The results of this study suggest that the fraction of the fast-relaxing water component may be more sensitive than single-component T2 for detection of cartilage degeneration in patients with osteoarthritis of the knee with greater diagnostic performance for distinguishing morphologically normal cartilage from morphologically degenerative cartilage at receiver operating characteristic curve analysis.
Abstract
Purpose
To compare multicomponent T2 parameters of the articular cartilage of the knee joint measured by using multicomponent driven equilibrium single-shot observation of T1 and T2 (mcDESPOT) in asymptomatic volunteers and patients with osteoarthritis.
Materials and Methods
This prospective study was performed with institutional review board approval and with written informed consent from all subjects. The mcDESPOT sequence was performed in the knee joint of 13 asymptomatic volunteers and 14 patients with osteoarthritis of the knee. Single-component T2 (T2Single), T2 of the fast-relaxing water component (T2F) and of the slow-relaxing water component (T2S), and the fraction of the fast-relaxing water component (FF) of cartilage were measured. Wilcoxon rank-sum tests and multivariate linear regression models were used to compare mcDESPOT parameters between volunteers and patients with osteoarthritis. Receiver operating characteristic analysis was used to assess diagnostic performance with mcDESPOT parameters for distinguishing morphologically normal cartilage from morphologically degenerative cartilage identified at magnetic resonance imaging in eight cartilage subsections of the knee joint.
Results
Higher cartilage T2Single (P < .001), lower cartilage FF (P < .001), and similar cartilage T2F (P = .079) and T2S (P = .124) values were seen in patients with osteoarthritis compared with those in asymptomatic volunteers. Differences in T2Single and FF remained significant (P < .05) after consideration of age differences between groups of subjects. Diagnostic performance was higher with FF than with T2Single for distinguishing between normal and degenerative cartilage (P < .05), with greater areas under the curve at receiver operating characteristic analysis.
Conclusion
Patients with osteoarthritis of the knee had significantly higher cartilage T2Single and significantly lower cartilage FF than did asymptomatic volunteers, and receiver operating characteristic analysis results suggested that FF may allow greater diagnostic performance than that with T2Single for distinguishing between normal and degenerative cartilage.
© RSNA, 2015
Introduction
Osteoarthritis is a highly prevalent and severely debilitating chronic disease (1,2). Characteristic changes in the cartilage macromolecular matrix occur with osteoarthritis, including a decrease in proteoglycan content and disruption of the highly organized collagen fiber network (3–5). Various quantitative magnetic resonance (MR) imaging techniques have been used to identify changes in the composition and ultrastructure of articular cartilage in patients with osteoarthritis (6). The T2 of cartilage is one of the most commonly used MR imaging parameters and has been shown to be sensitive for detection of cartilage degeneration in ex vivo specimens (7) and human subjects (8–12). However, cartilage T2 is a complex measurement that is influenced by multiple factors including water and macromolecular content (13–16), organization of the collagen fiber network (17–19), cartilage loading (20–22), and orientation of cartilage relative to the main magnetic field (23). Thus, changes in cartilage T2 may be difficult to interpret and sometimes challenging to detect because of the multiple potentially competing biologic factors that influence the measurement.
Quantitative MR imaging techniques that allow measurement of multicomponent T2 values have been used to improve the specificity of T2 analysis of cartilage (24–28). Nuclear MR spectroscopic studies have allowed identification of three exchangeable water components in cartilage: extremely fast-relaxing water tightly bound to collagen, fast-relaxing water tightly bound to proteoglycan, and slow-relaxing bulk water loosely bound to the hydrophilic glycosaminoglycan side chains of proteoglycan with T2 values of 2.2, 25.2, and 96.3 msec, respectively (24). Because of the low fraction and difficulty in measuring the water tightly bound to the collagen component, which has an extremely short T2 (24), most multicomponent T2 mapping techniques involve the use of bicomponent models that allow assessment of only the fast-relaxing water tightly bound to proteoglycan and slow-relaxing bulk water loosely bound to the hydrophilic glycosaminoglycan side chains of proteoglycan components of cartilage (25–28). Authors of previous studies have shown that the fraction of the fast-relaxing water tightly bound to the proteoglycan component is a sensitive and specific measure of the proteoglycan content of cartilage (24–26). However, authors of previous multicomponent T2 mapping studies (24–28) have been limited by their use of Carr-Purcell-Meiboom-Gill techniques with long acquisition times, which allowed for cartilage assessment on only a single section of ex vivo specimens.
Multicomponent driven equilibrium single-shot observation of T1 and T2 (mcDESPOT) is a rapid method for multicomponent T2 mapping (29–34). The use of mcDESPOT allows acquisition of three-dimensional (3D) voxel-based measurements of the fractions and T2 values of the fast-relaxing and slow-relaxing water components of the articular cartilage of the human knee joint at 3.0 T with high spatial resolution, large volume coverage, and relatively short imaging time, which makes it feasible for use in clinical practice and osteoarthritis research studies (35). Authors of few previous studies have investigated multicomponent T2 parameters in human articular cartilage. Therefore, it remains unknown how cartilage degeneration in human subjects leads to changes in the fractions and T2 values of the different water components of cartilage. Thus, this study was performed to compare multicomponent T2 parameters of the articular cartilage of the knee joint measured by using mcDESPOT in asymptomatic volunteers and patients with osteoarthritis.
Materials and Methods
Study Group
Our prospective study was performed between June 1, 2013, and February 1, 2014, in compliance with Health Insurance Portability and Accountability Act regulations and with approval from our institutional review board. All subjects signed written informed consent forms before their participation in the study. The study group consisted of 13 asymptomatic volunteers (10 men; average age, 26.6 years; range, 25–32 years; three women; average age, 32.0 years; range, 20–38 years) and 14 patients with osteoarthritis of the knee (nine men; average age, 51.5 years; range, 42–58 years; five women; average age, 54.5 years; range, 45–62 years). The sample size was selected by using a power analysis based on data obtained from a previously performed study (8) of cartilage T2 in which the authors compared volunteers and patients with osteoarthritis of the knee. The power analysis was performed by using two-sample Student t tests with 80% power and a significance level of .05 and was designed to allow detection of significant differences in cartilage T2 between volunteers and patients with osteoarthritis in at least three cartilage subsections in the knee joint.
All volunteers were selected from a database of individuals at our institution who had expressed interest in participating in MR imaging research and who had no history of knee pain, trauma, or surgery. All patients were recruited from the clinic of a fellowship-trained sports medicine specialist (J.J.W., with 8 years of clinical experience). All patients received a diagnosis of osteoarthritis during their routine clinical work-up according to standardized criteria that included chronic knee pain and stiffness for a minimum of 6 months and the presence of definitive grade 2 osteophytes on standing anterior-posterior knee radiographs (36,37). Exclusion criteria included a history of knee surgery, inflammatory arthritis, crystalline-induced arthritis, septic arthritis, and contraindication to MR imaging. Fourteen of 24 consecutive patients who received a diagnosis of osteoarthritis of the knee during their routine clinical work-up and who met the inclusion criteria were recruited, and 10 patients chose not to participate. Eight patients had Kellgren-Lawrence grade 2 osteoarthritis, while six patients had Kellgren-Lawrence grade 3 osteoarthritis (38).
MR Imaging Examination
All subjects underwent MR imaging examination of the knee joint with the same 3.0-T imager (Discovery MR750; GE Healthcare, Waukesha, Wis) and an eight-channel phased-array extremity coil (InVivo, Orlando, Fla). Foam padding was used to secure the knee firmly in the coil to minimize subject motion during the MR imaging examination. To assess the repeatability of cartilage multicomponent T2 measurements, the MR examination was performed twice on both knee joints in five of the volunteers (five men; average age, 29.2 years; range, 28–32 years). The subjects exited the imager and were allowed to rest in a sitting position for 10 min-utes between examinations.
