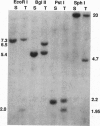
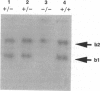
Abstract
Thalassemias are hereditary anemias caused by mutations that disturb the normal 1:1 balance of alpha- and beta-globin chains that form hemoglobin. We have disrupted the major adult beta-globin gene (b1) in mouse embryonic stem cells by using homologous recombination to insert selectable sequences into the gene. Mice homozygous for this insertional disruption of the b1 gene (Hbbth-2/Hbbth-2) are severely anemic and die perinatally. In contrast, approximately 60% of mice homozygous for deletion of the same gene (Hbbth-1/Hbbth-1) survive to adulthood and are much less anemic [Skow, L. C., Burkhart, B. A., Johnson, F. M., Popp, R. A., Goldberg, S. Z., Anderson, W. F., Barnett, L. B. & Lewis, S. E. (1983) Cell 34, 1043-1052]. These different phenotypes have implications for the control of beta-globin gene expression.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behringer R. R., Ryan T. M., Palmiter R. D., Brinster R. L., Townes T. M. Human gamma- to beta-globin gene switching in transgenic mice. Genes Dev. 1990 Mar;4(3):380–389. doi: 10.1101/gad.4.3.380. [DOI] [PubMed] [Google Scholar]
- Blom van Assendelft G., Hanscombe O., Grosveld F., Greaves D. R. The beta-globin dominant control region activates homologous and heterologous promoters in a tissue-specific manner. Cell. 1989 Mar 24;56(6):969–977. doi: 10.1016/0092-8674(89)90630-2. [DOI] [PubMed] [Google Scholar]
- Boguski M. S., Hardison R. C., Schwartz S., Miller W. Analysis of conserved domains and sequence motifs in cellular regulatory proteins and locus control regions using new software tools for multiple alignment and visualization. New Biol. 1992 Mar;4(3):247–260. [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. Developmental regulation of beta-globin gene switching. Cell. 1988 Oct 7;55(1):17–26. doi: 10.1016/0092-8674(88)90005-0. [DOI] [PubMed] [Google Scholar]
- Codrington J. F., Li H. W., Kutlar F., Gu L. H., Ramachandran M., Huisman T. H. Observations on the levels of Hb A2 in patients with different beta-thalassemia mutations and a delta chain variant. Blood. 1990 Sep 15;76(6):1246–1249. [PubMed] [Google Scholar]
- Curcio M. J., Kantoff P., Schafer M. P., Anderson W. F., Safer B. Compensatory increase in levels of beta minor globin in murine beta-thalassemia is under translational control. J Biol Chem. 1986 Dec 5;261(34):16126–16132. [PubMed] [Google Scholar]
- Enver T., Raich N., Ebens A. J., Papayannopoulou T., Costantini F., Stamatoyannopoulos G. Developmental regulation of human fetal-to-adult globin gene switching in transgenic mice. Nature. 1990 Mar 22;344(6264):309–313. doi: 10.1038/344309a0. [DOI] [PubMed] [Google Scholar]
- Goldberg S. Z., Kuebbing D., Trauber D., Schafer M. P., Lewis S. E., Popp R. A., Anderson W. F. A 66-base pair insert bridges the deletion responsible for a mouse model of beta-thalassemia. J Biol Chem. 1986 Sep 15;261(26):12368–12374. [PubMed] [Google Scholar]
- Hanscombe O., Vidal M., Kaeda J., Luzzatto L., Greaves D. R., Grosveld F. High-level, erythroid-specific expression of the human alpha-globin gene in transgenic mice and the production of human hemoglobin in murine erythrocytes. Genes Dev. 1989 Oct;3(10):1572–1581. doi: 10.1101/gad.3.10.1572. [DOI] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Kim C. G., Epner E. M., Forrester W. C., Groudine M. Inactivation of the human beta-globin gene by targeted insertion into the beta-globin locus control region. Genes Dev. 1992 Jun;6(6):928–938. doi: 10.1101/gad.6.6.928. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Smithies O. Recombinant fragment assay for gene targetting based on the polymerase chain reaction. Nucleic Acids Res. 1988 Sep 26;16(18):8887–8903. doi: 10.1093/nar/16.18.8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. L., Zhou B., Powers P., Enver T., Stamatoyannopoulos G. Beta-globin locus activation regions: conservation of organization, structure, and function. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8207–8211. doi: 10.1073/pnas.87.21.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Moon A. M., Ley T. J. Conservation of the primary structure, organization, and function of the human and mouse beta-globin locus-activating regions. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7693–7697. doi: 10.1073/pnas.87.19.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi A. K., Roginski R. S., Gregg R. G., Smithies O., Skoultchi A. I. Regulated expression of genes inserted at the human chromosomal beta-globin locus by homologous recombination. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3845–3849. doi: 10.1073/pnas.85.11.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skow L. C., Burkhart B. A., Johnson F. M., Popp R. A., Popp D. M., Goldberg S. Z., Anderson W. F., Barnett L. B., Lewis S. E. A mouse model for beta-thalassemia. Cell. 1983 Oct;34(3):1043–1052. doi: 10.1016/0092-8674(83)90562-7. [DOI] [PubMed] [Google Scholar]
- Steinberg M. H., Adams J. G., 3rd Hemoglobin A2: origin, evolution, and aftermath. Blood. 1991 Nov 1;78(9):2165–2177. [PubMed] [Google Scholar]
- Steinberg M. H., Coleman M. B., Adams J. G., 3rd Beta-thalassemia with exceptionally high hemoglobin A2. Differential expression of the delta-globin gene in the presence of beta-thalassemia. J Lab Clin Med. 1982 Oct;100(4):548–557. [PubMed] [Google Scholar]
- Townes T. M., Behringer R. R. Human globin locus activation region (LAR): role in temporal control. Trends Genet. 1990 Jul;6(7):219–223. doi: 10.1016/0168-9525(90)90182-6. [DOI] [PubMed] [Google Scholar]
- Whitelaw E., Tsai S. F., Hogben P., Orkin S. H. Regulated expression of globin chains and the erythroid transcription factor GATA-1 during erythropoiesis in the developing mouse. Mol Cell Biol. 1990 Dec;10(12):6596–6606. doi: 10.1128/mcb.10.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney J. B., 3rd Differential control of the synthesis of two hemoglobin beta chains in normal mice. Cell. 1977 Dec;12(4):863–871. doi: 10.1016/0092-8674(77)90150-7. [DOI] [PubMed] [Google Scholar]
- Whitney J. B., 3rd Simplified typing of mouse hemoglobin (Hbb) phenotypes using cystamine. Biochem Genet. 1978 Aug;16(7-8):667–672. doi: 10.1007/BF00484723. [DOI] [PubMed] [Google Scholar]