Abstract
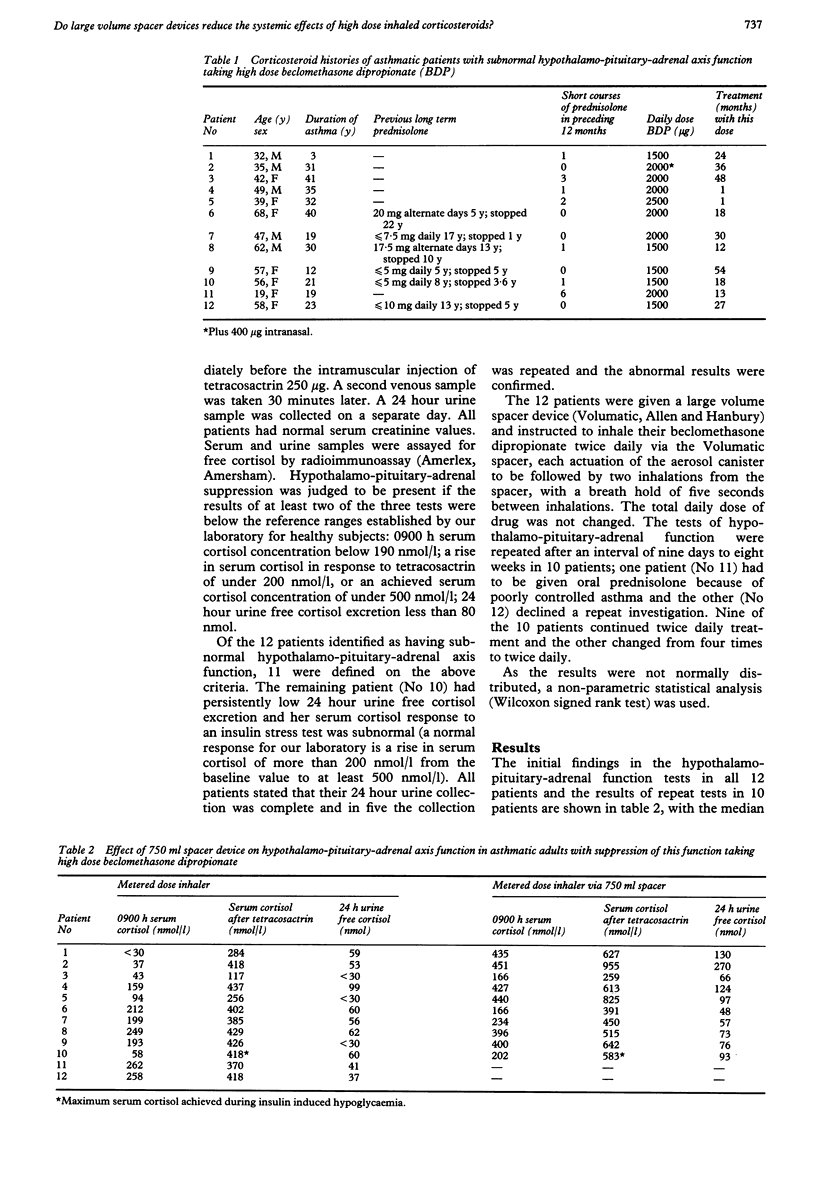
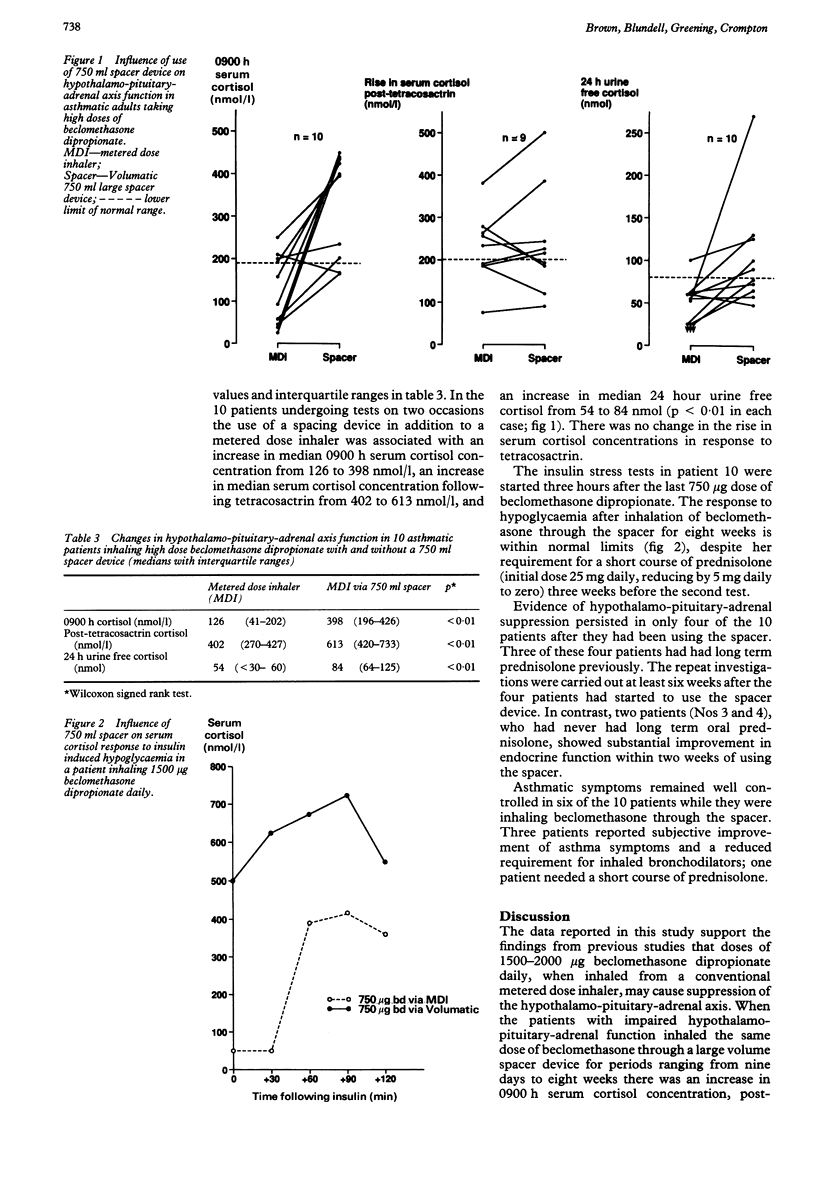
When used in high doses, inhaled corticosteroids may cause suppression of the hypothalamo-pituitary-adrenal axis. The influence of the mode of drug inhalation on the degree of this suppression is not clear. Hypothalamo-pituitary-adrenal function was assessed by measurement of 0900 h serum cortisol concentrations, a short tetracosactrin test, and 24 hour urine free cortisol excretion in 48 adults with asthma taking 1500-2500 micrograms beclomethasone dipropionate daily via a metered dose aerosol. Twelve patients had hypothalamo-pituitary-adrenal suppression, as judged by subnormal results from at least two of the three tests or (in one patient) by an abnormal insulin stress test response. These patients then changed to inhaling the same dose of beclomethasone dipropionate through a 750 ml spacer device (Volumatic). The endocrine tests were repeated from nine days to eight weeks later in 10 patients. Comparison with initial values showed that adding the spacing device caused an increase in the median 0900 h cortisol concentration from 126 nmol/l to 398 nmol/l, in the post-tetracosactrin cortisol concentration from 402 nmol/l to 613 nmol/l and in 24 hour urine free cortisol excretion from 54 nmol to 84 nmol. The rise in serum cortisol concentration in response to tetracosactrin did not change. Evidence of persisting hypothalamo-pituitary-adrenal axis suppression was present in only four of the 10 patients; the most pronounced improvements in function tended to occur in those who had never required long term oral corticosteroids. The results from this uncontrolled study suggest that asthmatic patients taking high dose beclomethasone dipropionate may minimise adverse effects by using a large volume spacer device.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ebden P., Jenkins A., Houston G., Davies B. H. Comparison of two high dose corticosteroid aerosol treatments, beclomethasone dipropionate (1500 micrograms/day) and budesonide (1600 micrograms/day), for chronic asthma. Thorax. 1986 Nov;41(11):869–874. doi: 10.1136/thx.41.11.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. C., McDonald C. F., Thomson S. A., Frame M. H., Pottage A., Crompton G. K. Dose of inhaled budesonide required to produce clinical suppression of plasma cortisol. Eur J Respir Dis. 1987 Jul;71(1):10–14. [PubMed] [Google Scholar]
