Abstract
Background and Purpose
Two new technologies are likely to revolutionize cardiac safety and drug development: in vitro experiments on human‐induced pluripotent stem cell‐derived cardiomyocytes (hiPSC‐CMs) and in silico human adult ventricular cardiomyocyte (hAdultV‐CM) models. Their combination was recently proposed as a potential replacement for the present hERG‐based QT study for pharmacological safety assessments. Here, we systematically compared in silico the effects of selective ionic current block on hiPSC‐CM and hAdultV‐CM action potentials (APs), to identify similarities/differences and to illustrate the potential of computational models as supportive tools for evaluating new in vitro technologies.
Experimental Approach
In silico AP models of ventricular‐like and atrial‐like hiPSC‐CMs and hAdultV‐CM were used to simulate the main effects of four degrees of block of the main cardiac transmembrane currents.
Key Results
Qualitatively, hiPSC‐CM and hAdultV‐CM APs showed similar responses to current block, consistent with results from experiments. However, quantitatively, hiPSC‐CMs were more sensitive to block of (i) L‐type Ca2+ currents due to the overexpression of the Na+/Ca2+ exchanger (leading to shorter APs) and (ii) the inward rectifier K+ current due to reduced repolarization reserve (inducing diastolic potential depolarization and repolarization failure).
Conclusions and Implications
In silico hiPSC‐CMs and hAdultV‐CMs exhibit a similar response to selective current blocks. However, overall hiPSC‐CMs show greater sensitivity to block, which may facilitate in vitro identification of drug‐induced effects. Extrapolation of drug effects from hiPSC‐CM to hAdultV‐CM and pro‐arrhythmic risk assessment can be facilitated by in silico predictions using biophysically‐based computational models.
Abbreviations
- AL
atrial‐like
- APD
AP duration
- APDratio
AP shape factor
- EAD
early after depolarizations
- hAdultV‐CM
human adult ventricular cardiomyocyte
- hiPSC
human‐induced pluripotent stem cell
- hiPSC‐CM
hiPSC‐derived cardiomyocyte
- MDP
maximum diastolic potential
- ORd
O'Hara–Rudy model of human adult ventricular cell
- Paci2013
Paci model of hiPSC‐CM
- Rate
rate of spontaneous beating
- VL
ventricular‐like
- VMax
maximum upstroke velocity
Tables of Links
| TARGETS |
|---|
| GPCRs b |
| β‐adrenoceptors |
| Transporters b |
| Na+/Ca2+ exchanger |
| LIGANDS | ||
|---|---|---|
| Chromanol 293B | Ivabradine | Nisoldipine |
| Dofetilide | Mexiletine | ORM10103 |
| E4031 | Nickel | TTX |
| HMR‐1556 | Nifedipine | Zatebradine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a, 2013b).
Introduction
Preclinical evaluation of the safety and efficacy of compounds before doing costly clinical trials is a major concern for pharmaceutical and cosmetic industry, regulators and society as a whole. Of major importance is the assessment of the effects of all new compounds on the heart, specifically for the identification of possible cardiotoxic side effects such as pro‐arrhythmia (Heist and Ruskin, 2010; Kannankeril, 2010; Sauer and Newton‐Cheh, 2012; Shah, 2013). Currently, pro‐arrhythmic risk is initially assessed preclinically using ion channel assays (especially hERG assays) and evaluating the prolongation of the cardiac action potential (AP) duration, which is manifested in the ECG as the QT interval (Leishman et al., 2012; Hamdam et al., 2013). In vivo and ex vivo experiments using animals (rats, rabbits, dogs and non‐human primates) are also used, in spite of two major limitations (Rodriguez et al., 2010): (i) impaired translation from animal experiments to humans due to inter‐species differences in the underlying physiology, and (ii) a lack of heterogeneity and diseased specimens in the samples considered in the experiments compared with the huge variability in the human population.
