Abstract
Metabolic disease encompasses several disorders including obesity, type 2 diabetes, and dyslipidemia. Recently, the incidence of metabolic disease has drastically increased, driven primarily by a worldwide obesity epidemic. Transgenerational inheritance remains controversial, but has been proposed to contribute to human metabolic disease risk based on a growing number of proof-of-principle studies in model organisms ranging from C. elegans to M. musculus to S. scrofa. Collectively, these studies demonstrate that heritable risk is epigenetically transmitted from parent to offspring over multiple generations in the absence of a continued exposure to the triggering stimuli. A diverse assortment of initial triggers can induce transgenerational inheritance including high-fat or high-sugar diets, low-protein diets, various toxins, and ancestral genetic variants. Although the mechanistic basis underlying the transgenerational inheritance of disease risk remains largely unknown, putative molecules mediating transmission include small RNAs, histone modifications, and DNA methylation. Due to the considerable impact of metabolic disease on human health, it is critical to better understand the role of transgenerational inheritance of metabolic disease risk to open new avenues for therapeutic intervention and improve upon the current methods for clinical diagnoses and treatment.
Keywords: Transgenerational inheritance, Intergenerational inheritance, epigenetics, epigenome, obesity, metabolism
1. Introduction
Metabolic disease includes obesity, type 2 diabetes (T2D), insulin resistance, atherosclerosis, hyperlipidemia, and hepatic steatosis. This class of disorders is driven by abnormalities in the conversion of food to energy, often as the result of prolonged under- or overnutrition. This shared etiology often results in the clustering of multiple metabolic risk factors in an individual, which is referred to as metabolic syndrome [1]. The prevalence of metabolic disease has increased dramatically over the past few decades, largely driven by a worldwide obesity epidemic [2,3]. Collectively, comorbidities of obesity now cause 3 million premature deaths annually worldwide and make obesity the sixth leading risk factor for loss of health and life [4,5]. Given the considerable impact on human health, it is critical to better understand the factors driving the recent increase in metabolic disease.
Metabolic disease can result from mutations in single genes such as leptin (obesity), peroxisome proliferator-activated receptor gamma (T2D), and apolipoprotein C-II (hypertriglyceridemia) [6–8]. However, monogenic disease causing mutations are relatively rare, and metabolic disease is more often the result of a complex and poorly understood relationship between multiple genetic risk factors and the environment. Estimates of the heritable contribution to adiposity-related traits are typically between 45-75%, with the remaining phenotypic variability the result of environmental factors or gene-environment interactions [9]. Heritability estimates of other metabolic traits including levels of glucose, insulin, triglycerides, cholesterol, and blood pressure are similar [10,11]. Unfortunately, pinpointing the exact genetic risk factors for most complex multifactorial traits and diseases has proven challenging [12,13]. Genome wide association studies for many complex traits and diseases, including height and body mass index (BMI), have estimated that all common single nucleotide polymorphisms (SNPs) collectively account for approximately half of trait heritability in an additive fashion [11,14–17]. The nature of the remaining half of the heritable risk remains unknown, but various hypotheses have been proposed that implicate epistasis, rare variants, allelic heterogeneity, locus heterogeneity, small effect sizes, copy number variants, and epigenetics [12,13,18]. It is likely that each of these mechanisms contributes to human variation and disease, with this review focusing on the subset of epigenetic effects that demonstrate transgenerational inheritance.
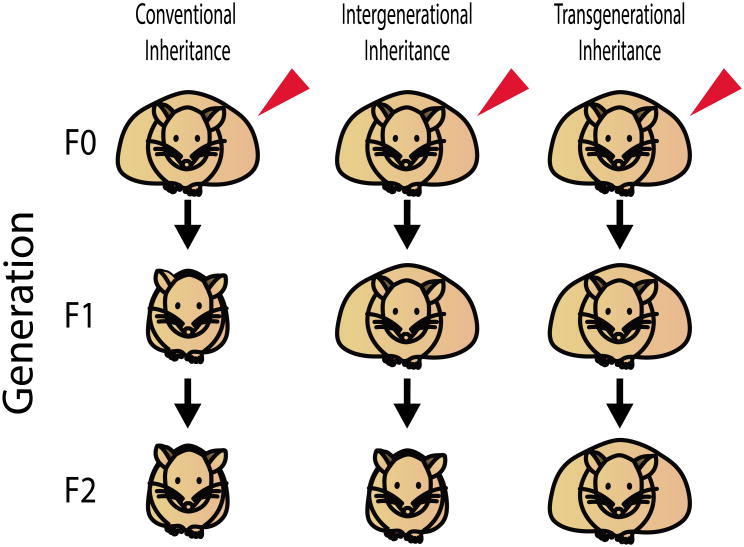
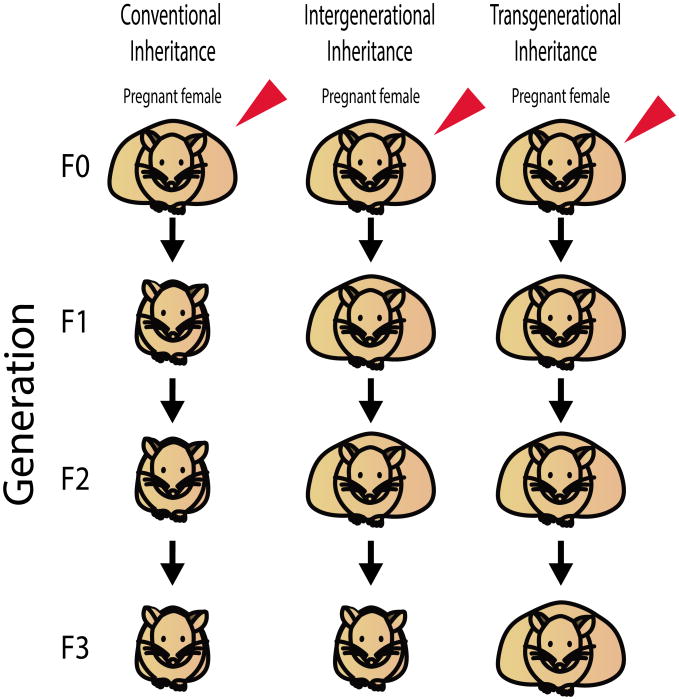
Transgenerational epigenetic inheritance refers to the transmission of phenotypes over generations that are not due to inherited changes in the primary DNA sequence [19,20]. Transgenerational effects are passed across generations in the absence of exposure to the original trigger to either the developing fetus or the germ cells that will eventually become the fetus [21]. Thus, when inherited through the paternal lineage, transgenerational inheritance is established by phenotypes transmitted for 2 generations, to the grandchildren (F2 generation) (Figure 1). When inherited through the maternal lineage, transgenerational inheritance is similarly established by phenotypes transmitted 2 generations to the grandchildren, unless the F0 female is pregnant during exposure to the triggering event. In this case, the presence of both the fetus in utero and the developing germ cells within the fetus require phenotypic transmission for 3 generations to the great-grandchildren (F3 generation) to establish transgenerational inheritance (Figure 2). This review is restricted to studies meeting this strict definition of transgenerational inheritance, with other excellent reviews provide a more comprehensive review of epigenetic inheritance, describing both transgenerational and intergenerational inheritance [22].
Figure 1. Characteristics of epigenetic and conventional inheritance patterns.
The red arrow indicates a triggering event (genetic or environmental) in the F0 generation. Obesity is shown as a representative phenotype. Although the F0 mice are represented as obese, the triggering event need not induce a phenotype in the F0 generation and may instead be restricted to future offspring. Phenotypic transmission to offspring is indicated by an obese mouse in the F1-F2 generations, whereas a lean mouse in the F1-F2 generations indicates failure to transmit the phenotype. Intergenerational inheritance describes phenotypic transmission to the F1 generation. Transgenerational inheritance describes phenotypic transmission to the F2 generation.
Figure 2. Characteristics of epigenetic and conventional inheritance patterns in pregnant females.
