77) | 16–18 weeks | EIA | 27–28 wks | IADPSG |
Abbreviations: ADA, American Diabetes Association; ADIPS, Australasian Diabetes in Pregnancy Society; EIA, Enzyme Immunoassay; N/A, not available or not reported; NDDG, National Diabetes Data Group; RIA, Radioimmunoassay.
Among the 13 included studies for adiponectin, six studies were conducted in Europe, four in North America, two in Asia and one in Australia. Five studies used the criteria proposed by Carpenter and Coustan or the American Diabetes Association (ADA) (these two criteria used the same procedure and cut points) for the diagnosis of GDM, three studies used the World Health Organization (WHO) criteria, three studies used the recently proposed criteria by the International Association of the Diabetes and Pregnancy Study Groups (IADPSG), and two used local criteria. GDM was usually diagnosed during the 24–28 weeks of gestation in these studies, with one exception. Blood samples for adiponectin measurement were collected at 5–20 weeks of gestation. No substantial differences were observed in the assay methods applied to measure adiponectin levels. The majority of included studies used enzyme immunoassays (EIA), while others employed radioimmunoassay (RIA). Several studies reported quality control measurements for adiponectin [15, 19, 20, 22, 23, 36], and the intra-assay and inter-assay coefficients of variation were acceptable (all < 10%).
Of the nine studies for leptin, three studies were conducted in North America, two in the Europe, two in Asia, one in Australia, and one in Mexico. GDM was usually diagnosed during the 24–28 weeks of gestation. The definition used for GDM was the criteria proposed by Carpenter and Coustan or the ADA in three studies, the IADPSG criteria in two studies, the National Diabetes Data Group criteria in one study, and local criteria in another two studies. Blood samples for leptin measurement were collected at 5–20 weeks of gestation. No substantial differences were observed in the methodology applied to assays; five studies used RIA, while the other four studies used EIA. Several studies reported quality control measurements for leptin [14, 16, 19, 26, 36], and the intra-assay and inter-assay coefficients of variation were acceptable (all < 10%).
3.2 Quantitative assessment of summary statistics
3.2.1 Adiponectin
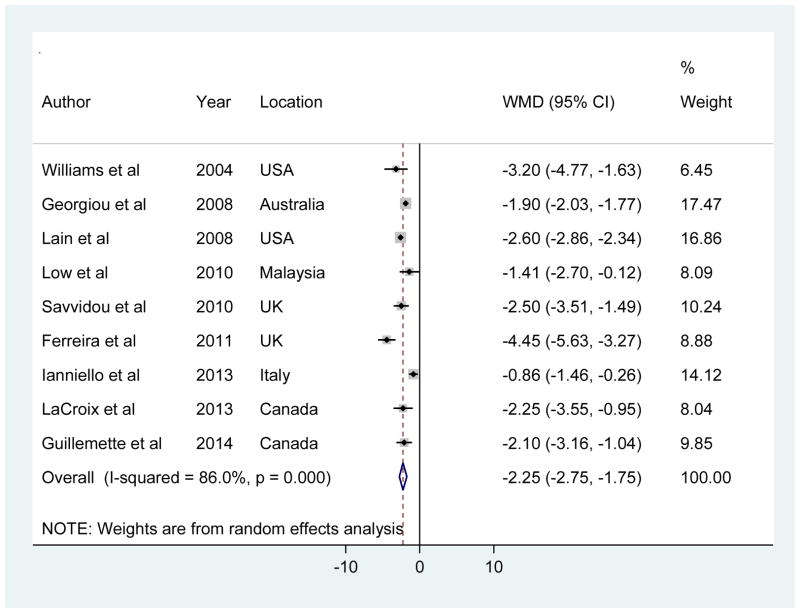
Adiponectin was the most widely studied adipokines in relation to GDM, and the available studies yielded relatively consistent results across different populations. Among the included studies, three studies reported a comparable value but different unit of adiponectin (i.e., ng/ml in two studies [18, 21] and mg/ml in the other study [34]) compared with others (i.e., μg/ml), and thus the reported values were unreasonably high or low after converting. We did not get confirmation from the original authors by email. Thus, to be conservative, we did not include these studies in the main analysis which calculated the pooled WMD. Instead, we included them in a sensitivity analysis using pooled SMD as the metric. In another study [36], adiponectin levels were reported as geometric means and ratios, without values in the absolute scale, and therefore it was omitted in the meta-analysis. Our meta-analysis of the other nine studies showed that adiponectin levels in the first or early second trimester were significantly lower in women who later developed GDM compared to women who later did not, yet with significant between-study heterogeneity (random-effects pooled WMD [95% CI], −2.25 [−2.75 to −1.75] μg/ml; I2 = 86%; Figure 2). Egger test for publication bias did not reach statistical significance (P = 0.49).