Two novel technologies have been proposed as a promising way to overcome the limitations of the existing methodologies used for preclinical safety evaluation of pharmaceutical compounds: in vitro experiments using human‐induced pluripotent stem cell‐derived cardiomyocytes (hiPSC‐CMs) and in silico simulations using human adult ventricular cardiomyocyte (hAdultV‐CM) models. The potential of both techniques, and especially their combination, has been acknowledged by the pharmaceutical industry, and also by the recent announcement by the US Food and Drug administration of a new paradigm for the evaluation of new molecular entities: ‘the comprehensive in vitro pro‐arrhythmia assay’ (Sager et al., 2014). The proposed scheme would include the following: (i) screening of drug action on multiple human cardiac currents (rather than just hERG) in heterologous expression systems; (ii) integration of ion channel/drug interaction data in in silico models of human ventricular electrophysiology to predict and evaluate changes in the human action potential; and (iii) in vitro evaluation of compound effects in a myocyte assay such as hiPSC‐CMs and comparison to the in silico results. A critical point not assessed so far is how electrophysiological differences between hiPSC‐CM and hAdultV‐CM may result in different drug responses. Identifying these differences is critical to ensure the interpretation of combined hiPSC‐CM and hAdultV‐CM in vitro/in silico assays.
In this study, we have conducted the first systematic in silico evaluation of the effects of selective ionic current block on the APs of ventricular‐like (VL) and atrial‐like (AL) hiPSC‐CMs and hAdultV‐CMs. Our ultimate aim was to inform the potential of in vitro and in silico hiPSC‐CMs technologies for preclinical drug evaluation. Our focus on selective ionic current block facilitates the systematic comparison of the different models and allows the quantification of the sensitivity of the AP to the inhibition of specific ionic currents. In our investigations we considered the most recent computational human models, which integrate existing biophysically detailed knowledge and datasets on the membrane kinetics of both hiPSC‐CMs and hAdultV‐CMs. We identified similarities and differences in the response of both types of cardiomyocytes to ionic current block and discuss them with regard to the underlying ionic mechanisms, in order to facilitate the interpretation of future experiments.
Methods
In silico models
Spontaneous and stimulated hiPSC‐CM APs were simulated using the Paci2013 model (Paci et al., 2013a), based on the comprehensive experimental dataset reported by Ma et al. (2011), obtained on isolated iCell hiPSC‐CMs (Cellular Dynamics International, Madison, WI, USA). The Paci2013 model allows the simulation of both VL and AL hiPSC‐CMs AP and includes formulations for the dynamics of ionic concentrations in the cytoplasm and sarcoplasmic reticulum, and for the kinetics of the main ionic currents and pumps, as described in the original paper. The VL and AL models were developed taking into account the differences between the main currents in ventricular and atrial phenotypes. This was performed by using scaling factors based on available data on known differences between ventricular and atrial cells. Importantly, the VL and AL models were independently validated against the morphological features of the APs for each cardiac phenotype reported previously (Ma et al., 2011) (Table 1). Slight improvements in the Paci2013 model were included, as described in the Supporting Information.
Table 1.
Comparison between the morphological biomarkers in spontaneous and stimulated VL hiPSC‐CM and AL hiPSC‐CM APs and Ca2+ transients
| EXP Non‐stimulated (Ma et al., 2011) | SIM | ||||||
|---|---|---|---|---|---|---|---|
| Non‐stimulated | Stimulated | ||||||
| hiPSC‐CM | hiPSC‐CM | hiPSC‐CM | hAdultV‐CM | ||||
| AL | VL | AL | VL | AL | VL | ||
| MDP (mV) | −73.5 ± 1.5 | −75.6 ± 1.2 | −72.2 | −77.4 | −71.3 | −76.2 | −88.0 |
| VMax (V s−1) | 26.2 ± 3.9 | 27.8 ± 4.8 | 24.9 | 26.3 | 33.9 | 47.4 | 259 |
| APA (mV) | 100 ± 2 | 104 ± 1 | 99.1 | 105 | 100 | 115 | 128 |
| Peak (mV) | 26.7 ± 1.4 | 28.3 ± 1.0 | 26.9 | 27.5 | 28.8 | 38.5 | 40.0 |
| APD30 (ms) | 123 ± 10 | 180 ± 11 | 137 | 212 | 167 | 258 | 166 |
| APD50 (ms) | – | – | 186 | 307 | 222 | 367 | 208 |
| APD70 (ms) | – | – | 229 | 358 | 267 | 418 | 240 |
| APD90 (ms) | 286 ± 21 | 415 ± 22 | 301 | 399 | 357 | 469 | 268 |
| APDratio | 1.1 ± 0.1 (<1.5) | 2.5 ± 0.2 (>1.5) | 1.09 | 3.16 | 1.09 | 3.41 | 1.60 |
| Rate (beats min−1) | 50.0 ± 10.0 | 35.3 ± 2.2 | 55.2 | 37.3 | – | – | – |
| Diastolic Ca2+ (nm) | – | – | 38 | 16 | 40 | 11 | 86 |
| Systolic Ca2+ (nm) | – | – | 296 | 281 | 241 | 150 | 368 |
| Amplitude (nm) | – | – | 258 | 265 | 201 | 140 | 282 |
EXP, experimental; SIM, simulated; APA, AP amplitude; Peak, peak voltage; Rate, rate of the spontaneous electrical activity; VL, ventricular‐like; AL, atrial‐like; hiPSC‐CM, human‐induced pluripotent stem cell‐derived cardiomyocyte; APs, action potentials; APD, action potential duration.
