Abstract
Epidemiological studies have been critical for estimating associations between exposure to ambient particulate matter (PM) air pollution and adverse health outcomes. Because total PM mass is a temporally and spatially varying mixture of constituents with different physical and chemical properties, recent epidemiological studies have focused on PM constituents. Most studies have estimated associations between PM constituents and health using the same statistical methods as in studies of PM mass. However, these approaches may not be sufficient to address challenges specific to studies of PM constituents, namely assigning exposure, disentangling health effects, and handling measurement error. We reviewed large, population-based epidemiological studies of PM constituents and health and describe the statistical methods typically applied to address these challenges. Development of statistical methods that simultaneously address multiple challenges, for example, both disentangling health effects and handling measurement error, could improve estimation of associations between PM constituents and adverse health outcomes.
Keywords: Particulate matter constituents, Health effects, Environmental epidemiology, Statistical methods, Chemical speciation
Introduction
Exposure to ambient particulate matter (PM) air pollution has been consistently associated with increased mortality and morbidity in epidemiological studies [1–7]. Most recent epidemiological studies focus on the mass concentration of particles of a specific size distribution, for example, PM less than 2.5 µm (PM2.5) in aerodynamic diameter; however, PM is a spatially and temporally varying mixture of chemical constituents including elemental carbon (EC) and organic carbon (OC) species, ions such as sulfate and nitrate, and elements such as silicon and nickel [8]. The spatial and temporal variation in PM composition may be driving previously observed spatial and temporal differences in estimated health effects of total PM mass [1, 4, 9]. Since studies of both short-term and long-term exposures to PM constituents have demonstrated that associations with adverse health outcomes vary by constituent [10–13], determining which chemical constituents of PM are most harmful is necessary in order to develop better strategies to protect human health.
Large-scale, epidemiological studies of PM constituents and health frequently use ambient, fixed-location monitors to assign exposure and then estimate associations with adverse health outcomes using single pollutant regression models, which is the same approach developed for and applied in epidemiological studies of total PM mass and other major ambient air pollutants. While this approach provides a general framework for estimating associations between air pollution and health, studies of PM constituents and health are faced with particular challenges. First, ambient monitors measuring PM constituents are particularly sparse across space and typically do not measure concentrations daily [14]. This presents problems for population-based epidemiological studies, which aim to assign exposures to populations spread over large areas (e.g., counties or cities), larger than reasonably represented by available monitoring data given the spatial heterogeneity of many PM constituents [15]; lack of daily monitoring data also limits investigation of multiday, lagged effects of PM constituent exposures. Second, PM constituents generated by the same source are often correlated with each other and also PM constituents, by definition fractions of total PM, may be correlated with total PM mass [8]. Thus, it can be difficult to disentangle the health effects of one PM constituent from the health effects of other PM constituents or total PM mass in epidemiological studies. Last, other sources of measurement error, such as error related to instruments or sampling may be greater for measurements of PM constituents than for total PM mass concentrations. As an example, many PM constituents (such as metals and specific organic species) contribute minimally to total PM by mass [8] and low concentrations of these constituents often cannot be confidently differentiated from zero when they fall below their method detection limits (MDL) [16–18]. Two previous papers outlined some of the challenges present in epidemiological studies of PM constituents [19, 20]. A review of current methods and challenges is needed as the number of studies of PM constituents and health has increased in recent years.
In this review, we summarize a literature search of how recent large, population-based epidemiological studies address these challenges and we highlight where further development of statistical methods is most needed. While our focus is large-scale epidemiological studies, smaller studies such as panel studies have a different set of challenges that would be useful to review in future work. We do not review the substantive findings of these epidemiological studies of PM constituents and health, which have been previously summarized elsewhere [21, 22].
Literature Search
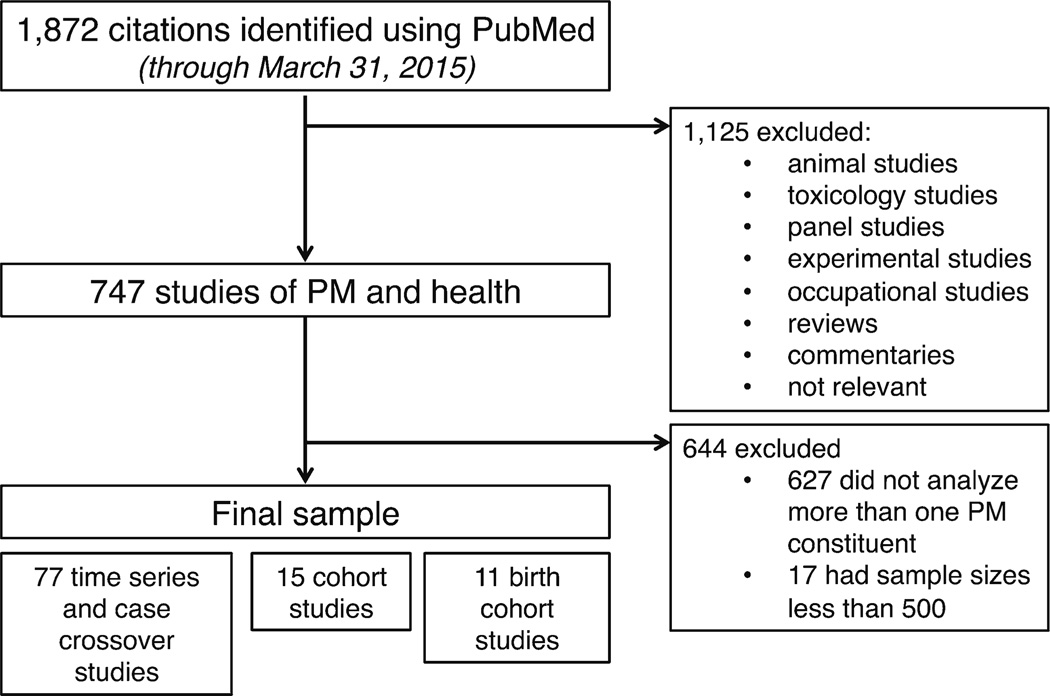
We systematically reviewed large, epidemiological studies of the associations between short-term and long-term exposures to PM constituents and adverse health outcomes. In our main search, we identified 1872 citations in PubMed through 31 March 2015 that contained at least one term from each of four categories: PM, constituents, health, and study design (Table 1). After reviewing 1872 citations, we identified 747 relevant, peer-reviewed, epidemiological studies of PM constituents and health. Studies were primarily excluded because they did not analyze PM constituents or health. We limited our review to only large (>500 individuals), population-based studies of multiple PM constituents. We did not review panel studies because they frequently are able to better characterize pollution exposure and they have a different set of challenges than those facing larger epidemiological studies. The process of our literature review is depicted in Fig. 1. Our review identified 15 cohort studies of long-term exposure to PM constituents, 77 time series and case-crossover studies of short-term exposure to PM constituents, and 11 birth cohort studies. From all these studies, we determined the statistical methods commonly used to address major challenges including assigning exposure in the presence of spatial and temporal heterogeneity, disentangling health effects of individual constituents, and instrument-related measurement error. Because of the large number of relevant studies identified (n = 103), we summarize recent studies that exemplify unique approaches to address these challenges in Table 2.
Table 1.
