βPIX (PAK1-interacting exchange factor beta, encoded by ARHGEF7) and PAK1 (p21 activated kinase) are interacting proteins that form a signaling module that mediates a number of cellular activities pertinent to cell migration (Kim et al., 2001; Koh et al., 2001; Webb et al., 2002). The interaction of βPIX with GIT1 provides a signaling anchor through a GIT1-paxillin interaction; this signaling anchor serves to sequester the complex to adhesions and to the leading edge of migrating cells (Brown et al., 2002; Manabe et al., 2002; West et al., 2001). This complex plays a pivotal role in adhesion turnover and lamellipodial protrusion during cell migration (Nayal et al., 2006; Webb et al., 2006). It is thought that the sequestration of PAK1 and βPIX to adhesions places the two proteins in proximity to membrane-anchored RAC1/CDC42, such that βPIX can activate RAC1/CDC42, and activated RAC1/CDC42 can activate PAK1 (Rosenberger and Kutsche, 2006; ten Klooster et al., 2006). Recent studies have also linked PAK1 to centrosomes, in which it is anchored by the GIT1-βPIX complex, induces Aurora A phosphorylation, and leads to a crucial check point in mitosis (Zhao et al., 2005). Although much is known about the functions of these proteins, the phosphorylations that regulate them have not been addressed in a systematic manner.
βPIX is a member of the DBL (diffuse B-cell lymphoma) family of Rho-GEFs (guanine nucleotide exchange factors) for RAC1/CDC42. The gene encoding βPIX, ARHGEF7, located on chromosome 13, has six known splice variants. They are: βPIX-a, βPIX-b, βPIX-bL, βPIX-c, βPIX-d, and p50 (Kim and Park, 2001). The primary 85 kD transcript, βPIX-a, is ubiquitously expressed and was first identified as a component of adhesions and a direct binding partner of PAK1 (Bagrodia et al., 1998; Koh et al., 2001; Manser et al., 1998). The other splice variants are primarily expressed in the CNS, in which they function in early-stage brain development (Kim et al., 2000; Kim and Park, 2001). βPIX is also regulated by post-splicing translation dynamics. For example, βPIX-bL has an internal ribosome entry site (IRES) within the 5′UTR (Rhee et al., 2004).
βPIX-a shares 62% similarity with another protein of the βPIX subgroup, αPIX (encoded by ARHGEF6), which is located on the X chromosome. Major differences between βPIXa and αPIX isoforms include the presence of an N-terminal calponin homology (CH) domain in αPIX. However, recent reports suggest that the βPIX-bL translation variant does contain an N-terminal CH domain (Rhee et al., 2004).
The function of βPIX is highly dependent on its binding interactions and on the regulation of key domains. The C-terminal coiled-coil domain is implicated in both βPIX dimerization and its translocation to microvillus-like structures and membrane ruffles (Koh et al., 2001). Additionally, βPIX activation of p38 MAP kinase is necessary for p38 translocation to the nucleus and for the formation of βPIX-dependent membrane ruffles (Lee et al., 2005; Lee et al., 2001). βPIX is also required for focal-complex formation and cell migration via its role in regulating FAK and p38 MAP kinase activation (Lee et al., 2005). Recently, the proline-rich region in the COOH terminus of RAC1 was shown to interact with the SH3 domain of βPIX and thereby target RAC1 to membrane ruffles and adhesions. In brief, at the leading edge of migrating cells, integrin ligation induces βPIX activation of CDC42, which activates PAK1. Following PAK1 activation, βPIX disassociates and binds RAC1, resulting in localized actin polymerization. In addition, RAC1 and PAK1 compete for binding to βPIX, and PAK1 controls the RAC1-βPIX interaction and adhesion-induced RAC1 activation (ten Klooster et al., 2006).
Structural studies of the βPIX-PAK1 interaction have revealed that, upon PAK1 activation, there is a sixfold decrease in binding affinity (Mott et al., 2005). Other studies suggest that the kinase activity of PAK1 is also regulated by its interaction with different βPIX isoforms: αPIX greatly enhances PAK1 activity, βPIX has permissive effects and the p50 βPIX demonstrates an inhibitory effect on PAK1 activity. This modulation of βPIX-dependent PAK1 activity is negatively regulated via an 18-amino acid T1 motif present in βPIX, but not in αPIX (Feng et al., 2002). The domain structure of mouse βPIX-a includes an N-terminal SH3 domain responsible for its interaction with PAK1 (aa 8-60), the DBL homology (DH) domain (aa 100-279) containing the Rho GEF activity, a plextrin homology (PH) domain (aa 295-400), a PxxP proline-rich region that encompasses the T1 motif (aa 408-493), the GIT1-binding domain (aa 496-555), and the coiled-coil domain (aa 587-634).
The diverse and highly regulated functions of βPIX indicate the importance of phosphorylation in its regulation. Some important sites have already been identified. In PC12 cells, bFGF and NGF induce phosphorylation of βPIX on S525 and T526; this phosphorylation results in activation of RAC1 (Shin et al., 2002). Moreover, inhibition of ERK or PAK2 prevents this βPIX-phosphorylation-dependent RAC1 activation (Shin et al., 2002; Shin et al., 2004). In human mesangial cells, endothelin-1 or cAMP activators lead to a PKA-dependent phosphorylation of βPIX at residues S516 and T526, resulting in βPIX translocation to adhesions and activation of CDC42 (Chahdi et al., 2005). A recent study demonstrates that FAK can phosphorylate βPIX and thereby enhance βPIX binding to RAC1 and the translocation of RAC1 to adhesions (Chang et al., 2007).
The phosphorylation of βPIX is also implicated in tumor progression. βPIX is phosphorylated via an SRC/FAK-dependent signal from the EGF receptor, and this phosphorylation activates the GEF activity of βPIX, which leads to activation of CDC42, to complex formation with the E3 ligase CBL and to the subsequent suppression of EGF degradation. This EGF-dependent phosphorylation of βPIX is required for viral SRC (v-SRC) transformation, aberrant cell growth and tumor formation in mice (Feng et al., 2006). βPIX, like its interacting partner PAK1, is upregulated in some breast cancer tissues, and promotes tumor growth in MCF-7 breast cancer cells by directly binding to the SH3 domain of PLCγ and enhancing phospholipase activity downstream of the PDGF receptor signal (Ahn et al., 2003; Bae et al., 2005).
PAK1, p21-activated kinase, is a serine/threonine kinase involved in cellular activities such as cytoskeletal dynamics, cell migration, neurogenesis, angiogenesis, mitosis, apoptosis and transformation (Jakobi et al., 2001; Kiosses et al., 2002; Manabe et al., 2002; Sells et al., 1997; Tang et al., 1997; Zhao et al., 2005). PAK1 was originally identified in a Rho GTPase screen, in which PAK1, PAK2 and PAK3 (68, 65 and 62 kD, respectively) were found to bind to GTP-bound forms of RAC1 and CDC42 (Manser et al., 1995; Manser et al., 1994; Teo et al., 1995). To date, six different isoforms have been discovered (Jaffer and Chernoff, 2002). Activation of PAK1 is initiated by the high-affinity binding of the small GTPases, RAC1-3 or CDC42, to the p21-binding domain (PBD/CRIB) (Knaus et al., 1998; Manser et al., 1994). Additionally, other Rho family GTPases, such as CHP (RHOV), TC10 (RHOQ) and WRCH-1 (RHOU), also activate PAK1 (Aronheim et al., 1998; Neudauer et al., 1998; Tao et al., 2001).