In silico simulations were also conducted using the hAdultV‐CM O'Hara–Rudy (ORd) endocardial model (O'Hara et al., 2011). The ORd model includes a similar level of biophysical detail as the Paci2013 model.
AP assessment
Action potentials were evaluated at steady‐state, after 900 s of simulation. The following morphological AP biomarkers were quantified: AP amplitude, AP duration at several repolarization percentages (APD30, APD50, APD70, and APD90), maximum diastolic potential (MDP), maximum upstroke velocity (VMax), peak voltage and spontaneous beating rate. The shape factor
discriminates between VL and AL hiPSC‐CM APs: as in Ma et al., (2011), an AP is considered VL if APDratio > 1.5 and AL if APDratio < 1.5. Details about the stimulation protocols are reported in the Supporting Information.
For each biomarker, the % variation induced by blocking each ionic current was computed (Tables 2, S1, S2 and S3). Then, considering only one model at a time, we normalized the variations in each specific biomarker with respect to the largest one (the most positive one for positive variations or the most negative one for negative variations) within all ionic current blocks (Figures 5, 6 and S2). To compare VL hiPSC‐CM and hAdultV‐CM, we repeated the previous procedure considering the % variations for both models (Figure 7).
Table 2.
Comparison between the % changes caused by each ionic current block in the AP biomarkers quantified by the VL hiPSC‐CM and the hAdultV‐CM AP models
| Variation (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MDP | VMax | APA | Peak | APD30 | APD50 | APD70 | APD90 | APDratio | ||
| INa | VL hiPSC‐CM | 1 | −53 | −7 | −23 | 14 | 3 | 0 | 1 | −20 |
| hAdultV‐CM | 0 | −69 | −3 | −8 | 3 | 2 | 2 | 1 | 0 | |
| ICaL | VL hiPSC‐CM | 0 | 5 | 0 | 2 | −82 | −73 | −68 | −66 | −35 |
| hAdultV‐CM | 0 | 3 | −2 | −5 | −24 | −21 | −17 | −16 | −5 | |
| IKrAd | VL hiPSC‐CM | 0 | −35 | −6 | −17 | 63 | 64 | 59 | 56 | 38 |
| hAdultV‐CM | 0 | 0 | 0 | 1 | 45 | 56 | 67 | 66 | 2 | |
| IKrSS | VL hiPSC‐CMa | −2 | −48 | −2 | −2 | 96 | 86 | 79 | 69 | 58 |
| hAdultV‐CMa | 0 | 1 | 0 | 1 | 32 | 39 | 45 | 44 | 16 | |
| IKs | VL hiPSC‐CM | 0 | −1 | 0 | −1 | 3 | 2 | 2 | 2 | 4 |
| hAdultV‐CM | 0 | 0 | 0 | 0 | 5 | 4 | 4 | 4 | −5 | |
| IK1 | VL hiPSC‐CM | −16 | −65 | −14 | −12 | 30 | 22 | 34 | 50 | −73 |
| hAdultV‐CM | 0 | 2 | 1 | 1 | 0 | 0 | 1 | 7 | −28 | |
| If | VL hiPSC‐CM | 1 | 21 | 4 | 9 | 1 | 3 | 3 | 4 | 6 |
| hAdultV‐CM | – | – | – | – | – | – | – | – | – | |
| INaCa | VL hiPSC‐CM | −1 | −1 | 2 | 6 | 11 | 1 | −3 | −8 | 24 |
| hAdultV‐CM | 0 | 1 | 0 | 1 | −10 | −11 | −10 | −9 | −18 | |
| Ito | VL hiPSC‐CM | 0 | −2 | 1 | 2 | 2 | 2 | 2 | 2 | 7 |
| hAdultV‐CM | 0 | 0 | 2 | 5 | −2 | −1 | 0 | 0 | 17 | |
The 2 × IC50 concentration was considered, except where specified. The % variations with respect to the control values of each biomarker are reported. Two rows are reported for IKr: Ad contains biomarkers 7 s after administration of the blocker, while SS refers to the steady‐state.