The red arrow indicates a triggering event (genetic or environmental) in the F0 generation. Obesity is shown as a representative phenotype. Although the F0 mice are represented as obese, the triggering event need not induce a phenotype in the F0 generation and may instead be restricted to future offspring. Phenotypic transmission to offspring is indicated by an obese mouse in the F1-F3 generations, whereas a lean mouse in the F1-F3 generations indicates failure to transmit the phenotype. Intergenerational inheritance describes phenotypic transmission to the F1 or F2 generation. Transgenerational inheritance describes phenotypic transmission to the F3 generation, due to the presence of germ cells in utero of the F0 female that will eventually develop in the F2 generation.
A trait can be transgenerationally inherited resulting from both environmental exposures such as diet or toxins or by ancestral genetic variants [23,24]. The phenotype can be passed from one generation to the next via cultural inheritance, microbiota, or through the germline [20]. Cultural inheritance refers to the often shared learning and choices of relatives, including smoking and alcohol consumption [25]. Another example is the prion-mediated inheritance of kuru, a lethal neurological disorder found among the Fore people of Papua New Guinea. Kuru was at first mistaken to be a Mendelian inherited genetic disorder, but was later shown to be mediated by a prion protein transmitted through the practice of cannibalism of deceased relatives [26]. Microbiota are also shared vertically within a family, as an individual's early microbiome in particular is in part acquired via maternal transmission during delivery, with long term effects on metabolism [27,28]. Finally, transgenerational inheritance can be transmitted through the germline, including through both male and female germ cells, which carry dozens of epigenetic marks on the genomic DNA and the histones upon which the genomic DNA is wrapped [19,20]. Importantly, although many epigenetic marks were largely considered absent in human sperm, many nucleosomes remain associated with chromatin in sperm and DNA methylation not only remains present at specific loci, but is hypermethylated at many loci relative to later embryonic stages [29,30]. Of note, it is unlikely that all epigenetic marks have been discovered, as highlighted by the recent identifications of many novel histone marks including lysine crotonylation and glutamine methylation [31]. In addition to the epigenetic marks, germ cells carry RNA molecules such as mRNAs, miRNA, and piRNAs, as well as metabolites and proteins that may be important for mediating transgenerational inheritance.
The epigenetic marks that modify histones and DNA are both responsive to nutrient status, acting as metabolic sensors that translate nutritional cues into gene expression changes [32–34]. For example, glucose availability regulates global histone acetylation levels in an ATP-citrate lyase dependent manner [35]. In addition, many micronutrients, such as choline, that regulate the folate cycle and therefore levels of the methyl donor S-adenosylmethionine have downstream effects on DNA methylation [36]. However, it remains controversial whether this information can be stably transmitted through the germline. Although it is easy to imagine an evolutionary benefit from signaling future offspring to alter their metabolism in response to current environmental cues, various experimental challenges have largely precluded the rigorous study of transgenerational inheritance of metabolic traits in humans [37]. These include a paucity of data from multi-generational pedigrees, the dynamic nature of the epigenome, and the relative inaccessibility of germ cells and key metabolic tissues. Thus, the contribution of transgenerational inheritance to metabolic disease in humans remains unclear [38,39]. Nonetheless, there is mounting evidence from model organisms ranging from nematodes to rodents demonstrating that transgenerational inheritance of disease risk can have phenotypic effects on par with risk factors inherited in a Mendelian fashion. These data, together with suggestive evidence of transgenerational inheritance in humans, hint that this unconventional mode of inheritance may represent an underappreciated contributor to metabolic disease susceptibility (Table 1).
Table 1. List of studies demonstrating transgenerational inheritance of metabolic disease.
| Species | Phenotype | Stimuli | Phenotype observed | Type of Epigenetic Modification (generation & tissue studied) | Reference |
|---|---|---|---|---|---|
| C. elegans | Increased lifespan | Starvation | F3 | Small RNAs (F0, F3 whole organism) | Rechavi et al. 2014 |
| C. elegans | Decreased fecundity | Glucose enriched diet | F2 | H3K4 methylation changes in F0, not F2 (F0-F2 whole organism) | Tauffenberger and Parker 2014 |
| D. melanogaster | Altered metabolism | Glucose enriched diet | F2 | N.D. | Buescher et al. 2013 |
| M. musculus | Obesity, T2D, tumor susceptibility, yellow fur | IAP retrotransposon | F2 | DNA methylation (F2 tail) | Morgan et al. 1999 |
| M. musculus | Obesity resistance | Gene x HFD interaction | F3 | N.D. | Yazbek et al. 2010 |
| M. musculus | Increased adiposity | HFD | F2 | mRNA, miRNA, DNA methylation (F0 testes, sperm) | Fullston et al. 2013 |
| M. musculus | Impaired glucose homeostasis | Low protein diet during lactation | F3 | N.D. | Frantz et al. 2011 |
| M. musculus | Improved glucose tolerance | Maternal HFD | Male F3 | N.D. | Dunn and Bale 2011 |
| M. musculus | Increased adiposity, NAFLD | TBT | F3 | mRNA (F1-F3 MSCs, liver) | Chamorro-Garcia et al. 2013 |
| M. musculus | Glucose intolerance | HFD and streptozocin | F2 | mRNA (F1), DNA methylation (F1, F2 sperm, pancreatic islets) | Wei et al. 2014 |
| R. rattus | Increased body weight, kidney and ovary disease | Methoxychlor | F4 | DNA methylation (F3 sperm) | Manikkam et al. 2014 |
| R. rattus | Increased body weight, testis/ovarian disease | Endocrine disruptors | F3 | DNA methylation (F3 sperm) | Manikkam et al. 2013 |
| R. rattus | Increased body weight | DDT | F4 | DNA methylation (F3 sperm) | Skinner et al. 2013 |
| S. scrofa | Decreased body weight | Methyl-supplemented diet | F2 | mRNA, DNA methylation (F2 muscle, liver, kidney) | Braunschweig et al. 2012 |
| H. sapiens | Increased diabetes mortality | Overnutrition | F2 | N.D. | Kaati et al. 2002 |
| H. sapiens | Prader-Willi Syndrome | Imprinting defect | F2 | DNA methylation (F2 sperm) | Buiting et al. 2003 |
TBT, tributyltin. DDT, dichlorodiphenyltrichloroethane. HFD, high fat diet. IAP, intracisternal A particle. NAFLD, non-alcoholic fatty liver disease. MSC, mesenchymal stem cell. T2D, type 2 diabetes. N.D., not done
2. Transgenerational Effects on Metabolic Disease in Model Organisms
Model organisms are valuable for studying transgenerational inheritance because of the precise control afforded over most aspects of their genetic makeup and environmental conditions. Experimental advantages when studying model organisms include invasive phenotyping, short generation times, low cost of maintenance, and precise control over diet, infection status, pedigree, and genetic background. Thus, a single genetic or environmental variable can be rigorously tested over multiple generations in a way that is not possible in human studies. It is also important to note that although questions have been raised regarding the ability to translate discoveries made in model organism to clinical benefits for humans [40,41], there is a high concordance between animal models and humans for both genotype-phenotype relationships for monogenic metabolic disease [42,43] and the effectiveness of weight loss compounds [44]. These similarities suggest that insight into transgenerational inheritance gained from studies of model organisms will translate to the study of human metabolic disease.
2.1 C. elegans
Despite difficulties associated with carrying out thorough metabolic phenotyping in C. elegans, many advances in understanding the pathophysiology of metabolic disease have been made using this powerful model organism. This power can be illustrated by the ability to conduct both high-throughput genetic and chemical screens. The utility of C. elegans in drug screening has improved considerably since the first high-throughput screen [45] thanks to advances in automation, liquid handling, and imaging analysis software [46]. Thus, a number of screens for compounds have been conducted including those for compounds that regulate food intake and for the evaluation of anti-obesity therapeutics [47,48]. Genomic siRNA-based screens have facilitated the identification of genes that regulate lipid content including those with similar functions in mammals such as Tubby, which is important for ciliary transport, as well as genes that encode fatty-acid and acyl-CoA-binding proteins and enzymes in the lipid biosynthesis pathway [49–51]. The three-day generation time of C. elegans also facilitates the rapid study of transgenerational effects. Importantly, C. elegans possesses several well conserved molecules with hypothesized involvement in epigenetic inheritance including transcription factors, histone modifications, and regulatory RNAs [52]. For example, post-translational histone modification signatures in C. elegans resemble that seen in D. melanogaster and mammals [53].