Structural data show that the PAK1 ‘off state’ is a trans-inhibited homodimer, in which the C-terminal catalytic domain of one kinase is auto-inhibited by the high-affinity binding (Kd=90 nM) of the N-terminal regulatory domain of the other kinase (Lei et al., 2000). A consequence of the anti-parallel dimerization is that the subunits overlap the PBD/CRIB domain and the inhibitory switch, preventing PAK1 activation without RAC1/CDC42 binding. Upon activation, GTPase binding liberates the trans-inhibition and frees the kinase to autophosphorylate T423 and S144 (Parrini et al., 2002; Pirruccello et al., 2006). In open conformation, prior to modification of the activation loop, PAK1 can phosphorylate substrates and thus resides in an ‘intermediate-active’ state (Lei et al., 2000; Lei et al., 2005). In the ‘active state’ conformation, activation-loop trans-phosphorylation is crucial for maintaining the full catalytic activity of PAK1 (Zenke et al., 1999).
Upon growth factor stimulation (e.g. PDGF), PAK1 translocates to the leading edge of actin-rich protrusions and membrane ruffles, with a concomitant increase in directed cell motility (Dharmawardhane et al., 1997; Sells et al., 1999; Sells et al., 1997; Zenke et al., 1999). In addition, PAK1 activity also mediates the formation of actin microspikes and the loss of large adhesions and stress fibers in an RAC1/CDC42-dependent manner (Manser et al., 1997). PAK1 is also involved in neurogenesis, neuronal guidance and non-syndromic mental retardation. A brain-specific mutation of R67C in PAK3, a residue that is crucial for GTPase binding and PAK1 activation, causes this congenital abnormality (Allen et al., 1998; Bienvenu et al., 2000; Boda et al., 2004).
PAK1 interacts directly with an assortment of proteins with differing functions. These proteins include the kinases [e.g. AKT (AKT1), PDK1, PI3K, CDK5, CDC2, SRC, ABL, PKA (PRKACA)] and the adapter proteins [e.g. NCK (NCK1), GRB2, βPIX and NESH (ABI3)] (Peri et al., 2003). NCK binding to PAK1 plays an important role in angiogenesis by coupling bFGF-induced angiogenic signals to the MAP kinase pathway (Hood et al., 2003). The PAK1-NCK interaction is linked to the TEK (TIE-2) angiopoietin receptor and to endothelial cell motility (Master et al., 2001). Moreover, in breast cancer epithelial cells, PAK1 activation by heregulin-β1 upregulates VEGF and the subsequent angiogenic response (Bagheri-Yarmand et al., 2000).
PAK1 is implicated as a key regulator of cell cycle G1 progression through PAK1-dependent upregulation of cyclin D1 (Nheu et al., 2004; Thullberg et al., 2006). Moreover, PAK1 also protects cells against intrinsic apoptotic signals. The current model suggests that PAK1 activity leads to a decrease in apoptosis by phosphorylation of BAD on S112 and S136, leading to disassociation of the BCL2-BAD inhibitory complex and to BAD–14-3-3 binding (Schurmann et al., 2000; Tang et al., 2000). Recently, PAK1 phosphorylation of RAF1 on S338/S339 has been linked to RAF1 translocation to the mitochondria, BAD phosphorylation, RAF1-BCL2 binding and inhibitory complex disassociation (Jin et al., 2005). Additionally, adhesion-dependent PAK1 phosphorylation of MEK1 on S298 is necessary for active MAPK signaling-complex formation (Slack-Davis et al., 2003).
Finally, PAK1 is implicated in tumor progression. PAK1 is overexpressed in breast, colon, ovarian, bladder, pancreas and T-cell lymphoma. Overexpression of PAK1 in cancer cells has been connected to enhanced invasive potential, increased cell motility, drug resistance, angiogenesis and anti-apoptotic cell survival (Kumar et al., 2006). Dominant-negative PAK1 inhibits Ras-induced transformation, whereas active PAK1 induces anchorage-independent growth in breast epithelial cells (Tang et al., 1997; Vadlamudi et al., 2000).
PAK1 mediates these diverse phenomena through its many substrates. One interesting substrate is the estrogen receptor (ER), which PAK1 phosphorylates on S305 (Wang et al., 2002). This phosphorylation is believed to be involved in breast tumor growth and tamoxifen resistance (Rayala et al., 2006a; Rayala et al., 2006b). The repertoire of PAK1 substrates include key regulators in cytoskeletal signaling pathways, such as RAF1, MEK1 (MAP2K1), MLCK (MYLK), MLC (MLC1), LIM kinase (LIMK2), GEF-H1 (ARHGEF2), Rho GDI (ARHGDIA), prolactin, paxillin, GIT1, cortactin, vimentin and filamin A (Bokoch, 2003; Nayal et al., 2006; Webb et al., 2006).
Several kinases have been reported to regulate PAK1. For example, AKT phosphorylates the PAK1 autophosphorylation site, S21, leading to NCK disassociation and increased PAK1 activity (Zhou et al., 2003). SRC kinases phosphorylate PAK1 on Y131 following CDC42/RAC1 activation (Renkema et al., 2002). CDK5, ERK2 (MAPK1) and CDC2A all phosphorylate T212 on PAK1 and regulate microtubule dynamics, adhesion signaling and post-mitotic spreading of fibroblasts, respectively (Banerjee et al., 2002; Sundberg-Smith et al., 2005; Thiel et al., 2002). The tyrosine kinase ABL activates PAK1 by phosphorylation (Roig et al., 2000); ETK (BMX) of the TEC family also phosphorylates PAK1 (Bagheri-Yarmand et al., 2001). In vitro studies have identified seven autophosphorylation sites for PAK1. They are: S21, S57, S144, S149, S199, S204 and T423 (Chong et al., 2001). The primary autophosphorylation sites that control enzymatic activity of PAK1 are S144 and T423, whereas S21, S57, S149, S199 and S204 autophosphorylation sites interfere with the interaction of SH3-PxxP binding proteins such as NCK and βPIX (Chong et al., 2001; Zenke et al., 1999). Phosphorylation of T423 by PDK1 is thought to be required for effective PAK1 activation in vivo (King et al., 2000; Zenke et al., 1999).
