Abstract
Background
Transfusion-related acute lung injury incidence remains the leading cause of posttransfusion mortality. The etiology may be related to leukocyte antibodies or biologically active compounds in transfused plasma, injuring susceptible recipient's lungs. We have hypothesized that transfusion could have less severe effects that are not always appreciated clinically, and have shown subtly decreased pulmonary oxygen gas transfer in healthy volunteers after transfusion of fresh and 21-day stored erythrocytes. Here we tested the same hypothesis in surgical patients.
Methods
Ninety-one patients undergoing elective major spine surgery with anticipated need for erythrocyte transfusion were randomly allocated to receive their first transfusion of erythrocytes as cell salvage (CS), washed stored, or unwashed stored. Clinicians were not blinded to group assignment. Pulmonary gas transfer and mechanics were measured 5 min before and 30 min after erythrocyte transfusion.
Results
The primary outcome variable, gas transfer, as assessed by change of PaO2/FIO2, with erythrocyte transfusion was not significant in any group: (CS: 9 ± 59, mean ± SD; washed, 10 ± 26; unwashed 15 ± 1), and did not differ among groups (P = 0.92). Pulmonary dead space (VD/VT) decreased with CS transfusion (−0.01 ± 0.04; P = 0.034), but did not change with other erythrocytes; the change from before to after erythrocyte transfusion did not differ among groups (−0.01 to +0.01; P = 0.28).
Conclusions
We did not find impaired gas exchange as assessed by PaO2/FIO2 with transfused erythrocytes that did or did not contain nonautologous plasma. This clinical trial did not support the hypothesis of erythrocyte transfusion-induced gas-exchange deficit that had been found in healthy volunteers.
Introduction
Since its original description,1,2 transfusion-related acute lung injury (TRALI) has been found to be the most common cause of transfusion related mortality.3 Recent mitigation efforts, such as the use of plasma from predominantly male donors, appear to have decreased the incidence of TRALI,4 although in 2013 TRALI continued to represent the largest single cause of transfusion related mortality reported to the Food and Drug Adminsitration.3
TRALI is defined as new acute lung injury (ALI) that develops during or within 6 hours of transfusion with no temporal relationship to an alternative risk factor of ALI.5 , The definition of ALI requires impaired gas exchange defined as a PaO2/FIO2 ratio of ≤ 300 mm Hg. The etiology of TRALI is thought to be related to leukocyte antibodies or biologically active compounds contained in the transfused plasma, which interact with susceptible recipient leukocytes to cause lung injury.4, 6–8 We hypothesized that transfusion could have a wider range of pulmonary effects, and that the definition of TRALI identifies only the most severe injury. We have identified small, but statistically significant, decrements in pulmonary gas exchange associated with transfusion of fresh and stored autologous erythrocytes in healthy volunteers.9 Active surveillance programs have been useful in identifying cases of TRALI that might otherwise have gone unnoticed,4 but cannot detect cases of more subtle pulmonary changes with blood transfusion.
In the current study, we sought to test our hypothesis that transfusion can cause pulmonary changes less severe than that defined by TRALI, by identifying diminished gas exchange in patients receiving blood transfusions during surgery. We studied patients undergoing elective major spine surgery who were anticipated to require erythrocyte transfusion. To identify subtle changes in gas exchange and pulmonary mechanics, we evaluated pulmonary function and mechanics in surgical patients immediately before and shortly after transfusion and compared groups randomly allocated to receive as their first transfusion autologous or allogeneic erythrocytes with or without the associated plasma. Transfusion of erythrocytes without associated plasma served as a control to test whether changes, if any, are related to any substance(s) contained in plasma.
Materials and Methods
After approval by the Institutional Review Board of the University of California, San Francisco and with each patient's informed written consent, we enrolled patients 16 to 75 yr of age undergoing elective major spinal surgery at a University Hospital with expected surgical blood loss sufficient to require erythrocyte transfusion from May 2006 through April 2010.* Patients were recruited in the preoperative clinic. We excluded patients who had pulmonary disease, abnormal pulmonary function or gas exchange by history or physical examination, and pre-operative measurement of oxyhemoglobin saturation (pulse oximetry); had undergone any operative procedure within one week of study; active infection; cardiac failure (defined as New York Heart Association Class III or IV failure, e.g., symptom free only at rest or with symptoms at rest). The original protocol sought to test unwashed autologous erythrocytes against washed autologous erythrocytes (including erythrocytes salvaged from the surgical field and washed) and washed and unwashed allogeneic erythrocytes. We did not seek to examine the effect of fresh frozen plasma (FFP). However, shortly after the initiation of the trial, clinical practice changed and very few patients subsequently donated and stored their autologous blood preoperatively in advance of their surgery. Leukoreduction of erythrocyte components also became standard, and there was no opportunity to test the effect of leukoreduction. Consequently, we report here the protocol and results as it was modified, shortly after enrollment was initiated. We tested the pulmonary effects of transfusion of allogeneic unwashed erythrocytes, that included the remaining autologous plasma, against the controls of allogeneic washed erythrocytes and autologous washed erythrocytes, and thus devoid of allogeneic plasma.