Figure 2.
Forest plot for mean difference in adiponectin levels (μg/ml) between GDM cases and controls
In stratified analyses (Supplementary Table 2), the pooled WMDs were not differentiated appreciably by geographical location (North America versus others), sample size (< 100 versus ≥100), average timing of blood samples collection for adiponectin measurement (first versus second trimester), assay methods for adiponectin measurement (EIA versus RIA), and diagnostic criteria for GDM (WHO criteria versus others). We did not stratify by time for GDM diagnosis because almost all included studies reported the diagnosis at 24–28 weeks of gestation. Meta-regression analysis including the above variables in the model also did not identify significant contributors to the source of heterogeneity. Sensitivity analysis by omitting one study at a time did not alter the results (Supplementary Figure 1). In addition, we calculated the pooled SMD which allows including the results from all studies regardless of units. The pooled random-effects SMD (95% CI) was −1.20 (−1.63 to −0.78) (Supplementary Figure 2).
3.2.2 Leptin
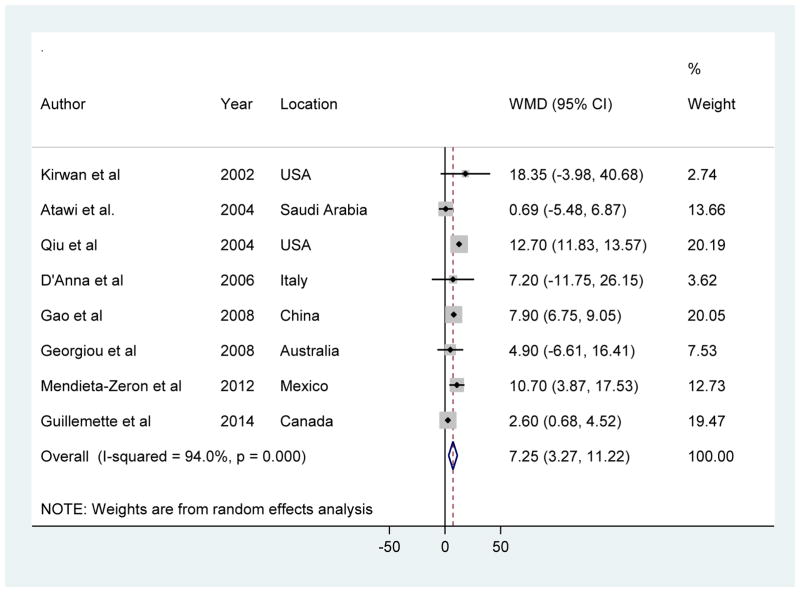
Among the nine studies, one study [36] reported leptin adiponectin levels as geometric means and ratios, without values in the absolute scale, and therefore it was omitted in the meta-analysis. A meta-analysis of the remaining eight prospective studies showed that leptin levels in the first or early second trimester were significantly higher in women who later developed GDM compared to women who later did not, yet with significant between-study heterogeneity (random-effects WMD [95% CI], 7.25 [3.27 to 11.22] ng/ml; I2 = 94%; Figure 3). Egger test for publication bias did not provide evidence of significant effect (P = 0.41).
