Abstract
Current acellular pertussis vaccines have various shortcomings, which may contribute to their suboptimal efficacy and waning immunity in vaccinated populations. This calls for the development of new pertussis vaccines capable of inducing long-lived protective immunity. Immunization with whole cell pertussis vaccines and natural infection with Bordetella pertussis induce distinct and more protective immune responses when compared with immunization with acellular pertussis vaccines. Therefore, the immune responses induced with whole cell vaccine or after infection can be used as a benchmark for the development of third-generation vaccines against pertussis. Here, we review the literature on the immunology of B. pertussis infection and vaccination and discuss the lessons learned that will help in the design of improved pertussis vaccines.
Keywords: Bordetella pertussis, vaccine, T cell, Toll-like receptor agonist, immunology
To develop improved pertussis vaccines capable of inducing long-lived protective immunity, lessons have to be learned from immunology of Bordetella pertussis infection and current vaccination.
Graphical Abstract Figure.
To develop improved pertussis vaccines capable of inducing long-lived protective immunity, lessons have to be learned from immunology of Bordetella pertussis infection and current vaccination.
Introduction
Bordetella pertussis is a respiratory tract pathogen that causes the severe disease whooping cough (pertussis) in infants and young children. It can also infect adolescents and adults. The disease was largely controlled in developed countries following the introduction of whole cell pertussis (wP) vaccines in the 1940s/1950s. However, pertussis still kills over 200 000 infants yearly worldwide, mostly in developing countries, but the incidence of disease is also increasing in many developed counties (Black et al. 2010). This has been attributed to the introduction of acellular pertussis (aP) vaccines in the 1990s, which were developed in response to concerns around the safety of wP vaccines. Unlike wP vaccines, which are composed of killed bacteria with a wide range of antigens and pathogen-associated molecular patterns (PAMPs) that bind to pattern recognition receptors (PRRs) and activate innate immune cells, aP vaccines are composed of three to five B. pertussis antigens absorbed to alum as the adjuvant. A monocomponent vaccine based on hydrogen peroxide-detoxified pertussis toxin (PT) was also developed (Trollfors et al. 1995), but had lower efficacy than the three and five component aP vaccines. Although initial reports from phase 3 clinical trials carried out in the 1990s suggested that aP vaccines were at least as effective as wP vaccines (Greco et al. 1996; Gustafsson et al. 1996), recent studies have demonstrated a rapid and alarming drop in protection over time, suggesting a failure to sustain immunity (Klein et al. 2012).
There have been a number of explanations put forward to account for recent epidemics of pertussis in highly vaccinated populations. First, there is evidence of antigen variation in some of the key protective antigens and components of aP vaccines, namely pertussis toxin (PT), pertactin (Prn) and Fimbriae (Fim2 and Fim3) (van Loo et al. 2002; Tsang et al. 2004; Bart et al. 2014). Moreover, Prn-deficient strains have emerged following introduction of aP vaccination in Europe (Bouchez et al. 2009; Barkoff et al. 2012; Hegerle et al. 2012; Zeddeman et al. 2014), Australia (Lam et al. 2014), Japan (Otsuka et al. 2012) and the USA (Queenan, Cassiday and Evangelista 2013; Pawloski et al. 2014; Martin et al. 2015). Therefore, it is possible that the immune responses induced by three to five antigens from the single isolate used to manufacture the aP vaccine do not protect against at least some of the current circulating strains. Secondly, the waning protective immunity observed following immunization with aP vaccines suggests that these vaccines fail to generate effective immunological memory (Klein et al. 2012, 2013; Sheridan et al. 2012; Liko, Robison and Cieslak 2013; Witt et al. 2013).
Finally, there is convincing evidence that the aP vaccines do not induce the optimum profile of immune responses required for protection against infection with B. pertussis and that these differ significantly from those induced by natural infection or immunization with wP vaccines. The consensus view from studies in the mouse model is that wP vaccines and previous infection confer better protective immunity than aP vaccines because they induce Th1 cells and associated opsonizing antibodies, with a minor contribution by Th17 cells (Table 1). In contrast, the less effective aP vaccines induce a mixed Th2 and Th17 response (Ross et al. 2013), but the Th2 component appears to be redundant to protection and, together with associated IgE (Ryan et al. 2000), may even be the culprits in rare type hypersensitivity reactions seen in children after a fourth or fifth dose of the aP vaccines (Rennels et al. 2008). This pattern of immune responses induced by the pertussis vaccines is generally similar in humans and mice. Recent evidence from the new animal model of experimental pertussis in baboons has uncovered a major deficit in protective immunity induced by aP vaccination. While the immune responses induced by immunization with wP vaccines were able to prevent disease and enhanced clearance of the infection (though not as effectively as previous infection), immunization with the aP vaccine prevented disease, but did not prevent infection or transmission of B. pertussis to naive baboons (Warfel, Zimmerman and Merkel 2014). Whilst it has not been conclusively proven in the baboon, it appears that the failure of aP vaccines to prevent infection reflects its failure to induce appropriate cellular immune responses, especially Th1 cells. In this minireview, immunological evidence from infection and vaccination studies will be discussed in order to guide the development of improved pertussis vaccines.
Table 1.
The putative roles of different immune cells in protective immunity to B. pertussis.
| Cell Type | Infectiona | wP | aP | Species | Function | Reference |
|---|---|---|---|---|---|---|
| Th1 cell | +++ | ++ | +/– | Man | IFNγ production, macrophage activation, opsonizing Ab, prevents dissemination of Bp | (Ryan et al. 1997, 1998b; Ausiello et al. 1999; Mascart et al. 2003; Mascart et al. 2007; Schure et al. 2012) |
| +++ | + | + | Baboon | (Warfel and Merkel 2013) | ||
| +++ | ++ | +/– | Mouse | (Mills et al. 1993; Ross et al. 2013; Raeven et al. 2014; Brummelman et al. 2015) | ||
| Th2 cell | +/– | +/– | +++ | Man | No identified role in mice; unknown role in baboons and humans | (Ryan et al. 1997, 1998b; Ausiello et al. 1999; Mascart et al. 2003; Mascart et al. 2007; Schure et al. 2012), van Twillert unpublished |
| – | – | +++ | Baboon | (Warfel and Merkel 2013) | ||
| – | – | +++ | Mouse | (Mills et al. 1993; Ross et al. 2013; Raeven et al. 2014; Brummelman et al. 2015) | ||
| Th17 cell | +++ | ++ | +/– | Man | IL-17 production, neutrophil recruitment and activation | (Schure et al. 2012), van Twillert, unpublished |
| +++ | ++ | – | Baboon | (Warfel, Zimmerman and Merkel 2014) | ||
| +++ | ++ | +/– | Mouse | (Ross et al. 2013; Raeven et al. 2014; Brummelman et al. 2015) | ||
| TRM cell | +++ | ++ | – | Mouse | Sustains local cellular immunity in the respiratory tract? | Wilk and Mills, unpublished |
| TFH cell | +++ | ++ | + | Mouse | Activates Ab production and memory B cells? | Wilk, Allen and Mills, unpublished |
| TCM cell | ? | + | + | Man and Mouse | Maintains long-term immunity | (Brummelman et al. 2015; de Rond et al. 2015). |
| TEM cell | ? | + | ++ | Man and Mouse | Immediate effector function | (Brummelman et al. 2015, de Rond et al. 2015). |
| TTD cell | ? | + | ++ | Man | Waning immunity? | (de Rond et al. 2015). |
| γδ T cell | ++ | ? | ? | Mouse | Immune regulation early in infection | (Zachariadis et al. 2006) |
| B cell | + | ++ | +++ | Mouse | Ab production | (Mahon et al. 1997; Leef et al. 2000; Stenger et al. 2010) |
| Alveolar Mac | +++ | + | – | Mouse | Early response to infection, phagocytosis and killing of Bp | (Bernard et al. 2015) |
| DC | +++ | ? | ? | Mouse | Activation of naive T cells in lymph nodes | (Dunne et al. 2009) |
| NK cell | +++ | ? | ? | Mouse | Early IFNγ, prevents dissemination of Bp | (Byrne et al. 2004) |
| Neutrophil | +++ | ++ | – | Mouse | Ab-dependent phagocytosis and killing of opsonized Bp | (Andreasen and Carbonetti 2009; Ross et al. 2013; Eby, Gray and Hewlett 2014). |
aResponses denoted as +++, ++, +, +/–, – and ? equate to strong, medium, weak, weak/ inconsistent, undetectable responses or not tested respectively; Bp, B. pertussis, Ab, antibody.
