Abstract
The zebrafish/tumor xenograft angiogenesis assay is used to approach tumor angiogenesis, a pivotal step in cancer progression and target for anti-tumor therapies. Here, we evaluated whether the assay could allow the identification of microRNAs having an anti-angiogenic potential. For that, we transfected DU-145 prostate cancer cells with four microRNAs (miR-125a, miR-320, miR-487b, miR-492) responsive to both anti- and pro-angiogenic stimuli applied to human umbilical vein endothelial cells. After transfection, DU-145 cells were injected close to the developing subintestinal vessels of transgenic Tg(Kdrl:eGFP)s843 zebrafish embryos that express green fluorescent protein under the control of Kdrl promoter. At 72 h post-fertilization, we observed that green fluorescent protein–positive neo-vessels infiltrated the graft of DU-145 transfected with miR-125a, miR-320, and miR-487b. Vice versa, neo-vessel formation and tumor cell infiltration were inhibited when DU-145 cells transfected with miR-492 were used. These results indicated that the zebrafish/tumor xenograft assay was adequate to identify microRNAs able to suppress the release of angiogenic growth factors by angiogenic tumor cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-014-9735-y) contains supplementary material, which is available to authorized users.
Keywords: microRNAs, Tumor angiogenesis, Zebrafish/tumor xenografts, Prostate tumor cells
Introduction
Angiogenesis plays a critical role in cancer growth and progression (Carmeliet and Jain 2000; Hanahan and Weinberg 2000). Since tumors cannot grow without development of a new blood supply, their growth and expansion are thought to be totally dependent on angiogenesis (Folkman 2002). The angiogenic growth factors released by neoplastic cells, including members of the vascular endothelial growth factor (Ferrara 2004) and fibroblast growth factor (Presta et al. 2005) families, are responsible for tumor neo-vascularization. Therefore, the identification of anti-angiogenic molecules may have significant implications for the development of antineoplastic therapies.
Rodents and chick embryo have been developed as model systems to investigate the angiogenic process and to screen pro-angiogenic and anti-angiogenic compounds, each with its own unique characteristics and disadvantages (Hasan et al. 2004). Several reports proposed zebrafish (Danio rerio) angiogenesis as a new model for drug screening (Serbedzija et al. 1999) and tumor angiogenesis (Tobia et al. 2011). When compared with other vertebrate model systems, the zebrafish offers many advantages for the study of angiogenesis, including small size, low cost, ease of experimentation and amenability to in vivo manipulation (Thisse and Zon 2002). It is noteworthy that the zebrafish embryos are nearly transparent until 5 days post fertilization (dpf) and this feature, coupled to the availability of reporter fluorescent transgenic lines for endothelial markers, permits the direct in vivo observation of the blood vessels during development without instrumentation or manipulation other than fluorescent microscopy (Santoro 2011). All together such characteristics rise the zebrafish (Danio rerio)/tumor xenograft model a promising alternative model in cancer research (Lam et al. 2006). The use of tumor cell engrafts in animal models may allow the continuous delivery of angiogenic factors produced by a limited number of tumor cells, thus mimicking the initial stages of tumor angiogenesis and metastasis. Recent studies have shown the feasibility of injecting human melanoma cells in zebrafish embryos to follow their fate and study their impact on zebrafish vessel development (Topczewska et al. 2006). The basic concept is that pro-angiogenic factors released locally by the tumor graft will affect the normal developmental pattern of the sub intestinal veins (SIVs) by stimulating the migration and growth of sprouting vessels toward the implant (Nicoli et al. 2007). When compared with the rabbit cornea and chick embryo chorioallantoic membrane assays, the zebrafish/tumor xenograft model showed a similar capacity to discriminate between highly angiogenic and poorly angiogenic tumor cell lines (Nicoli and Presta 2007).
Pro-angiogenesis and anti-angiogenesis microRNAs are attractive drugs for therapy of cardiovascular and cancer diseases (Patella and Rainaldi 2012). In this work, we exploited the zebrafish/tumor xenograft model to evaluate whether the angiogenic response elicited by tumor cells was affected when microRNAs (miRNAs) related to angiogenesis were over expressed.