Terms used to search PubMed for epidemiological studies of particulate matter constituents and adverse health outcomes
| Particulate matter | Constituents | Health | Study design |
|---|---|---|---|
| Particulate matter | Constituent(s) | Health | Epidemiologic(al) |
| Airborne particles | Component(s) | Mortality | Time series |
| PM | Source(s) | Morbidity | Cohort |
| PM2.5 | Species | Hospitalizations | Case crossover |
| Carbon | Birth | Bayesian | |
| Sulfate | Emergency | ||
| Metal(s) |
Fig. 1.
Depiction of literature search conducted on PubMed for epidemiological studies of particulate matter constituents and adverse health outcomes through 31 March 2015
Table 2.
Summary of current methods used to handle different challenges in studies of particulate matter (PM) constituents and adverse health outcomes
| Issue | Approach | References |
|---|---|---|
| Assigning exposure in the presence of spatial and temporal heterogeneity | ||
| Spatial variability in cohort studies | Closest or average of closest monitors | [13, 23–30] |
| Spatial models and land use regression models | [31–38] | |
| Additional monitoring campaigns | [32–38] | |
| Air quality modeling | [39–42] | |
| Multiday exposure in time series, case-crossover studies | Average exposure across days | [48, 49] |
| Distributed lag models | [50–56] | |
| Spatial misalignment in time series, case-crossover studies | Closest or average of closest monitors | [11, 47•, 48, 57–61, 65•, 66] |
| Spatial models | [63, 64] | |
| Air quality modeling | [44] | |
| Disentangling health effects of individual constituents | ||
| Confounding by total PM mass | Standard adjustment | [36, 38, 41, 65•, 67, 68] |
| Control for non-constituent PM | [51, 53] | |
| Regress out effect of PM mass | [65•, 69] | |
| Constituents modify PM mass health effect | [44, 65•, 70, 71] | |
| Bayesian hierarchical model with PM mass | [64, 72, 73] | |
| Confounding by other PM constituents | Standard adjustment | [11, 26, 30, 37, 53, 60] |
| Compare posterior distributions | [74] | |
| Variable selection | [13, 75•] | |
| Estimate joint effects | [61, 76] | |
| Estimate effects of sources | [25, 29, 42, 50, 59, 66, 77–79] | |
| Cluster days | [80, 81] | |
| Instrument-related measurement error | ||
| Concentrations below MDL | Substitute 1/2 MDL | [69, 75•, 82] |
| Inclusion criteria | [23, 25, 34, 53, 68–71, 76, 80, 83–85] | |
| Other instrument-related measurement error | Coarsen measurements using quantiles | [44, 88] |
| Air quality models | [39–42, 44] |
For methods that were used in many studies, a selection of recent studies that exemplify the issue are included
Assigning Exposure in the Presence of Spatial and Temporal Heterogeneity
Cohort, time series, and case-crossover studies rely on spatial and temporal variability in pollutant concentrations to estimate associations with adverse health outcomes. Cohort studies frequently estimate long-term exposure to pollution (e.g., over years) and compare the spatial distributions between pollutant exposures and adverse health outcomes across an area. In time series studies, temporal (e.g., day-to-day) variability is compared between the pollutant and adverse health outcome for a given area. Time-stratified case-crossover studies also compare temporal variability in the pollutant and outcome, but exposure is assigned to each individual and compared between the case day, when the outcome occurred, and control days, when the outcome did not occur. In some cases, cohort studies, and in particular birth cohort studies, use both spatial and temporal variability in the pollutant and outcome to estimate associations.
Fixed-location networks of ambient pollution monitors provide information about the spatial and temporal variability of pollutant concentrations and these data are commonly used to assign exposure in epidemiological studies; however, as noted above, ambient monitors that measure PM constituent concentrations are frequently spatially sparse. For example, the EPA Chemical Speciation Network (CSN) is a network of approximately 250 sites across the entire USA with only a small number (generally ≤3) of monitors in each urban area, compared with over 2000 monitors across the USA for PM2.5 [11, 14]. In addition, ambient monitoring of PM constituents generally is only conducted every third or sixth day [10, 14]. It is challenging to estimate the true spatial and temporal variation of PM constituent concentrations using the available monitoring data. In this section, we discuss the various ways epidemiological studies have assigned exposure to ambient PM constituents, which are frequently temporally and spatially heterogeneous due to their chemical and physical properties.
Cohort Studies
Because cohort and birth cohort studies rely primarily on spatial variability to estimate associations with health, predicting the spatial surface of local pollutant concentrations is important. Incorrect prediction of the spatial surface can lead to biases in estimated health effects. The spatial surface is commonly estimated using the observed pollutant concentrations at ambient monitors. Frequently, these locations do not match the locations where we observe health outcomes (e.g., residential addresses), and this difference in spatial locations between pollution data and health outcome data is referred to as spatial misalignment in the spatial statistical literature. One method for assigning exposure to PM constituents and accounting for spatial misalignment in cohort studies is to use the long-term average concentration from the closest ambient monitor within a certain distance of an individual’s residential address [13, 23–25]. Other studies of PM constituents have estimated long-term exposure using unweighted or distance-weighted average concentration of several neighboring monitors [26–29]. A comparison of these three approaches (the closest ambient monitor, the unweighted mean of closest monitors, and the weighted mean of closest monitors) found estimated health effects were mostly consistent across approaches [30]. These approaches to predict the exposure surface are both easy to understand and apply, but they often will fail to fully characterize the true concentrations of highly variable PM constituents at residential addresses.
Some cohort studies apply spatial models to ambient monitoring data to assign exposure to PM constituents. To obtain exposure at locations without monitoring data, some simple spatial models predict the full exposure surface by modeling the dependence between monitoring locations as a function of distance between monitors, possibly incorporating spatially varying covariates. Kriging methods are commonly applied spatial models that use a Gaussian process to model the correlation between monitoring locations. Instead of modeling the dependence between monitors directly, land use regression models use spatially varying covariates to predict the exposure surface at locations where covariate information is available but constituent concentrations are not. The predictors are derived using Geographic Information Systems (GIS) data and may include characteristics such as traffic intensity, population density, and land use. Many cohort studies have used spatial or land use regression models to predict exposure to PM constituents [31–38]. To characterize a pollutant’s spatial variation, data from many ambient monitors with good spatial coverage over the area of interest are usually necessary, though such data are not always available in smaller studies. Hence, spatial models and land use models cannot generally be used in single community studies unless supplemental data are collected.
While national monitoring networks are frequently used in cohort studies, some cohort studies have conducted additional measurement campaigns for ambient PM constituents. The European Study of Cohorts for Air Pollution Effects (ESCAPE) study sampled ambient pollution in 20 major European cities at 20 sites each [33, 34, 37, 38]. In the USA, the Multi-Ethnic Study of Atherosclerosis (MESA) included fixed-site ambient monitors placed in densely populated areas underrepresented by other monitoring networks as well as rotating monitors placed outside a sample of subjects’ homes [32, 35, 36]. The large number of ambient monitors allowed these studies to fit complex spatio-temporal models for PM constituents; however, these campaigns are expensive and time consuming.