CURRENT STATE OF THE ART ON THE MECHANISMS OF NATURAL IMMUNITY TO B. PERTUSSIS
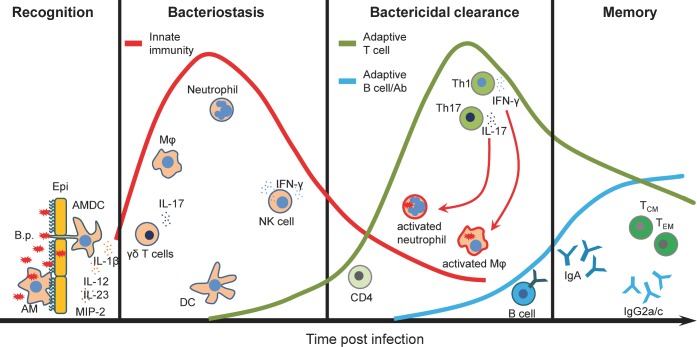
The innate immune response plays a crucial role in controlling the initial stages of B. pertussis infection and helps to shape the subsequent adaptive immune responses. B. pertussis attaches to ciliated epithelial cells on the upper respiratory tract (Coutte et al. 2003; Carbonetti et al. 2007). These cells may also provide a first barrier, by preventing pathogen penetration and through production of mucine by secretory goblet cells which can protect the lungs from pathogens that are inhaled into the respiratory track. Two resident innate immune cell types, airway mucosal dendritic cells (AMDCs) and alveolar macrophages (AMs), which are in close contact with the lung epithelium, mediate the first cellular response to B. pertussis. AMDCs are strategically positioned between epithelial cells to take up antigen directly from the airway lumen and prime T cells after migration to the lymph node (Stumbles et al. 1998; Jahnsen et al. 2006), whereas AMs reside within the mucous layer and appear to be important for phagocytosis and killing of B. pertussis (Lambrecht 2006). These lung-resident innate immune cells provide a first line of immediate immune defense against B. pertussis infection. Additionally, they initiate and orchestrate complex and tightly regulated processes that involve activation and recruitment of other immune cells and generation of long-lasting adaptive immunity (Fig. 1 and Table 1).
Figure 1.
Relative contribution of the cell subtypes to the induction of immune response to B. pertussis. The immune response to B. pertussis is a complex process that involves activation and recruitment of immune cells to the respiratory tract and generation of long-lasting adaptive immunity. Attachment of B. pertussis to ciliated epithelial cells and recognition by AMDCs and AMs provide a first line of immediate defense against B. pertussis infection. Secretion of cytokines and chemokines promotes recruitment of innate immune cells. Innate IL-17 together with CXCL2 (or MIP-2) secreted by activated macrophages and epithelial cells promote neutrophils recruitment. NK cells play a protective role through the secretion of IFNγ, which enhances the antimicrobial activity of macrophages as well as induces Th1 cells. Activated neutrophils and macrophages participate in an antibody-mediated phagocytosis and intracellular killing of B. pertussis. DCs migrate to the lymph nodes to present the antigen to the naive T cells. Primed T cells proliferate and differentiate into Th1 and Th17 cells that migrate to the lungs to further activate neutrophils and macrophages by production of IFNγ and IL-17, respectively. Activated B cells differentiate into plasma cells that produce B. pertussis-specific IgA or IgG2a/c antibodies (mouse). Finally, a small fraction of T and B cells become memory cells providing an effective protection after reinfection. B.p., Bordetella pertussis; Epi, epithelium; AMs, alveolar macrophages; AMDCs, airway mucosal dendritic cells; DCs, dendritic cells; Mφ, macrophages; Neu, neutrophils; Th, T helper cells; TCM, central memory T cells; TEM, effector memory T cells.
B. pertussis was classically considered to be an extracellular pathogen that infects the upper respiratory tract, but it can also penetrate the lungs and has been found inside ciliated respiratory epithelial cells and in lung macrophages (Paddock et al. 2008; Lamberti et al. 2010, 2013). The recognition of B. pertussis PAMPs and virulence factors including lipooligosacharide (LOS), adenylate cyclase toxin (ACT), filamentous hemagglutinin (FHA) and TLR2 lipoproteins by PPRs expressed by macrophages and DCs (and other cells of the innate immune system) leads to their maturation and production of cytokines and chemokines that mediate and regulate immune responses to the bacteria. It was also shown that PT can act as PAMP, triggering the TLR4 signaling pathway (Wang et al. 2006; Nishida et al. 2010) and enhancing IL-12 production, thereby acting as an adjuvant to promote Th1 responses (Ryan et al. 1998a; Ausiello et al. 2002; Tonon et al. 2002). Binding of LOS to TLR4 results in DC maturation and production of IL-12 and IFNγ, cytokines required for development of Th1 responses (Higgins et al. 2003). TLR4 activation of DCs with LOS can also induce IL-10 production, consequently generating IL-10-secreting type-1 regulatory T cells (Tr1) that may dampen Th1 responses and limit inflammatory pathology in B. pertussis-infected lungs (Higgins et al. 2003). DCs can also be activated by intrinsic recognition of other B. pertussis virulence factors and toxins. It was shown that ACT activates the NLRP3 inflammasome and caspase-1, leading to production of mature bioactive IL-1β. Active IL-1β together with IL-23 promotes expansion of murine Th17 cells, which help to recruit neutrophils that promote killing of B. pertussis (Dunne et al. 2010). Furthermore, studies with human DCs have demonstrated that ACT enhances Th17 responses but suppresses Th1 responses by inhibiting IL-12p70 production (Spensieri et al. 2006). ACT also modulates TLR-induced signaling to upregulate IL-10 production and promote the development of Treg cells (Hickey, Brereton and Mills 2008).
It was shown that TLR4-deficient C3H/HeJ mice had a more severe course of B. pertussis infection when compared with TLR4-sufficient C3H/HeN mice (Mann et al. 2005; Higgins et al. 2006; Banus et al. 2008). TLR4 expressed by DCs and macrophages plays a central role in protective immunity to this pathogen. Furthermore, an early burst of proinflammatory cytokines and chemokines produced by AMs soon after infection depends on TLR4 signaling pathway. Mice that lack MyD88 adaptor-like protein (Mal; also known as TIRAP) were unable to control infection (Bernard et al. 2015). The bacteria disseminated from the lungs causing lethality in nearly 50% of MAL−/− mice. Additionally, AMs were completely depleted from the lungs of Mal−/− mice early after infection, suggesting that these cells are critical for mediating protection to B. pertussis (Bernard et al. 2015).
The initial recruitment of DCs and macrophages into the lungs of B. pertussis-infected mice is followed by recruitment or expansion of γδ T cells and infiltration of neutrophils and NK cells. It is thought that γδ T cells orchestrate the early cell trafficking into the lungs by producing IL-17 (Zachariadis et al. 2006). This cytokine together with CXCL2 (MIP-2) secreted by activated macrophages and epithelial cells is required for neutrophil recruitment as well as induction of Th1 and Th17 responses to B. pertussis.
A primary role of neutrophils during the microbial infection is antibody-mediated phagocytosis and intracellular killing of pathogenic bacteria and formation of neutrophil extracellular traps (Andreasen and Carbonetti 2009; Eby, Gray and Hewlett 2014). It was shown that ACT can inhibit neutrophil function but does not impact on protective immunity to B. pertussis. It was suggested that neutrophils are important, but not essential, in the controlling of bacteriostasis of B. pertussis, but are critical for clearance of B. bronchiseptica; infection of neutrophil-deficient mice with B. bronchseptica had a lethal outcome (Harvill, Cotter and Miller 1999). It was proposed that B. bronchiseptica can kill resident and recruited phagocytes in the lungs or AMs are sufficient to control B. pertussis infection (Harvill, Cotter and Miller 1999).
NK cells play a protective role in immunity to B. pertussis through secretion of IFNγ, which enhances the antimicrobial activity of macrophages. Production of IFNγ by NK cells is dependent on IL-12 from B. pertussis-activated DCs. Depletion of NK cells from B. pertussis-infected mice resulted in the dissemination of the bacteria from the respiratory tract to the liver and this was associated with reduced IFNγ production and Th1 responses (Byrne et al. 2004). Thus, NK cells play a critical role in early innate immune control of infection and in shaping the adaptive immune response to B. pertussis (Fig. 1).