- Martin L. E., Harrison C., Tanner R. J. Metabolism of beclomethasone dipropionate by animals and man. Postgrad Med J. 1975;51 (Suppl 4):11–20. [PubMed] [Google Scholar]
- Martin L. E., Tanner R. J., Clark T. J., Cochrane G. M. Absorption and metabolism of orally administered beclomethsone dipropionate. Clin Pharmacol Ther. 1974 Mar;15(3):267–275. doi: 10.1002/cpt1974153267. [DOI] [PubMed] [Google Scholar]
- Newman S. P., Millar A. B., Lennard-Jones T. R., Morén F., Clarke S. W. Improvement of pressurised aerosol deposition with Nebuhaler spacer device. Thorax. 1984 Dec;39(12):935–941. doi: 10.1136/thx.39.12.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. P., Pavia D., Morén F., Sheahan N. F., Clarke S. W. Deposition of pressurised aerosols in the human respiratory tract. Thorax. 1981 Jan;36(1):52–55. doi: 10.1136/thx.36.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahl P., Jensen T. Decreased adreno-cortical suppression utilizing the Nebuhaler for inhalation of steroid aerosols. Clin Allergy. 1987 Sep;17(5):393–398. doi: 10.1111/j.1365-2222.1987.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Hodson M. E. Effects of long term inhaled high dose beclomethasone dipropionate on adrenal function. Thorax. 1983 Sep;38(9):676–681. doi: 10.1136/thx.38.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. J., Hodson M. E. High-dose beclomethasone inhaler in the treatment of asthma. Lancet. 1983 Feb 5;1(8319):265–269. doi: 10.1016/s0140-6736(83)91686-0. [DOI] [PubMed] [Google Scholar]
- Tarlo S. M., Broder I., Davies G. M., Leznoff A., Mintz S., Corey P. N. Six-month double-blind, controlled trial of high dose, concentrated beclomethasone dipropionate in the treatment of severe chronic asthma. Chest. 1988 May;93(5):998–1002. doi: 10.1378/chest.93.5.998. [DOI] [PubMed] [Google Scholar]
- Toogood J. H., Baskerville J., Jennings B., Lefcoe N. M., Johansson S. A. Use of spacers to facilitate inhaled corticosteroid treatment of asthma. Am Rev Respir Dis. 1984 May;129(5):723–729. doi: 10.1164/arrd.1984.129.5.723. [DOI] [PubMed] [Google Scholar]
- Toogood J. H., Jennings B., Baskerville J., Johansson S. A. Clinical use of spacer systems for corticosteroid inhalation therapy: a preliminary analysis. Eur J Respir Dis Suppl. 1982;122:100–107. [PubMed] [Google Scholar]