All MR imaging examinations consisted of the mcDESPOT sequence and a frequency-selective fat-suppressed 3D fast spin-echo (FSE) sequence performed on the sagittal plane through the knee joint. The 3D FSE sequence was performed with the following parameters: repetition time msec/echo time msec, 2216/23.6; field of view, 16 cm; matrix, 384 × 384; section thickness, 1.0 mm; bandwidth, 31.2 kHz; number of sections, 96; signal average, one; and imaging time, 7 minutes. The mcDESPOT sequence was a custom-made MR imaging pulse sequence consisting of a series of conventional spoiled gradient-echo and balanced steady-state free-precession sequences that are commercially available on the MR imaging platforms of all vendors. Eight spoiled gradient-echo sequences were performed with the following parameters: 4.9/2.3; and flip angles, α = 3°, 4°, 5°, 6°, 7°, 9°, 13°, and 18°. Eight balanced steady-state free-precession sequences were performed with the following parameters: 5.6/2.8; and flip angles, α = 2°, 5°, 10°, 15°, 20°, 30°, 40°, and 50°. Two balanced steady-state free-precession sequences were performed at each flip angle with radiofrequency phase cycling (φ = 0° and 180°) to remove the effects of balanced steady-state free-precession banding artifacts and to provide an estimate of the B0 field. An additional inversion recovery spoiled gradient-echo sequence was performed with the following parameters: 4.9/2.3; inversion time msec, 450; and flip angle, α = 5° to estimate the transmit B1 field. All sequences were performed by using the following parameters: field of view, 16 cm; matrix, 256 × 256; section thickness, 3 mm; bandwidth, 83.3 kHz; number of sections, 32; number of signals acquired, one. Total imaging time for the mcDESPOT sequence was 17 minutes.
Cartilage T2 Single- and Multicomponent Map Reconstruction
Multicomponent T2 maps of articular cartilage were reconstructed from the mcDESPOT source images with in-house software developed by using a high-level technical computing language and interactive environment (MATLAB 2010b; MathWorks, Natick, Mass). Single-component T2 (T2Single) maps were created by using the driven equilibrium single-shot observation of T2 full-modeling reconstruction method (33). Results of previous studies have shown high pixel-by-pixel correlation between cartilage T2Single measurements obtained by using mcDESPOT and those obtained by using conventional Carr-Purcell-Meiboom-Gill techniques (35). Multicomponent T2 maps for the fast-relaxing water component (T2F) and the slow-relaxing water component (T2S) and maps for the fraction of the fast-relaxing water component (FF) were created by using the two-pool mcDESPOT reconstruction method (29–31). The mcDESPOT method fits the observed spoiled gradient-echo and balanced steady-state free-precession signal at various flip angles with the use of the mathematical model described in the Appendix E1(Fig E1 [online]). Image registration software (Flexible Image Registration Toolbox; Functional Magnetic Resonance Imaging of the Brain Analysis Group, Oxford University, England) was used during the reconstruction process to correct for any subject motion that may have occurred between the multiple sequences.
Image Analysis
Quantitative cartilage analysis was performed by a research assistant (F.L., with 4 years of experience in segmentation) under the supervision of a fellowship-trained musculoskeletal radiologist (R.K., with 12 years of clinical experience) by using in-house software developed in the computing language and interactive environment (MATLAB, Mathworks). To investigate depth-dependent variations in multicomponent T2 parameters, pixel-by-pixel measurements of T2Single, T2F, T2S, and FF were plotted along a linear region of interest extending from the cartilage-bone interface to the articular surface in a single section through patellar cartilage in a randomly chosen asymptomatic volunteer. The articular cartilage on all sagittal 3D FSE sections through the knee joint in all subjects was segmented semiautomatically. Eight cartilage subsections were defined on the 3D FSE images, including the patella, trochlea, central medial femoral condyle, posterior medial femoral condyle, central lateral femoral condyle, posterior lateral femoral condyle, medial tibial plateau, and lateral tibial plateau as shown in Figure 1. The anterior margins of the anterior horn of the menisci were used to separate the trochlea from the central femoral condyles, while the posterior margins of the posterior horn of the menisci were used to separate the central femoral condyles from the posterior femoral condyles.
Figure 1:
Schematic illustration shows 3D cartilage segmentation of knee joint in a 25-year-old asymptomatic male volunteer. Colored 3D contours created from 3D FSE images were used to delineate articular cartilage of patella (PAT), trochlea (TROC), central medial femoral condyle (MFCC), posterior medial femoral condyle (MFCP), central lateral femoral condyle (LFCC), posterior lateral femoral condyle (LFCP), medial tibial plateau (MTP), and lateral tibial plateau (LTP). Contours were superimposed over cartilage T2Single, T2F T2S, and FF maps to measure average T2Single, T2F, T2S, and FF values in each cartilage subsection.
Cartilage thickness was calculated by exploring the 3D pixel data of the contours placed around articular cartilage on the 3D FSE images. The x and y coordinates of points were extracted on the basis of the locations of these pixels in each image, while the z coordinates were extracted on the basis of the section thickness and section indexes. The points in each image were patched into 3D triangular meshes, and the meshes were expressed as lists of triangular faces and sets of three vertices that form the triangles. The cartilage thickness was measured by calculating the minimum Euclidian distance from each triangle on the inner and outer cartilage boundary. The mean thickness was calculated as the average value of all MR image sections for each cartilage subsection. The average T2Single, T2F, T2S, and FF in each cartilage subsection was measured by superimposing the 3D FSE contours of each subsection over the cartilage T2Single, T2F, T2S, and FF maps. Cartilage T2Single, T2F, T2S, FF, and thickness of the entire knee joint were calculated by averaging the values measured in all eight cartilage subsections.
Morphologic joint analysis was performed by a fellowship-trained musculoskeletal radiologist (R.K., with 12 years of clinical experience) who was blinded to whether a subject was an asymptomatic volunteer or a patient with osteoarthritis. The radiologist used the sagittal 3D FSE images and axial and coronal reformatted 3D FSE images generated by using the volumetric source data to grade the severity of degeneration in each cartilage subsection in the knee joint by using Boston-Leeds Osteoarthritis Knee Scoring (BLOKS) system (39).
Statistical Analysis
Statistical analysis was performed by using MATLAB and the R programming environment (R programming environment, Version 2.3.1; R Foundation of Statistical Imaging; Vienna, Austria; http://www.R-project.org). For all tests, a statistically significant difference in MR imaging parameters between groups of subjects was defined as a P value less than .05. The Holm-Bonferroni correction method was used to adjust all P values to account for multiple comparisons (40).
Coefficients of variation were used to assess the repeatability of cartilage multicomponent T2 measurements on each cartilage subsection of the knee joint. Coefficients of variation were defined as the mean divided by the standard deviation of the multicomponent T2 measurements obtained by using the two repeat mcDESPOT sequences performed in the same knee of the same volunteers. Coefficients of variation for repeat cartilage multicomponent T2 measurements of the entire knee joint were also calculated by averaging the values measured in all eight cartilage subsections.