The domain structure of PAK1 includes five N-terminal canonical PxxP SH3-binding motifs and one nonclassical SH3-binding motif. The first PxxP site is responsible for NCK binding (aa 13-21); the second site binds the adaptor protein, GRB2 (aa ~42-50); and the noncanonical PxP site is responsible for βPIX binding (aa 182-203). The p21-binding domain (PBD) is the primary high-affinity binding site for RAC1 and CDC42 (aa 67-113), and encompasses the CRIB (CDC42 and RAC1 interactive binding) domain, which minimally binds RAC1 and CDC42 (aa 75-90). Overlapping this p21-binding domain is the autoinhibitory region (aa 83-149) that regulates activity of the kinase domain (aa 249-529). The remaining C-terminus (aa 530-545) of PAK1 contains a conserved heterodimeric G protein complex binding site for the Gβ subunit (Leeuw et al., 1998).
In this study, we used mass spectrometry to generate a map of βPIX and PAK1 phosphorylation sites. HEK-293 cells were transiently transfected with either FLAG-βPIX or FLAG-PAK1 for 48 hours at low expression levels in the presence of 10% serum. To minimize phosphatase activity, the cells were incubated for 30 minutes with peroxovanadate and calyculin A prior to lysis; phosphatase inhibitor cocktails were present during lysis and immunopurification. To optimize coverage, immunoprecipitates were digested with either trypsin (βPIX) or a trypsin/chymotrypsin combination (PAK1) and analyzed via C18 column chromatography or enriched for phosphorylation-specific sequences with IMAC chromatography (Schroeder et al., 2005) by nanoflow HPLC interfaced to microcapillary ESI mass spectrometry. For βPIX, we analyzed peptides corresponding to 91% of the protein and detected 16 phosphorylation sites. We analyzed peptides on PAK1 corresponding to 83% of the protein and detected 13 total phosphorylation sites, including nine novel phosphorylation sites.
A summary of the βPIX analysis is shown in Fig. 1. Interestingly, no phosphorylation sites were detected in the SH3 domain, which is responsible for protein binding to the PAK1-PxP binding domain. However, two sites, S71 and S79, were present in the N-terminal spacer region (aa 61-99), between the SH3 and DH domains. Six phosphorylated serines (S131, S135, S145, S146, S192 and S195) reside in the DH/Rho-GEF domain. These phosphorylation sites are positioned to regulate the GEF activity of βPIX. We identified one phosphorylation site, S340, in the PH domain, and the ambiguous site S427/S428 in the PxxP proline-rich region. Phosphorylation sites S498, S516 and Y542 reside within the GIT1-binding domain. Our analyses confirm the phosphorylation previously described at S516; but we did not observe the previously reported phosphorylation on S525 or T526 (Chahdi et al., 2005; Shin et al., 2002; Shin et al., 2004). These sites either might not be phosphorylated under our conditions, or might be present at levels that we cannot detect or on peptides that we did not observe. The single tyrosine phosphorylation site that we identified, Y542, could serve as a FAK substrate and might play an important role in GIT1 binding and βPIX translocation (Chang et al., 2007; Feng et al., 2006). The C-terminal coiled-coil domain contains three identified phosphorylation sites: S559, S588 and T592. These are likely to be involved in βPIX dimerization and translocation to membrane ruffles. Sites S340, T592 and the ambiguous site S427/S428 were identified in EGF-treated cells but not in serum-cultured cells.
Fig. 1.
Phosphorylation sites detected in serum-only and EGF-stimulated murine βPIXa. (A) Ser, Thr and Tyr coverage of the FLAG-βPIXa sequence (tag not shown) generated with trypsin. Detected peptides are bold and underlined. Residues not covered are shaded in gray. Observed phosphorylation sites are red. Red brackets above residues indicate that a phosphorylation site could not be unambiguously assigned to the specific amino acid. In total, 91% of the amino acid sequence and 89% of the Ser, Thr and Tyr sites were covered. A total of 16 phosphorylation sites were identified. (B) Mapping of domains and identified phosphorylation sites. MS/MS-identified phosphorylation sites are labeled. Sites labeled with red text were detected only with the treatment of EGF to cells. Sites within red brackets specify ambiguity between two sites.
The mouse βPIXa protein shares 90% identity with the rat species and 86% homology with the human species. Table 1 shows that all phosphorylated βPIXa residues that we identified are conserved in the rat, whereas S145 and S195 are not present in the human sequence. βPIXa shares 62% identity with αPIX; 10 of the 16 detected phosphorylation sites are conserved between the two isoforms (Table 1). The conserved S71 might be important for SH3 and/or GEF function, S192 might regulate the RhoGEF domain and S340 might regulate the PH domain. The other conserved sites between α/β isoforms reside in the PxxP domain, GIT1-binding domain and the coiled-coil domains.
Table 1.
Phosphorylation sites of murine βPIXa detected by mass spectrometry
| Site | Detected in serum-incubated sample | Detected in EGF-stimulated sample | Homologs/other family members | Putative kinases |
|---|---|---|---|---|
| S71 | + | + | R, M, H/αPIX | CDC2, CDK5 |
| S79 | Not covered | + | R, M, H | CDC2, CDK5 |
| S131 | + | + | R, M, H | |
| S135 | + | R, M, H | ||
| S145 | + | R, M | ||
| S146 | + | R, M, H | ||
| S192 | + | + | R, M, H/αPIX | |
| S195 | + | + | R, M | |
| S340 | + | R, M, H/αPIX | ||
| S427/S428 | + | R, M, H/αPIX (S428 only) | ||
| S498 | IMAC | Not covered | R, M, H/αPIX | CDC2, CDK5 |
| S516 | + | + | R, M, H/αPIX | PKA |
| Y542 | IMAC | R, M, H/αPIX | ||
| S559 | IMAC | IMAC | R, M, H/αPIX | PKA, AKT, 14-3-3 |
| S588 | Not covered | + | R, M, H/αPIX | PKCmu |
| T592 | + | R, M, H/αPIX |
Serum-incubated and EGF-stimulated samples were both treated with calyculin A and peroxovanadate to inhibit phosphatases. Phosphorylation sites labeled ‘IMAC’ were only detected after sample enrichment by immobilized metal affinity chromatography (IMAC). Sites detected without enrichment are denoted with a ‘+’. ‘Not covered’ signifies that the residue was not observed during mass spectrometry analysis, therefore phosphorylation status could not be determined. Homologous phosphorylation sites were determined via sequence comparison analysis using FASTA (http://fasta.bioch.virginia.edu/) (Pearson, 1990). Consensus phosphorylation motifs for putative kinases were analyzed using the Scansite scoring algorithm (http://scansite.mit.edu) (Obenauer et al., 2003). For additional data regarding βPIXa phosphorylation, see the Cell Migration Consortium website: http://www.cellmigration.org. R, rat; M, mouse; H, human.
