Abstract
Chloroplast protrusions (CPs) have frequently been observed in plants, but their significance to plant metabolism remains largely unknown. We investigated in the alpine plant Ranunculus glacialis L. treated under various CO2 concentrations if CP formation is related to photorespiration, specifically focusing on hydrogen peroxide (H2O2) metabolism. Immediately after exposure to different CO2 concentrations, the formation of CPs in leaf mesophyll cells was assessed and correlated to catalase (CAT) and ascorbate peroxidase (APX) activities. Under natural irradiation, the relative proportion of chloroplasts with protrusions (rCP) was highest (58.7 %) after exposure to low CO2 (38 ppm) and was lowest (3.0 %) at high CO2 (10,000 ppm). The same relationship was found for CAT activity, which decreased from 34.7 nkat mg−1 DW under low CO2 to 18.4 nkat mg−1 DW under high CO2, while APX activity did not change significantly. When exposed to natural CO2 concentration (380 ppm) in darkness, CP formation was significantly lower (18.2 %) compared to natural solar irradiation (41.3 %). In summary, CP formation and CAT activity are significantly increased under conditions that favour photorespiration, while in darkness or at high CO2 concentration under light, CP formation is significantly lower, providing evidence for an association between CPs and photorespiration.
Electronic supplementary material
The online version of this article (doi:10.1007/s00709-015-0778-5) contains supplementary material, which is available to authorized users.
Keywords: Ascorbate peroxidase, Hydrogen peroxide, Photorespiration, Stromules, Ultrastructure
Introduction
Extensions of different organelles, including protrusions and stromules, are a frequently observed phenomenon (Gray et al. 2001; Hanson and Sattarzadeh 2008, 2011; Mathur et al. 2012), but their physiological functions remain largely unknown. In chloroplasts, different forms of stroma-filled extensions of the plastid envelope were described more than a century ago (e.g. Senn 1908; Heitz 1937; reviewed by Gray et al. 2001), referred to as protuberances and later as proliferations (Lütz and Moser 1977; Lütz 1987), stromules (Köhler and Hanson 2000) or chloroplast protrusions (Buchner et al. 2007a,b; Holzinger et al. 2007a,b). Stromules were described as long and thin stroma-filled tubules (diameter 0.4–0.8 μm, length up to 65 μm; Gray et al. 2001), similar to beak-like chloroplast protrusions (CPs) of the chloroplast envelope (diameter 3–5 μm, length 3–5 μm; Holzinger et al. 2007a) but significantly narrower. Both stromules and CPs may form and withdraw rapidly. For comprehensive literature concerning stromule activity and dynamics, see Köhler and Hanson (2000), Kwok and Hanson (2003, 2004a,b) and Hanson and Sattarzadeh (2008).
Stromules have been suggested to be involved in the protein trafficking (Köhler et al. 1997; Gray et al. 2001), and the mechanisms are currently being investigated (see Hanson and Sattarzadeh 2011, 2013; Schattat et al. 2012, 2015). Stromules differ from CPs in shape, and therefore, possibly in function. Moser et al. (2015) demonstrated that under natural environmental conditions CP formation in leaves of Ranunculus glacialis follows a pronounced diurnal rhythm, and that CPs are most abundant in the afternoon and not related to temperature or irradiation stress. However, it has been suggested that CPs may contribute to the adaptation mechanisms of plants in extreme habitats such as in alpine and polar regions with short vegetation periods (Lütz and Engel 2007; Lütz 2010; Lütz et al. 2012).
Formation of CPs was shown to increase after acid mist treatment in Sitka spruce (Wulff et al. 1996) and in salt-stressed Mesembryanthemum crystallinum (Paramanova et al. 2004) and rice leaves, the latter of which contained crystalline inclusions within CPs alongside immunolabelled ribulose 1,5-bisphosphate carboxylase/oxigenase (rubisco) (Yamane et al. 2012). In early TEM studies on R. glacialis (Lütz 1987) and later in other high alpine and polar plant species (Gielwanowska and Szczuka 2005; Lütz et al. 2006; Holzinger et al. 2007b; Lütz and Engel 2007; Lütz 2010; Lütz et al. 2012), CPs were frequently found to be located in close spatial proximity to mitochondria and peroxisomes, suggesting a link between photorespiration and CP formation. However, quantitative evidence for this link is still missing. During photosynthesis, rubisco catalyses CO2 fixation; however, under increasing temperatures, rubisco increasingly reacts with O2 (photorespiration) and the resulting oxidation of ribulose-1,5-bisphosphate (RuBP) produces glycolate. This is broken down by glycolate oxidase in peroxisomes producing H2O2 which is detoxified by catalase (Mhamdi et al. 2012). Moser et al. (2015) found that CP formation was significantly reduced after exposure of R. glacialis to 2000 ppm CO2 and 2 % O2, suggesting that a restriction of photorespiration, as achieved under these conditions, is involved in the withdrawal of CPs.
