Abstract
Vertebrate erythrocytes and thrombocytes arise from the common bipotent thrombocytic-erythroid progenitors (TEPs). Even though nonmammalian erythrocytes and thrombocytes are phenotypically very similar to each other, mammalian species have developed some key evolutionary improvements in the process of erythroid and thrombocytic differentiation, such as erythroid enucleation, megakaryocyte endoreduplication, and platelet formation. This brings up a few questions that we try to address in this review. Specifically, we describe the ontology of erythro-thrombopoiesis during adult hematopoiesis with focus on the phylogenetic origin of mammalian erythrocytes and thrombocytes (also termed platelets). Although the evolutionary relationship between mammalian and nonmammalian erythroid cells is clear, the appearance of mammalian megakaryocytes is less so. Here, we discuss recent data indicating that nonmammalian thrombocytes and megakaryocytes are homologs. Finally, we hypothesize that erythroid and thrombocytic differentiation evolved from a single ancestral lineage, which would explain the striking similarities between these cells.
1. Introduction
Hematopoiesis is mediated by self-renewal and differentiation of hematopoietic stem cells (HSCs) and their progenies, which is tightly controlled through a complex array of extrinsic and intrinsic factors [1, 2]. Dysregulation of some of these pathways can lead to distinct hematopoietic disorders, such as anemia, thrombocytopenia, and myelogenous leukemia, predominantly caused by defects in the erythroid-megakaryocytic compartment [3, 4]. It is well accepted that mammalian megakaryocytes and erythrocytes are generated from common bipotent megakaryocyte-erythrocyte progenitors (MEPs) [5]. In mammals, megakaryocytes are formed by endoreduplication of megakaryoblasts to generate polyploid cells. Once the ploidy state of 8–64N is reached, megakaryocytes produce thrombocytes (in mammals also referred to as platelets) [6]. The key mediator of this process is thrombopoietin (TPO) [7, 8]. Red blood cells (RBCs) do likewise develop from MEPs through several stages of committed progenitors, termed burst-forming units-erythroid (BFU-E), colony-forming units-erythroid (CFU-E), and erythroblasts. The most prominent factors regulating erythropoiesis in vivo and ex vivo are erythropoietin (EPO) and stem cell factor (SCF, or KIT ligand, KITL) [9, 10]. Notably, mammalian erythroblasts undergo chromatin condensation and nucleus extrusion, giving rise to enucleated mature erythrocytes [11, 12]. In contrast, nonmammalian vertebrates possess nucleated oval-shaped diploid thrombocytes [13, 14] and RBCs [15] (Figure 1). Similarly to mammals, both of these lineages have been demonstrated to arise from bipotent progenitors, termed thrombocyte-erythrocyte progenitors (TEPs), cells equivalent to mammalian MEPs [16, 17].
Figure 1.
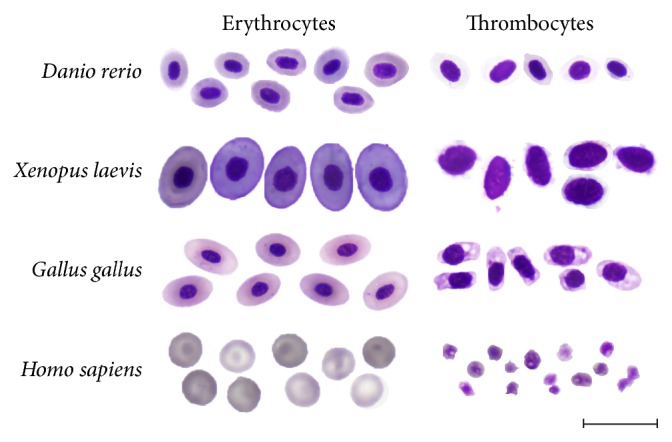
A comparative view of erythrocytes and thrombocytes from zebrafish (Danio rerio), xenopus (Xenopus laevis), chicken (Gallus gallus), or human (Homo sapiens) peripheral blood. Cells were smeared on glass slides and stained with May-Grünwald Giemsa. Photomicrographs were taken at 1000x magnification. Scale bar is 20 μm.
The present review aims to summarize the ontology and phylogeny of erythro-thrombocytic differentiation in vertebrates. Here, we highlight the relationship between mammalian and nonmammalian erythroid and thrombocytic cells. Moreover, despite the morphological and functional differences between erythroid and thrombocytic cell lineages, we provide a model underlining the common evolutionary origin of these two cell lineages from a single ancestral precursor.
2. Ontogeny of Thrombocytes and Erythrocytes
2.1. Models of Adult Hematopoiesis
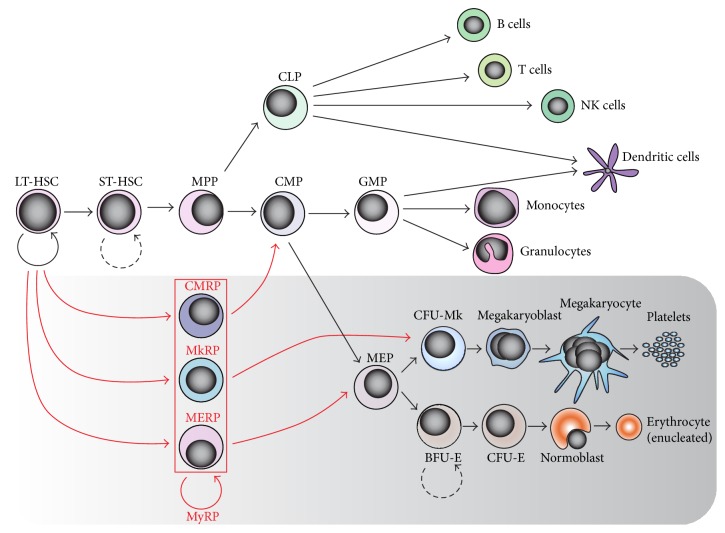
Both in vivo and ex vivo, all terminally differentiated blood cells in adult organisms arise from long-term HSCs (LT-HSC) [18, 19] that have unlimited self-renewal capacity. Direct downstream progenies of HSCs, termed short-term HSCs (ST-HSCs) and multipotent progenitor cells (MPPs), are progressively losing their self-renewal capability upon commitment. According to the most prevalent classical hierarchical model of hematopoiesis (Figure 2) [20, 21], the MPPs further give rise to common lymphoid progenitors (CLPs) and common myeloid progenitors (CMPs). CLPs are responsible for production of lymphoid cells, whereas CMPs differentiate into granulocyte-monocyte progenitors (GMPs) and bipotent MEPs/TEPs that are responsible for generation of thrombocytes and erythrocytes [6, 16, 17], the most abundant and specialized cell types in the adult organism.