Several birth cohort studies in our review used the Community Multiscale Air Quality (CMAQ) model, which simulates spatially resolved PM2.5 constituent concentrations using meteorology, state-of-the-art knowledge on atmospheric chemistry, and emission information [39–41]. One of these studies applied a spatio-temporal model using CMAQ data to identify critical exposure windows in the associations between PM2.5 constituents and congenital anomalies [39]. In a study of low birth weight, a chemical transport model was applied to estimate spatially resolved exposure to PM constituents [42]. While using modeled PM constituent concentrations provides more spatially resolved exposure information than using available ambient monitoring data alone, these simulated concentrations can be biased and need to be calibrated using ambient monitoring data within the study area [41, 43, 44].
Time Series and Case-Crossover Studies
Associations between short-term exposure to PM constituents and adverse health outcomes are frequently estimated using time series or time-stratified case-crossover methods. In these epidemiological designs, the selected exposure time frame generally ranges from same-day exposure to exposure several days preceding the outcome. Most studies in our review estimated associations with adverse health outcomes for a single day of exposure (e.g., same-day exposure), often because they did not have daily PM constituent concentrations. Previous studies of total PM mass have shown that the estimated effects on adverse health outcomes can span multiple exposure days [1, 45, 46]. Estimating PM constituent health effects corresponding to multiple days of exposure using non-daily data requires temporal imputation, which has not been previously attempted and is challenging because observed PM constituent concentrations may be substantially different than unobserved concentrations on subsequent and previous days. A non-daily sampling schedule can impact health effect estimation, possibly driven both by decreased power and by random variability across the sampled subsets of health and pollution data [47•]. When daily data are available, studies have averaged exposure over multiple days preceding the outcome of interest [48, 49]. Epidemiological studies of PM constituents with access to daily data have also estimated associations using unconstrained and constrained distributed lag models, which simultaneously estimate associations for multiple exposure days and allow estimation of cumulative effects [50–56].
Spatial variability of the exposure in time series studies is also important to consider because pollution is often measured at fixed-site ambient monitors while adverse health outcomes are aggregated across administrative regions such as cities or counties, a problem of spatial misalignment. To account for spatial misalignment between concentrations and outcomes in time series designs, most studies used either one or an average of ambient monitors to represent concentrations for an entire administrative region (e.g., [11, 47•, 57–59]). Other studies have also used population-weighted averages that utilize spatially interpolated PM2.5 constituents at a finer spatial resolution within the administrative region [48, 60, 61]. A study of pediatric asthma emergency department visits in Atlanta found estimated health effects of PM2.5 constituents did not vary substantially when the ambient average was estimated in three ways; using one designated central monitor, the unweighted average of ambient monitors, or a population-weighted average [48].
These approaches, while straightforward, will not provide a good estimate of the true ambient average for an administrative region if concentrations of the PM constituent are spatially heterogeneous and only a small number of monitors are available. Spatial heterogeneity exacerbates the spatial misalignment problem for time series studies because the true ambient average pollution concentration frequently has less temporal variability than the observed concentrations of a pollutant at a single ambient monitor. Therefore, even for spatially heterogeneous constituents with high temporal correlations across space, spatial misalignment can lead to error in the estimated ambient average concentration, which is needed when linking to aggregated health outcomes. An investigation of various sources of exposure error in time series studies found that attenuation in estimated health effects driven by spatial error was greater for EC, a spatially heterogeneous constituent, than for sulfate, a spatially homogeneous constituent [62•].
When data are available from many monitors, spatiotemporal models can be used to account for some spatial misalignment. In a study of PM2.5 constituents and hospital admissions in 20 US counties, spatial models were used to estimate county-wide ambient concentrations and the corresponding spatial misalignment error variance for each PM2.5 constituent [63]. Another national-level US study represented constituents as time-varying proportions of PM2.5 and fitted a spatial model to PM2.5 mass to account for spatial misalignment [64]. Because these models rely solely on monitoring data, which provide limited spatial information and are not randomly placed throughout an administrative region, they may not fully capture a pollutant’s spatial variability.
In case-crossover studies, exposure to pollution is assigned to each individual. The most common approach to account for spatial misalignment between the individual’s residential address and the ambient constituent monitor is to use only residential addresses located within a certain distance of an ambient monitor [65•, 66], which will be most effective for spatially homogeneous pollutants such as sulfate. A study of myocardial infarction in New Jersey assigned exposure to PM2.5 constituents using monitor-calibrated CMAQ concentrations based on the nearest CMAQ grid-cell containing an ambient monitor [44]. CMAQ yields PM constituent concentrations with more complete spatio-temporal coverage than ambient monitors alone provide; however proper calibration of CMAQ outputs requires data from more ambient monitors than are generally available for PM constituents.
Disentangling Health Effects of Individual Constituents
Disentangling health effects corresponding to PM constituents from those corresponding to total PM mass within epidemiological studies is challenging. Previous studies have consistently demonstrated that total PM mass is associated with adverse health outcomes [1, 2, 5] and epidemiological studies of PM constituents may estimate significant health effects solely because PM constituents are correlated with total PM mass. Many studies used standard covariate adjustment for total PM mass in models of PM constituents and adverse health outcomes (e.g., [36, 38, 41, 67, 68]), though this approach can lead to large standard errors for constituents highly correlated with total PM. Instead of adjusting for total PM2.5 directly, several studies estimated associations between PM2.5 constituents and health while controlling for the leftover PM2.5 mass (total PM2.5 mass−PM2.5 constituent) [51, 53]. This approach minimizes multicollinearity between the constituent and total PM2.5, but the interpretation of health effects can be challenging since increases in the PM2.5 constituent correspond to decreases in the leftover PM2.5 fraction. Another alternative is to first isolate the PM constituent from total PM mass by adjusting for total PM mass, which yields the portion of the PM constituent uncorrelated with total PM. Then the resulting residuals can be used in health effect regression models [65•, 69], though these estimated health effects do not represent the total effect of a PM constituent on adverse health outcomes [65•]. Studies have also estimated health effects for total PM2.5 mass and determined whether the estimated health effect was modified by each PM2.5 constituent [44, 70, 71]. This approach is appealing because the results indicate whether PM2.5 toxicity increases with a higher proportion of a particular constituent, but it does not allow direct estimation of constituent health effects. Mostofsky et al. [65•] discussed the relative advantages and disadvantages of approaches for controlling for total PM mass in epidemiological studies of PM constituents.
Other epidemiological studies have used Bayesian models to account for the relationships between PM constituents and total PM mass. One approach is to estimate community-level health effects of total PM and then apply a Bayesian hierarchical model to determine whether community- and season-specific fractions of PM2.5 constituents explain variability in estimated health effects of PM [72]. Similarly, fully Bayesian hierarchical models can be developed to allow long-term average PM2.5 constituent concentrations to impact the associations between PM2.5 and adverse health outcomes [73]. Constituent health effects can also be modeled simultaneously by representing each as a time-varying proportion of total PM2.5 mass in a Bayesian hierarchical model [64]. Though these Bayesian models require a considerable number of ambient monitors, these models leverage the spatially and temporally resolved PM2.5 ambient monitoring data in their analysis of PM2.5 constituents.
