Abstract
Diabetic nephropathy (DN) is one of the most serious microvascular complications of diabetes and may lead to end-stage renal disease (ESRD) and chronic renal failure. The aim of this study was to determine whether low-molecular-weight fucoidan (LMWF) can reduce harmful transforming growth factor-β (TGF-β)-mediated renal fibrosis in DN using in vitro and in vivo experimental models. The experimental results showed that LMWF significantly reversed TGF-β1-induced epithelial-mesenchymal transition and dose-dependently inhibited accumulation of extracellular matrix proteins, including connective tissue growth factor and fibronectin. It was found that LMWF significantly reduced blood urea nitrogen and blood creatinine in both type 1 and type 2 diabetic rat models. H&E, PAS and Masson’s trichrome staining of kidney tissue showed LMWF significantly reduced renal interstitial fibrosis. Treatment with LMWF significantly increased E-cadherin expression and reduced α-SMA, CTGF and fibronectin expression in both type 1 and type 2 diabetic models. LMWF also decreased the phosphorylation of Akt, ERK1/2, p38 and Smad3 in vitro and in vivo. These data suggest that LMWF may protect kidney from dysfunction and fibrogenesis by inhibiting TGF-β pathway and have the potential benefit to slow down the progression of DN.
Keywords: Fucoidan, diabetic nephropathy, epithelialto-mesenchymal transition, transforming growth factor-β1, extracellular matrix, tubulointerstitial fibrosis
Introduction
Diabetic nephropathy (DN) is one of the most serious microvascular complications of diabetes. DN may lead to end-stage renal disease (ESRD) and chronic renal failure that makes the patients have to receive dialytic treatment or kidney replacement therapy [1]. DN is morphologically characterized by thickening of the glomerular and tubular basement membrane, expanded mesangial extracellular matrix, microvascular damage, and fibrotic changes in the tubulo interstitium [2,3]. Tubulointerstitial fibrosis (TIF) is a common pathway that leads to progressive renal injury in DN.
It is widely accepted that matrix-producing myofibroblasts contribute to the renal fibrosis in DN by facilitating deposition of interstitial extracellular matrix (ECM) [2,4,5]. However Iwano et al. found that up to 36% of all interstitial fibroblasts derived from tubular epithelial cells by epithelial-mesenchymal transition (EMT) during tissue fibrosis in a TIF model [6]. The increased concentrations of transforming growth factor-β1 (TGF-β1) have been mainly identified as a key mediator of the fibrotic response in DN, also for EMT [7-10]. Furthermore, it was found that TGF-β1 acts directly via either Smad-dependent or Smad-independent pathways, together with the connective tissue growth factor (CTGF) [11]. CTGF is well known to regulate ECM related gene expression, which is exclusively induced by TGF-β1 in fibroblasts, and contributes to TGF-β1-dependent renal interstitial fibrosis [12,13]. There still is no effective drug and therapy to prevent and treat the patients with renal fibrosis.
Fucoidan represents an intriguing group of natural fucose-enriched sulfated polysaccharides. Marine brown algae are one of the richest sources of sulfated polysaccharides. A fraction of low-molecular-weight fucoidan (LMWF) (~7 kDa) is obtained by radical depolymerization of extracts from brown seaweed. We have previously reported LMWF could prevent renal ischemia reperfusion injury via inhibition of the MAPK signaling pathway in in vivo and in vitro models [14]. In the literature, fucoidan had renoprotective effects in chronic renal failure model [15,16], ameliorated metabolic dysfunctions in db/db mice [17], and inhibited α-amylase and α-glucosidase activities [18]. In recent studies, fucoidan administration increased insulin secretion in overweight or obese adults [19] and delay the progression of diabetic renal complications in STZ rats [20]. LMWF improved the action of insulin via AMPK stimulation [21], attenuated retinopathy [22] and endothelial dysfunction [23] in diabetic animal models. Therefore, we proposed that LMWF might be a possible candidate drug for preventing and treating DN.
In present study, we studied whether LMWF exerted a protective role against DN as well as its possible protective mechanisms using in vitro cell model and in vivo diabetic rat models. The experimental results showed LMWF could prevent the progression of renal fibrosis in DN, which indicates that LMWF may be developed as a candidate drug for preventing DN.