Figure 2.
Models of mammalian adult hematopoiesis with respect to the megakaryocytic-erythroid compartment (grey box). Hierarchical [20, 21] (black arrows) and myeloid bypass [25] (red arrowheads) models of hematopoiesis are shown. According to the conventional hierarchical model of hematopoiesis, the bipotent megakaryocyte-erythroid progenitors (MEPs) are able to give rise to megakaryocytes and erythrocytes. The alternative myeloid bypass model predicts the existence of various myeloid repopulating progenitors (MyRPs) as a subset of long-term hematopoietic stem cells (LT-HSCs), such as common myeloid repopulating progenitors (CMRPs), megakaryocyte repopulating progenitors (MkRPs), and megakaryocyte-erythroid repopulating progenitors (MERPs). These progenitors are capable of long-term repopulation and differentiation into the particular cell lineages. ST-HSC: short-term HSC; MPP: multipotent progenitor cell; CLP: common lymphoid progenitor; GMP: granulocyte/monocyte progenitor; CFU-Mk: colony-forming unit-megakaryocyte; BFU-E: burst-forming units-erythroid; CFU-E: colony-forming units-erythroid.
Although the hierarchical model of hematopoiesis has been generally accepted over years, recent development of state-of-the-art technologies led to discoveries of alternative hematopoietic pathways that are either bias or bypass certain multipotent progenitors. This includes the myeloid/lymphoid biased model [22], revised model for adult hematopoiesis [23], myeloid based model [24], or myeloid bypass model [25]. Some of these models are in accordance with the classical hierarchical model and provide alternative pathways for development of more specialized cell types at a much higher hierarchical level than previously realized. Although detailed examination of these pathways goes beyond the scope of this review, we would like to highlight those alternative models that refer to production of erythroid and megakaryocytic cells (Figure 2).
First evidence suggesting a direct pathway leading from HSCs to MEPs was based on the identification of a subset of HSCs, marked by Lineage−Sca1+c-Kithigh (LSK) and Flt3− antibodies, which may have given rise directly to MEPs [22]. This is in agreement with recent findings, further proving that the LSK CD150+CD48−CD34− subset of HSCs is capable of short-term and long-term platelet reconstitution as well as reconstitution of other erythro-myeloid, but not lymphoid, cell lineages [26]. Extensive single-cell transplantation experiments revealed the presence of long-term megakaryocyte repopulating progenitors (MkRPs), megakaryocyte-erythroid repopulating progenitors (MERPs), and common myeloid repopulating progenitors (CMRPs) within the CD150+/−CD41+/−CD34−LSK cells (Figure 2) [25]. While CMRPs were shown to be generally present within the CD34−LSK fraction of cells, MkRPs and MERPs seem to be present only within the CD150+CD41−CD34−LSK or CD150−CD41+CD34−LSK fraction of cells. As a follow-up, the intermediate pathway bridging MkRP and megakaryocytes was identified, and fully restricted unipotent megakaryocyte progenitors CD41+CD42b+LSK were characterized recently [27].
Importantly, the experimental data suggesting alternative erythroid and megakaryocytic pathways are solely based on experiments performed in mammalian hematopoietic models and there is a lack of evidence of their existence in nonmammalian vertebrate species. We can only speculate whether these pathways evolved in mammals only or whether they are evolutionarily conserved. One may presume that these mechanisms might play an important physiological role in the steady-state and emergency hematopoiesis.
2.2. Extrinsic Factors Involved in Erythro-Thrombopoiesis
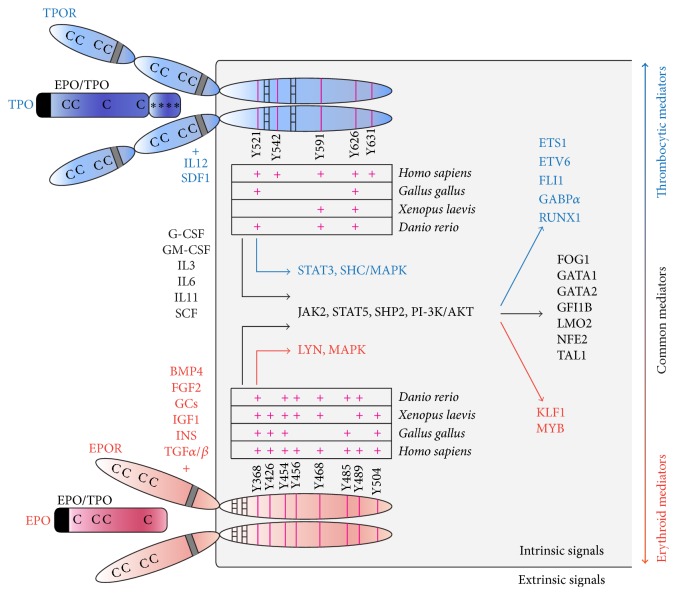
Erythro-thrombocytic differentiation has been shown to be regulated by multiple cytokines (Figure 3), many of which have broad effects on all hematopoietic lineages. The most important factors regulating erythropoiesis are EPO and SCF [9, 10]. EPO interacts with its cognate receptor, EPOR, and promotes erythroid progenitor self-renewal, survival, and differentiation, while SCF mediates proliferation of these progenitors. Other important factors that control erythropoiesis include fibroblast growth factor 2 (FGF2) [28], insulin (INS), insulin-like growth factor 1 (IGF1) [29], transforming growth factor α (TGFα) and TGFβ family members (TGFβ, bone morphogenetic protein 4, BMP4) [30–34], and glucocorticoids (GCs, such as dexamethasone, Dex) [35]. These factors could either promote erythroid progenitor self-renewal or take part in their differentiation, depending on the cooperating signals. Further cytokines that act synergistically with the lineage-restricted factors and that could instrument both erythroid and thrombocytic differentiation pathways are interleukin 3 (IL3), IL6, IL11, granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage CSF (GM-CSF) [36, 37], and the previously mentioned SCF [10, 38]. These factors act as early modulators of upstream progenitors in erythroid and thrombocytic differentiation, driving their self-renewal, or promote megakaryocytic maturation. Other cytokines involved in thrombocytic differentiation, megakaryocytic maturation, or platelet biogenesis include, besides TPO, also IL12 and SDF1 [36]. TPO interacts with its cognate receptor, TPOR (c-MPL) [39, 40]. Its signalization seems to be strongly required for thrombopoiesis, since mice lacking c-MPL signaling are highly thrombocytopenic [41].