To minimize type I error due to comparison of multiple MR imaging parameters on multiple cartilage subsections, average cartilage T2Single, T2F, T2S, FF, and thickness of the entire knee joint were first compared between the groups of subjects. Wilcoxon rank-sum tests were used to compare average cartilage T2Single, T2F, T2S, FF, and thickness of the entire knee joint between volunteers and patients. Kruskal-Wallis one-way analysis of variance was used to compare average cartilage T2Single, T2F, T2S, FF, and thickness of the entire knee joint among volunteers, patients with Kellgren-Lawrence grade 2 osteoarthritis, and those with grade 3 osteoarthritis of the knee. Multivariate linear regression models were then used to account for age differences between groups of subjects in the analysis by using both MR imaging parameters and age as continuous variables to distinguish between groups of subjects. Wilcoxon rank-sum tests were used to compare MR imaging parameters on each cartilage subsection between volunteers and patients for those MR imaging parameters found to be significantly different for the entire knee joint.
Receiver operating characteristic analysis was used to assess diagnostic performance with T2Single, T2F, T2S, and FF values on MR images of each cartilage subsection for differentiation of morphologically normal cartilage from cartilage with morphologic degeneration and for differentiation of morphologically normal cartilage from cartilage with mild morphologic degeneration. To determine the severity of morphologic cartilage degeneration on MR images, a normalized BLOKS score was calculated by dividing the BLOKS score of each cartilage subsection by the maximum possible BLOKS score in the subsection. Morphologically normal cartilage was defined as a cartilage subsection with a BLOKS score of 0 (104 subsections in volunteers and 24 subsections in patients), cartilage with morphologic degeneration was defined as a cartilage subsection with a BLOKS score greater than 0 (88 subsections in patients), and cartilage with mild morphologic degeneration was defined as a cartilage subsection with a normalized BLOKS score greater than 0 but less than 0.3 (64 subsections in patients). Areas under the curve (AUC) were calculated to assess diagnostic performance. Differences in AUCs among T2Single, T2F, T2S, and FF were compared by using a previously described methodology (41).
Results
The coefficients of variation for multicomponent T2 measurements obtained by using the two repeat mcDESPOT sequences on each cartilage subsection of the knee joint ranged from 2.6% for T2F measurements in the posterior medial femoral condyle to 10.9% for T2F measurements in the lateral tibial plateau. The coefficients of variation for repeat multicomponent T2 measurements of the entire knee joint were 3.1% for T2Single, 1.8% for T2F, 2.2% for T2S, and 1.7% for FF (Table 1).
Table 1.
Coefficients of Variation for Repeat Multicomponent T2 Measurements
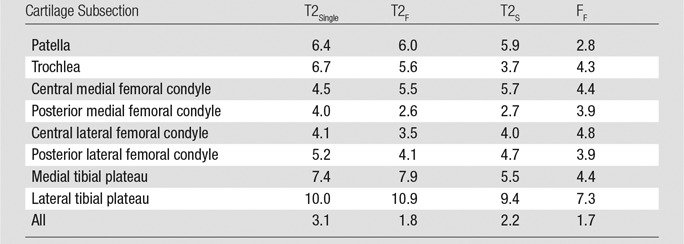
Note.—Data are percentages.
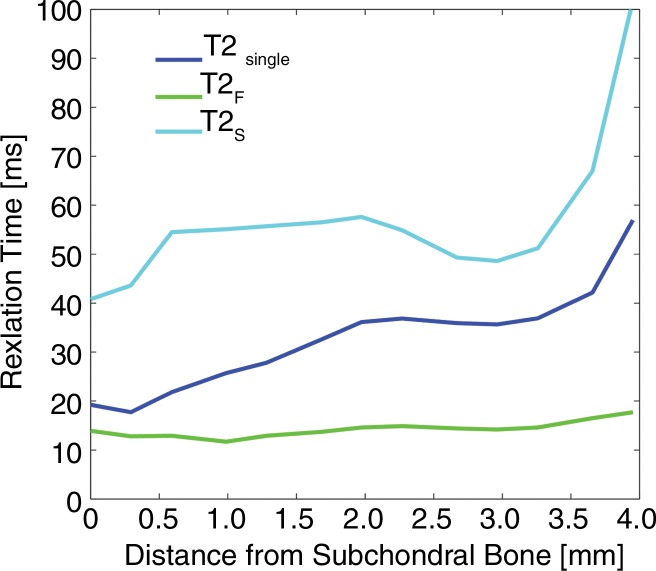
Figure 2 shows pixel-by-pixel measurements of T2Single, T2F, T2S, and FF plotted as a function of distance from the bone-cartilage interface to the articular surface. T2Single, T2F, and T2S increased from values of 19.2, 13.9, and 40.8 msec, respectively, at the bone-cartilage interface to values of 56.9, 17.7, and 101.7 msec, respectively, at the articular surface. FF decreased from a value of 43.0% at the bone-cartilage interface to a value of 16.9% at the articular surface. The percentage of change throughout the depth of the articular cartilage for T2Single, T2F, T2S, and FF was 66.3%, 21.5%, 59.9%, and 60.7%, respectively.
Figure 2a:
Line graphs show pixel-by-pixel measurements of (a) T2Single, T2F, and T2S and (b) FF plotted along linear region of interest extending from cartilage-bone interface to articular surface on single sagittal section through patellar cartilage in randomly chosen 27-year-old asymptomatic male volunteer. Note depth-dependent variations in single component and multicomponent T2 parameters.
Figure 2b:
Line graphs show pixel-by-pixel measurements of (a) T2Single, T2F, and T2S and (b) FF plotted along linear region of interest extending from cartilage-bone interface to articular surface on single sagittal section through patellar cartilage in randomly chosen 27-year-old asymptomatic male volunteer. Note depth-dependent variations in single component and multicomponent T2 parameters.
There were higher cartilage T2Single (P < .001) and lower cartilage FF (P < .001) values in the entire knee joint in patients with osteoarthritis than in asymptomatic volunteers. There were no significant differences in cartilage T2F (P = .079), T2S (P = .124), and thickness (P = .143) values of the entire knee joint between the groups of subjects. The difference in cartilage T2Single and cartilage FF values between volunteers and patients with osteoarthritis remained significant (P < .05) when multivariate analysis was used to account for age differences between the groups of subjects. There were higher cartilage T2Single values in the central medial femoral condyle (P = .007), posterior medial femoral condyle (P = .015), central lateral femoral condyle (P = .040), posterior lateral femoral condyle (P = .007), and medial tibial plateau (P = .009) in patients than in volunteers. There were lower cartilage FF values in the trochlea (P = .032), central medial femoral condyle (P = .014), posterior medial femoral condyle (P = .040), central lateral femoral condyle (P = .004), posterior lateral femoral condyle (P = .001), medial tibial plateau (P = .041), and lateral tibial plateau (P = .040) in patients than in volunteers (Table 2). There was a significant difference among volunteers, patients with Kellgren-Lawrence grade 2 knee osteoarthritis, and patients with Kellgren-Lawrence grade 3 knee osteoarthritis for T2Single (P < .001), FF (P < .001), and thickness (P = .014) values, but no significant difference for T2F (P = .173) and T2S (P = .074) values (Table 3). When multivariate analysis was used to account for age differences among groups of subjects, the difference among volunteers, patients with Kellgren-Lawrence grade 2 osteoarthritis, and patients with Kellgren-Lawrence grade 3 osteoarthritis remained significant for FF (P < .05) values but was no longer significant for T2Single (P = .061) and thickness (P = .355) values. Figures 3 and 4 show T2Single, T2F, T2S, and FF maps of patellar cartilage in an asymptomatic volunteer and a patient with osteoarthritis.
Table 2.
Average Cartilage Multicomponent T2 Parameters and Cartilage Thickness

Note.—Data are averages ± standard deviation. OA = osteoarthritis, VOL = asymptomatic volunteers.
*Indicates significant difference in values between the groups of subjects, P < .05.
Table 3.