Materials and methods
Cells and growth conditions
Human Umbilical Vein Endothelial Cells (HUVECs), obtained from human umbilical cords, were grown on gelatine-coated plates in M199 medium supplemented with 10 % fetal bovine serum (FBS), penicillin and streptomycin 1 % and l-glutamine 1 % (Euroclone, Milan, Italy), epithelial growth factor (20 ng/ml), heparin (12.5 U/ml) (Sigma-Aldrich, Buchs SG, Switzerland). DU-145 prostate cancer cell line, kindly provided by Dr. Maria De Angioletti (Istituto Toscano Tumori – ITT, Florence, Italy), was grown in RPMI 1640 with 10 % FBS (Euroclone, Milan, Italy). Cells were incubated at 37 °C in a humidified atmosphere containing 6 % CO2.
qRT-PCR
To detect the expression level of miRNAs, total RNA was extracted from either HUVECs or DU-145 using miRNeasy mini kit (Qiagen, Hilden, Germany). To quantify miRNAs, 1 µg of total RNA was retrotranscribed with miScript Reverse Transcription Kit and qRT-PCR was carried out using miScript SYBR Green PCR Kit (Qiagen, Hilden, Germany). All reactions were performed in triplicate with Rotor-Gene Q 2Plex (Qiagen). Relative quantification of miRNAs expression was normalized with those obtained from the amplification of U6 as internal control.
Transfection of ribooligonucleotides
Approximately 2x105 cells were seeded in each well of a 6-well plate. After 24 h, HUVECs were transfected using Gene Silencer Transfection Reagent (Genlantis, San Diego, CA, USA) according to the manufacturer's instructions. DU-145 cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The following double strand ribooligonucleotides (GenePharma, Shangai, China) were transfected: miR-125a (5′-UCCCUGAGACCCUUUAACCUGUGAUU-3′; 5′-UCACAGGUUAAAGGGUCUCAGUUAUU-3′), miR-320 (5′AAAAGCUGGGUUGAGAGGGCGAUUU-3′; 5′-AUCGCCCUCUCAACCCAGCUAAUUU-3′), miR-487b (5′-AAUCGUACAGGGUCAUCCACUUUU-3′;5′-AAGUGGAUGACCCUGUACGUAUUU-3′), miR-492 (5′-AGGACCUGCGGGACAAGAUUCUUUU-3′; 5′-AAGAAUCUUGUCCCGCAGGUAAUUU-3′). As control we used miR-Ct (5′-UUCUCCGAACGUGUCACGUTT-3′; 5′-ACGUGACACGUUCGGAGAATT-3′). After 6 h from transfection fresh growth medium was added. Cells were collected 48 h after transfection and tube formation assay was used to classify miRNAs as pro- or anti-angiogenesis.
Tube formation assay
HUVECs (7x104) transfected with miRNAs were seeded in 30 mm diameter dishes on a Matrigel matrix (Sigma-Aldrich, Buchs SG, Switzerland) prepared as the manufacturer’s recommendation. Crystal violet 4 % (Sigma-Aldrich, Buchs SG, Switzerland) was added 6 h after seeding for 20 min and finally dishes were rinsed in water. Tube formation was evaluated by using ImageJ software.
Animal model, tumor cells and grafting procedure
Tg(Kdrl:eGFP)s843 was the Zebrafish transgenic line used in this study. Fishes of this transgenic cell line express the eGFP under the control of endothelial Kdrl promoter allowing the imaging of vessels. Fishes were raised in a ZEBTECH zebrafish Housing System (Tecniplast, Varese, Italy). Following current Italian national rules no approval needs to be given for research on zebrafish embryos.