Figure 3.
Composite summary of the most prominent factors and signals involved in the regulation of erythro-thrombopoiesis. Erythroid signals are in red, thrombocytic signals are in blue, and signals involved in both differentiation pathways are depicted in black. Human TPO ligand and TPOR are shown in blue; human EPO and EPOR are shown in red. Tyrosine residues (Y, pink lines) in TPOR/EPOR intracellular domains important for receptor signaling are shown. Some of them are highly conserved throughout the vertebrate species as demonstrated in the table. Cytokines' signal peptides are in black and EPO/TPO domains are shown. Receptors' WSXSW motifs are in grey and squared boxes represent Box1/Box2. Cs represent conserved Cys residues; ∗s represent glycosylation sites.
It is interesting that EPO and TPO signaling share many common features. Both ligands belong to the four-helix bundle cytokine family and share a highly conserved amino-terminal EPO/TPO domain [42]. In mammals, TPO's C-terminal portion encodes a highly glycosylated domain [43, 44] that is missing in nonmammalian vertebrates [16, 17] and its role in mammals is to regulate the half-life of TPO in the circulation [41]. EPO and TPO share four conserved cysteine (Cys) residues that form disulfide bonds [16, 17, 45] responsible for keeping the ligand's tertiary structure. Importantly, EPOR and TPOR are also reminiscent of each other. Both receptors belong to the family of type I cytokine receptors [46, 47]. The extracellular domains of these receptors [48] are characterized by the presence of four conserved Cys residues and a tryptophan-serine-x-serine-tryptophan (WSXSW) motif, involved in ligand binding and receptor signaling. Class I receptors possess one transmembrane domain, and their intracellular region consists of two conserved domains, Box1/Box2, involved in mediating downstream signals. Other important features of these receptors are intracellular tyrosine residues, many of which are conserved throughout the vertebrate species (Figure 3). The only structural difference between EPOR and TPOR is that the extracellular domain has been duplicated in TPOR, having eight conserved Cys residues and two WSXSW motifs [46]. The activation of EPOR as well as TPOR occurs through the receptor homodimerization upon ligand binding, which in turn triggers similar downstream signaling pathways. These similarities between EPO/TPO ligands and their receptors suggest that they might have evolved from a single ligand/receptor by a duplication event during evolution.
2.3. Intrinsic Factors Involved in Erythro-Thrombopoiesis
The intracellular signaling pathways mediated by EPOR and TPOR overlap to a large extent (Figure 3). Neither EPOR nor TPOR have an intrinsic enzymatic activity and their signaling is primarily dependent on associated Janus kinase 2 (JAK2) [49, 50]. Particularly, receptor homodimerization leads to autophosphorylation of JAK2 that is bound to Box1/2 and that in turn phosphorylates the receptor itself as well as other signaling molecules. Both EPOR and TPOR stimulate JAK2-mediated phosphorylation of STAT5 (signal transducers and activators of transcription), activate the phosphatidylinositol 3-kinase (PI-3K)/AKT pathway, and promote mitogen-activated protein kinase (MAPK) signaling [49, 50]. This is achieved by recruitment of GRB2 either directly or indirectly via the adaptor molecule SHC, while GRB2 further activates SOS, RAF, and MEK proteins, finally triggering MAPK activation [51, 52]. In contrast to EPOR, TPOR is a much more potent activator of the MAPK pathway and STAT3 signaling [53]. Conversely, it has been shown that EPOR interacts with LYN kinase, which can bind to JAK2 and affects STAT5 [54]. EPOR and TPOR signaling is limited by a negative feedback loop employing SHP1 and SHIP phosphatases and suppressors of cytokine signaling (SOCS1, SOCS3) [49, 55, 56].
The described signaling networks work either in concert or antagonistically to drive specification of erythroid and thrombocytic cell lineages. This is mainly governed by the balanced activity of transcription factors binding to GATA or ETS motifs and others [57], including GATA binding factors, GATA1, GATA2; ETS factors, ETS1, ETS Variant 6 (ETV6/TEL), friend leukemia virus integration 1 (FLI1), GA-binding protein transcription factor (GABPα); and other factors, runt-related transcription factor 1 (RUNX1/AML1), c-MYB (MYB), friend of GATA1 (FOG1), growth factor independent 1B (GFI1B), nuclear factor-erythroid2 complex (NFE2, NFE2, and MAFK subunits), LIM domain only 2 (LMO2), T-cell acute lymphocytic leukemia 1 (TAL1/SCL), and Krüppel-like factor (KLF1) [3, 57–59]. It is the interaction and crosstalk between these transcription factors that makes the system complex. A number of these transcription factors, such as FOG1, GATA1/2, GFI1B, LMO2, NFE2, and TAL1, are critical for both erythroid and thrombocytic development, whereas others are rather dedicated to unilineage differentiation, such as the erythroid EKLF and MYB or the thrombocytic ETS1, ETV6, FLI1, GABPα, and RUNX1 (Figure 3). From this overview, it is more than apparent that erythroid and thrombocytic signaling share many common features, further suggesting a common evolutionary origin of EPO/TPO signaling pathways.