IC50 concentration is considered.
EXP, experimental; SIM, simulated; APA, AP amplitude; Peak, peak voltage; Rate, rate of the spontaneous electrical activity; VL, ventricular‐like; AL, atrial‐like; hiPSC‐CM, human‐induced pluripotent stem cell‐derived cardiomyocyte; APs, action potentials; APD, action potential duration.
Figure 5.
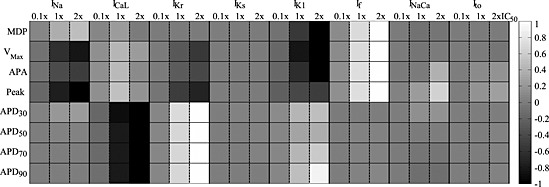
Global comparison of the effect of ionic current blocks on the morphological AP biomarkers simulated by the VL hiPSC‐CM model. Stimulated APs were considered (pacing rate 60 beats min‐1). Grey levels represent the % variation of each biomarker (normalized in the interval [−1, 1], black: greatest reduction, white: greatest increment) for each block level. For IKr, biomarkers were computed 7 s after block, because for 2 × IC50 the AP was prolonged over the next stimulation pulse. APDxx, action potential duration at XX% of repolarization.
Figure 7.
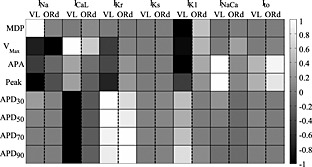
Comparison of the normalized % variation of the biomarkers induced by the blocker concentration 2 × IC50 in the VL hiPSC‐CM and the hAdultV‐CM (ORd) models. Biomarkers for IKr blocks were computed after 7 s from administration. APDxx, action potential duration at XX% of repolarization.
In silico simulation of ionic current block
Specific block of INa, ICaL, If, IK1, IKr, IKs, Ito and INaCa was assessed on stimulated APs. As in previous studies (Brennan et al., 2009; Davies et al., 2012; Zemzemi et al., 2013), drug block was simulated using a simple pore block model affecting the relevant maximum conductances based on a dose–response curve for each of the ionic currents, I, as follows:
where [D] represents the current blocker concentration, IC50 is the drug concentration leading to 50% of block and the Hill coefficient is assumed to be one. This particular model aids in the interpretation of our results for selective drugs. For each current, four different degrees of block were tested, 0.1 × IC50, IC50, 2 × IC50 and full block, corresponding to current block levels of 9, 50, 67 and 100%. From steady‐state conditions, the maximum conductance of each current was reduced according to the specific concentration of the blocker. Simulations were then run for an additional 300 s, after which the APs were assessed and the aforementioned biomarkers were quantified. In the case of IKr block, APs were assessed after 7 s from block, because for longer simulations, repolarization failure was observed in some cases (at 2 × IC50 in AL hiPSC‐CM and at full block in VL and AL hiPSC‐CM and hAdultV‐CM).
Numerical simulations
The Paci2013 model was implemented in MATLAB Simulink (The MathWorks, Inc., Natick, MA, USA), whereas the ORd model was implemented in MATLAB. Both models were solved using ode15s.
Results
Spontaneous and stimulated control hiPSC‐CM APs
The time course of the simulated VL and AL hiPSC‐CM APs and the underlying ionic currents is illustrated in Figures 1 and 2 at steady‐state, for spontaneous and stimulated APs, respectively. Table 1 presents the quantitative comparison between the biomarkers obtained from experimental spontaneous APs as reported in Ma et al., (2011) and those quantified from the in silico simulations. Both models yielded values for the biomarkers consistent with experimentally‐reported ranges. Moreover, the main differences between VL and AL APs were successfully simulated, including an APDratio of 3.16 and 1.09 and the higher spontaneous beating rate of AL compared with VL hiPSC‐CMs, as reported by Moretti et al., (2010), Ma et al., (2011) and Lahti et al., (2012).