Figure 3.
Forest plot for mean difference in leptin levels (ng/ml) between GDM cases and controls
Similar to adiponectin, there was no evidence of heterogeneity in pooled WMDs for leptin levels by average timing of blood collection for leptin measurement (first versus second trimester), assay methods for leptin measurement (EIA versus RIA), and diagnostic criteria for GDM (Carpenter and Coustan criteria versus others) (Supplementary Table 3). Sensitivity analysis by omitting one study at a time did not alter the results materially (Supplementary Figure 3).
3.3 Findings of adipokines not suitable for a pooling of summary statistics
We did not pool studies that investigated visfatin, RBP-4, resistin, TNF-α, interleukin-6 (IL-6), or vaspin, because too few independent prospective studies have been published on these adipokines. Findings on their association with GDM risk have been conflicting (Table 3). For instance, three studies have been conducted about RBP-4 levels and GDM risk [28, 29, 32]. Although a significant association was reported in a U.S. population [29], there was no significant association in other studies from UK [32] and China [28]. Conflicting results were also reported in the four studies about TNF-α levels and risk of GDM, with one study reporting a significant association [18] while another two reporting no significant association [12, 19, 35]. Recently, adipocyte fatty acid-binding protein (AFABP) has been suggested to be a probable candidate involved in the pathophysiology of GDM [37]. However, no prospective study has conducted so far on the association between AFABP and GDM. The only study, which was cross-sectional, reported an elevated AFABP level in women with GDM as compared with healthy pregnant controls [38].
Table 3.
Summary of the results from available prospective studies relating adipokine levels to GDM risk
| Adipokines | Number of studies | Results* |
|---|---|---|
| Adiponectin | 13 | ↑ 0 study, ↓ 12 studies, ↔ 1 study |
| Leptin | 9 | ↑ 4 studies, ↓ 0 study, ↔ 5 studies |
| Visfatin | 2 | ↑ 1 study, ↓ 0 study, ↔ 1 study |
| RBP-4 | 3 | ↑ 1 study, ↓ 0 study, ↔ 2 studies |
| Resistin | 2 | ↑ 0 study, ↓ 0 study, ↔ 2 studies |
| TNF-α | 4 | ↑ 1 study, ↓ 0 study, ↔ 3 studies |
| IL-6 | 3 | ↑ 0 study, ↓ 0 study, ↔ 3 studies |
| Vaspin | 1 | ↑ 1 study, ↓ 0 study, ↔ 0 study |
↑ positive association, ↓ inverse association, ↔ non-significant difference (p > 0.05)
Abbreviations: IL-6, interleukin-6; RBP-4, retinol binding protein-4; TNF-α, tumor necrosis factor-α.
4. DISCUSSION
In this systematic review and quantitative analysis of available data regarding adipokines and GDM, we observed that adiponectin levels in the first or second trimester of pregnancy are lower among pregnant women who later develop GDM than non-GDM women, whereas leptin levels are higher. Prospective data were sparse and findings were inconsistent for visfatin, RBP-4, resistin, TNF-α, IL-6, and vaspin. We did not identify prospective studies for several novel adipokines, including chemerin, apelin, omentin, or AFABP.
The observed associations of GDM with adiponectin and leptin levels are biologically plausible. GDM develops when pancreatic β cells of pregnant women are unable to increase insulin secretion enough to counteract the corresponding fall in tissue sensitivity to insulin during pregnancy [39]. Adiponectin is a signaling protein that is synthesized and secreted by adipose tissue and is one of the most abundant plasma proteins in humans [40]. Adiponectin can reduce ectopic fat storage through stimulating lipid oxidation and inhibiting lipolysis in adipose tissue [41]. In addition, adiponectin may also display anti-inflammatory properties by suppressing TNF-α production [42]. Intravenous administration of recombinant adiponectin in rodent models of insulin resistance restored normal insulin sensitivity [6]. In humans, adiponectin levels were inversely associated with fasting glucose, insulin, and insulin resistance [43], and the risk of developing T2DM [44]. Thus, a reduction in adiponectin levels may be associated with the development of GDM through decreased insulin sensitivity and attenuated anti-inflammatory capacity.