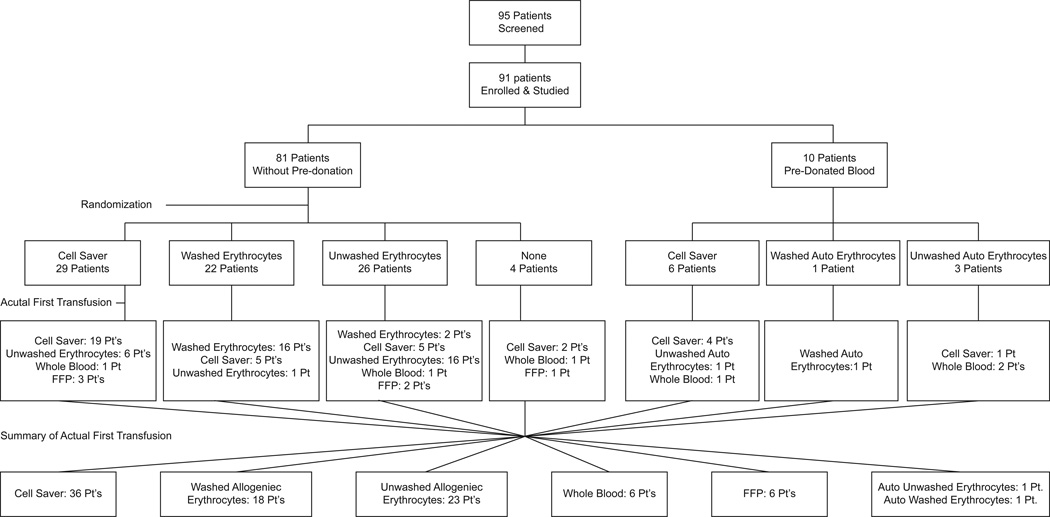
Patients were randomly allocated (computer generated, sealed opaque envelopes produced by the blinded study statistician) to receive either erythrocytes that had been salvaged from the surgical field and washed (“cell salvage,” [CS]), stored and washed allogeneic erythrocytes (washed packed erythrocytes) or unwashed stored allogeneic erythrocytes (unwashed packed erythrocytes) as their first transfusion in a 1:1:1 ratio (fig. 1). All allogeneic erythrocytes were leukoreduced. Washing and cell salvaging were performed with a Fresenius-Kabi C.A.T.S. (Continuous Auto Transfusion System) centrifugal disk system set to "quality wash mode" with a wash ratio of 7:1. Erythrocytes were first diluted with approximately 400 mL of normal saline to prevent excessive cell lysis, then processed and washed with about 1,200 mL of saline. For cell salvaging, blood was collected continuously from the surgical field, diluted during collection, then processed and washed in a similar fashion. We studied the first transfusion for each patient to avoid possible confounding effects of any previous transfusion that day. Clinicians were not blinded as to the nature of the erythrocytes transfused. It was recognized, a priori, before the initiation of the trial, that some patients might require erythrocyte transfusion before the type of erythrocytes dictated by protocol randomization were available (e.g., CS). In those cases, transfusion of stored erythrocytes was permitted at the discretion of the anesthesiologist. We further recognized that this might represent a substantial fraction of the patients; thus, a priori, we decided to analyze data according to actual type of erythrocytes received ("as treated"), rather than according to the randomization scheme ("as randomized"), judging that the former would introduce less bias and distortion than the latter. An intention-to-treat analysis was also performed and is provided in Supplemental Digital Content 1.
Figure 1.
Trial patient flow.
No other clinical decisions were dictated by the research protocol. The exact FIO2 was not dictated by the study protocol. However, clinicians were encouraged to not alter FIO2 or ventilation parameters during the period extending from the measurements before transfusion, through transfusion, until following the measurements after transfusion.
All patients underwent general anesthesia according to the preference of the attending anesthesiologist. Standard patient care usually included somatosensory and motor evoked potential monitoring. Thus, general anesthesia usually consisted of intravenous infusions of propofol and an opioid, and an inhaled anesthetic at ≤ 0.5 minimum alveolar concentration. All patients had an indwelling arterial catheter, most commonly in the radial artery, as part of their standard care. A central venous catheter was placed in some patients, as decided by the anesthesiologist.
Baseline data were obtained after induction of general anesthesia, insertion of the radial arterial catheter, and final surgical positioning. PaO2, PCO2, and pH (ABG) were measured using ABL800 FLEX blood gas analyzer (Radiometer Medical A/S, Copenhagen, Denmark). Pulmonary mechanics data included respiratory rate, tidal volume, minute ventilation, peak and plateau airway pressure, fraction inspired oxygen concentration (FIO2) and end-tidal partial pressure of carbon dioxide (PETCO2), (GE Datex Ohmeda Aisis). Physiologic or total dead space fraction (VD/VT) was measured with the NICO® 7600 (Novametrix Medical Systems Inc., Wallingford, CT).
The primary outcome measure was the change in PaO2/FIO2 (P/F ratio) from before to after transfusion between groups. Secondary outcomes were the changes in VD/VT and PaO2 from before to after transfusion. To determine the effects of transfusion, measurements were performed 5 min before and 30 min after each blood transfusion. Patients were followed for 48 h for clinical diagnosis of acute lung injury.
Statistics
Power Analysis
Sample size was calculated for the outcome variable, VD/VT, from preliminary data in this population (healthy patients transfused during elective major posterior spinal surgery). We did not have sufficient data from these patients, with unchanging FIO2 to enable us to use P/F for the analysis. A power analysis for unpaired samples (two-tailed), with an alpha of 0.05 and a power of 0.8 indicated a requirement of 66 patients per group.
Demographic data were compared between the transfusion groups using ANOVA for continuous variables. For categorical data, Fisher's exact test was used for 2 × 2 tables and chi-square approximation for larger tables.
Data were analyzed only when available for both before and after blood transfusions. Imputation of data was not performed. Data before and after blood transfusion were compared for each transfusion group separately, by paired t-test. ANOVA was used to compare the transfusion groups separately before and after blood transfusion. Multiple comparisons were performed when ANOVA demonstrated statistical significance using the Tukey-Kramer honest significant difference. A mixed-effects model was performed using all data for the repeated-measures effect before versus after transfusion, and the between transfusion group effect, and their interaction (2-way repeated-measures ANOVA).
The primary and secondary outcomes, PaO2/FIO2 ratio, PaO2 and VD/VT, were analyzed for all patients where complete data existed for before and after transfusion. PaO2/FIO2 ratio and PaO2 were only analyzed in patients in whom FIO2 did not change (less than 10%).
Data are reported as mean ± SD, with 95% confidence intervals (CI) where indicated. All statistical tests were two-tailed. P < 0.05 was considered statistically significant. All data analyses were performed with JMP 10.0 (SAS Institute, Cary, NC).
Results
Demographics and Transfusion
Ninety-five patients were screened; 91 were enrolled and studied (fig. 1). Table 1 shows demographic information according to the actual first blood transfusion received. Three patients had surgery in the supine position, 7 patients had surgery in both the supine and prone positions, and 81 patients had surgery in only the prone position. Transfusions were given only during stable periods in a single position, either supine or prone.
Table 1.