We used R. glacialis as a model alpine species to analyse the suggested link between photorespiration and CP formation in more detail. To achieve this, we measured CP formation in leaves exposed to solar irradiation at varying CO2 concentrations to favour or restrict photorespiration, and assessed the activity of catalase (CAT).
Material and methods
Plant material and study site
R. glacialis is one of the highest ascending (>4000 m a.s.l.) seed plants in the European Alps. As a pioneer species it prefers scree and humid siliceous substrates in the sub-nival and nival zone and is also present in arctic and subarctic regions (Schönswetter et al. 2003). Individuals of R. glacialis were carefully excavated near the Timmelsjoch pass (2563 m a.s.l.; Ötztal Alps, Tyrol, 46° 54′ N/11° 09′ E; 18 July 2013), potted and left in their natural habitat for 3 weeks. The potted plants were transported to the ‘Alpine Garden Patscherkofel’ near Innsbruck (1950 m a.s.l.). For acclimation, the plants were partially shaded and carefully watered for 1 week until the experiments started on 15 August 2013.
Exposure to different CO2 concentrations
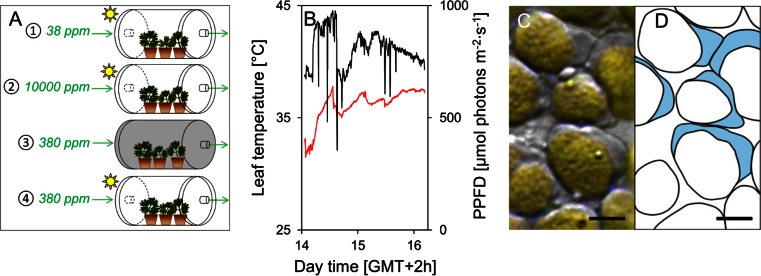
To determine the impact of different CO2 concentrations on CP formation under natural solar irradiation or darkness, four experimental conditions (ECs) were applied for 2.5 h. The CO2 concentrations in EC1 and EC2 were controlled to stimulate and prevent photorespiration, respectively. EC1 and EC2 comprised CO2 concentrations of 38 and 10,000 ppm, respectively, under natural solar irradiation. EC3 and EC4 comprised atmospheric CO2 concentrations (380 ppm) either kept in the dark using metal cylinders or under natural solar irradiation, respectively (Fig. 1a). Environmental conditions were maintained using highly transparent Plexiglas cylinders (200 × 350 mm, XT 29070, Röhm, Darmstadt, Germany; spectral transmittance: see Suppl. 1). Each cylinder contained five individuals of R. glacialis that were provided with variable CO2 concentrations (Airliquide, Schwechat, Austria) at a constant flow rate of 4000 ml min−1. Leaf temperatures of four individual leaves in EC1, EC2 and EC4 were monitored every 5 s by software-controlled heat tolerance testing system (HTTS; Buchner et al. 2013) to enable regulating EC3 to the same temperature of EC1, EC2 and EC4.
Fig. 1.
Experimental set-up for determining the effect of different CO2 concentrations on the formation of chloroplast protrusions (CPs) in leaves of R. glacialis. On 15 August 2013, a potted plants (n = 5) were placed inside cylindrical exposure chambers made of highly transparent Plexiglas and exposed to natural solar irradiation with the exception of (3) which was kept in darkness. During the 2.5 h exposure, air with different CO2 concentrations was streamed through the chambers. 1 10,000 ppm, 2 38 ppm, while 3 and 4 had normal CO2 concentration (380 ppm). b Mean leaf temperature (red line; n = 16) and photosynthetically active photon flux density (PPFD; black line) for the whole duration of the exposure. c Typical CPs in leaf mesophyll of R. glacialis immediately after the 2.5 h exposure at 38 ppm CO2 and at natural solar irradiation. The DIC microscopy image clearly shows CPs as broad and beak-like, stroma-filled extensions of the chloroplast envelope. d Schematic drawing of c; light blue areas indicate CPs. Horizontal bars 2 μm
Sampling and preservation
At the end of a 2.5-h exposure, the chambers were quickly opened and leaf samples were taken (one per individual) and cut into 2 × 2 mm pieces that were fixed in 2.5 % glutaraldehyde (GA) in sodium cacodylate buffer (50 mM, pH 7.0). After 1.5 h of immersion in the fixative, leaf pieces were rinsed with and subsequently stored in the same buffer at 5 °C in darkness. For determining enzymatic activities, the remaining leaves were frozen in liquid nitrogen (LN2), intermediately stored at −80 °C and lyophilized (Lyovac GT 2, Leybold-Heraeus, Köln, Germany) for 5 days. Prior to chemical analysis, dry samples were ground (Tissue Lyser II, Qiagen, Venlo, the Netherlands) at a speed of 30 Hz for 2 × 45 s and cooled with LN2.