Figure 1.
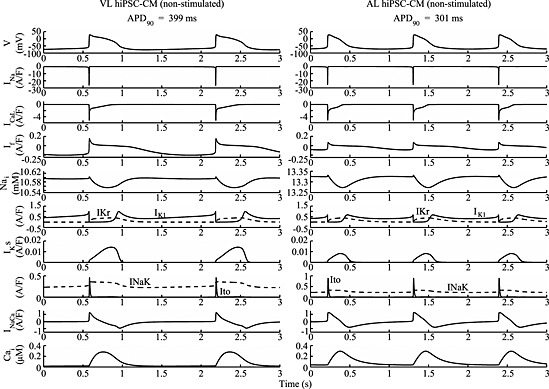
Simulated spontaneous VL (left) and AL (right) hiPSC‐CM action potentials, ionic currents and concentrations. No external stimulus was used (Istim = 0).
Figure 2.

Simulated VL (left) and AL (right) hiPSC‐CM action potentials, ionic currents and concentrations under stimulation at 60 (VL) and 80 beats min‐1 (AL).
The last three columns of Table 1 provide a comparison between the biomarkers from stimulated hiPSC‐CM and hAdultV‐CM APs simulations. Simulated hiPSC‐CM exhibited depolarized MDP values (by ~12 mV), decreased AP amplitude and longer APD than hAdultV‐CM, with values consistent with the experimental data reported by Li et al. (1998) and Moretti et al. (2010).
Effects of ionic current block on hiPSC‐CM and hAdultV‐CM AP
Figures 3 and 4 show the stimulated APs for VL hiPSC‐CM, AL hiPSC‐CM and hAdultV‐CM in control conditions and for the four degrees of block of INa, ICaL, If, IK1 and IKr, IKs, Ito INaCa. Biomarker values are summarized in Figures 5 and 6 for VL and AL hiPSC‐CM and in Figure S2 for hAdultV‐CM. A specific comparison of the variations induced by 2 × IC50 block in VL hiPSC‐CM and hAdultV‐CM APs is presented in Figure 7. Numerical values are presented in Table 2, and in the Supporting Information, Tables S1–S3. Stimulated APs are also compared with spontaneous ones in Tables S4 and S5, showing that the biomarker changes are quite consistent.
Figure 3.
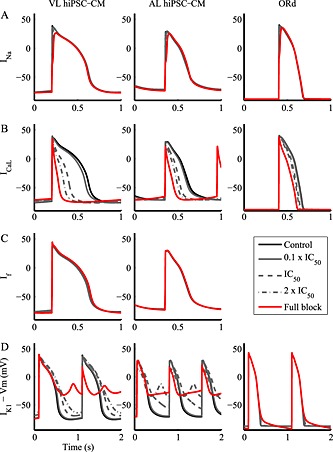
Comparison of the steady‐state effects on the action potential of ionic current block in the VL (left) and AL (centre) hiPSC‐CM and hAdultV‐CM (right) models. The four blocker concentrations considered were as follows: 0.1 × IC50 = 9%, IC50 = 50%, 2 × IC50 = 67% and full block. If block was not simulated for hAdultV‐CM model because it does not contain this current. VL and AL hiPSC‐CM IK1 full block trace refers to the first APs after the block.
Figure 4.
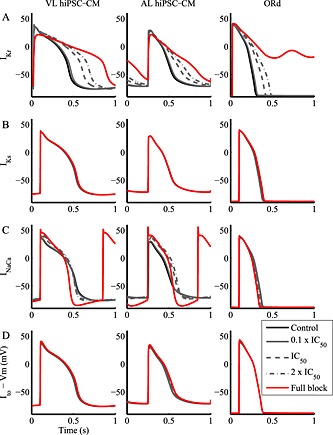
Comparison of the steady‐state effects on the action potential of ionic current block in the VL (left) and AL (centre) hiPSC‐CM and hAdultV‐CM (right) models. The four blocker concentrations considered were as follows: 0.1 × IC50 = 9%, IC50 = 50%, 2 × IC50 = 67% and full. For all the models, IKr traces report AP after 7 s from block. INaCa full block traces refer to 10 beats after block.