DU-145 prostate cancer cells were transfected with each miRNA and after 48 h transfected cells were collected and grafted as described by Nicoli and Presta (2007). Briefly, DU-145 transfected cells were mixed with Matrigel and samples of 1000 cells were grafted into the perivitelline space of 48 hpf (hours post fertilization) transgenic Tg(Kdrl:eGFP)s843 embryos. Xenografts were performed by microinjection in a constant injection volume of ~ 5 nl (confirmed by volume analysis) using a microinjector (Tritech Research, Los Angeles, CA, USA). After 24 h post xenografting the neo-vascular response was imaged by confocal fluorescence microscopy. For each experiment more than 20 xenografted transgenic Tg(Kdrl:eGFP)s843 zebrafish embryos were imaged as followings: embryos were treated with 0.01 mM tricaine solution (Sigma-Aldrich, Buchs SG, Switzerland) and embedded in 1 % low melting agarose medium (Bio-Rad, Hercules, CA, USA). Stacks were acquired with a Leica TCS-SL DM IRE 2 confocal microscope, image stacks were processed with FIJI-WIN32 by projection.
Statistical analyses
Results are expressed as mean ± SD of at least three biological replicates and data were analyzed by Student t test. P < 0.05 was considered statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001). Microarray Z score values are calculated as (log2 (R/G)–μ)/σ, where R/G is the fold change of the intensity of each microarray spot, μ and σ are the mean value and the standard deviation of the global intensity.
Results and Discussion
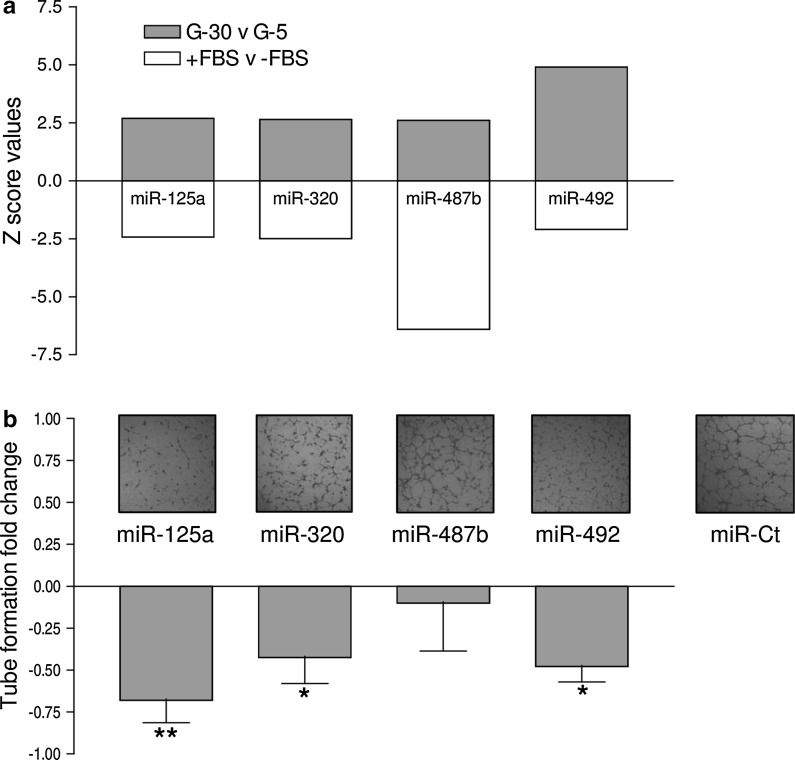
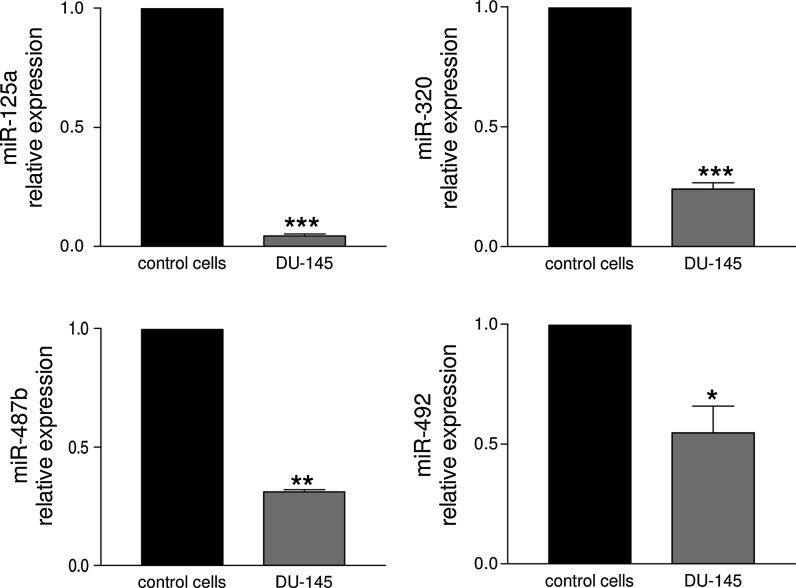
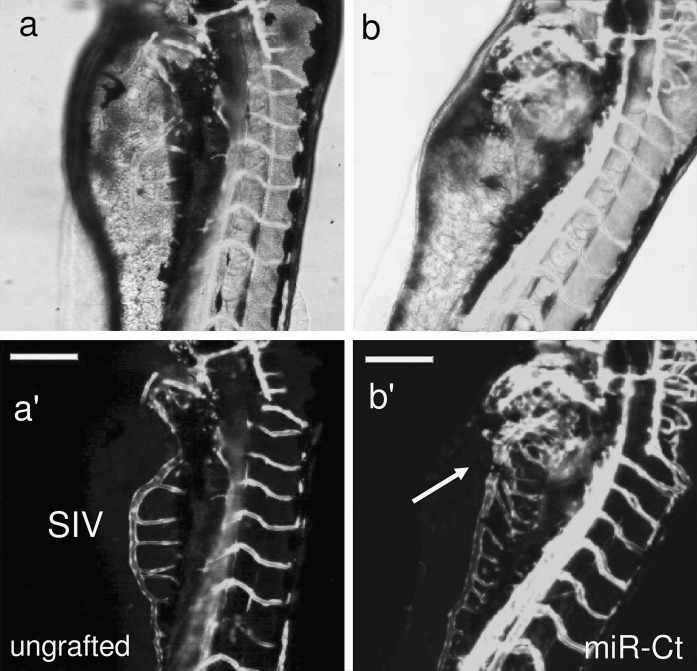
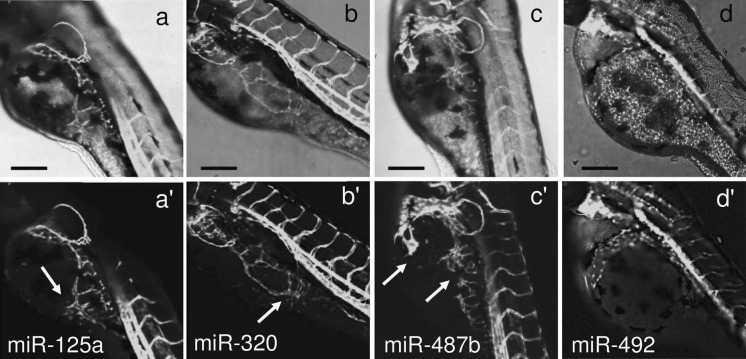
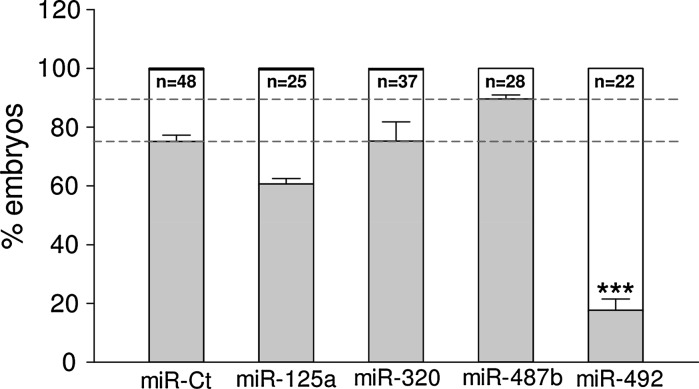
The angiogenesis status is defined by a balance between pro- and anti-angiogenic molecules. This homeostasis is perfectly controlled by a tight regulation of the level of angiogenesis inducers and inhibitors, and any dysregulation is often associated with pathological disorders. Metabolic and physico-chemical stresses have been reported to either promote or impair the angiogenic properties of endothelial cells. Possible mediators of stress responses are microRNAs (miRNAs) (Patella and Rainaldi 2012), endogenous 21-25 nucleotides non-coding single-stranded