Numeric assessment of chloroplast protrusions
Semi-thin sections (30 μm) were sliced from the GA-fixed leaf samples and analysed using an inverted microscope with differential interference contrast (DIC) optics (Axiovert 200 M; Plan-Apochromat 63 × 1.4 NA; Carl Zeiss, Jena, Germany). According to the method of Moser et al. (2015), the palisade parenchyma was photographed (Axiocam MCR 5, Carl Zeiss, Jena, Germany) and for each sample 10 individual cells were randomly selected. Stacks of images showing the same cell were analysed at different focal planes (Adobe Photoshop CS2, Adobe Systems Inc., San José, CA, USA). For each cell, 10 chloroplasts were thoroughly screened for CPs. Only chloroplasts positioned slightly off the cell wall, and not concealed by cell-wall fragments or other structures, were selected for further investigation. The relative proportion of chloroplasts with CPs (rCP) was calculated for each cell screened (1).
| 1 |
- n(CP)
number of chloroplasts showing at least one CP
- n
number of chloroplasts inspected
Determination of enzyme activities
Sample preparation
Twenty milligrams of lyophilized and ground leafs were extracted in 1 ml 50 mM Sørensen’s buffer, pH 7, with 1 mM EDTA and vortexed for 15 s. The suspension was centrifuged at 4 °C for 5 min at 12,000 g and 600 μl of the supernatant was diluted with 1400 μl of extraction buffer. Enzymes were purified from low molecular weight compounds that interfered with enzyme assays with PD10 Sephadex® G-25 desalting columns (GE Healthcare, Chalfont St Giles, UK) with centrifugation (4 °C, 1000 g, 2 min). The resulting extract was kept on ice prior to measurements.
Catalase (CAT; 1.11.1.6) and ascorbate peroxidase (APX; EC 1.1.11.1) activities
CAT activity was measured by combining 100 μl of the extract with 620 μl of extraction buffer and 80 μl of 150 mM H2O2. The breakdown of H2O2 was measured by following the absorbance decrease at 240 nm (ε = 43.6 M−1 cm−1) for 2 min. APX activity was measured by combining 150 μl of extract with 820 μl extraction buffer, 20 μl of 10 mM ascorbate solution and 15 μl of 15 mM H2O2. The breakdown of ascorbate was measured by following the absorbance decrease at 265 nm (ε = 7.0 mM−1 cm−1) for 2 min. For CAT and APX activities, three technical replicates were measured for each biological replicate (n = 5), and activity was normalized to dry mass.
Statistics
Correlation analysis and one-way ANOVA followed by related post hoc tests (Duncan, Games-Howell) to determine significant differences between means were calculated by statistical software (SPSS 21, IBM, Armonk, NY, USA).
Results
Leaf temperature and irradiation during the exposure phase
During the treatment, the photosynthetically active photon flux density (PPFD) varied from 360 to 967 μmol photons m−2 s−1 (mean 715), which is below the maxima that may occur in the field (>2500 μmol photons m−2 s−1) but in the range of mean PPFD during daytime (Buchner, unpublished data). Leaf temperatures of the four different ECs were around 36 °C and almost identical (Table 1) and never fell below 31 °C (Fig. 1b), whereas short leaf temperature maxima up to 41.9 °C occurred. At natural growing sites of R. glacialis mean leaf temperatures during daytime are typically lower, but maximum half hourly mean values around 37–38 °C occur occasionally (Buchner et al. 2015; Moser et al. 2015) and do not cause any leaf damage (Larcher et al. 1997; Buchner et al. 2015). Even short exposure to 41.9 °C as applied here does not induce lethal leaf damage (Buchner et al. 2015), but it stimulates photorespiration because the specificity of rubisco to CO2 over O2 is reduced as is the solubility of CO2 (Brooks and Farquhar 1985).
Table 1.
