Abstract
Key points
Childhood obesity is associated with precocious oxidative stress, which can contribute to future diseases.
Early overfed rats have increased oxidative stress in liver and plasma at weaning.
The model of litter size reduction causes adipocyte hypertrophy and lower PPARγ at weaning, suggesting a decrease in adipocyte proliferation.
Abstract
Neonatal overfeeding induced by litter size reduction leads to further obesity and other metabolic disorders, such as liver oxidative stress and microsteatosis at adulthood. We hypothesized that overfeeding causes an early redox imbalance at weaning, which could programme the animals to future liver dysfunction. Thus, we studied lipogenesis, adipogenesis, catecholamine status and oxidative balance in weaned overfed pups. To induce early overfeeding, litters were adjusted to three pups at the 3rd day of lactation (SL group). The control group contained 10 pups per litter until weaning (NL group). Peripheral autonomic nerve function was determined in vivo at 21 days old. Thereafter, pups were killed for further analysis. Differences were considered significant when P < 0.05. The SL pups presented with a higher visceral adipocyte area, higher content of lipogenic enzymes (ACC, FAS) and with a lower content of adipogenic factors (CEBP, PPARγ) in visceral adipose tissue (VAT). Although autonomic nerve activity and adrenal catecholamine production were not significantly altered, catecholamine receptor (β3ADR) content was lower in VAT. The SL pups also presented with higher triglyceride, PPARγ, PPARα and PGC1α contents in liver. In plasma and liver, the SL pups showed an oxidative imbalance, with higher lipid peroxidation and protein oxidation. The SL group presented with a higher serum alanine aminotransferase (ALT). The early increase in lipogenesis in adipose tissue and liver in weaned overfed rats suggests that the higher oxidative stress and lower catecholamine content in VAT are associated with the early development of liver dysfunction and adipocyte hypertrophy.
Abbreviations
- ACC
acetyl‐CoA carboxylase
- β2ADR
β2 adrenergic receptor
- β3ADR
β3 adrenergic receptor
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CAT
catalase
- C/EBPs
CCAAT‐enhancer‐binding proteins
- FAS
fatty acid synthase
- GPx
glutathione peroxidase
- MDA
malondialdehyde
- NL
normal litter
- PGC1α
peroxisome proliferator‐activated receptor‐gamma coactivator 1 α
- PPARγ
nuclear receptor peroxisome proliferator‐activated receptor γ
- ROS
reactive oxygen species
- SL
small litter
- SOD
superoxide dismutase
- TG
triglyceride
- TH
tyrosine hydroxylase
- VAT
visceral adipose tissue
Introduction
Obesity is an epidemic health problem (Ng et al. 2014) associated with metabolic syndrome, which is characterized by central obesity, dyslipidaemia, type 2 diabetes, cardiovascular diseases, and non‐alcoholic fatty liver disease (Shin et al. 2013). Childhood obesity is especially concerning because it causes precocious metabolic syndrome (Ozturk & Soylu, 2014) and high rates of long‐standing obesity (Tounian, 2011). Currently it is known that disturbances arising from the obesity are influenced by pro‐oxidative conditions (Codoñer‐Franch et al. 2011; Aroor & DeMarco, 2014).
Experimental studies have shown that precocious nutritional interventions are able to modulate tissue‐specific endocrine and physiological functions, as an adaptation mechanism. This physiological phenomenon is named ontogenetic plasticity or metabolic programming (de Moura & Passos, 2005; Hanson & Gluckman, 2014). It has been observed in overnutrition during lactation by litter size reduction, leading to a higher milk offer (Cunha et al. 2009) and long‐term obesity and their comorbidities (Habbout et al. 2013 b). This precocious obesity increases serum triacylglycerol, visceral and total fat body content and produces high serum insulin at weaning (Rodrigues et al. 2011).
The development of obesity is related to adipocyte hypertrophy and ectopic lipid accumulation. The adipocyte differentiation and lipid storage is stimulated by specific transcription factors, such as CCAAT‐enhancer‐binding proteins (C/EBPs) and a nuclear receptor, peroxisome proliferator‐activated receptor gamma (PPARγ) (Ali et al. 2013). Another factor involved in lipid metabolism is the peroxisome proliferator‐activated receptor alpha (PPARα); its reduced activation is related to reduction of fatty acid oxidation and increased lipid accumulation (Gao et al. 2015). It has been demonstrated that the proliferation capacity of adipose tissue from obese mammals can reach a limit, and the hypertrophied adipocytes release high amounts of free fatty acids, which are stored in the liver (Ameer et al. 2014). Donnelly and colleagues (2005) showed that patients with liver steatosis present with 59% of triglycerides in the livers originating from adipocyte lipolysis, 26% from de novo lipogenesis and only 15% from the diet. This suggests that a dysfunction of the visceral adipose tissue may be the first step to liver steatosis. In the liver, de novo lipogenesis is stimulated by PPARγ expression, which overexpression is related to lipid accumulation (Sanders & Griffin, 2015). Acetyl CoA carboxylase (ACC) and fatty acid synthase (FAS) are essential enzymes for lipogenesis; the first enzyme catalyses the malonyl‐CoA synthesis while the second enzyme catalyses the synthesis of long chains fatty acid from acetyl‐CoA and malonil‐CoA (Sanders & Griffin, 2015). Adipocyte hypertrophy and liver lipid accumulation are associated with increased expression and activity of these enzymes in both tissues (Sanders & Griffin, 2015).
The increase in lipid influx into the liver from visceral adipocytes might be favoured by increased activity of the catecholaminergic system, leading to higher sympathetic noradrenaline (norepinephrine) or adrenal medullar adrenaline release, or through increased catecholamine sensitivity in adipocytes (Nielsen et al. 2014). Similarly, liver lipid storage might be favoured by reduced catecholamine sensitivity in this tissue. Since the model of litter size reduction leads to an adrenal catecholamine dysfunction at adulthood (Conceição et al. 2013 b), it is important to identify whether this disruption occurs early in life, contributing to the long‐term metabolic disturbances found in this programming model of obesity.
Some studies associate metabolic illness, such as obesity and their comorbidities, with an increase in oxidative stress (Bullon et al. 2000; Tariq et al. 2014). However, there are few reports showing the close association between neonatal oxidative stress and the development of diseases at adulthood (Holloway et al. 2005; Bruin et al. 2008; Niu et al. 2013).
Habbout and colleagues (2012 and 2013 a) showed that increased plasma and cardiac oxidative stress were associated with higher susceptibility to cardiac injury at adulthood . Our group demonstrated that rats that were early overfed due to reducing litter size presented with long‐term obesity, hyperphagia, hepatic microsteatosis and oxidative stress at adulthood (Conceição et al. 2013 a). However, there are no reports on whether the early obesity induced by litter size reduction is related to precocious oxidative imbalance. Thus, the aim of the present study is to determine if early postnatal overnutrition is able to induce fat accumulation and oxidative stress in liver and adipocytes during the weaning period. We also evaluated visceral adipocyte morphology, the expression of adipogenic/lipogenic proteins in visceral adipose tissue and/or liver and autonomic nervous system activity and catecholamine status in 21‐day‐old rats.