Figure 6.
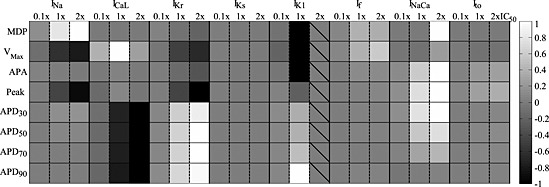
Global comparison of the effect of ionic current blocks on the morphological AP biomarkers simulated by the atrial‐like hiPSC‐CM model. Stimulated APs were considered (pacing rate 80 beats min‐1). Grey levels represent the % variation of each biomarker (normalized in the interval [−1, 1], black: greatest reduction, white: greatest increment) for each block level. 2 × IC50 was not considered for IK1 due to repolarization failure. For IKr, biomarkers were computed 7 s after block, because for 2 × IC50 the AP was prolonged over the next stimulation pulse. APDxx, action potential duration at XX% of repolarization.
INa block
Specific INa block is usually obtained by means of tetrodotoxin (Ma et al., 2011) or mexiletine (Ma et al., 2013). As expected, in all cell types INa block affected primarily the AP upstroke, and, therefore, VMax and AP amplitude (Figures 3A, S1 and Tables 2, S1–S5). VMax was strongly reduced by INa block in both hiPSC‐CMs (up to −78%). The peak voltage was affected as well, and the VL hiPSC‐CM was found to be more sensitive than the AL hiPSC‐CM.
Similar effects of INa block were observed in the hAdultV‐CM upstroke (Figure 3A and Tables 2 and S3), including a dramatic decrease in VMax with the highest levels of block (up to −69%). The peak voltage was slightly less sensitive to INa block in simulated hAdultV‐CM than in VL hiPSC‐CM. For full block, the stimulus current had to be increased from 80 to 140 pA·pF−1 to trigger an AP due to a significant reduction in excitability.
ICaL block
ICaL has a critical role in sustaining the AP plateau and therefore the APD. In in vitro experiments, it is selectively blocked by using nifedipine (Ma et al., 2011) or nisoldipine. In our simulations, the ICaL block‐induced APD shortening was evident in all models, although it was larger in hiPSC‐CMs than in hAdultV‐CM (over 68% vs. less than 30% for full block) (Figure 3B and Tables 2, S1–S5). Of note, due to a marked APD shortening, the spontaneous beating prevailed on pacing at 80 beats·min−1 for the AL hiPSC‐CMs, therefore the pacing rate was increased to 100 beats·min−1.
We hypothesized that such a different AP shortening in VL hiPSC‐CM and hAdultV‐CM is induced by differences in the INaCa between the two models. To test this hypothesis, we replaced the INaCa formulation in the hAdultV‐CM model by the one in the VL hiPSC‐CM. The simulations then showed that APD after ICaL block was close to that obtained in VL hiPSC‐CM (Figure S4, green vs. blue curves). Comparing the time course of INaCa during the AP in the original hiPSC‐CM model and in the hAdultV‐CM model with the hiPSC‐CM INaCa formulation under ICaL full block conditions (not shown) shows that the current profiles are similar but the amplitude of the current (both in outward and inward modes) is 3.2 times larger in hiPSC‐CM than in hAdultV‐CM. We then increased the maximal INaCa current by 3.2 times and tested the ICaL full block again, which showed that the INaCa increase was almost fully responsible for the APD shortening obtained within the hAdultV‐CM model with the hiPSC‐CM INaCa formulation (see in Figure S4 the red curve almost superimposed to the green one). Finally, we also tested the effect of the persistent component of the sodium current INaL (not present in the hiPSC‐CM models) in preserving the APD in hAdultV‐CM in conditions of ICaL block. By fully blocking the INaL and ICaL at the same time in the hAdultV‐CM model with the hiPSC‐CM INaCa formulation, an even larger APD90 reduction was achieved that almost equalled the shortening shown in hiPSC‐CM after ICaL block (see in Figure S4 the magenta curve almost superimposed to the blue one). The analysis therefore suggests that the differences in the response of hAdultV‐CM and hiPSC‐CM to ICaL block are due to differences in INaCa and INaL currents.