Similar to the issues for accounting for PM2.5 mass, many studies have simultaneously included multiple constituents in health effect regression models in an attempt to disentangle the health effect of one PM constituent from other constituents (e.g., [11, 26, 30, 37, 53, 60). However, there is substantial correlation between PM constituents, driven by shared sources and meteorology, and simultaneously estimating their health effects can lead to multicollinearity and unstable estimates of association. Because of this multicollinearity, health effects may be observed for a non-toxic PM constituent in single pollutant models when another unobserved, but correlated, constituent is truly toxic. A study of 119 US counties compared the estimated health effect magnitudes between PM2.5 constituents by comparing the posterior probabilities that one constituent’s effect exceeded another using a multivariate Bayesian hierarchical model [74]. While this approach helps to disentangle the health effects of PM2.5 constituents, it does not directly address multicollinearity because the approach still fits multivariable regression models to each county. Other studies used variable selection procedures to determine the PM constituents most associated with health. Forward selection in a multipollutant model has been applied to determine the PM2.5 constituents that were most associated with mortality [13]. Spatial variable selection models can be used to simultaneously estimate health effects of multiple PM2.5 constituents while imposing some regularity constraints to account for multicollinearity [75•]. In variable selection models, only those constituents that best predict the outcome are included in the final model. In this strategy, the selection of constituents may be driven by those that are best measured.
Instead of estimating health effects of individual PM constituents, some studies estimated health effects of groups of PM constituents. To estimate joint effects of PM constituents, two studies first estimated individual effects in a multivariate regression model and then summed the resulting estimated coefficients [61] or applied a second-stage regression model [76]. Many studies estimated associations between exposure to PM sources, which represent groups of constituents, and adverse health outcomes (e.g., [25, 29, 42, 50, 59, 66, 77, 78]). In most cases, sources are not directly measured and must be inferred from PM chemical constituent concentrations using source apportionment models [25, 29, 77, 78]. A simpler alternative to source apportionment modeling is to recreate sources of PM (e.g., soil dust) using a linear function of constituents associated with that source [79]. Other studies have used k-means clustering [80] and Bayesian modeling [81] to cluster study days based on properties of PM, including chemical composition, and determined whether the association between PM2.5 and health varied by cluster. Estimating health effects for groups of PM constituents reduces multicollinearity at the expense of not differentiating the effects of one constituent from other constituents.
Instrument-Related Measurement Error
PM can consist of over 50 chemical constituents and many of these constituents, such as transition metals, contribute minimally to PM2.5 by mass, but may be associated with adverse health outcomes [8, 23, 26]. These constituents may be more prone to instrument-related measurement error than major ambient pollutants in part because they frequently have low concentrations in ambient air, and depending on the sensitivity of the specific measurement methods used, their concentrations can often be below the MDL [16–18]. In our literature review, most studies did not mention how data below the MDL were treated. In several studies, concentrations below the MDL were replaced by half the MDL, a common practice in environmental studies [69, 75•, 82]. A study of hourly PM2.5 constituent concentrations in Seoul, South Korea found that estimated associations between PM2.5 constituents and mortality did not vary substantially between using the raw data below the MDL, substituting 1/2 MDL, and omitting values below the MDL, though the percentage below the MDL varied across constituents from 0.0 % (OC) to 43.4 % (sodium) [82]. In studies of constituents with a larger percentage of concentrations below the MDL or studies of daily concentrations, the estimated health effects may be more sensitive to the method used to adjust data below the MDL. A large number of studies included only those constituents with few observations below the MDL [23, 25, 34, 53, 68–71, 76, 80, 83–85], though the proportion allowed varied between studies. Development of imputation approaches [86] or incorporating modeled estimates from dispersion or emissions models may aid health effect estimation for constituents with concentrations below the MDL.
Measurement error in PM2.5 constituent data from ambient monitors can also be driven by the sampling method, the amount of humidity in the air during measurements, loss of volatile particles, electrostatic charge, deposition of additional particles on the filter before or after sampling, or other artifacts [87]. Studies of PM2.5 constituents have minimized measurement error by using only concentration quantiles [44, 88]. Air quality models including CMAQ and chemical transport models do not suffer from the same sources of measurement error as monitoring data because they simulate pollutant concentrations instead of measuring them directly in the air [40–42, 44]. However, these models still rely on monitoring data for bias calibration, and thus accurate and precise monitoring data remain a key part of exposure assessment.
Discussion
In this review, we identified three challenges present in epidemiological studies of PM constituents and adverse health outcomes, including assigning exposure in the presence of spatial and temporal heterogeneity, disentangling health effects of individual constituents, and overcoming instrument-related measurement error. One additional consideration is the development and application of statistical methods that address several of these issues simultaneously. As an example, some PM constituents are emitted by the same, locally generated source (e.g., EC and OC from traffic emissions) and this can drive both the spatio-temporal heterogeneity and the correlation between the PM constituents. To address spatial heterogeneity for multiple PM2.5 constituents, spatial models can be combined with a Bayesian hierarchical model that allows PM2.5 constituents to impact the associations between total PM2.5 mass and mortality [73]. Using a spatial model to estimate PM constituents can introduce measurement error in health effect models and a bootstrap-based measurement error correction model can be used to correct some of this error [31]. If the exposure lag most associated with a health outcome varies between PM constituents, which are not measured daily, it is unclear how to estimate associations in multipollutant models [29]. Additional statistical methods are still needed to address other combinations of these challenges.
When comparing estimated health effects from single pollutant models across constituents, differential measurement error between constituents (driven by differences in spatial heterogeneity, sampling method errors, etc.) can lead to observed associations only for the pollutant measured with smaller error [62•]. A common approach in studies of multiple PM constituents is to estimate their respective health effects in multipollutant models. However, in the case of differential measurement error, the health effects can be transferred from the truly toxic pollutant to one that is correlated with the toxic pollutant, but measured with lower error [21, 89]. Few epidemiological studies of multiple PM constituents and health have incorporated methods for handling measurement error, though methods have been developed for and applied to ambient pollutants [90–92].
We focused on statistical challenges specific to epidemiological studies of PM constituents and health, though previous studies of ambient pollutants have discussed exposure estimation [62•, 93–95], confounding [96], measurement error [91, 92, 97, 98], and spatio-temporal modeling [99–101]. Epidemiological studies of total PM mass are often not as sensitive as constituency-based studies to the issues we outline above. While PM2.5 can also be spatially and temporally heterogeneous [8], there are more total PM2.5 mass monitors than constituent monitors in most communities and PM2.5 monitors often collect daily data [14]. PM monitoring data with sufficient spatio-temporal coverage can be used to calibrate air quality models like CMAQ for use in subsequent health effect studies [101, 102]. Epidemiological studies of total PM often control for other pollutants such as ozone, sulfur dioxide, and nitrogen dioxide [4, 103], but the correlation between PM and these pollutants may not be as large as correlations found in studies of PM constituents. Last, because total PM concentrations are by definition larger than concentrations of PM constituents, total PM may be less prone to some sources of measurement error, such as concentrations below the MDL.
Time series, case-crossover and cohort studies of PM constituents and health are necessary for estimating population-level health effects that can guide regulation. This review discusses important considerations for such studies, but we acknowledge that other types of studies are needed to fully understand the pathway from pollution exposure to mortality and morbidity. Panel studies generally follow a relatively small number of individuals over time, and while less generalizable than large population-based studies, these studies often conduct better exposure measurement and provide an opportunity to assess air pollution health associations under specific contexts [104–107]. Occupational studies measure workplace pollutants and therefore have better exposure measurement for occupational exposures than many population-based studies have for ambient exposures [108, 109]. However, depending on the occupation, occupational exposures to specific PM constituents can exceed those experienced by the general population and these studies are primarily focused on indoor and not ambient pollution. Natural experiments, such as the reduction in ambient pollution levels in Beijing, China during the 2008 Olympics, can provide a unique opportunity to characterize health effects associated with a specific reduction in pollution, but may not be as useful to characterize short-term health effects associated with typical day-to-day variability [105]. Other experimental study designs, such as controlled exposure studies, toxicological studies, and animal studies, are also necessary to determine biological mechanisms and infer causal associations. Epidemiological studies are critical to fully characterize ambient air pollution health effects in the general population and should continue to be used, along with other study designs, to better understand how PM constituents impact human health.
