ABSTRACT
Bacillus anthracis, a spore-forming pathogen, replicates as chains of vegetative cells by regulating the separation of septal peptidoglycan. Surface (S)-layer proteins and B. anthracis S-layer-associated proteins (BSLs) function as chain length determinants and are assembled in the envelope by binding to the secondary cell wall polysaccharide (SCWP). B. anthracis expresses six different genes encoding LytR-CpsA-Psr (LCP) enzymes (lcpB1 to -4, lcpC, and lcpD), which when expressed in Staphylococcus aureus promote attachment of wall teichoic acid to peptidoglycan. Mutations in B. anthracis lcpB3 and lcpD cause aberrations in cell size and chain length that can be explained as discrete defects in SCWP assembly; however, the function of the other lcp genes is not known. By deleting combinations of lcp genes from the B. anthracis genome, we generated variants with single lcp genes. B. anthracis expressing lcpB3 alone displayed physiological cell size, vegetative growth, spore formation, and S-layer assembly. Strains expressing lcpB1 or lcpB4 displayed defects in cell size and shape, S-layer assembly, and spore formation yet sustained vegetative growth. In contrast, the lcpB2 strain was unable to grow unless the gene was expressed from a multicopy plasmid (lcpB2++), and variants expressing lcpC or lcpD displayed severe defects in growth and cell shape. The lcpB2++, lcpC, or lcpD strains supported neither S-layer assembly nor spore formation. We propose a model whereby LCP enzymes fulfill partially overlapping functions in transferring SCWP molecules to discrete sites within the bacterial envelope.
IMPORTANCE Products of genes essential for bacterial envelope assembly represent targets for antibiotic development. The LytR-CpsA-Psr (LCP) enzymes tether bactoprenol-linked intermediates of secondary cell wall polymers to the C6 hydroxyl of N-acetylmuramic acid in peptidoglycan; however, the role of LCPs as a target for antibiotic therapy is not defined. We show here that LCP enzymes are essential for the cell cycle, vegetative growth, and spore formation of Bacillus anthracis, the causative agent of anthrax disease. Furthermore, we assign functions for each of the six LCP enzymes, including cell size and shape, vegetative growth and sporulation, and S-layer and S-layer-associated protein assembly.
INTRODUCTION
Bacillus anthracis, a Gram-positive spore-forming bacterium, infects and replicates within vertebrates, thereby precipitating anthrax disease. Without therapy, anthrax can be fatal, which triggers formation of infectious B. anthracis spores within the carcass (1, 2). During vegetative replication, B. anthracis grows as chains of rod-shaped bacteria that are tethered at septal peptidoglycan, a developmental program that protects bacteria from engulfment by phagocytes (3, 4). Earlier work identified the S-layer protein Sap, the S-layer-associated protein BslO, and the CsaB pyruvyl-transferase as determinants of B. anthracis chain length (3, 5, 6). Both S-layer proteins (Sap and EA1) and BslO are endowed with three S-layer homology (SLH) domains, promoting association of proteins with pyruvylated secondary cell wall polysaccharide (SCWP) in the B. anthracis cell wall envelope (7). Mutants lacking bslO are defective in their ability to release cells from growing B. anthracis chains and thus exhibit exaggerated chain length (3). BslO, an N-acetylglucosaminidase, is deposited into the S-layer at the septum, where it contributes to septal peptidoglycan cleavage (3). Subcellular localization of BslO is dependent on Sap, which forms an S-layer throughout the cylindrical envelope of B. anthracis vegetative forms, limiting the deposition of EA1 and BslO to the cell poles in the vicinity of the septal peptidoglycan (8).
The SCWP of B. anthracis is composed of the repeat unit [→4)-β-ManNAc-(1→4)-β-GlcNAc-(1→6)-α-GlcNAc-(1→], where α-GlcNAc is substituted with α-Gal and β-Gal at O-3 and O-4, respectively, and β-GlcNAc is substituted with α-Gal at O-3 (9). CsaB-mediated pyruvylation of the terminal N-acetylmannosamine (ManNAc) is a prerequisite for SCWP binding to S-layer (Sap and EA1) and B. anthracis S-layer-associated proteins (BSLs) (5, 6, 10). The genetic determinants for SCWP synthesis in B. anthracis remain largely unknown. Nonetheless, it has been demonstrated that the ManNAc residues are supplied by GneY and GneZ, two epimerases that catalyze the stereochemical inversion at the C-2 position of UDP-GlcNAc to UDP-ManNAc (11, 12). Earlier work revealed that the SCWP is covalently attached to B. anthracis peptidoglycan, at least in part via murein linkage units composed of the disaccharide (GlcNAc-ManNAc) (6, 12, 13). This model is based on the observations that tagO, encoding undecaprenyl-phosphate N-acetylglucosaminyl 1-phosphate transferase, the first of two enzymes in the synthesis pathway for murein linkage units, is required for SCWP synthesis in B. anthracis (6). Furthermore, hydrofluoric acid (HF) treatment hydrolyzes phosphodiester bonds that tether SCWP to peptidoglycan (6). At least two LytR-CpsA-Psr (LCP) enzymes contribute to SCWP attachment to the B. anthracis peptidoglycan and the assembly of S-layers (13). B. anthracis encodes six LCP proteins, which have been named LcpB1 to -4, LcpC, and LcpD based on sequence comparisons with Staphylococcus aureus and Bacillus subtilis LCP enzymes (13). LCP enzymes are universal catalysts for the transfer of undecaprenyl-phosphate polymers to peptidoglycan in Gram-positive bacteria (14–17).
LCP proteins were originally identified in B. subtilis as TagT, TagU, and TagV and proposed to catalyze wall teichoic acid (WTA) attachment to peptidoglycan (14), specifically the formation of HF-sensitive phosphodiester bonds between the C-6 hydroxyl of N-acetylmuramic acid and WTA (18, 19). This activity has been shown to be shared by S. aureus LcpA (LcpASa), LcpBSa, and LcpCSa (16). In addition, LCP enzymes of S. aureus catalyze the attachment of capsular polysaccharide to peptidoglycan (17). Heterologous expression of B. anthracis lcpB2, lcpB3, lcpB4, lcpC, or lcpD but not lcpB1 was shown to restore to various extents defects in staphylococcal growth and WTA attachment to peptidoglycan in S. aureus variants lacking all three lcp genes (ΔlcpABC) (13). Loss of lcpB3 or lcpD in B. anthracis produced cells with altered size and chain-length phenotypes that can be explained by defects in S-layer and SCWP assembly (13). Together these observations suggest that LCP enzymes recognize undecaprenolpyrophosphate-linked amino sugars as the substrates for transfer to peptidoglycan.
The phenotypic analyses of mutants lacking individual lcp genes could not explain why B. anthracis evolved six LCP enzymes when B. subtilis and S. aureus elaborate only three. Unlike B. subtilis or S. aureus, B. anthracis does not synthesize WTA or capsular polysaccharide. Here, we developed B. anthracis variants expressing only one of the six genes for LCP enzymes. Based on the phenotypic analyses of these variants, we propose a model to account for the expanded repertoire of B. anthracis LCP enzymes and their contribution to SCWP attachment to peptidoglycan.
MATERIALS AND METHODS
Bacterial growth and reagents.