IKr and IKs block
As shown in Figure 4A and Tables 2, S1–S5, IKr block induces a strong APD prolongation for all cell types, at all repolarization phases. A similar APD prolongation was obtained using E4031 (Ma et al., 2011) or dofetilide. At the IC50 concentration, the APD90 increased by about +40% for all the three models. Full IKr block induced an even more dramatic prolongation of APD90, +90% and more for both hiPSC‐CMs, and for hAdultV‐CM, early after depolarizations (EADs) were triggered. Note the strong decrease in VMax caused by IKr block in hiPSC‐CMs, which was due to the fact that APD prolongation reduces the diastolic interval and therefore limits the INa recovery from inactivation and its availability to trigger the next AP. In VL hiPSC‐CM, for 2 × IC50 IKr block, only 6% of INa current was available (computed as product of the inactivation gating variables h and j) at the end of the diastolic phase. The VMax drop observed in VL hiPSC‐CM was not seen for hAdultV‐CM, in which INa had sufficient time to recover from inactivation even after IKr block‐induced AP prolongation because, in spite of the similar % prolongation, the absolute APD90 value was significantly shorter in hAdultV‐CM than in VL hiPSC‐CM (446 ms vs. 732 ms at 2 × IC50).
In Ma et al, (2011), 3R4S‐Chromanol 293B was used for IKs block but its influence on Ito was also reported (Thomas et al., 2003). In Thomas et al., (2003) and So et al. (2008), HMR‐1556 resulted a more selective blocker of IKs, with influence on other currents only at high concentrations. Simulations demonstrated only a minor effect of IKs block on the AP shape for both hiPSC‐CMs and hAdultV‐CM (Figure 4B and Tables 2, S1–S5), with APD90 shortening <3% for full block. This is also due to the fact that in our in silico analysis, the effect of stimulation of β‐adrenoceptors was not considered.
IK1 block
Simulations show the importance of IK1 in maintaining the MDP in all cell types but with significant quantitative differences (Figure 3D and Tables 2, S1, S2, S4 and S5). IK1 block, which can be performed in vitro by means of BaCl2 (Sartiani et al., 2007), resulted in an elevated MDP (over 9%) in both hiPSC‐CMs, and as a consequence, reduced INa availability and a severe reduction in VMax by over 50% for IC50 block. The influence of IK1 on APD was also strong for both hiPSC‐CMs, especially for APD90, which was prolonged at the IC50 by 28% in VL hiPSC‐CM and by 58% in AL hiPSC‐CM. Figure 3D shows the repolarization failure that immediately followed the full IK1 block in both the hiPSC‐CM models (and 2 × IC50 block only for AL hiPSC‐CM).
The hAdultV‐CM simulations show a very stable MDP for the three lowest degrees of IK1 block (Figure 3D and Tables 2, S3). In contrast, a complete block induced a marked slowdown of the late repolarization with consequent APD90 prolongation, and this was followed by a slight hyperpolarization (+5%) of MDP.
To understand the different behaviours in VL hiPSC‐CM and hAdultV‐CM, we performed additional in silico analyses to evaluate the role of IKr in compensating for the lack of a repolarizing effect of IK1 in hAdultV‐CM versus VL hiPSC‐CM. As reported in Figure S5A and S5B, the peak IKr under AP was half in VL hiPSC‐CM with respect to hAdultV‐CM (0.4 vs. 0.86 pA·pF−1) and it was not enough to repolarize the AP in the case of IK1 block. To confirm this result, we simulated, in conditions of full block of IK1, the effect of doubling IKr in hiPSC‐CM (Figure S5C) and halving the IKr in hAdultV‐CM (Figure S5D). Figure S5C shows in VL hiPSC‐CM how a doubled IKr partly compensates for the absence of IK1, and restores repolarization. In contrast, Figure S5D illustrates that in hAdultV‐CM, reducing IKr enhances the effects of IK1 block, especially during the late repolarization.