Average Cartilage Multicomponent T2 Parameters and Cartilage Thickness by Subject Group
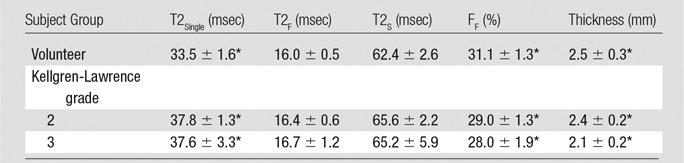
Note.—Data are averages ± standard deviation.
*Indicates significant difference in values among the groups of subjects, P < .05.
Figure 3a:

T2Single, T2F, T2S, and FF maps show articular cartilage of knee joint in a 25-year-old male asymptomatic volunteer. (a) 3D FSE image shows morphologically normal patellar cartilage. Corresponding (b) T2Single, (c) T2F, (d) T2S, and (e) FF maps show normal depth-dependent distribution of single-component and multicomponent T2 parameters of patellar cartilage, with lower T2Single, T2F, and T2S, and higher FF in the deep cartilage than in superficial cartilage.
Figure 4a:

T2Single, T2F, T2S, and FF maps show articular cartilage of knee joint in a 49-year-old man with Kellgren-Lawrence grade 2 osteoarthritis of the knee. (a) 3D FSE image shows focal area of superficial partial-thickness cartilage loss on patella (arrow). Corresponding (b) T2Single, (c) T2F, (d) T2S, and (e) FF maps show increased T2Single, T2F, and T2S, and decreased FF in patellar cartilage (arrow) at the site of superficial cartilage lesion. Note that changes in FF are more pronounced and extend deeper into cartilage than other single-component and multicomponent T2 parameters.
Figure 3b:

T2Single, T2F, T2S, and FF maps show articular cartilage of knee joint in a 25-year-old male asymptomatic volunteer. (a) 3D FSE image shows morphologically normal patellar cartilage. Corresponding (b) T2Single, (c) T2F, (d) T2S, and (e) FF maps show normal depth-dependent distribution of single-component and multicomponent T2 parameters of patellar cartilage, with lower T2Single, T2F, and T2S, and higher FF in the deep cartilage than in superficial cartilage.
Figure 3c:
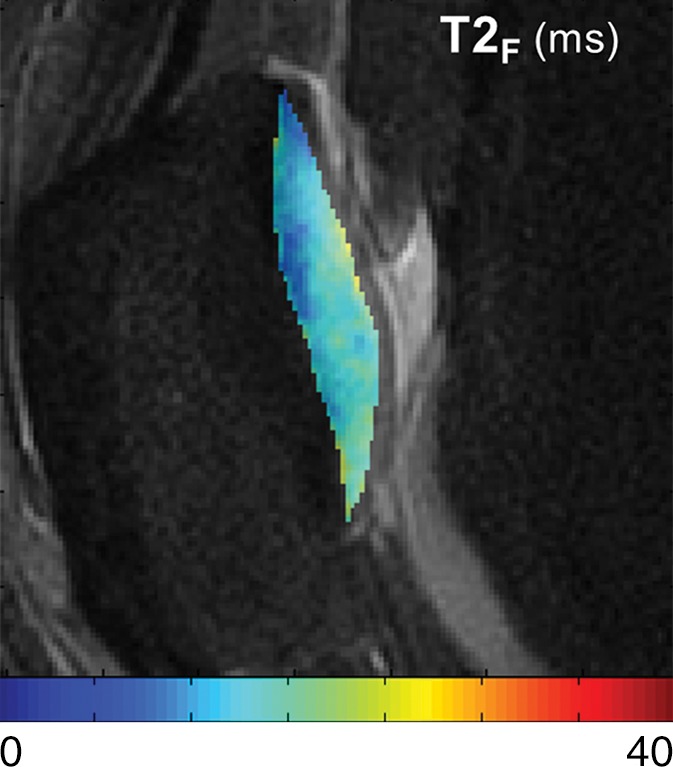
T2Single, T2F, T2S, and FF maps show articular cartilage of knee joint in a 25-year-old male asymptomatic volunteer. (a) 3D FSE image shows morphologically normal patellar cartilage. Corresponding (b) T2Single, (c) T2F, (d) T2S, and (e) FF maps show normal depth-dependent distribution of single-component and multicomponent T2 parameters of patellar cartilage, with lower T2Single, T2F, and T2S, and higher FF in the deep cartilage than in superficial cartilage.
Figure 3d:
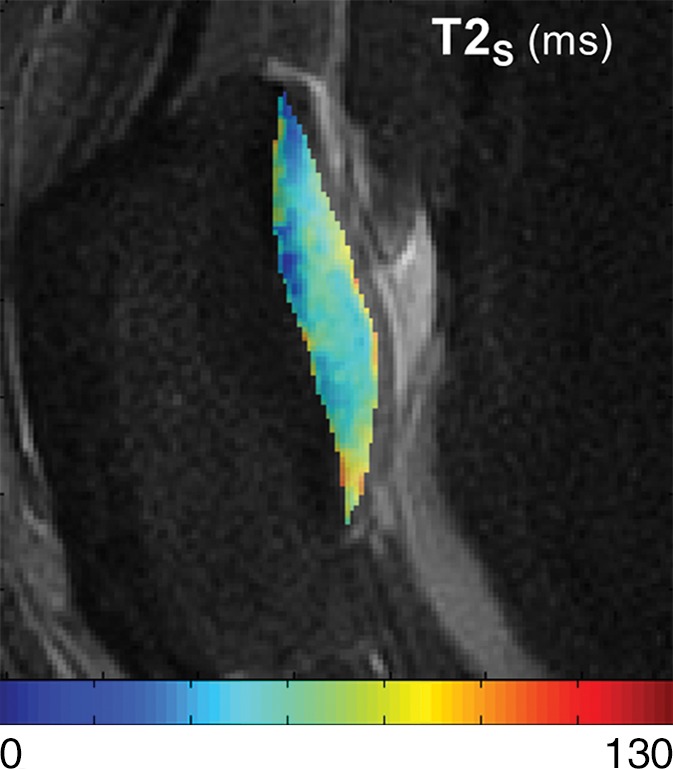
T2Single, T2F, T2S, and FF maps show articular cartilage of knee joint in a 25-year-old male asymptomatic volunteer. (a) 3D FSE image shows morphologically normal patellar cartilage. Corresponding (b) T2Single, (c) T2F, (d) T2S, and (e) FF maps show normal depth-dependent distribution of single-component and multicomponent T2 parameters of patellar cartilage, with lower T2Single, T2F, and T2S, and higher FF in the deep cartilage than in superficial cartilage.
Figure 3e:

T2Single, T2F, T2S, and FF maps show articular cartilage of knee joint in a 25-year-old male asymptomatic volunteer. (a) 3D FSE image shows morphologically normal patellar cartilage. Corresponding (b) T2Single, (c) T2F, (d) T2S, and (e) FF maps show normal depth-dependent distribution of single-component and multicomponent T2 parameters of patellar cartilage, with lower T2Single, T2F, and T2S, and higher FF in the deep cartilage than in superficial cartilage.