Impact of different CO2/O2 ratios under elevated temperature on the formation of CPs and CAT and APX activities
| Experimental condition [EC] | CO2 [ppm] | PPFD [μmol photons m−2 s−1] | TL [°C] | rCP [%] | CAT [nkat mg−1 DW] | APX [nkat mg−1 DW] |
|---|---|---|---|---|---|---|
| EC 1 | 38 | 715 | 35.9/39.2 | 58.7 | 34.7 | 0.34 |
| EC 2 | 10,000 | 715 | 35.9/39.8 | 3.0 | 18.4 | 0.27 |
| EC 3 | 380 | 0 | 36.3/41.9 | 18.2 | 20.4 | 0.29 |
| EC 4 | 380 | 715 | 36.6/41.0 | 41.3 | 22.2 | 0.31 |
Individual R. glacialis plants were exposed to different experimental conditions [EC]. During exposure, CO2 concentration and leaf temperature (TL; mean/maximum) were controlled, and mean solar irradiation (PPFD) was monitored. After 2.5 h of exposure, the relative frequency of chloroplast protrusions (rCP) and the activities of catalase (CAT) and ascorbate peroxidase (APX) were determined
Impact of CO2 concentration on the formation of CPs
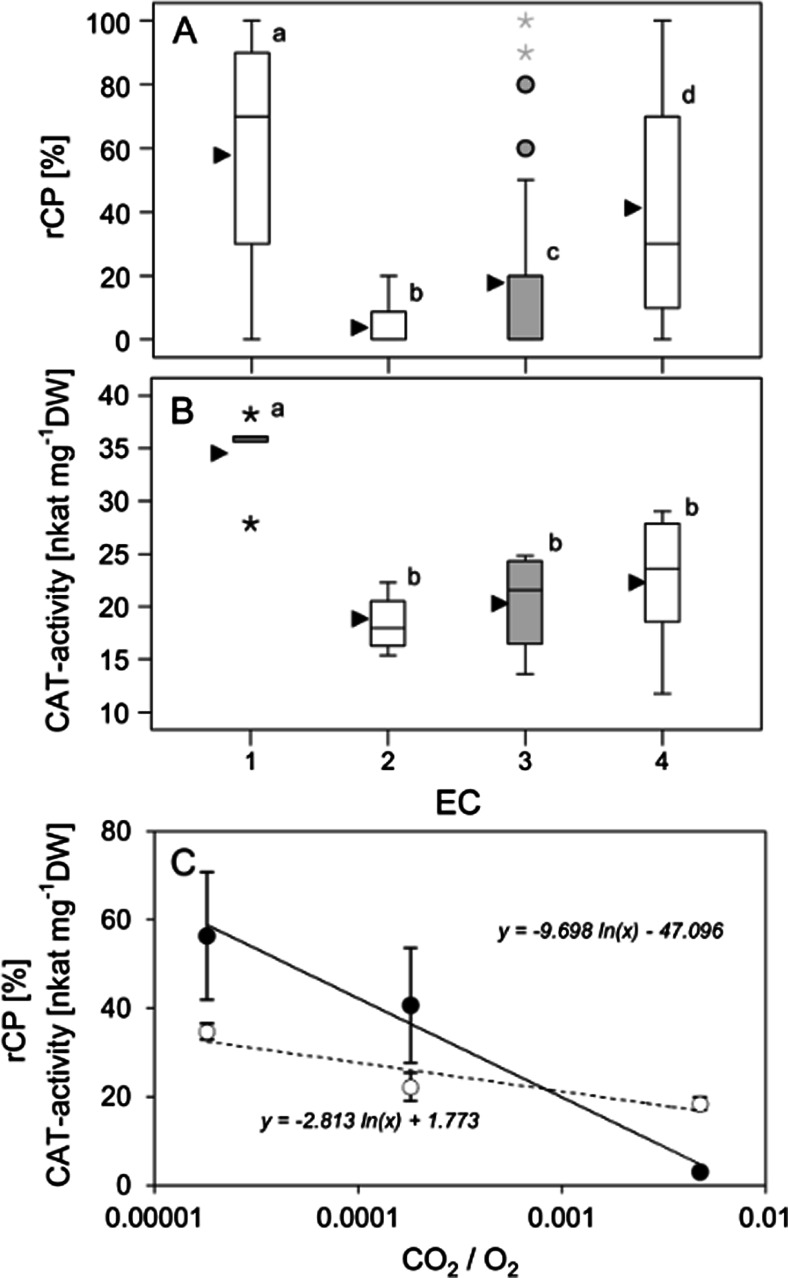
In DIC images, CPs were easily identifiable as broad, stroma-filled lobes (Fig. 1c, d). Although CPs were present in all ECs, they were most abundant (mean ± SE) after exposure to light under 38 ppm CO2 (58.7 % ± 4.6). In contrast, rCP was lowest after exposure to light under 10,000 ppm CO2 (3.0 % ± 0.7). Exposure to solar irradiation at the natural ambient CO2 concentration (380 ppm) led to an rCP of 41.3 % ± 4.4, while the same treatment in the dark led to an rCP of 18.2 % ± 4.2. Mean values of rCP differed significantly (P < 0.05) between all ECs (Fig. 2a).
Fig. 2.