Materials and methods
Source of LMWF
As previously described, LMWF was produced from the seaweed L. japonica commercially cultured in Qingdao, China, and analyzed as the followings: fucose content 29.5%; uronic acid content 7.5%; and sulfate content 30.1% [15]. Its average molecular weight is about 7000 Da determined by high performance steric exclusion chromatography analysis. LMWF was dissolved in physiological saline for animal treatment and in PBS for cell incubation.
Cell culture
HK2 cells (human kidney proximal tubular cells) were purchased from Cell Culture Centre, Institute of Basic Medical Science Chinese Academy of Medical Sciences (Beijing, China). Briefly, HK2 cells were cultured in DMEM/F12 containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin, in a humidified atmosphere with 5% CO2 at 37°C.
For TGF-β1 treatments, cells were treated with vehicle (PBS) or 10 ng/ml recombinant human TGF-β1 (R&D Systems, Minneapolis, MN) for 48 h to induce cellular fibrogenesis and different concentrations of LMWF (1~20 μg/ml) for the same duration.
Animal models and drug treatment
Male Goto-Kakizaki (GK) rats (Shanghai SLAC laboratory animal Co., LTD.) and age-matched control Wistar rats were used. The animals were housed with a 12/12 h light/dark cycle, food and water available ad libitum. At 12 weeks of age, fasting blood glucose was determined in each rat, and they were divided into three groups: Wistar rats (control group, n=10); untreated GK rats (GK group, n=10); LMWF-treated GK rats (GK-F group, n=10). In LMWF-treated groups, 100 mg/kg/day of LMWF was administered by gavage for 12 weeks. At 24 weeks of age, rats were sacrificed under anesthesia.
Male Sprague-Dawley rats, weighting 200~220 g, were purchased from the Animal Center of Peking University Health Science Center. Experimental diabetes was induced by a single intraperitoneal injection of 65 mg/kg body weight Streptozotocin (STZ; Amresco, Solon, OH) in citrate buffer after a 14 h overnight fasting. Induction of the diabetes was confirmed by measuring the blood glucose 3 days after STZ administration. The rats with fasting blood glucose concentration > 16.5 mM were classified as successful diabetes model and used in the study. The rats were randomly divided into three groups: Normal control rats (control group, n=10), STZ-induced diabetic rats (STZ group, n=10); LMWF-treated diabetic rats (STZ-F group, n=10). Rats in LMWF-treated group received 100 mg/kg/day of LMWF by gavage for 12 weeks. Rats in control group and STZ group received physiological saline.
The study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of China Association for Laboratory Animal Science. All animal care and protocols were approved by the Animal Care Committee of Peking University Health Science Center. All sacrifice was performed under pentobarbitone anesthesia, and every effort was made to minimize animal suffering.
Morphological analysis
Kidneys were harvested and fixed with 4% formaldehyde for paraffin embedding.
After being embedded in paraffin, several sections of 7 μm were obtained and stained with hematoxylin and eosin and periodic acid-Schiff (PAS) for histological evaluation, and also stained with Masson’s trichrome to demonstrate fibrosis in kidney tissues.
The tubulointerstitial fibrosis index (TFI) was assessed in Masson’s trichrome-stained sections in 10 randomly selected fields of view at 400× magnification using alight microscope. The degree of fibrosis was graded using a semi-quantitative scoring method. The degree of fibrosis was on a scale of 0-3 (grade 0, normal; grade 1, lesion area < 25%; grade 2, lesion area 25-50%; grade 3, lesion area > 50%).
Measurement of blood creatinine and urea concentration
Renal function was monitored by measuring blood creatinine and urea nitrogen (BUN). Blood samples were collected for determination of urea and creatinine. Blood creatinine concentration was measured with a commercial kit (NJJC Bio, Nanjing, China), according to the manufacturer’s instructions. BUN concentration was measured using the QuantiChrom Urea Assay Kit (DIUR-500; BioAssay Systems, Hayward, CA).