Demographics for the as Treated Population
| Cell Salvage | Washed Allogeneic | Unwashed Allogeneic | Other | All | P value | |
|---|---|---|---|---|---|---|
| n | 36 (39.6%) | 18 (19.8%) | 23 (25.3%) | 14 (15.4%) | 91 (100%) | |
| Age (years) | 57 ± 11 | 62± 8 | 56 ± 12 | 45 ± 17 | 56 ± 13 | 0.0020 |
| Gender | 0.17 | |||||
| Female | 25 (69%) | 16 (89%) | 14 (61%) | 8 (57%) | 63 (69%) | |
| Male | 11 (31%) | 2 (11%) | 9 (39%) | 6 (43%) | 28 (31%) | |
| Height (cm) | 167 ± 6 | 167 ± 6 | 167 ± 6 | 167 ± 6 | 167 ± 6 | 0.13 |
| Weight (Kg) | 83.9 ± 20.0 | 72.4 ± 14.5 | 80.5 ± 23.8 | 75.6 ± 20.1 | 79.5 ± 20.3 | 0.22 |
| Body Mass Index (kg/m2) | 30.2 ± 7.0 | 28.2 ± 5.6 | 29.5 ± 7.1 | 27.3 ± 7.1 | 29.2 ± 6.7 | 0.53 |
| Tobacco Use | ||||||
| Any | 15 (42%) | 11 (61%) | 7 (30%) | 3 (21%) | 36 (40%) | 0.10 |
| Current | 0 (0%) | 0 (0%) | 0 (0%) | 2 (14%) | 2 (2%) | 0.011 |
| Ethnicity | 0.87 | |||||
| Caucasian | 30 (83.3%) | 16 (88.9%) | 21 (91.3%) | 11 (78.6%) | 78 (85.7%) | |
| Asian | 1 (2.8%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.1%) | |
| African American | 1 (2.8%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.1%) | |
| Native American/Alaskan | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Hawaiin/Pacific Islander | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Other/Multiethnic | 4 (11.1%) | 2 (11.1%) | 2 (8.7%) | 3 (21.4%) | 11 (12.1%) | |
| Hispanic | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (4.4%) |
Data are mean ± SD or n (%); treatment groups are all red blood cells as described in the text; "Other" includes fresh frozen plasma, whole blood, washed and unwashed autologous erythrocytes.
Primary Outcome Variable
P/F Ratio
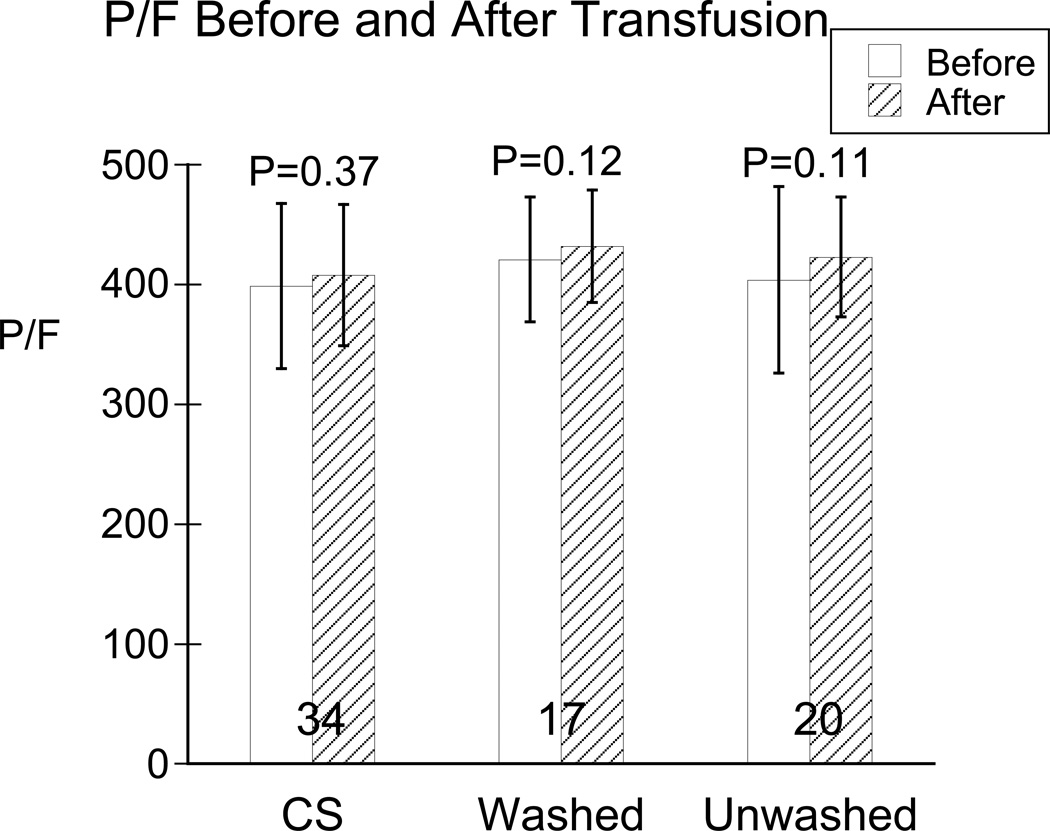
Baseline P/F ratio did not differ among groups (P = 0.19; table 2). Change of P/F from before to after transfusion (∆P/F, mean ± SD) did not differ among groups (CS, 9 ± 59 (95% CI: −11 to 29) mmHg; washed allogeneic, 10 ± 26 (95% CI: −3 to 24) mm Hg; unwashed allogeneic, 15 ± 51 (95% CI: −11 to 38) mmHg; P = 0.92). There were no P/F differences among erythrocyte types either before (P = 0.55) or after (P = 0.45) transfusion (table 3). No significant changes in P/F ratio occurred from before to after transfusion for any type of erythrocytes (P = 0.12 - 0.37; table 3, fig. 2). These results were confirmed by analysis by the mixed-effects model: there were no differences before and after transfusion (P = 0.42) or between transfusion groups (P = 0.80). The comparable intention-to-treat analysis is shown in the Supplemental Digital Content 1, tables 2 and 3.
Table 2.