Figure 4b:
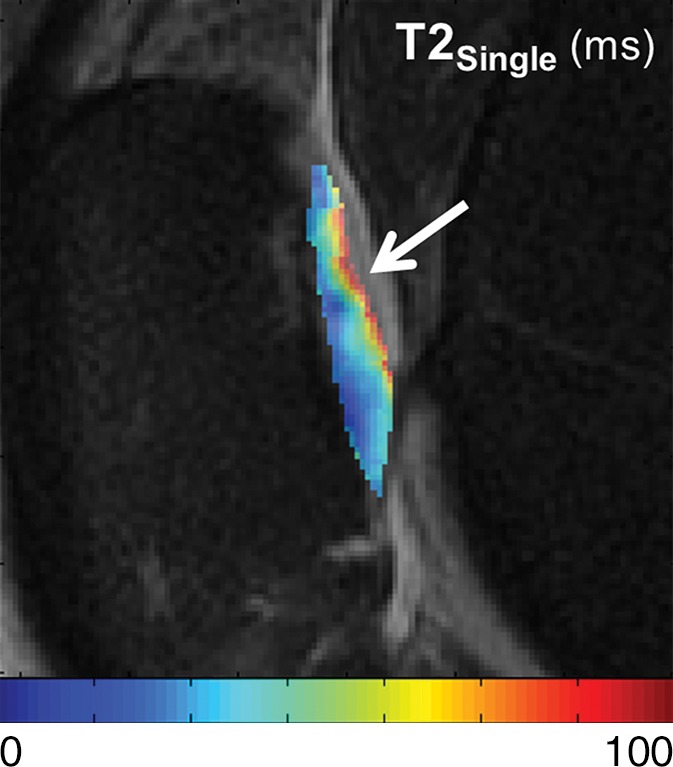
T2Single, T2F, T2S, and FF maps show articular cartilage of knee joint in a 49-year-old man with Kellgren-Lawrence grade 2 osteoarthritis of the knee. (a) 3D FSE image shows focal area of superficial partial-thickness cartilage loss on patella (arrow). Corresponding (b) T2Single, (c) T2F, (d) T2S, and (e) FF maps show increased T2Single, T2F, and T2S, and decreased FF in patellar cartilage (arrow) at the site of superficial cartilage lesion. Note that changes in FF are more pronounced and extend deeper into cartilage than other single-component and multicomponent T2 parameters.
Figure 4c:
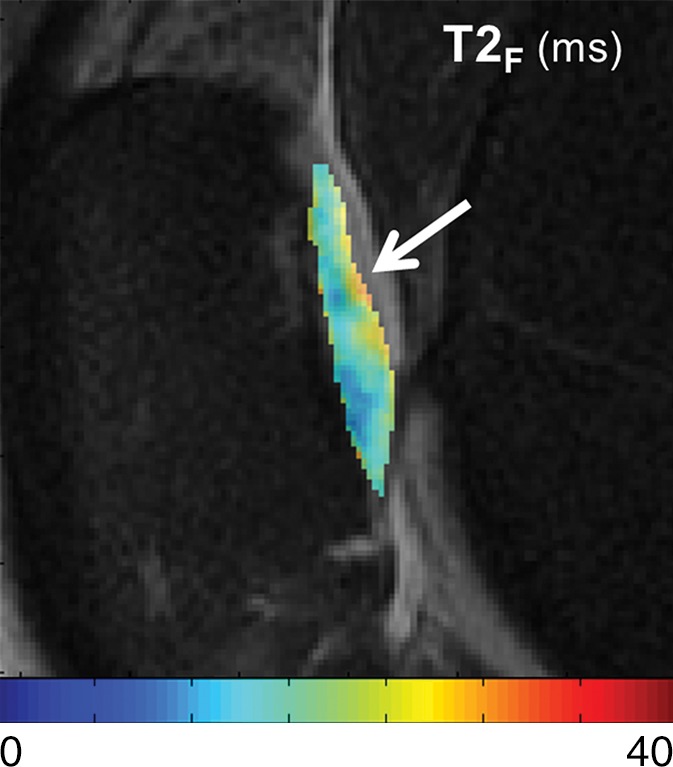
T2Single, T2F, T2S, and FF maps show articular cartilage of knee joint in a 49-year-old man with Kellgren-Lawrence grade 2 osteoarthritis of the knee. (a) 3D FSE image shows focal area of superficial partial-thickness cartilage loss on patella (arrow). Corresponding (b) T2Single, (c) T2F, (d) T2S, and (e) FF maps show increased T2Single, T2F, and T2S, and decreased FF in patellar cartilage (arrow) at the site of superficial cartilage lesion. Note that changes in FF are more pronounced and extend deeper into cartilage than other single-component and multicomponent T2 parameters.
Figure 4d:

T2Single, T2F, T2S, and FF maps show articular cartilage of knee joint in a 49-year-old man with Kellgren-Lawrence grade 2 osteoarthritis of the knee. (a) 3D FSE image shows focal area of superficial partial-thickness cartilage loss on patella (arrow). Corresponding (b) T2Single, (c) T2F, (d) T2S, and (e) FF maps show increased T2Single, T2F, and T2S, and decreased FF in patellar cartilage (arrow) at the site of superficial cartilage lesion. Note that changes in FF are more pronounced and extend deeper into cartilage than other single-component and multicomponent T2 parameters.
Figure 4e:

T2Single, T2F, T2S, and FF maps show articular cartilage of knee joint in a 49-year-old man with Kellgren-Lawrence grade 2 osteoarthritis of the knee. (a) 3D FSE image shows focal area of superficial partial-thickness cartilage loss on patella (arrow). Corresponding (b) T2Single, (c) T2F, (d) T2S, and (e) FF maps show increased T2Single, T2F, and T2S, and decreased FF in patellar cartilage (arrow) at the site of superficial cartilage lesion. Note that changes in FF are more pronounced and extend deeper into cartilage than other single-component and multicomponent T2 parameters.
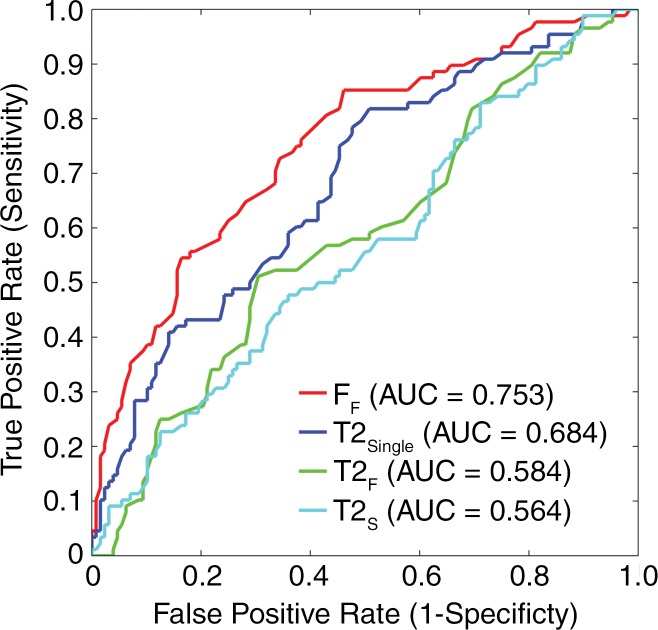
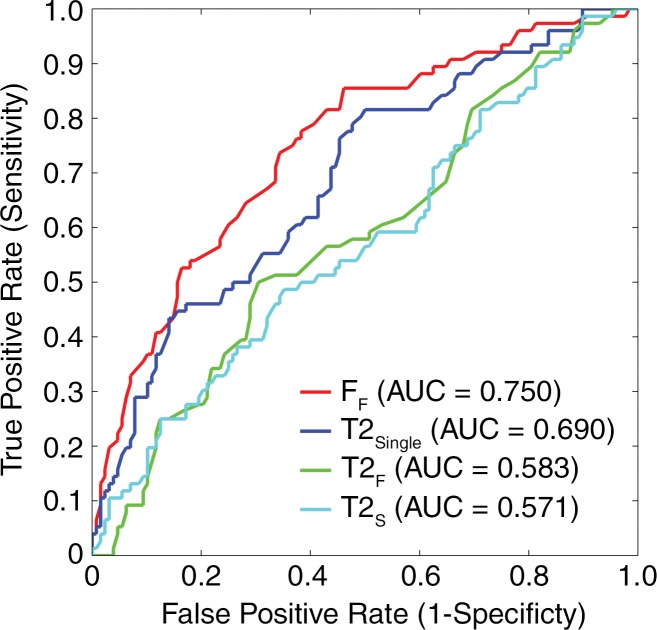
AUC values for FF, T2Single, T2F, and T2S were 0.753 (95% confidence interval [CI]: 0.690, 0.809), 0.684 (95% CI: 0.618, 0.745), 0.584 (95% CI: 0.515, 0.651), and 0.564 (95% CI: 0.495, 0.631), respectively, for distinguishing between morphologically normal cartilage and cartilage with morphologic degeneration and 0.750 (95% CI: 0.689, 0.801), 0.690 (95% CI: 0.631, 0.748), 0.583 (95% CI: 0.512, 0.652), and 0.571 (95% CI: 0.500, 0.640), respectively, for distinguishing between morphologically normal cartilage and cartilage with mild morphologic degeneration (Fig 5). The use of FF values showed higher (P < .05) AUC values than did the use of T2Single, T2F, and T2S values for distinguishing between normal cartilage and cartilage with degeneration and between normal cartilage and cartilage with mild degeneration. T2Single values showed higher (P < .05) AUCs than did T2F and T2S values for distinguishing between normal cartilage and cartilage with degeneration and between normal cartilage and cartilage with mild degeneration. However, there was no significant difference in AUC values between T2F and T2S for distinguishing between normal cartilage and cartilage with degeneration (P = .227) and between normal cartilage and cartilage with mild degeneration (P = .489).