B. anthracis strain Sterne 34F2 (20) and its variants were grown in brain heart infusion (BHI) broth or agar at 37°C. Escherichia coli K1077 (21) was grown in Luria-Bertani (LB) broth or agar at 37°C. Where necessary, ampicillin (Amp [100 μg/ml]), chloramphenicol (Cm [10 μg/ml]), spectinomycin (Sp [200 μg/ml]), or kanamycin (Kan [20 μg/ml]) was added to cultures. B. anthracis strains were sporulated in modified G medium (modG) (22). Spore preparations were heat treated at 68°C for 30 min to kill vegetative bacilli. Spore titers were determined by plating aliquots of serially diluted spore preparations on LB agar; CFU were enumerated after overnight incubation at 30°C. To examine growth, overnight cultures were normalized to an absorbance at 600 nm (A600) of 5 and diluted 1:100 into 100 μl fresh BHI medium, and growth at 37°C was monitored every 30 min for 12 h in a Synergy HT plate reader (BioTek) by recording the A600. For measurements of growth rate, r, the exponential growth phase for each resulting growth curve was fit to the equation At = A0 × ert, where At is absorbance at time t, A0 is the initial absorbance, and t is the time elapsed between A0 and At recordings. To assess viability, aliquots were removed at 6 h, serially diluted, plated on LB agar, and grown overnight at 30°C.
Bacterial strains and plasmids.
The bacterial strains and plasmids utilized in this study are listed in Table 1. B. anthracis Sterne(pXO1+, pXO2−) was used as the parent wild-type (WT) strain. The alleles lcpB2::kan and lcpD::spec were obtained by transposon mutagenesis with bursa aurealis (23) and have been described earlier (13). Deletion of lcpB1, lcpB3, lcpB4, or lcpC was achieved by allelic replacement using the temperature-sensitive vector pLM4 (13, 24, 25). The process was repeated as needed to replace genes with unmarked alleles in a single background. For marked alleles such as lcpB2::kan and lcpD::spec, bacteriophage CP51 was used to transduce the resistance traits into wild-type B. anthracis Sterne and isogenic lcp allelic variants (26). Transductants were verified by DNA sequencing of inverse PCR products as previously described (23). Unmarked mutant alleles were verified by PCR using primers flanking the cloning sites. The sequences of the primers used for this study were described previously (13).
TABLE 1.
B. anthracis strains used in this study
| Strain genotype | Designation | Descriptiona | Reference or source |
|---|---|---|---|
| WT | Sterne 34F2 | WT (pXO1+, pXO2−) | 20 |
| Variants | |||
| lcpB2::aphA3 ΔlcpB3B4C lcpD::aad9 | lcpB1+ strain | lcpB3 (BAS0746) (deletion, nt 798568–799746), lcpB4 (BAS3381) (deletion, nt 3356447–3357475), lcpC (BAS5115) (deletion, nt 4995461–4994610), lcpB2 (BAS0572)::aphA3 (insertion, nt 619533), lcpD (BAS5047)::aad9 (insertion, nt 4919831) | This work |
| ΔlcpB1B3B4C lcpD::aad9/plcpB2 | lcpB2++ strain | lcpB1 (BAS1830) (deletion, nt 1856993–1857982), lcpB3 (BAS0746) (deletion, nt 798568–799746), lcpB4 (BAS3381) (deletion, nt 3356447–3357475), lcpC (BAS5115) (deletion, nt 4995461–4994610), lcpD (BAS5047)::aad9 (insertion, nt 4919831); plcpB2 is pWWW412 with lcpB2 (BAS0572) | This work |
| ΔlcpB1 lcpB2::aphA3 ΔlcpB4C lcpD::aad9 | lcpB3+ strain | lcpB1 (BAS1830) (deletion, nt 1856993–1857982), lcpB4 (BAS3381) (deletion, nt 3356447–3357475), lcpC (BAS5115) (deletion, nt 4995461–4994610), lcpB2 (BAS0572)::aphA3 (insertion, nt 619533), lcpD (BAS5047)::aad9 (insertion, nt 4919831) | This work |
| ΔlcpB1 lcpB2::aphA3 ΔlcpB3C lcpD::aad9 | lcpB4+ strain | lcpB1 (BAS1830) (deletion, nt 1856993–1857982), lcpB3 (BAS0746) (deletion, nt 798568–799746), lcpC (BAS5115) (deletion, nt 4995461–4994610), lcpB2 (BAS0572)::aphA3 (insertion, nt 619533), lcpD (BAS5047)::aad9 (insertion, nt 4919831) | This work |
| ΔlcpB1 lcpB2::aphA3 ΔlcpB3B4 lcpD::aad9 | lcpC+ strain | lcpB1 (BAS1830) (deletion, nt 1856993–1857982), lcpB3 (BAS0746) (deletion, nt 798568–799746), lcpB4 (BAS3381) (deletion, nt 3356447–3357475), lcpB2 (BAS0572)::aphA3 (insertion, nt 619533), lcpD (BAS5047)::aad9 (insertion, nt 4919831) | This work |
| ΔlcpB1 lcpB2::aphA3 ΔlcpB3B4C | lcpD+ strain | lcpB1 (BAS1830) (deletion, nt 1856993–1857982), lcpB3 (BAS0746) (deletion, nt 798568–799746), lcpB4 (BAS3381) (deletion, nt 3356447–3357475), lcpC (BAS5115) (deletion, nt 4995461–4994610), lcpB2 (BAS0572)::aphA3 (insertion, nt 619533) | This work |
| Δsap | sap strain | sap (BAS0841) (deletion, nt 896758–899063) | 27 |
| Δeag | eag strain | eag (BAS0842) (deletion, nt 899843–902414) | 27 |
| bslO::aad9 | bslO strain | bslO (BAS1683)::aad9 (insertion, nt 1703151) | 3 |
The nucleotide numbers provided in this column correspond to nucleotide positions in the sequenced genome of the Sterne strain as described in the NCBI databank under accession no. NC_005945.
Microscopy.
The chain lengths of bacilli were determined by analyzing microscopy images (3). Briefly, B. anthracis cultures were normalized by measuring an A600 of 5 and diluted 1:100 into BHI broth at 37°C. At defined time points, aliquots of cells were removed and then fixed with 4% formalin, and images were captured with a charge-coupled device (CCD) camera on an Olympus IX81 microscope using 20× or 40× objectives. Chain lengths of bacilli were measured from acquired differential interference contrast (DIC) images using ImageJ and converted to lengths in micrometers using reference images of an objective micrometer (3). Cell lengths were assessed in the same manner, except that cell septa were stained with boron dipyrromethene vancomycin (B-vancomycin [Invitrogen]), and images were obtained with a 100× objective. The statistical significance of length distributions between different strains was analyzed using a one-way analysis of variance (ANOVA).
For fluorescence microscopy, B. anthracis cultures were normalized to an A600 of 5, diluted 1:100 into BHI, and incubated for either 2 or 8 h at 37°C before cells were fixed with 4% formalin. After extensive rinsing, samples were incubated with rabbit antisera raised against purified Sap, EA1, or BslO, and bound antibodies were detected with DyLight594-conjugated anti-rabbit IgG secondary antibody. Cells were counterstained with B-vancomycin. A Leica SP5 STED-CW superresolution laser scanning confocal microscope with a 63× objective was used to analyze the fluorescence of B. anthracis cells as described previously (27).