Baseline Blood Gas and Gas Exchange Data for the as Treated Population
| Cell Salvage | Washed Allogeneic | Unwashed Allogeneic | P value | |
|---|---|---|---|---|
| n | 36 (8.5%) | 18 (4.3%) | 23 (5.4%) | |
| pH | 7.42 ± 0.04 | 7.44 ± 0.05 | 7.44 ± 0.04 | 0.097 |
| PaCO2 (mm Hg) | 39.3 ± 3.3 | 38.2 ± 4.3 | 39.5 ± 3.0 | 0.46 |
| PaO2 (mm Hg) | 364 ± 84 | 375 ± 60 | 364 ± 83 | 0.88 |
| HCO3− (mmol/L) | 25.7 ± 2.0 | 26.1 ± 2.0 | 27.1 ± 2.3 | 0.047 |
| Base Excess (mmol/L) | 1.6 ± 2.3 | 2.4 ± 2.5 | 3.4 ± 2.4 | 0.021 |
| SaO2 (%) | 100.0 ± 0.2 | 99.9 ± 0.2 | 100.0 ± 0.0 | 0.55 |
| FIO2 | 0.91 ± 0.13 (31) | 0.89 ± 0.15 (14) | 0.92 ± 0.12 | 0.80 |
| P/F Ratio | 409 ± 60 (31) | 435 ± 88 (14) | 389 ± 73 (19) | 0.19 |
| VD/VT | 0.39 ± 0.07 | 0.39 ± 0.09 | 0.39 ± 0.07 | 0.93 |
Data are mean ± SD, or mean ± SD (n) if n different from total; P/F ratio = PaO2/FIO2; VD/VT = measured physiological dead space; treatment groups are all erythrocytes as described in the text.
Table 3.
P/F, VD/VT, and PaO2 before and after Transfusion of first Erythrocyte Unit
| Cell Salvage | Washed Allogeneic | Unwashed Allogeneic | P value (between) | |
|---|---|---|---|---|
| P/F Ratio | ||||
| n pairs | 34 (47.2%) | 17 (23.6%) | 21 (29.2%) | |
| Pre | 399 ± 69 | 421 ± 52 | 405 ± 76 | 0.55 |
| Post | 408 ± 59 | 432 ± 47 | 420 ± 76 | 0.45 |
| Δ | 9 ± 59 | 10 ± 26 | 15 ± 51 | 0.92 |
| P (within) | 0.37 | 0.12 | 0.20 | |
| VD/VT | ||||
| n pairs | 34 (47.2%) | 18 (25.0%) | 22 (30.6%) | |
| Pre | 0.38 ± 0.08 | 0.38 ± 0.08 | 0.36 ± 0.09 | 0.72 |
| Post | 0.37 ± 0.08 | 0.37 ± 0.08 | 0.37 ± 0.06 | 0.99 |
| Δ | −0.01 ± 0.04 | −0.01 ± 0.04 | 0.01 ± 0.06 | 0.28 |
| P (within) | 0.034 | 0.53 | 0.62 | |
| PaO2 | ||||
| n pairs | 31 (45.6%) | 16 (23.5%) | 21 (30.9%) | |
| Pre | 356 ± 82 | 322 ± 90 | 362 ± 83 | 0.31 |
| Post | 360 ± 75 | 328 ± 90 | 372 ± 82 | 0.26 |
| Δ | 4 ± 55 | 6 ± 23 | 10 ± 40 | 0.90 |
| P (within) | 0.68 | 0.30 | 0.27 |
Data are Mean ± SD. P/F Ratio = PaO2/FIO2; VD/VT = measured physiological dead space; PaO2 data only for patients at constant FIO2; Δ = difference from pre- to post-transfusion; P values are for paired comparisons from before to after transfusion (within), and unpaired comparisons between transfusion groups; treatment groups are all erythrocytes as described in the text.
Figure 2.
P/F (PaO2/FIO2) before (unfilled bars) and after (diagonal-filled bars) in the three transfusion groups: cells saver (CS), washed and unwashed allogeneic erythrocytes. Data are mean ± SD, with “n” for each group show at the base of the bars. P values are those comparing P/F before to after transfusion within each group. There were no statistical differences between groups before or after transfusion or for changes from before to after transfusion.
Secondary Outcome Variables
Dead Space (VD/VT)
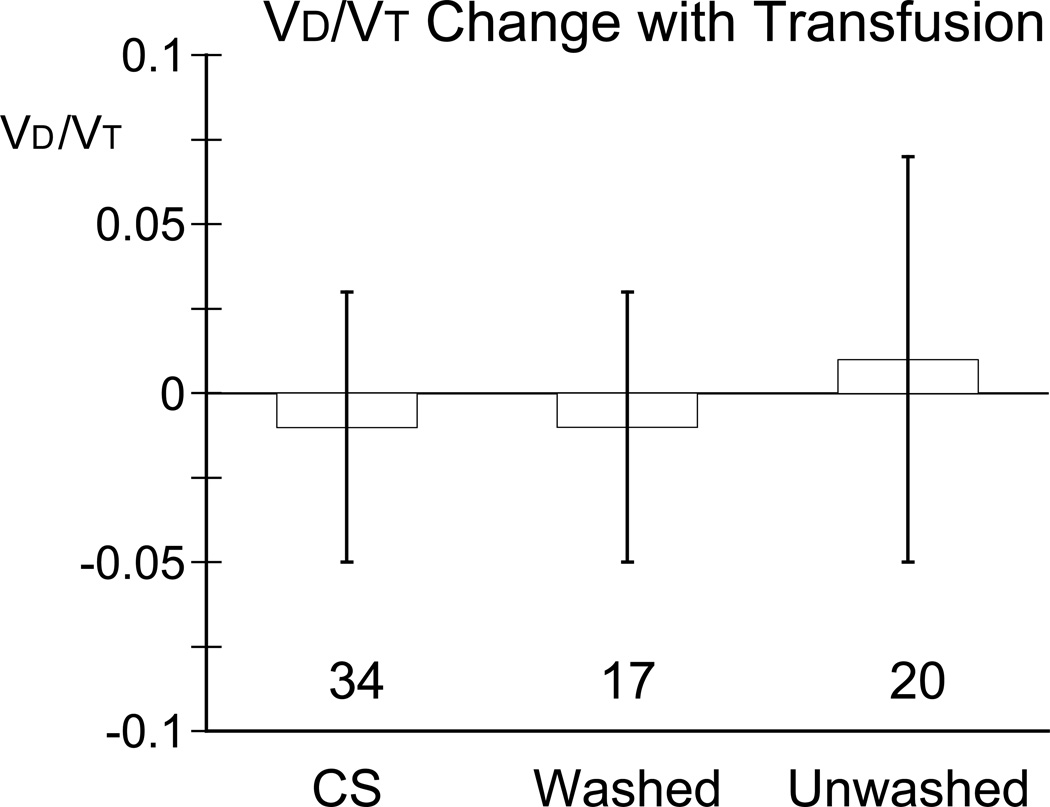
Baseline VD/VT did not differ between groups (P = 0.92, table 2). ∆VD/VT did not differ among groups (P = 0.28). VD/VT decreased slightly, but statistically significantly in the cell salvage group from before to after transfusion (0.38 ± 0.08 to 0.37 ± 0.08; P = 0.034, table 3, fig. 3), but not change significantly in the other two groups (washed allogeneic 0.38 ± 0.08 to 0.37 ± 0.08, P = 0.53 and unwashed allogeneic 0.36 ± 0.09 to 0.37 ± 0.06, P = 0.62). VD/VT did not differ among groups either before (P = 0.72) or after (P = 0.99) transfusion. These results were confirmed by analysis by the mixed-effects model: the statistical significance within the CS group was no longer apparent and no differences were found by multiple comparisons.