Two recent studies illustrate the power of C. elegans for investigating transgenerational inheritance of metabolic disease. The first is an elegant set of experiments by Rechavi and colleagues demonstrating that starvation alters metabolism for three subsequent generations via the inheritance of small RNAs [54]. Just six days of starvation, restricted to the L1 larvae developmental stage, increased lifespan of the F3 progeny by 37% relative to the F3 offspring of continuously fed worms [54]. The small RNA profiles of the F3 progeny from the starved line more closely resembled those of the starved F0 line than the continuously fed F0 line. Those differentially expressed small RNAs between the starved and fed lines were significantly enriched for targets associated with nutrient reservoir activity and thus represent a potential mechanism for the increased lifespan [54]. In contrast to the effects of starvation on lifespan, Tauffenberger and Parker discovered that worms from the commonly used wild-type strain N2 that were fed a glucose enriched diet had fewer progeny and a shorter lifespan relative to control fed N2 worms [55]. The F2 offspring of these worms also exhibited decreased fecundity relative to offspring of control worms, although lifespan was unchanged [55]. This study demonstrates how a single metabolic input (glucose) can have pleiotropic effects that can be separately inherited in either a transgenerational (fecundity) or non-transgenerational (lifespan) manner in subsequent generations.
2.2 D. melanogaster
Drosophila research has a long and rich history of developing and fine-tuning methodologies for genetic manipulation [56]. Many of these methodologies, such as unbiased forward genetic screens and the Gal4/UAS system, can be applied to allow researchers to use Drosophila as a means of studying transgenerational epigenetic inheritance of metabolic phenotypes [57]. To engineer models of metabolic disease, Drosophila strains have been generated with phenotypes resembling obesity and diabetes by genetically manipulating pathways associated with the Drosophila fat body, the main glycogen storage depot in Drosophila [58,59]. Additionally, dietary modifications such as high sugar diets are sufficient to induce obesity and insulin resistance in Drosophila [60,61]. Many of the pathways and genes involved in Drosophila metabolism have human orthologs, which, along with the short lifespan of Drosophila, make it a good model system for studying transgenerational effects [58,62–64]. There are however, limitations such as the lack of conclusive evidence for DNA methylation, despite the identification of a putative DNA methyltransferase Dmnt2 [65]. Whether Dnmt2 functions as either a DNA or RNA methyltransferase, or both, remains unclear, with this distinction important for the heritability of these marks by subsequent generations [66–68]. The existence of 5-methylcytosine in Drosophila genomic DNA was recently found in limited quantities using a sensitive high performance liquid chromotography and mass spectrophotometry-based approach, but other studies, including by whole-genome bisulfite sequencing, have failed to replicate these findings [69–74].
Evidence for transgenerational inheritance of metabolic disease comes from a study of newly hatched virgin females from the standard laboratory strain w1118 that were fed a diet containing either 0.15 mol/L sucrose or 1 mol/L sucrose for 7 days [75]. The females fed the high-sugar diet had elevated triacylglycerol (TAG), glycogen, and trehalose levels, although body weight was slightly decreased, perhaps as a consequence of the increased morbidity. F1 generation adult male offspring of high-sugar fed mothers exhibited reduced body weight and glucose levels even when fed the low-sugar diet. Challenging these F1 flies with a high-sugar diet further decreased their body weight, as well as increased their trehalose and TAG levels. To test for transgenerational effects, F1 virgin females fed the low sugar diet were mated and the F2 progeny were reared on the low sugar diet. F2 female larvae from the high-sugar lineage exhibited increased whole body trehalose and decreased TAG levels, whereas male larvae exhibited increased glucose and trehalose levels relative to the low-sugar lineage. This altered body composition of F2 larvae indicates that maternal diet effects metabolic programming for at least two generations.
2.3 Rodents
Perhaps the most intensively studied model of transgenerational inheritance is the agouti viable yellow mouse strain (Avy), with the resulting data highlighting both the promise and challenges associated with studying non-Mendelian inheritance. The Avy mutation is due to an intracisternal A particle (IAP) insertion 100 kilobases upstream of the agouti coding sequence [76]. Avy is a dominant allele that causes ectopic agouti expression resulting in yellow fur, obesity, T2D, and increased tumor susceptibility [76]. The severity of the phenotype is variable, and is correlated with the DNA methylation status of the IAP insertion such that Avy mice with yellow fur have decreased DNA methylation and increased agouti expression relative to Avy mice with brown fur [77]. The coat color, adiposity, and methylation status are maintained when inherited through the female germline, with the phenotypes of offspring correlated with the phenotypes of the dam [77,78]. In contrast, the paternally inherited allele is demethylated more rapidly and there is no epigenetic inheritance through the paternal lineage [79]. However, it remains controversial whether DNA methylation or other chromatin marks are the causal inherited mark [79,80].
Treatment of Avy mice with epigenetic modifying compounds highlights how targeting therapeutics towards the mechanism of transgenerational inheritance can be used to break the generational cycle of metabolic disease. For example, feeding Avy dams a methyl-supplemented diet that induces hypermethylation prevents the transgenerational inheritance of obesity [78]. However, the effect of diet-induced hypermethylation is itself not inherited in a transgenerational manner suggesting a distinct epigenetic mechanism [81]. In addition to methyl-donor supplementation, other therapeutic modifiers of the Avy phenotype include ethanol, genistein, and bisphenol A. Each, when fed to pregnant dams, modified the expression of the agouti coat color or obesity phenotype of Avy offspring [82–84]. However, whereas the effect of methyl-donor supplementation has been consistently replicated [85–87], the effects of bisphenol A and genistein were not [88].
IAP insertions are more resistant to epigenetic erasure than other DNA sequences, which may explain why the Avy mutation exhibits such a strong transgenerational inheritance pattern [89,90]. However, there are many other examples of transgenerational inheritance of metabolic disease beyond the Avy mouse model that are not related to IAP insertions. Obesity risk is one such phenotype that is transgenerationally transmitted with multiple triggering events, including both genetic and environmental exposures to specialized diets or toxins. Examples include a quantitative trait loci, Obrq2a, for which the allele derived from strain C57BL/6J is associated with increased adiposity and food intake relative to the allele derived from strain A/J [24]. F2 offspring of congenic mice carrying the Obrq2aA/J allele on an otherwise C57BL/6J background, but that did not inherit the A/J-derived allele of Obrq2a and were thus genetically identical to C57BL/6J mice, had reduced food intake and adiposity relative to control C57BL/6J mice. The transgenerational effects of the ancestral Obrq2aA/J allele were only observed when transmitted through the paternal lineage, with the body weight and food intake in the F2 generation equivalent to that of the F0 generation [24]. Environmental factors also influence adiposity in the F2 generation, with grandpaternal high-fat diet (HFD) feeding increasing adiposity in both male and female offspring 2 generations removed from the obesogenic diet [91]. The grandpaternal HFD feeding was associated with alterations in mRNA and miRNA expression in sperm, as well as a global reduction in DNA methylation [91]. These examples implicate paternal transgenerational transmission of obesity susceptibility to offspring, but maternal transmission of obesity risk has been demonstrated as well. Compounds such as dichlorodiphenyltrichloroethane (DDT), the pesticide methoxychlor, a mixture of plastic-derived endocrine disruptors, or the banned biocide tributyltin (TBT) when administered to pregnant rodents increased the body weight of both male and female offspring in the F3 generation [92– 96]. TBT exposure not only increased lipid accumulation in adipose tissue, but in hepatocytes as well, resulting in a phenotype resembling nonalcoholic fatty liver disease [92].
Glucose intolerance is also transgenerationally transmitted to offspring through both the maternal and paternal lineage. Male mice were made insulin resistant through a combination of HFD feeding and low dose streptozotocin (STZ) treatment, with the resulting F2 male offspring incurring similar impairments in glucose tolerance and insulin sensitivity [97]. DNA methylation near insulin signaling genes in islets was consistently increased (Pik3ca and Pik3r1) or decreased (Ptpn1) in both the F1 and F2 offspring of the F0 prediabetic male mice, suggesting a potential physiological explanation and a mechanism of inheritance [97]. Maternal HFD feeding during pregnancy and lactation had the reverse effect, with increased glucose tolerance in F3 mice, although the effect was restricted to male F3 offspring [98]. The improved glucose tolerance was inherited via both the paternal and maternal lineage, whereas a transgenerationally inherited increase in body size and body weight in the F3 female mice was solely inherited through the paternal lineage. The transgenerational inheritance of body size solely through the paternal lineage was correlated with increased variability in paternally expressed imprinted genes in the liver of F3 generation mice relative to maternally expressed imprinted genes [98]. A low protein diet fed to pregnant rats and maintained during lactation impaired glucose homeostasis in offspring from the F1 through F3 generations, although the phenotypic effect was diminished in the F3 generation [99]. The F1 though F3 generations of offspring from pregnant mice fed a low protein diet also had altered glucose homeostasis as reflected by decreased plasma insulin levels in the absence of changes in glucose levels [100]. The lower insulin levels were associated with decreased pancreatic mass and islet volume, although again the effects on pancreatic morphology began to diminish in the F3 generation [100].