Conclusions
Recent epidemiological studies of PM constituents and health have used a variety of statistical methods to assign exposure in the presence of spatial and temporal heterogeneity, disentangle health effects of individual constituents, and address instrument-related measurement error. Spatial and temporal heterogeneity in PM constituent concentrations has been estimated by spatio-temporal models and more recently by monitor-calibrated air quality model simulations. Commonly multipollutant models of PM constituents include adjustment for total PM mass or other PM constituents, but more advanced statistical techniques, such as source apportionment modeling, have also been applied to estimate health effects corresponding to multiple correlated constituents. Instrument-related and model-based measurement errors have not been extensively investigated in epidemiological studies of PM constituents and health and additional methods may need to be developed. Methods that simultaneously address several issues presented by PM constituent data are critical for future epidemiological studies of PM constituents and health and remain an active area of statistical and epidemiological research.
Acknowledgments
This publication was made possible by US EPA grant R834799 (HHC, LAW, and SES), NIEHS grants T32 ES012160 (LAW and JRK), 5R21ES022795-02 (HHC and SES), R01ES019560 (RDP), and Electric Power Research Institute grants EP-P45572/C19698 (SES) and 10002467 (HHC and SES) This publication’s contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA, NIH, or NIEHS. Further, US EPA, NIH, and NIEHS do not endorse the purchase of any commercial products or services mentioned in the publication.
Roger D. Peng serves on the Health Review Committee of the Health Effects Institute, a non-profit organization that funds air pollution and health research.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Jenna R. Krall, Howard H. Chang, Stefanie Ebelt Sarnat, and Lance A. Waller declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Jenna R. Krall, Email: jenna.krall@emory.edu.
Howard H. Chang, Email: howard.chang@emory.edu.
Stefanie Ebelt Sarnat, Email: sebelt@emory.edu.
Roger D. Peng, Email: rdpeng@jhu.edu.
Lance A. Waller, Email: lwaller@emory.edu.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. J Am Med Assoc. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pope CA, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. J Am Med Assoc. 2002;287(9):1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng RD, Chang HH, Bell ML, et al. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among medicare patients. J Am Med Assoc. 2008;299(18):2172–2179. doi: 10.1001/jama.299.18.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng RD, Dominici F, Pastor-Barriuso R, et al. Seasonal analyses of air pollution and mortality in 100 US cities. Am J Epidemiol. 2005;161(6):585–594. doi: 10.1093/aje/kwi075. [DOI] [PubMed] [Google Scholar]
- 5.Stafoggia M, Samoli E, Alessandrini E, et al. Short-term associations between fine and coarse particulate matter and hospitalizations in Southern Europe: results from the MED-PARTICLES project. Environ Health Perspect. 2013;121(9):1026–1033. doi: 10.1289/ehp.1206151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsouyanni K, Touloumi G, Spix C, et al. Short term effects of ambient sulphur dioxide and particulate matter on mortality in 12 European cities: results from time series data from the APHEA project. Br Med J. 1997;314(7095):1658–1663. doi: 10.1136/bmj.314.7095.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong C-M, Vichit-Vadakan N, Kan H, et al. Public health and air pollution in Asia (PAPA): a multicity study of short-term effects of air pollution on mortality. Environ Health Perspect. 2008;116(9):1195–1202. doi: 10.1289/ehp.11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell ML, Dominici F, Ebisu K, et al. Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies. Environ Health Perspect. 2007;115(7):989–995. doi: 10.1289/ehp.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect. 2009;117(6):898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng RD, Bell ML, Geyh AS, et al. Emergency admissions for cardiovascular and respiratory diseases and the chemical composition of fine particle air pollution. Environ Health Perspect. 2009;117(6):957–963. doi: 10.1289/ehp.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krall JR, Anderson GB, Dominici F, et al. Short-term exposure to particulate matter constituents and mortality in a national study of US urban communities. Environ Health Perspect. 2013;121(10):1148–1153. doi: 10.1289/ehp.1206185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darrow LA, Klein M, Flanders WD, et al. Ambient air pollution and preterm birth: a time-series analysis. Epidemiology. 2009;20(5):689–698. doi: 10.1097/EDE.0b013e3181a7128f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostro B, Lipsett M, Reynolds P, et al. Long-term exposure to constituents of fine particulate air pollution and mortality: results from the California Teachers Study. Environ Health Perspect. 2010;118(3):363–369. doi: 10.1289/ehp.0901181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Environmental Protection Agency. Integrated science assessment for particulate matter. 2009 [PubMed] [Google Scholar]
- 15.Bell ML, Ebisu K, Peng RD. Community-level spatial heterogeneity of chemical constituent levels of fine particulates and implications for epidemiological research. J Expo Sci Environ Epidemiol. 2011;21(4):372–384. doi: 10.1038/jes.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polissar AV, Hopke PK, Poirot RL. Atmospheric aerosol over Vermont: chemical composition and sources. Environ Sci Technol. 2001;35(23):4604–4621. doi: 10.1021/es0105865. [DOI] [PubMed] [Google Scholar]
- 17.Kim E, Hopke PK, Paatero P, et al. Incorporation of parametric factors into multilinear receptor model studies of Atlanta aerosol. Atmos Environ. 2003;37(26):5009–5021. [Google Scholar]
- 18.Maykut NN, Lewtas J, Kim E, et al. Source apportionment of PM2.5 at an urban IMPROVE site in Seattle, Washington. Environ Sci Technol. 2003;37(22):5135–5142. doi: 10.1021/es030370y. [DOI] [PubMed] [Google Scholar]
- 19.Lipfert FW, Wyzga RE. Statistical considerations in determining the health significance of constituents of airborne particulate matter. J Air Waste Manage Assoc. 1999;49(9):182–191. doi: 10.1080/10473289.1999.10463896. [DOI] [PubMed] [Google Scholar]
- 20.Dominici F, Peng RD, Barr CD, et al. Protecting human health from air pollution: shifting from a single-pollutant to a multipollutant approach. Epidemiology. 2010;21(2):187–194. doi: 10.1097/EDE.0b013e3181cc86e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohr AC, Wyzga RE. Attributing health effects to individual particulate matter constituents. Atmos Environ. 2012;62:130–152. [Google Scholar]