Figure 5a:
Receiver operating characteristic curves with AUCs showing diagnostic performance with average T2Single, T2F, T2S, and FF values in each cartilage subsection to differentiate between (a) morphologically normal cartilage and cartilage with morphologic degeneration and between (b) morphologically normal cartilage and cartilage with mild morphologic degeneration. FF showed significantly higher (P < .05) AUC values than did other single-component and multicomponent T2 parameters, indicating greater diagnostic performance.
Figure 5b:
Receiver operating characteristic curves with AUCs showing diagnostic performance with average T2Single, T2F, T2S, and FF values in each cartilage subsection to differentiate between (a) morphologically normal cartilage and cartilage with morphologic degeneration and between (b) morphologically normal cartilage and cartilage with mild morphologic degeneration. FF showed significantly higher (P < .05) AUC values than did other single-component and multicomponent T2 parameters, indicating greater diagnostic performance.
Discussion
The results of our study demonstrated differences in both single-component and multicomponent T2 parameters of the articular cartilage of the knee joint between volunteers and patients with osteoarthritis of the knee. Cartilage T2Single was significantly higher in patients with osteoarthritis of the knee than in volunteers in our study, which is similar to the findings of previous studies in which the Carr-Purcell-Meiboom-Gill techniques were used to measure T2Single (8–12). Increased T2Single of degenerative cartilage is thought to be due to multiple factors including increased hydration (13), decreased macromolecular content (14–16), and changes in tissue anisotropy due to disruption of the highly organized collagen fiber network (17–19). Cartilage FF was also significantly lower in patients than volunteers in our study. Previous multicomponent T2-mapping studies (24–26) in which authors used nuclear MR spectroscopy have shown that FF is strongly correlated with the proteoglycan content of cartilage. Thus, the decreased FF in patients in our study may be the result of proteoglycan loss in degenerative cartilage that occurs during the early stages of osteoarthritis and gradually worsens as the disease progresses (3,4,42).
Although mcDESPOT and the Carr-Purcell-Meiboom-Gill techniques used in previous nuclear MR spectroscopic studies involve the use of different pulse sequences and reconstruction algorithms to measure multicomponent T2 parameters, there is evidence to suggest that FF measured by using mcDESPOT also may reflect the proteoglycan content of cartilage. The T2F values of cartilage measured by using mcDESPOT in our study are similar to values measured by using Carr-Purcell-Meiboom-Gill techniques in previous nuclear MR imaging studies (24–26). In addition, the depth-dependent decrease in FF from the bone-cartilage interface to the articular surface in our study correlates well with the depth-dependent decrease in the proteoglycan content of cartilage (43–46). Furthermore, proteoglycan loss due to trypsin degradation of ex vivo bovine cartilage specimens has been shown to decrease FF significantly in superficial cartilage measured by using mcDESPOT (47). However, additional studies are needed to allow direct correlation of FF measured by using mcDESPOT with the proteoglycan content of cartilage to determine whether the MR parameter can serve as a sensitive and specific biomarker of proteoglycan in cartilage.
Our study results suggest that FF may be more sensitive than T2Single for detection of cartilage degeneration in patients with osteoarthritis of the knee and may allow greater diagnostic performance for distinguishing morphologically normal cartilage from morphologically degenerative cartilage at receiver operating characteristic analysis. The improved diagnostic performance of FF for detection of cartilage degeneration may be due to the fact that FF is primarily influenced by the proteoglycan content of cartilage, while T2Single is a nonspecific parameter influenced by multiple potentially competing biologic changes that occur during cartilage degeneration. For example, changes in degenerative cartilage such as a reduction in macromolecular content (14–16) and loss of tissue anisotropy due to disruption of the highly organized collagen fiber network (17–19) would result in an increase in T2Single. However, this increase in T2Single may be offset partially by simultaneous changes that decrease T2Single such as collagen denaturation, which creates additional sites of interaction between water and collagen molecules (48). Although results of previous studies have shown that T2Single can allow detection of cartilage degeneration in both ex vivo specimens (7) and human subjects (8–12), our results suggest that FF allows greater diagnostic performance for distinguishing normal from degenerative cartilage. FF measured by using mcDESPOT has the additional advantage of being relatively uninfluenced by the magic angle effect, which leads to spurious increases in T2Single when cartilage is oriented at 55° relative to the main magnetic field (49).
In our study, we found that cartilage T2F and T2S values were higher in patients with osteoarthritis of the knee than in volunteers, but the differences between the groups of subjects were not statistically significant. Authors of previous nuclear MR spectroscopic studies (24–26) have reported significant increases in T2F and T2S after trypsin degeneration of ex vivo cartilage specimens. The increase in T2F and T2S may be due to that fact that water tightly and loosely bound to degraded proteoglycan in degenerative cartilage would likely have higher T2 than water bound to the intact macromolecule. The absence of significant differences in cartilage T2F and T2S between volunteers and patients in our study may be due to inadequate statistical power. However, our results clearly indicate that the FF measured by using mcDESPOT has greater sensitivity for detection of cartilage degeneration than does the T2 of each individual water component. The T2 of cartilage is a composite measure of the T2 values and fractions of the individual water components of cartilage, which are independent characteristics of the tissue. The limited diagnostic performance of T2F and T2S for distinguishing normal from degenerative cartilage may be responsible for the decreased diagnostic performance with T2Single compared with that with FF.
Studies to investigate how cartilage degeneration in human subjects leads to changes in the fractions and T2 values of the different water components of cartilage have not been performed previously because of the long imaging times of Carr-Purcell-Meiboom-Gill techniques currently used for multicomponent T2 mapping. Multicomponent T2*-mapping techniques have been used to assess the fast and slow-relaxing water components of human articular cartilage (50,51). In a previous study (50), authors investigated multicomponent T2* parameters of human cadaveric patellar cartilage specimens and showed an increased FF in degenerative cartilage, which the authors attributed to greater binding between water and degraded collagen fibers. However, multicomponent T2*-mapping techniques use extremely short echo times which can detect signal from the extremely fast-relaxing water tightly bound to the collagen component of cartilage. Furthermore, multicomponent T2* parameters of cartilage are influenced by magnetic field inhomogeneity and inherent differences in tissue susceptibility that may change with varying degrees of degeneration and may not reflect the true T2 characteristics of the different water components of cartilage. Thus, multicomponent T2- and T2*-mapping techniques likely provide uniquely different but perhaps complementary information regarding the composition and ultrastructure of articular cartilage.