Methods
Experimental model
Our protocol was approved by the Animal Care and Use Committee of the Biology Institute of the State University of Rio de Janeiro. The Wistar rats employed in the experiment were housed under controlled temperature (23 ± 3°C), light (12 h light–dark cycle), and had free access to water and food. Twenty nulliparous female Wistar rats were placed with (10) male rats (both groups 120 days old) at a 2‐to‐1 ratio for 5 days. After the mating, pregnant females were housed in individual cages until delivery. After birth, all litters were adjusted to 10 male pups for each dam. Female pups were substituted by male pups from other litters. To induce early overfeeding (SL group, n = 10), at the third day of life, the litter size was reduced to three male pups per dam. The control group (NL group, n = 10) was kept with ten pups per dam until weaning at post‐natal day 21 (PN21). We carried out the experiments with male pups only because it had already been demonstrated that there are no gender differences at weaning with this model of litter size reduction, especially in regard of body weight, fat mass, lean mass, leptinaemia, energy expenditure and respiratory exchange ratio (Stefanidis & Spencer, 2012).
At weaning, the pups were fasted for 2 h and then anesthetized (pentobarbital sodium, 90 mg (kg body weight)−1) to allow in vivo autonomic nerve activity assessment. After the experiments, the pups were killed by exsanguination. Visceral fat tissue (epididymal, mesenteric and retroperitoneal depots) was dissected and weighed. Blood samples were centrifuged (1000 × g, 4°C, 20 min) to collect the plasma and stored (−20°C) for later analysis. Liver and visceral adipose tissue (VAT) was collect for further analysis.
Autonomic nerve activity assessment
To evaluate the autonomic activity, a parasympathetic nerve, the left superior branch of the upper vagus nerve, and a sympathetic nerve, the left intrascapular nerve, were exposed under a dissection microscope. The branches were placed on a pair of hook platinum electrodes connected to an electronic device (Bio‐Amplificator, Insight, Ribeirão Preto, SP, Brazil) to record electrical signals. The nerve activity was amplified (10,000) and filtered (1–60 kHz). Data were analysed using Insight software (Insight). All nerve acquisition was processed inside a Faraday cage to avoid electromagnetic interference and the rats were kept under a warming light. After 10 min of stabilization, the average of number of spikes during 15 min was counted and the nerve firing rate was expressed as number of spikes (5 s)–1) (Scomparin et al. 2009).
Morphological evaluation of the adipose tissue
Adipose tissue samples were fixed in formaldehyde 0.1 m phosphate‐buffered saline (pH 7.2). The tissues were then dehydrated, cleared and paraffin‐embedded. Non‐consecutive slices 10 μm thick were obtained and stained with Haematoxylin and Eosin to assess morphology. Digital images were obtained from histological laminas with the Olympus BX40 microscope (Olympus, Tokyo, Japan) using a ×40 objective. Three laminas were obtained from each rat, and 10 pictures of different fields were taken from each lamina. From each picture, the area of five adipocytes was measured using the software Image‐Pro Plus 5.0 (Media Cybernetics, Silver Spring, MD, USA) (Conceição et al. 2011).
Triglyceride content
Triglyceride (TG) content was determined by colorimetric assay after total liver lipid content extraction with isopropanol (Vetec, Duque de Caxias, RJ, Brazil) and centrifugation (740 × g, 10 min, 4°C) (Folch et al. 1957). The TG content in the supernatant was evaluated using a commercial kit (Bioclin, BH, MG, Brazil), and the absorbance was measured at 500 nm (Hidex, Turku, Finland).
Western blotting analysis
The protein content of peroxisome proliferator‐activated receptor α and γ (PPARα and PPARγ; ab8934 from Abcam, Cambridge, MA, USA and sc‐7273 from Santa Cruz Biotechnology Inc., Dallas, Texas, USA. Wembley, UK, respectively), peroxisome proliferator‐activated receptor‐gamma coactivator 1 α (PGC1; Santa Cruz, sc‐13067), acetyl‐CoA carboxylase (ACC; Santa Cruz, sc‐26817), fatty acid synthase (FAS; Abcam, ab150508), β2 adrenergic receptor (β2ADR; Abcam, ab36956), β3 adrenergic receptor (β3ADR; Santa Cruz, sc‐50436), tyrosine hydroxylase (TH; Sigma‐Aldrich Inc., MO, USA, T2928), superoxide dismutase 1 and 2 (SOD1 and SOD2; Santa Cruz, sc‐11407 and sc‐133134, respectively), glutathione peroxidase (GPx; Santa Cruz, sc‐133152), catalase (CAT; Sigma‐Aldrich, C0979), 4‐hydroxynonenal protein adduct (4‐HNE; Santa Cruz, sc‐130083), and 3‐nitrotyrosin (Santa Cruz, sc‐55256) were evaluated by Western blotting in liver and VAT. Briefly, tissues were homogenized in RIPA buffer (50 mm Tris‐HCl (pH 7.4), 1% NP‐40, 150 mm NaCl, 1 mm EDTA, 1 mm PMSF, 1 mm Na3VO4, 1 mm NaF), and protease inhibitor cocktail (F. Hoffmann‐La Roche Ltd, Basel, Switzerland). Homogenates were centrifuged for 5 min (1,120 × g, 4°C). The protein concentration in the supernatants was determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, Carlsbad, CA, USA) and aliquots of homogenate containing 10 mg total protein were then analysed by SDS–PAGE. Samples were electroblotted onto nitrocellulose membranes (Hybond ECL; Amersham Pharmacia Biotech, Amersham, London, UK). Membranes were incubated with Tris‐buffered saline (TBS) containing 2% albumin for 90 min. Membranes were then washed with TBS and incubated with a specific primary antibody (dilution 1:500) overnight at 4°C, after which they were washed and incubated with secondary antibody diluted to 1:5000 (anti‐mouse, B8520 from Sigma‐Aldrich; anti‐goat, 62–6540, and anti‐rabbit, 65–6140, from Invitrogen, Carlsbad, CA, USA) and then conjugated with HRP at an adequate dilution for 1 h at room temperature. The protein bands were visualized by chemiluminescence (Kit ECL plus, Amersham Biosciences, London, UK) followed by exposure to ImageQuant LAS (GE Healthcare, Buckinghamshire, Chalfont St Giles, UK). Area and density of the bands were quantified by Image J software (Wayne Rasband National Institute of Health, Bethesda, MA, USA). Results were expressed relative (%) to the control group.