3. Phylogeny of Erythrocytes and Thrombocytes in Vertebrates
Mammalian and nonmammalian erythro-thrombocytic cells appear to be phenotypically very different as a result of divergent evolution. It has been shown that mammals and birds split off from their lizard-like ancestors 310 million years ago [60]. Since that time, certain aspects of erythroid and thrombocytic differentiation have changed; adult mammalian RBCs possess the unique feature of being enucleated, and mammalian thrombocytes are not individual cells but fragments of megakaryocytes. These adaptations likely enhanced the biological performance of the corresponding cells, which could be demonstrated on a few examples. Enucleated erythrocytes are more flexible and the lack of the nucleus creates more intracellular space for hemoglobin. This provides a typical biconcave shape, increasing the surface area for an efficient oxygen exchange [61]. Mammalian platelets are generated in very high numbers (thousands of platelets per one megakaryocyte), as compared to nonmammalian thrombocytes, and are much smaller and more flexible. These features ensure their efficient spreading and increased resistance to fluid shear forces [62]. Both of these improvements in erythrocytes and thrombocytes allowed development of thinner capillaries in mammals, preventing their possible blockage [61, 62]. It is likely that these enhancements provided a survival advantage to early mammalian species.
However, these enhancements also bring up the question of the evolutionary origin of these cells. Hypothetically, mammalian erythrocytes and megakaryocytes could have evolved de novo, functioning as analogs of nonmammalian erythrocytes and thrombocytes [17]. Conversely, they might have evolved as a possible improvement from ancestral erythro-thrombocytic cells, as previously discussed, indicating that mammalian and nonmammalian erythrocytes and thrombocytes are homologs. Indeed, the following lines of evidence suggest that the latter hypothesis may be more probable (Figure 4).
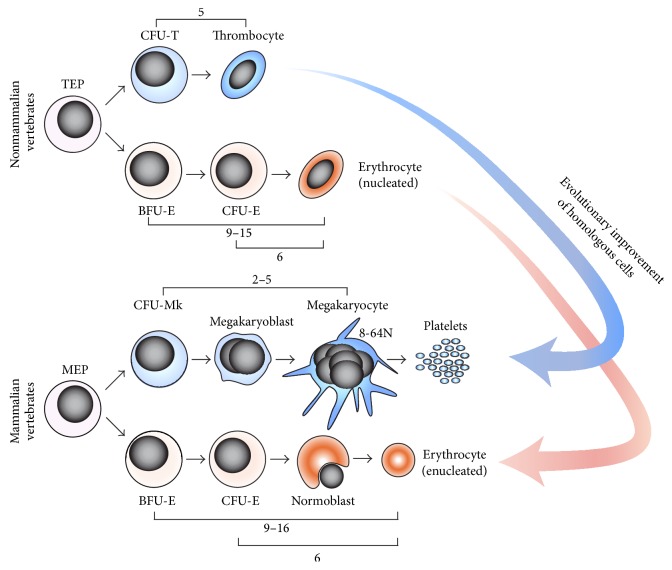
Figure 4.
Nonmammalian and mammalian model of erythropoiesis and thrombopoiesis. According to the integrated model of hematopoiesis, mammalian erythrocytes and megakaryocytes have likely evolved from their nonmammalian erythroid and thrombocytic homologs as an evolutionary improvement. Nonmammalian erythrocytes and thrombocytes are phenotypically similar (nucleated, diploid oval-shaped cells), whereas mammalian megakaryocytes and erythrocytes are very different from each other. Numbers indicate the proliferation potential of particular progenitors. TEP: thrombocyte-erythroid progenitor; CFU-T: colony-forming unit-thrombocyte. Modified from Bartunek et al. [16] and Svoboda et al. [17].
First, the initial commitment of both lineages requires involvement of similar signaling pathways and transcription factors throughout the vertebrate phylum. The most prominent factors required for erythroid differentiation that were found to be functionally conserved from fish to man are FOG1, GATA1, GATA2, KLF1 (zebrafish ortholog Klf4), LMO2, MYB, NFE2, TAL1, and others [17, 63–66]. Similarly, the list of conserved factors that are involved in vertebrate thrombopoiesis includes ETS1, FLI1, FOG1, GATA1, GATA2, NFE2, RUNX1, TAL1, and others [17, 64, 67–69]. Second, multiple zebrafish mutant lines or knockdowns have been generated that recapitulate common human disorders, such as various types of anemia, protoporphyria, or thrombocytopenia [64, 70, 71]. Many of these mutants and knockdowns are affected in the same loci that are relevant to human diseases, which further highlights the similar mechanisms underpinning these processes. Third, the processes involved in hemostasis are highly conserved among mammalian and nonmammalian vertebrates; platelets and thrombocytes are activated by the same stimuli, and blood clotting takes place in an almost identical way [62, 71].
Finally, the last piece of evidence favoring the hypothesis that megakaryocytes likely evolved as a thrombocytic improvement is based on characterization of the relationships between zebrafish hematopoietic progenitors and on mapping their proliferation kinetics. Multiple studies indicate that mammalian BFU-E progenitors are capable of 9 to 16 cell divisions during their maturation, depending on the presence of cooperating factors [72]. The CFU-E progenitors are capable of at most 6 cell divisions [72] and megakaryocytes endoreduplicate approximately 2 to 5 times [73] to form 8–64N cells. In line with this observation is the study indicating that the number of cell divisions during zebrafish erythroid and thrombocytic terminal differentiation is closely matched to mammalian species [17]: the zebrafish BFU-E progenitors are capable of 9 to 15 cell divisions, depending on cooperative signals, the CFU-E progenitors can undergo 6 cell divisions, and thrombocytes can undergo 5 cell divisions during their terminal differentiation.
Taken together, these data led to the establishment of the “integrated model of hematopoiesis” [17] (Figure 4), proposing that despite striking phenotypic differences between mammalian megakaryocytes and nonmammalian thrombocytes, there is a clear link between mammalian and nonmammalian erythroid and thrombocytic cells in terms of their molecular control and their proliferation potential. This model further suggests that mammalian erythrocytes and megakaryocytes have evolved from nonmammalian erythrocytes and thrombocytes as their possible improvements, which implies their homologous relationship.