Figure 3.
Changes in physiological dead space (VD/VT) from before to after transfusion for each group: cells saver (CS), washed and unwashed allogeneic erythrocytes. Data are mean ± SD, with “n” for each group show at the base of the bars. VD/VT in the CS group changed significantly with transfusion (P = 0.034); but did not change in the washed allogeneic (P = 0.53) or the unwashed allogeneic (P = 0.62) transfusion groups. There were no differences among groups for changes from before to after transfusion (P = 0.28).
PaO2
Baseline PaO2 did not differ among groups (table 2). For patients in whom FIO2 did not change >10% from before to after transfusion there were no differences in PaO2 among groups before (P = 0.31) or after (P = 0.26) transfusion or from before to after transfusion (∆PaO2; P = 0.90; table 3). The mixed-effects model produced similar results.
Ventilatory Mechanics
Baseline values were not significantly different between groups. No differences were found in minute ventilation, respiratory rate, tidal volume, peak and plateau airway pressure, and static and dynamic compliance before or after transfusion, or between the different transfusion groups (table 4).
Table 4.
Ventilatory Data for the as Treated Population
| Cell Salvage | Washed Allogeneic | Unwashed Allogeneic | P value (between) | |
|---|---|---|---|---|
| n | 36(46.8%) | 18(23.4%) | 23(29.9%) | |
| Minute Ventilation (L/min) | ||||
| Pre | 5.3 ± 1.3 | 4.4 ± 1.1 | 5.3 ± 1.6 | 0.10 |
| Post | 5.3 ± 1.1 | 4.4 ± 1.0 | 5.3 ± 1.4 | 0.051 |
| P (within) | 0.80 | 0.83 | 0.86 | |
| Tidal Volume (mL) | ||||
| Pre | 524 ± 108 | 492 ± 57 | 512 ± 96 | 0.61 |
| Post | 524 ± 99 | 479 ± 58 | 516 ± 114 | 0.25 |
| P (within) | 0.91 | 0.19 | 0.75 | |
| Respiratory Rate (breaths/min) | ||||
| Pre | 10 ± 2 | 9 ± 2 | 10 ± 2 | 0.26 |
| Post | 10 ± 2 | 9 ± 2 | 10 ± 2 | 0.19 |
| P (within) | 1.00 | 0.59 | 0.42 | |
| Peak Inspiratory Pressure (cm H2O) | ||||
| Pre | 23 ± 4 | 22 ± 4 | 22 ± 5 | 0.14 |
| Post | 23 ± 4 | 21 ± 4 | 22 ± 5 | 0.16 |
| P (within) | 0.50 | 0.25 | 0.64 | |
| Plateau Pressure (cm H2O) | ||||
| Pre | 20 ± 4 | 19 ± 4 | 19 ± 4 (22) | 0.32 |
| Post | 20 ± 4 | 18 ± 4 | 18 ± 5 (22) | 0.28 |
| P (within) | 0.34 | 0.26 | 0.69 | |
| PEEP (cm H2O) | ||||
| Pre | 5 ± 1 | 5 ± 1 | 5 ± 1 | 0.48 |
| Post | 5 ± 1 | 4 ± 1 | 4 ± 1 | 0.10 |
| P (within) | 1.0 | 0.096 | 0.16 | |
| Dynamic Compliance (mL/cm H2O) | ||||
| Pre | 29 ± 6 | 30 ± 6 | 31 ± 8 | 0.52 |
| Post | 29 ± 6 | 29 ± 6 | 32 ± 8 | 0.57 |
| P (within) | 0.61 | 0.91 | 0.66 | |
| Static Compliance (mL/cm H2O) | ||||
| Pre | 38 ± 18 | 36 ± 8 | 39 ± 16 (22) | 0.54 |
| Post | 38 ± 16 | 37 ± 13 | 40 ± 15 (22) | 0.68 |
| P (within) | 0.84 | 0.68 | 0.73 |
Data are mean ± SD or mean ± SD (n) if n differs. Minute ventilation calculated from respiratory rate and tidal volume. PEEP = positive end-expiratory pressure. P values are for paired comparisons from before to after transfusion (within), and unpaired comparisons between transfusion groups; treatment groups are all erythrocytes as described in the text.
Hemodynamics
There were no differences in baseline hemodynamic variables between the groups. Patients receiving salvaged erythrocytes had small (4 – 6 mmHg), but statistically significant increases in systolic, diastolic and mean blood pressure (P = 0.008 to 0.029), and central venous pressure (P = 0.0026), but only diastolic blood pressure differed among groups after transfusion, (table 5). Patients receiving allogeneic washed and unwashed erythrocytes had no statistically significant changes in blood pressure. Heart rate did not change significantly in any group. All groups demonstrated small (0.1 – 0.2 °C), but statistically significant increases in core body temperature.
Table 5.