Effect of a 2.5-h exposure of R. glacialis individuals at different CO2 concentrations on the formation of CPs and on catalase activity. Depending on the experimental conditions (ECs) the following CO2 concentrations were applied: 1 38 ppm, 2 10,000 ppm, and 3 and 4 380 ppm. 1, 2 and 4 where exposed to natural solar irradiation (mean PPFD = 715 μmol photons m−2 s−1), while 3 was kept in darkness (grey boxes). a Relative proportion of chloroplasts with at least one chloroplast protrusion (rCP); b catalase activity in response to the different treatments; c rCP (black circles) of the light-exposed samples significantly correlates with the CO2/O2 ratio in accordance with a logarithmic function (solid line). This is also true for CAT activity (white circles, dashed line). Box plots: horizontal lines indicate the median and the 25th and the 75th percentiles; whiskers extend to 1.5 times box-height; circles represent outliers, asterisks indicate extreme outliers that have values more than three times box-height; black triangles indicate arithmetic means. Significant differences (P < 0.05) between groups are indicated by different characters (one-way ANOVA followed by Duncan’s (a) and Games-Howell (b) post hoc tests)
Impact of CO2 concentration on CAT and APX activities
CAT activity was significantly higher (P < 0.05) after light exposure under 38 ppm CO2 (34.7 nkat mg−1 DW ± 1.8) compared to light exposure under 10,000 ppm CO2 (18.4 nkat mg−1 DW ± 1.5). Exposure to natural CO2 concentrations (380 ppm) resulted in an enzyme activity of 22.2 nkat mg−1 DW ± 3.2 in the light and 20.4 nkat mg−1 DW ± 2.6 in darkness (Fig. 2b). Results of EC2, EC3 and EC4 did not differ significantly from each other (P > 0.05). No significant differences were found for APX activity between the four ECs. An overview of rCP, CAT and APX activities subsequent to the exposure to the different ECs is given in Table 1.
rCP and CAT activities in relation to CO2/O2 ratio
The CO2/O2 ratio significantly affected CP formation and CAT activity. In the light (EC1, EC2, EC4), rCP and CAT activities significantly (P = 0.001) correlated negatively with the CO2/O2 ratio (Spearman’s rho = −0.756 and −0.771, respectively) (Fig. 2c). Furthermore, rCP and CAT activities were positively correlated (Spearman’s rho = 0.666, P = 0.011). However, no correlations were found between APX activity and the CO2/O2 ratio or rCP (data not shown).
Discussion
Photorespiration and CP formation
It is believed that photorespiration requires close spatial proximity of chloroplasts, peroxisomes and mitochondria to allow transport of metabolites between these organelles (Douce and Neuburger 1999; Eisenhut et al. 2013). It has been suggested that the formation of CPs supports photorespiration by bridging gaps between organelles (Lütz et al. 2012; Hanson and Sattarzadeh 2011) and by enlarging the chloroplast surface to facilitate envelope-bound transport (Lütz 1987; Lütz 2010; Holzinger et al. 2007b; Lütz and Engel 2007). Furthermore, Sage and Sage (2009) suggested that CPs (or stromules) may also operate as a photorespiratory CO2-scavenging system that supports re-fixation of photorespiratory-released CO2. Catalase, which is essential in scavenging H2O2 produced from photorespiration, is almost exclusively located in peroxisomes and also plays a role in stress response (Feierabend 2005; Wingler et al. 2000; Mhamdi et al. 2012). Accumulation of H2O2 was shown to occur in microbodies of R. glacialis using diaminobenzidine (Lütz 1987), which is a stain commonly used for H2O2 in relation to peroxidase activity (e.g. Roach et al. 2010). However, it was not known if CAT activity was related to CP formation.
Hydrogen peroxide can be scavenged by several enzymes, including CAT and peroxidases. APX plays a key role in the ascorbate-glutathione cycle, which serves to scavenge H2O2 (Foyer and Noctor 2011). However, only CAT activity but not APX activity correlated with CP formation, which suggests that there was a need for enhanced H2O2 scavenging in peroxisomes rather than chloroplasts. Interestingly, this indicates that the low CO2 treatment used to promote photorespiration apparently did not induce the Mehler reaction, agreeing with a recent rethinking that the Mehler reaction is restricted under low CO2 conditions (Noctor et al. 2014; Roach et al. 2015). The exposure to varying CO2/O2 ratios allowed us to modulate photorespiration, showing that CP formation positively correlates with CAT activity (Fig. 2a, b), supporting the hypothesis that CP formation and photorespiration are linked, although a causal relationship is still to be confirmed. Furthermore, it will be interesting to study if organelle extensions such as stromules and CPs also support signalling pathways (Noctor et al. 2007), such as retrograde signalling, which is essential for coordinating cellular activities during plant stress response (Kwok and Hanson 2004a; Fernández and Strand 2008).