- 22.Atkinson RW, Mills IC, Walton HA, et al. Fine particle components and health—a systematic review and meta-analysis of epidemiological time series studies of daily mortality and hospital admissions. J Expo Sci Environ Epidemiol. 2014;25(2):208–214. doi: 10.1038/jes.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basu R, Harris M, Sie L, et al. Effects of fine particulate matter and its constituents on low birth weight among full-term infants in California. Environ Res. 2014;128:42–51. doi: 10.1016/j.envres.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahimou B, Salihu HM, Aliyu MH, et al. Risk of preeclampsia from exposure to particulate matter (PM2.5) speciation chemicals during pregnancy. J Occup Environ Med. 2014;56(2):1228–1234. doi: 10.1097/JOM.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 25.Pereira G, Bell ML, Lee HJ, et al. Sources of fine particulate matter and risk of preterm birth in Connecticut, 2000–2006: a longitudinal study. Environ Health Perspect. 2014;122(10):1117–1122. doi: 10.1289/ehp.1307741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebisu K, Bell ML. Airborne PM2.5 chemical components and low birth weight in the northeastern and mid-atlantic regions of the United States. Environ Health Perspect. 2012;120(12):1746–1752. doi: 10.1289/ehp.1104763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilhelm M, Ghosh JK, Su J, et al. Traffic-related air toxics and preterm birth: a population-based case-control study in Los Angeles county, California. Environ Heal. 2011;10:89. doi: 10.1186/1476-069X-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilhelm M, Ghosh JK, Su J, et al. Traffic-related air toxics and term low birth weight in Los Angeles county, California. Environ Health Perspect. 2012;120(1):132–138. doi: 10.1289/ehp.1103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lippmann M, Chen L-C, Gordon T, et al. Research report 171. Boston: Health Effects Institute; 2013. National particle component toxicity (NPACT) initiative: integrated epidemiologic and toxicologic studies of the health effects of particulate matter components. [PubMed] [Google Scholar]
- 30.Sun M, Kaufman JD, Kim S-Y, et al. Particulate matter components and subclinical atherosclerosis: common approaches to estimating exposure in a Multi-Ethnic Study of Atherosclerosis cross-sectional study. Environ Heal. 2013;12:39. doi: 10.1186/1476-069X-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergen S, Sheppard L, Sampson PD, et al. A national prediction model for PM2.5 component exposures and measurement error-corrected health effect inference. Environ Health Perspect. 2013;121(9):1017–1025. doi: 10.1289/ehp.1206010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vedal S, Kim S-Y, Miller K, et al. Research Report 178. Boston: Health Effects Institute; 2013. Section I. NPACT epidemiologic study of components of fine particulate matter and cardiovascular disease in the MESA and WHI-OS cohorts. [Google Scholar]
- 33.Wang M, Beelen R, Stafoggia M, et al. Long-term exposure to elemental constituents of particulate matter and cardiovascular mortality in 19 European cohorts: results from the ESCAPE and TRANSPHORM projects. Environ Int. 2014;66:97–106. doi: 10.1016/j.envint.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 34.Fuertes E, MacIntyre E, Agius R, et al. Associations between particulate matter elements and early-life pneumonia in seven birth cohorts: results from the ESCAPE and TRANSPHORM projects. Int J Hyg Environ Health. 2014;217(8):819–829. doi: 10.1016/j.ijheh.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Adar SD, D’Souza J, Mendelsohn-Victor K, et al. Markers of inflammation and coagulation after long-term exposure to coarse particulate matter: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis. Environ Health Perspect. 2015;123(6):541–548. doi: 10.1289/ehp.1308069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S-Y, Sheppard L, Kaufman JD, et al. Individual-level concentrations of fine particulate matter chemical components and subclinical atherosclerosis: a cross-sectional analysis based on 2 advanced exposure prediction models in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2014;180(7):718–728. doi: 10.1093/aje/kwu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beelen R, Hoek G, Raachou-Nielsen O, et al. Natural cause mortality and long-term exposure to particle components: an analysis of 19 European cohorts within the multi-center ESCAPE project. Environ Health Perspect. 2015;123(6):525–533. doi: 10.1289/ehp.1408095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eeftens M, Hoek G, Gruzieva O, et al. Elemental composition of particulate matter and the association with lung function. Epidemiology. 2014;25(5):648–657. doi: 10.1097/EDE.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 39.Warren J, Fuentes M, Herring A, et al. Bayesian spatial–temporal model for cardiac congenital anomalies and ambient air pollution risk assessment. Environmetrics. 2012;23(8):673–684. doi: 10.1002/env.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robledo CA, Mendola P, Yeung E, et al. Preconception and early pregnancy air pollution exposures and risk of gestational diabetes mellitus. Environ Res. 2015;137:316–322. doi: 10.1016/j.envres.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Männistö T, Mendola P, Liu D, et al. Acute air pollution exposure and blood pressure at delivery among women with and without hypertension. Am J Hypertens. 2015;28(1):58–72. doi: 10.1093/ajh/hpu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laurent O, Hu J, Li L, et al. Sources and contents of air pollution affecting term low birth weight in Los Angeles county, California, 2001–2008. Environ Res. 2014;134:488–495. doi: 10.1016/j.envres.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Appel KW, Bhave PV, Gilliland AB, et al. Evaluation of the community multiscale air quality (cMAQ) model version 4.5 sensitivities impacting model performance; part ii-particulate matter. Atmos Environ. 2008;42(24):6057–6066. [Google Scholar]
- 44.Rich DQ, Ozkaynak H, Crooks J, et al. The triggering of myocardial infarction by fine particles is enhanced when particles are enriched in secondary species. Environmental Science & Technology. 2013;47(16):9414–9423. doi: 10.1021/es4027248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz J. The distributed lag between air pollution and daily deaths. Epidemiology. 2000;11(3):320–326. doi: 10.1097/00001648-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 46.Ostro B, Broadwin R, Green S, et al. Fine particulate air pollution and mortality in nine California counties: results from CALFINE. Environ Health Perspect. 2006;114(1):29–33. doi: 10.1289/ehp.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim S-Y, Sheppard L, Hannigan MP, et al. The sensitivity of health effect estimates from time-series studies to fine particulate matter component sampling schedule. J Expo Sci Environ Epidemiol. 2013;23(5):481–486. doi: 10.1038/jes.2013.28. The authors imposed multiple non-daily sampling schedules on available daily PM2.5 constituent concentrations and then assessed the associations between PM2.5 constituents and hospital admissions. They found the power to detect associations was decreased for non-daily sampling schedules and that differences in random variability between sampled datasets could lead to different inferences about associations between PM2.5 constituents and health.