The lungs of naïve mice contain very low numbers of T cells. However, during the course of infection with B. pertussis the adaptive immune response slowly develops and T cells are recruited to or expanded in the respiratory tract and mediate subsequent bacterial clearance of B. pertussis from the respiratory tract. Human infants without fully developed adaptive immunity are particularly susceptible to the severe symptoms of whooping cough, which can be lethal. Studies in mice have demonstrated that CD4+ T cells play a key role in the clearance of B. pertussis infection. Primary infection of athymic nu/nu mice, which lack all T cells, results in a persistent or even lethal infection with B. pertussis (Mills et al. 1993). Adoptive transfer of immune splenic T cells into athymic mice reversed chronic infection and resulted in bacterial clearance within 14 to 21 days post-challenge. Similarly, transfer of B. pertussis-specific CD4+ T cells into immunosuppressed sublethally irradiated naïve BALB/c mice before infection resulted in clearance of the pathogen. In contrast, sublethally irradiated mice were not able to clear primary infection after transfer of B. pertussis-specific CD8+ cells (Mills et al. 1993). In addition, antigen-specific CD4+ T cells from infected mice produce IFNγ and/or IL-17, but not Th2-type cytokines, suggesting that Th1 and Th17 cells are involved in protective immunity to B. pertussis infection. Indeed, IFNγ−/− mice exhibit impaired ability to clear the pathogen from the lungs (Barbic et al. 1997), whereas IFNγR−/− mice develop a lethal disseminated infection. Interestingly, IFNγR−/− mice that survive the initial infection develop neither Th1 nor Th2 response, suggesting that Th17 cells might be involved in developing protective immunity to B. pertussis (Mahon et al. 1997). Indeed, it has been demonstrated that IL-17−/− mice infected with B. pertussis have a significantly greater bacterial load in comparison to wild-type (WT) mice (Ross et al. 2013). This impaired ability of IL-17−/− mice to clear the bacteria correlated with a significant decreased frequency of neutrophils in the lungs during the course on infection (Ross et al. 2013). Taken together, the published studies demonstrate that Th1 and Th17 cells both contribute to the protective immunity induced by primary B. pertussis infection in mice (Fig. 1).
Humoral immunity may play an auxiliary role in the clearance of infection from the lungs and contribute to a development of the long-lasting, cell-mediated immunity to B. pertussis. The number of B cells significantly increases in the lungs during the course of infection reaching the highest number when the infection is almost cleared. Similarly, B. pertussis-specific IgG antibodies are only detectable in the serum at significant levels when the pathogen is almost cleared from the lungs (Mills et al. 1993; Kirimanjeswara, Mann and Harvill 2003). However, IgA was detected in the lungs 2 weeks after challenge (Mills et al. 1993). This suggests that IgG antibodies do not play a major role in the clearance of a primary natural infection, but may be more important in adaptive immunity induced by previous infection or vaccination. Leef et al. showed that B cells may contribute to the protective immunity by different mechanisms than specific antibody production. Although, B. pertussis-infected Ig−/− mice developed a chronic infection and could not clear the infection, these mice also lack mature B cells and fail to generate an effective T-cell response to B. pertussis (Mahon et al. 1997). It is also possible that B cells may contribute to the induction of memory CD4+ T cells by acting as the antigen-presenting cells (Leef et al. 2000; Linton, Harbertson and Bradley 2000).
Convalescent mice are able to clear the pathogen from the lungs as soon as 3–7 days after reinfection (Mills et al. 1993). Baboons recovered from a B. pertussis infection are protected from disease and do not transmit the pathogen upon subsequent challenge and this was associated with a long-lived mixed Th1/Th17-cell response (Warfel and Merkel 2013; Warfel, Zimmerman and Merkel 2014). Hence, natural infection induces strong and persistent immune protection against reinfection with B. pertussis, although it does not result in life-long immunity.
MECHANISMS OF VACCINE-INDUCED IMMUNITY AND LIMITATIONS OF CURRENT AP VACCINES
Due to their high reactogenicity, wP vaccines were replaced in the 1990s in most developed countries by aP vaccines containing a high dose of three to five immunogenic B. pertussis antigens, namely PT, Prn, FHA, Fim2 and Fim3. Both vaccine types provide protection against severe disease, however, with important underlying differences. aP vaccine-induced protective immune responses are less durable, and in contrast to wP vaccine-induced immunity only protect against clinical symptoms of pertussis but not against colonization and transmission of B. pertussis (Warfel, Zimmerman and Merkel 2014).
Besides its narrow specificity, various other features of aP vaccine-induced immunity may account for these differences. First, aP-induced T-cell responses have a distinct functional programming, compared to those induced by wP vaccines, as evidenced by both humoral and cell-mediated responses. While the two vaccine types readily induce significant levels of B. pertussis antigen-specific IgG antibodies, as reported in humans (Edwards et al. 1995; Hendrikx et al. 2011) and in mice (Redhead et al. 1993; Stenger et al. 2010; Raeven et al. 2015), there is a difference in class switching patterns. In mice, aP vaccines induce predominantly antibodies of the IgG1 subclass (Stenger et al. 2010; Brummelman et al. 2015), reflecting the induction of a Th2-type response, whereas wP vaccines induce predominantly IgG2a/b/c, as well as IgG1, and IgG3 (Raeven et al. 2015), which is consistent with the strong induction of Th1 cells. A comparison of the antibody isotype in cohorts of children that had been primed with either wP or aP vaccines, and followed between 3 and 9 years of age, showed that IgG1 was the predominant IgG subclass induced by both vaccines (Hendrikx et al. 2011). In humans, however, this complement activating and opsonizing IgG subclass is thought to be driven by Th1 cells (Hjelholt et al. 2013). Only low levels of the IgG2 subclass were observed in both wP- and aP-primed children (Hendrikx et al. 2011). The Th2-associated B. pertussis-specific IgG4 subclass was detected only after aP vaccination, with higher concentration after a greater number of doses (Hendrikx et al. 2011). Moreover, these IgG4 antibodies strongly correlate with the total IgE production in these aP-vaccinated children (Hendrikx et al. 2011), indicating that Th2-skewed immune responses are induced by aP vaccination in humans as well as in mice.
Further evidence for different programming of immune responses induced with aP and wP vaccines comes from studies focused on CD4+ T-cell responses and assessing the importance of their secreted cytokine profiles in the context of protective immunity in different species (Table 1). In mice, aP vaccination is associated with induction of CD4+ T cells that produce IL-4, IL-5 and IL-17, but relatively lower concentrations of IFNγ (Ross et al. 2013; Brummelman et al. 2015), representing a mixed Th2/Th17 response, while wP vaccines induce a mixed IFNγ/IL17A (Th1/Th17) response (Ross et al. 2013). Studies using cytokine-defective mice have shown that protection against B. pertussis induced by immunization with an aP vaccine is as effective in IL-4−/− or IFNγ−/− mice as in WT mice, whereas protection is significantly diminished in IL-17A−/− mice (Ross et al. 2013). This indicates that Th17 cells play an essential role in the protective immune response induced by the aP vaccine, while the Th2 cells do not contribute to protection. In contrast, Th1 are required for protection induced with wP vaccines (Ross et al. 2013).
In the baboon model, immunization with an aP vaccine, which conferred protection against disease but not infection or transmission, induces a mixed Th1/Th2 type of CD4+ T-cell response. In contrast, wP vaccination, providing protection against colonization and host-to-host transmission, induced a mixed Th1/Th17 memory response (Warfel, Zimmerman and Merkel 2014), suggesting that both Th1 and Th17 cells may be important in prevention of B. pertussis colonization in this model.
In humans, aP vaccination has been shown to induce a Th2-dominated, yet mixed Th2/Th1/Th17 type of CD4+ T-cell response in children (Ryan et al. 1998b; Ausiello et al. 1999; Mascart et al. 2007; Schure et al. 2012). In contrast, wP vaccines induce a mostly mixed Th1/Th17-type CD4+ T-cell response, similar to natural infection (Ryan et al. 1998b, 2000; Mascart et al. 2003, 2007; Rowe et al. 2005; Vermeulen et al. 2010; Ross et al. 2013). Notably, some of the Th1 responses in certain aP-vaccinated children may be attributed to exposure to or subclinical infections with B. pertussis (Ryan et al. 1998b; Ausiello et al. 1999; Mascart et al. 2003; Schure et al. 2012). In general, the studies in humans are consistent with the data from animal models, i.e. indicating Th2 dominance associated with aP vaccination and a mixed Th1/Th17 profile after wP vaccination, implying that aP vaccines may have a poorly protective functional T-cell profile in humans.
A second feature of the immune response that might contribute to the difference in long-term effectiveness of aP and wP vaccines has recently emerged from the series of head-to-head comparisons of the cellular immunity in aP- and wP-primed cohorts of children by Buisman and coworkers. It was found that PBMC from aP-primed 4-year-old children, three years after their primary pertussis vaccine series, produced higher specific levels of CD4+ T-cell cytokines than those from wP-primed children; however, this response was not boosted after a fifth dose of aP vaccine, in contrast to the response in wP-primed children (Schure et al. 2012). Yet, at the age of 6, two years after this booster dose, PBMC from aP-primed children produced lower levels B. pertussis-specific IL-17 when compared with PBMC from wP-primed children (Schure et al. 2013). Furthermore, assessment of CCR7 and CD45RA expression, which allowed CD4+ T cells to be categorized as naive T cells (TN), effector memory T cells (TEM), central memory T cells (TCM) or terminally differentiated T cells (TTD), showed that the proportion of B. pertussis antigen-specific TTD cells was higher, in the aP-primed cohort (de Rond et al. 2015). Together, these data suggest that aP-primed CD4+ T-cell responses might be more end-stage differentiated than those induced by wP vaccines. The high antigen dose in aP vaccines compared to wP vaccines may contribute to this phenomenon. High antigen and adjuvant dose may induce vigorous short-term immunity, but can also exhaust the immune system and impede long-term immunity (Darrah et al. 2007; Joshi et al. 2007; Gattinoni, Klebanoff and Restifo 2012; Tubo and Jenkins 2014). For example, in the Leishmania model, high dose vaccination with a Leishmania protein-expressing adenoviral vector in mice elicited higher numbers of total IFNγ-producing CD4+ T cells compared to low dose vaccination; however, high dose vaccination resulted in poor protective immunity (Darrah et al. 2007).