Vital Signs for the as Treated Population
| Cell Salvage | Washed Allogeneic | Unwashed Allogeneic | P value (between) | |
|---|---|---|---|---|
| n | 36(46.8%) | 18(23.4%) | 23(29.9%) | |
| Heart Rate (beats/min) | ||||
| Pre | 69 ± 14 | 66 ± 12 | 70 ± 11 | 0.48 |
| Post | 68 ± 10 | 66 ± 12 | 70 ± 12 | 0.53 |
| P (within) | 0.28 | 0.95 | 0.94 | |
| BP Systolic (mm Hg) | ||||
| Pre | 109 ± 15 | 102 ± 13 | 104 ± 16 | 0.39 |
| Post | 115 ± 18 | 106 ± 8 | 107 ± 17 | 0.055 |
| P (within) | 0.018 | 0.36 | 0.34 | |
| BP Diastolic (mm Hg) | ||||
| Pre | 60 ± 11 | 55 ± 6 | 58 ± 10 | 0.42 |
| Post | 64 ± 12 | 57 ± 5 | 59 ± 8 | 0.031 |
| P (within) | 0.029 | 0.15 | 0.30 | |
| BP Mean (mm Hg) | ||||
| Pre | 76 ± 12 | 75 ± 13 | 75 ± 12 | 0.86 |
| Post | 83 ± 15 | 77 ± 8 | 77 ± 12 | 0.099 |
| P (within) | 0.008 | 0.67 | 0.60 | |
| CVP (mm Hg) | ||||
| Pre | 10 ± 4 (19) | 11 ± 3 (17) | 9 ± 4 (17) | 0.17 |
| Post | 11 ± 4 (19) | 11 ± 4 (17) | 10 ± 4 (17) | 0.85 |
| P (within) | 0.0026 | 0.49 | 0.12 | |
| Temperature (°C) | ||||
| Pre | 36.3 ± 0.8 | 36.1 ± 0.8 | 36.1 ± 0.6 (22) | 0.22 |
| Post | 36.4 ± 0.9 | 36.2 ± 0.8 | 36.3 ± 0.6 (22) | 0.26 |
| P (within) | < 0.0001 | 0.047 | 0.0010 |
Data are mean ± SD or mean ± SD (n) if n differs. BP = arterial blood pressure; CVP = central venous pressure. P values are for paired comparisons from before to after transfusion (within), and unpaired comparisons between transfusion groups; treatment groups are all erythrocytes as described in the text.
Blood Gases
Baseline arterial blood gas values did not differ between the groups (table 2). With transfusion, although a few were statistically significant, changes in blood gas values were small and of little physiologic consequence, (table 6).
Table 6.
Arterial Blood Gas Values for the as Treated Population
| Cell Salvage | Washed Allogeneic | Unwashed Allogeneic | P value (between) | |
|---|---|---|---|---|
| n | 34(47.2%) | 17(23.6%) | 21(29.2%) | |
| pH | ||||
| Pre | 7.41 ± 0.04 | 7.41 ± 0.05 | 7.42 ± 0.04 | 0.55 |
| Post | 7.40 ± 0.05 | 7.39 ± 0.04 | 7.41 ± 0.04 | 0.45 |
| P Value (within) | 0.058 | 0.055 | 0.057 | |
| PaCO2 (mm Hg) | ||||
| Pre | 38.9 ± 3.6 | 40.4 ± 3.9 | 38.6 ± 2.5 | 0.24 |
| Post | 39.8 ± 4.3 | 39.6 ± 4.2 | 39.4 ± 2.3 | 0.94 |
| P Value (within) | 0.10 | 0.47 | 0.18 | |
| PaO2 (mm Hg) | ||||
| Pre | 355 ± 78 | 328 ± 90 | 362 ± 83 | 0.42 |
| Post | 358 ± 81 | 326 ± 88 | 372 ± 82 | 0.23 |
| P Value (within) | 0.79 | 0.81 | 0.27 | |
| HCO3− (mmol/L) | ||||
| Pre | 24.5 ± 2.0 | 25.5 ± 2.2 | 25.8 ± 3.5 | 0.17 |
| Post | 24.4 ± 2.1 | 24.0 ± 1.8 | 25.0 ± 2.5 | 0.40 |
| P Value (within) | 0.32 | 0.0002 | 0.11 | |
| Base Excess (mmol/L) | ||||
| Pre | 0.5 ± 2.2 | 1.5 ± 2.5 | 1.5 ± 2.6 | 0.24 |
| Post | 0.0 ± 2.4 | −0.2 ± 1.9 | 1.0 ± 2.8 | 0.27 |
| P Value (within) | 0.025 | < 0.0001 | 0.045 | |
| SaO2 (%) | ||||
| Pre | 99.9 ± 0.4 | 100.0 ± 0.0 | 99.9 ± 0.4 | 0.65 |
| Post | 100.0 ± 0.2 | 99.9 ± 0.2 | 99.9 ± 0.4 | 0.71 |
| P Value (within) | 0.32 | 0.33 | 1.0 | |
| FIO2 | ||||
| Pre | 0.89 ± 0.14 | 0.78 ± 0.20 | 0.89 ± 0.13 | 0.047 |
| Post | 0.88 ± 0.17 | 0.76 ± 0.20 | 0.88 ± 0.14 | 0.037 |
| P Value (within) | 0.65 | 0.25 | 0.56 |
Data are mean ± SD. P values are for paired comparisons from before to after transfusion (within), and unpaired comparisons between transfusion groups; treatment groups are all erythrocytes as described in the text.
Lung Injury
Three patients developed acute lung injury. One of these patients developed TRALI in the Postanesthesia Care Unit, and had received FFP as his/her first transfusion, with a total transfusion of 3 units FFP, 1 unit of whole blood (family member donor designated10) and 1 unit of cell salvaged blood. Another patient developed bacteremia, and multisystem organ failure with lung injury, related to a platelet transfusion.11 These cases have been reported separately,10,11 including the evidence for diagnosis. The third patient developed hypoxemia postoperatively owing to a pulmonary embolus, and remained mechanically ventilated after surgery.