In contrast to adiponectin, leptin may contribute to the pathogenesis of GDM through elevated insulin resistance. Leptin is an adipose tissue-derived hormone that plays a key role in the regulation of energy intake and energy expenditure. Circulating leptin levels are elevated with increasing adiposity [45], reflecting a state of leptin resistance [46]. Several studies have positively associated leptin levels with insulin resistance independent of body mass index (BMI), whereas other studies suggest that the relationship between leptin levels and insulin resistance is mainly accounted for by obesity [47, 48]. Elevated leptin levels were also independently associated with a higher risk of incident GDM [14] and T2DM [49]. Animal and human studies have demonstrated that a circulating soluble form of the leptin receptor influences the amount of free versus bound leptin in serum and plays a part in modulating availability and biological function of leptin [50]. In humans, an increasing BMI was associated with a lower concentration of soluble leptin receptor, whereas fasting increased its concentration [51]. Lewandowski et al [52] reported a significantly higher level of soluble leptin receptor in women with type 1 diabetic pregnancy than women with normal pregnancy. However, there is no published study on the association between soluble leptin receptor and GDM.
Interestingly, there is evidence, although still limited, implicating that the association between adipokines and GDM may be independent of adiposity measures. For example, Williams et al [15] have shown a significantly inverse association between adiponectin levels in early pregnancy and subsequent risk of GDM after controlling for prepregnancy BMI. Similar results were reported by Lain et al [20] and LaCroix et al [31]. For leptin, Qiu et al [14] found a linear association between leptin levels in early pregnancy and GDM risk independent of prepregnancy BMI and other confounders; each 10 ng/mL increase in the leptin concentration was associated with a 20% higher in GDM risk. These results indicate that there could be other pathways linking adipokines to the development of GDM, although BMI is not an accurate measure of adiposity [53]. Future studies using objective measures of adiposity (e.g., dual-energy X-ray absorptiometry) may help clarify the adipokines-GDM relations independent of adiposity. Few studies have investigated the interaction between adiposity measures and adipokines among pregnancy women. No significant interaction between BMI and adiponectin in association with risk of developing GDM was found in a nested case-control study among US pregnant women [20]. Similarly, non-significant interactions of adiposity measures and adiponectin levels in relation to T2DM risk were observed in some [54], although not all [55], studies conducted in the general population.
There are several critical data gaps that require additional research. First, the trajectories and dynamic associations of adipokines with subsequent risk of GDM remain unclear. Unlike diabetes among non-pregnant individuals, the dynamic patterns of adipokine levels in women with GDM are influenced not only by GDM status, but also by the profound and yet time-dependent metabolic challenges related to pregnancy. Pregnancy is normally associated with progressive insulin resistance, and there is a two-thirds decrease in insulin sensitivity in late pregnancy [39]. In parallel with the changes in insulin sensitivity, adipokines exhibit different dynamic patterns during normal pregnancy [56]. For instance, adiponectin levels progressively decline [57] while leptin levels progressively increase during pregnancy [58]. Longitudinal studies with blood samples collected at multiple time points before the onset of GDM (e.g., before pregnancy or during early pregnancy) would provide insights to improve understanding of the roles of adipokines in the pathogenesis of GDM. No such study was identified in our systematic review. Second, the causal relation between adipokines and GDM warrants further investigation. During the past decades, Mendelian randomization analysis simultaneously considering the triangle associations of genetic variants, intermediate phenotypes, and disease status has been increasingly utilized to inform causal inference in observational studies [59]. However, we are not aware of such studies investigating associations of both adipokine levels and genetic variants relevant to adipokine levels with GDM risk using Mendelian randomization analysis approach.