Reverse transcription polymerase chain reaction (RT‐PCR) analysis
Total RNA was extracted from 100 mg of VAT, under RNAse‐free conditions, with TRIzol Reagent (Invitrogen), and quantified via Nano Vue TM Plus Spectrophotometer (GE Healthcare, Buckinghamshire, UK). The cDNA was prepared from total RNA using the Moloney murine leukemia virus reverse transcriptase (M‐MLV RT) for RT‐PCR and oligo(dT)15 primer (Promega, WI, USA). The mRNA levels of C/EBPβ (forward (F) primer: 5′‐ATGCAATCCGGATCAAACGT‐3′; reverse (R) primer: 5′‐CCGCAGGAACATCTTTAAGTGA‐3′) and PPARγ (forward (F) primer: 5′‐ATTCTGGCCCACCAACTTCGG‐3′; reverse (R) primer: 5′‐TGGAAGCCTGATGCTTTATCCCCA‐3′) in visceral fat mass were amplified on Applied Biosystems 7500 Real‐Time PCR System (Life Technologies Co. Carlsbad, CA, USA) using SYBR Green PCR Master Mix (Applied BioSystems, Foster City, CA, USA) according to the recommendations of the manufacturer. The oligonucleotide primers and probes were designed and prepared by ABI Applied Biosystems. The co‐amplification of mouse 36β‐4 Mrna (F primer: 5′‐TGTTTGACAACGGCAGCATTT‐3′; R primer: 5′‐CCGAGGCAACAGTTGGGT A‐3′), a variant internal control, was performed in all of the samples. Assays were performed in triplicate, and the results were normalized to the 36β‐4 mRNA levels using the 2ΔΔCT method (Livak & Schmittgen, 2001).
Adrenal catecholamine measurement
Total catecholamines were quantified by the trihydroxyindole method (Conceição et al. 2013 b). Right adrenal glands were homogenized in 10% acetic acid and centrifuged (1120 × g, 5 min). Briefly, 50 μl of adrenaline standard and the adrenal supernatant were mixed with 250 μl of buffer phosphate (0.5 m, pH 7.0) and 25 μl of potassium ferricyanate (0.5%), and incubated for 20 min in an ice bath. The reaction was stopped with 500 μl of ascorbic acid (60 mg ml−1)–NaOH (5 n) solution, and diluted with 2 ml of water distilled. The fluorescence was determined at 420 nm to excitation and 510 nm to emission (Hidex, Turku, Finland).
Antioxidant enzymes activities
Liver aliquots (200 mg) were homogenized in potassium phosphate buffer with EDTA. After centrifugation (3398 × g, 10 min, 4°C), homogenates were stored at −80°C until each oxidative parameters assay. The total protein content was determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, CA, USA). Total superoxide dismutase (SOD) activity was assayed by measuring the inhibition of adrenaline auto‐oxidation as absorbance at 480 nm (Bannister & Calabrese, 1987). Glutathione peroxidase (GPx) activity was evaluated according to Flohé & Günzler (1984) by measuring the oxidation of NADPH in the presence of H2O2 at 340 nm. Catalase (CAT) activity was measured by the rate of decrease in H2O2 at 240 nm according to the Aebi method (1984). Enzymatic activities were expressed as U (mg of protein)–1).
The SOD/GPx and SOD/(GPx+CAT) ratio activities were calculated, since previous data show that the ratio of the activities of these enzymes is more important than the absolute activity and is related to cellular oxidative status (Amstad et al. 1991; de Haan et al. 1996).
Antioxidant capacity assay
The antioxidant capacity of the sample was measured by the reduction of the DPPH• (2,2‐diphenyl‐1‐picrylhydrazyl) radical at 517 nm (Amarowicz et al. 2000). Plasma or liver homogenate samples (400 μl) were mixed with 400 μl of DPPH• (0.25 mm) in ethanol solution and incubated (30 min) at darkroom and room temperature. They were then centrifuged (212 × g, 5 min, 4°C), and the supernatant absorbance (Abs) was read in spectrophotometer (517 nm). The sample scavenging capacity for DPPH• free radicals was evaluated by the following equation:
where AA is antioxidant activity. The results were expressed as percentage DPPH• scavenging.
MDA content
The lipid peroxidation was estimated by quantification of malondialdehyde (MDA) using the thiobarbituric acid reactive substances method (Vynche, 1970). The liver was homogenized in phosphate buffer with EDTA. The liver homogenate was mixed with 10% trichloroacetic acid (1:2) and centrifuged (212 × g, 10 min, 4ºC). After this, 500 μl of supernatant was incubated with 500 μl of thiobarbituric acid (0.67%) for 30 min (95°C). The absorbance was read in a spectrophotometer (532 nm). The results were expressed as μmol (mg protein)–1).
Plasma transaminases
The plasma activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were quantified by the Reitman & Frankel method (1957) using colorimetric kits (Doles, Goiânia, GO, Brazil).
Statistical analysis
The statistical analyses were carried out using Graph Pad Prism 5.0 for Windows statistical software (GraphPad Software, La Jolla, CA, USA). Comparisons between the groups were performed with Student's unpaired t test. For all analyses, the data were given as means and standard error of the mean (SEM) and were considered significant when P < 0.05.
Results
As expected, the SL group presented with higher body mass from PN07 until PN21 (PN21: NL = 35.3 ± 0.5 g vs. SL = 44.3 ± 1.0 g; P < 0.0001), higher visceral fat mass (PN21: NL = 180.0 ± 17.0 mg vs. SL = 390.0 ± 34.0 mg; P < 0.0001) and higher relative visceral fat mass (PN21: NL = 4.76 ± 0.3 vs. SL = 8.25 ± 0.6; mg tissue (g body weight)–1), P < 0.0001) compared with NL group. These pups also had a higher adipocyte area (+148%; P < 0.05; Fig. 1).
Figure 1. The effect of reducing litter size on adipocyte morphology at 21 days old .
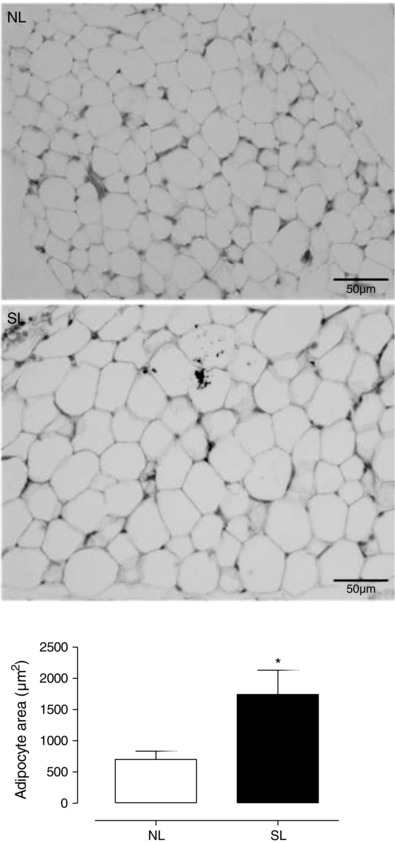
Representative photomicrography of the visceral adipocyte from rats raised in normal litters (NL) and small litters (SL) during lactation. The quantitative analysis of the adipocyte area is shown below. Values are expressed as means ± SEM; n = 4 per group; *P < 0.05.
In VAT, we detected lower adipogenic transcription factor C/EBPβ mRNA (−57%; P = 0.0082; Fig. 2 A), lower PPARγ mRNA (−62%; P = 0.0003; Fig. 2 B) and lower PPARγ protein content (−29%; P = 0.025; Fig. 2 C) in SL pups compared with the NL pups. The lipogenic enzymes ACC and FAS were higher (+252%; P = 0.01; Fig. 2 D and +223%; P = 0.002; Fig. 2 E, respectively) in VAT in the SL group.
Figure 2. The effect of reducing litter size on adipogenic and lipogenic biomarkers in visceral adipose tissue at 21 days old .