2.4 Nonstandard model organisms
Although most biomedical research involving model organisms is focused on a limited number of species, other animal species not typically found in a laboratory setting can also provide a rich resource for studies of transgenerational inheritance. This includes many species, such as cows and pigs, that have significant economic value and often detailed pedigree information [101,102]. For example, Braunschweigh et al. has demonstrated that male pigs (boars) fed a diet to induce hypermethylation have F2 offspring with decreased adiposity [103]. The F2 offspring had changes in gene expression and DNA methylation detected in the liver and muscle tissue that were correlated with the reduced adiposity [103]. This suggests that the use of nonstandard model organisms is a relatively untapped resource for investigating transgenerational inheritance of metabolic disease.
3. Transgenerational Effects on Metabolic Disease in Humans
Significant experimental hurdles limit the ability to rigorously test for transgenerational inheritance of metabolic disease in humans. Thus, the question of whether transgenerational inheritance contributes to metabolic disease susceptibility remains unanswered [39,104]. Nonetheless, it is possible to partially circumvent these experimental challenges by taking advantage of natural experiments of human history, for example by examining the metabolic phenotypes of offspring from relatives that experienced a severe famine relative to those when food was abundant. The Överkalix region of northern Sweden provides one such natural experiment, with records spanning the 1800s of crop yields and overall mortality from cardiovascular disease and diabetes mellitus [105]. The analysis showed that if the paternal grandfather was exposed to excess food during the prepubertal growth period, the grandchildren had increased risk of diabetes mortality [105]. Suggestive data (0.05 < p < 0.11) from the same study also found that exposure of the paternal grandmother to famine during this same growth period protected from cardiovascular disease, but had increased risk of diabetes mortality [105]. Interestingly, these effects were found to be sex-specific, with the effect of the paternal grandfather's food supply limited to the mortality of grandsons, whereas the effect of the paternal grandmother's food supply was restricted to the mortality of granddaughters [106]. Of note, the multigenerational effects were highly dependent on the age at which the modified diets were eaten, with all effects observed when diet was modified during the prepubertal growth stage, but not during the pubertal growth stage [104]. Although these effects are consistent with transgenerational inheritance, whether they are inherited via the germline or through cultural inheritance remains unclear [107].
Another example of potentially transgenerational inheritance of a metabolic disorder in humans comes from the study of Prader-Willi Syndrome (PWS), which is a neurological disorder due to imprinting abnormalities linked to the proximal arm of chromosome 15 [108,109]. PWS is characterized by early onset obesity and hyperphagia [109]. A study of 19 PWS patients with no identified mutation in the imprinting center at 15q11-13, found that the imprinting defect always occurred on the chromosome inherited from the paternal grandmother [110]. This is highly unlikely to be due to chance and thus suggests that PWS resulted from a failure to erase the grandmaternal imprint during paternal spermatogenesis, and illustrates how abnormal inheritance of epigenetic marks over multiple generations in humans can lead to disease [110].
4. Mechanism of inheritance
In contrast to studies of conventionally inherited disease, studies of transgenerational inheritance have thus far been largely observational, providing few causal links between epigenomic variants and phenotypic effects. There are many reasons for this, including the experimental limitations related to quantitatively measuring the dynamic epigenome relative to cataloging static DNA sequence variants. Additionally, it is not known whether transgenerational inheritance is typically caused by a single or small number of changes in the epigenome or whether it is more often due to global changes in the epigenome. Finally, the lack of widely available methods for engineering changes to the epigenome has restricted the ability to test candidate variants. Nonetheless, progress has been made in correlating various epigenetic marks or inherited RNAs with transgenerational transmission of metabolic disease risk. These studies can be divided into 2 distinct categories. The first category investigates the mechanism of disease transmission to offspring by measuring the epigenome in germ cells. The second category investigates the physiological mechanism of disease through measurements of the epigenome in relevant somatic tissue such as liver, pancreas, or adipose. Whether epigenetic variation is consistently altered in both germ cells and somatic tissue, or whether germline variants trigger a signaling cascade resulting in distinct epigenetic modifications in somatic tissue remains unclear as few studies have investigated both germline and somatic tissue.
Epigenome analysis of the germline for metabolic disease has been limited to measurements of DNA methylation and thus likely presents a very limited picture of the potential mechanisms underlying the transgenerational inheritance of metabolic disease (Table 1). This is likely for technical reasons related to the relative simplicity of quantitatively measuring DNA methylation relative to other epigenetic marks rather than a strong biological reason supporting DNA methylation as the primary mechanism for the multi-generational transmission of disease risk. Nonetheless, multiple studies have identified variation in the levels of DNA methylation in sperm that are correlated with altered disease susceptibility in subsequent generations. For example, DNA methylation analysis of all promoter regions in the genome of F3 offspring from gestating female rats exposed to endocrine disrupting compound identified 197 promoters that were differentially methylated (DMRs) relative to control rats [93]. The 197 promoter regions were distributed throughout the genome, but were functionally clustered in the glial derived neurotrophic factor (Gdnf) and neurotrophin 3 (Ntf3) signaling pathways, and included 5 obesity-related genes (Tnfrsf12a, Esrra, Fgf19, Wnt10b, Gdnf) that could be related to the increased body weight in the F3 generation from the chemical-exposed lineage [93]. A similar analysis of sperm from F3 generation rats from a lineage treated with either DDT or the pesticide methoxychlor identified 39 and 37 DMRs of the genome respectively [94,95]. There was little overlap between the DMRs induced by the endocrine disrupting compounds, DDT, and methoxychlor suggesting that the effects of each compound on the epigenome are specific, despite the fact that all 3 compounds increased the body weight of F3 offspring [94]. Genome-wide DNA methylation was also profiled in sperm and pancreatic islets from prediabetic F1 offspring of male mice treated with STZ and HFD [97]. There were 6,021 intragenic DMRs identified in sperm and 7,681 DMRs in islets, of which 2,269 DMRs (38%) were in common. A functional role for these intragenic DMRs is strongly suggested by a comparison to 542 intergenic DMRs identified in sperm and 782 DMRs identified in islets, of which only 16 DMRs (3%) were shared between the 2 tissues. A subset of 10 DMRs with the most significant differences between the STZ/HFD treated and control lineages were also tested in sperm from the F2 generation, with consistent differences observed for each of the 10 DMRs in both the F1 and F2 generations [97].
Considering together the analysis of both germ cells and somatic tissue (Table 1), there remains primarily only correlative evidence linking specific classes of epigenomic variation with transmission of disease risk. The difficulty of gaining insight into the causal relationship between specific epigenomic changes and phenotype variation is highlighted by studies of the Avy mouse strain. Analysis of the Avy epigenome has the advantage that unlike other examples of transgenerational inheritance, the exact molecular lesion is known, an IAP insertion 100 kb upstream of the agouti promoter [76]. The degree of methylation of the IAP insertion correlates with the phenotypic expressivity of the mutant agouti allele as reflected by coat color [85]. However, DNA methylation of the maternally inherited Avy allele is absent at the blastocyst stage, suggesting that DNA methylation cannot be the sole inherited mark [79]. In addition, DNA methylation is not the only epigenetic mark correlated with coat color in Avy mice. Avy mice with hypomethylation of the IAP insertion also exhibit enrichment of H3 and H4 di-acetylation, whereas hypermethylation of the IAP insertion is also enriched for H4K20me3 [80]. Thus, despite significant progress made during decades of study, it remains unclear what the epigenetic mark(s) is that mediates the transgenerational inheritance stemming from the Avy mutation.