Western blot analysis
Tissues or cells were homogenized in RIPA lysis buffer containing protease inhibitor cocktail (Roche, Indianapolis, IN). Total protein was measured by BCA (Pierce, Rockford, IL) and size separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were blotted to polyvinylidene difluoride membranes (Amersham Biosciences, Piscataway, NJ). Blots were incubated with antibodies against p-ERK1/2, ERK2, p-Akt1/2/3, Akt1, α-SMA, CTGF and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA), p-p38, p38, Smad3, vimentin, Snail, ZEB1 (Cell Signaling Technology, Beverly, MA), E-cadherin, TGF-β1, p-Smad3 (Bioworld Technology, Louis Park, MN), fibronectin (Abcam, Cambridge, MA). Goat anti-rabbit IgG and goat anti-mouse IgG (Santa Cruz Biotechnology) were added and the blots were developed with ECL plus kit (Amersham Biosciences).
Statistical analyses
All results are expressed as mean ± SEM. For multiple comparisons, the statistical analysis was performed by using one-way ANOVA followed by the Tukey’s multiple comparison tests. P-values < 0.05 were considered statistically significant.
Results
Effect of LMWF on EMT induced by TGF-β1 in HK2 cells
As shown in Figure 1A, HK2 cells displayed epithelial-like appearance in the control group. Following stimulation with 10 ng/ml of TGF-β1, cells demonstrated a decrease in cell-cell contact and adopted a spindle-shaped, fibroblastic appearance. These changes were significantly apparent with TGF-β1 treatment for 48 h. LMWF (5, 10, 20 μg/ml) significantly attenuated the morphological changes.
Figure 1.

LMWF reversed TGF-β1-induced epithelial-mesenchymal transition in HK2 cells. HK2 cells were incubated with 10 ng/ml of TGF-β1 and different concentrations of LMWF (5, 10, 20 μM) for 48 h. A. Morphology of the HK2 cells cultured in indicated conditions. B. Expression levels of E-cadherin, α-SMA, vimentin, CTGF, fibronectin, β-actin of HK2 cells incubated with 10 ng/ml of TGF-β1 and 0, 5, 10, 20 μg/ml LMWF for 48 h was detected by Western blot analysis. Representative blotting (left) and quantification of protein levels (right) are shown. Mean ± SEM. n=3; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. control group; *P < 0.05, **P < 0.01 vs. TGF-β1 group.
The expression of epithelial phenotypic marker, E-cadherin, as well as the mesenchymal phenotypic markers, α-SMA and vimentin, were detected by Western blot analysis (Figure 1B). In TGF-β1 group, 10 ng/ml of TGF-β1 resulted in a significant increase in α-SMA and vimentin expression, and a decrease in E-cadherin expression. Furthermore, the expression of CTGF and fibronectin was also altered, indicating a switch to an EMT phenotype in HK2 cells (Figure 1B). Those changes in protein expression were inhibited by treatment with LMWF in a dose-dependent manner (Figure 1B).
Effect of LMWF on downstream pathways of TGF-β1 in HK2 cells
Next, we investigated whether the Akt, ERK, p38 and Smad3 pathways (crucial downstream pathways of TGF-β1) were affected by LMWF in the cell model. Figure 2 shows that the phosphorylation of Akt, ERK1/2, p38, Smad3 and the expression of Snail, ZEB1 were significantly increased in HK2 cells incubated with TGF-β1 for 48 h. LMWF significantly decreased the phosphorylation of Akt, ERK1/2, p38, Smad3 and the expression of Snail, ZEB1 in a dose dependent manner.
Figure 2.

LMWF inhibited downstream pathways of TGF-β1 in HK2 cells. Expression and phosphorylation levels of p-Akt, Akt, p-ERK1/2, ERK2, p-p38, p38, p-Smad3, Smad3, Snail, ZEB1 and β-actin of HK2 cells incubated with 10 ng/ml TGF-β1 and 0, 5, 10, 20 μg/ml LMWF for 48 h were detected by Western blot analysis. Representative blotting (left) and quantification of protein levels (right) are shown. Mean ± SEM. n=3; ##P < 0.01, ###P < 0.001 vs. control group; *P < 0.05 vs. TGF-β1 group.