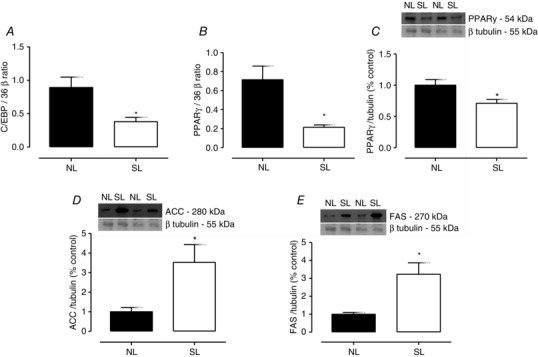
RNAm level of C/EBPβ (A) and PPARγ (B), protein content of PPARγ (C), ACC (D) and FAS (D) in visceral adipose tissue from rats raised in normal litters (NL) and small litters (SL) during lactation. The representative blots of proteins are shown above graphs C, D and E. β‐Tubulin content was used as control loading. Results are expressed as relative (%) to the control group and as means ± SEM; n = 4–7 per group; *P < 0.05. The PCR analyses were normalized to the 36 β mRNA level.
At weaning, the liver mass of the SL group was not different from the NL group (Fig. 3 A), although they presented with a higher triglyceride liver content (+22%; P < 0.0001; Fig. 3 B). Whereas PPARγ content was higher (+56%; P = 0.034; Fig. 3 C) in SL pups, FAS content was unchanged (Fig. 3 D). The lipolitic transcription factors, PPARα and PGC1α, were higher in SL group (+98%; P = 0.03; Fig. 3 E and +82%; P = 0.0004; Fig. 3 F, respectively) than in controls.
Figure 3. The effect of reducing litter size on lipid metabolism in liver at 21 days old .
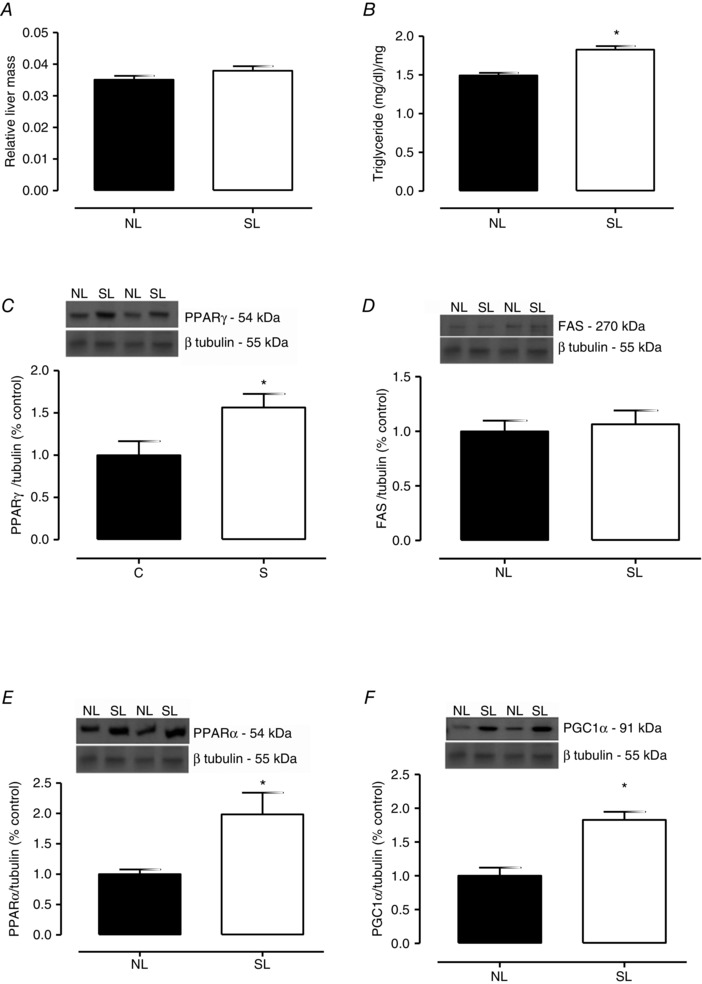
Relative mass (A), triglyceride content (B), PPARγ (C), FAS (D), PPARα (E) and PGC1α (F) protein contents from rats raised in normal litters (NL) and small litters (SL) during lactation. The representative blots of proteins are shown above graphs C, D, E and F. β‐Tubulin content was used as control loading. Results are expressed as relative (%) to the control group and as means ± SEM; n = 6–10 per group; *P < 0.05.
The parameters related to autonomic nervous function, catecholamine production and receptors in rats at weaning are depicted in Table 1. Both the sympathetic and the parasympathetic nerve activities were unaltered in the SL group. In the adrenal glands, neither the catecholamine content nor the tyrosine hydroxylase content was different between groups. Regarding the adrenergic receptor analysis, the β3ADR content was lower in VAT in SL pups than in NL pups, while the β2ADR content in liver was unchanged.
Table 1.
Effect of reducing litter size on parameters related to autonomic nervous, catecholamine production and receptors in rats at PN21
| NL | SL | P | |
|---|---|---|---|
| Sympathetic nerve activity (spikes (5 s)–1) | 23.14 ± 2.9 | 20.53 ± 3.2 | 0.55 |
| Parasympathetic nerve activity (spikes (5 s)–1) | 18.13 ± 2.1 | 18.80 ± 2.3 | 0.83 |
| Catecholamine content in adrenal (μm mg–1) | 5.78 ± 1.4 | 8.36 ± 1.4 | 0.20 |
| TH content in adrenal medulla (% of control) | 1.00 ± 0.1 | 0.90 ± 0.1 | 0.70 |
| β3ADR content in VAT (% of control) | 1.00 ± 0.1 | 0.55 ± 0.1* | 0.01 |
| β2ADR content in liver (% of control) | 1.00 ± 0.2 | 1.17 ± 0.3 | 0.58 |
Data are presented as means ± SEM of 5–10 pups for NL and SL groups with level of significance (*) set at P < 0.05. NL, normal litter; SL, small litter; TH, tyrosine hydroxylase; β3ADR, β3 adrenergic receptor; β2ADR, β2 adrenergic receptor; VAT, visceral adipose tissue.
In the liver, SOD1, SOD2 and CAT contents were increased but the GPx content was decreased (Fig. 4 A). In liver, SOD and CAT activities were unchanged, and GPx activity was decreased in SL group (−33%; P = 0.04; Table 2). The total antioxidant capacity assayed as the DPPH• scavenging capacity was decreased (−50%; P = 0.03; Table 2). The ratio between the antioxidant enzyme activities, SOD/GPx (Table 2) and SOD/(GPx+CAT) (Table 2), were unchanged. The MDA content and protein carbonyl content were increased in SL pups (+77%, P = 0.02, and +50%, P = 0.02, respectively, Table 2), as were the 4‐HNE protein adducts and 3‐nitrotyrosin liver content (Fig. 4 B and C, respectively).
Figure 4. The effect of reducing litter size on antioxidant protein content and oxidative biomarkers in liver at 21 days old .