- 48.Strickland MJ, Darrow LA, Mulholland JA, et al. Implications of different approaches for characterizing ambient air pollutant concentrations within the urban airshed for time-series studies and health benefits analyses. Environ Heal. 2011;10:36. doi: 10.1186/1476-069X-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinclair AH, Tolsma D. Associations and lags between air pollution and acute respiratory visits in an ambulatory care setting: 25-month results from the aerosol research and inhalation epidemiological study. J Air Waste Manage Assoc. 2004;54(9):1212–1218. doi: 10.1080/10473289.2004.10470979. [DOI] [PubMed] [Google Scholar]
- 50.Lall R, Ito K, Thurston GD. Distributed lag analyses of daily hospital admissions and source-apportioned fine particle air pollution. Environ Health Perspect. 2010;119(4):455–460. doi: 10.1289/ehp.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winquist A, Schauer JJ, Turner JR, et al. Impact of ambient fine particulate matter carbon measurement methods on observed associations with acute cardiorespiratory morbidity. J Expo Sci Environ Epidemiol. 2015;25(2):215–221. doi: 10.1038/jes.2014.55. [DOI] [PubMed] [Google Scholar]
- 52.Cakmak S, Dales RE, Rubio MA, et al. The risk of dying on days of higher air pollution among the socially disadvantaged elderly. Environ Res. 2011;111(3):388–393. doi: 10.1016/j.envres.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Sarnat SE, Winquist A, Schauer JJ, et al. Fine particulate matter components and emergency department visits for cardiovascular and respiratory diseases in the St. Louis, Missouri–Illinois, metropolitan area. Environ Health Perspect. 2015;123(5):437–444. doi: 10.1289/ehp.1307776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim S-Y, Peel JL, Hannigan MP, et al. The temporal lag structure of short-term associations of fine particulate matter chemical constituents and cardiovascular and respiratory hospitalizations. Environ Health Perspect. 2012;120(8):1094–1099. doi: 10.1289/ehp.1104721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou J, Ito K, Lall R, et al. Time-series analysis of mortality effects of fine particulate matter components in Detroit and Seattle. Environ Health Perspect. 2011;119(4):461–466. doi: 10.1289/ehp.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang W, Cao J, Tao Y, et al. Seasonal variation of chemical species associated with short-term mortality effects of PM2.5 in Xi’an, a central city in China. Am J Epidemiol. 2012;175(6):556–566. doi: 10.1093/aje/kwr342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Metzger KB, Tolbert PE, Klein M, et al. Ambient air pollution and cardiovascular emergency department visits. Epidemiology. 2004;15(1):46–56. doi: 10.1097/01.EDE.0000101748.28283.97. [DOI] [PubMed] [Google Scholar]
- 58.Lipfert FW, Morris SC, Wyzga RE. Daily mortality in the Philadelphia metropolitan area and size-classified particulate matter. J Air Waste Manage Assoc. 2000;50(8):1501–1513. doi: 10.1080/10473289.2000.10464185. [DOI] [PubMed] [Google Scholar]
- 59.Pun VC, Yu IT-s, Ho K-f, et al. Differential effects of source-specific particulate matter on emergency hospitalizations for ischemic heart disease in Hong Kong. Environ Health Perspect. 2014;122(4):391–396. doi: 10.1289/ehp.1307213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Darrow LA, Klein M, Flanders WD, et al. Air pollution and acute respiratory infections among children 0–4 years of age: an 18-year time-series study. Am J Epidemiol. 2014;180(10):968–977. doi: 10.1093/aje/kwu234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winquist A, Kirrane E, Klein M, et al. Joint effects of ambient air pollutants on pediatric asthma emergency department visits in Atlanta, 1998–2004. Epidemiology. 2014;25(5):666–673. doi: 10.1097/EDE.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dionisio KL, Baxter LK, Chang HH. An empirical assessment of exposure measurement error and effect attenuation in bipollutant epidemiologic models. Environ Health Perspect. 2014;122(11):1216–1224. doi: 10.1289/ehp.1307772. This study estimated the effects of spatial measurement error for two PM2.5 constituents using a combination of air quality modeling and ambient monitoring and found the resulting attenuation in estimated health effects was greater for elemental carbon, a spatially heterogeneous pollutant, than sulfate, which is relatively spatially homogeneous.
- 63.Peng RD, Bell ML. Spatial misalignment in time series studies of air pollution and health data. Biostatistics. 2010;11(4):720–740. doi: 10.1093/biostatistics/kxq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuentes M, Song H-R, Ghosh SK, et al. Spatial association between speciated fine particles and mortality. Biometrics. 2006;62(3):855–863. doi: 10.1111/j.1541-0420.2006.00526.x. [DOI] [PubMed] [Google Scholar]
- 65. Mostofsky E, Schwartz J, Coull BA, et al. Modeling the association between particle constituents of air pollution and health outcomes. Am J Epidemiol. 2012;176(4):317–326. doi: 10.1093/aje/kws018. This study reviewed methods to adjust for total PM mass in epidemiologic studies of PM constituents and adverse health outcomes. While each method has advantages and disadvantages in interpretation, the authors did not find that the estimated relative toxicity of PM2.5 constituents varied substantially between methods to adjust for total PM2.5 mass in a study of ischemic stroke in Boston, MA.
- 66.Kioumourtzoglou M-A, Coull BA, Dominici F, et al. The impact of source contribution uncertainty on the effects of source-specific PM2.5 on hospital admissions: a case study in Boston, MA. J Expo Sci Environ Epidemiol. 2014;24(4):365–371. doi: 10.1038/jes.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heal MR, Elton RA, Hibbs LR, et al. A time-series study of the health effects of water-soluble and total-extractable metal content of airborne particulate matter. Occup Environ Med. 2009;66(9):636–638. doi: 10.1136/oem.2008.045310. [DOI] [PubMed] [Google Scholar]
- 68.Pun VC, Yu IT-S, Qiu H, et al. Short-term associations of cause-specific emergency hospitalizations and particulate matter chemical components in Hong Kong. Am J Epidemiol. 2014;179(9):1086–1095. doi: 10.1093/aje/kwu026. [DOI] [PubMed] [Google Scholar]
- 69.Basagaña X, Jacquemin B, Karanasiou A, et al. Short-term effects of particulate matter constituents on daily hospitalizations and mortality in five South-European cities results from the MED-PARTICLES project. Environ Int. 2015;75:151–158. doi: 10.1016/j.envint.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 70.Dai L, Zanobetti A, Koutrakis P, et al. Associations of fine particulate matter species with mortality in the United States: a multicity time-series analysis. Environ Health Perspect. 2014;122(8):837–842. doi: 10.1289/ehp.1307568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valdés A, Zanobetti A, Halonen JI, et al. Elemental concentrations of ambient particles and cause specific mortality in Santiago, Chile: a time series study. Environ Heal. 2012;11:82. doi: 10.1186/1476-069X-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bell ML. Research report 161. Boston: Health effects Institute; 2012. Assessment of the health impacts of particulate matter characteristics. [PubMed] [Google Scholar]
- 73.Chung Y, Dominici F, Wang Y, et al. Associations between long-term exposure to chemical constituents of fine particulate matter (PM2.5) and mortality in Medicare enrollees in the eastern United States. Environ Health Perspect. 2015;123(5):467–474. doi: 10.1289/ehp.1307549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levy JI, Diez D, Dou Y, et al. A meta-analysis and multisite time-series analysis of the differential toxicity of major fine particulate matter constituents. Am J Epidemiol. 2012;175(11):1091–1099. doi: 10.1093/aje/kwr457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Boehm Vock LF, Reich BJ, et al. Spatial variable selection methods for investigating acute health effects of fine particulate matter components. Biometrics. 2014;71(1):167–177. doi: 10.1111/biom.12254. The authors proposed a national spatial variable selection model to simultaneously estimate associations between cardiovascular emergency room admissions and 22 PM2.5 constituents, which is a much larger number of constituents than generally analyzed in one model. This model allows estimation of associations for highly correlated pollutants by imposing regularity constraints and also permits the associations to vary over space, which may occur because of differences in population susceptibility or pollutant sources.