Effect of LMWF on renal function and injury of GK rats
To confirm the protective effects of LMWF on EMT found in in vitro model, GK rats were used as a DN model. The physiological metabolic parameters in GK and control groups were observed at 24 weeks of age. The GK rats had reduced body weight (418.6 ± 7.0 vs. 629.8 ± 8.1 g, P < 0.01), increased the blood glucose (16.2 ± 1.2 vs. 6.5 ± 0.1 mM, P < 0.01), increased kidney index (KW/BW; 4.17 ± 0.04 vs. 2.59 ± 0.05 mg/g, P < 0.01), proteinuria (460.3 ± 42.1 vs. 84.6 ± 16.3 mg/24 h/kg body weight, P < 0.001) compared to control rats, indicating a successful DN rat model. Treatment of LMWF significantly decreased kidney index and urine total protein compared with those in GK group (3.70 ± 0.07 vs. 4.17 ± 0.04 mg/g and 265.6 ± 30.3 vs. 460.3 ± 42.1 mg/24 h/kg body weight, P < 0.01), but had no effect on body weight change (400.3 ± 6.4 g) and blood glucose (17.7 ± 1.4 mM).
GK rats had the renal dysfunction at 24 weeks of age, and LMWF significantly protected renal function, as demonstrated by changes of BUN and blood creatinine levels in different groups (Figure 3A and 3B).
Figure 3.

LMWF attenuated renal functional defect and tissue damage in GK rats. GK rats were administered with vehicle or LMWF daily for 12 weeks from 12 weeks of age. Serum and kidneys were collected for renal function test and histological examination. A. Serum BUN. B. Serum creatinine concentration. C. Representative images of H&E, periodic acid-Schiff and Masson’s trichrome staining of kidney (original magnification ×400). Data are presented as mean ± SEM. n=5-8 for each group. *P < 0.05; **P < 0.01.
The kidney injury was determined using hematoxylin and eosin, PAS and Masson’s trichrome staining of the renal tissue (Figure 3C). The renal tissue appeared normal in controls. In contrast, it was observed that diabetes-induced histopathological changes in the renal tissues, including the expanded mesangial matrix and thicken glomerular basement. LMWF could prevent the glomerulosclerosis as demonstrated by reduced mesangial expansion and extracellular matrix deposition. Treatment with LMWF also reduced the tubulointerstitial fibrosis compared with GK group (TFI: 0.21 ± 0.02 vs. 0.68 ± 0.17, P < 0.05).
Effect of LMWF on renal fibrosis in GK rats
We detected the expression of TGF-β1 by Western blot analysis (Figure 4). GK rats at 24 weeks of age had increased TGF-β1 protein expression that was attenuated by LMWF. α-SMA protein expression increased significantly, whereas E-cadherin protein expression decreased significantly. The expression of CTGF and fibronectin increased significantly in diabetic kidneys, suggesting increased ECM. These results indicate that EMT may occur in GK rat kidneys. Treatment with LMWF effectively reduced α-SMA, CTGF and fibronectin expression and increased E-cadherin expression.
Figure 4.
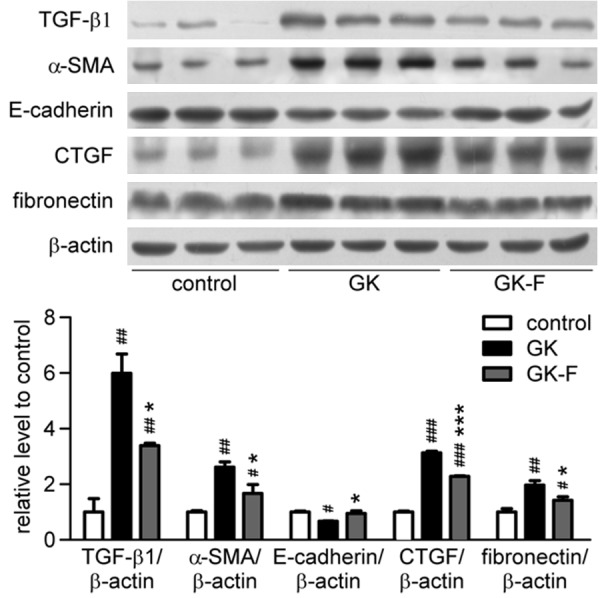
LMWF reduced renal interstitial fibrosis in GK rats. Expression of TGF-β1, E-cadherin, α-SMA, CTGF, fibronectin and β-actin of kidneys at 24 weeks of age was detected by Western blot analysis. Representative blotting (up) and quantification of protein levels (down) are shown. Mean ± SEM. n=3; #P < 0.05, ##P <0.01, ###P < 0.001 vs. control group; *P < 0.05, ***P < 0.001 vs. GK group.