Chloroplast protrusion—a multifaceted phenomenon
Chloroplast protrusions are not solely formed during photorespiration, but also seem to have other roles. We show that CPs were also formed under conditions that do not induce photorespiration (Fig. 2a). If the only role of CPs was in photorespiration, no CPs would be formed in the dark. However, rCP was not zero after exposure to 380 ppm CO2 in darkness. In R. glacialis, Moser et al. (2015) observed highest rCP values at moderately solar irradiation and moderately elevated leaf temperatures with a significant correlation between leaf temperature and rCP. Furthermore, rCP at 50 ppm did not differ significantly from that at 370 ppm CO2, indicating that photorespiration was not the main reason for CP formation, because leaf temperature was only 25 °C (compared to ~36 °C used here). In Arabidopsis, CP formation increased with temperature, likely supporting the increased transport of metabolites required for increased metabolic rates at high temperature (Holzinger et al. 2007a). Even in darkness CP formation apparently may support metabolite transport out of the chloroplast during the degradation of transitory starch (Schleucher et al. 1998).
Recent results indicate that increased CP formation could also be related to stress factors. Our results and those of Moser et al. (2015) do not strongly indicate that CPs are formed in response to temperature and irradiation stress. On the other hand, in rice (Yamane et al. 2012) and soybean (He et al. 2014), high salt concentration promoted the formation of CPs and rubisco-containing bodies. In wheat seedlings, protrusions of the chloroplast envelope were shown to be increased during water stress (Freeman and Duysen 1975). Chloroplast swelling and the occurrence of large thylakoid-free areas have also been described in context with chilling or sublethal freezing (Ciamporová and Trginová 1999; Stefanowska et al. 2002) or after heat stress (Larcher et al. 1997). Furthermore, Ishida et al. (2014) showed that vesicles originating from stromules or CPs may be involved in autophagic processes in context with nutrient recycling and chloroplast function maintenance.
In summary, this short communication shows a strong correlation between CP formation and CAT activity, in support of the hypothesis that photorespiration is linked with CP formation, and that CP formation is a multifaceted phenomenon with more than one physiological role.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 28 kb)
Acknowledgments
We wish to thank Ines Bauer and Siegfried Aigner for the technical support and Cornelius Lütz for stimulating discussions on CP formation. Financial support of project P 22158-B16 to O. Buchner by the Austrian Science Fund (FWF) is gratefully acknowledged.
References
- Brooks A, Farquhar GD. Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta. 1985;165:397–406. doi: 10.1007/BF00392238. [DOI] [PubMed] [Google Scholar]
- Buchner O, Holzinger A, Lütz C. Effects of temperature and light on the formation of chloroplast protrusions in leaf mesophyll cells of high alpine plants. Plant Cell Environ. 2007;30:1347–1356. doi: 10.1111/j.1365-3040.2007.01707.x. [DOI] [PubMed] [Google Scholar]
- Buchner O, Lütz C, Holzinger A. Design and construction of a new temperature-controlled chamber for light and confocal microscopy under monitored conditions: biological application for plant samples. J Microsc (Oxford) 2007;225:183–191. doi: 10.1111/j.1365-2818.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Buchner O, Karadar M, Bauer I, Neuner G. A novel system for in situ determination of heat tolerance of plants: first results on alpine dwarf shrubs. Plant Methods. 2013;9:7. doi: 10.1186/1746-4811-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner O, Stoll M, Karadar M, Kranner I, Neuner Application of heat stress in situ demonstrates a protective role of irradiation on photosynthetic performance in alpine plants. Plant Cell Environ. 2015 doi: 10.1111/pce.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čiamporová M, Trgiňová I. Modifications of plant cell ultrastructure accompanying metabolic responses to low temperatures. Biologia. 1999;54:349–360. [Google Scholar]
- Douce R, Neuburger M. Biochemical dissection of photorespiration. Curr Opin Plant Biol. 1999;2:214–222. doi: 10.1016/S1369-5266(99)80038-7. [DOI] [PubMed] [Google Scholar]
- Eisenhut M, Pick TR, Bordych C, Weber APM. Towards closing the remaining gaps in photorespiration—the essential but unexplored role of transport proteins. Plant Biol. 2013;15:676–685. doi: 10.1111/j.1438-8677.2012.00690.x. [DOI] [PubMed] [Google Scholar]