- 76.Kioumourtzoglou M-A, Zanobetti A, Schwartz JD, et al. The effect of primary organic particles on emergency hospital admissions among the elderly in 3 US cities. Environ Heal. 2013;12:68. doi: 10.1186/1476-069X-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bell ML, Ebisu K, Leaderer BP, et al. Associations of PM2.5 constituents and sources with hospital admissions: analysis of four counties in Connecticut and Massachusetts (USA) for persons ≥65 years of age. Environ Health Perspect. 2013;122(2):138–144. doi: 10.1289/ehp.1306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park ES, Hopke PK, Oh M-S, et al. Assessment of source-specific health effects associated with an unknown number of major sources of multiple air pollutants: a unified Bayesian approach. Biostatistics. 2014;15(3):484–497. doi: 10.1093/biostatistics/kxu004. [DOI] [PubMed] [Google Scholar]
- 79.Mar TF, Norris GA, Koenig JQ, et al. Associations between air pollution and mortality in Phoenix, 1995–1997. Environ Health Perspect. 2000;108(4):347–353. doi: 10.1289/ehp.00108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zanobetti A, Austin E, Coull BA, et al. Health effects of multipollutant profiles. Environ Int. 2014;71:13–19. doi: 10.1016/j.envint.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pirani M, Best N, Blangiardo M, et al. Analysing the health effects of simultaneous exposure to physical and chemical properties of airborne particles. Environ Int. 2015;79:56–64. doi: 10.1016/j.envint.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Son J-Y, Lee J-T, Kim K-H, et al. Characterization of fine particulate matter and associations between particulate chemical constituents and mortality in Seoul, Korea. Environ Health Perspect. 2012;120(6):872–878. doi: 10.1289/ehp.1104316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ito K, Mathes R, Ross Z, et al. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ Health Perspect. 2011;119(4):467–473. doi: 10.1289/ehp.1002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ostro B, Feng W-Y, Broadwin R, et al. The impact of components of fine particulate matter on cardiovascular mortality in susceptible subpopulations. Occup Environ Med. 2008;65(11):750–756. doi: 10.1136/oem.2007.036673. [DOI] [PubMed] [Google Scholar]
- 85.Cakmak S, Dales RE, Blanco Vida C. Components of particulate air pollution and mortality in Chile. Int J Occup Environ Health. 2009;15(2):152–158. doi: 10.1179/oeh.2009.15.2.152. [DOI] [PubMed] [Google Scholar]
- 86.Krall JR, Simpson CH, Peng RD. A model-based approach for imputing censored data in source apportionment studies. Environ Ecol Stat. 2015 doi: 10.1007/s10651-015-0319-6. (to appear) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Environmental Protection Agency. Particulate matter (PM2.5) speciation guidance document. 1999 [Google Scholar]
- 88.Hirshon JM, Shardell M, Alles S, et al. Elevated ambient air zinc increases pediatric asthma morbidity. Environ Health Perspect. 2008;116(6):826–831. doi: 10.1289/ehp.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zidek JV, Wong H, Le ND, et al. Causality, measurement error and multicollinearity in epidemiology. Environmetrics. 1996;7(4):441–451. [Google Scholar]
- 90.Schwartz J, Coull BA. Control for confounding in the presence of measurement error in hierarchical models. Biostatistics. 2003;4(4):539–553. doi: 10.1093/biostatistics/4.4.539. [DOI] [PubMed] [Google Scholar]
- 91.Gryparis A, Paciorek CJ, Zeka A, et al. Measurement error caused by spatial misalignment in environmental epidemiology. Biostatistics. 2009;10(2):258–274. doi: 10.1093/biostatistics/kxn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang HH, Peng RD, Dominici F. Estimating the acute health effects of coarse particulate matter accounting for exposure measurement error. Biostatistics. 2011;12(4):637–652. doi: 10.1093/biostatistics/kxr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Özkaynak H, Baxter LK, Dionisio KL, et al. Air pollution exposure prediction approaches used in air pollution epidemiology studies. J Expo Sci Environ Epidemiol. 2013;23:566–572. doi: 10.1038/jes.2013.15. [DOI] [PubMed] [Google Scholar]
- 94.Chang HH, Warren JL, Darrow LA, et al. Assessment of critical exposure and outcome windows in time-to-event analysis with application to air pollution and preterm birth study. Biostatistics, advance publication. 2015 doi: 10.1093/biostatistics/kxu060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fuentes M. Statistical issues in health impact assessment at the state and local levels. Air Qual Atmos Health. 2009;2(1):47–55. doi: 10.1007/s11869-009-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eftim S, Dominici F. Multisite time-series studies versus cohort studies: methods, findings, and policy implications. J Toxicol Environ Health A: Curr Issues. 2005;68(13–14):1191–1205. doi: 10.1080/15287390590936076. [DOI] [PubMed] [Google Scholar]
- 97.Zeger SL, Thomas D, Dominici F, et al. Exposure measurement error in time-series studies of air pollution concepts and consequences. Environ Health Perspect. 2000;108(5):419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Szpiro AA, Paciorek CJ, Sheppard L. Does more accurate exposure prediction necessarily improve health effect estimates? Epidemiology. 2011;22(5):680–685. doi: 10.1097/EDE.0b013e3182254cc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Puett RC, Hart JE, Yanosky JD, et al. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ Health Study. Environ Health Perspect. 2009;117(11):1697–1701. doi: 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Warren J, Fuentes M, Herring A, et al. Spatio-temporal modeling of the association between air pollution exposure and preterm birth: Identifying critical windows of exposure. Biometrics. 2012;68(4):1157–1167. doi: 10.1111/j.1541-0420.2012.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi J, Fuentes M, Reich BJ. Spatial–temporal association between fine particulate matter and daily mortality. Comput Stat Data Anal. 2009;53(8):2989–3000. doi: 10.1016/j.csda.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chang HH, Reich BJ, Miranda ML. Time-to-event analysis of fine particle air pollution and preterm birth: results from North Carolina, 2001–2005. Am J Epidemiol. 2012;175(2):91–98. doi: 10.1093/aje/kwr403. [DOI] [PubMed] [Google Scholar]
- 103.Tolbert PE, Klein M, Peel JL, et al. Multipollutant modeling issues in a study of ambient air quality and emergency department visits in Atlanta. J Expo Sci Environ Epidemiol. 2007;17:S29–S35. doi: 10.1038/sj.jes.7500625. [DOI] [PubMed] [Google Scholar]
- 104.Lagorio S, Forastiere F, Pistelli R, et al. Air pollution and lung function among susceptible adult subjects: a panel study. Environ Heal. 2006;5:11. doi: 10.1186/1476-069X-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang J, Zhu T, Kipen H, et al. Research report 174. Boston: Health Effects Institute; 2013. Cardiorespiratory biomarker responses in healthy young adults to drastic air quality changes surrounding the 2008 Beijing olympics. [PMC free article] [PubMed] [Google Scholar]
- 106.Strak M, Janssen NA, Godri KJ, et al. Respiratory health effects of airborne particulate matter the role of particle size, composition, and oxidative potential-The RAPTES Project. Environ Health Perspect. 2012;120(8):1183–1189. doi: 10.1289/ehp.1104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cakmak S, Dales R, Kauri LM, et al. Metal composition of fine particulate air pollution and acute changes in cardiorespiratory physiology. Environ Pollut. 2014;189:208–214. doi: 10.1016/j.envpol.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 108.Neophytou AM, Hart JE, Cavallari JM, et al. Traffic-related exposures and biomarkers of systemic inflammation, endothelial activation and oxidative stress: a panel study in the US trucking industry. Environ Heal. 2013;12:105. doi: 10.1186/1476-069X-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hou L, Zhang X, Zheng Y, et al. Altered methylation in tandem repeat element and elemental component levels in inhalable air particles. Environ Mol Mutagen. 2014;55(3):256–265. doi: 10.1002/em.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]