4. Origin of Erythrocytes and Thrombocytes in Vertebrates
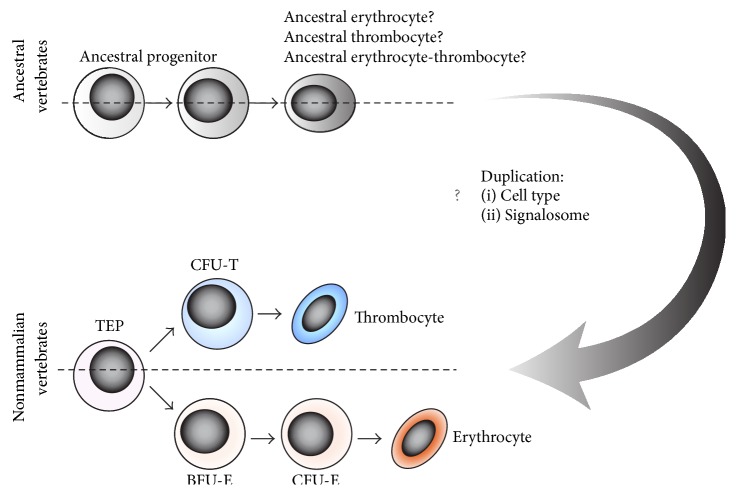
Up to now, we have discussed the evolutionary development of mammalian erythrocytes and megakaryocytes from nonmammalian homologous cells. However, in this chapter we would like to focus on the hypothetical origin of erythroid and thrombocytic differentiation programs from ancestral vertebrates. According to the generally well-accepted evolutionary hypothesis, the invertebrate and vertebrate species bifurcated approximately 520–550 million years ago [60]. This resulted in enormous divergence of these species and led to de novo parallel formation of various analogous features. Even though many invertebrate animals possess both erythrocyte-like and thrombocyte-like analogous cells, commonly referred to as amebocytes, coelomocytes, hemocytes, or thrombocytoids, these cells are not considered to be the progenitors of vertebrate erythro-thrombocytic cells [74–77]. Therefore, erythrocytes and thrombocytes found in cyclostomates and fish are the first cells that have evolved to be particularly specialized in oxygen transport or hemostasis [13, 78–80]. Both cell lineages likely first appeared in direct fish ancestors and it is highly probable that both differentiation programs split from one ancestral differentiation program after its duplication (Figure 5). This view is supported both by similar cell characteristics (similar oval shape, condensed nuclei, and proliferation coupled to differentiation) and by similar or shared regulatory molecules, as previously discussed. This includes the structural and functional resemblance between EPO and TPO signalosomes, likely derived from a single ligand-receptor complex due to a duplication event. As discussed above, both EPO and TPO mediate substantially redundant signaling and activate similar signaling pathways and transcription factors. This has been well illustrated experimentally as TPO expanded erythroid progenitors [7, 81] and, strikingly, TPO in combination with SCF and IL11 was shown to substitute for EPO signaling in the erythroid progenitors derived from Epor deficient mice [82]. Conversely, EPO was shown to synergize with TPO to promote megakaryocyte colony growth and maturation [36, 83].
Figure 5.
The common ancestral erythro-thrombocytic model predicts the existence of unilineage differentiation in ancestral vertebrates, leading to ancient erythroid or thrombocytic cells or erythro-thrombocytic cells with dual function. Hypothetical duplication of cell types and their signalosomes led to the origin of erythrocytes with EPO signaling and to the origin of thrombocytes together with TPO signaling.
In summary, based on the integrated model of hematopoiesis we propose the “Common ancestral erythro-thrombocytic hypothesis.” This hypothesis predicts the existence of ancestral vertebrate organisms with unilineage differentiation, leading to ancestral erythrocytes/thrombocytes or erythro-thrombocytes with dual function. This unilineage differentiation program might have been further duplicated during the evolution of early vertebrates, giving rise to specialized erythroid and thrombocytic differentiation programs in conjunction with EPO/TPO signaling.
5. Conclusions
Erythroid and thrombocytic differentiation share many common features. Besides phenotypic similarities between erythrocytes and thrombocytes found in nonmammalian vertebrates, this includes the common progenitors of these cells (TEPs/MEPs), similarities between EPO and TPO signaling, and shared signaling mechanisms mediating the lineage commitment. These similarities are also present in mammalian species, while the basic molecular mechanisms driving erythro-thrombocytic lineage commitment seem to be evolutionarily highly conserved. The integrated model of hematopoiesis (Figure 5) suggests that mammalian megakaryocytes and erythrocytes likely evolved as an improvement of their ancestral counterparts (found in nonmammalian vertebrates) to increase their biological performance during oxygen transport and hemostasis. This indicates that mammalian and nonmammalian erythrocytes and thrombocytes did not evolve de novo but instead are homologous.
Finding the actual relationship between the mammalian and nonmammalian blood cells might have a major impact on hematopoietic research. Since the employment of mammalian model organisms brings only partial progress due to the interference with sophisticated mammalian megakaryocytic and erythroid enhancements, nonmammalian model organisms, such as chicken or zebrafish, could then be efficiently utilized to identify novel key regulators of cell fate determination.
In addition to this and based on the described similarities between erythroid and thrombocytic differentiation, we suggest that both cell lineages have evolved from a single ancestral differentiation program. This was likely mediated by the duplication of the ancestral cell type and its signalosome during the evolution of early vertebrates.