S-layer fractionation.
B. anthracis cultures normalized to an A600 of 5 were diluted 1:100 into BHI broth and grown at 37°C until cultures reached an A600 of 2. One milliliter of culture was sedimented by centrifugation, and the supernatant (medium fraction) was transferred to a new tube. Cells in the sediment were washed with phosphate-buffered saline (PBS) twice, sedimented, and suspended in PBS buffer containing 3 M urea. The samples were heated at 95°C for 10 min and centrifuged (10,000 × g, 10 min), and the supernatants containing S-layer and S-layer-associated proteins (S-layer fraction) were transferred to a new tube. The bacterial sediment was washed with PBS and mechanically lysed by silica bead beating. After sedimentation of the beads (1,000 ×g, 1 min), the lysate (cellular fraction) was transferred to a third tube. Proteins in the medium, S-layer, and cellular fractions were precipitated by addition of trichloroacetic acid (TCA [10% final concentration]). Precipitates were collected by centrifugation (10,000 × g, 10 min), washed with acetone, air dried, suspended in 100 μl of 1 M Tris-HCl (pH 8.0)–4% SDS, and mixed with an equal volume of SDS-PAGE loading buffer. Proteins in samples were separated by SDS-PAGE and visualized by Coomassie staining or transferred to polyvinylidene difluoride membrane for immunoblot analysis using rabbit antisera raised against purified antigens. Immunoreactive products were revealed by chemiluminescent detection after incubation with horseradish peroxidase (HRP)-conjugated secondary antibody (Cell Signaling Technology).
RESULTS
LytR-CpsA-Psr proteins are required for B. anthracis growth.
To analyze the function of different LCP enzymes in B. anthracis, we chose to generate six strains that each express a single lcp gene. The coding sequences of four genes—lcpB1 (BAS1830), lcpB3 (BAS0746), lcpB4 (BAS3381), and lcpC (BAS5115)—were deleted from the chromosome of B. anthracis Sterne 34F2 by allelic replacement either alone or as combinations with two, three, or four deletions. Bacteriophage CP51 was used to generate lysates of B. anthracis lcpB2::kan (lcpB2 = BAS0572) and lcpD::spec (lcpD = BAS5047) strains isolated following mutagenesis with the bursa aurealis minitransposon carrying kanamycin (aphA3) and spectinomycin (aad9) resistance markers, respectively. lcpB2::aphA3 and/or lcpD::aad9 was then transduced into B. anthracis mutants with deletions in three or four lcp genes. As a result, five new strains of the genotypes lcpB1+, lcpB3+, lcpB4+, lcpC+, and lcpD+ were generated (Table 1). Each strain was designated after the only remaining lcp gene. For example, the lcpB1+ strain carries lcpB1 on the chromosome but no longer carries lcpB2, lcpB3, lcpB4, lcpC, and lcpD. We were unable to transduce lcpD::aad9 into the ΔlcpB1 ΔlcpB3 ΔlcpB4 ΔlcpC background unless the variant had been transformed with plcpB2, a high-copy-number plasmid for lcpB2 overexpression (Fig. 1A; Table 1). As a control, the B. anthracis ΔlcpB1 ΔlcpB3 ΔlcpB4 ΔlcpC strain transformed with the control vector (pWWW412) could not be transduced with CP51 lysate from the B. anthracis lcpD::aad9 strain (Fig. 1A). The sixth variant is thus a merodiploid strain that carries two copies of the lcpB2 gene and thus is designated by the genotype lcpB2++ (Table 1). Together, these data indicate that expression of chromosomal lcpB1+, lcpB3+, lcpB4+, lcpC+ or lcpD+ alone was sufficient to support B. anthracis vegetative growth, whereas expression of lcpB2+ was not. Nevertheless, when lcpB2 was overexpressed from a plasmid, lcpB2++ supported B. anthracis vegetative growth in the absence of the other five lcp genes. The data suggest further that expression of lcp genes is essential for B. anthracis vegetative growth.
FIG 1.
Growth and viability of B. anthracis variants expressing single lcp genes. (A) The ΔlcpB1B3B4C quadruple mutant, which still carries the lcpB2 and lcpD genes, was transformed with a plasmid containing lcpB2 (plcpB2) or the cloning vector, and these strains were used as recipient strains for transduction of the lcpD::aad9 allele using bacteriophage CP51. The plcpB2-bearing strain is merodiploid for lcpB2. Following transduction, bacteria were plated on spectinomycin-containing medium and incubated at 30°C for 30 h. Recombination of the lcpD::aad9 allele was verified by DNA sequencing and occurred only in the merodiploid strain. (B) Overnight cultures of wild-type (WT) B. anthracis Sterne and isogenic variants expressing a single lcp gene were normalized to A600 of 5, diluted 1:100 into fresh medium, and grown at 37°C. Growth was monitored by recording the change in cell density (absorbance) at 600 nm every 30 min over 12 h. (C) After 6 h following dilution in fresh medium (as described for panel B), cultures were serially diluted, and 5-μl aliquots of each serial 10-fold dilution (0 through −7) were plated on agar medium. An image of the plate after overnight incubation at 30°C is shown.
To quantify vegetative growth, overnight cultures of B. anthracis were normalized for A600, diluted 100× into fresh medium, and incubated with rotation at 37°C while monitoring vegetative expansion as increases in A600 (Fig. 1B). These growth curves were used to deduce growth rates of 0.488, 0.498, 0.542, 0.496, 0.471, 0.350, and 0.253 h−1, respectively, for the wild-type and lcpB1+, lcpB2++, lcpB3+, lcpB4+, lcpC+, and lcpD+ strains. An aliquot of cultures was removed at 6 h, diluted serially, and spotted on agar plates for enumeration of colonies (Fig. 1C). The lcpB3+ variant grew at the same rate as the wild type and displayed similar plating efficiency (Fig. 1B and C). In contrast, the lcpD+ variant displayed a severe growth defect, with an extended lag phase (Fig. 1B) and diminished vegetative expansion (Fig. 1C). The lcpC+ and lcpB4+ variants grew more slowly than the wild type but expanded faster than the lcpD+ mutant. Of note, the 6-h plating efficiency of the lcpC+ and lcpB4+ strains was reduced to the same level as the lcpD+ mutant, suggesting that not all vegetative expansion of lcpC+ and lcpB4+ generates viable cells. Finally, the lcpB1+ and lcpB2++ variants displayed slight reductions in vegetative expansion and plating efficiency compared to the B. anthracis wild type or the lcpB3+ variant. These data indicate that, in the absence of the other LCP enzymes, only LcpB3 is sufficient to support B. anthracis vegetative growth at wild-type levels.
Cell size and chain length of lcp variant B. anthracis.