We also investigated whether the Akt, ERK, p38, Smad pathways were affected by LMWF in diabetic kidneys. As shown in Figure 5, the GK rats at 24 weeks of age had increased phosphorylation of Akt, ERK1/2, p38 and Smad3 in kidneys, which was significantly reduced by LMWF treatment. The result was consistent with the reduced TGF-β1 in LMWF group.
Figure 5.

LMWF inhibited Akt, ERK, p38, Smad signaling pathway in kidney of GK rats. Expression of p-Akt, p-ERK1/2, p-p38, p-Smad3 of kidneys at 24 weeks of age was detected by Western blotting, and then normalized versus nonphosphorylated forms (total forms). Representative blotting (up) and quantification of protein levels (down) are shown. Mean ± SEM. n=3; #P < 0.05, ##P < 0.01 vs. control group; *P < 0.05 vs. GK group.
Effect of LMWF on DN of STZ rats
To confirm the effects of LMWF on DN found in GK rats, we used another in vivo DN model induced by STZ. We detected physiological metabolic parameters in STZ rats after 12 weeks of induced experimental diabetes. Compared with control rats, STZ rats had reduced body weight (503.3 ± 16.2 vs. 275.5 ± 12.1 g, P < 0.01), increased the blood glucose (7.56 ± 0.13 vs. 26.70 ± 0.91 mM, P < 0.01), renal dysfunction with significant increases in blood creatinine and BUN (Figure 6A and 6B), indicating a successful diabetic model with DN. Treatment of LMWF protected renal function, as demonstrated by reduced BUN and blood creatinine, but had no effect on body weight change (315.3 ± 12.3 g) and blood glucose (23.23 ± 1.63 mM) compared with STZ group.
Figure 6.

LMWF alleviated DN of STZ rats. Rats were treated for 12 weeks with LMWF daily. A. Serum BUN. B. Serum creatinine. Data are presented as mean ± SEM. n=8 for each group. *P < 0.05, **P < 0.01. C. Representative images of H&E, periodic acid-Schiff (PAS) and Masson’s trichrome staining of kidney (original magnification ×400, scale bar: 100 mm). D. Expression of TGF-β1, α-SMA, E-cadherin, CTGF, fibronectin, β-actin, p-Akt, Akt, p-ERK1/2, ERK2, p-p38, p38, p-Smad3, Smad3 in kidneys treated for 12 weeks were detected by Western blot analysis. Representative blotting (left) and quantification of protein levels (right) are shown. Mean ± SEM, n=3; #P < 0.05, ##P < 0.01, ###P < 0.001 vs control group; *P < 0.05 vs STZ group.
We also detected renal fibrosis in STZ rats. As shown in Figure 6C, STZ rats had increased TGF-β1 expression, which was attenuated by LMWF. The α-SMA expression increased significantly, whereas the E-cadherin expression decreased significantly in kidneys after 12 weeks of STZ administration. Treatment with LMWF significantly enhanced E-cadherin expression and reduced α-SMA, CTGF and fibronectin expression. As we predicted, the Akt, ERK, p38, Smad pathways were also affected by LMWF in diabetic kidneys. Figure 6C shows that the STZ rats had increased phosphorylation of Akt, ERK1/2, p38 and Smad3 in kidneys, which was significantly reduced by LMWF treatment.
Discussion
Renal fibrosis is the pivotal process underlying the progression of CKD to ESRD. It is characterized by a series of responses involving glomerulosclerosis, TIF and alterations in renal vasculature. Of these, TIF is a manifestation of ESRD and is an important determinant of progressive renal injury. EMT is thought to be a critical event in the pathogenesis of TIF. So, the current study aimed to investigate the possible protective effects of LMWF against DN, especially TIF and EMT using different in vitro and in vivo experimental models.
HK2 cells are a proximal tubular cell line derived from a normal kidney. It has been reported that the activation of TGF-β signaling is sufficient to induce EMT and several signaling pathways have been implicated in TGF-β-induced EMT in HK2 cells [24,25].In present study, TGF-β1 decreased expression of E-cadherin, and increased expression of α-SMA, vimentin and synthesized extracellular matrix molecules, such as fibronectin in HK2 cells. Treatment with LMWF remarkably preserved the epithelial-like morphology, restored the expression of EMT marker protein. These results suggest that LMWF may exert negative regulation on TGF-β induced EMT.