- Feierabend J. Catalases in plants: molecular and functional properties and role in stress defence. In: Smirnoff N, editor. Antioxidants and reactive oxygen species in plants. Oxford: Blackwell; 2005. pp. 101–140. [Google Scholar]
- Fernández AP, Strand Å. Retrograde signaling and plant stress: plastid signals initiate cellular stress responses. Curr Opin Plant Biol. 2008;11:509–513. doi: 10.1016/j.pbi.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman TP, Duysen ME. The effect of imposed water stress on the development and ultrastructure of wheat chloroplasts. Protoplasma. 1975;83:131–145. doi: 10.1007/BF01289336. [DOI] [Google Scholar]
- Gielwanowska I, Szczuka E. New ultrastructural features of organelles in leaf cells of Deschampsia antarctica Desv. Polar Biol. 2005;28:951–955. doi: 10.1007/s00300-005-0024-2. [DOI] [Google Scholar]
- Gray JC, Sullivan JA, Hibberd JM, Hanson MR. Stromules: mobile protrusions and interconnections between plastids. Plant Biol. 2001;3:223–233. doi: 10.1055/s-2001-15204. [DOI] [Google Scholar]
- Hanson MR, Sattarzadeh A. Dynamic morphology of plastids and stromules in angiosperm plants. Plant Cell Environ. 2008;31:646–657. doi: 10.1111/j.1365-3040.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- Hanson MR, Sattarzadeh A. Stromules: recent insights into a long neglected feature of plastid morphology and function. Plant Physiol. 2011;155:1486–1492. doi: 10.1104/pp.110.170852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MR, Sattarzadeh A. Trafficking of proteins through plastid stromules. Plant Cell. 2013;25:2774–2782. doi: 10.1105/tpc.113.112870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Yu C, Zhou L, Chen Y, Liu A, Jin J, Hong J, Qi Y, Jiang D. Rubisco decrease is involved in chloroplast protrusion and rubisco-containing body formation in soybean (Glycine max.) under salt stress. Plant Physiol Biochem. 2014;74:118–124. doi: 10.1016/j.plaphy.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Heitz E. Untersuchungen über den Bau der Plastiden I. Die gerichteten Chlorophyllscheiben der Chloroplasten. Planta. 1937;26:134–163. doi: 10.1007/BF01913844. [DOI] [Google Scholar]
- Holzinger A, Buchner O, Lütz C, Hanson MR. Temperature-sensitive formation of chloroplast protrusions and stromules in mesophyll cells of Arabidopsis thaliana. Protoplasma. 2007;230:23–30. doi: 10.1007/s00709-006-0222-y. [DOI] [PubMed] [Google Scholar]
- Holzinger A, Wasteneys GO, Lütz C. Investigating cytoskeletal function in chloroplast protrusion formation in the Arctic-Alpine plant Oxyria digyna. Plant Biol. 2007;9:400–410. doi: 10.1055/s-2006-924727. [DOI] [PubMed] [Google Scholar]
- Ishida H, Izumi M, Wada S, Makino A. Roles of autophagy in chloroplast recycling. BBA Bioenerg. 2014;1837:512–521. doi: 10.1016/j.bbabio.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Köhler RH, Hanson MR. Plastid tubules of higher plants are tissue-specific and developmentally regulated. J Cell Sci. 2000;113:81–89. doi: 10.1242/jcs.113.1.81. [DOI] [PubMed] [Google Scholar]
- Köhler RH, Cao J, Zipfel WR, Webb WW, Hanson MR. Exchange of protein molecules through connections between higher plant plastids. Science. 1997;276:2039–2042. doi: 10.1126/science.276.5321.2039. [DOI] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR. Microfilaments and microtubules control the morphology and movement of non-green plastids and stromules in Nicotiana tabacum. Plant J. 2003;35:16–26. doi: 10.1046/j.1365-313X.2003.01777.x. [DOI] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR. Plastids and stromules interact with the nucleus and cell membrane in vascular plants. Plant Cell Rep. 2004;23:188–195. doi: 10.1007/s00299-004-0824-9. [DOI] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR. Stromules and the dynamic nature of plastid morphology. J Microsc (Oxford) 2004;214:124–137. doi: 10.1111/j.0022-2720.2004.01317.x. [DOI] [PubMed] [Google Scholar]
- Larcher W, Wagner J, Lütz C. The effect of heat on photosynthesis, dark respiration and cellular ultrastructure of the arctic-alpine psychrophyte Ranunculus glacialis. Photosynthetica. 1997;34:219–232. doi: 10.1023/A:1006840623763. [DOI] [Google Scholar]
- Lütz C (1987) Cytology of high alpine plants II. Microbody activity in leaves of Ranunculus glacialis L. Cytologia 52:679-686
- Lütz C. Cell physiology of plants growing in cold environments. Protoplasma. 2010;244:53–73. doi: 10.1007/s00709-010-0161-5. [DOI] [PubMed] [Google Scholar]
- Lütz C, Engel L. Changes in chloroplast ultrastructure in some high-alpine plants: adaptation to metabolic demands and climate? Protoplasma. 2007;231:183–192. doi: 10.1007/s00709-007-0249-8. [DOI] [PubMed] [Google Scholar]
- Lütz C, Moser W (1977) Beiträge zur Cytologie hochalpiner Pflanzen. I. Untersuchungen zur Ultrastruktur von Ranunculus glacialis L. Flora 166:21-34