RNA molecules that generally bind to the 3′UTR of target genes and inhibit their translation. Our approach was to compare the global miRNA expression profiles of HUVECs challenged with either high glucose as anti-angiogenic stimuli (30 mM glucose, G-30) or FBS addition as pro-angiogenic stimuli (Supplemental material). We identified four miRNAs (miR-125a, miR-320, miR-487b, miR-492) that moved in opposite directions depending on the applied stimulus (Fig. 1a). Hyperglycemia, mimicked in vitro by high glucose, is recognized as a determinant of endothelial dysfunction and insulin resistance, two conditions leading to severe cardiovascular problems (Kim et al. 2006). In response to high glucose, the PI3 K/Akt/NO pathway plays an important role in the protection of endothelial cells from apoptosis at early stage (< 24 h), whereas reduced proliferation and increase in apoptosis is observed at later stages (> 48 h) (Varma et al. 2005; Ho et al. 2006). An effect of high glucose on migration and angiogenic potential has been also reported as well as impairment of eNOS functionality and expression (Ding et al. 2000; Hamuro et al. 2002). To ascertain whether the four miRNAs were involved in the HUVECs angiogenesis, we transfected each miRNA and used the tube formation assay to test if they were angiogenesis inducers or inhibitors. The tube formation while unaffected by miR-487b was reduced by miR-125a, miR-320 and miR-492 (Fig. 1b), thus indicating that three out of four miRNAs up regulated by high glucose behaved as anti-angiogenic molecules in HUVECs. Then we extended the functional analysis in zebrafish model as this could help in the development of strategies to harness the dynamics of blood vessels in human health and diseases. The zebrafish/tumor xenograft angiogenesis assay is based on the grafting of mammalian tumor cells in the proximity of the developing SIV plexus at 48 hpf. To evaluate whether the assay could be used for the validation of anti-angiogenic miRNAs, we transfected them in DU-145 prostate cancer cell line often used for investigating tumor angiogenesis (Connolly and Rose 1998; Arbiser et al. 2002; Zhang et al. 2013). Firstly we evaluated the miR-125a, miR-320, miR-487b and miR-492 endogenous expression levels in DU-145 cells in comparison