5. Epigenetic Based Treatment of Metabolic Disease
Current recommendations for treating metabolic disease are centered around lifestyle modifications, potentially coupled with therapeutic interventions depending on disease severity [111]. The combination of drug therapy with improved diet and increased physical activity can improve clinical outcomes relative to lifestyle modifications alone [112]. However, many of the currently available therapeutics have limited efficacy or significant adverse effects, and thus there remains a significant unmet need for new medications [113]. Currently available drugs typically target a key molecule in the pathophysiology of disease, whereas targeting the transgenerational inheritance of metabolic disease risk instead aims at breaking the generational cycle of disease. These efforts would likely focus on compounds that modify the epigenome such as ‘writers’ that attach epigenetic marks to histones or DNA (i.e. DNA methyltransferases) and ‘erasers’ that remove epigenetic marks (i.e. histone deacetylases) [114,115]. A number of compounds targeting the epigenome are already FDA approved or are in various stages of clinical trials, primarily for the treatment of cancer [114].
In many ways, the use of epigenetic modulators to reduce the risk of metabolic disease in offspring would not represent a profound shift relative to current treatment strategies for reducing the risk of neural tube defects. Current recommendations of the United States Public Health Service and the Center for Disease Control are for all women of childbearing age to consume 400 μg of folic acid daily to reduce the occurrence of neural tube defects in offspring [116]. The methyl group from dietary folic acid is metabolized in a multistep pathway to provide a methyl donor for DNA and histone methylation and thus supplementation with folic acid leads to global increases in methylation [117]. Folate is not alone as many other common components of nutritional supplements also alter the epigenome including vitamin B6, vitamin B12, betaine, methionine, and choline [32]. Evidence that targeting the epigenome may represent a powerful approach for treating metabolic disease comes from landmark studies of dietary interventions in Avy mice. Maternal diets supplemented with compounds that induce global hypermethylation reduced the transgenerational transmission of obesity from dams carrying the Avy mutation, resulting in offspring with reduced adiposity [78,81,87].
Non-pharmacological treatment strategies could also be used that focus on removing the triggering event in a way that is analogous to the cessation of smoking to reduce the incidence of lung cancer. However, this requires not only identifying a triggering compound or event amid a complex environment, but also convincing current generations to modify behavior for the sake of reducing risk to future generations. Nonetheless, towards this end, studies in animal models have identified specific dietary changes and environmental pollutants that alter risk of metabolic disease for future generations (Table 1). These compounds range from sugar to pesticides to endocrine disrupting compounds, all of which humans have had increased continual or isolated exposures to over the past century. For example, the average per capita consumption of sugar in the United States increased from 12-13 pounds per year in the early 1800s to 109 pounds per year in the 1929, to a peak of nearly 150 pounds per year in 1999 [118,119]. The association between metabolic disease and sugar intake of previous generations is of course strictly correlative in nature, but it remains an intriguing hypothesis that the current epidemic of metabolic disease has its roots partially in dietary changes in the early 20th century. Needless to say, there have been many other significant environmental changes over the same time period such that identifying individual causative factors may prove impossible, highlighting the need for continued research in model organisms.
6. The Glass Is Half Empty
The idea of transgenerational inheritance remains highly controversial for a number of reasons including that the supporting evidence is almost exclusively from animal models. Animal research-based experimental designs throughout biomedical research have come under fire recently for an apparent lack of reproducibility. Thus, it is important to maintain a healthy dose of skepticism when dealing with extraordinary scientific claims of all sorts, including transgenerational inheritance [120]. It is possible that the existing literature demonstrating transgenerational inheritance does not represent true supporting evidence, but is instead the result of publication bias, where experiments obtaining positive outcomes are more likely to be published [121]. These apparently positive outcomes may instead be due to chance observations as the result of underpowered experimental designs [122].
There have also been a number of studies published that failed to find transgenerational inheritance of metabolic disease, using stimuli similar to those that induced transgenerational inheritance in other publications. These include studies of low-protein diets in pregnant rats, which increased systolic blood pressure and reduced nephron number in F1 and F2 generation rats, but had no effect on the F3 generation, thus demonstrating intergenerational but not transgenerational inheritance [123]. Pregnant rats fed low-protein diet through gestation also had offspring that were insulin resistant in the F2, but not F3, generation when maintained on energy-restricted diets [124]. Similarly, treatment of pregnant rats with dexamethasone resulted in low birth weight and glucose intolerance in the F1 and F2 generations, again with no effect on the F3 generation [125]. These studies highlight why experimental validation by multiple research groups of putative positive results is critical for evaluating claims of transgenerational inheritance.
Apparent transgenerational inheritance could also be instead explained by conventional modes of inheritance if the original triggering event induced a stable genetic mutation in the genomic DNA. Methylated cytosine residues have elevated mutation rates relative to unmodified cytosines, providing a potential mechanism for how epigenetic modifiers that induce DNA hypermethylation could induce DNA mutations that would be stably inherited by future generations according to the laws of classical Mendelian inheritance [126,127].
7. Outstanding Questions Surrounding Transgenerational Inheritance
Classical Mendelian inheritance has long been the backbone of genetics research [128]. The fundamental concept of linkage and association mapping studies is predicated on the physical inheritance of disease causing mutations in affected individuals [129]. This basic framework has directly led to the identification of mutations in thousands of genes that result in monogenic disease and thousands of variants associated with complex traits and diseases [128,130]. While many additional genetic causes of phenotypic variation remain to be discovered, it has largely been assumed that these variants would follow the basic tenants of Mendelian inheritance. As transgenerational inheritance involves the transmission of disease risk in the absence of inherited genetic variation, it does not follow these basic rules. However, what the rules are that govern the mode and mechanism of transgenerational inheritance are poorly understood. Insights gained from the limited number of studies to date have left unanswered many of the most fundamental questions. For example, for how many generations is disease risk transmitted transgenerationally? Maternal malnourishment impaired the glucose homeostasis of offspring in the F3 generation, however the effect was smaller relative to the F2 generation, thus trending towards a return to baseline levels [99,100]. In contrast, maternal TBT exposure increased adiposity equally among offspring in the F1, F2, and F3 generations [92]. Thus, it remains unclear how many generations of future offspring can be impacted by transgenerational inheritance and how and whether the effects will diminish across generations.
The molecular nature of inherited epigenetic marks responsible for phenotypic transmission of disease risk also remains largely unknown. Most studies investigating this question have focused on DNA methylation, only scratching the surface of the complete landscape of epigenomic variation. Histone marks such as H3K27me and H3K9me are stably inherited, but are not yet widely analyzed in association with transgenerational inheritance [131,132]. As the costs of DNA sequencing and the amount of template DNA required for sequencing both continue to fall, it will only further increase the feasibility of genome-wide unbiased investigations of the complete epigenome [133]. Of course, a complete map of the epigenome does not by itself address the key question of causality for specific epigenetic marks, however there has been tremendous progress of late developing a toolbox of reagents to address this important question. It is now possible to engineer site-specific modifications in the epigenome in a targeted and reproducible manner, opening new avenues for functionally testing epigenetic marks in a way not previously possible. The methods for engineering epigenomic modifications are based on fusing the transcription-activator-like effector (TALE) repeat domains, thus providing DNA binding specificity, to an epigenetic writer or eraser. TALE fusions with the TET1 catalytic domain induce site-specific DNA demethylation whereas TALE fusions with LSD1 induce site-specific histone demethylation [134,135]. This class of site-specific epigenetic modifiers can also be induced optically, and therefore reversibly, by incorporating the light-sensitive cryptochrome 2 protein [136]. This unprecedented toolbox of site-specific epigenetic modifiers can be used to establish causal relationship between transgenerational transmission of disease and specific epigenetic marks, thus finally enabling a host of important questions surrounding the tissue specificity, developmental timing, and stability of inherited epigenetic marks to be answered.
Highlights.
We discuss examples of transgenerational inheritance of metabolic disease in both humans and model organisms.