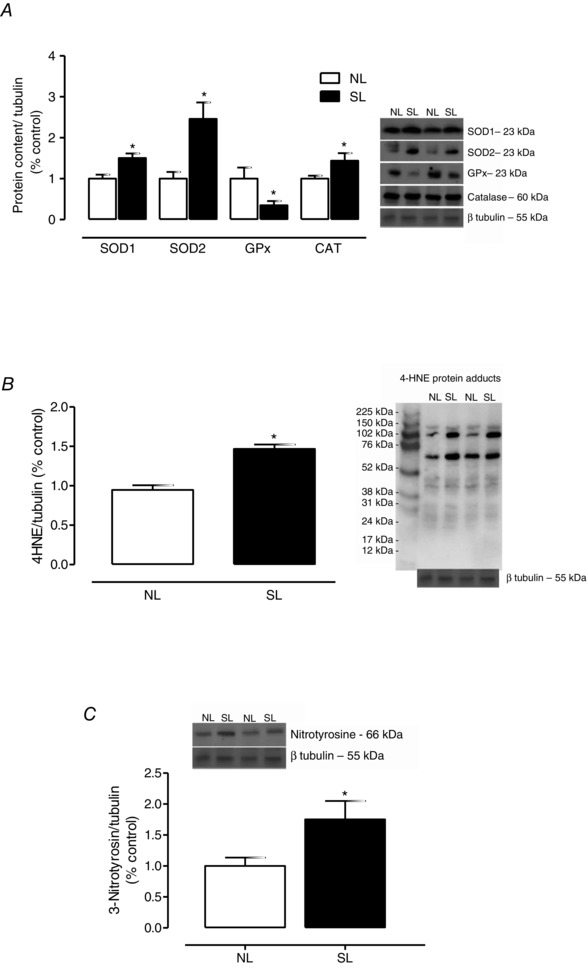
SOD1, SOD2, GPx and CAT protein contents (A), 4HNE protein adducts (B), and 3‐nitrotyrosine content (C) from rats raised in normal litters (NL) and small litters (SL) during lactation. The representative blots of proteins are shown next to the graphs. β‐Tubulin content was used as control loading. Results are expressed as relative (%) to the control group and as means ± SEM; n = 6–7 per group; *P < 0.05.
Table 2.
Effect of reducing litter size on oxidative parameters in liver and plasma of rats at PN21
| NL | SL | P | ||
|---|---|---|---|---|
| Liver | SOD (U (mg protein)–1) | 2138 ± 280 | 1975 ± 222 | 0.67 |
| GPx (U (mg protein)–1) | 0.332 ± 0.05 | 0.220 ± 0.01* | 0.04 | |
| CAT (U (mg protein)–1) | 0.107 ± 0.01 | 0.104 ± 0.01 | 0.82 | |
| SOD/GPx ratio | 8187 ± 1708 | 8099 ± 757 | 0.96 | |
| SOD/(GPx+CAT) ratio | 5772 ± 1033 | 5493 ± 427 | 0.82 | |
| DPPH• scavenging (%) | 15.68 ± 2.1 | 7.81 ± 2.6* | 0.03 | |
| MDA (μmol mg–1) | 73.0 ± 11.0 | 129.0 ± 17.0* | 0.02 | |
| Total protein bound carbonyl (nmol (mg protein)–1) | 150.7 ± 14.0 | 226.7 ± 24.0* | 0.02 | |
| Plasma | SOD (U (mg protein)–1) | 1861 ± 203 | 1790 ± 191 | 0.8 |
| GPx (U (mg protein)–1) | 0.037 ± 0.01 | 0.041 ± 0.01 | 0.72 | |
| CAT (U (mg protein)–1) | 0.048 ± 0.01 | 0.028 ± 0.01* | 0.04 | |
| SOD/GPx ratio | 3316 ± 805 | 7496 ± 821* | 0.006 | |
| SOD/(GPx+CAT) ratio | 3091 ± 781 | 6363 ± 697* | 0.01 | |
| MDA plasma (μmol mg–1) | 0.09 ± 0.01 | 0.22 ± 0.04* | 0.02 | |
| Total protein bound carbonyl (nmol (mg protein)–1) | 310.4 ± 60.4 | 813.5 ± 140.9* | 0.01 |
Data are presented as means ± SEM of 10 pups for NL and SL groups with level of significance (*) set at P < 0.05. SOD, superoxide dismutase; GPx, glutathione peroxidase; CAT, catalase; MDA, malondialdehyde; NL, normal litter; SL, small litter; DPPH, 2,2‐diphenyl‐1‐picrylhydrazyl.
Regarding the plasma transaminase analysis, the SL group showed an increase in ALT activity (+25%; P = 0.006; Fig. 5 B) compared with the NL group, despite having normal AST activity (Fig. 5 A).
Figure 5. The effect of reducing litter size on liver function enzymes at 21 days old .
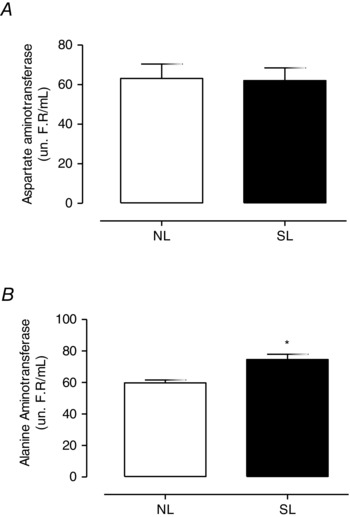
Plasma activity of aspartate aminotransferase (AST; A) and alanine aminotransferase (ALT; B) from rats raised in normal litters (NL) and small litters (SL) during lactation. Results are expressed as means ± SEM; n = 6–10 per group; *P < 0.05. un R.F./mL refers to the Reitman Frankel unit used to quantify plasma activity of aspartate aminotransferase and alanine aminotransferase through the Reitman and Frankel method.
In the plasma analysis (Table 2), SOD and GPx activities were unaltered (Table 2) in SL rats, while CAT activity was lower than controls (−42%; P = 0.04). These animals also had higher SOD/GPx (+126%; P = 0.006) and SOD/(GPx+CAT) (+106%; P = 0.01), indicating an increased cellular oxidative condition. Both MDA and protein carbonyl contents were increased in the SL group compared with the NL group (+130%, P = 0.02, and +162%, P = 0.01, respectively).
Discussion
As expected, at weaning the SL pups showed higher body weight and visceral adipose tissue. The increases in central adiposity and adipocyte hypertrophy were associated with a higher content of the lipogenic enzymes ACC, which converts acetyl‐CoA to malonil‐CoA, and FAS, which catalyses the synthesis of long chain fatty acid from acetyl‐CoA and malonil‐CoA, in the VAT of SL pups (Ameer et al. 2014). Aubert and colleagues (1980) showed that litter size reduction caused adipocyte hypertrophy and hyperplasia in adult mice. However, the present study shows that both the mRNA level and protein content of the adipogenic factors C/EBPβ and PPARγ were lower in visceral adipose tissue from the SL group. In agreement, Akyürek and colleagues (2013) reported lower serum PPARγ levels in obese 8‐ to 12‐year‐old children. The maternal obesity model also is associated with lower PPARγ levels in visceral adipose tissue of adult mice offspring (Magliano et al. 2013). PPARγ activation causes mature adipocyte apoptosis and increases adipocyte proliferation, which is more sensitive to insulin anti‐lipolitic action (Okuno et al. 1998). However, Akyürek et al. (2013) and Magliano et al. (2013) support the conclusion that PPARγ is not necessary for mature adipocyte function in obesity. Apparently, increased lipogenesis in the adipocytes is associated with a reduction in PPARγ and C/EBP in the SL group, which suggests that the adipocyte storage capacity reaches a limit, because there is less stimulus for adipocyte proliferation, favouring adipocyte hypertrophy and hypertriglyceridaemia (Rodrigues et al. 2011), and ectopic lipid storage, as observed in the higher triglyceride content in the liver.