to normal prostate cells (FirstChoice Human Prostate Total RNA, Ambion). The fact that all miRNAs were down regulated in DU-145 (Fig. 2) render these cells a good cellular model for gain of function experiments. DU-145 cells were transfected with each miRNA and then grafted by injection in Tg(kdrl:eGFP)s843 zebrafish embryos. At microscopic level, in comparison to ungrafted embryos (Fig. 3a, a’), DU-145/miR-Ct grafts (cells transfected with miR-Ct) favored the neo-vessel formation (Fig. 3b, b’), suggesting that the pro-angiogenic factors were released locally by DU-145 cells. Then, we investigated whether DU-145 cells transfected with miR-125a, miR-320, miR-487b and miR-492 modified their capability to stimulate the neo-vessel formation. The results are shown in Fig. 4. DU-145/miR-125a (Fig. 4a, a’), DU-145/miR-320 (Fig. 4b, b’) and DU-145/miR-487b (Fig. 4c, c’) grafts affected the normal developmental pattern of the SIVs by stimulating the migration and growth of sprouting vessels toward the implant like DU-145/miR-Ct grafts (Fig. 3b, b’), thus indicating that miR-125a, miR-320 and miR-487b were not angiogenesis inhibitors. Vice versa, neo vessels formation was strongly inhibited by DU-145/miR-492 grafts (Fig. 4d, d’) and this confirmed previous data showing that miR-492 impairs the angiogenic potential of HUVECs (Patella et al. 2013). To give a measure of the grafting efficiency we determined the frequency of embryos with neo-vessel formation over the injected embryos: 60–90 % of the injected embryos showed neo-vessel formation with the exception of DU-145/miR-492 grafts which induced neo-vessel formation in <15 % of injected embryos (Fig. 5). The discovery that neo-vessel formation was suppressed by overexpressing miR-492 in angiogenic tumor cells implies that the zebrafish/tumor xenograft assay can be used to validate microRNAs with potential anti-angiogenesis activity. If so, the development of anti neoplastic therapies based on anti-angiogenic miRNAs might be tempted. So far, miRNA-based therapy was successful in few cases (Janssen et al. 2013; Thum 2013), but we are confident that the resolving power of the zebrafish/tumor xenograft assay in identifying anti-angiogenic miRNAs could greatly contribute to extend their use as therapeutic molecules.