Discussion
The major finding of this clinical trial is a lack of impaired pulmonary exchange for oxygen with transfusion of a single unit of erythrocytes. This pertained to all groups, whether plasma was part of the erythrocyte transfusion (unwashed group) or not (washed groups). Thus, this clinical trial failed to support our hypothesis that an erythrocyte transfusion subtly impairs pulmonary gas exchange and that erythrocyte transfusion can induce a spectrum of pulmonary injury more broad than is described by the definition of TRALI.
This differs from our study in volunteers where we detected a decrement in oxygenation with transfusion of both fresh and stored erythrocytes.9 Those changes were extremely small but were detectable by employing a cross-over design and a repeated measures analysis. We had thought that carefully assessing patients would also be sufficiently robust to detect small changes, but the design and statistical analysis of this clinical trial was less powerful than that of the volunteer study. Furthermore, small differences may be difficult to detect at high FIO2. The standard deviation of PaO2 greatly exceeded the increase in A-a gradient that we were able to detect in our volunteer study. Other factors, such as changes in cardiac output and mechanical ventilation with positive end-expiratory pressure, may have also obscured small changes in oxygenation.
In a similarly sized randomized trial in mechanically ventilated critically ill patients, Kor et al.12 examined pulmonary changes from before to after transfusion of a single unit of erythrocytes, comparing the changes for fresh versus standard issue erythrocytes. They found no differences in changes between groups; the before to after changes were small and likely not statistically significant (only changes presented, without within group analysis).
In our trial, a clinically very small, but statistically significant, decrease in dead space (physiological improvement) was detected when transfusing salvaged erythrocytes, but not when transfusing erythrocytes of the other groups. There are several possible reasons for this finding. This could reflect an improvement in pulmonary perfusion that accompanied the greater increase in mean arterial blood pressure with erythrocyte transfusion in this group. The mean arterial pressure increase might have been greater in the CS group owing to a number of patients having received CS first according not to the randomization scheme, but rather to clinical need. Consistent with this, no decrease in dead space was found in the intention to treat analysis. Respiratory mechanics, including compliance, do not appear to have been a contributing factor, as no differences were found before and after transfusion for any related variables. Alternatively, the failure of the other two groups to improve VD/VT with transfusion could represent an indication that some detrimental factor prevented the improvement that was seen with CS transfusion. However, we do not have any data to support or refute this theory.
The lack of significant findings may also be an indication of the difficulty in attempting to study TRALI prospectively. Despite having studied 91 patients, one might consider the trial as having been underpowered. However, the observed effect was actually in the opposite direction from the changes we hypothesized would occur with subclinical TRALI. The lower 95% confidence limit for change in P/F ratio was −11 mm Hg, which we do not believe is clinically meaningful. A larger trial would narrow confidence limits, but not change the fact that our results suggest no statistically or clinical significant effect. Therefore, our lack of finding does not appear to be due to a type II error.
By definition, TRALI, captures only severe cases of pulmonary function impairment.5 We sought to detect more subtle changes, but failed to do so. The etiology of more subtle changes, as we found previously,9 may differ from that of TRALI. TRALI is hypothesized to result from transfusion of plasma, rather than erythrocytes per se. In a series of laboratory experiments, Silliman et al. showed that lung injury in rats6 8 can be caused by infusion/transfusion of biologically active compounds, such as lysophosphatidyl cholines,6,13 arachidonic acid, or 5- and 12- hydroxyeicosatetranoic acid.8 They hypothesized that the clinical syndrome of TRALI resulted from initial neutrophil priming (so called "first hit") that could result from a number of clinical circumstances that induce inflammation, such as surgery. Looney et al, similarly were able to produce pulmonary injury in an immune-mediated mouse model with priming of hematopoetic cells and deposition of activated neutrophils and platelets.7 Our previous finding of subtle changes in pulmonary gas exchange with autologous erythrocyte transfusion in healthy adults could be interpreted as support for the nonimmune based "two hit" hypothesis, as no allogeneic antibodies could have been involved. However, in those healthy volunteers, there was no "first hit" or reason for neutrophil priming, and we also found no difference between fresh erythrocytes and those stored for 21 days. Impugned biologically active compounds would have been expected to increase during such storage period, and if they are a cause of such changes, then the changes should have been larger with transfusion of blood stored for a longer period. This cast some doubt on that theorized etiology as the cause of the finding. Toy et al.,4 in a prospective clinical observational trial conducted concurrently with the trial we report here, determined that both donor and recipient characteristics are associated with TRALI. The most important transfusion factors were the load of strong cognate HLA class II and granulocyte antibodies transfused. They found that biologically active compounds were not a risk factor, although the confidence limits were sufficiently wide to preclude their elimination as a risk factor. In support of the importance of priming as the first hit in the "two hit" hypothesis, Toy et al found elevated patient interleukin-8 level before transfusion to be an independent risk factor. The work presented here, in failing to find subtle changes of pulmonary function in erythrocyte transfusion with or without associated plasma and thus, either antibodies or biologically active compounds that might be contained in plasma, cannot address the veracity of either hypothesis.
Our trial had some important weaknesses. First, a substantial fraction of patients received their first transfusion that differed from the randomization scheme. This was dictated by clinical need, and could not have been otherwise. We have no reason to believe that there was another systematic reason for this, related to randomization, but cannot know that with certainty. An intention-to-treat analysis did not suggest any different results (Supplemental Digital Content 1, tables 1 to 6). The second major weakness is that we did not study as many patients as had been suggested by the a priori power analysis, owing the end of the grant period (and, thus, funding). However, P/F ratio actually increased numerically, (but not statistically) in all transfusion groups, thus offering no suggestion of pulmonary injury. Furthermore, the numerical (but not statistical) difference we found is so small as to be of unlikely clinical significance. The size of the effect for both our primary and secondary outcomes, and the confidence limits, suggests that our conclusions are not due to an underpowered study (type II error).
We tested the effects of transfusion of a single, first unit of erythrocytes, finding no pulmonary effects. This was done to avoid any possible confounding effects of earlier transfusion that could have been with a different sort (CS, washed, unwashed) of erythrocytes. It is possible that transfusion of a greater number of erythrocyte units would have an effect. However, in a large prospective surveillance, case-controlled study, the number of units of all components (not just erythrocytes) transfused was not a risk factor for the development of clinical TRALI, although the volumes of transfused HLA class II antibody, and anti-human neutrophil antigen were.4 The volume of lysophosphatidyl cholines transfused was not a risk factor in the multivariate analysis of that study.