Transgenerational inheritance can be triggered by both genetic and environmental stimuli
Paternal transgenerational inheritance is correlated with DNA methylation changes in sperm
We present examples of therapeutically targeting the epigenome to prevent the transgenerational transmission of metabolic disease
New tools for site-specifically modifying the epigenome will enable unprecedented evaluation of the role of inherited epigenetic marks in disease
Acknowledgments
This work was supported by NIH grants DK084079 and DK099533. We thank the Biochemistry Media Lab at the University of Wisconsin for providing clip art used in the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. The Lancet. 2011;377:557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. JAMA. 2014;312:189–90. doi: 10.1001/jama.2014.6228. [DOI] [PubMed] [Google Scholar]
- 4.Murray CJL, Lopez AD. Measuring the Global Burden of Disease. N Engl J Med. 2013;369:448–57. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 5.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2014;384:766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barroso I, Gurnell M, Crowley VEF, Agostini M, Schwabe JW, Soos MA, et al. Dominant negative mutations in human PPARg associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–3. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 7.Breckenridge WC, Little JA, Steiner G, Chow A, Poapst M. Hypertriglyceridemia associated with deficiency of apolipoprotein C-II. N Engl J Med. 1978;298:1265–73. doi: 10.1056/NEJM197806082982301. [DOI] [PubMed] [Google Scholar]
- 8.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 9.Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJF, et al. Variability in the Heritability of Body Mass Index: A Systematic Review and Meta-Regression. Front Endocrinol. 2012;3 doi: 10.3389/fendo.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lusis AJ, Attie AD, Reue K. Metabolic syndrome: from epidemiology to systems biology. Nat Rev Genet. 2008;9:819–30. doi: 10.1038/nrg2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vattikuti S, Guo J, Chow CC. Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits. PLoS Genet. 2012;8:e1002637. doi: 10.1371/journal.pgen.1002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eichler EE, Flint J, Gibson G, Kong A, Leal SM, Moore JH, et al. Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet. 2010;11:446–50. doi: 10.1038/nrg2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golan D, Lander ES, Rosset S. Measuring missing heritability: Inferring the contribution of common variants. Proc Natl Acad Sci. 2014;111:E5272–81. doi: 10.1073/pnas.1419064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46:1173–86. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Manolio TA, Pasquale LR, Boerwinkle E, Caporaso N, Cunningham JM, et al. Genome partitioning of genetic variation for complex traits using common SNPs. Nat Genet. 2011;43:519–25. doi: 10.1038/ng.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuk O, Hechter E, Sunyaev SR, Lander ES. The mystery of missing heritability: Genetic interactions create phantom heritability. Proc Natl Acad Sci U S A. 2012;109:1193–8. doi: 10.1073/pnas.1119675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet. 2012:153–62. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- 20.Heard E, Martienssen RA. Transgenerational Epigenetic Inheritance: Myths and Mechanisms. Cell. 2014;157:95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skinner MK. What is an epigenetic transgenerational phenotype? Reprod Toxicol. 2008;25:2–6. doi: 10.1016/j.reprotox.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aiken CE, Ozanne SE. Transgenerational developmental programming. Hum Reprod Update. 2014;20:63–75. doi: 10.1093/humupd/dmt043. [DOI] [PubMed] [Google Scholar]
- 23.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21:214–22. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yazbek SN, Spiezio SH, Nadeau JH, Buchner DA. Ancestral paternal genotype controls body weight and food intake for multiple generations. Hum Mol Genet. 2010;19:4134–44. doi: 10.1093/hmg/ddq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gluckman PD, Hanson MA, Beedle AS. Non-genomic transgenerational inheritance of disease risk. BioEssays. 2007;29:145–54. doi: 10.1002/bies.20522. [DOI] [PubMed] [Google Scholar]
- 26.Lindenbaum S. KURU, PRIONS, AND HUMAN AFFAIRS: Thinking About Epidemics. Annu Rev Anthropol. 2001;30:363–85. doi: 10.1146/annurev.anthro.30.1.363. [DOI] [Google Scholar]
- 27.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–70. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell. 2014;158:705–21. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–8. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith ZD, Chan MM, Humm KC, Karnik R, Mekhoubad S, Regev A, et al. DNA methylation dynamics of the human preimplantation embryo. Nature. 2014;511:611–5. doi: 10.1038/nature13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Law C, Cheung P, Adhvaryu K. Chemical “Diversity” of Chromatin Through Histone Variants and Histone Modifications. Curr Mol Biol Rep. 2015 doi: 10.1007/s40610-015-0005-3. [DOI] [Google Scholar]
- 32.Anderson OS, Sant KE, Dolinoy DC. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem. 2012;23:853–9. doi: 10.1016/j.jnutbio.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katada S, Imhof A, Sassone-Corsi P. Connecting Threads: Epigenetics and Metabolism. Cell. 2012;148:24–8. doi: 10.1016/j.cell.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Soubry A. Epigenetic inheritance and evolution: a paternal perspective on dietary influences. Prog Biophys Mol Biol. 2015 doi: 10.1016/j.pbiomolbio.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-Citrate Lyase Links Cellular Metabolism to Histone Acetylation. Science. 2009;324:1076–80. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J Off Publ Fed Am Soc Exp Biol. 2006;20:43–9. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stöger R. The thrifty epigenotype: An acquired and heritable predisposition for obesity and diabetes? BioEssays. 2008;30:156–66. doi: 10.1002/bies.20700. [DOI] [PubMed] [Google Scholar]
- 38.Bird A. Genome Biology: Not Drowning but Waving. Cell. 2013;154:951–2. doi: 10.1016/j.cell.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Grossniklaus U, Kelly B, Ferguson-Smith AC, Pembrey M, Lindquist S. Transgenerational epigenetic inheritance: how important is it? Nat Rev Genet. 2013;14:228–35. doi: 10.1038/nrg3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6:114–8. [PMC free article] [PubMed] [Google Scholar]
- 41.McGonigle P, Ruggeri B. Animal models of human disease: Challenges in enabling translation. Biochem Pharmacol. 2014;87:162–71. doi: 10.1016/j.bcp.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Brockmann GA, Bevova MR. Using mouse models to dissect the genetics of obesity. Trends Genet. 2002;18:367–76. doi: 10.1016/s0168-9525(02)02703-8. [DOI] [PubMed] [Google Scholar]
- 43.Kunej T, Skok DJ, Zorc M, Ogrinc A, Michal JJ, Kovac M, et al. Obesity Gene Atlas in Mammals. J Genomics. 2013;1:45–55. doi: 10.7150/jgen.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vickers SP, Jackson HC, Cheetham SC. The utility of animal models to evaluate novel anti-obesity agents: Animal models of obesity: a review. Br J Pharmacol. 2011;164:1248–62. doi: 10.1111/j.1476-5381.2011.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwok TCY, Ricker N, Fraser R, Chan AW, Burns A, Stanley EF, et al. A small-molecule screen in C. elegans yields a new calcium channel antagonist. Nature. 2006;441:91–5. doi: 10.1038/nature04657. [DOI] [PubMed] [Google Scholar]
- 46.O'Reilly LP, Luke CJ, Perlmutter DH, Silverman GA, Pak SC. C. elegans in high- throughput drug discovery. Adv Drug Deliv Rev. 2014;69-70:247–53. doi: 10.1016/j.addr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aitlhadj L, Stürzenbaum SR. The toxicological assessment of two anti-obesity drugs in C. elegans. Toxicol Res. 2013;2:145. doi: 10.1039/c2tx20096a. [DOI] [Google Scholar]
- 48.Lemieux GA, Keiser MJ, Sassano MF, Laggner C, Mayer F, Bainton RJ, et al. In Silico Molecular Comparisons of C. elegans and Mammalian Pharmacology Identify Distinct Targets That Regulate Feeding. PLoS Biol. 2013;11:e1001712. doi: 10.1371/journal.pbio.1001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–72. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- 50.Borman AD, Pearce LR, Mackay DS, Nagel-Wolfrum K, Davidson AE, Henderson R, et al. A Homozygous Mutation in the TUB Gene Associated with Retinal Dystrophy and Obesity. Hum Mutat. 2014;35:289–93. doi: 10.1002/humu.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mukhopadhyay S, Jackson PK. The tubby family proteins. Genome Biol. 2011;12:225. doi: 10.1186/gb-2011-12-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rankin CH. A review of transgenerational epigenetics for RNAi, longevity, germline maintenance and olfactory imprinting in Caenorhabditis elegans. J Exp Biol. 2015;218:41–9. doi: 10.1242/jeb.108340. [DOI] [PubMed] [Google Scholar]