The strengths of this systematic review include comprehensive literature search and meticulous protocol for study selection and data analysis. There are several limitations. First, prospective studies of adipokines and GDM risk among non-Caucasian populations are sparse. This limited the capacity of exploring the adipokines-GDM association by race/ethnicity groups. Compared with Caucasian women, Asian, Hispanic, and Native American women have an increased risk of GDM [3]. In addition, the associations between adipokines and insulin sensitivity varied by race/ethnicity [60]. Future studies among non-Caucasian populations are warranted. Second, the number of included studies for most adipokines was small. Even for adiponectin and leptin, the pooled sample size was limited. Thus it may compromise the statistical power of the meta-analysis. Although the statistical test showed no indication of publication bias for the two adipokines included in the meta-analysis, we cannot rule out the possibility of publication bias due to the small number of studies.
5. Conclusions
In summary, in this systematic review, we observed that adiponectin levels in the first or second trimester of pregnancy are lower among pregnant women who later develop GDM than non-GDM women, whereas leptin levels are higher. Future studies are warranted to clarify the association of other adipokines and GDM. Moreover, well-designed prospective studies with longitudinal assessment of adipokines during pregnancy are needed to understand the trajectories and dynamic associations of adipokines with GDM risk.
Supplementary Material
Acknowledgments
Funding: This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (to C.Z., W.B., and M.K.), research grants R01-DK-062290 (to S. L.), R01-DK-58845 and R01-DK-088078 (to Y.S.) from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Abbreviations
- ADA
American Diabetes Association
- AFABP
adipocyte fatty acid-binding protein
- BMI
body mass index
- EIA
enzyme immunoassays
- GDM
Gestational diabetes mellitus
- IADPSG
International Association of the Diabetes and Pregnancy Study Groups
- IL-6
interleukin-6
- RBP4
retinol binding protein 4
- RIA
radioimmunoassay
- SD
standard deviation
- SMD
standardized mean difference
- T2DM
type 2 diabetes mellitus
- TNF-α
tumor necrosis factor-α
- WHO
World Health Organization
- WMD
weighted mean difference
Footnotes
Author contributions: WB and CZ designed the study and wrote the manuscript. AB and WB collected and analyzed the data. YS, MK, SL, and CZ interpreted the results and reviewed and edited the manuscript. W.B. and C.Z. had primary responsibility for final content. All authors provided intellectual input into the paper, and all authors read and approved the final manuscript.
Conflicts of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S88–90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 2.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31(2):340–6. doi: 10.2337/dc07-1596. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet Med. 2004;21(2):103–13. doi: 10.1046/j.1464-5491.2003.00985.x. [DOI] [PubMed] [Google Scholar]
- 4.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409(6821):729–33. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 7.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 8.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 12.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51(7):2207–13. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 13.Atawi FA, Warsy AS, Babay Z, Addar M. Leptin concentration during different stages of pregnancy. Clin Exp Obstet Gynecol. 2004;31(3):211–6. [PubMed] [Google Scholar]