Lastly, measurements were made 30 min after transfusion. It is possible that that is an insufficient period of time to allow for development of lung injury. However, we found subtle gas exchange after autologous transfusion in this period of time in healthy volunteers,9 and in their perfused rat lung model, Silliman et al.6 found that perfusion with plasma from erythrocytes stored for 42 days increased pulmonary artery pressure within 10 min, and mortality begins within 30 min in a two-hit mouse model of TRALI.7 In the clinical circumstance of surgery with substantial bleeding, it was not possible to construct a prospective protocol that would have reliably allowed us to make measurements at a later time in all patients, without the confounding effect of additional transfusions.
In summary, in a trial of healthy patients undergoing elective major spinal surgery, we did not find any indication of erythrocyte-induced pulmonary injury, independent of whether the erythrocytes did or did not have associated allogeneic plasma. Thus, we could not support our hypothesis that TRALI might have a more broad range than as indicated by the accepted definition. This finding differs from a previous study in healthy volunteers, where transfusion of autologous erythrocytes induced very subtle decrements in pulmonary gas exchange.
Supplementary Material
Final Boxed Summary Statement.
What we already know about this topic:
* Transfusions are associated with the potential for producing varying degrees of acute lung injury that is an important cause of morbidity and mortality
What this article tells us that is new:
* In this study, transfused erythrocytes in surgical patients did not impair gas exchange as assessed by PaO2/FIO2
Acknowledgments
The project was supported by National Heart, Lung and Blood Institute Transfusion Medicine SCCOR P50HL081027 (PT), Bethesda, Maryland.
Footnotes
The study was not registered at clinicaltrial.gov. Registration for this type of study was not required, or even routine, at the time it was initiated.
Conflicts of Interest: JF has received research funding from Masimo, Inc. (Irvine, California,), Bluepoint Medical (Selmsdorf, Germany), Nonin Medical (Plymouth, Minnesota), CAS Medical Systems (Branford, Connecticut), Covidien (Minneapolis, Minnesota), Mespere Lifesciences (Waterloo, Ontario, Canada), Pacific Medico (Tokyo, Japan), Xhale Inc. (Gainesville, Florida), and Anamedical (Tel Aviv, Israel). MG, JL, PT, and JT report no conflicts of interest. RBW has a relationship with or consults for the following organizations/companies that have an interest in red cell transfusion: United States Food and Drug Administration, National Heart, Lung, and Blood Institute/National Institute of Health (Bethesda, Maryland), United States Department of Defense (Frederick, Maryland), TerumoBCT (Lakewood, Colorado). RBW has also consulted for Sangart (San Diego, California), OPK Biotech (Cambridge, Massachusetts), HbO2 Therapeutics (Souderton, Pennsylvania), Octapharma USA (Hoboken, New Jersey) within the past 3 years.
References
- 1.Popovsky MA, Abel MD, Moore SB. Transfusion-related acute lung injury associated with passive transfer of antileukocyte antibodies. Am Rev Respir Dis. 1983;128:185–189. doi: 10.1164/arrd.1983.128.1.185. [DOI] [PubMed] [Google Scholar]
- 2.Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25:573–577. doi: 10.1046/j.1537-2995.1985.25686071434.x. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed October 26, 2014];US FDA: Fatalities Reported to FDA Following Blood Collection and Transfusion: Annual Summary for Fiscal Year 2013. http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/ucm346639.htm.
- 4.Toy P, Gajic O, Bacchetti P, Looney MR, Gropper MA, Hubmayr R, Lowell CA, Norris PJ, Murphy EL, Weiskopf RB, Wilson G, Koenigsberg M, Lee D, Schuller R, Wu P, Grimes B, Gandhi MJ, Winters JL, Mair D, Hirschler N, Sanchez Rosen R, Matthay MA. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119:1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toy P, Popovsky MA, Abraham E, Ambruso DR, Holness L, Kopko P, McFarland J, Nathens A, Silliman CC, Stroncek D. National Heart Lung and Blood Institute Working Group on TRALI: Transfusion-related acute lung injury: Definition and review. Crit Care Med. 2005;33:721–726. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 6.Silliman C, Voelkel N, Allard JD, Eklzi D, Tuder R, Johnson J, Ambrusco D. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–1467. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119:3450–3461. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silliman CC, Moore EE, Kelher MR, Khan SY, Gellar L, Elzi DJ. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549–2554. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiskopf RB, Feiner J, Toy P, Twiford J, Shimabukuro D, Lieberman J, Looney MR, Lowell CA, Gropper MA. Fresh and stored red blood cell transfusion equivalently induce subclinical pulmonary gas exchange deficit in normal humans. Anesth Analg. 2012;114:511–519. doi: 10.1213/ANE.0b013e318241fcd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunbar N, Cooke M, Diab M, Toy P. Transfusion-related acute lung injury after transfusion of maternal blood: A case-control study. Spine (Phila Pa 1976) 2010;35:E1322–E1327. doi: 10.1097/BRS.0b013e3181e3dad2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rollins MD, Molofsky AB, Nambiar A, Pandey S, Weiskopf RB, Toy P. Two septic transfusion reactions presenting as transfusion-related acute lung injury from a split plateletpheresis unit. Crit Care Med. 2012;40:2488–2491. doi: 10.1097/CCM.0b013e3182544f85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kor DJ, Kashyap R, Weiskopf RB, Wilson GA, van Buskirk CM, Winters JL, Malinchoc M, Hubmayr RD, Gajic O. Fresh red blood cell transfusion and short-term pulmonary, immunologic, and coagulation status: A randomized clinical trial. Am J Respir Crit Care Med. 2012;185:842–850. doi: 10.1164/rccm.201107-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelher MR, Masuno T, Moore EE, Damle S, Meng X, Song Y, Liang X, Niedzinski J, Geier SS, Khan SY, Gamboni-Robertson F, Silliman CC. Plasma from stored packed red blood cells and MHC class I antibodies causes acute lung injury in a 2-event in vivo rat model. Blood. 2009;113:2079–2087. doi: 10.1182/blood-2008-09-177857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.