The identified βPIXa phosphorylation sites in this study were compared to known consensus kinase phos phorylation motifs (Table 1). S516 is a consensus phosphorylation site for PKA; this has been confirmed by biochemical determinations (Chahdi et al., 2005). Some of the other sites also match predicted consensus phos phorylation motifs for known kinases. For example, S71, S79 and S498 are predicted phosphorylation sites for cyclin-dependent kinase 1 and 5 [CDK1 (CDC2A) and CDK5, respectively). Interestingly, PKA and AKT are predicted to phosphorylate S559, which serves as a binding site for 14-3-3 – a mediator of GEF activity (Tzivion et al., 2006; Zenke et al., 2004). Considering the interdependence of PAK1 and βPIX binding on activity, the S559 docking site for 14-3-3 in βPIX could serve as another regulator of its GEF activity. Protein kinase C mu (PKCμ) is the predicted kinase for S588 phosphorylation.
A summary of our PAK1 analysis is shown in Fig. 2. We detected nine phosphorylation sites in the regulatory N-terminus of PAK1, including S2, S21 and S57 in the NCK/GRB2 binding region. The close proximity of S57 to the GRB2 PxxP binding site suggests a role in GRB2 binding, similar to NCK disassociation by S21 phosphorylation (Zhou et al., 2003). Interestingly, we did not detect phosphorylation sites in the CRIB/PBD domain. However, we identified two sites, Y131 and Y142, within the autoinhibitory domain that partially overlaps the CRIB/PBD domain. Y131 is a SRC phosphorylation site in the autoinhibitory domain that regulates the kinase activity of PAK1 (Renkema et al., 2002). Y142 phosphorylation might also regulate kinase activity via modulation of the inhibitory domain. Several sites are phosphorylated between the inhibitory and kinase domains. They include: Y153, the ambiguous site S155 or S156, S174, T185 and T212. It is possible that phosphorylation of T185 in the βPIX-binding domain modulates βPIX binding. The C-terminal kinase domain contains three phosphorylation sites – Y285, Y429 and Y464 – that could regulate catalytic function. Some autophosphorylation sites known to be important in PAK1 function, such as S144 and T423, were not identified in this study. One autophosphorylation site, S199, was not covered in our analysis, and another, S204, resides near a protease cleavage site and might have altered its accessibility. S144 and S149 autophosphorylation sites could be affected by their neighboring phospho-tyrosines Y142 and Y153. Recently, a comparison study between three common phospho-enrichment assays revealed differences in their sets of isolated phospho-peptides, pointing to differing efficiencies of retention using various enrichment methods (Bodenmiller et al., 2007). Despite these limitations, important known sites (e.g. S21 and S57) along with many novel sites were observed.
Fig. 2.
Phosphorylation sites detected in human PAK1. (A) Ser, Thr and Tyr coverage of the FLAG-PAK1 sequence (tag not shown) generated with trypsin/chymotrypsin. Detected peptides are bold and underlined. Residues not covered are shaded in gray. Observed phosphorylation sites are red. In total, 83% of the amino acid sequence was covered. Thirteen phosphorylation sites were identified. (B) Mapping of domains and identified phosphorylation sites. MS/MS-identified phosphorylation sites are labeled. N, G and P denote the NCK-, GRB2- and βPIX-binding sites, respectively; AI, auto-inhibitory domain. Red brackets indicate the ambiguous site.
Human PAK1 shares 98% and 99% identity with mouse and rat PAK1, respectively. Table 2 shows that all of the residues phosphorylated in human PAK1 are conserved in the mouse and rat sequences. PAK1 shares 77% identity with PAK2 and 81% with PAK3. Seven of the 13 identified residues that are phosphorylated in PAK1 are also present in PAK2 and PAK3 (Table 2). They include S2, and the autophosphorylation sites S21 and S57; Y131 and Y142 of the autoinhibitory region; and Y429 and Y464 of the kinase domain.
Table 2.
Phosphorylation sites of human PAK1 detected by mass spectrometry
| Site | Detected without enrichment (+) or with IMAC | Homologs/other family members | Putative (roman) and known (italics) kinases |
|---|---|---|---|
| S2 | +/IMAC | R, M, H/PAK2, PAK3 | |
| S21* | IMAC | R, M, H/PAK2, PAK3 | AUTO/AKT |
| S57* | IMAC | R, M, H/PAK2, PAK3 | AUTO |
| Y131* | +/IMAC | R, M, H/PAK2, PAK3 | SRC |
| Y142 | +/IMAC | R, M, H/PAK2, PAK3 | PDGF-R |
| Y153 | +/IMAC | R, M, H/PAK2, PAK3 | |
| S155/S156 | +/IMAC | R, M, H/(S155) PAK 2 only | |
| S174 | + | R, M, H/PAK 3 | Casein kinase 1 |
| T185 | + | R, M, H/PAK 2 | ERK1/casein kinase 1 |
| T212* | +/IMAC | R, M, H | ERK1, CDK5, CDC2 |
| Y285 | + | R, M, H/PAK 3 | PY-SH2 motif |
| Y429 | +/IMAC | R, M, H/PAK2, PAK3 | |
| Y464 | IMAC | R, M, H/PAK2, PAK3 | EGF-R/PY-SH2 motif |
Serum-stimulated samples were treated with peroxovanadate and calyculin A to inhibit phosphatases. Numbered phosphorylation sites marked with (*) represents previously confirmed phosphorylation. Phosphorylation sites labeled ‘IMAC’ were only detected after sample enrichment by immobilized metal affinity chromatography. Sites detected without enrichment (C18) are denoted with a ‘+’. Homologous phosphorylation sites among species (R, rat; M, mouse; H, human) and PAK1 isoforms 1-3 were determined via sequence-comparison analysis using FASTA (http://fasta.bioch.virginia.edu/) (Pearson, 1990). Consensus phosphorylation motifs for putative kinases were analyzed using the Scansite scoring algorithm (http://scansite.mit.edu) (Obenauer et al., 2003). For additional data regarding PAK1 phosphorylation, see the Cell Migration Consortium web site: http://www.cellmigration.org
The PAK1 phosphorylation sites identified in this study were compared to known consensus kinase phosphorylation motifs (Table 2). The conserved Y142 site in the autoinhibitory domain is a predicted target for the PDGF receptor. Casein kinase is the predicted kinase for S174 and T185; ERK1 shows preference to sites T185 and T212; and Y464 is the predicted target for the EGF receptor. In addition, Y285 and Y464 are SH2-binding motifs similar to those in LCK and GRB2.