- 14.Qiu C, Williams MA, Vadachkoria S, Frederick IO, Luthy DA. Increased maternal plasma leptin in early pregnancy and risk of gestational diabetes mellitus. Obstet Gynecol. 2004;103(3):519–25. doi: 10.1097/01.AOG.0000113621.53602.7a. [DOI] [PubMed] [Google Scholar]
- 15.Williams MA, Qiu C, Muy-Rivera M, Vadachkoria S, Song T, Luthy DA. Plasma adiponectin concentrations in early pregnancy and subsequent risk of gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89(5):2306–11. doi: 10.1210/jc.2003-031201. [DOI] [PubMed] [Google Scholar]
- 16.D’Anna R, Baviera G, Cannata ML, De Vivo A, Di Benedetto A, Corrado F. Midtrimester amniotic fluid leptin and insulin levels and subsequent gestational diabetes. Gynecol Obstet Invest. 2007;64(2):65–8. doi: 10.1159/000099149. [DOI] [PubMed] [Google Scholar]
- 17.Dasanayake AP, Chhun N, Tanner AC, Craig RG, Lee MJ, Moore AF, et al. Periodontal pathogens and gestational diabetes mellitus. J Dent Res. 2008;87(4):328–33. doi: 10.1177/154405910808700421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao XL, Yang HX, Zhao Y. Variations of tumor necrosis factor-alpha, leptin and adiponectin in mid-trimester of gestational diabetes mellitus. Chin Med J (Engl) 2008;121(8):701–5. [PubMed] [Google Scholar]
- 19.Georgiou HM, Lappas M, Georgiou GM, Marita A, Bryant VJ, Hiscock R, et al. Screening for biomarkers predictive of gestational diabetes mellitus. Acta Diabetol. 2008;45(3):157–65. doi: 10.1007/s00592-008-0037-8. [DOI] [PubMed] [Google Scholar]
- 20.Lain KY, Daftary AR, Ness RB, Roberts JM. First trimester adipocytokine concentrations and risk of developing gestational diabetes later in pregnancy. Clin Endocrinol (Oxf) 2008;69(3):407–11. doi: 10.1111/j.1365-2265.2008.03198.x. [DOI] [PubMed] [Google Scholar]
- 21.Paradisi G, Ianniello F, Tomei C, Bracaglia M, Carducci B, Gualano MR, et al. Longitudinal changes of adiponectin, carbohydrate and lipid metabolism in pregnant women at high risk for gestational diabetes. Gynecol Endocrinol. 2010;26(7):539–45. doi: 10.3109/09513591003632084. [DOI] [PubMed] [Google Scholar]
- 22.Savvidou M, Nelson SM, Makgoba M, Messow CM, Sattar N, Nicolaides K. First-trimester prediction of gestational diabetes mellitus: examining the potential of combining maternal characteristics and laboratory measures. Diabetes. 2010;59(12):3017–22. doi: 10.2337/db10-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira AF, Rezende JC, Vaikousi E, Akolekar R, Nicolaides KH. Maternal serum visfatin at 11–13 weeks of gestation in gestational diabetes mellitus. Clin Chem. 2011;57(4):609–13. doi: 10.1373/clinchem.2010.159806. [DOI] [PubMed] [Google Scholar]
- 24.Low CF, Mohd Tohit ER, Chong PP, Idris F. Adiponectin SNP45TG is associated with gestational diabetes mellitus. Arch Gynecol Obstet. 2011;283(6):1255–60. doi: 10.1007/s00404-010-1548-4. [DOI] [PubMed] [Google Scholar]
- 25.Nanda S, Savvidou M, Syngelaki A, Akolekar R, Nicolaides KH. Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat Diagn. 2011;31(2):135–41. doi: 10.1002/pd.2636. [DOI] [PubMed] [Google Scholar]
- 26.Mendieta Zeron H, Garcia Solorio VJ, Nava Diaz PM, Garduno Alanis A, Santillan Benitez JG, Dominguez Garcia V, et al. Hyperleptinemia as a prognostic factor for preeclampsia: a cohort study. Acta Medica (Hradec Kralove) 2012;55(4):165–71. doi: 10.14712/18059694.2015.41. [DOI] [PubMed] [Google Scholar]