- Lütz C, Blassnigg M, di Piazza L, Remias D. Deschampsia antarctica and Colobanthus quitensis from Antarctica: a physiological and ultrastructural comparison. Lyon: FESPB Congress; 2006. pp. RAS03–015. [Google Scholar]
- Lütz C, Bergweiler P, DiPiazza L, Holzinger A. Cell organelle structure and function in Alpine and Polar plants are influenced by growth conditions and climate. In: Lütz C, editor. Plants in Alpine Regions. Cell physiology of adaption and survival strategies. Vienna: Springer; 2012. [Google Scholar]
- Mathur J, Mammone A, Barton KA. Organelle extensions in plant cells. J Integr Plant Biol. 2012;54:851–867. doi: 10.1111/j.1744-7909.2012.01175.x. [DOI] [PubMed] [Google Scholar]
- Mhamdi A, Noctor G, Baker A. Plant catalases: peroxisomal redox guardians. Arch Biochem Biophys. 2012;525:181–194. doi: 10.1016/j.abb.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Moser T, Holzinger A, Buchner O. Chloroplast protrusions in leaves of Ranunculus glacialis L. respond significantly to different ambient conditions but are not related to temperature stress. Plant Cell Environ. 2015 doi: 10.1111/pce.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, de Paepe R, Foyer CH. Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci. 2007;12:125–134. doi: 10.1016/j.tplants.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Foyer CH. The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol. 2014;164:1636–1648. doi: 10.1104/pp.113.233478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramonova NV, Shevyakova NI, Kuznetsov VV. Ultrastructure of chloroplasts and their storage inclusions in the primary leaves of Mesembryanthemum crystallinum affected by putrescine and NaCl. Russ J Plant Physiol. 2004;51:86–96. doi: 10.1023/B:RUPP.0000011307.95130.8f. [DOI] [Google Scholar]
- Roach T, Beckett RP, Minibayeva FV, Colville L, Whitaker C, Chen H, Bailly C, Kranner I. Extracellular superoxide production, viability and redox poise in response to desiccation in recalcitrant Castanea sativa seeds. Plant Cell Environ. 2010;33:59–75. doi: 10.1111/j.1365-3040.2009.02053.x. [DOI] [PubMed] [Google Scholar]
- Roach T, Na CS, Krieger-Liszkay A. High-light-induced hydrogen peroxide production in Chlamydomonas reinhardtii is increased under high CO2. Plant J. 2015 doi: 10.1111/tpj.12768. [DOI] [PubMed] [Google Scholar]
- Sage TL, Sage RF. The functional anatomy of rice leaves: Implications for refixation of photorespiratory CO2 and efforts to engineer C4 Photosynthesis into rice. Plant Cell Physiol. 2009;50:756–772. doi: 10.1093/pcp/pcp033. [DOI] [PubMed] [Google Scholar]
- Schattat MH, Griffiths S, Mathur N, Barton K, Wozny MR, Dunn N, Greenwood JS, Mathur J. Differential coloring reveals that plastids do not form networks for exchanging macromolecules. Plant Cell. 2012;24:1465–1477. doi: 10.1105/tpc.111.095398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattat MH, Barton KA, Mathur J. The myth of interconnected plastids and related phenomena. Protoplasma. 2015;252:359–371. doi: 10.1007/s00709-014-0666-4. [DOI] [PubMed] [Google Scholar]
- Schleucher J, Vanderveer PJ, Sharkey TD. Export of carbon from chloroplasts at night. Plant Physiol. 1998;118:1439–1445. doi: 10.1104/pp.118.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönswetter P, Paun O, Tribsch A, Niklfeld H. Out of the Alps: colonization of northern Europe by east alpine populations of the glacier buttercup Ranunculus glacialis L. (Ranunculaceae) Mol Ecol. 2003;12:3373–3381. doi: 10.1046/j.1365-294X.2003.01984.x. [DOI] [PubMed] [Google Scholar]
- Senn G. Die Gestalts- und Lageveränderung der Pflanzen-Chromatophoren. Leipzig: Wilhelm Engelmann; 1908. [Google Scholar]
- Stefanowska M, Kuras M, Kacperska A. Low temperature induced modifications in cell ultrastructure and localization of phenolics in winter oilseed rape (Brassica napus L. var. oleifera L.) leaves. Ann Bot. 2002;90:637–645. doi: 10.1093/aob/mcf241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A, Lea PJ, Quick WP, Leegood RC. Photorespiration: metabolic pathways and their role in stress protection. Philos T Roy Soc B. 2000;355:1517–1529. doi: 10.1098/rstb.2000.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff A, Crossley A, Sheppard LJ. Fine structure of acid mist treated Sitka spruce needles: open-top chamber and field experiments. Ann Bot. 1996;77:1–10. doi: 10.1006/anbo.1996.0001. [DOI] [Google Scholar]
- Yamane K, Mitsuya S, Taniguchi M, Miyake H. Salt-induced chloroplast protrusion is the process of exclusion of ribulose-1,5-bisphosphate carboxylase /oxygenase from chloroplasts into cytoplasm in leaves of rice. Plant Cell Environ. 2012;35:1663–1671. doi: 10.1111/j.1365-3040.2012.02516.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 28 kb)