It is recognized that TGF-β/Smad signaling plays an essential role in renal fibrosis by stimulating fibrogenic cells to produce ECM proteins and inducing transformation of tubular epithelial cells to myofibroblasts through EMT. TGF-β binds to the receptor, which interacts with Smad2 and Smad3, and then forms a complex with Co-Smad4. The complex binds to the promoters of TGF-β target genes and regulates their transcription [26]. In addition to activating Smad signals, TGF-β signaling can regulate activity of a number of downstream signaling molecules, such as mitogen activated protein kinases (MAPKs) and PI3K-Akt [27]. In addition, these non-canonical signals can crosstalk with the Smad pathways. MAPKs involved in hyperglycemia-induced TGF-β signaling include extracellular signal regulated kinases (ERK1 and 2, p44/p42 MAPKs) and p38 MAP kinase. Activation of Akt pathway is required for induction of TGF-β-dependent EMT [28]. Akt can increase Snail expression and induce EMT through phosphorylation of IKKα [29] and inhibition of GSK-3β that has been characterized as a main kinase responsible for the subcellular location and protein stability of Snail [30,31].
Previous studies suggested fucoidan could have an anti-fibrotic effect through the TGF-β1/Smad3 pathway on DMN-induced liver fibrosis [32] and on CCl4-inducedliverfibrosis in rats [33]. A recent study found that fucoidan could suppress proliferation of breast cancer cells and expression of EMT biomarkers [34]. Our results show that all these pathways, Smad, ERK, p38 and Akt, were activated in both renal tissues of diabetic animal models and HK2 cells incubated with TGF-β1. However, the changes of these signaling pathways were all attenuated by LMWF. Interestingly, LMWF reduced the expression of transcription factor snail and ZEB1 induced by TGF-β1. The snail zinc-finger protein is related to the numerous transcription factors regulating EMT [35], and ZEB1 is able to initiate EMT by binding to E-boxes within the E-cadherin promoter and repressing its transcription [36]. Together, these results indicate that LMWF might suppress EMT and TIF by blocking TGF-β activated downstream signals.
Numerous studies have shown that TGF-β is a key mediator of fibrosis in both experimental and clinical CKD. Thus, in vitro results prompted us further to evaluate the efficacy of LMWF by using in vivo diabetic models. Unlike other diabetic models, the GK rat has been claimed to be a model of genetic non-obese type 2 diabetes mellitus that exhibits mild hyperglycemia, known to develop DN, and has been widely used in diabetes research [37,38]. In this study, the untreated 24-week-old GK rats showed several signs of DN. LMWF did not influence blood glucose. However, urinary protein, kidney index, blood creatinine and BUN were decreased in LMWF treated GK rats compared with untreated GK rats. LMWF treatment significantly decreased fibronectin, TGF-β1 and CTGF expression in the kidney of GK rats. In addition, the activation of renal Akt, ERK, p38, Smad pathways was significantly inhibited by LMWF treatment. STZ rats are another classical diabetic model used for studying the pharmacological activities of drugs on DN and related mechanisms. The present study shows that LMWF treatment also attenuated progressive diabetic renal injury in STZ rats, consistent with the results obtained in GK rats. However, there was no effect of LWMF on existing diabetic renal injury in GK rats and STZ rats treated with LMWF after DN occurring (data not shown).
Considering the complex pathogenic factors contributing to renal damage in DN, as well as the various pharmacological effects of LMWF, it is possible that other factors or pathways might mediate the renal beneficial effects of LMWF.LMWF may, therefore, be able to modulate endothelial cell function to attenuate inflammation, fibrosis, and further injury in DN. Moreover, fucoidan [17,19,20] and LMWF [21] were previously reported to improve glucose homeostasis and lipid profiles in some experimental models. In our study, LMWF did not reduce plasma glucose concentration in two diabetic animal models.