Fig. 1.
Positive and negative Z score values of miR-125a, miR-320, miR-487b and miR-492 resulting from the miRNA microarray comparative analysis of 30 mM (G-30) versus 5 mM (G-5) glucose treated HUVECs and FBS plus (+FBS) versus FBS minus (−FBS) treated HUVECs (a). Tube formation of HUVECs transfected with miR-125a, miR-320, and miR-487b and miR-492 (green bar chart): tube formation was reduced by miR-125, miR-320 and miR-492; representative pictures of tube formation are shown above the relative bar (b). (Color figure online)
Fig. 2.
Detection by qRT-PCR of the expression levels of miR-125a, miR-320, miR-487b and miR-492 in prostate cancer cell DU-145. In comparison to normal prostate cells (control cells) the four miRNAs were down regulated
Fig. 3.
Tg(kdrl:eGFP)s843 zebrafish embryos were ungrafted (a, a’) or grafted with DU-145 cells transfected with miR-Ct (b, b’). Images representative of transgenic embryos showing that neo-vessel formation was favored in embryos engrafted with DU-145/miR-Ct
Fig. 4.
Tg(kdrl:eGFP)s843 zebrafish embryos were grafted with DU-145 cells transfected with miR-125a (a, a’), miR-320 (b, b’), miR-487b (c, c’) and miR-492 (d, d’). In comparison to miR-Ct xenografts, only miR-492 xenografts reduced the vessel formation
Fig. 5.
DU-145 prostate cancer cells transfected with miR-125a, miR-320, miR-487b and miR-492 were injected in Tg(kdrl:eGFP)s843 zebrafish embryos. The percentage of embryos positive for neo-vessel formation was calculated on the total number of injected embryos
Electronic supplementary material
Acknowledgments
This work was partly supported by RSTL-CNR Grant, Code 783.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Elena Chiavacci and Milena Rizzo have contributed equally to the work.
References
- Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci USA. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Connolly JM, Rose DP. Angiogenesis in two human prostate cancer cell lines with differing metastatic potential when growing as solid tumors in nude mice. J Urol. 1998;160:932–936. doi: 10.1016/S0022-5347(01)62835-0. [DOI] [PubMed] [Google Scholar]
- Ding Y, Vaziri ND, Coulson R, Kamanna VS, Roh DD. Effects of simulated hyperglycemia, insulin, and glucagon on endothelial nitric oxide synthase expression. Am J Physiol Endocrinol Metab. 2000;279:E11–E17. doi: 10.1152/ajpendo.2000.279.1.E11. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9(Suppl 1):2–10. doi: 10.1634/theoncologist.9-suppl_1-2. [DOI] [PubMed] [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- Hamuro M, Polan J, Natarajan M, Mohan S. High glucose induced nuclear factor kappa B mediated inhibition of endothelial cell migration. Atherosclerosis. 2002;162:277–287. doi: 10.1016/S0021-9150(01)00719-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hasan J, Shnyder SD, Bibby M, Double JA, Bicknel R, Jayson GC. Quantitative angiogenesis assays in vivo- -a review. Angiogenesis. 2004;7:1–16. doi: 10.1023/B:AGEN.0000037338.51851.d1. [DOI] [PubMed] [Google Scholar]