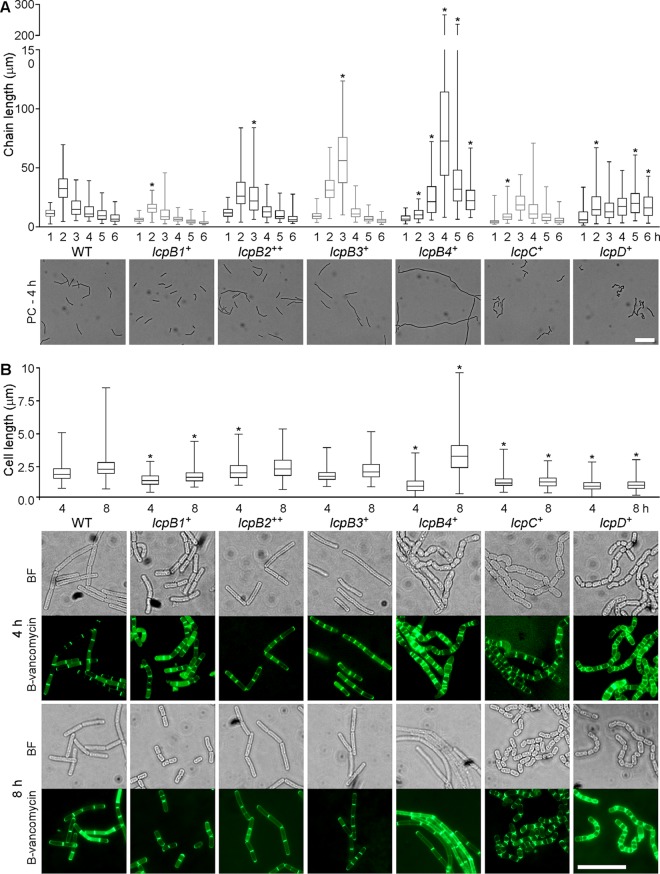
B. anthracis varies the chain length of vegetative cells by regulating the separation of septal peptidoglycan. As reported before, chain lengths of wild-type bacilli were increased 2 h following dilution of overnight cultures into fresh medium and were subsequently reduced in a stepwise fashion, as vegetative bacilli approached stationary phase. Earlier work showed that the B. anthracis lcpD mutant displayed an increased chain length, whereas the lcpB3 variant formed shorter chains with cells that are smaller than their wild-type counterparts (13). Overnight cultures of B. anthracis variants were diluted 100-fold, and fixed aliquots, withdrawn at hourly intervals, were analyzed by DIC microscopy for chain length (Fig. 2A; see Fig. S1 in the supplemental material). The average chain length of the lcpB1+, lcpB4+, lcpC+, and lcpD+ variants was diminished at 2 h, whereas the lcpB2++, lcpB3+, lcpB4+, and lcpD+ strains displayed increased chain lengths at one or more time points (marked with asterisks in Fig. 2A). The average chain length of the lcpB4+ variant was particularly exaggerated; at the 4-h time interval, the median chain length of lcpB4+ bacilli was 73 μm, compared to a median of 11 μm for wild-type B. anthracis. Thus, none of the six LCP enzymes alone is sufficient to impose physiological control of B. anthracis chain length, which appears to require multiple LCP proteins, if not a full complement of these enzymes.
FIG 2.
Chain length and cell length of B. anthracis variants expressing single lcp genes. (A) Aliquots of cultures grown as described for Fig. 1B were removed and fixed at hourly intervals for up to 6 h following inoculation in fresh medium. Images were captured and used to measure the lengths of 100 chains per time point using the ImageJ software. Results are presented in a box-and-whisker plot, where the box denotes the 25th and 75th percentiles, the whiskers denote the minimum and maximum values, and the center bar indicates the median value. Representative phase-contrast (PC) microscopy images from the 4-h time point are shown beneath the plot. The scale bar represents 20 μm. (B) Aliquots of cultures fixed at 4 and 8 h following inoculation in fresh medium were stained with BODIPY-vancomycin (B-vancomycin). The lengths of individual cells (n > 150) were measured using the ImageJ software and are presented in a box-and-whisker plot as in panel A. Representative images from both time points are shown below the plot, with bright-field (BF) microscopy images on top and images of the specimen stained with B-vancomycin below. The scale bar represents 10 μm. Data were analyzed by ANOVA for significant differences compared to the WT at the same time point. *, P < 0.05.
Microscopy experiments revealed also aberrant cell shapes of the lcp variants. To quantify these differences, the length of 150 individual cells was measured at the 4- and 8-h time intervals after specimens were stained with BODIPY-vancomycin to visualize peptidoglycan and cell septa (Fig. 2B). The lcpB4+ variant displayed the greatest variation in cell shape and size from wild-type bacilli. At 4 h, the lcpB4+ strain placed septal peptidoglycan in irregular intervals, generating cells that were either very short, of extended length, or with a widened, deformed shape (Fig. 2B). By 8 h, the lcpB4+ strain formed cells that were significantly longer than wild-type bacilli; indeed some cells reached a length of 10 μm and did not form septal peptidoglycan (Fig. 2B). Cells from the lcpB1+, lcpC+, and lcpD+ strains were significantly shorter than wild-type bacilli at both 4 and 8 h postinoculation (Fig. 2B). Shortened cell size was associated with cell rounding, and the lcpB1+, lcpC+, and lcpD+ mutants appeared to form chains of cocci (Fig. 2B). lcpB2++ cells were only slightly elongated at 4 h but otherwise retained the morphological features of wild-type B. anthracis. Finally, the lcpB3+ strain formed cells with a physiological size that could not be distinguished from wild-type bacilli.
lcpC+ and lcpD+ variants cannot assemble B. anthracis S-layers.
The ability of single lcp variants to assemble an S-layer was analyzed by fractionating B. anthracis cultures. Cultures were centrifuged, and the extracellular medium (M) was separated from the bacterial sediment. The S-layer (S) was solubilized by extraction with 3 M urea, which, following sample centrifugation, was separated with the supernatant from bacterial cells. Murein sacculi of B. anthracis were disrupted by bead beating to generate a cell lysate (C). Proteins in all fractions were precipitated with TCA, separated by SDS-PAGE, and analyzed by Coomassie blue staining (Fig. 3A). In wild-type B. anthracis, S-layer proteins Sap and EA1 are the most abundant polypeptides in fractionated cultures in the S-layer and medium fractions of Coomassie-stained SDS-PAGE (Fig. 3A, arrow). The lcpC+ and lcpD+ strains did not assemble detectable S-layers, whereas the lcpB2++ strain assembled dramatically reduced amounts of Sap and EA1 in the envelope, and the lcpB4+ strain assembled a diminished EA1 S-layer (Fig. 3A). The lcpB1+ and lcpB3+ strains assembled S-layer proteins in the bacterial envelope in a manner similar to that of wild-type B. anthracis (Fig. 3A).
FIG 3.
Subcellular fractionation of proteins with SLH domains. (A) Cultures of wild-type (WT) B. anthracis, carrying all six lcp genes or of variants with a single lcp gene (lcpB1+, lcpB2++, lcpB3+, lcpB4+, lcpC+, or lcpD+) were grown to an A600 of 2. Cultures were centrifuged to separate the extracellular medium (M) from the bacterial sediment. Proteins in the S layers (S) were extracted by boiling cells in 3 M urea. Stripped cells were broken mechanically to release cytosolic extracts (C). Proteins in all fractions were precipitated with TCA, washed in acetone, and separated by 10% SDS-PAGE. The gel was stained with Coomassie brilliant blue. The positions of Sap and EA1 that migrate with the same mobility on SDS-PAGE and of molecular mass markers are indicated by an arrow, and masses are given in kilodaltons on the right. (B) B. anthracis cultures fractionated as described for panel A were subjected to immunoblotting with rabbit antisera raised against purified Sap, EA1, BslO, BslR, BslS, BslT, BslU, or PrsA1.