- 27.Nanda S, Poon LC, Muhaisen M, Acosta IC, Nicolaides KH. Maternal serum resistin at 11 to 13 weeks’ gestation in normal and pathological pregnancies. Metabolism. 2012;61(5):699–705. doi: 10.1016/j.metabol.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Ping F, Xiang HD, Li M, Li W, Liu JT, Nie M, et al. Effects of variation in retinol binding protein 4 gene and adipose specific expression of gestational diabetes in Beijing, China. Diabetes Res Clin Pract. 2012;97(2):283–9. doi: 10.1016/j.diabres.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Abetew DF, Qiu C, Fida NG, Dishi M, Hevner K, Williams MA, et al. Association of retinol binding protein 4 with risk of gestational diabetes. Diabetes Res Clin Pract. 2013;99(1):48–53. doi: 10.1016/j.diabres.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gkiomisi A, Makedou KG, Anastasilakis AD, Polyzos SA, Kourtis A, Gerou S, et al. Serum vaspin levels in women with and without gestational diabetes mellitus during pregnancy and postpartum. Cytokine. 2013;61(1):127–32. doi: 10.1016/j.cyto.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Lacroix M, Battista MC, Doyon M, Menard J, Ardilouze JL, Perron P, et al. Lower adiponectin levels at first trimester of pregnancy are associated with increased insulin resistance and higher risk of developing gestational diabetes mellitus. Diabetes Care. 2013;36(6):1577–83. doi: 10.2337/dc12-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nanda S, Nikoletakis G, Markova D, Poon LC, Nicolaides KH. Maternal serum retinol-binding protein-4 at 11–13 weeks’ gestation in normal and pathological pregnancies. Metabolism. 2013;62(6):814–9. doi: 10.1016/j.metabol.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Ianniello F, Quagliozzi L, Caruso A, Paradisi G. Low adiponectin in overweight/obese women: association with diabetes during pregnancy. Eur Rev Med Pharmacol Sci. 2013;17(23):3197–205. [PubMed] [Google Scholar]
- 34.Rasanen JP, Snyder CK, Rao PV, Mihalache R, Heinonen S, Gravett MG, et al. Glycosylated fibronectin as a first-trimester biomarker for prediction of gestational diabetes. Obstet Gynecol. 2013;122(3):586–94. doi: 10.1097/AOG.0b013e3182a0c88b. [DOI] [PubMed] [Google Scholar]
- 35.Guillemette L, Lacroix M, Battista MC, Doyon M, Moreau J, Menard J, et al. TNFalpha dynamics during the oral glucose tolerance test vary according to the level of insulin resistance in pregnant women. J Clin Endocrinol Metab. 2014;99(5):1862–9. doi: 10.1210/jc.2013-4016. [DOI] [PubMed] [Google Scholar]
- 36.Maitland RA, Seed PT, Briley AL, Homsy M, Thomas S, Pasupathy D, et al. Prediction of gestational diabetes in obese pregnant women from the UK Pregnancies Better Eating and Activity (UPBEAT) pilot trial. Diabet Med. 2014;31(8):963–70. doi: 10.1111/dme.12482. [DOI] [PubMed] [Google Scholar]
- 37.Fasshauer M, Blüher M, Stumvoll M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol. 2013 doi: 10.1016/S2213-8587(13)70176-1. [DOI] [PubMed] [Google Scholar]
- 38.Kralisch S, Stepan H, Kratzsch J, Verlohren M, Verlohren HJ, Drynda K, et al. Serum levels of adipocyte fatty acid binding protein are increased in gestational diabetes mellitus. Eur J Endocrinol. 2009;160(1):33–8. doi: 10.1530/EJE-08-0540. [DOI] [PubMed] [Google Scholar]
- 39.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115(3):485–91. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stumvoll M, Haring H. Resistin and adiponectin--of mice and men. Obes Res. 2002;10(11):1197–9. doi: 10.1038/oby.2002.162. [DOI] [PubMed] [Google Scholar]
- 41.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–46. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 42.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112(1):91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol. 2002;13(1):51–9. doi: 10.1097/00041433-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361(9353):226–8. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- 45.Chessler SD, Fujimoto WY, Shofer JB, Boyko EJ, Weigle DS. Increased plasma leptin levels are associated with fat accumulation in Japanese Americans. Diabetes. 1998;47(2):239–43. doi: 10.2337/diab.47.2.239. [DOI] [PubMed] [Google Scholar]