The liver triglyceride content was increased in SL rats, followed by increased PPARγ protein content. The association between liver lipid accumulation and increases in PPARγ was previously reported in an experimental model of obesity and diabetes (Satoh et al. 2013; Alfaradhi et al. 2014). The precocious triglyceride accumulation may be due to increased de novo hepatic lipogenesis, since the increase in milk intake in the litter size reduction model provides elevated amounts of carbohydrate for fatty acid synthesis (Moreira et al. 2009; Cunha et al. 2009). The lipolitic factors PPARα and PGC1 were increased and the FAS content was unchanged in the SL group, suggesting increased oxidative metabolism in the liver. These results have already been reported (Lin et al. 2005); a high fat diet intake was able to increase the PGC‐1β content in mice liver. These findings suggest that in short‐term obesity the liver is able preferentially to use fatty acids as an energy source, which could decrease the contribution of glycolysis to the insulin resistance observed in this model at this age (Rodrigues et al. 2011).
Some studies show that autonomic dysfunction makes an important contribution to obesity development (Lima et al. 2007; Messina et al. 2013). Brown adipose tissue (BAT) has a thermogenic function, dissipating energy as heat, stimulated by sympathetic nerve activity. A reduction in sympathetic activity is reported in obesity models (Lima et al. 2007; Scomparin et al. 2009). Here, we report evidence that the firing rates of BAT (sympathetic nerve) and vagus (parasympathetic nerve) nerves were not changed in the SL group at weaning. The long‐term outcomes of reducing litter size upon the sympathetic and parasympathetic nerve activity have already been reported (de Almeida et al. 2013). At PN90, the SL rats showed an increase in vagal nerve superior branch activity, which was related to higher insulin secretion, but these SL rats did not show a change in BAT sympathetic nerve activation. In addition, the adrenal medulla catecholamine content and TH content were not affected by early postnatal obesity. These findings suggest that precocious onset of obesity may be not related to changes in autonomic peripheral nerve activity as demonstrated previously in other obese models. Scomparin and colleagues (2009) showed a reduction in sympathetic firing rate in MSG obese model adult rats. Besides alterations in firing rate activity, the sympathetic function can also be modulated by adrenergic tissue sensitivity (Lima et al. 2007). In the present study, SL rats had lower β3ADR in VAT, suggesting reduced adrenergic sensitivity, which leads to a decrease in lipolitic action and accords with higher central adiposity. In the liver, the SL rats showed unchanged β2ADR at weaning. Thus, the adipocyte changes can, at least in part, be explained by the reduction in adrenergic action, and not by changes seen in the liver. Curiously, on reaching adulthood (PN180) these SL animals had a lower β2ADR content in liver, but showed no changes in β3ADR content in VAT (Conceição et al.2013 b). These liver alterations are more remarkable, when we consider the higher liver steatosis in the adult SL animal. Thus, we suggest that the programming effect is seen first in the adipocytes, rather than in the liver, and this effect is aggravated during development.
Obesity and a high‐fat diet are related to an increase in reactive oxygen species production (Roberts et al. 2006; Aroor & DeMarco, 2014). In our plasma analysis, SOD and GPx activities were unchanged, CAT activity was decreased and SOD/GPx and SOD/(GPx+CAT) ratios were increased in the SL group. The SOD/GPx activity ratio is associated with higher production of hydrogen peroxide and lipid peroxidation (Amstad et al. 1991; de Haan et al. 1996). Accordingly, the lipid peroxidation and protein carbonyl content were increased in the plasma of the SL group; this oxidative condition in plasma has already been observed as an outcome of reducing litter size at adulthood (PN180 and PN210) (Conceição et al. 2013 a; Habbout et al. 2012 and 2013 b).
The common hepatic manifestation of metabolic syndrome is non‐alcoholic steatosis. Its development involves increased free fatty acid influx and de novo lipogenesis followed by oxidative stress conditions (Rolo et al. 2012). Besides lipid accumulation in the liver, early overfeeding is able to induce oxidative stress at weaning. The SOD and CAT content in the liver was higher in our SL group, despite no change in their activities, and GPx content and activity were decreased. These findings may contribute to the decrease in antioxidant capacity confirmed by a decrease in DPPH• scavenging activity, indicating oxidative damage in the liver, with higher MDA content and 4‐HNE protein adducts, which are parameters of lipid peroxidation, followed by carbonylated protein content and 3‐nitrotyrosin, which are biomarkers of protein oxidation by free radical species. Furthermore the SL rats showed a higher plasma level of ALT activity. This finding is indicative of cellular liver damage (Rinella, 2015; Sanal, 2015), in agreement with our present data. Additionally, it is known that high ROS content is correlated to apoptosis (Bruin et al. 2008), immune system activation (Cohen et al. 2011) and epigenetic modifications (Milagro et al. 2012). This early disturbance may contribute to increased lipid accumulation at weaning and the other disturbances developed by this programming model at adulthood, such as microsteatosis, oxidative stress and insulin resistance in the liver (Conceição et al. 2013 a).
Early obesity also may be result of an imbalance in formula feeding prescription, which may cause a high caloric intake. In fact both litter size reduction and formula feeding increase the body fat content and oxidative stress, but each condition has its own particular physiological profile. The lack of maternal hormones in formula feeding may prejudice normal adipose tissue and neuronal development (Melnik, 2014); formula feeding has no antioxidants which are important for adaptation to the hyperoxygenated environment after birth (Friel et al. 2011). This precocious exposure to a hyperoxidative status affects the physiological mechanism related to epigenetic modifications of important metabolism‐related genes, such as the lipogenic, adipogenic and antioxidant genes, which can cause future diseases (Yara et al. 2015).
Our data help to elucidate the sequence of mechanisms by which early overfeeding induces both adipocyte hypertrophy and liver dysfunction. The reduction in adrenergic sensitivity, despite an apparently normal sympathetic activation, may contribute to early adipocyte lipid accumulation. The postnatal obesity we observed was related to an increase in lipogenic capacity in adipose tissue and liver. Furthermore, the present results provide evidence that early overfeeding is able to induce the precocious onset of oxidative imbalance. This early exposure to a pro‐oxidative condition might favour liver dysfunction and obesity. Thus further experimental studies must be designed to help us understand whether the early prevention of oxidative stress might be a good strategy to prevent future disease.
Additional information
Competing interests
The authors declare that there is no competing interest that could be perceived as prejudicing the impartiality of the research reported.