Immunoblotting experiments revealed that the lcpC+ and lcpD+ strains assembled small amounts of Sap and EA1 in the bacterial envelope (Fig. 3B). Immunoblotting experiments otherwise confirmed Coomassie-stained SDS-PAGE data on S-layer protein assembly. We also used immunoblotting to analyze the assembly of S-layer-associated proteins in the envelope of B. anthracis strains (Fig. 3B). The assembly of the murein hydrolases BslO, BslS, BslT, and BslU was diminished in the lcpD+ strain yet unaffected in the lcpC+ strain or in any of the other single-lcp-gene-expressing strains. Immunoblotting with anti-BslR revealed the reciprocal phenotype with increased envelope assembly of the BslR murein hydrolase in lcpC+ and lcpD+ strains compared to wild-type and lcpB1+, lcpB2++, lcpB3+, or lcpB4+ strains (Fig. 3B). As a control, immunoblotting experiments suggested that the abundance and subcellular distribution of PrsA, a secreted lipoprotein, were similar between wild-type and lcp variant strains. Together, these data showed that the capacity for S-layer assembly is greater in B. anthracis variants expressing single LcpB-type enzymes (lcpB1+, lcpB2++, lcpB3+, or lcpB4+) than in strains producing LcpC or LcpD.
The lcpB3+ variant generates a physiological distribution of S-layer and S-layer-associated proteins.
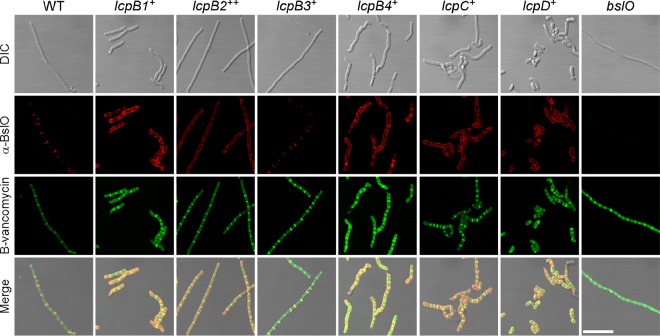
Next, microscopy was used to visualize the deposition and localization of S-layer and S-layer-associated proteins on the surface of bacilli. Overnight cultures of B. anthracis were diluted into fresh medium and incubated with rotation, and aliquots were withdrawn at timed intervals. Cells were fixed and incubated with rabbit immune sera raised against purified Sap, EA1, or BslO, as well as secondary antibody conjugated to fluorophore (Fig. 4 and 5). B-vancomycin staining was used to reveal cell septa. As expected, wild-type B. anthracis assembled homogeneous Sap S-layers along the cylindrical envelope of bacteria, whereas EA1 S-layers as well as BslO were deposited in the vicinity of septal peptidoglycan (Fig. 4 and 5). The lcpD+ strain assembled neither cylindrical S-layers with Sap nor EA1 S-layers in the vicinity of septal peptidoglycan; instead, lcpD+ cells revealed punctate EA1 deposits, irregular Sap assemblies (Fig. 4), and a cylindrical deposition of BslO (Fig. 5). The lcpC+ variant generated cylindrical Sap assemblies and punctate EA1 deposits that colocalized with B-vancomycin staining of shortened bacilli (Fig. 4). Nevertheless, the lcpC+ variant mislocalized BslO, which was found throughout the cylindrical envelope (Fig. 5). A similar phenotype was revealed for the lcpB1+ variant, albeit the overall amount of EA1 staining in lcpB1+ cells was higher than that of lcpC+ bacilli (Fig. 4). The lcpB2+ and lcpB4+ variants mislocalized EA1 and BslO, which were detected in large patches throughout the cylindrical envelope; this phenotype was associated with areas of diminished Sap S-layer assembly (Fig. 4 and 5). Of note, the lcpB4+ variant formed aberrant cells with increased diameter and irregular shape, which were generally devoid of Sap or EA1 assemblies. Only the lcpB3+ variant assembled cylindrical Sap and septal EA1 S-layers with septal BslO deposits in a manner similar to wild-type B. anthracis (Fig. 4 and 5).
FIG 4.
Deposition of S-layer proteins in the envelope of lcp variants. B. anthracis Sterne (WT) and isogenic variants carrying a single lcp gene were fixed in 4% buffered formalin at 8 h following inoculation of cultures in fresh medium. The localization of Sap (A) and EA1 (B) was observed with specific rabbit antisera as well as secondary antibody conjugates and counterstained with B-vancomycin to reveal the septal peptidoglycan. As controls, sap and eag mutants were also stained with their respective antisera. DIC, fluorescence, and merged microscopy images are shown for each strain. Scale bars represent 10 μm.
FIG 5.
Deposition of S-layer-associated proteins in the envelope of lcp variants. B. anthracis Sterne (WT) and isogenic variants carrying a single lcp gene were fixed in 4% buffered formalin at 2 h following inoculation of cultures in fresh medium. The localization of BslO was observed with specific rabbit antisera as well as secondary antibody conjugates and counterstained with B-vancomycin to reveal the septal peptidoglycan. As a control, bslO mutant bacilli were also stained with their respective antisera. DIC, fluorescence, and merged microscopy images are shown for each strain. Scale bars represent 10 μm.
Only lcpB3+ is fully sufficient for B. anthracis spore formation.
During sporulation, B. anthracis directs septum formation in the vicinity of one of the two cell poles, thereby generating large mother cells and smaller forespores, which are then developed further into heat-resistant spores. We wondered whether LCP enzymes are required for this process. Overnight cultures of wild-type B. anthracis and its lcp+ variants were washed and suspended in modG medium at an A600 of 1 to initiate sporulation. At timed intervals, culture aliquots were withdrawn, heat treated (68°C to kill vegetative bacilli), serially diluted, and spread on agar plates to enumerate CFU of heat-resistant spores. At time zero, B. anthracis cultures in modG medium harbored similar amounts of vegetative bacilli without spores (Fig. 6A). After 24 h of incubation in modG medium, spore formation in wild-type B. anthracis had been completed, and further incubation for 48 or 96 h did not result in elevated spore counts (Fig. 6B). The lcpB3+ variant sporulated with similar efficiency to wild-type B. anthracis, whereas the lcpB2++, lcpC+, and lcpD+ strains failed to produce spores (Fig. 6A and B). The lcpB1+ and lcpB4+ strains generated spores, albeit with 1,000- to 10,000-fold reduced efficacy (Fig. 6A and B). Microscopic examination of B. anthracis incubated in modG medium confirmed these observations. Refractile spores were readily visualized in samples from wild-type and lcpB3+ B. anthracis, whereas spores could rarely be detected in samples of the lcpB1+ and lcpB4+ variants (Fig. 6C).
FIG 6.