- 53.Wenzel D, Palladino F, Jedrusik-Bode M. Epigenetics in C. elegans: facts and challenges. Genes N Y N 2000. 2011;49:647–61. doi: 10.1002/dvg.20762. [DOI] [PubMed] [Google Scholar]
- 54.Rechavi O, Houri-Ze'evi L, Anava S, Goh WSS, Kerk SY, Hannon GJ, et al. Starvation-induced transgenerational inheritance of small RNAs in C. elegans. Cell. 2014;158:277–87. doi: 10.1016/j.cell.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tauffenberger A, Parker JA. Heritable Transmission of Stress Resistance by High Dietary Glucose in Caenorhabditis elegans. PLoS Genet. 2014;10:e1004346. doi: 10.1371/journal.pgen.1004346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venken KJT, Bellen HJ. Emerging technologies for gene manipulation in Drosophila melanogaster. Nat Rev Genet. 2005;6:167–78. doi: 10.1038/nrg1553. [DOI] [PubMed] [Google Scholar]
- 57.Bharucha KN. The epicurean fly: using Drosophila melanogaster to study metabolism. Pediatr Res. 2009;65:132–7. doi: 10.1203/PDR.0b013e318191fc68. [DOI] [PubMed] [Google Scholar]
- 58.Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 2007;6:257–66. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith WW, Thomas J, Liu J, Li T, Moran TH. From fat fruit fly to human obesity. Physiol Behav. 2014;136:15–21. doi: 10.1016/j.physbeh.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Musselman LP, Fink JL, Narzinski K, Ramachandran PV, Hathiramani SS, Cagan RL, et al. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Model Mech. 2011;4:842–9. doi: 10.1242/dmm.007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–90. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- 63.Chien S, Reiter LT, Bier E, Gribskov M. Homophila: human disease gene cognates in Drosophila. Nucleic Acids Res. 2002;30:149–51. doi: 10.1093/nar/30.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schlegel A, Stainier DYR. Lessons from “lower” organisms: what worms, flies, and zebrafish can teach us about human energy metabolism. PLoS Genet. 2007;3:e199. doi: 10.1371/journal.pgen.0030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kunert N, Marhold J, Stanke J, Stach D, Lyko F. A Dnmt2-like protein mediates DNA methylation in Drosophila. Dev Camb Engl. 2003;130:5083–90. doi: 10.1242/dev.00716. [DOI] [PubMed] [Google Scholar]
- 66.Durdevic Z, Hanna K, Gold B, Pollex T, Cherry S, Lyko F, et al. Efficient RNA virus control in Drosophila requires the RNA methyltransferase Dnmt2. EMBO Rep. 2013;14:269–75. doi: 10.1038/embor.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goll MG, Kirpekar F, Maggert KA, Yoder JA, Hsieh CL, Zhang X, et al. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–8. doi: 10.1126/science.1120976. [DOI] [PubMed] [Google Scholar]
- 68.Schaefer M, Pollex T, Hanna K, Tuorto F, Meusburger M, Helm M, et al. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24:1590–5. doi: 10.1101/gad.586710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boffelli D, Takayama S, Martin DIK. Now you see it: Genome methylation makes a comeback in Drosophila: Think again. BioEssays. 2014;36:1138–44. doi: 10.1002/bies.201400097. [DOI] [PubMed] [Google Scholar]
- 70.Capuano F, Mülleder M, Kok R, Blom HJ, Ralser M. Cytosine DNA methylation is found in Drosophila melanogaster but absent in Saccharomyces cerevisiae, Schizosaccharomyces pombe, and other yeast species. Anal Chem. 2014;86:3697–702. doi: 10.1021/ac500447w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dunwell TL, McGuffin LJ, Dunwell JM, Pfeifer GP. The mysterious presence of a 5-methylcytosine oxidase in the Drosophila genome: possible explanations. Cell Cycle Georget Tex. 2013;12:3357–65. doi: 10.4161/cc.26540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lyko F, Ramsahoye BH, Jaenisch R. DNA methylation in Drosophila melanogaster. Nature. 2000;408:538–40. doi: 10.1038/35046205. [DOI] [PubMed] [Google Scholar]
- 73.Raddatz G, Guzzardo PM, Olova N, Fantappie MR, Rampp M, Schaefer M, et al. Dnmt2-dependent methylomes lack defined DNA methylation patterns. Proc Natl Acad Sci. 2013;110:8627–31. doi: 10.1073/pnas.1306723110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zemach A, McDaniel IE, Silva P, Zilberman D. Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science. 2010;328:916–9. doi: 10.1126/science.1186366. [DOI] [PubMed] [Google Scholar]
- 75.Buescher JL, Musselman LP, Wilson CA, Lang T, Keleher M, Baranski TJ, et al. Evidence for transgenerational metabolic programming in Drosophila. Dis Model Mech. 2013;6:1123–32. doi: 10.1242/dmm.011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duhl DM, Vrieling H, Miller KA, Wolff GL, Barsh GS. Neomorphic agouti mutations in obese yellow mice. Nat Genet. 1994;8:59–65. doi: 10.1038/ng0994-59. [DOI] [PubMed] [Google Scholar]
- 77.Morgan HD, Sutherland HG, Martin DI, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23:314–8. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- 78.Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes. 2008;32:1373–9. doi: 10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blewitt ME, Vickaryous NK, Paldi A, Koseki H, Whitelaw E. Dynamic Reprogramming of DNA Methylation at an Epigenetically Sensitive Allele in Mice. PLoS Genet. 2006;2:e49. doi: 10.1371/journal.pgen.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dolinoy DC, Weinhouse C, Jones TR, Rozek LS, Jirtle RL. Variable histone modifications at the Avy metastable epiallele. Epigenetics. 2010;5:637–44. doi: 10.4161/epi.5.7.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Waterland RA, Travisano M, Tahiliani KG. Diet-induced hypermethylation at agouti viable yellow is not inherited transgenerationally through the female. FASEB J. 2007;21:3380–5. doi: 10.1096/fj.07-8229com. [DOI] [PubMed] [Google Scholar]
- 82.Anderson OS, Nahar MS, Faulk C, Jones TR, Liao C, Kannan K, et al. Epigenetic responses following maternal dietary exposure to physiologically relevant levels of bisphenol A. Environ Mol Mutagen. 2012;53:334–42. doi: 10.1002/em.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–72. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaminen-Ahola N, Ahola A, Maga M, Mallitt KA, Fahey P, Cox TC, et al. Maternal Ethanol Consumption Alters the Epigenotype and the Phenotype of Offspring in a Mouse Model. PLoS Genet. 2010;6:e1000811. doi: 10.1371/journal.pgen.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132:2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- 86.Cropley JE, Suter CM, Beckman KB, Martin DIK. From The Cover: Germ-line epigenetic modification of the murine Avy allele by nutritional supplementation. Proc Natl Acad Sci. 2006;103:17308–12. doi: 10.1073/pnas.0607090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12:949–57. [PubMed] [Google Scholar]
- 88.Rosenfeld CS, Sieli PT, Warzak DA, Ellersieck MR, Pennington KA, Roberts RM. Maternal exposure to bisphenol A and genistein has minimal effect on Avy/a offspring coat color but favors birth of agouti over nonagouti mice. Proc Natl Acad Sci. 2013;110:537–42. doi: 10.1073/pnas.1220230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hackett JA, Sengupta R, Zylicz JJ, Murakami K, Lee C, Down TA, et al. Germline DNA Demethylation Dynamics and Imprint Erasure Through 5-Hydroxymethylcytosine. Science. 2013;339:448–52. doi: 10.1126/science.1229277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lane N, Dean W, Erhardt S, Hajkova P, Surani A, Walter J, et al. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis. 2003;35:88–93. doi: 10.1002/gene.10168. [DOI] [PubMed] [Google Scholar]
- 91.Fullston T, Ohlsson Teague EMC, Palmer NO, DeBlasio MJ, Mitchell M, Corbett M, et al. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 2013;27:4226–43. doi: 10.1096/fj.12-224048. [DOI] [PubMed] [Google Scholar]