Acknowledgments
The authors would like to thank Vit Karafiat for assistance with blood smear preparation and Ondrej Machon and Jana Oltova for critical reading of the paper. This work was supported by the Ministry of Education, Youth and Sports, Program NPU I, Project LO1419.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Orkin S. H. Diversification of haematopoietic stem cells to specific lineages. Nature Reviews Genetics. 2000;1(1):57–64. doi: 10.1038/35049577. [DOI] [PubMed] [Google Scholar]
- 2.Kaushansky K. Lineage-specific hematopoietic growth factors. The New England Journal of Medicine. 2006;354(19):2034–2045. doi: 10.1056/nejmra052706. [DOI] [PubMed] [Google Scholar]
- 3.Wickrema A., Crispino J. D. Erythroid and megakaryocytic transformation. Oncogene. 2007;26(47):6803–6815. doi: 10.1038/sj.onc.1210763. [DOI] [PubMed] [Google Scholar]
- 4.Crispino J. D., Weiss M. J. Erythro-megakaryocytic transcription factors associated with hereditary anemia. Blood. 2014;123(20):3080–3088. doi: 10.1182/blood-2014-01-453167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debili N., Coulombel L., Croisille L., et al. Characterization of a bipotent erythro-megakaryocytic progenitor in human bone marrow. Blood. 1996;88(4):1284–1296. [PubMed] [Google Scholar]
- 6.Broudy V. C., Kaushansky K. Thrombopoietin, the c-mpl ligand, is a major regulator of platelet production. Journal of Leukocyte Biology. 1995;57(5):719–725. doi: 10.1002/jlb.57.5.719. [DOI] [PubMed] [Google Scholar]
- 7.Kaushansky K., Broudy V. C., Grossmann A., et al. Thrombopoietin expands erythroid progenitors, increases red cell production, and enhances erythroid recovery after myelosuppressive therapy. The Journal of Clinical Investigation. 1995;96(3):1683–1687. doi: 10.1172/jci118210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato T., Matsumoto A., Ogami K., Tahara T., Morita H., Miyazaki H. Native thrombopoietin: structure and function. Stem Cells. 1998;16(5):322–328. doi: 10.1002/stem.160322. [DOI] [PubMed] [Google Scholar]
- 9.Krantz S. B. Erythropoietin. Blood. 1991;77(3):419–434. [PubMed] [Google Scholar]
- 10.Broudy V. C. Stem cell factor and hematopoiesis. Blood. 1997;90(4):1345–1364. [PubMed] [Google Scholar]
- 11.Muir A. R., Kerr D. N. Erythropoiesis: an electron microscopical study. Quarterly Journal of Experimental Physiology and Cognate Medical Sciences. 1958;43(1):106–114. doi: 10.1113/expphysiol.1958.sp001295. [DOI] [PubMed] [Google Scholar]
- 12.Simpson C. F., Kling J. M. The mechanism of denucleation in circulating erythroblasts. Journal of Cell Biology. 1967;35(1):237–245. doi: 10.1083/jcb.35.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratnoff O. D. The evolution of hemostatic mechanisms. Perspectives in Biology and Medicine. 1987;31(1):4–33. doi: 10.1353/pbm.1987.0003. [DOI] [PubMed] [Google Scholar]
- 14.Schneider W., Gattermann N. Megakaryocytes: origin of bleeding and thrombotic disorders. European Journal of Clinical Investigation. 1994;24(1):16–20. doi: 10.1111/j.1365-2362.1994.tb02420.x. [DOI] [PubMed] [Google Scholar]
- 15.Gulliver G. On the size and shape of red corpuscles of the blood of vertebrates, with drawings of them to a uniform scale, and extended and revised tables of measurements. Proceedings of the Zoological Society of London. 1875;1875:474–495. [Google Scholar]
- 16.Bartunek P., Karafiat V., Bartunkova J., et al. Impact of chicken thrombopoietin and its receptor c-Mpl on hematopoietic cell development. Experimental Hematology. 2008;36(4):495–505. doi: 10.1016/j.exphem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Svoboda O., Stachura D. L., Machoňová O., et al. Dissection of vertebrate hematopoiesis using zebrafish thrombopoietin. Blood. 2014;124(2):220–228. doi: 10.1182/blood-2014-03-564682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith L. G., Weissman I. L., Heimfeld S. Clonal analysis of hematopoietic stem-cell differentiation in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(7):2788–2792. doi: 10.1073/pnas.88.7.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81(11):2844–2853. [PubMed] [Google Scholar]
- 20.Akashi K., Traver D., Miyamoto T., Weissman I. L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404(6774):193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 21.Reya T., Morrison S. J., Clarke M. F., Weissman I. L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 22.Adolfsson J., Månsson R., Buza-Vidas N., et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential: a revised road map for adult blood lineage commitment. Cell. 2005;121(2):295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Luc S., Buza-Vidas N., Jacobsen S. E. W. Delineating the cellular pathways of hematopoietic lineage commitment. Seminars in Immunology. 2008;20(4):213–220. doi: 10.1016/j.smim.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Kawamoto H., Wada H., Katsura Y. A revised scheme for developmental pathways of hematopoietic cells: the myeloid-based model. International Immunology. 2010;22(2):65–70. doi: 10.1093/intimm/dxp125.dxp125 [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto R., Morita Y., Ooehara J., et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154(5):1112–1126. doi: 10.1016/j.cell.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Sanjuan-Pla A., Macaulay I. C., Jensen C. T., et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502(7470):232–236. doi: 10.1038/nature12495. [DOI] [PubMed] [Google Scholar]
- 27.Nishikii H., Kanazawa Y., Umemoto T., et al. Unipotent megakaryopoietic pathway bridging hematopoietic stem cells and mature megakaryocytes. Stem Cells. 2015;33(7):2196–2207. doi: 10.1002/stem.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartůněk P., Pajer P., Karafiát V., Blendinger G., Dvořák M., Zenke M. bFGF signaling and v-Myb cooperate in sustained growth of primitive erythroid progenitors. Oncogene. 2002;21(3):400–410. doi: 10.1038/sj/onc/1205103. [DOI] [PubMed] [Google Scholar]
- 29.Miyagawa S.-I., Kobayashi M., Konishi N., Sato T., Ueda K. Insulin and insulin-like growth factor I support the proliferation of erythroid progenitor cells in bone marrow through the sharing of receptors. British Journal of Haematology. 2000;109(3):555–562. doi: 10.1046/j.1365-2141.2000.02047.x. [DOI] [PubMed] [Google Scholar]