Our study had several limitations. One limitation was the relatively small number of subjects, which may have been responsible for the failure to find statistically significant differences in cartilage T2F and T2S between volunteers and patients. In addition, all patients in our study were much older than the volunteers and had well-established Kellgren-Lawrence 2 or advanced Kellgren-Lawrence 3 grades of the disease. The presence of definitive osteophytes is the radiographic hallmark of osteoarthritis (36,37). We felt that it was important that the initial validation of mcDESPOT for detection of differences between volunteers and patients should include volunteers without senescence-related changes in cartilage composition and ultrastructure and patients whose osteoarthritis was diagnosed by using standard clinical and radiographic criteria. Another limitation of our study was the use of the relatively insensitive reference standard of morphologic MR imaging results for determining the presence or absence of cartilage degeneration in the knee joint. Additional studies with surgical or histologic correlation are needed to determine whether multicomponent T2 parameters measured by using mcDESPOT can allow detection of cartilage degeneration during the earliest stages of the disease process. Furthermore, the repeatability of cartilage multicomponent T2 measurements obtained by using mcDESPOT was assessed only in volunteers and not in patients. Another limitation of our study was that global regions of interest within cartilage were used to compare multicomponent T2 parameters between volunteers and patients. Because of the depth-dependent variations of multicomponent T2 parameters, comparison of MR parameters in the superficial and deep layers of cartilage may have provided higher discriminatory power between groups of subjects. However, dividing the cartilage into superficial and deep layers would have been extremely difficult and the results would have been prone to partial volume artifacts, given the 0.6-mm in-plane spatial resolution of the multicomponent T2 maps, especially in the evaluation of the thin articular cartilage in patients. A final limitation was relatively long imaging time of the mcDESPOT sequence, which may limit its use in the clinical setting. However, we are currently investigating various methods to reduce the imaging time of the mcDESPOT sequence, including use of a smaller number of optimized spoiled gradient-echo and balanced steady-state free-precession sequences, more rapid methods for estimating the B0 and B1 fields, and use of parallel imaging.
In conclusion, our study results have shown that patients with osteoarthritis of the knee have significantly higher cartilage T2Single and significantly lower cartilage FF values than do asymptomatic volunteers. Our results suggest that FF measured by using mcDESPOT may be more sensitive than T2Single for detection of cartilage degeneration in patients with osteoarthritis of the knee and may allow greater diagnostic performance for distinguishing between morphologically normal cartilage and morphologically degenerative cartilage at receiver operating characteristic analysis. The mcDESPOT sequence may provide an improved quantitative MR imaging technique for evaluating articular cartilage at 3.0 T. However, further studies are needed to better understand the mechanisms responsible for changes in multicomponent T2 parameters in patients with osteoarthritis and to investigate the ability of mcDESPOT to allow detection of cartilage degeneration in clinical practice and to monitor changes in cartilage composition and ultrastructure in osteoarthritis research studies.
Advances in Knowledge
■ Single-component T2 and the fraction of the fast-relaxing water component were significantly different (P < .05) between asymptomatic volunteers and patients with osteoarthritis of the knee.
■ Use of the fraction of the fast-relaxing water component value showed significantly higher (P < .05) diagnostic performance than did use of single-component T2 for distinguishing morphologically normal cartilage from morphologically degenerative cartilage in the knee joint.
Implication for Patient Care
■ The fraction of the fast-relaxing water component measured at 3.0 T by using multicomponent-driven equilibrium single-shot observation of T1 and T2 may allow greater diagnostic performance than does single-component T2 for detection of cartilage degeneration in the human knee joint.
APPENDIX
SUPPLEMENTAL FIGURES
Received September 14, 2014; revision requested October 22; revision received January 15, 2015; accepted February 5; final version accepted February 19.
Funding: This research was supported by the National Institutes of Health (grant R01NS065034).
Disclosures of Conflicts of Interest: F.L. disclosed no relevant relationships. K.W.C. disclosed no relevant relationships. A.S. disclosed no relevant relationships. R.G.S. disclosed no relevant relationships. J.J.W. disclosed no relevant relationships. W.F.B. Activities related to the present article: nonfinancial support from GE Healthcare. Activities not related to the present article: ownership interest in inseRT MRI. Other relationships: disclosed no relevant relationships. R.K. disclosed no relevant relationships.
Abbreviations:
- AUC
- area under the curve
- BLOKS
- Boston-Leeds osteoarthritis knee scoring
- CI
- confidence interval
- FF
- fraction of the fast-relaxing water component
- FSE
- fast spin echo
- mcDESPOT
- multicomponent driven equilibrium single-shot observation of T1 and T2
- T2F
- T2 of the fast-relaxing water component
- T2Single
- single-component T2
- T2s
- T2 of the slow-relaxing water component
- 3D
- three-dimensional
References
- 1.Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am 2004;42(1):1–9, v. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum 1998;41(8):1343–1355. [DOI] [PubMed] [Google Scholar]
- 3.Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Ann Rheum Dis 1977;36(2):121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Squires GR, Okouneff S, Ionescu M, Poole AR. The pathobiology of focal lesion development in aging human articular cartilage and molecular matrix changes characteristic of osteoarthritis. Arthritis Rheum 2003;48(5):1261–1270. [DOI] [PubMed] [Google Scholar]
- 5.Billinghurst RC, Dahlberg L, Ionescu M, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest 1997;99(7):1534–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold GE, Burstein D, Dardzinski B, Lang P, Boada F, Mosher T. MRI of articular cartilage in OA: novel pulse sequences and compositional/functional markers. Osteoarthritis Cartilage 2006;14(Suppl A):A76–A86. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Cheng J, Lin K, et al. Quantitative MRI using T1ρ and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging 2011;29(3):324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology 2004;232(2):592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apprich S, Welsch GH, Mamisch TC, et al. Detection of degenerative cartilage disease: comparison of high-resolution morphological MR and quantitative T2 mapping at 3.0 Tesla. Osteoarthritis Cartilage 2010;18(9):1211–1217. [DOI] [PubMed] [Google Scholar]
- 10.Koff MF, Amrami KK, Kaufman KR. Clinical evaluation of T2 values of patellar cartilage in patients with osteoarthritis. Osteoarthritis Cartilage 2007;15(2):198–204. [DOI] [PubMed] [Google Scholar]
- 11.Stahl R, Blumenkrantz G, Carballido-Gamio J, et al. MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthritis Cartilage 2007;15(11):1225–1234. [DOI] [PubMed] [Google Scholar]
- 12.Kijowski R, Blankenbaker DG, Munoz Del Rio A, Baer GS, Graf BK. Evaluation of the articular cartilage of the knee joint: value of adding a T2 mapping sequence to a routine MR imaging protocol. Radiology 2013;267(2):503–513. [DOI] [PubMed] [Google Scholar]