In conclusion, our results demonstrate that LMWF treatment attenuated renal dysfunction and fibrogenesis in kidneys of type 1 and type 2 diabetic rats. The renal beneficial effect of LMWF might be associated with its blockage of TIF via repressing TGF-β1 and the downstream pathways, which was proved by both in vivo and in vitro models. The present study suggests that LMWF may be developed as a candidate drug to prevent or inhibit fibrotic progression in DN.
Acknowledgements
This work was supported by National Natural Science Foundation of China grants 81370783, 41376166 and 81170632, the 111 Project, International Science & Technology Cooperation Program of China 2012DFA11070 and Shanghai Pharmaceutical Association grant 2014-YY-03-04.
Disclosure of conflict of interest
None.
References
- 1.Gray SP, Cooper ME. Diabetic nephropathy in 2010: Alleviating the burden of diabetic nephropathy. Nat Rev Nephrol. 2011;7:71–73. doi: 10.1038/nrneph.2010.176. [DOI] [PubMed] [Google Scholar]
- 2.Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol. 2000;15:290–301. doi: 10.1007/s004670000461. [DOI] [PubMed] [Google Scholar]
- 3.Fioretto P, Caramori ML, Mauer M. The kidney in diabetes: dynamic pathways of injury and repair. The Camillo Golgi Lecture 2007. Diabetologia. 2008;51:1347–1355. doi: 10.1007/s00125-008-1051-7. [DOI] [PubMed] [Google Scholar]
- 4.Meran S, Steadman R. Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol. 2011;92:158–167. doi: 10.1111/j.1365-2613.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeisberg M, Kalluri R. The role of epithelialto-mesenchymal transition in renal fibrosis. J Mol Med (Berl) 2004;82:175–181. doi: 10.1007/s00109-003-0517-9. [DOI] [PubMed] [Google Scholar]
- 6.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hills CE, Squires PE. TGF-beta1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. Am J Nephrol. 2010;31:68–74. doi: 10.1159/000256659. [DOI] [PubMed] [Google Scholar]
- 8.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 9.Sharma K, Ziyadeh FN. The emerging role of transforming growth factor-beta in kidney diseases. Am J Physiol. 1994;266:F829–842. doi: 10.1152/ajprenal.1994.266.6.F829. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava SP, Koya D, Kanasaki K. MicroRNAs in kidney fibrosis and diabetic nephropathy: roles on EMT and EndMT. Biomed Res Int. 2013;2013:125469. doi: 10.1155/2013/125469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35:83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Okada H, Kikuta T, Kobayashi T, Inoue T, Kanno Y, Takigawa M, Sugaya T, Kopp JB, Suzuki H. Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J Am Soc Nephrol. 2005;16:133–143. doi: 10.1681/ASN.2004040339. [DOI] [PubMed] [Google Scholar]
- 13.Lee HS. Paracrine role for TGF-beta-induced CTGF and VEGF in mesangial matrix expansion in progressive glomerular disease. Histol Histopathol. 2012;27:1131–1141. doi: 10.14670/HH-27.1131. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Wang W, Zhang Q, Li F, Lei T, Luo D, Zhou H, Yang B. Low molecular weight fucoidan against renal ischemia-reperfusion injury via inhibition of the MAPK signaling pathway. PLoS One. 2013;8:e56224. doi: 10.1371/journal.pone.0056224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q, Li Z, Xu Z, Niu X, Zhang H. Effects of fucoidan on chronic renal failure in rats. Planta Med. 2003;69:537–541. doi: 10.1055/s-2003-40634. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Wang F, Yun H, Zhang H, Zhang Q. Effect and mechanism of fucoidan derivatives from Laminaria japonica in experimental adenine-induced chronic kidney disease. J Ethnopharmacol. 2012;139:807–813. doi: 10.1016/j.jep.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Kim KJ, Yoon KY, Lee BY. Fucoidan regulate blood glucose homeostasis in C57BL/KSJ m+/+db and C57BL/KSJ db/db mice. Fitoterapia. 2012;83:1105–1109. doi: 10.1016/j.fitote.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Kim KT, Rioux LE, Turgeon SL. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry. 2014;98:27–33. doi: 10.1016/j.phytochem.