Author contributions
Conception and design: E.P.S.C., E.O., E.G.M., P.C.L. Animal treatment, collection and measurements: E.P.S.C., J.C.C. Analysis and interpretation of data: E.P.S.C., E.O., J.C.C., E.G.M., P.C.L. Drafting and/or revising the article critically for important intellectual content: E.P.S.C., E.O., J.C.C., E.G.M., P.C.L. All authors read and approved the final version of the manuscript.
Funding
This research was supported by the ‘National Council for Scientific and Technological Development’ (Conselho Nacional de Desenvolvimento Científico e Tecnológico‐CNPq) and the ‘Carlos Chagas Filho Research Foundation of the State of Rio de Janeiro’ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro‐FAPERJ). E.P.S.C. is recipient of a FAPERJ fellowship and J.C.C. is recipiente of a CNPq fellowship.
Acknowledgment
All authors are grateful to Miss Monica Moura and Mr Ulisses Risso Siqueira for technical assistance.
References
- Aebi H (1984). Catalase in vitro methods. Enzymol 105, 121–126. [DOI] [PubMed] [Google Scholar]
- Akyürek N, Aycan Z, Cetinkaya S, Akyürek O, Yilmaz Ağladioğlu S & Ertan U (2013). Peroxisome proliferator activated receptor (PPAR)‐gamma concentrations in childhood obesity. Scand J Clin Lab Invest 73, 355–360. [DOI] [PubMed] [Google Scholar]
- Alfaradhi MZ, Fernandez‐Twinn DS, Martin‐Gronert MS, Musial B, Fowden A & Ozanne SE (2014). Oxidative stress and altered lipid homeostasis in the programming of offspring fatty liver by maternal obesity. Am J Physiol Regul Integr Comp Physio 307, R26–R34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AT, Hochfeld WE, Myburgh R & Pepper MS (2013). Adipocyte and adipogenesis. Eur J Cell Biol 92, 229–236 [DOI] [PubMed] [Google Scholar]
- Amarowicz R, Naczk M & Shahidi F (2000). Antioxidant activity of various fractions of non‐tannin phenolics of canola hulls. J Agric Food Chem 48, 2755–2759. [DOI] [PubMed] [Google Scholar]
- Ameer F, Scandiuzz, L , Hasnain S, Kalbacher H & Zaidi N (2014). De novo lipogenesis in health and disease. Metabolism 63, 895–902. [DOI] [PubMed] [Google Scholar]
- Amstad P, Peskin A, Shah G, Mirault ME, Moret R, Zbinden I & Cerutti P (1991). The balance between Cu,Zn‐superoxide dismutase and catalase affects the sensitivity of mouse epidermal cells to oxidative stress. Biochemistry 24, 9305–9313. [DOI] [PubMed] [Google Scholar]
- Aroor AR & DeMarco VG (2014). Oxidative stress and obesity: the chicken or the egg? Diabetes 63, 2216–2218. [DOI] [PubMed] [Google Scholar]
- Aubert R, Suquet JP & Lemonnier D (1980). Long‐term morphological and metabolic effects of early under‐ and over‐nutrition in mice. J Nutr 110, 649–661. [DOI] [PubMed] [Google Scholar]
- Bannister JV & Calabrese L (1987). Assays for superoxide dismutase. Methods Biochem Anal 32, 279–312. [DOI] [PubMed] [Google Scholar]
- Bruin JE, Petre M, Lehman M, Raha S, Gerstein HC, Morrison KM & Holloway AC (2008). Maternal nicotine exposure increases oxidative stress in the offspring. Free Radic Biol Med 44, 1919–1925. [DOI] [PubMed] [Google Scholar]
- Bullon P, Newman HN & Battino M (2000). Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: a shared pathology via oxidative stress and mitochondrial dysfunction? Periodontol 2000 64, 139–153. [DOI] [PubMed] [Google Scholar]
- Codoñer‐Franch P, Valls‐Bellés V, Arilla‐Codoñer A & Alonso‐Iglesias E (2011). Oxidant mechanisms in childhood obesity: the link between inflammation and oxidative stress. Transl Res 158, 369–384. [DOI] [PubMed] [Google Scholar]
- Cohen JI, Chen X & Nagy LE (2011). Redox signaling and the innate immune system in alcoholic liver disease. Antioxid Redox Signals 15, 523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conceição EP, Franco JG, Oliveira E, Resende AC, Amaral TA, Peixoto‐Silva N, Passos MC, Moura EG & Lisboa PC (2013. a). Oxidative stress programming in a rat model of postnatal early overnutrition–role of insulin resistance. J Nutr Biochem 24, 81–87. [DOI] [PubMed] [Google Scholar]
- Conceição EP, Moura EG, Trevenzoli IH, Peixoto‐Silva N, Pinheiro CR, Younes‐Rapozo V, Oliveira E & Lisboa PC (2013. b). Neonatal overfeeding causes higher adrenal catecholamine content and basal secretion and liver dysfunction in adult rats. Eur J Nutr 52, 1393–1404. [DOI] [PubMed] [Google Scholar]
- Conceição EP, Trevenzoli IH, Oliveira E, Franco JG, Carlos AS, Nascimento‐Saba CC, Moura EG & Lisboa PC (2011). Higher white adipocyte area and lower leptin production in adult rats overfed during lactation. Horm Metab Res 43, 513–516. [DOI] [PubMed] [Google Scholar]
- Cunha ACDSR, Pereira RO, Pereira MJDS, Soares VDM, Martins MR, Teixeira MT & Moura AS (2009). Long‐term effects of overfeeding during lactation on insulin secretion – the role of GLUT‐2. J Nutr Biochem 20, 435–442. [DOI] [PubMed] [Google Scholar]
- de Almeida DL, Fabrício GS, Trombini AB, Pavanello A, Tófolo LP, da Silva Ribeiro TA, de Freitas Mathias PC & Palma‐Rigo K (2013). Early overfeed‐induced obesity leads to brown adipose tissue hypoactivity in rats. Cell Physiol Biochem 32, 1621–1630. [DOI] [PubMed] [Google Scholar]
- de Haan JB, Cristiano F, Iannello R, Bladier C, Kelner MJ & Kola I (1996). Elevation in the ratio of Cu/Zn‐superoxide dismutase to glutathione peroxidase activity induces features of cellular senescence and this effect is mediated by hydrogen peroxide. Hum Mol Genet 5, 283–292. [DOI] [PubMed] [Google Scholar]
- de Moura EG & Passos MC (2005). Neonatal programming of body weight regulation and energetic metabolism. Biosci Rep 25, 251–269. [DOI] [PubMed] [Google Scholar]
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD & Parks EJ (2005). Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115, 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel JK, Diehl‐Jones B, Cockell KA, Chiu A, Rabanni R, Davies SS & Roberts LJ 2nd (2011). Evidence of oxidative stress in relation to feeding type during early life in premature infants. Pediatr Res 69, 160–164. [DOI] [PubMed] [Google Scholar]
- Flohé L & Günzler WA (1984). Assays of glutathione peroxidase. Methods Enzymol 105, 114–121. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M & Sloane Stanley GH (1957). A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 115, 1343–1351. [PubMed] [Google Scholar]
- Gao Q, Jia Y, Yang G, Zhang X, Boddu PC, Petersen B, Narsingam S, Zhu YJ, Thimmapaya B, Kanwar YS & Reddy JK (2015). PPARα‐deficient ob/ob obese mice become more obese and manifest severe hepatic steatosis due to decreased fatty acid oxidation. Am J Pathol 185, 1396–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habbout A, Delemasure S, Goirand F, Guilland JC, Chabod F, Sediki M, Rochette L & Vergely C (2012). Postnatal overfeeding in rats leads to moderate overweight and to cardiometabolic and oxidative alterations in adulthood. Biochimie 94, 117–124. [DOI] [PubMed] [Google Scholar]