Sporulation efficiency of lcp variants. Bacilli from overnight cultures of B. anthracis Sterne (WT) and isogenic variants carrying a single lcp gene were washed, suspended in modG sporulation medium at an A600 of 1, and incubated at 30°C with shaking. (A) Culture aliquots were taken immediately following inoculation into modG medium, serially diluted, and plated to enumerate viable bacteria at time 0 (CFU). (B) Culture aliquots were collected at 24, 48, and 96 h following inoculation into modG medium, heat treated at 68°C for 30 min to kill vegetative bacilli, serially diluted, and plated to enumerate spores. (C) Culture aliquots from the 48-h time point were also fixed and visualized by phase-contrast microscopy. Representative images are shown. The scale bar represents 10 μm.
Aliquots of sporulating B. anthracis that had been removed 3 and 8 h following suspension in modG medium at an A600 of 5 were stained with FM4-64 and B-vancomycin for visualization of membranes and septal peptidoglycan (see Fig. S2 in the supplemental material). In the wild-type and lcpB3+ strains of B. anthracis, these experiments revealed asymmetric cell division sites and forespore engulfment after 3 h of incubation as well as spore formation after 8 h. Asymmetric cell division and forespore engulfment were not detected in the lcpB2++, lcpC+, and lcpD+ strains, suggesting that these variants did not initiate the early stages of spore formation.
DISCUSSION
B. subtilis, a spore-forming Gram-positive microbe, has been studied in depth as a model organism for bacterial development, cell biology, and industrial production of enzymes. Earlier work characterized the chemical structure of peptidoglycan and attached secondary cell wall polymers, including polyglycerol-phosphate wall teichoic acid (WTA), minor polyribitol-phosphate teichoic acid (TA), and teichuronic acid (TU) (reviewed in reference 28). Other studies identified genetic determinants for the synthesis of WTA, TA, and TU, which are located within a 50-kb region on the B. subtilis genome (reviewed in reference 29). B. subtilis employs three genes for LCP enzymes to assemble secondary wall polymers in the bacterial envelope. The genetic determinants—tagT, tagU, and tagV—are located in the 50-kb region for secondary cell wall polymer synthesis (14). B. subtilis strains lacking all three genes (ΔtagTUV) cannot grow; however, this growth phenotype is suppressed by mutations in tagO (14). As TagO catalyzes the first step in the synthesis pathway for murein linkage units (30), B. subtilis tagO strains suppress the growth phenotype of ΔtagTUV mutants via feedback inhibition of undecaprenol-phosphate-linked intermediates in the synthesis pathway for secondary cell wall polymers. B. subtilis mutants lacking combinations of two genes for LCP enzymes do not display significant phenotypes (14). These data suggest that LCP enzymes of B. subtilis display overlapping and redundant functions in tethering murein linkage units with appended WTA, TA, or TU to the bacterial peptidoglycan.
S. aureus, a Gram-positive pathogen that does not form spores, attaches polyribitol-phosphate wall teichoic acid (WTA) and capsular polysaccharide to the peptidoglycan envelope (16, 17, 31). Three LCP enzymes—encoded by lcpA, lcpB, and lcpC—fulfill overlapping and partially redundant functions, anchoring murein linkage units with appended WTA or capsular polysaccharide to peptidoglycan. Functional redundancy has also been observed for LCP-mediated attachment of capsular polysaccharide in Streptococcus pneumoniae (15). In S. aureus, deletion of lcpC primarily affects cell wall attachment of the capsular polysaccharide (16), whereas deletion of lcpA and lcpB imposes defects in S. aureus WTA synthesis (17). Similar to B. subtilis, S. aureus mutants with defects in WTA synthesis machinery downstream of the TagO/TagA-derived murein linkage unit cannot replicate, and this phenotype is suppressed by mutations in tagO (32, 33). Nevertheless, S. aureus mutants lacking all three genes for LCP enzymes (ΔlcpABC) are capable of growth, albeit at a reduced rate (16, 31). The reduced growth phenotype of S. aureus ΔlcpABC mutants is not suppressed by tagO mutations (16). Expression of B. anthracis lcpB2, lcpB3, lcpB4, lcpC, or lcpD but not of lcpB1 can restore some of the defects in staphylococcal growth and WTA assembly in S. aureus ΔlcpABC mutants (13). These data suggest that LCP enzymes of S. aureus and B. anthracis have evolved substrate preferences, recognizing murein linkage units with attached carbohydrate polymers or undecaprenol-phosphate-linked polysaccharides without murein linkage units.
B. anthracis does not synthesize WTA (34), and a thorough search of its genome does not identify homologs of the B. subtilis genes for TU synthesis. Unlike S. aureus and B. subtilis, B. anthracis attaches SCWP in the bacterial cell wall envelope and uses its CsaB-pyruvylated and PatA1/PatB1- or PatA2/PatB2-acetylated derivatives for the assembly of S-layer (Sap and EA1) and S-layer-associated proteins (BslA-U and AmiA) (5, 6, 35). Earlier work proposed that the genes for SCWP synthesis were located in the surface polysaccharide synthesis (sps) gene cluster (BAS5116 to BAS5127) (36). In agreement with this model, gneZ (BAS5117), which encodes a UDP-ManNAc epimerase, is essential for B. anthracis growth and SCWP synthesis (12). Nevertheless, gneY (BAS5048), a homolog of gneZ, can substitute for loss of gneZ function to restore B. anthracis growth and SCWP synthesis. gneY is part of another gene cluster also involved in SCWP synthesis; it includes lcpD (BAS5047) and tagO (BAS5050). lcpC (BAS5115) is also part of the sps gene cluster, whereas lcpB1 (BAS1830), lcpB2 (BAS0572), lcpB3 (BAS0746), and lcpB4 (BAS3381) are not associated with chromosomal genes known to be required for SCWP synthesis or assembly.
Previous work generated mutations that abolished the expression of individual lcp genes in B. anthracis, examining mutants for growth, cell size, chain length, SCWP synthesis, and S-layer assembly phenotypes. The lcpD mutant displayed increased chain length, diminished assembly of SCWP, and an S-layer protein assembly defect (13). In contrast, the lcpB3 mutant formed smaller cells with reduced chain length and defects in BSL deposition into S-layer structures (13). The remaining mutants (lcpB1, lcpB2, lcpB4, and lcpC genotypes) did not reveal phenotypic defects. Here we show that deletions of several different lcp genes from the chromosome can be tolerated by B. anthracis, albeit all attempts to generate a strain expressing only genome-contained lcpB2 failed. This could be circumvented by cloning lcpB2 on a plasmid under the control of the constitutive hprK promoter (37). These data suggest that, as observed for gneY, lcpB2 may not be expressed under laboratory conditions. Furthermore, lcp genes, similar to gneZ and tagO, are essential for B. anthracis vegetative growth (6, 12, 38). With the exception of the lcpB3+ variant, all B. anthracis strains expressing single lcp gene were impaired for growth and displayed defects in cell size, chain length, and spore formation. Of note, the lcpB3+ variant still assembled S-layers from Sap and EA1. These data suggest that LcpB3 likely transfers murein linkage units and SCWP with or without a ketal-pyruvyl modification to peptidoglycan, maintaining the housekeeping functions of the B. anthracis cell cycle and supporting assembly of the S-layer but not of BSLs. The lcpC+ and lcpD+ variants displayed severe growth, cell size, chain length, and S-layer assembly defects. Nonetheless, lcpC+ and lcpD+ variants were able to deposit BSLs into the bacterial envelope. We propose that LcpC and LcpD assemble SCWP with (LcpC) and without (LcpD) murein linkage units and PatA1/PatB1- or PatA2/PatB2-mediated acetylation of SCWP in the B. anthracis envelope. Finally, B. anthracis expressing only lcpB1 (lcpB1+) or only lcpB4 (lcpB4+) displayed moderate defects in cell size or shape, chain length, and spore formation, yet these variants sustained vegetative growth. Because the lcpB1+ and lcpB4+ strains, unlike the lcpB3+ strain, display defects in S-layer and BSL assembly, we speculate that the preferred substrate of LcpB1 and LcpB4 may be specialized SCWP (Fig. 7). For example, specialized SCWP could carry terminal repeats that bear chemical modifications distinct from the known pyruvyl and acetyl groups (Fig. 7). Finally, we cannot rule out the possibility that some LCPs transfer as of yet unknown polymers in the envelope of B. anthracis.