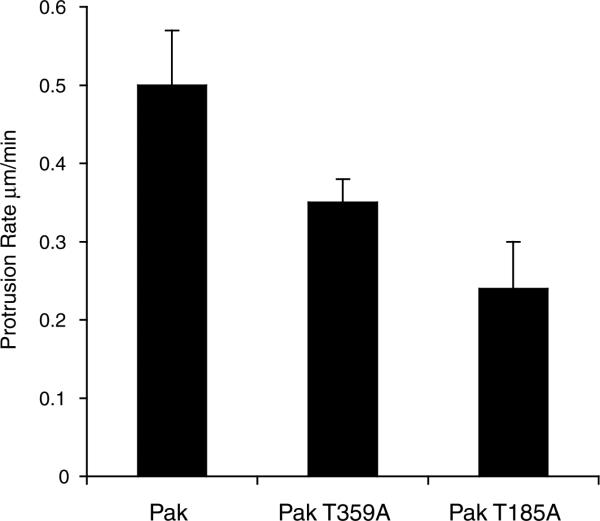
Two sites, T359 and T185, appear likely to be important for βPIX function. We tested this by expressing mutant proteins that cannot be phosphorylated on these sites and assaying for protrusion, which is a βPIX/PAK1-mediated function. Threonine 359 resides in the kinase domain of PAK1. The regulation of its phosphorylation might directly affect the kinase activity of PAK1. The PAK1 T359A mutation was generated and a GFP fusion protein was transfected into CHO-K1 cells. Protrusion rates were then determined using kymography. Cells containing the T359A mutation showed a 30% reduction in the rate of protrusion when compared to wild-type PAK1 (Fig. 3). Threonine 185 resides in the βPIX-binding domain of PAK1. It might alter βPIX binding or affect the structure of the nearby auto-inhibitory domain, resulting in altered PAK1 kinase activity. The non-phosphorylatable PAK1 T185A mutation was generated and a GFP fusion protein was transfected into CHO-K1 cells. Cells containing the T359A mutation showed a 50% reduction in protrusion rates when compared to wild-type PAK1 (Fig. 3). These preliminary observations confirm the importance of these phosphorylations.
Fig. 3.
Effect of PAK1 T359 and T185 on protrusion. GFP-PAK1 mutants were expressed in CHO cells and protrusion rates determined by kymography (Nayal et al., 2006). Rates are in μm/minute. Rates were determined using at least ten cells per mutant.
In summary, we have identified a number of potentially interesting phosphorylation sites in βPIX and PAK1. Since βPIX and PAK1 are important binding partners, these sites should provide a useful starting point for investigating the function and regulation of βPIX and PAK1 during migration and other cellular processes.
Materials and Methods
Sample preparation
HEK cells were transfected with FLAG-βPIX or FLAG-PAK1 (100 ng per 100 mm plate; five plates) using lipofectamine. After 48 hours of culture in 10% FBS, cells were incubated with fresh 10% FBS, 1 mM peroxovanadate and 5 nM calyculin A for 30 minutes, and then harvested (25 mM Tris-HCl, 200 mM NaCl, 0.5% NP-40, pH 7.4). The lysates were pre-cleared with mouse IgG-agarose for 1 hour at 4°C and immunoprecipitated with 100 μl FLAG-agarose (Sigma) for 1 hour at 4°C. Samples were washed (4×) with 25 mM Tris-HCl, 200 mM NaCl, pH 7.4, and FLAG-tagged PAK1 was eluted by incubating the beads with 0.2 mg/ml FLAG peptide in 25 mM Tris-HCl for 20 minutes at 4°C.
Sample analysis
FLAG-βPIXa immunoprecipitates (IP), one from EGF-stimulated cells and one from serum-only cells, were reduced and alkylated with DTT/iodoacetamide in 100 mM ammonium bicarbonate buffer. Samples were digested with 50 ng trypsin and a 2.5% aliquot of the resulting peptides was analyzed by a combination of nanoflow HPLC (C18 column) interfaced to ESI tandem mass spectrometry (LTQ-FTMS, Thermo Electron, San Jose, CA). To obtain better quality MS/MS spectra of highly charged and phosphopeptides, an additional aliquot (6%) of the sample was analyzed by nanoflow HPLC interfaced to electron transfer dissociation (ETD) mass spectrometry (Coon et al., 2005; Syka et al., 2004). A third aliquot (50%) of the sample was desalted by loading it onto a fused-silica, microcapillary HPLC column (360×100 μm) packed with 5 cm of 5-20 μm C18 packing material and washing it with 0.1 M acetic acid. The sample was gradient eluted to an Eppendorf tube, solvent was removed on a speed-vac, and the resulting dried peptides were converted to their corresponding methyl esters, enriched for phosphopeptides by IMAC (Ficarro et al., 2002) and then analyzed by nanoflow HPLC interfaced with ESI on a modified LTQ instrument equipped for both CAD and ETD (Coon et al., 2005; Syka et al., 2004).
FLAG-PAK1 immunoprecipitates from 10% FBS conditioned cells were reduced and alkylated with DTT/iodoacetamide in 100 mM ammonium bicarbonate buffer. Samples were subsequently digested with 500 ng trypsin and 500 ng chymotrypsin overnight at room temperature. An aliquot of the resulting peptides was separated by C18 RP-HPLC coupled with nano-electrospray tandem mass spectrometry (LTQ-FTMS instrument, Thermo Electron, San Jose, CA). To enrich for phosphopeptides, a 50% aliquot of the samples was desalted by loading onto a 360×75 μm capillary column packed with 5 cm of R3 packing material. This column was then rinsed with 0.1% acetic acid and the peptides were eluted with 70% acetonitrile into Eppendorf tubes. The peptides were dried via speed-vac and then subjected to esterification with methanol followed by enrichment of phosphopeptides via IMAC (Ficarro et al., 2002). In brief, the phosphopeptides were captured on an IMAC column (360×150 μm) at low pressure (1-2 μl/minute) and then eluted from the IMAC column directly to a pre-column (360×75 μm), which was then butt-connected to an analytical column (360×50 μm) and analyzed by nanoflow (50-100 nl/minute) HPLC-MS/MS using a 1-hour gradient. The instrument was operated in the ‘top ten’ mode, in which the top ten most-intense peaks detected in the FT were selected for CID fragmentation in the LTQ. These ten peaks were then placed on an exclusion list for 30 seconds, enabling greater dynamic range of sample detection. The mass spectra were searched using SEQUEST and TPP-tools (Keller et al., 2005; Nesvizhskii et al., 2003), with manual verification of all phosphopeptides.
Acknowledgments
We thank Donna Webb for her help in the initial phase of these studies and for her comments on the manuscript; Jonathan Chernoff for the PAK1 construct and the helpful comments; Li Ma and Andrea Bradfield for technical support with the PAK1 study; and Sean S. Cheon for help with mutant PAK1 plasmids. This work was funded by the Cell Migration consortium NIH U54 GM064346, GM37537 (D.F.H.) and by the Cancer Training grant to the University of Virginia (S.J.P.).