- 30.Krystal G., Lam V., Dragowska W., et al. Transforming growth factor β1 is an inducer of erythroid differentiation. The Journal of Experimental Medicine. 1994;180(3):851–860. doi: 10.1084/jem.180.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandrillon O., Schmidt U., Beug H., Samarut J. TGF-β cooperates with TGF-α to induce the self-renewal of normal erythrocytic progenitors: evidence for an autocrine mechanism. EMBO Journal. 1999;18(10):2764–2781. doi: 10.1093/emboj/18.10.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huber T. L., Zhou Y., Mead P. E., Zon L. I. Cooperative effects of growth factors involved in the induction of hematopoietic mesoderm. Blood. 1998;92(11):4128–4137. [PubMed] [Google Scholar]
- 33.Fuchs O., Simakova O., Klener P., et al. Inhibition of Smad5 in human hematopoietic progenitors blocks erythroid differentiation induced by BMP4. Blood Cells, Molecules, and Diseases. 2002;28(2):221–233. doi: 10.1006/bcmd.2002.0487. [DOI] [PubMed] [Google Scholar]
- 34.Harandi O. F., Hedge S., Wu D.-C., Mckeone D., Paulson R. F. Murine erythroid short-term radioprotection requires a BMP4-dependent, self-renewing population of stress erythroid progenitors. Journal of Clinical Investigation. 2010;120(12):4507–4519. doi: 10.1172/jci41291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Lindern M., Zauner W., Mellitzer G., et al. The glucocorticoid receptor cooperates with the erythropoietin receptor and c-Kit to enhance and sustain proliferation of erythroid progenitors in vitro. Blood. 1999;94(2):550–559. [PubMed] [Google Scholar]
- 36.Gordon M. S., Hoffman R. Growth factors affecting human thrombocytopoiesis: potential agents for the treatment of thrombocytopenia. Blood. 1992;80(2):302–307. [PubMed] [Google Scholar]
- 37.McNiece I. K., Langley K. E., Zsebo K. M. Recombinant human stem cell factor synergises with GM-CSF, G-CSF, IL-3 and epo to stimulate human progenitor cells of the myeloid and erythroid lineages. Experimental Hematology. 1991;19(3):226–231. [PubMed] [Google Scholar]
- 38.Steinlein P., Wessely O., Meyer S., Deiner E.-M., Hayman M. J., Beug H. Primary, self-renewing erythroid progenitors develop through activation of both tyrosine kinase and steroid hormone receptors. Current Biology. 1995;5(2):191–204. doi: 10.1016/S0960-9822(95)00040-6. [DOI] [PubMed] [Google Scholar]
- 39.de Sauvage F. J., Hass P. E., Spencer S. D., et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the C-MpI ligand. Nature. 1994;369(6481):533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- 40.Alexander W. S. Thrombopoietin. Growth Factors. 1999;17(1):13–24. doi: 10.3109/08977199909001059. [DOI] [PubMed] [Google Scholar]
- 41.Alexander W. S. Thrombopoietin and the c-Mpl receptor: insights from gene targeting. International Journal of Biochemistry and Cell Biology. 1999;31(10):1027–1035. doi: 10.1016/s1357-2725(99)00079-5. [DOI] [PubMed] [Google Scholar]
- 42.Park H., Park S. S., Jin E. H., et al. Identification of functionally important residues of human thrombopoietin. The Journal of Biological Chemistry. 1998;273(1):256–261. doi: 10.1074/jbc.273.1.256. [DOI] [PubMed] [Google Scholar]
- 43.Foster D., Lok S. Biological roles for the second domain of thrombopoietin. Stem Cells. 1996;14(supplement 1):102–107. doi: 10.1002/stem.5530140712. [DOI] [PubMed] [Google Scholar]
- 44.Linden H. M., Kaushansky K. The glycan domain of thrombopoietin enhances its secretion. Biochemistry. 2000;39(11):3044–3051. doi: 10.1021/bi991756h. [DOI] [PubMed] [Google Scholar]
- 45.Erslev A. J., Caro J. Physiologic and molecular biology of erythropoietin. Medical Oncology and Tumor Pharmacotherapy. 1986;3(3-4):159–164. doi: 10.1007/BF02934992. [DOI] [PubMed] [Google Scholar]
- 46.Mignotte V., Vigon I., de Crevecoeur E. B., Romeo P.-H., Lemarchandel V., Chretien S. Structure and transcription of the human c-mpl gene (MPL) Genomics. 1994;20(1):5–12. doi: 10.1006/geno.1994.1120. [DOI] [PubMed] [Google Scholar]
- 47.Youssoufian H., Longmore G., Neumann D., Yoshimura A., Lodish H. F. Structure, function, and activation of the erythropoietin receptor. Blood. 1993;81(9):2223–2236. [PubMed] [Google Scholar]
- 48.Robb L. Cytokine receptors and hematopoietic differentiation. Oncogene. 2007;26(47):6715–6723. doi: 10.1038/sj.onc.1210756. [DOI] [PubMed] [Google Scholar]
- 49.Constantinescu S. N., Ghaffari S., Lodish H. F. The erythropoietin receptor: structure, activation and intracellular signal transduction. Trends in Endocrinology & Metabolism. 1999;10(1):18–23. doi: 10.1016/s1043-2760(98)00101-5. [DOI] [PubMed] [Google Scholar]
- 50.Drachman J. G., Kaushansky K. Dissecting the thrombopoietin receptor: functional elements of the Mpl cytoplasmic domain. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(6):2350–2355. doi: 10.1073/pnas.94.6.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tilbrook P. A., Klinken S. P. The erythropoietin receptor. The International Journal of Biochemistry & Cell Biology. 1999;31(10):1001–1005. doi: 10.1016/s1357-2725(99)00071-0. [DOI] [PubMed] [Google Scholar]
- 52.Hitchcock I. S., Kaushansky K. Thrombopoietin from beginning to end. British Journal of Haematology. 2014;165(2):259–268. doi: 10.1111/bjh.12772. [DOI] [PubMed] [Google Scholar]
- 53.Kota J., Caceres N., Constantinescu S. N. Aberrant signal transduction pathways in myeloproliferative neoplasms. Leukemia. 2008;22(10):1828–1840. doi: 10.1038/leu.2008.236. [DOI] [PubMed] [Google Scholar]
- 54.Chin H., Arai A., Wakao H., Kamiyama R., Miyasaka N., Miura O. Lyn physically associates with the erythropoietin receptor and may play a role in activation of the Stat5 pathway. Blood. 1998;91(10):3734–3745. [PubMed] [Google Scholar]
- 55.Kaushansky K. Molecular mechanisms of thrombopoietin signaling. Journal of Thrombosis and Haemostasis. 2009;7(supplement 1):235–238. doi: 10.1111/j.1538-7836.2009.03419.x. [DOI] [PubMed] [Google Scholar]
- 56.Ingley E. Integrating novel signaling pathways involved in erythropoiesis. IUBMB Life. 2012;64(5):402–410. doi: 10.1002/iub.1024. [DOI] [PubMed] [Google Scholar]