FIG 7.
A model of possible functions of LytR-CpsA-Psr (LCP) proteins in B. anthracis. LCP proteins function to attach secondary cell wall polysaccharide (SCWP) to the peptidoglycan of B. anthracis cells. LcpB3 is the housekeeping LCP and attaches the majority of the SCWP. LcpB2, LcpC, and LcpD attach specialized SCWP to the cell poles and septa to enable proper deposition of S-layer and S-layer-associated proteins. LcpB1 and LcpB4 attach specialized SCWP along the midcell to allow for proper asymmetrical division during sporulation.
lcpB1+, lcpB2++, and lcpB4+ variants display reduced viability compared to lcpB3+ bacteria. In addition, lcpB1+, lcpB2++, and lcpB4+ variants fail to regulate chain length during the life cycle, and while their overall capacity to bind proteins with SLH domains is not affected, the deposition of these proteins is altered in the envelope. Deposition of proteins appears to be favored along the cylinder of lcpB1+ and lcpB4+ cells. This preferential deposition suggests that assembly of pyruvylated and acetylated SCWP, the receptor of SLH proteins, occurs at distinct sites in an LcpB-dependent manner when bacteria undergo binary fission, i.e., during vegetative replication, as illustrated in the model of Fig. 7. The lcpC+ and lcpD+ variants replicate poorly and display a marked reduction in their ability to retain SLH proteins in the envelope. Interestingly, these variants cannot sporulate, a phenotype not previously observed for genes involved in S-layer or SCWP assembly (6, 27, 35, 38). The inability to form spores (i.e., to shift the division site to undergo asymmetric cell division) may correlate with the failure to maintain the typical rod-shape morphology of vegetative cells. This defect may be caused by the inherent defect of lcpC+ and lcpD+ variants to homogenously assemble SCWP in the envelope of vegetative bacilli. We propose that all LCPs carry out attachment of SCWP, with LcpB3 being the most effective enzyme, followed by LcpB1, LcpB4, LcpB2, LcpC, and LcpD. This in turn correlates with the ability to form spores. Thus, we favor a model whereby LcpB2, LcpC, and LcpD influence biochemical reactions that are linked to the division site, whereas LcpB1 and LcpB4 promote reactions along the cylinder of rod-shaped bacilli.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of our laboratory for discussion and comments on the manuscript, Derek Elli and Yating Wang for sharing reagents, and So-Young Oh for experimental support.
M.L.Z. and J.M.L. are trainees of the Medical Scientist Training Program at the University of Chicago and are supported by National Institutes of Health (NIH) Training Grant GM07281. J.M.L. is a recipient of NIH Ruth L. Kirschstein National Research Service Award 1F30AI110036-01. This research was supported by grant AI069227 from the National Institute of Allergy and Infectious Diseases, Infectious Disease Branch (to O.S. and D.M.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00656-15.
REFERENCES
- 1.Turnbull PC. 1991. Anthrax vaccines: past, present and future. Vaccine 9:533–539. doi: 10.1016/0264-410X(91)90237-Z. [DOI] [PubMed] [Google Scholar]
- 2.Mock M, Fouet A. 2001. Anthrax. Annu Rev Microbiol 55:647–671. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- 3.Anderson VJ, Kern JW, McCool JW, Schneewind O, Missiakas D. 2011. The SLH-domain protein BslO is a determinant of Bacillus anthracis chain length. Mol Microbiol 81:192–205. doi: 10.1111/j.1365-2958.2011.07688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruthel G, Ribot WJ, Bavari S, Hoover T. 2004. Time-lapse confocal imaging of development of Bacillus anthracis in macrophages. J Infect Dis 189:1313–1316. doi: 10.1086/382656. [DOI] [PubMed] [Google Scholar]
- 5.Mesnage S, Fontaine T, Mignot T, Delepierre M, Mock M, Fouet A. 2000. Bacterial SLH domain proteins are non-covalently anchored to the cell surface via a conserved mechanism involving wall polysaccharide pyruvylation. EMBO J 19:4473–4484. doi: 10.1093/emboj/19.17.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kern J, Ryan C, Faull K, Schneewind O. 2010. Bacillus anthracis surface-layer proteins assemble by binding to the secondary cell wall polysaccharide in a manner that requires csaB and tagO. J Mol Biol 401:757–775. doi: 10.1016/j.jmb.2010.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kern J, Wilton R, Zhang R, Binkowski TA, Joachimiak A, Schneewind O. 2011. Structure of surface layer homology (SLH) domains from Bacillus anthracis surface array protein. J Biol Chem 286:26042–26049. doi: 10.1074/jbc.M111.248070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kern VJ, Kern JW, Theriot JA, Schneewind O, Missiakas D. 2012. Surface-layer (S-layer) proteins Sap and EA1 govern the binding of the S-layer-associated protein BslO at the cell septa of Bacillus anthracis. J Bacteriol 194:3833–3840. doi: 10.1128/JB.00402-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhury B, Leoff C, Saile E, Wilkins P, Quinn CP, Kannenberg EL, Carlson RW. 2006. The structure of the major cell wall polysaccharide of Bacillus anthracis is species specific. J Biol Chem 281:27932–27941. doi: 10.1074/jbc.M605768200. [DOI] [PubMed] [Google Scholar]
- 10.Forsberg LS, Abshire TG, Friedlander A, Quinn CP, Kannenberg EL, Carlson RW. 2012. Localization and structural analysis of a conserved pyruvylated epitope in Bacillus anthracis secondary cell wall polysaccharides and characterization of the galactose deficient wall polysaccharide from avirulent B. anthracis CDC 684. Glycobiology 22:1103–1117. doi: 10.1093/glycob/cws080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velloso LM, Bhaskaran SS, Schuch R, Fischetti VA, Stebbins CE. 2008. A structural basis for the allosteric regulation of non-hydrolysing UDP-GlcNAc 2-epimerases. EMBO Rep 9:199–205. doi: 10.1038/sj.embor.7401154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang YT, Missiakas D, Schneewind O. 2014. GneZ, a UDP-GlcNAc 2-epimerase, is required for S-layer assembly and vegetative growth of Bacillus anthracis. J Bacteriol 196:2969–2978. doi: 10.1128/JB.01829-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liszewski Zilla M, Chan YG, Lunderberg JM, Schneewind O, Missiakas D. 2015. LytR-CpsA-Psr enzymes as determinants of Bacillus anthracis secondary cell wall polysaccharide assembly. J Bacteriol 197:343–353. doi: 10.1128/JB.02364-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai Y, Marles-Wright J, Cleverley RM, Emmins R, Ishikawa S, Kuwano M, Heinz N, Bui NK, Hoyland CN, Ogasawara N, Lewis RJ, Vollmer W, Daniel RA, Errington J. 2011. A widespread family of bacterial cell wall assembly proteins. EMBO J 30:4931–4941. doi: 10.1038/emboj.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberhardt A, Hoyland CN, Vollmer D, Bisle S, Cleverley RM, Johnsborg O, Havarstein LS, Lewis RJ, Vollmer W. 2012. Attachment of capsular polysaccharide to the cell wall in Streptococcus pneumoniae. Microb Drug Resist 18:240–255. doi: 10.1089/mdr.2011.0232. [DOI] [PubMed] [Google Scholar]