References
- Ahn SJ, Chung KW, Lee RA, Park IA, Lee SH, Park DE, Noh DY. Overexpression of betaPix-a in human breast cancer tissues. Cancer Lett. 2003;193:99–107. doi: 10.1016/s0304-3835(03)00004-1. [DOI] [PubMed] [Google Scholar]
- Allen KM, Gleeson JG, Bagrodia S, Partington MW, MacMillan JC, Cerione RA, Mulley JC, Walsh CA. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat. Genet. 1998;20:25–30. doi: 10.1038/1675. [DOI] [PubMed] [Google Scholar]
- Aronheim A, Broder YC, Cohen A, Fritsch A, Belisle B, Abo A. Chp, a homologue of the GTPase Cdc42Hs, activates the JNK pathway and is implicated in reorganizing the actin cytoskeleton. Curr. Biol. 1998;8:1125–1128. doi: 10.1016/s0960-9822(98)70468-3. [DOI] [PubMed] [Google Scholar]
- Bae JY, Ahn SJ, Lee JE, Kim JE, Han MR, Han W, Kim SW, Shin HJ, Lee SJ, Park D, et al. BetaPix-a enhances the activity of phospholipase Cgamma1 by binding SH3 domain in breast cancer. J. Cell. Biochem. 2005;94:1010–1016. doi: 10.1002/jcb.20357. [DOI] [PubMed] [Google Scholar]
- Bagheri-Yarmand R, Vadlamudi RK, Wang RA, Mendelsohn J, Kumar R. Vascular endothelial growth factor up-regulation via p21-activated kinase-1 signaling regulates heregulin-beta1-mediated angiogenesis. J. Biol. Chem. 2000;275:39451–39457. doi: 10.1074/jbc.M006150200. [DOI] [PubMed] [Google Scholar]
- Bagheri-Yarmand R, Mandal M, Taludker AH, Wang RA, Vadlamudi RK, Kung HJ, Kumar R. Etk/Bmx tyrosine kinase activates Pak1 and regulates tumorigenicity of breast cancer cells. J. Biol. Chem. 2001;276:29403–29409. doi: 10.1074/jbc.M103129200. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Jordon KA, Van Aelst L, Cerione RA. A novel regulator of p21-activated kinases. J. Biol. Chem. 1998;273:23633–23636. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- Banerjee M, Worth D, Prowse DM, Nikolic M. Pak1 phosphorylation on t212 affects microtubules in cells undergoing mitosis. Curr. Biol. 2002;12:1233–1239. doi: 10.1016/s0960-9822(02)00956-9. [DOI] [PubMed] [Google Scholar]
- Bienvenu T, des Portes V, McDonell N, Carrie A, Zemni R, Couvert P, Ropers HH, Moraine C, van Bokhoven H, Fryns JP, et al. Missense mutation in PAK3, R67C, causes X-linked nonspecific mental retardation. Am. J. Med. Genet. 2000;93:294–298. doi: 10.1002/1096-8628(20000814)93:4<294::aid-ajmg8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Boda B, Alberi S, Nikonenko I, Node-Langlois R, Jourdain P, Moosmayer M, Parisi-Jourdain L, Muller D. The mental retardation protein PAK3 contributes to synapse formation and plasticity in hippocampus. J. Neurosci. 2004;24:10816–10825. doi: 10.1523/JNEUROSCI.2931-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenmiller B, Mueller LN, Mueller M, Domon B, Aebersold R. Reproducible isolation of distinct, overlapping segments of the phosphoproteome. Nat. Methods. 2007;4:231–237. doi: 10.1038/nmeth1005. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-activated kinases. Annu. Rev. Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Brown MC, West KA, Turner CE. Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol. Biol. Cell. 2002;13:1550–1565. doi: 10.1091/mbc.02-02-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahdi A, Miller B, Sorokin A. Endothelin 1 induces beta 1Pix translocation and Cdc42 activation via protein kinase A-dependent pathway. J. Biol. Chem. 2005;280:578–584. doi: 10.1074/jbc.M411130200. [DOI] [PubMed] [Google Scholar]
- Chang F, Lemmon CA, Park D, Romer LH. FAK potentiates Rac1 activation and localization to matrix adhesion sites: a role for {beta}PIX. Mol. Biol. Cell. 2007;18:253–264. doi: 10.1091/mbc.E06-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong C, Tan L, Lim L, Manser E. The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J. Biol. Chem. 2001;276:17347–17353. doi: 10.1074/jbc.M009316200. [DOI] [PubMed] [Google Scholar]
- Coon JJ, Ueberheide B, Syka JE, Dryhurst DD, Ausio J, Shabanowitz J, Hunt DF. Protein identification using sequential ion/ion reactions and tandem mass spectrometry. Proc. Natl. Acad. Sci. USA. 2005;102:9463–9468. doi: 10.1073/pnas.0503189102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmawardhane S, Sanders LC, Martin SS, Daniels RH, Bokoch GM. Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J. Cell Biol. 1997;138:1265–1278. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Albeck JG, Cerione RA, Yang W. Regulation of the Cool/Pix proteins: key binding partners of the Cdc42/Rac targets, the p21-activated kinases. J. Biol. Chem. 2002;277:5644–5650. doi: 10.1074/jbc.M107704200. [DOI] [PubMed] [Google Scholar]
- Feng Q, Baird D, Peng X, Wang J, Ly T, Guan JL, Cerione RA. Cool-1 functions as an essential regulatory node for EGF receptor- and Src-mediated cell growth. Nat. Cell Biol. 2006;8:945–956. doi: 10.1038/ncb1453. [DOI] [PubMed] [Google Scholar]
- Ficarro SB, McCleland ML, Stukenberg PT, Burke DJ, Ross MM, Shabanowitz J, Hunt DF, White FM. Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat. Biotechnol. 2002;20:301–305. doi: 10.1038/nbt0302-301. [DOI] [PubMed] [Google Scholar]
- Hood JD, Frausto R, Kiosses WB, Schwartz MA, Cheresh DA. Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J. Cell Biol. 2003;162:933–943. doi: 10.1083/jcb.200304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffer ZM, Chernoff J. p21-activated kinases: three more join the Pak. Int. J. Biochem. Cell Biol. 2002;34:713–717. doi: 10.1016/s1357-2725(01)00158-3. [DOI] [PubMed] [Google Scholar]
- Jakobi R, Moertl E, Koeppel MA. p21-activated protein kinase gamma-PAK suppresses programmed cell death of BALB3T3 fibroblasts. J. Biol. Chem. 2001;276:16624–16634. doi: 10.1074/jbc.M007753200. [DOI] [PubMed] [Google Scholar]
- Jin S, Zhuo Y, Guo W, Field J. p21-activated Kinase 1 (Pak1)-dependent phosphorylation of Raf-1 regulates its mitochondrial localization, phosphorylation of BAD, and Bcl-2 association. J. Biol. Chem. 2005;280:24698–24705. doi: 10.1074/jbc.M413374200. [DOI] [PubMed] [Google Scholar]
- Keller A, Eng J, Zhang N, Li XJ, Aebersold R. A uniform proteomics MS/MS analysis platform utilizing open XML file formats. Mol. Syst. Biol. 2005;1 doi: 10.1038/msb4100024. PMID: 16729052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim T, Lee D, Park SH, Kim H, Park D. Molecular cloning of neuronally expressed mouse betaPix isoforms. Biochem. Biophys. Res. Commun. 2000;272:721–725. doi: 10.1006/bbrc.2000.2845. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee SH, Park D. Leucine zipper-mediated homodimerization of the p21-activated kinase-interacting factor, beta Pix. Implication for a role in cytoskeletal reorganization. J. Biol. Chem. 2001;276:10581–10584. doi: 10.1074/jbc.C000806200. [DOI] [PubMed] [Google Scholar]