Thus, aP vaccine-induced immunity has various shortcomings, including immune responses of narrow specificity and with the wrong type of Th-cell subtypes and end-stage differentiated memory T cells which may collectively contribute to its suboptimal efficacy.
APPROACHES TO IMPROVE EFFICACY OF CURRENT AP VACCINATION BY TLR LIGATION
The lesson from studies on T cells in mouse models and humans to date have suggested that next-generation aP vaccines should induce CD4+ T cells with a TCM cell phenotype a mixed Th1/Th17-cell profile, like those induced by infection or immunization with wP vaccine. An important factor in natural infection with B. pertussis and immunization with wP vaccines that steers protective adaptive immune responses is the presence of PAMPs, including LOS that binds to TLR4 and lipoproteins that bind to TLR2 (Dunne et al. 2015). These PAMPs activate innate cells via PRRs and thereby promote DC maturation and production of proinflammatory cytokines that direct the induction of Th1 and Th17 cells (Fedele et al. 2008). Current aP vaccines do not contain classic PAMPs. Although active PT has adjuvant properties and might be considered to be a PAMP, the PT preparations in aP vaccines are aldehyde treated which destroys immunostimulatory activity (Ryan et al. 1998a). Overall, PT-containing aP vaccine induces a Th2-dominated response, likely due to the Th2-promoting properties of the adjuvant alum. One approach to enhance the efficacy of aP vaccines could be to address this shortfall by replacing or complementing alum with PAMPs, which are known to induce Th1 responses. Indeed, there is evidence from mouse studies that several TLR agonists may be effective Th1 promoting adjuvants for experimental aP vaccines.
A natural TLR2 ligand of B. pertussis, BP1569, was recently identified (Dunne et al. 2015). Immunization of mice with an experimental aP vaccine containing a corresponding synthetic lipopeptide LP1569 induced a higher level of protection against aerosol B. pertussis challenge when compared with the same vaccine formulated with alum. Furthermore, when compared with an alum-adjuvanted vaccine, the same vaccine formulated with the TLR2 lipoprotein was found to induce significantly higher IgG2a antibodies and promote higher IL-17, IFNγ and lower IL-5 production by spleen cells after antigen stimulation ex vivo.
The TLR4 agonist LOS is an important PAMP in wP vaccines enhancing Th1 responsiveness, but was also the main mediator of their high reactogenicity (Donnelly et al. 2001). Although mice are less sensitive to endotoxin than humans (Munford 2010), LOS from B. pertussis is less effective in activating human macrophages and DCs than LPS from other bacteria (Fedele et al. 2008). Therefore, well-defined detoxified LPS derivatives from other Gram-negative bacteria with Th1-promoting capacity have been tested as adjuvant for an experimental aP vaccine. Immunization of mice with an aP vaccine adjuvanted with monophosphoryl lipid A (MPL), the hydrophobic biologically active Lipid A part of Salmonella minnesota LPS, was more effective in protecting against B. pertussis challenge than the aP vaccine adjuvanted with alum (Geurtsen et al. 2008). Furthermore, B. pertussis-specific IL-5 production by spleen cells was lower following immunization with MPL-adjuvanted vaccine, indicating that this adjuvant can skew the immune response away from a Th2 response. Substantiating these data, the authors recently found, at the single T-cell level, that addition of the water-soluble genetically engineered derivative from Neisseria meningitidis LPS, LpxL1 (Zariri and van der Ley 2015), to the aP vaccine increased the percentage of specific IFNγ and IL-17-producing CD4+ T cells and diminished the percentage of IL-5-producing CD4+ T cells (Brummelman et al. 2015). Combining direct MHC class II tetramer staining of Prn-specific CD4+ T cells with the memory markers CD44 and CD62L in this model indicated that the ratio of TCM and TEM was not altered by addition of the adjuvant; however, there was a net benefit in the number of TCM, since higher numbers of B. pertussis-specific memory CD4+ T cells were detected after vaccination with the LpxL1-containing aP vaccine. Hence, this analysis confirmed that there was no trade-off for the altered cytokine profile after TLR4 engaging aP vaccination by a reduction of induced TCM cells. Since there are species differences in specificity of ligands for TLR4 (Marr et al. 2010; Bryant and Monie 2012), caution should be exercised in translating studies on LPS-based adjuvants from mice to humans.
TLR9 agonists, including CpG oligonucleotides from bacterial DNA, can also induce Th1 and Th17 responses and have been used as adjuvants for experimental aP vaccines tested in mice. The complementation or replacement of alum in an aP vaccine by CpG increased the anti-PT IgG2a titers, and the IgG2a/IgG1 ratio, providing indirect evidence for induction of Th1 responses (Sugai et al. 2005). In addition, B. pertussis-specific IFNγ production by spleen cells was found to increase when PT, FHA and Prn were administered intranasally with both CpG and alum, but not with CpG alone (Asokanathan, Corbel and Xing 2013). Finally, in another report substituting alum with CpG in an experimental parenterally delivered aP vaccine enhanced its efficacy against aerosol challenge with B. pertussis and was associated with stronger B. pertussis-specific IFNγ and IL-17, but lower IL-4 and IL-13 production by spleen cells, and higher IgG2a concentrations in sera (Ross et al. 2013). The failure of the study by Asokanathan et al. to detect IFNγ following immunization with pertussis antigens and CpG (without alum) may be due to the route of immunization (i.n.) and the lower dose of CpG (30 μg) employed. Together, these studies demonstrate that TLR agonists such as TLR2, TLR4 and TLR9 ligands can steer the aP vaccine-induced CD4+ T-cell response towards a more favorable protective Th1/Th17 response, and that pertussis vaccine formulations containing experimentally selected adjuvants could be considered as more effective alternatives for current aP vaccines.
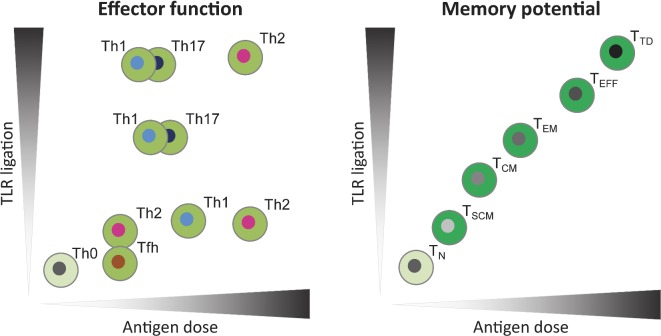
In addition to the type of adjuvant, the antigen composition and dose of both adjuvant and antigen may influence the induction of T-cell response with aP vaccines. Therefore, careful evaluation of choice of adjuvant and antigen and their dose will be required to ensure that a vaccine induces the appropriate functional T-cell subtype and profile of memory CD4+ T cells required for complete and sustained protective immunity against infection (Fig. 2).
Figure 2.
Models for the influence of antigen dose and the strength of TLR ligation on CD4+ T-cell differentiation. Vaccine-induced CD4+ T-cell responses can be evaluated based on effector function i.e. Th type (left panel) and memory potential i.e. memory differentiation stage (right panel). Antigen dose can affect TCR signal strength, thereby influencing Th type and memory T-cell differentiation (Gattinoni, Klebanoff and Restifo 2012; Tubo and Jenkins 2014). Inflammatory signals, such as induced by TLR ligation, during T-cell priming can also influence the Th type and memory potential of antigen-specific T cells via innate mechanisms (Joshi et al. 2007; Tubo and Jenkins 2014). The x axes depict the range of antigen dose and the y axes show the amount of TLR ligation present during vaccination or infection. In vivo, antigen and TLR ligand dose may form a gradient of local concentrations, possibly inducing an array of differentiation states. In the presence of low dose TLR ligands, low antigen dose will steer towards Th2 or Tfh cells, while an intermediate dose promotes Th1 cells and a high dose Th2 cells. Increasing TLR ligation induces Th17 and Th1 cells, yet high exposure to both TLR ligation as antigen dose will promote Th2 cells (Tubo and Jenkins 2014). In parallel, both low and high antigen dose and TLR ligation strength may also induce regulatory functions in cell populations (not shown). The memory differentiation stage is correlated with both the antigen dose and TLR ligation, meaning that the memory potential of the cells decreases when the cells are exposed to increasing TLR ligation and antigen dose (Joshi et al. 2007; Gattinoni, Klebanoff and Restifo 2012). The color gradient of memory phenotypes from light to dark indicates loss of long-term memory potential. TLR: Toll-like-receptor; TN: naïve T cell; TSCM: stem-cell memory T cell; TCM: central memory T cell; TEM: effector memory T cell; TEFF: effector T cell; TTD: terminally differentiated T cell.