- 92.Chamorro-García R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. Transgenerational Inheritance of Increased Fat Depot Size, Stem Cell Reprogramming, and Hepatic Steatosis Elicited by Prenatal Exposure to the Obesogen Tributyltin in Mice. Environ Health Perspect. 2013;121:359–66. doi: 10.1289/ehp.1205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics Derived Endocrine Disruptors (BPA, DEHP and DBP) Induce Epigenetic Transgenerational Inheritance of Obesity, Reproductive Disease and Sperm Epimutations. PLoS ONE. 2013;8:e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Manikkam M, Haque MM, Guerrero-Bosagna C, Nilsson EE, Skinner MK. Pesticide Methoxychlor Promotes the Epigenetic Transgenerational Inheritance of Adult-Onset Disease through the Female Germline. PLoS ONE. 2014;9:e102091. doi: 10.1371/journal.pone.0102091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Skinner MK, Manikkam M, Tracey R, Guerrero-Bosagna C, Haque M, Nilsson EE. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med. 2013;11:228. doi: 10.1186/1741-7015-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tracey R, Manikkam M, Guerrero-Bosagna C, Skinner MK. Hydrocarbons (jet fuel JP-8) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. Reprod Toxicol Elmsford N. 2013;36:104–16. doi: 10.1016/j.reprotox.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wei Y, Yang CR, Wei YP, Zhao ZA, Hou Y, Schatten H, et al. Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals. Proc Natl Acad Sci. 2014;111:1873–8. doi: 10.1073/pnas.1321195111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dunn GA, Bale TL. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology. 2011;152:2228–36. doi: 10.1210/en.2010-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Benyshek DC, Johnston CS, Martin JF. Glucose metabolism is altered in the adequately-nourished grand-offspring (F3 generation) of rats malnourished during gestation and perinatal life. Diabetologia. 2006;49:1117–9. doi: 10.1007/s00125-006-0196-5. [DOI] [PubMed] [Google Scholar]
- 100.Frantz EDC, Aguila MB. Pinheiro-Mulder A da R, Mandarim-de-Lacerda CA. Transgenerational endocrine pancreatic adaptation in mice from maternal protein restriction in utero. Mech Ageing Dev. 2011;132:110–6. doi: 10.1016/j.mad.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 101.Bähr A, Wolf E. Domestic Animal Models for Biomedical Research: Domestic Animal Models. Reprod Domest Anim. 2012;47:59–71. doi: 10.1111/j.1439-0531.2012.02056.x. [DOI] [PubMed] [Google Scholar]
- 102.Feeney A, Nilsson E, Skinner MK. Epigenetics and transgenerational inheritance in domesticated farm animals. J Anim Sci Biotechnol. 2014;5:48. doi: 10.1186/2049-1891-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Braunschweig M, Jagannathan V, Gutzwiller A, Bee G. Investigations on Transgenerational Epigenetic Response Down the Male Line in F2 Pigs. PLoS ONE. 2012;7:e30583. doi: 10.1371/journal.pone.0030583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pembrey M, Saffery R, Bygren LO, Carstensen J, Edvinsson S, et al. Network in Epigenetic Epidemiology. Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. J Med Genet. 2014;51:563–72. doi: 10.1136/jmedgenet-2014-102577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kaati G, Bygren LO, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents' and grandparents' slow growth period. Eur J Hum Genet EJHG. 2002;10:682–8. doi: 10.1038/sj.ejhg.5200859. [DOI] [PubMed] [Google Scholar]
- 106.Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjöström M, et al. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2005;14:159–66. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- 107.Bygren LO, Kaati G, Edvinsson S, Pembrey ME. Reply to Senn. Eur J Hum Genet. 2006;14:1149–50. doi: 10.1038/sj.ejhg.5201685. [DOI] [Google Scholar]
- 108.Bird LM. Angelman syndrome: review of clinical and molecular aspects. Appl Clin Genet. 2014;7:93–104. doi: 10.2147/TACG.S57386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med Off J Am Coll Med Genet. 2012;14:10–26. doi: 10.1038/gim.0b013e31822bead0. [DOI] [PubMed] [Google Scholar]
- 110.Buiting K, Groß S, Lich C, Gillessen-Kaesbach G, El-Maarri O, Horsthemke B. Epimutations in Prader-Willi and Angelman Syndromes: A Molecular Study of 136 Patients with an Imprinting Defect. Am J Hum Genet. 2003;72:571–7. doi: 10.1086/367926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaur J. A Comprehensive Review on Metabolic Syndrome. Cardiol Res Pract. 2014;2014:1–21. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112.Yanovski SZ, Yanovski JA. Long-term Drug Treatment for Obesity: A Systematic and Clinical Review. JAMA. 2014;311:74. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moller DE. Metabolic Disease Drug Discovery— “Hitting the Target” Is Easier Said Than Done. Cell Metab. 2012;15:19–24. doi: 10.1016/j.cmet.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 114.Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov. 2012;11:384–400. doi: 10.1038/nrd3674. [DOI] [PubMed] [Google Scholar]
- 115.Helin K, Dhanak D. Chromatin proteins and modifications as drug targets. Nature. 2013;502:480–8. doi: 10.1038/nature12751. [DOI] [PubMed] [Google Scholar]
- 116.Scholl TO, Johnson WG. Folic acid: influence on the outcome of pregnancy. Am J Clin Nutr. 2000;71:1295S–303S. doi: 10.1093/ajcn/71.5.1295s. [DOI] [PubMed] [Google Scholar]
- 117.Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2012 doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- 118.Pelto GH, Pelto PJ. Diet and delocalization: dietary changes since 1750. J Interdiscip Hist. 1983;14:507–28. [PubMed] [Google Scholar]
- 119.Putnam J, Allshouse J, Kantor LS. U.S. Per Capita Food Supply Trends: More Calories, Refined Carbohydrates, and Fats. Food Rev. 2002;25:2–15. [Google Scholar]
- 120.Churchill GA. When Are Results Too Good to Be True? Genetics. 2014;198:447–8. doi: 10.1534/genetics.114.169912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64:1277–82. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 122.Ioannidis JPA. How to Make More Published Research True. PLoS Med. 2014;11:e1001747. doi: 10.1371/journal.pmed.1001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harrison M, Langley-Evans SC. Intergenerational programming of impaired nephrogenesis and hypertension in rats following maternal protein restriction during pregnancy. Br J Nutr. 2009;101:1020–30. doi: 10.1017/S0007114508057607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Benyshek DC, Johnston CS, Martin JF, Ross WD. Insulin sensitivity is normalized in the third generation (F3) offspring of developmentally programmed insulin resistant (F2) rats fed an energy-restricted diet. Nutr Metab. 2008;5:26. doi: 10.1186/1743-7075-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R34–8. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- 126.Coulondre C, Miller JH, Farabaugh PJ, Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978;274:775–80. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- 127.Walsh CP, Xu GL. Cytosine methylation and DNA repair. Curr Top Microbiol Immunol. 2006;301:283–315. doi: 10.1007/3-540-31390-7_11. [DOI] [PubMed] [Google Scholar]
- 128.Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A. OMIM. org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders Nucleic Acids Res. 2015;43:D789–98. doi: 10.1093/nar/gku1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lander ES, Botstein D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989;121:185–99. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42:D1001–6. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gaydos LJ, Wang W, Strome S. H3K27me and PRC2 transmit a memory of repression across generations and during development. Science. 2014;345:1515–8. doi: 10.1126/science.1255023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ragunathan K, Jih G, Moazed D. Epigenetic inheritance uncoupled from sequence-specific recruitment. Science. 2014 doi: 10.1126/science.1258699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–30. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Maeder ML, Angstman JF, Richardson ME, Linder SJ, Cascio VM, Tsai SQ, et al. Targeted DNA demethylation and activation of endogenous genes using programmable TALE-TET1 fusion proteins. Nat Biotechnol. 2013;31:1137–42. doi: 10.1038/nbt.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mendenhall EM, Williamson KE, Reyon D, Zou JY, Ram O, Joung JK, et al. Locus-specific editing of histone modifications at endogenous enhancers. Nat Biotechnol. 2013;31:1133–6. doi: 10.1038/nbt.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Konermann S, Brigham MD, Trevino AE, Hsu PD, Heidenreich M, Cong L, et al. Optical control of mammalian endogenous transcription and epigenetic states. Nature. 2013;500:472–6. doi: 10.1038/nature12466. [DOI] [PMC free article] [PubMed] [Google Scholar]