- Ho FM, Lin WW, Chen BC, Chao CM, Yang CR, Lin LY, Lai CC, Liu SH, Liau CS. High glucose-induced apoptosis in human vascular endothelial cells is mediated through NF-kappaB and c-Jun NH2-terminal kinase pathway and prevented by PI3 K/Akt/eNOS pathway. Cell Signal. 2006;18:391–399. doi: 10.1016/j.cellsig.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction - Molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- Lam SH, Wu YL, Vega VB, Miller LD, Spitsbergen J, Tong Y, Zhan H, Govindarajan KR, Lee S, Mathavan S, Murthy KR, Buhler DR, Liu ET, Gong Z. Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat Biotechnol. 2006;24:73–75. doi: 10.1038/nbt1169. [DOI] [PubMed] [Google Scholar]
- Nicoli S, Presta M. The zebrafish/tumor xenograft angiogenesis assay. Nat Protoc. 2007;2:2918–2923. doi: 10.1038/nprot.2007.412. [DOI] [PubMed] [Google Scholar]
- Nicoli S, Ribatti D, Cotelli F, Presta M. Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res. 2007;67:2927–2931. doi: 10.1158/0008-5472.CAN-06-4268. [DOI] [PubMed] [Google Scholar]
- Patella F, Rainaldi G. microRNAs mediate metabolic stresses and angiogenesis. Cell Mol Life Sci. 2012;69:1049–1065. doi: 10.1007/s00018-011-0775-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patella F, Leucci E, Evangelista M, Parker B, Wen J, Mercatanti A, Rizzo M, Chiavacci E, Lund AH, Rainaldi G. MiR-492 impairs the angiogenic potential of endothelial cells. J Cell Mol Med. 2013;17:1006–1015. doi: 10.1111/jcmm.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitto L, Rizzo M, Simili M, Colligiani D, Evangelista M, Mercatanti A, Mariani L, Cremisi F, Rainaldi G. miR- 290 acts as a physiological effector of senescence in mouse embryo fibroblasts. Physiol Genomics. 2009;39:210–218. doi: 10.1152/physiolgenomics.00085.2009. [DOI] [PubMed] [Google Scholar]
- Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Rainer J, Sanchez-Cabo F, Stocker G, Sturn A, Trajanoski Z. CARMAweb: comprehensive R- and bioconductor- based web service for microarray data analysis. Nucleic Acids Res. 2006;34:W498–W503. doi: 10.1093/nar/gkl038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MM. “Fishing” for endothelial microRNA functions and dysfunction. Vascul Pharmacol. 2011;55:60–68. doi: 10.1016/j.vph.2011.08.224. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Flynn E, Willett CE. Zebrafish angiogenesis: a new model for drug screening. Angiogenesis. 1999;3:353–359. doi: 10.1023/A:1026598300052. [DOI] [PubMed] [Google Scholar]
- Thisse C, Zon LI. Organogenesis–heart and blood formation from the zebrafish point of view. Science. 2002;295:457–462. doi: 10.1126/science.1063654. [DOI] [PubMed] [Google Scholar]
- Thum T. MicroRNA therapeutics in cardiovascular medicine. EMBO Mol Med. 2013;4:3–14. doi: 10.1002/emmm.201100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobia C, De Sena G, Presta M. Zebrafish embryo, a tool to study tumor angiogenesis. Int J Dev Biol. 2011;55:505–509. doi: 10.1387/ijdb.103238ct. [DOI] [PubMed] [Google Scholar]
- Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- Varma S, Lal BK, Zheng R, Breslin JW, Saito S, Pappas PJ, Hobson RW, 2nd, Duran WN. Hyperglycemia alters PI3k and Akt signaling and leads to endothelial cell proliferative dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H1744–H1751. doi: 10.1152/ajpheart.01088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Lee YW, Rui YF, Cheng TY, Jiang XH, Li G. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res Ther. 2013;4:70. doi: 10.1186/scrt221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.