- Kim T, Park D. Molecular cloning and characterization of a novel mouse betaPix isoform. Mol. Cells. 2001;11:89–94. [PubMed] [Google Scholar]
- King CC, Gardiner EM, Zenke FT, Bohl BP, Newton AC, Hemmings BA, Bokoch GM. p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1). J. Biol. Chem. 2000;275:41201–41209. doi: 10.1074/jbc.M006553200. [DOI] [PubMed] [Google Scholar]
- Kiosses WB, Hood J, Yang S, Gerritsen ME, Cheresh DA, Alderson N, Schwartz MA. A dominant-negative p65 PAK peptide inhibits angiogenesis. Circ. Res. 2002;90:697–702. doi: 10.1161/01.res.0000014227.76102.5d. [DOI] [PubMed] [Google Scholar]
- Knaus UG, Wang Y, Reilly AM, Warnock D, Jackson JH. Structural requirements for PAK activation by Rac GTPases. J. Biol. Chem. 1998;273:21512–21518. doi: 10.1074/jbc.273.34.21512. [DOI] [PubMed] [Google Scholar]
- Koh CG, Manser E, Zhao ZS, Ng CP, Lim L. Beta1PIX, the PAK-interacting exchange factor, requires localization via a coiled-coil region to promote microvillus-like structures and membrane ruffles. J. Cell Sci. 2001;114:4239–4251. doi: 10.1242/jcs.114.23.4239. [DOI] [PubMed] [Google Scholar]
- Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat. Rev. Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- Lee J, Jung ID, Chang WK, Park CG, Cho DY, Shin EY, Seo DW, Kim YK, Lee HW, Han JW, et al. p85 beta-PIX is required for cell motility through phosphorylations of focal adhesion kinase and p38 MAP kinase. Exp. Cell Res. 2005;307:315–328. doi: 10.1016/j.yexcr.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Lee SH, Eom M, Lee SJ, Kim S, Park HJ, Park D. BetaPix-enhanced p38 activation by Cdc42/Rac/PAK/MKK3/6-mediated pathway. Implication in the regulation of membrane ruffling. J. Biol. Chem. 2001;276:25066–25072. doi: 10.1074/jbc.M010892200. [DOI] [PubMed] [Google Scholar]
- Leeuw T, Wu C, Schrag JD, Whiteway M, Thomas DY, Leberer E. Interaction of a G-protein beta-subunit with a conserved sequence in Ste20/PAK family protein kinases. Nature. 1998;391:191–195. doi: 10.1038/34448. [DOI] [PubMed] [Google Scholar]
- Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, Harrison SC. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102:387–397. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- Lei M, Robinson MA, Harrison SC. The active conformation of the PAK1 kinase domain. Structure. 2005;13:769–778. doi: 10.1016/j.str.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Manabe R, Kovalenko M, Webb DJ, Horwitz AR. GIT1 functions in a motile, multi-molecular signaling complex that regulates protrusive activity and cell migration. J. Cell Sci. 2002;115:1497–1510. doi: 10.1242/jcs.115.7.1497. [DOI] [PubMed] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- Manser E, Chong C, Zhao ZS, Leung T, Michael G, Hall C, Lim L. Molecular cloning of a new member of the p21-Cdc42/Rac-activated kinase (PAK) family. J. Biol. Chem. 1995;270:25070–25078. doi: 10.1074/jbc.270.42.25070. [DOI] [PubMed] [Google Scholar]
- Manser E, Huang HY, Loo TH, Chen XQ, Dong JM, Leung T, Lim L. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol. Cell. Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- Master Z, Jones N, Tran J, Jones J, Kerbel RS, Dumont DJ. Dok-R plays a pivotal role in angiopoietin-1-dependent cell migration through recruitment and activation of Pak. EMBO J. 2001;20:5919–5928. doi: 10.1093/emboj/20.21.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott HR, Nietlispach D, Evetts KA, Owen D. Structural analysis of the SH3 domain of beta-PIX and its interaction with alpha-p21 activated kinase (PAK). Biochemistry. 2005;44:10977–10983. doi: 10.1021/bi050374a. [DOI] [PubMed] [Google Scholar]
- Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. Paxillin phosphorylation at Ser273 localizes a GIT1-PIXPAK complex and regulates adhesion and protrusion dynamics. J. Cell Biol. 2006;173:587–589. doi: 10.1083/jcb.200509075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- Neudauer CL, Joberty G, Tatsis N, Macara IG. Distinct cellular effects and interactions of the Rho-family GTPase TC10. Curr. Biol. 1998;8:1151–1160. doi: 10.1016/s0960-9822(07)00486-1. [DOI] [PubMed] [Google Scholar]
- Nheu T, He H, Hirokawa Y, Walker F, Wood J, Maruta H. PAK is essential for RAS-induced upregulation of cyclin D1 during the G1 to S transition. Cell Cycle. 2004;3:71–74. [PubMed] [Google Scholar]
- Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrini MC, Lei M, Harrison SC, Mayer BJ. Pak1 kinase homodimers are autoinhibited in trans and dissociated upon activation by Cdc42 and Rac1. Mol. Cell. 2002;9:73–83. doi: 10.1016/s1097-2765(01)00428-2. [DOI] [PubMed] [Google Scholar]
- Pearson WR. Rapid and sensitive sequence comparison with FASTP and FASTA. Meth. Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- Peri S, Navarro JD, Amanchy R, Kristiansen TZ, Jonnalagadda CK, Surendranath V, Niranjan V, Muthusamy B, Gandhi TK, Gronborg M, et al. Development of human protein reference database as an initial platform for approaching systems biology in humans. Genome Res. 2003;13:2363–2371. doi: 10.1101/gr.1680803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirruccello M, Sondermann H, Pelton JG, Pellicena P, Hoelz A, Chernoff J, Wemmer DE, Kuriyan J. A dimeric kinase assembly underlying autophosphorylation in the p21 activated kinases. J. Mol. Biol. 2006;361:312–326. doi: 10.1016/j.jmb.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Rayala SK, Molli PR, Kumar R. Nuclear p21-activated kinase 1 in breast cancer packs off tamoxifen sensitivity. Cancer Res. 2006a;66:5985–5988. doi: 10.1158/0008-5472.CAN-06-0978. [DOI] [PubMed] [Google Scholar]
- Rayala SK, Talukder AH, Balasenthil S, Tharakan R, Barnes CJ, Wang RA, Aldaz M, Khan S, Kumar R. P21-activated kinase 1 regulation of estrogen receptor-alpha activation involves serine 305 activation linked with serine 118 phosphorylation. Cancer Res. 2006b;66:1694–1701. doi: 10.1158/0008-5472.CAN-05-2922. [DOI] [PubMed] [Google Scholar]