Several pertussis vaccine candidates in development address the absence of functional PRR triggering by current aP vaccines by expressing endogenous PAMPs. BPZE1, a live-attenuated B. pertussis vaccine candidate has been shown to induce FHA- and PT-specific Th1 responses in immunized neonatal mice and confer protection against challenge with virulent B. pertussis (Feunou et al. 2014). Furthermore, BPZE1 has been shown to induce maturation of human DCs and subsequent induction of IFNγ- and IL-17- producing T cells, indicating that BPZE1 should be capable of inducing the favorable Th1/Th17 response in humans (Schiavoni et al. 2014). This vaccine is currently being tested in the baboon model and is in clinical development (Thorstensson et al. 2014). Another vaccine concept based on B. pertussis outer membrane vesicles also has been shown to induce a mixed Th1/Th17-type response in mice and to induce protection against B. pertussis challenge (Gaillard et al. 2014) (Raeven and Brummelman et al. in preparation). These vaccine candidates also address other limitations of aP vaccines, by expressing ‘natural’ (low) doses antigen (preventing an exhausted immune response) and a broader panel of antigens, important to ensure maintenance of long-lasting protective immunity against circulating B. pertussis strains with altered antigenic make up.
IMMUNOLOGICAL EVALUATION OF NEW PERTUSSIS VACCINES IN (PRE-) CLINICAL DEVELOPMENT
The development of new pertussis vaccines requires immunological tools to determine the functional differentiation, memory potential, breadth and protective capacity of the induced immune responses in reliable preclinical animal models, important to predict efficacy in humans. Mouse and more recently baboon models have been used to evaluate candidate pertussis vaccines. One of the problems with these models is that the level of protection is usually assessed shortly after vaccination (van der Ark et al. 2012). Furthermore, mice are not the natural host of B. pertussis and relatively high vaccine doses are often used in these challenge models to induce measureable immune responses; thus, the results may be difficult to translate to the humans. Future studies in these models should address the issue of waning immunity by assessing the long-term protection of experimental aP vaccines, and the protection induced by lower doses. While it is possible to do medium-term (6–12 month) studies in the mouse model, studies of greater than 1 year will require experiments in the recently developed baboon model. Warfel and Merkel (2013) have used species-specific tools to assess waning of the vaccine induced CD4+ T-cell response up to 15–24 month after B. pertussis infection in baboons. However, cost and animal availability will be significant limitations to extensive use of this model. In both mice and baboons, identifying early biomarkers that predict long-term immunity would be valuable.
One such biomarker of long-term immunity in pre-clinical and clinical vaccination settings is the memory phenotype of the induced specific CD4+ T cells, since TCM are important for long-term immunity (Sallusto et al. 1999; Kaech, Wherry and Ahmed 2002; Seder and Ahmed 2003; Sallusto, Geginat and Lanzavecchia 2004) (Fig. 2). Furthermore, there is emerging evidence from other infectious diseases that follicular helper T (TFH) cells (Yoo, Fish and Braciale 2012; Zimara et al. 2014) and tissue resident memory (TRM) (Teijaro et al. 2011; Iijima and Iwasaki 2014) may be key to long-term immunity and these have yet to be addressed in the field of pertussis vaccines. Memory phenotypes can be determined in the early stage after vaccination. Recently, we showed that MHC class II-tetramers specific for the immunodominant Prn epitope can be used to evaluate the CD4+ T-cell memory phenotype on a single cell base in both mouse and humans (Brummelman et al. 2015; Han et al. 2015). The knowledge on human pertussis MHC class II T-cell epitopes is increasing by studies using prediction methods, and advanced immunoproteomics analysis (Han et al. 2013; Stenger et al. 2014), however, is still very limited (De Magistris et al. 1989; Peppoloni et al. 1991; Vaughan et al. 2014). However, routine assessment of memory T cells induced by pertussis vaccine will require optimization and standardization of techniques. The availability of MHC class II tetramer with peptides from B. pertussis antigens together with single cell analysis using intracellular cytokine staining is one approach that may facilitate accurate quantification of B. pertussis-specific memory T cells induced with pertussis vaccines.
Furthermore, systems biology approaches could aid in identifying novel early biomarkers that predict the outcome of the adaptive immune response. Using this approach, a recent study revealed molecular and cellular immunological processes in response to live B. pertussis infection and may provide guidance in evaluating new candidate vaccines (Raeven et al. 2014). State-of-the art tools, such as MHC class II tetramers and systems biology, are needed for mouse, baboon and humans to evaluate the quality of the immune response induced by current and potential new pertussis vaccines.
CONCLUSIONS AND FUTURE PERSPECTIVE
The potential shortcomings of current aP vaccines are still a subject of debate. While the switch from wP to less effective aP vaccines is one explanation for the resurgence of pertussis, it has also been argued that recent epidemics may be part of natural cycles of disease that have always been with us, even in the wP vaccine era (Riolo, King and Rohani 2013). Nevertheless, the fact remains that the incidence of pertussis is higher than of any other vaccine-preventable infectious disease. There is now growing acceptance that we do have a problem and must come up with short- and long-term solutions. While a rationally designed third-generation pertussis vaccine would appear to be the ideal solution to improve the aP vaccine in use today, the development and especially the clinical evaluation, regulation, licensing and implementation of new aP vaccines into pediatric immunization programs will face many logistic as well as scientific hurdles.
In the interim, there are a number of short-term measures that are being introduced or considered. These include (1) maternal immunization to protect disease in the newborn by passive transfer of antibody, which has proved very effective in prevent infant death in certain countries (e.g. UK); (2) additional booster vaccination with current aP vaccines in adolescents, to tackle the problem of waning immunity; (3) immunization of adults with a vaccine that prevented infection as well as diseases to reduce the carriage and spread of the bacteria; and (4) cocooning strategies to contain local outbreaks of disease.
Before considering a new pertussis vaccine for infants, the first step may be to develop an alternative aP formulation that includes a more powerful new generation adjuvant, such as a TLR agonists, instead of or in addition to alum, and to evaluate it as part of a pertussis only vaccine for boosting adolescents and maybe adults. While this will still require clear evidence of improved immunogenicity over the existing vaccine and stringent safety data before being licensed, it would be logistically less difficult than introducing a new pertussis vaccine for pediatric use. However, one scientific issue that may mitigate against success of this approach is that there is evidence that the immune responses is ‘set’ after the initial immunization and attempts to induce B. pertussis-specific Th1 responses in individuals that have received a full course of aP vaccination may be difficult even through the use of Th1-inducing adjuvants. So switching from Th2 to Th1 by changing the adjuvants may not be so straightforward in a primed population, but should be less of an issue in naive individuals. Although it has been argued that the immune responses is Th2 skewed in neonates (Zepp et al. 1996), it has been demonstrated that infection with B. pertussis or immunization with wP vaccines can induce potent Th1-skewed CD4 T-cells responses in human infants (Ryan et al. 1998b, 2000; Rowe et al. 2005; Mascart et al. 2007), suggesting that it should be possible to induce Th1 responses in infants with an aP vaccine formulated with the appropriate Th1-promoting adjuvant.
In the longer term, a new pertussis vaccine will be required for primary and booster vaccination of infants and children. There is now convincing evidence from clinical studies that the current aP vaccine does not induce sustained protective immunity (Klein et al. 2012), and that B. pertussis is evolving to escape immune responses that the vaccines generate (Mooi, Van Der Maas and De Melker 2014). Furthermore, studies in mice complemented by those in humans have indicated that the aP vaccine fails to induce protective Th1 responses (Ryan et al. 1998b; Ross et al. 2013). The development of a new aP vaccine should address these limitations and should be capable of inducing Th1 responses and sustained immunity mediated by effective T- and B-cell memory. Preliminary studies have suggested that current aP vaccines are less effective than wP vaccines in inducing TFH and TRM cells (Wilk and Mills, unpublished). TFH play a crucial role in T-cell help for antibody production and in memory B-cell formation, whereas TRM appear to be critical for sustained cellular immunity at mucosal surfaces. Therefore, new generation vaccines also need to consider the induction of these T-cell types. The inclusion of additional antigen(s), such as ACT (Sebo, Osicka and Masin 2014) or the autotransporter BrkA (Marr et al. 2008), could help to broaden the immune responses and reduce the problem associated with immune escape but will add further complexity, especially for manufacturer and regulatory agency approval.