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Corona DM, Martinez-Abundis E, Gonzalez-Ortiz M. Effect of Fucoidan administration on insulin secretion and insulin resistance in overweight or obese adults. J Med Food. 2014;17:830–832. doi: 10.1089/jmf.2013.0053. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Liu H, Li N, Zhang Q, Zhang H. The protective effect of fucoidan in rats with streptozotocin-induced diabetic nephropathy. Mar Drugs. 2014;12:3292–3306. doi: 10.3390/md12063292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong YT, Kim YD, Jung YM, Park DC, Lee DS, Ku SK, Li X, Lu Y, Chao GH, Kim KJ, Lee JY, Baek MC, Kang W, Hwang SL, Chang HW. Low molecular weight fucoidan improves endoplasmic reticulum stress-reduced insulin sensitivity through AMP-activated protein kinase activation in L6 myotubes and restores lipid homeostasis in a mouse model of type 2 diabetes. Mol Pharmacol. 2013;84:147–157. doi: 10.1124/mol.113.085100. [DOI] [PubMed] [Google Scholar]
- 22.Yang W, Yu X, Zhang Q, Lu Q, Wang J, Cui W, Zheng Y, Wang X, Luo D. Attenuation of streptozotocin-induced diabetic retinopathy with low molecular weight fucoidan via inhibition of vascular endothelial growth factor. Exp Eye Res. 2013;115:96–105. doi: 10.1016/j.exer.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Cui W, Zheng Y, Zhang Q, Wang J, Wang L, Yang W, Guo C, Gao W, Wang X, Luo D. Low-molecular-weight fucoidan protects endothelial function and ameliorates basal hypertension in diabetic Goto-Kakizaki rats. Lab Invest. 2014;94:382–393. doi: 10.1038/labinvest.2014.12. [DOI] [PubMed] [Google Scholar]
- 24.Hills CE, Siamantouras E, Smith SW, Cockwell P, Liu KK, Squires PE. TGFbeta modulates cell-to-cell communication in early epithelial-to-mesenchymal transition. Diabetologia. 2012;55:812–824. doi: 10.1007/s00125-011-2409-9. [DOI] [PubMed] [Google Scholar]
- 25.Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E, Bottinger EP. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci U S A. 2001;98:6686–6691. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng XM, Chung AC, Lan HY. Role of the TGF-beta/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond) 2013;124:243–254. doi: 10.1042/CS20120252. [DOI] [PubMed] [Google Scholar]
- 27.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3’ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 29.Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A, Larue L. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26:7445–7456. doi: 10.1038/sj.onc.1210546. [DOI] [PubMed] [Google Scholar]
- 30.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 31.Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelialmesenchymal transition. J Cell Biol. 2005;168:29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong SW, Jung KH, Lee HS, Zheng HM, Choi MJ, Lee C, Hong SS. Suppression by fucoidan of liver fibrogenesis via the TGF-beta/Smad pathway in protecting against oxidative stress. Biosci Biotechnol Biochem. 2011;75:833–840. doi: 10.1271/bbb.100599. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi S, Itoh A, Isoda K, Kondoh M, Kawase M, Yagi K. Fucoidan partly prevents CCl4-induced liver fibrosis. Eur J Pharmacol. 2008;580:380–384. doi: 10.1016/j.ejphar.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu HY, Lin TY, Hwang PA, Tseng LM, Chen RH, Tsao SM, Hsu J. Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent TGFbeta receptor degradation in breast cancer. Carcinogenesis. 2013;34:874–884. doi: 10.1093/carcin/bgs396. [DOI] [PubMed] [Google Scholar]
- 35.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 36.Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol. 2004;48:365–375. doi: 10.1387/ijdb.041794hp. [DOI] [PubMed] [Google Scholar]
- 37.Goto Y, Suzuki K, Ono T, Sasaki M, Toyota T. Development of diabetes in the non-obese NIDDM rat (GK rat) Adv Exp Med Biol. 1988;246:29–31. doi: 10.1007/978-1-4684-5616-5_4. [DOI] [PubMed] [Google Scholar]
- 38.Phillips AO, Baboolal K, Riley S, Grone H, Janssen U, Steadman R, Williams J, Floege J. Association of prolonged hyperglycemia with glomerular hypertrophy and renal basement membrane thickening in the Goto Kakizaki model of non-insulin-dependent diabetes mellitus. Am J Kidney Dis. 2001;37:400–410. doi: 10.1053/ajkd.2001.21322. [DOI] [PubMed] [Google Scholar]