- Habbout A, Guenancia C, Lorin J, Rigal E, Fassot C, Rochette L & Vergely C (2013. a). Postnatal overfeeding causes early shifts in gene expression in the heart and long‐term alterations in cardiometabolic and oxidative parameters. PLoS One 8, e56981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habbout A, Li N, Rochette L & Vergely C (2013. b). Postnatal overfeeding in rodents by litter size reduction induces major short‐ and long‐term pathophysiological consequences. J Nutr 143, 553–562. [DOI] [PubMed] [Google Scholar]
- Hanson MA & Gluckman PD (2014). Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev 94, 1027–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway AC, Lim GE, Petrik JJ, Foster WG, Morrison KM & Gerstein HC (2005). Fetal and neonatal exposure to nicotine in Wistar rats results in increased beta cell apoptosis at birth and postnatal endocrine and metabolic changes associated with type 2 diabetes. Diabetologia 48, 2661–2666. [DOI] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S & Stadtman ER (1990). Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186, 464–478. [DOI] [PubMed] [Google Scholar]
- Lima JJ, Feng H, Duckworth L, Wang J, Sylvester JE, Kissoon N & Garg H (2007). Association analyses of adrenergic receptor polymorphisms with obesity and metabolic alterations. Metabolism 56, 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, Newgard CB & Spiegelman BM (2005). Hyperlipidemic effects of dietary saturated fats mediated through PGC‐1beta coactivation of SREBP. Cell 120, 261–273. [DOI] [PubMed] [Google Scholar]
- Livak KJ & Schmittgen TD (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the 2 method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Magliano DC, Bargut TC, de Carvalho SN, Aguila MB, Mandarim‐de‐Lacerda CA, & Souza‐Mello V (2013). Peroxisome proliferator‐activated receptors‐alpha and gamma are targets to treat offspring from maternal diet‐induced obesity in mice. PloS One 20, e64258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnik BC (2014). The potential mechanistic link between allergy and obesity development and infant formula feeding. Allergy Asthma Clin Immunol 10, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina G, De Luca V, Viggiano A, Ascione A, Iannaccone T, Chieffi S & Monda M (2013). Autonomic nervous system in the control of energy balance and body weight: personal contributions. Neurol Res Int 2013, 639280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milagro FI, Mansego ML, De Miguel C & Martínez JA (2012). Dietary factors, epigenetic modifications and obesity outcomes: Progresses and perspectives. Mol Aspects Med 34, 782–812. [DOI] [PubMed] [Google Scholar]
- Moreira SB, Teixeira Teixeira M, da Silveira Osso F, Pereira RO, de Oliveira Silva‐Junior G, Garcia de Souza EP & Moura AS (2009). Left ventricular hypertrophy induced by overnutrition early in life. Nutr Metab Cardiovasc Dis 19, 805–810. [DOI] [PubMed] [Google Scholar]
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF et al (2014). Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen TS, Jessen N, Jørgensen JO, Møller N & Lund S (2014). Dissecting adipose tissue lipolysis: molecular regulation and implications for metabolic disease. J Mol Endocrinol 52, R199–222. [DOI] [PubMed] [Google Scholar]
- Niu Y, Herrera EA, Evans RD & Giussani DA (2013). Antioxidant treatment improves neonatal survival and prevents impaired cardiac function at adulthood following neonatal glucocorticoid therapy. J Physiol 591, 5083–5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H, Yazaki Y & Kadowaki T (1998). Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest 101, 1354–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk Y & Soylu OB (2014). Fatty liver in childhood. World J Hepatol 26, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman S, Frankel S (1957). A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28, 56–63. [DOI] [PubMed] [Google Scholar]
- Rinella ME (2015). Nonalcoholic fatty liver disease: a systematic review. JAMA 313, 2263–2273. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A & Vaziri ND (2006). Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet‐induced metabolic syndrome. Metabolism 55, 928–934. [DOI] [PubMed] [Google Scholar]
- Rodrigues AL, de Moura EG, Passos MC, Trevenzoli IH, da Conceição EP, Bonono IT, Neto JF & Lisboa PC (2011). Postnatal early overfeeding induces hypothalamic higher SOCS3 expression and lower STAT3 activity in adult rats. J Nutr Biochem 22, 109–117. [DOI] [PubMed] [Google Scholar]
- Rolo AP, Teodoro JS & Palmeira CM (2012). Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med 52, 59–69. [DOI] [PubMed] [Google Scholar]
- Sanal MG (2015). Biomarkers in nonalcoholic fatty liver disease‐the emperor has no clothes? World J Gastroenterol 21, 3223–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders FW & Griffin JL (2015). De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol Rev Camb Philos Soc (in press; DOI: 10.1111/brv.12178). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Ide N, Kagawa Y & Maeda T (2013). Hepatic steatosis with relation to increased expression of peroxisome proliferator‐activated receptor‐γ in insulin resistant mice. Biol Pharm Bull 36, 616–623. [DOI] [PubMed] [Google Scholar]
- Scomparin DX, Gomes RM, Grassiolli S, Rinaldi W, Martins AG, de Oliveira JC, Gravena C & de Freitas Mathias PC (2009). Autonomic activity and glycemic homeostasis are maintained by precocious and low intensity training exercises in MSG‐programmed obese mice. Endocrine 36, 510–517. [DOI] [PubMed] [Google Scholar]
- Shin JA, Lee JH, Lim SY, Ha HS, Kwon HS, Park YM, Lee WC, Kang MI, Yim HW, Yoon KH & Son HY (2013). Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J Diabetes Investig 8, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanidis A & Spencer SJ (2012). Effects of neonatal overfeeding on juvenile and adult feeding and energy expenditure in the rat. PLoS One 7, e52130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq Z, Green CJ & Hodson L (2014). Are oxidative stress mechanisms the common denominator in the progression from hepatic steatosis towards non‐alcoholic steatohepatitis (NASH)? Liver Int 34, e180–190. [DOI] [PubMed] [Google Scholar]
- Tounian P (2011). Programming towards childhood obesity. Ann Nutr Metab 58 (Suppl. 2), 30–41. [DOI] [PubMed] [Google Scholar]
- Vynche W (1970). Direct determination of the thiobarbituric acid value in thricloracetic acid extracts of fish as a measure of oxidative rancidity. Fett Wiss Technol 72, 1084–1087. [Google Scholar]
- Yara S, Lavoie JC & Levy E (2015). Oxidative stress and DNA methylation regulation in the metabolic syndrome. Epigenomics 7, 283–300. [DOI] [PubMed] [Google Scholar]