- 46.Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med. 1999;130(8):671–80. doi: 10.7326/0003-4819-130-8-199904200-00014. [DOI] [PubMed] [Google Scholar]
- 47.Zuo H, Shi Z, Yuan B, Dai Y, Wu G, Hussain A. Association between serum leptin concentrations and insulin resistance: a population-based study from China. PLoS One. 2013;8(1):e54615. doi: 10.1371/journal.pone.0054615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimmet PZ, Collins VR, de Courten MP, Hodge AM, Collier GR, Dowse GK, et al. Is there a relationship between leptin and insulin sensitivity independent of obesity? A population-based study in the Indian Ocean nation of Mauritius. Mauritius NCD Study Group. Int J Obes Relat Metab Disord. 1998;22(2):171–7. doi: 10.1038/sj.ijo.0800559. [DOI] [PubMed] [Google Scholar]
- 49.Sun Q, van Dam RM, Meigs JB, Franco OH, Mantzoros CS, Hu FB. Leptin and soluble leptin receptor levels in plasma and risk of type 2 diabetes in U.S. women: a prospective study. Diabetes. 2010;59(3):611–8. doi: 10.2337/db09-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mantzoros CS, Flier JS. Editorial: leptin as a therapeutic agent--trials and tribulations. J Clin Endocrinol Metab. 2000;85(11):4000–2. doi: 10.1210/jcem.85.11.7062. [DOI] [PubMed] [Google Scholar]
- 51.Yannakoulia M, Yiannakouris N, Bluher S, Matalas AL, Klimis-Zacas D, Mantzoros CS. Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J Clin Endocrinol Metab. 2003;88(4):1730–6. doi: 10.1210/jc.2002-021604. [DOI] [PubMed] [Google Scholar]
- 52.Lewandowski K, Horn R, O’Callaghan CJ, Dunlop D, Medley GF, O’Hare P, et al. Free leptin, bound leptin, and soluble leptin receptor in normal and diabetic pregnancies. J Clin Endocrinol Metab. 1999;84(1):300–6. doi: 10.1210/jcem.84.1.5401. [DOI] [PubMed] [Google Scholar]
- 53.Rahman M, Berenson AB. Accuracy of current body mass index obesity classification for white, black, and Hispanic reproductive-age women. Obstet Gynecol. 2010;115(5):982–8. doi: 10.1097/AOG.0b013e3181da9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoon SJ, Lee HS, Lee SW, Yun JE, Kim SY, Cho ER, et al. The association between adiponectin and diabetes in the Korean population. Metabolism. 2008;57(6):853–7. doi: 10.1016/j.metabol.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto S, Matsushita Y, Nakagawa T, Hayashi T, Noda M, Mizoue T. Circulating adiponectin levels and risk of type 2 diabetes in the Japanese. Nutr Diabetes. 2014;4:e130. doi: 10.1038/nutd.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miehle K, Stepan H, Fasshauer M. Leptin, adiponectin and other adipokines in gestational diabetes mellitus and pre-eclampsia. Clin Endocrinol (Oxf) 2012;76(1):2–11. doi: 10.1111/j.1365-2265.2011.04234.x. [DOI] [PubMed] [Google Scholar]
- 57.Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, et al. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49(7):1677–85. doi: 10.1007/s00125-006-0264-x. [DOI] [PubMed] [Google Scholar]
- 58.Schubring C, Englaro P, Siebler T, Blum WF, Demirakca T, Kratzsch J, et al. Longitudinal analysis of maternal serum leptin levels during pregnancy, at birth and up to six weeks after birth: relation to body mass index, skinfolds, sex steroids and umbilical cord blood leptin levels. Horm Res. 1998;50(5):276–83. doi: 10.1159/000023290. [DOI] [PubMed] [Google Scholar]
- 59.Sheehan NA, Didelez V, Burton PR, Tobin MD. Mendelian randomisation and causal inference in observational epidemiology. PLoS Med. 2008;5(8):e177. doi: 10.1371/journal.pmed.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mente A, Razak F, Blankenberg S, Vuksan V, Davis AD, Miller R, et al. Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care. 2010;33(7):1629–34. doi: 10.2337/dc09-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.