The clinical evaluation and regulatory approval of a new pertussis vaccine will not be straightforward. At this stage, the criteria for testing and licensing of a new vaccine are not clear. Large-scale phase 3 clinical trials, such as those carried out in Sweden and Italy in the 1990s, to estimate the efficacy of current aP vaccines (Greco et al. 1996; Gustafsson et al. 1996) will not be possible, because there is no equivalent cohort of non-vaccinated individuals, such as those available in Sweden and Italy in the 1990s when these countries had stopped using wP vaccines. Evidence of immunogenicity and safety in smaller clinical trials combined with estimates of efficacy from an animal model is one approach that may be considered. Alternatively, human challenge studies are being considered and have the obvious advantage over mice and baboons of being in the target species, albeit in adults rather than in children. However, most, if not all, adult volunteers, even if they lack circulating anti-B. pertussis antibodies, are likely to have memory T and B cells specific for B. pertussis antigens induced either by previous immunization or exposure to live B. pertussis through clinical or subclinical infections. Therefore, while human challenge may be useful for comparing immune responses and protection against infection induced by new compared with existing aP vaccines in primed individuals, they are unlikely to tell us what type of immune responses these vaccines will induce in unprimed individuals or whether they will protect naive individuals and more importantly naıve children. Regardless of the complexity of the task, the obstacles to development, assessment and regulation of new pertussis vaccines must be overcome using lessons from immunology in order to tackle the growing threat from this vaccine-preventable infectious disease.
Acknowledgments
Kingston Mills is funded by Science Foundation Ireland (PI grant #11/PI/1036). Jolanda Brummelman is funded by a strategic research grant of the Ministry of Health (S/000193).
Conflict of interest. None declared.
REFERENCES
- Andreasen C, Carbonetti NH. Role of neutrophils in response to Bordetella pertussis infection in mice. Infect Immun. 2009;77:1182–8. doi: 10.1128/IAI.01150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokanathan C, Corbel M, Xing D. A CpG-containing oligodeoxynucleotide adjuvant for acellular pertussis vaccine improves the protective response against Bordetella pertussis. Human Vaccines Immunother. 2013;9:325–31. doi: 10.4161/hv.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausiello CM, Fedele G, Urbani F, et al. Native and genetically inactivated pertussis toxins induce human dendritic cell maturation and synergize with lipopolysaccharide in promoting T helper type 1 responses. J Infect Dis. 2002;186:351–60. doi: 10.1086/341510. [DOI] [PubMed] [Google Scholar]
- Ausiello CM, Lande R, Urbani F, et al. Cell-mediated immune responses in four-year-old children after primary immunization with acellular pertussis vaccines. Infect Immun. 1999;67:4064–71. doi: 10.1128/iai.67.8.4064-4071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banus S, Stenger RM, Gremmer ER, et al. The role of Toll-like receptor-4 in pertussis vaccine-induced immunity. BMC Immunol. 2008;9:21. doi: 10.1186/1471-2172-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbic J, Leef MF, Burns DL, et al. Role of gamma interferon in natural clearance of Bordetella pertussis infection. Infect Immun. 1997;65:4904–8. doi: 10.1128/iai.65.12.4904-4908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoff AM, Mertsola J, Guillot S, et al. Appearance of Bordetella pertussis strains not expressing the vaccine antigen pertactin in Finland. Clin Vaccine Immunol. 2012;19:1703–4. doi: 10.1128/CVI.00367-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart MJ, Harris SR, Advani A, et al. Global population structure and evolution of Bordetella pertussis and their relationship with vaccination. mBio. 2014;5:e01074. doi: 10.1128/mBio.01074-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard NJ, Finlay CM, Tannahill GM, et al. A critical role for the TLR signaling adapter Mal in alveolar macrophage-mediated protection against Bordetella pertussis. Mucosal Immunol. 2015;8:982–92. doi: 10.1038/mi.2014.125. [DOI] [PubMed] [Google Scholar]
- Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- Bouchez V, Brun D, Cantinelli T, et al. First report and detailed characterization of B. pertussis isolates not expressing Pertussis Toxin or Pertactin. Vaccine. 2009;27:6034–41. doi: 10.1016/j.vaccine.2009.07.074. [DOI] [PubMed] [Google Scholar]
- Brummelman J, Helm K, Hamstra HJ, et al. Modulation of the CD4(+) T cell response after acellular pertussis vaccination in the presence of TLR4 ligation. Vaccine. 2015;33:1483–91. doi: 10.1016/j.vaccine.2015.01.063. [DOI] [PubMed] [Google Scholar]
- Bryant CE, Monie TP. Mice, men and the relatives: cross-species studies underpin innate immunity. Open Biol. 2012;2:120015. doi: 10.1098/rsob.120015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne P, McGuirk P, Todryk S, et al. Depletion of NK cells results in disseminating lethal infection with Bordetella pertussis associated with a reduction of antigen-specific Th1 and enhancement of Th2, but not Tr1 cells. Eur J Immunol. 2004;34:2579–88. doi: 10.1002/eji.200425092. [DOI] [PubMed] [Google Scholar]
- Carbonetti NH, Artamonova GV, Van Rooijen N, et al. Pertussis toxin targets airway macrophages to promote Bordetella pertussis infection of the respiratory tract. Infect Immun. 2007;75:1713–20. doi: 10.1128/IAI.01578-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutte L, Alonso S, Reveneau N, et al. Role of adhesin release for mucosal colonization by a bacterial pathogen. J Exp Med. 2003;197:735–42. doi: 10.1084/jem.20021153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- De Magistris MT, Romano M, Bartoloni A, et al. Human T cell clones define S1 subunit as the most immunogenic moiety of pertussis toxin and determine its epitope map. J Exp Med. 1989;169:1519–32. doi: 10.1084/jem.169.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rond L, Schure RM, Ozturk K, et al. Identification of pertussis-specific effector memory T cells in preschool children. Clin Vaccine Immunol. 2015;22:561–9. doi: 10.1128/CVI.00695-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly S, Loscher CE, Lynch MA, et al. Whole-cell but not acellular pertussis vaccines induce convulsive activity in mice: evidence of a role for toxin-induced interleukin-1beta in a new murine model for analysis of neuronal side effects of vaccination. Infect Immun. 2001;69:4217–23. doi: 10.1128/IAI.69.7.4217-4223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne A, Mielke LA, Allen AC, et al. A novel TLR2 agonist from Bordetella pertussis is a potent adjuvant that promotes protective immunity with an acellular pertussis vaccine. Mucosal Immunol. 2015;8:607–17. doi: 10.1038/mi.2014.93. [DOI] [PubMed] [Google Scholar]
- Dunne PJ, Moran B, Cummins RC, et al. CD11c+CD8{alpha}+ dendritic cells promote protective immunity to respiratory infection with Bordetella pertussis. J Immunol. 2009;183:400–10. doi: 10.4049/jimmunol.0900169. [DOI] [PubMed] [Google Scholar]
- Dunne A, Ross PJ, Pospisilova E, et al. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J Immunol. 2010;185:1711–9. doi: 10.4049/jimmunol.1000105. [DOI] [PubMed] [Google Scholar]
- Eby JC, Gray MC, Hewlett EL. Cyclic AMP-mediated suppression of neutrophil extracellular trap formation and apoptosis by the Bordetella pertussis adenylate cyclase toxin. Infect Immun. 2014;82:5256–69. doi: 10.1128/IAI.02487-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KM, Meade BD, Decker MD, et al. Comparison of 13 acellular pertussis vaccines: overview and serologic response. Pediatrics. 1995;96:548–57. [PubMed] [Google Scholar]
- Fedele G, Nasso M, Spensieri F, et al. Lipopolysaccharides from Bordetella pertussis and Bordetella parapertussis differently modulate human dendritic cell functions resulting in divergent prevalence of Th17-polarized responses. J Immunol. 2008;181:208–16. doi: 10.4049/jimmunol.181.1.208. [DOI] [PubMed] [Google Scholar]
- Feunou PF, Kammoun H, Debrie AS, et al. Heterologous prime-boost immunization with live attenuated B. pertussis BPZE1 followed by acellular pertussis vaccine in mice. Vaccine. 2014;32:4281–8. doi: 10.1016/j.vaccine.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Gaillard ME, Bottero D, Errea A, et al. Acellular pertussis vaccine based on outer membrane vesicles capable of conferring both long-lasting immunity and protection against different strain genotypes. Vaccine. 2014;32:931–7. doi: 10.1016/j.vaccine.2013.12.048. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer. 2012;12:671–84. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurtsen J, Fransen F, Vandebriel RJ, et al. Supplementation of whole-cell pertussis vaccines with lipopolysaccharide analogs: modification of vaccine-induced immune responses. Vaccine. 2008;26:899–906. doi: 10.1016/j.vaccine.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Greco D, Salmaso S, Mastrantonio P, et al. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. Progetto Pertosse Working Group. New Engl J Med. 1996;334:341–8. doi: 10.1056/NEJM199602083340601. [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Hallander HO, Olin P, et al. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. New Engl J Med. 1996;334:349–55. doi: 10.1056/NEJM199602083340602. [DOI] [PubMed] [Google Scholar]