- Renkema GH, Pulkkinen K, Saksela K. Cdc42/Rac1-mediated activation primes PAK2 for superactivation by tyrosine phosphorylation. Mol. Cell. Biol. 2002;22:6719–6725. doi: 10.1128/MCB.22.19.6719-6725.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S, Yang SJ, Lee SJ, Park D. betaPix-b(L), a novel isoform of betaPix, is generated by alternative translation. Biochem. Biophys. Res. Commun. 2004;318:415–421. doi: 10.1016/j.bbrc.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Roig J, Tuazon PT, Zipfel PA, Pendergast AM, Traugh JA. Functional interaction between c-Abl and the p21-activated protein kinase gamma-PAK. Proc. Natl. Acad. Sci. USA. 2000;97:14346–14351. doi: 10.1073/pnas.97.26.14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger G, Kutsche K. AlphaPIX and betaPIX and their role in focal adhesion formation. Eur. J. Cell Biol. 2006;85:265–274. doi: 10.1016/j.ejcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Schroeder MJ, Webb DJ, Shabanowitz J, Horwitz AF, Hunt DF. Methods for the detection of paxillin post-translational modifications and interacting proteins by mass spectrometry. J. Proteome Res. 2005;4:1832–1841. doi: 10.1021/pr0502020. [DOI] [PubMed] [Google Scholar]
- Schurmann A, Mooney AF, Sanders LC, Sells MA, Wang HG, Reed JC, Bokoch GM. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol. Cell. Biol. 2000;20:453–461. doi: 10.1128/mcb.20.2.453-461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- Sells MA, Boyd JT, Chernoff J. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J. Cell Biol. 1999;145:837–849. doi: 10.1083/jcb.145.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin EY, Shin KS, Lee CS, Woo KN, Quan SH, Soung NK, Kim YG, Cha CI, Kim SR, Park D, et al. Phosphorylation of p85 beta PIX, a Rac/Cdc42-specific guanine nucleotide exchange factor, via the Ras/ERK/PAK2 pathway is required for basic fibroblast growth factor-induced neurite outgrowth. J. Biol. Chem. 2002;277:44417–44430. doi: 10.1074/jbc.M203754200. [DOI] [PubMed] [Google Scholar]
- Shin EY, Woo KN, Lee CS, Koo SH, Kim YG, Kim WJ, Bae CD, Chang SI, Kim EG. Basic fibroblast growth factor stimulates activation of Rac1 through a p85 betaPIX phosphorylation-dependent pathway. J. Biol. Chem. 2004;279:1994–2004. doi: 10.1074/jbc.M307330200. [DOI] [PubMed] [Google Scholar]
- Slack-Davis JK, Eblen ST, Zecevic M, Boerner SA, Tarcsafalvi A, Diaz HB, Marshall MS, Weber MJ, Parsons JT, Catling AD. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J. Cell Biol. 2003;162:281–291. doi: 10.1083/jcb.200212141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg-Smith LJ, Doherty JT, Mack CP, Taylor JM. Adhesion stimulates direct PAK1/ERK2 association and leads to ERK-dependent PAK1 Thr212 phosphorylation. J. Biol. Chem. 2005;280:2055–2064. doi: 10.1074/jbc.M406013200. [DOI] [PubMed] [Google Scholar]
- Syka JE, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. USA. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Chen Z, Ambrose D, Liu J, Gibbs JB, Chernoff J, Field J. Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Mol. Cell. Biol. 1997;17:4454–4464. doi: 10.1128/mcb.17.8.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zhou H, Chen A, Pittman RN, Field J. The Akt proto-oncogene links Ras to Pak and cell survival signals. J. Biol. Chem. 2000;275:9106–9109. doi: 10.1074/jbc.275.13.9106. [DOI] [PubMed] [Google Scholar]
- Tao W, Pennica D, Xu L, Kalejta RF, Levine AJ. Wrch-1, a novel member of the Rho gene family that is regulated by Wnt-1. Genes Dev. 2001;15:1796–1807. doi: 10.1101/gad.894301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J. Cell Biol. 2006;172:759–769. doi: 10.1083/jcb.200509096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo M, Manser E, Lim L. Identification and molecular cloning of a p21cdc42/rac1-activated serine/threonine kinase that is rapidly activated by thrombin in platelets. J. Biol. Chem. 1995;270:26690–26697. doi: 10.1074/jbc.270.44.26690. [DOI] [PubMed] [Google Scholar]
- Thiel DA, Reeder MK, Pfaff A, Coleman TR, Sells MA, Chernoff J. Cell cycle-regulated phosphorylation of p21-activated kinase 1. Curr. Biol. 2002;12:1227–1232. doi: 10.1016/s0960-9822(02)00931-4. [DOI] [PubMed] [Google Scholar]
- Thullberg M, Gad A, Beeser A, Chernoff J, Stromblad S. The kinase-inhibitory domain of p21-activated kinase 1 (PAK1) inhibits cell cycle progression independent of PAK1 kinase activity. Oncogene. 2006;26:1820–1828. doi: 10.1038/sj.onc.1209983. [DOI] [PubMed] [Google Scholar]
- Tzivion G, Gupta VS, Kaplun L, Balan V. 14-3-3 proteins as potential oncogenes. Semin. Cancer Biol. 2006;16:203–213. doi: 10.1016/j.semcancer.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Vadlamudi RK, Adam L, Wang RA, Mandal M, Nguyen D, Sahin A, Chernoff J, Hung MC, Kumar R. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J. Biol. Chem. 2000;275:36238–36244. doi: 10.1074/jbc.M002138200. [DOI] [PubMed] [Google Scholar]
- Wang RA, Mazumdar A, Vadlamudi RK, Kumar R. P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium. EMBO J. 2002;21:5437–5447. doi: 10.1093/emboj/cdf543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells – over and over and over again. Nat. Cell Biol. 2002;4:E97–E100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Kovalenko M, Whitmore L, Horwitz AF. Phosphorylation of serine 709 in GIT1 regulates protrusive activity in cells. Biochem. Biophys. Res. Commun. 2006;346:1284–1288. doi: 10.1016/j.bbrc.2006.06.036. [DOI] [PubMed] [Google Scholar]
- West KA, Zhang H, Brown MC, Nikolopoulos SN, Riedy MC, Horwitz AF, Turner CE. The LD4 motif of paxillin regulates cell spreading and motility through an interaction with paxillin kinase linker (PKL). J. Cell Biol. 2001;154:161–176. doi: 10.1083/jcb.200101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke FT, King CC, Bohl BP, Bokoch GM. Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J. Biol. Chem. 1999;274:32565–32573. doi: 10.1074/jbc.274.46.32565. [DOI] [PubMed] [Google Scholar]
- Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J. Biol. Chem. 2004;279:18392–18400. doi: 10.1074/jbc.M400084200. [DOI] [PubMed] [Google Scholar]
- Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol. Cell. 2005;20:237–249. doi: 10.1016/j.molcel.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Zhou GL, Zhuo Y, King CC, Fryer BH, Bokoch GM, Field J. Akt phosphorylation of serine 21 on Pak1 modulates Nck binding and cell migration. Mol. Cell. Biol. 2003;23:8058–8069. doi: 10.1128/MCB.23.22.8058-8069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]