- 57.Doré L. C., Crispino J. D. Transcription factor networks in erythroid cell and megakaryocyte development. Blood. 2011;118(2):231–239. doi: 10.1182/blood-2011-04-285981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klimchenko O., Mori M., DiStefano A., et al. A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood. 2009;114(8):1506–1517. doi: 10.1182/blood-2008-09-178863. [DOI] [PubMed] [Google Scholar]
- 59.Shivdasani R. A. Molecular and transcriptional regulation of megakaryocyte differentiation. Stem Cells. 2001;19(5):397–407. doi: 10.1634/stemcells.19-5-397. [DOI] [PubMed] [Google Scholar]
- 60.Kumar S., Hedges S. B. A molecular timescale for vertebrate evolution. Nature. 1998;392(6679):917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- 61.Ji P., Murata-Hori M., Lodish H. F. Formation of mammalian erythrocytes: chromatin condensation and enucleation. Trends in Cell Biology. 2011;21(7):409–415. doi: 10.1016/j.tcb.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmaier A. A., Stalker T. J., Runge J. J., et al. Occlusive thrombi arise in mammals but not birds in response to arterial injury: evolutionary insight into human cardiovascular disease. Blood. 2011;118(13):3661–3669. doi: 10.1182/blood-2011-02-338244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bartůněk P., Králová J., Blendinger G., Dvořák M., Zenke M. GATA-1 and c-myb crosstalk during red blood cell differentiation through GATA-1 binding sites in the c-myb promoter. Oncogene. 2003;22(13):1927–1935. doi: 10.1038/sj.onc.1206281. [DOI] [PubMed] [Google Scholar]
- 64.de Jong J. L. O., Zon L. I. Use of the zebrafish system to study primitive and definitive hematopoiesis. Annual Review of Genetics. 2005;39:481–501. doi: 10.1146/annurev.genet.39.073003.095931. [DOI] [PubMed] [Google Scholar]
- 65.Dolznig H., Bartunek P., Nasmyth K., Mullner E. W., Beug H. Terminal differentiation of normal chicken erythroid progenitors: shortening of G1 correlates with loss of D-cyclin/cdk4 expression and altered cell size control. Cell Growth & Differentiation. 1995;6(11):1341–1352. [PubMed] [Google Scholar]
- 66.Kulkeaw K., Sugiyama D. Zebrafish erythropoiesis and the utility of fish as models of anemia. Stem Cell Research and Therapy. 2012;3(6, article 55) doi: 10.1186/scrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amigo J. D., Ackermann G. E., Cope J. J., et al. The role and regulation of friend of GATA-1 (FOG-1) during blood development in the zebrafish. Blood. 2009;114(21):4654–4663. doi: 10.1182/blood-2008-12-189910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kulessa H., Frampton J., Graf T. GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts. Genes and Development. 1995;9(10):1250–1262. doi: 10.1101/gad.9.10.1250. [DOI] [PubMed] [Google Scholar]
- 69.Lin H.-F., Traver D., Zhu H., et al. Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood. 2005;106(12):3803–3810. doi: 10.1182/blood-2005-01-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson H. J., Gandhi M. J., Shafizadeh E., et al. In vivo inactivation of MASTL kinase results in thrombocytopenia. Experimental Hematology. 2009;37(8):901–908. doi: 10.1016/j.exphem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lang M. R., Gihr G., Gawaz M. P., Müller I. Hemostasis in Danio rerio: is the zebrafish a useful model for platelet research? Journal of Thrombosis and Haemostasis. 2010;8(6):1159–1169. doi: 10.1111/j.1538-7836.2010.03815.x. [DOI] [PubMed] [Google Scholar]
- 72.Hattangadi S. M., Wong P., Zhang L., Flygare J., Lodish H. F. From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood. 2011;118(24):6258–6268. doi: 10.1182/blood-2011-07-356006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tomer A., Harker L. A., Burstein S. A. Purification of human megakaryocytes by fluorescence-activated cell sorting. Blood. 1987;70(6):1735–1742. [PubMed] [Google Scholar]
- 74.Hartenstein V. Blood cells and blood cell development in the animal kingdom. Annual Review of Cell and Developmental Biology. 2006;22:677–712. doi: 10.1146/annurev.cellbio.22.010605.093317. [DOI] [PubMed] [Google Scholar]
- 75.Glomski C. A., Tamburlin J. The phylogenetic odyssey of the erythrocyte. I. Hemoglobin: the universal respiratory pigment. Histology and Histopathology. 1989;4(4):509–514. [PubMed] [Google Scholar]
- 76.Glomski C. A., Tamburlin J. The phylogenetic odyssey of the erythrocyte. II. The early or invertebrate prototypes. Histology and Histopathology. 1990;5(4):513–525. [PubMed] [Google Scholar]
- 77.Needham A. E. Haemostatic mechanisms in the invertebrata. Symposia of the Zoological Society of London. 1970;27:19–44. [Google Scholar]
- 78.Davidson C. J., Tuddenham E. G., McVey J. H. 450 million years of hemostasis. Journal of Thrombosis and Haemostasis. 2003;1(7):1487–1494. doi: 10.1046/j.1538-7836.2003.00334.x. [DOI] [PubMed] [Google Scholar]
- 79.Doolittle R. F., Jiang Y., Nand J. Genomic evidence for a simpler clotting scheme in jawless vertebrates. Journal of Molecular Evolution. 2008;66(2):185–196. doi: 10.1007/s00239-008-9074-8. [DOI] [PubMed] [Google Scholar]
- 80.Glomski C. A., Tamburlin J., Chainani M. The phylogenetic odyssey of the erythrocyte. III. Fish, the lower vertebrate experience. Histology and Histopathology. 1992;7(3):501–528. [PubMed] [Google Scholar]
- 81.Kobayashi M., Laver J. H., Kato T., Miyazaki H., Ogawa M. Recombinant human thrombopoietin (Mpl ligand) enhances proliferation of erythroid progenitors. Blood. 1995;86(7):2494–2499. [PubMed] [Google Scholar]
- 82.Kieran M. W., Perkins A. C., Orkin S. H., Zon L. I. Thrombopoietin rescues in vitro erythroid colony formation from mouse embryos lacking the erythropoietin receptor. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(17):9126–9131. doi: 10.1073/pnas.93.17.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Broudy V. C., Lin N. L., Kaushansky K. Thrombopoietin (c-mpl ligand) acts synergistically with erythropoietin, stem cell factor, and interleukin-11 to enhance murine megakaryocyte colony growth and increases megakaryocyte ploidy in vitro. Blood. 1995;85(7):1719–1726. [PubMed] [Google Scholar]