- Han WG, Helm K, Poelen MM, et al. Ex vivo peptide-MHC II tetramer analysis reveals distinct end-differentiation patterns of human pertussis-specific CD4(+) T cells following clinical infection. Clin Immunol. 2015;157:205–15. doi: 10.1016/j.clim.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Han WG, van Twillert I, Poelen MC, et al. Loss of multi-epitope specificity in memory CD4(+) T cell responses to B. pertussis with age. PloS One. 2013;8:e83583. doi: 10.1371/journal.pone.0083583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvill ET, Cotter PA, Miller JF. Pregenomic comparative analysis between bordetella bronchiseptica RB50 and Bordetella pertussis tohama I in murine models of respiratory tract infection. Infect Immun. 1999;67:6109–18. doi: 10.1128/iai.67.11.6109-6118.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegerle N, Paris AS, Brun D, et al. Evolution of French Bordetella pertussis and Bordetella parapertussis isolates: increase of Bordetellae not expressing pertactin. Clin Microbiol Infect. 2012;18:E340–6. doi: 10.1111/j.1469-0691.2012.03925.x. [DOI] [PubMed] [Google Scholar]
- Hendrikx LH, Schure RM, Ozturk K, et al. Different IgG-subclass distributions after whole-cell and acellular pertussis infant primary vaccinations in healthy and pertussis infected children. Vaccine. 2011;29:6874–80. doi: 10.1016/j.vaccine.2011.07.055. [DOI] [PubMed] [Google Scholar]
- Hickey FB, Brereton CF, Mills KH. Adenylate cycalse toxin of Bordetella pertussis inhibits TLR-induced IRF-1 and IRF-8 activation and IL-12 production and enhances IL-10 through MAPK activation in dendritic cells. J Leukocyte Biol. 2008;84:234–43. doi: 10.1189/jlb.0208113. [DOI] [PubMed] [Google Scholar]
- Higgins SC, Jarnicki AG, Lavelle EC, et al. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177:7980–9. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- Higgins SC, Lavelle EC, McCann C, et al. Toll-like receptor 4-mediated innate IL-10 activates antigen-specific regulatory T cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J Immunol. 2003;171:3119–27. doi: 10.4049/jimmunol.171.6.3119. [DOI] [PubMed] [Google Scholar]
- Hjelholt A, Christiansen G, Sorensen US, et al. IgG subclass profiles in normal human sera of antibodies specific to five kinds of microbial antigens. Pathog Dis. 2013;67:206–13. doi: 10.1111/2049-632X.12034. [DOI] [PubMed] [Google Scholar]
- Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346:93–8. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen FL, Strickland DH, Thomas JA, et al. Accelerated antigen sampling and transport by airway mucosal dendritic cells following inhalation of a bacterial stimulus. J Immunol. 2006;177:5861–7. doi: 10.4049/jimmunol.177.9.5861. [DOI] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–95. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- Kirimanjeswara GS, Mann PB, Harvill ET. Role of antibodies in immunity to Bordetella infections. Infect Immun. 2003;71:1719–24. doi: 10.1128/IAI.71.4.1719-1724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein NP, Bartlett J, Fireman B, et al. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics. 2013;131:e1716–22. doi: 10.1542/peds.2012-3836. [DOI] [PubMed] [Google Scholar]
- Klein NP, Bartlett J, Rowhani-Rahbar A, et al. Waning protection after fifth dose of acellular pertussis vaccine in children. New Engl J Med. 2012;367:1012–9. doi: 10.1056/NEJMoa1200850. [DOI] [PubMed] [Google Scholar]
- Lam C, Octavia S, Ricafort L, et al. Rapid increase in pertactin-deficient Bordetella pertussis isolates, Australia. Emerg Infect Dis. 2014;20:626–33. doi: 10.3201/eid2004.131478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberti Y, Gorgojo J, Massillo C, et al. Bordetella pertussis entry into respiratory epithelial cells and intracellular survival. Pathog Dis. 2013;69:194–204. doi: 10.1111/2049-632X.12072. [DOI] [PubMed] [Google Scholar]
- Lamberti YA, Hayes JA, Perez Vidakovics ML, et al. Intracellular trafficking of Bordetella pertussis in human macrophages. Infect Immun. 2010;78:907–13. doi: 10.1128/IAI.01031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN. Alveolar macrophage in the driver's seat. Immunity. 2006;24:366–8. doi: 10.1016/j.immuni.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Leef M, Elkins KL, Barbic J, et al. Protective immunity to Bordetella pertussis requires both B cells and CD4(+) T cells for key functions other than specific antibody production. J Exp Med. 2000;191:1841–52. doi: 10.1084/jem.191.11.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liko J, Robison SG, Cieslak PR. Priming with whole-cell versus acellular pertussis vaccine. New Engl J Med. 2013;368:581–2. doi: 10.1056/NEJMc1212006. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–65. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- Mahon BP, Sheahan BJ, Griffin F, et al. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-gamma receptor or immunoglobulin mu chain genes. J Exp Med. 1997;186:1843–51. doi: 10.1084/jem.186.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann PB, Wolfe D, Latz E, et al. Comparative toll-like receptor 4-mediated innate host defense to Bordetella infection. Infect Immun. 2005;73:8144–52. doi: 10.1128/IAI.73.12.8144-8152.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr N, Novikov A, Hajjar AM, et al. Variability in the lipooligosaccharide structure and endotoxicity among Bordetella pertussis strains. J Infect Dis. 2010;202:1897–906. doi: 10.1086/657409. [DOI] [PubMed] [Google Scholar]
- Marr N, Oliver DC, Laurent V, et al. Protective activity of the Bordetella pertussis BrkA autotransporter in the murine lung colonization model. Vaccine. 2008;26:4306–11. doi: 10.1016/j.vaccine.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Martin SW, Pawloski L, Williams M, et al. Pertactin-negative Bordetella pertussis strains: evidence for a possible selective advantage. Clin Infect Dis. 2015;60:223–7. doi: 10.1093/cid/ciu788. [DOI] [PubMed] [Google Scholar]
- Mascart F, Hainaut M, Peltier A, et al. Modulation of the infant immune responses by the first pertussis vaccine administrations. Vaccine. 2007;25:391–8. doi: 10.1016/j.vaccine.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Mascart F, Verscheure V, Malfroot A, et al. Bordetella pertussis infection in 2-month-old infants promotes type 1 T cell responses. J Immunol. 2003;170:1504–9. doi: 10.4049/jimmunol.170.3.1504. [DOI] [PubMed] [Google Scholar]
- Mills KH, Barnard A, Watkins J, et al. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993;61:399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi FR, Van Der Maas NA, De Melker HE. Pertussis resurgence: waning immunity and pathogen adaptation—two sides of the same coin. Epidemiol Infect. 2014;142:685–94. doi: 10.1017/S0950268813000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford RS. Murine responses to endotoxin: another dirty little secret? J Infect Dis. 2010;201:175–7. doi: 10.1086/649558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Suda R, Nagamatsu Y, et al. Pertussis toxin up-regulates angiotensin type 1 receptors through Toll-like receptor 4-mediated Rac activation. J Biol Chem. 2010;285:15268–77. doi: 10.1074/jbc.M109.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka N, Han HJ, Toyoizumi-Ajisaka H, et al. Prevalence and genetic characterization of pertactin-deficient Bordetella pertussis in Japan. PloS One. 2012;7:e31985. doi: 10.1371/journal.pone.0031985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Sanden GN, Cherry JD, et al. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin Infect Dis. 2008;47:328–38. doi: 10.1086/589753. [DOI] [PubMed] [Google Scholar]
- Pawloski LC, Queenan AM, Cassiday PK, et al. Prevalence and molecular characterization of pertactin-deficient Bordetella pertussis in the United States. Clin Vaccine Immunol. 2014;21:119–25. doi: 10.1128/CVI.00717-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppoloni S, Nencioni L, Di Tommaso A, et al. Lymphokine secretion and cytotoxic activity of human CD4+ T-cell clones against Bordetella pertussis. Infect Immun. 1991;59:3768–73. doi: 10.1128/iai.59.10.3768-3773.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenan AM, Cassiday PK, Evangelista A. Pertactin-negative variants of Bordetella pertussis in the United States. New Engl J Med. 2013;368:583–4. doi: 10.1056/NEJMc1209369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeven RH, Brummelman J, Pennings JL, et al. Molecular signatures of the evolving immune response in mice following a Bordetella pertussis infection. PloS One. 2014;9:e104548. doi: 10.1371/journal.pone.0104548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeven RH, van der Maas L, Tilstra W, et al. Immunoproteomic profiling of Bordetella pertussis outer membrane vesicle vaccine reveals broad and balanced humoral immunogenicity. J Proteome Res. 2015;14:2929–42. doi: 10.1021/acs.jproteome.5b00258. [DOI] [PubMed] [Google Scholar]