- 16.Chan YG, Frankel MB, Dengler V, Schneewind O, Missiakas D. 2013. Staphylococcus aureus mutants lacking the LytR-CpsA-Psr family of enzymes release cell wall teichoic acids into the extracellular medium. J Bacteriol 195:4650–4659. doi: 10.1128/JB.00544-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan YG, Kim HK, Schneewind O, Missiakas D. 2014. The capsular polysaccharide of Staphylococcus aureus is attached to peptidoglycan by the LytR-CpsA-Psr (LCP) family of enzymes. J Biol Chem 289:15680–15690. doi: 10.1074/jbc.M114.567669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kojima N, Arakai Y, Ito E. 1985. Structure of the linkage units between ribitol teichoic acids and peptidoglycan. J Bacteriol 161:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokoyama K, Miyashita T, Arakai Y, Ito E. 1986. Structure and functions of linkage unit intermediates in the biosynthesis of ribitol teichoic acids in Staphylococcus aureus H and Bacillus subtilis W23. Eur J Biochem 161:479–489. doi: 10.1111/j.1432-1033.1986.tb10469.x. [DOI] [PubMed] [Google Scholar]
- 20.Sterne M. 1937. Avirulent anthrax vaccine. Onderstepoort J Vet Sci Anim Ind 21:41–43. [PubMed] [Google Scholar]
- 21.Fulford W, Model P. 1984. Specificity of translational regulation by two DNA-binding proteins. J Mol Biol 173:211–226. doi: 10.1016/0022-2836(84)90190-6. [DOI] [PubMed] [Google Scholar]
- 22.Kim HU, Goepfert JM. 1974. A sporulation medium for Bacillus anthracis. J Appl Bacteriol 37:265–267. doi: 10.1111/j.1365-2672.1974.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 23.Tam C, Glass EM, Anderson DM, Missiakas D. 2006. Transposon mutagenesis of Bacillus anthracis strain Sterne using bursa aurealis. Plasmid 56:74–77. doi: 10.1016/j.plasmid.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Marraffini LA, Schneewind O. 2006. Targeting proteins to the cell wall of sporulating Bacillus anthracis. Mol Microbiol 62:1402–1417. doi: 10.1111/j.1365-2958.2006.05469.x. [DOI] [PubMed] [Google Scholar]
- 25.Gaspar AH, Marraffini LA, Glass EM, Debord KL, Ton-That H, Schneewind O. 2005. Bacillus anthracis sortase A (SrtA) anchors LPXTG motif-containing surface proteins to the cell wall envelope. J Bacteriol 187:4646–4655. doi: 10.1128/JB.187.13.4646-4655.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green BD, Battisti L, Koehler TM, Thorne CB, Ivins BE. 1985. Demonstration of a capsule plasmid in Bacillus anthracis. Infect Immun 49:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen-Mau SM, Oh SY, Kern VJ, Missiakas DM, Schneewind O. 2012. Secretion genes as determinants of Bacillus anthracis chain length. J Bacteriol 194:3841–3850. doi: 10.1128/JB.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuhaus FC, Baddiley J. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol Mol Biol Rev 67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazarevic V, Pooley HM, Mauel C, Karamata D. 2002. Teichoic and teichuronic acids from Gram-positive bacteria, p 465–492. In Vandamme EJ, De Baets S, Steinbuchel A (ed), Polysaccharides from prokaryotes, vol 5 Wiley-VCH Verlag, Weinheim, Germany. [Google Scholar]
- 30.Soldo B, Lazarevic V, Karamata D. 2002. tagO is involved in the synthesis of all anionic cell-wall polymers in Bacillus subtilis 168. Microbiology 148:2079–2087. doi: 10.1099/00221287-148-7-2079. [DOI] [PubMed] [Google Scholar]
- 31.Dengler V, Meier PS, Heusser R, Kupferschmied P, Fazekas J, Friebe S, Staufer SB, Majcherczyk PA, Moreillon P, Berger-Bachi B, McCallum N. 2012. Deletion of hypothetical wall teichoic acid ligases in Staphylococcus aureus activates the cell wall stress response. FEMS Microbiol Lett 333:109–120. doi: 10.1111/j.1574-6968.2012.02603.x. [DOI] [PubMed] [Google Scholar]
- 32.Brown S, Santa Maria JP Jr, Walker S. 2013. Wall teichoic acids of Gram-positive bacteria. Annu Rev Microbiol 67:313–336. doi: 10.1146/annurev-micro-092412-155620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia G, Kohler T, Peschel A. 2010. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int J Med Microbiol 300:148–154. doi: 10.1016/j.ijmm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Molnár J, Prágai B. 1971. Attempts to detect the presence of teichoic acid in Bacillus anthracis. Acta Microbiol Acad Sci Hung 18:105–108. [PubMed] [Google Scholar]
- 35.Lunderberg JM, Nguyen-Mau SM, Richter GS, Wang YT, Dworkin J, Missiakas DM, Schneewind O. 2013. Bacillus anthracis acetyltransferases PatA1 and PatA2 modify the secondary cell wall polysaccharide and affect the assembly of S-layer proteins. J Bacteriol 195:977–989. doi: 10.1128/JB.01274-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuch R, Pelzek AJ, Raz A, Euler CW, Ryan PA, Winer BY, Farnsworth A, Bhaskaran SS, Stebbins CE, Xu Y, Clifford A, Bearss DJ, Vankayalapati H, Goldberg AR, Fischetti VA. 2013. Use of a bacteriophage lysin to identify a novel target for antimicrobial development. PLoS One 8:e60754. doi: 10.1371/journal.pone.0060754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bubeck Wardenburg J, Williams WA, Missiakas D. 2006. Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc Natl Acad Sci U S A 103:13831–13836. doi: 10.1073/pnas.0603072103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lunderberg JM, Liszewski Zilla M, Missiakas D, Schneewind O. 31 August 2015. Bacillus anthracis tagO is required for vegetative growth and secondary cell wall polysaccharide synthesis. J Bacteriol doi: 10.1128/JB.00494-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.