- 13.Liess C, Lüsse S, Karger N, Heller M, Glüer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage 2002;10(12):907–913. [DOI] [PubMed] [Google Scholar]
- 14.Nishioka H, Hirose J, Nakamura E, et al. T1ρ and T2 mapping reveal the in vivo extracellular matrix of articular cartilage. J Magn Reson Imaging 2012;35(1):147–155. [DOI] [PubMed] [Google Scholar]
- 15.Watrin-Pinzano A, Ruaud JP, Olivier P, et al. Effect of proteoglycan depletion on T2 mapping in rat patellar cartilage. Radiology 2005;234(1):162–170. [DOI] [PubMed] [Google Scholar]
- 16.Wong CS, Yan CH, Gong NJ, Li T, Chan Q, Chu YC. Imaging biomarker with T1ρ and T2 mappings in osteoarthritis - in vivo human articular cartilage study. Eur J Radiol 2013;82(4):647–650. [DOI] [PubMed] [Google Scholar]
- 17.Goodwin DW, Wadghiri YZ, Zhu H, Vinton CJ, Smith ED, Dunn JF. Macroscopic structure of articular cartilage of the tibial plateau: influence of a characteristic matrix architecture on MRI appearance. AJR Am J Roentgenol 2004;182(2):311–318. [DOI] [PubMed] [Google Scholar]
- 18.Nieminen MT, Rieppo J, Töyräs J, et al. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med 2001;46(3):487–493. [DOI] [PubMed] [Google Scholar]
- 19.Xia Y, Moody JB, Burton-Wurster N, Lust G. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis Cartilage 2001;9(5):393–406. [DOI] [PubMed] [Google Scholar]
- 20.Apprich S, Mamisch TC, Welsch GH, et al. Quantitative T2 mapping of the patella at 3.0T is sensitive to early cartilage degeneration, but also to loading of the knee. Eur J Radiol 2012;81(4):e438–e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosher TJ, Liu Y, Torok CM. Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthritis Cartilage 2010;18(3):358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosher TJ, Smith HE, Collins C, et al. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology 2005;234(1):245–249. [DOI] [PubMed] [Google Scholar]
- 23.Xia Y. Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol 2000;35(10):602–621. [DOI] [PubMed] [Google Scholar]
- 24.Reiter DA, Lin PC, Fishbein KW, Spencer RG. Multicomponent T2 relaxation analysis in cartilage. Magn Reson Med 2009;61(4):803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiter DA, Roque RA, Lin PC, et al. Mapping proteoglycan-bound water in cartilage: Improved specificity of matrix assessment using multiexponential transverse relaxation analysis. Magn Reson Med 2011;65(2):377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiter DA, Roque RA, Lin PC, Doty SB, Pleshko N, Spencer RG. Improved specificity of cartilage matrix evaluation using multiexponential transverse relaxation analysis applied to pathomimetically degraded cartilage. NMR Biomed 2011;24(10):1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang N, Xia Y. Dependencies of multi-component T2 and T1ρ relaxation on the anisotropy of collagen fibrils in bovine nasal cartilage. J Magn Reson 2011;212(1):124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng S, Xia Y. On the measurement of multi-component T2 relaxation in cartilage by MR spectroscopy and imaging. Magn Reson Imaging 2010;28(4):537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deoni SC, Rutt BK, Arun T, Pierpaoli C, Jones DK. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med 2008;60(6):1372–1387. [DOI] [PubMed] [Google Scholar]
- 30.Deoni SC, Rutt BK, Jones DK. Investigating the effect of exchange and multicomponent T(1) relaxation on the short repetition time spoiled steady-state signal and the DESPOT1 T(1) quantification method. J Magn Reson Imaging 2007;25(3):570–578. [DOI] [PubMed] [Google Scholar]
- 31.Deoni SC, Peters TM, Rutt BK. High-resolution T1 and T2 mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. Magn Reson Med 2005;53(1):237–241. [DOI] [PubMed] [Google Scholar]
- 32.Deoni SC. High-resolution T1 mapping of the brain at 3T with driven equilibrium single pulse observation of T1 with high-speed incorporation of RF field inhomogeneities (DESPOT1-HIFI). J Magn Reson Imaging 2007;26(4):1106–1111. [DOI] [PubMed] [Google Scholar]
- 33.Deoni SC. Transverse relaxation time (T2) mapping in the brain with off-resonance correction using phase-cycled steady-state free precession imaging. J Magn Reson Imaging 2009;30(2):411–417. [DOI] [PubMed] [Google Scholar]
- 34.Deoni SC. Correction of main and transmit magnetic field (B0 and B1) inhomogeneity effects in multicomponent-driven equilibrium single-pulse observation of T1 and T2. Magn Reson Med 2011;65(4):1021–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu F, Chaudhary R, Hurley SA, et al. Rapid multicomponent T2 analysis of the articular cartilage of the human knee joint at 3.0T. J Magn Reson Imaging 2014;39(5):1191–1197. [DOI] [PubMed] [Google Scholar]
- 36.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29(8):1039–1049. [DOI] [PubMed] [Google Scholar]
- 37.Felson DT, McAlindon TE, Anderson JJ, et al. Defining radiographic osteoarthritis for the whole knee. Osteoarthritis Cartilage 1997;5(4):241–250. [DOI] [PubMed] [Google Scholar]
- 38.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score). Ann Rheum Dis 2008;67(2):206–211. [DOI] [PubMed] [Google Scholar]
- 40.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6(2):65–70. [Google Scholar]
- 41.Parker CB, DeLong ER. ROC methodology within a monitoring framework. Stat Med 2003;22(22):3473–3488. [DOI] [PubMed] [Google Scholar]
- 42.Rizkalla G, Reiner A, Bogoch E, Poole AR. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis. Evidence for molecular heterogeneity and extensive molecular changes in disease. J Clin Invest 1992;90(6):2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia Y, Zheng S, Bidthanapally A. Depth-dependent profiles of glycosaminoglycans in articular cartilage by microMRI and histochemistry. J Magn Reson Imaging 2008;28(1):151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nieminen MT, Rieppo J, Silvennoinen J, et al. Spatial assessment of articular cartilage proteoglycans with Gd-DTPA-enhanced T1 imaging. Magn Reson Med 2002;48(4):640–648. [DOI] [PubMed] [Google Scholar]
- 45.Kurkijärvi JE, Nissi MJ, Kiviranta I, Jurvelin JS, Nieminen MT. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and T2 characteristics of human knee articular cartilage: topographical variation and relationships to mechanical properties. Magn Reson Med 2004;52(1):41–46. [DOI] [PubMed] [Google Scholar]
- 46.Froimson MI, Ratcliffe A, Gardner TR, Mow VC. Differences in patellofemoral joint cartilage material properties and their significance to the etiology of cartilage surface fibrillation. Osteoarthritis Cartilage 1997;5(6):377–386. [DOI] [PubMed] [Google Scholar]
- 47.Liu F, Chaudhary R, Hurley S, Samsonov A, Block W, Kijowski R. Multi-component T2 analysis of cartilage degradation model using mcDESPOT at 3.0T [abstr]. In: Proceedings of the Twenty-First Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2013; 1621. [Google Scholar]
- 48.Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med 2004;51(3):503–509. [DOI] [PubMed] [Google Scholar]
- 49.Chaudhary R, Liu F, Kaiser J, Block W, Kijowski R. Sensitivity of multi-component driven equilibrium single pulse observation of T1 and T2 (mcDESPOT) to the magic angle effect [abstr]. In: Proceedings of the Twenty-Second Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2014; 1697. [Google Scholar]
- 50.Pauli C, Bae WC, Lee M, et al. Ultrashort-echo time MR imaging of the patella with bicomponent analysis: correlation with histopathologic and polarized light microscopic findings. Radiology 2012;264(2):484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qian Y, Williams AA, Chu CR, Boada FE. Multicomponent T2* mapping of knee cartilage: technical feasibility ex vivo. Magn Reson Med 2010;64(5):1426–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.