- Redhead K, Watkins J, Barnard A, et al. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect Immun. 1993;61:3190–8. doi: 10.1128/iai.61.8.3190-3198.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennels MB, Black S, Woo EJ, et al. Safety of a fifth dose of diphtheria and tetanus toxoid and acellular pertussis vaccine in children experiencing extensive, local reactions to the fourth dose. Pediatr Infect Dis J. 2008;27:464–5. doi: 10.1097/INF.0b013e31816591f7. [DOI] [PubMed] [Google Scholar]
- Riolo MA, King AA, Rohani P. Can vaccine legacy explain the British pertussis resurgence? Vaccine. 2013;31:5903–8. doi: 10.1016/j.vaccine.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PJ, Sutton CE, Higgins S, et al. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog. 2013;9:e1003264. doi: 10.1371/journal.ppat.1003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Yerkovich ST, Richmond P, et al. Th2-associated local reactions to the acellular diphtheria-tetanus-pertussis vaccine in 4- to 6-year-old children. Infect Immun. 2005;73:8130–5. doi: 10.1128/IAI.73.12.8130-8135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M, McCarthy L, Rappuoli R, et al. Pertussis toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7–1, B7–2 and CD28. Int Immunol. 1998a;10:651–662. doi: 10.1093/intimm/10.5.651. [DOI] [PubMed] [Google Scholar]
- Ryan M, Murphy G, Gothefors L, et al. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J Infect Dis. 1997;175:1246–50. doi: 10.1086/593682. [DOI] [PubMed] [Google Scholar]
- Ryan M, Murphy G, Ryan E, et al. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology. 1998b;93:1–10. doi: 10.1046/j.1365-2567.1998.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EJ, Nilsson L, Kjellman N, et al. Booster immunization of children with an acellular pertussis vaccine enhances Th2 cytokine production and serum IgE responses against pertussis toxin but not against common allergens. Clin Exp Immunol. 2000;121:193–200. doi: 10.1046/j.1365-2249.2000.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Schiavoni I, Fedele G, Quattrini A, et al. Live attenuated B. pertussis BPZE1 rescues the immune functions of Respiratory Syncytial virus infected human dendritic cells by promoting Th1/Th17 responses. PloS One. 2014;9:e100166. doi: 10.1371/journal.pone.0100166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schure RM, Hendrikx LH, de Rond LG, et al. T-cell responses before and after the fifth consecutive acellular pertussis vaccination in 4-year-old Dutch children. Clin Vaccine Immunol. 2012;19:1879–86. doi: 10.1128/CVI.00277-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schure RM, Hendrikx LH, de Rond LG, et al. Differential T- and B-cell responses to pertussis in acellular vaccine-primed versus whole-cell vaccine-primed children 2 years after preschool acellular booster vaccination. Clin Vaccine Immunol. 2013;20:1388–95. doi: 10.1128/CVI.00270-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebo P, Osicka R, Masin J. Adenylate cyclase toxin-hemolysin relevance for pertussis vaccines. Expert Rev Vaccines. 2014;13:1215–27. doi: 10.1586/14760584.2014.944900. [DOI] [PubMed] [Google Scholar]
- Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–42. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- Sheridan SL, Ware RS, Grimwood K, et al. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA. 2012;308:454–6. doi: 10.1001/jama.2012.6364. [DOI] [PubMed] [Google Scholar]
- Spensieri F, Fedele G, Fazio C, et al. Bordetella pertussis inhibition of interleukin-12 (IL-12) p70 in human monocyte-derived dendritic cells blocks IL-12 p35 through adenylate cyclase toxin-dependent cyclic AMP induction. Infect Immun. 2006;74:2831–8. doi: 10.1128/IAI.74.5.2831-2838.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger RM, Meiring HD, Kuipers B, et al. Bordetella pertussis proteins dominating the major histocompatibility complex class II-presented epitope repertoire in human monocyte-derived dendritic cells. Clin Vaccine Immunol. 2014;21:641–50. doi: 10.1128/CVI.00665-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger RM, Smits M, Kuipers B, et al. Impaired long-term maintenance and function of Bordetella pertussis specific B cell memory. Vaccine. 2010;28:6637–46. doi: 10.1016/j.vaccine.2010.06.118. [DOI] [PubMed] [Google Scholar]
- Stumbles PA, Thomas JA, Pimm CL, et al. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J Exp Med. 1998;188:2019–31. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai T, Mori M, Nakazawa M, et al. A CpG-containing oligodeoxynucleotide as an efficient adjuvant counterbalancing the Th1/Th2 immune response in diphtheria-tetanus-pertussis vaccine. Vaccine. 2005;23:5450–6. doi: 10.1016/j.vaccine.2004.09.041. [DOI] [PubMed] [Google Scholar]
- Teijaro JR, Turner D, Pham Q, et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187:5510–4. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorstensson R, Trollfors B, Al-Tawil N, et al. A phase I clinical study of a live attenuated Bordetella pertussis vaccine–BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PloS One. 2014;9:e83449. doi: 10.1371/journal.pone.0083449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonon S, Goriely S, Aksoy E, et al. Bordetella pertussis toxin induces the release of inflammatory cytokines and dendritic cell activation in whole blood: impaired responses in human newborns. Eur J Immunol. 2002;32:3118–25. doi: 10.1002/1521-4141(200211)32:11<3118::AID-IMMU3118>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Trollfors B, Taranger J, Lagergard T, et al. A placebo-controlled trial of a pertussis-toxoid vaccine. New Engl J Med. 1995;333:1045–50. doi: 10.1056/NEJM199510193331604. [DOI] [PubMed] [Google Scholar]
- Tsang RS, Lau AK, Sill ML, et al. Polymorphisms of the fimbria fim3 gene of Bordetella pertussis strains isolated in Canada. J Clin Microbiol. 2004;42:5364–7. doi: 10.1128/JCM.42.11.5364-5367.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubo NJ, Jenkins MK. TCR signal quantity and quality in CD4 T cell differentiation. Trends Immunol. 2014;35:591–6. doi: 10.1016/j.it.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ark AA, Hozbor DF, Boog CJ, et al. Resurgence of pertussis calls for re-evaluation of pertussis animal models. Exp Rev Vaccines. 2012;11:1121–37. doi: 10.1586/erv.12.83. [DOI] [PubMed] [Google Scholar]
- van Loo IH, Heuvelman KJ, King AJ, et al. Multilocus sequence typing of Bordetella pertussis based on surface protein genes. J Clin Microbiol. 2002;40:1994–2001. doi: 10.1128/JCM.40.6.1994-2001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan K, Seymour E, Peters B, et al. Substantial gaps in knowledge of Bordetella pertussis antibody and T cell epitopes relevant for natural immunity and vaccine efficacy. Human Immunol. 2014;75:440–51. doi: 10.1016/j.humimm.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen F, Verscheure V, Damis E, et al. Cellular immune responses of preterm infants after vaccination with whole-cell or acellular pertussis vaccines. Clin Vaccine Immunol. 2010;17:258–62. doi: 10.1128/CVI.00328-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Yang D, Chen Q, et al. Induction of dendritic cell maturation by pertussis toxin and its B subunit differentially initiate Toll-like receptor 4-dependent signal transduction pathways. Exp Hematol. 2006;34:1115–24. doi: 10.1016/j.exphem.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Warfel JM, Merkel TJ. Bordetella pertussis infection induces a mucosal IL-17 response and long-lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal Immunol. 2013;6:787–96. doi: 10.1038/mi.2012.117. [DOI] [PubMed] [Google Scholar]
- Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model; P Natl Acad Sci USA; 2014. pp. 787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt MA, Arias L, Katz PH, et al. Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin Infect Dis. 2013;56:1248–54. doi: 10.1093/cid/cit046. [DOI] [PubMed] [Google Scholar]
- Yoo JK, Fish EN, Braciale TJ. LAPCs promote follicular helper T cell differentiation of Ag-primed CD4+ T cells during respiratory virus infection. J Exp Med. 2012;209:1853–67. doi: 10.1084/jem.20112256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariadis O, Cassidy JP, Brady J, et al. gammadelta T cells regulate the early inflammatory response to bordetella pertussis infection in the murine respiratory tract. Infect Immun. 2006;74:1837–45. doi: 10.1128/IAI.74.3.1837-1845.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariri A, van der Ley P. Biosynthetically engineered lipopolysaccharide as vaccine adjuvant. Exp Rev Vaccines. 2015;14:861–76. doi: 10.1586/14760584.2015.1026808. [DOI] [PubMed] [Google Scholar]
- Zeddeman A, van Gent M, Heuvelman CJ, et al. Investigations into the emergence of pertactin-deficient Bordetella pertussis isolates in six European countries, 1996 to 2012. Euro Surveill. 2014;19:pii:20881. doi: 10.2807/1560-7917.es2014.19.33.20881. [DOI] [PubMed] [Google Scholar]
- Zepp F, Knuf M, Habermehl P, et al. Pertussis-specific cell-mediated immunity in infants after vaccination with a tricomponent acellular pertussis vaccine. Infect Immun. 1996;64:4078–84. doi: 10.1128/iai.64.10.4078-4084.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimara N, Florian C, Schmid M, et al. Langerhans cells promote early germinal center formation in response to Leishmania-derived cutaneous antigens. Eur J Immunol. 2014;44:2955–67. doi: 10.1002/eji.201344263. [DOI] [PubMed] [Google Scholar]