If block
If can be blocked by means of zatebradine (Sartiani et al., 2007) or ivabradine. Figure 3C and S3 show that If block has only minor effects on the AP shape in both hiPSC‐CMs, whereas it cannot be simulated in hAdultV‐CM because it lacks If. However, in VL hiPSC‐CM, it has some enhancing effects on MDP, VMax and peak voltage (Figure 5 and Tables 2, S1, S2, S4 and S5). As shown in Figure 2, If is stronger in VL than in AL hiPSC‐CMs, especially its inward component, which could explain the greater sensitivity of VL hiPSC‐CM to If changes. By blocking If, the reduced inward component hyperpolarizes MDP up to 3%; especially for VL hiPSC‐CM: this is reflected in an increased VMax and peak voltage.
INaCa block
In Paci et al., (2012), nickel was used to block the INaCa, but it also affected IKr and the T‐type Ca2+ current. Recently, the effect of the more specific INaCa blocker ORM10103 was tested on canine ventricular cells, but its effects in human ventricular myocytes are still unknown (Kormos et al., 2014; Nagy et al., 2014). The most important effect caused by INaCa block (Figure 4C and Tables 2, S1, S2, S4 and S5) in in silico hiPSC‐CMs and in hAdultV‐CM, especially at full block, is the APD90 shortening, up to 20% in VL hiPSC‐CM. In VL and AL hiPSC‐CMs, we also observed a lot of additional effects. At full block, MDP was hyperpolarized because of the lack of the inward component of INaCa; this hyperpolarization induced a greater activation of INa and consequently a greater VMax; moreover, as a consequence of the hyperpolarized MDP the INaCa block affected the whole AP morphology, with an increased peak voltage and the plateau phase moved towards more positive potentials and a slower repolarization, which made the APD30 and APD50 longer. Conversely, the phase 3 repolarization was faster and induced APD90 shortening. The phase 2 change was even more pronounced in AL hiPSC‐CM, leading to a longer plateau duration, which almost compensated for the shortening obtained during the late repolarization. The full block traces shown in Figure 4C for both the hiPSC‐CM models and the hAdultV‐CM model are illustrative of the INaCa block occurring 10 beats after blocker administration: traces at the steady‐state were not considered because of reduced intracellular ion concentrations. Finally, Figures S6 and S7 show the effect of INaCa block on the intracellular Na+ and Ca2+ concentrations.
Ito block
Results using selective Ito blockers are scarce. In de Haan et al. (2006), AVE0118 was reported to be specific for Ito but it also affects the ultrarapid K+ current. Ito block resulted in only minor effects in VL and AL hiPSC‐CMs (Figure 4D and Tables 2, S1, S2, S4 and S5), specifically it increased the peak voltage and APD90, especially for AL hiPSC‐CM. Also for the hAdultV‐CM (Table S3), Ito block mainly induced an increased peak voltage. Ito block would be expected to induce stronger effects in epicardial hAdultV‐CM with a stronger Ito than the endocardial hAdultV‐CM considered here.
Discussion
The present study provides a first systematic in silico evaluation of the effect of selective ionic current block on the AP shape of VL and AL hiPSC‐CMs, and their comparison to hAdultV‐CM. Quantitative results on AP biomarkers for the three cell types are obtained for four block levels of the eight most important transmembrane cardiac currents. Our main findings are as follows: (i) specific ionic current block induces similar qualitative changes in hiPSC‐CMs and hAdultV‐CM AP biomarkers for most ionic currents; (ii) given the importance of IKr block in preclinical drug evaluation, our results highlight that IKr block results in nearly identical AP prolongation in VL hiPSC‐CM and hAdultV‐CM for 0.1 × IC50 and IC50, and consistent for 2 × IC50; (iii) hiPSC‐CMs were more sensitive to ICaL and IK1 block, compared with hAdultV‐CMs, for example, APD shortening/prolongation was up to more than four times larger after ICaL/IK1 block; (iv) high levels of IK1 block induced repolarization failure in hiPSC‐CMs, which was not observed in hAdult‐CM (this increased sensitivity can be attributed to a reduced repolarization reserve of hiPSC‐CMs); (v) our mechanistic study revealed the importance of differences in INaCa, INaL and IK1 between hAdult‐CM and hiPSC‐CMs in modulating their response to ionic current block. Although AL cells are present in in vitro preparations, they are a less common phenotype compared with the VL ones, and they are rarely used for drug tests. Therefore, greater attention was placed in this study on the VL hiPSC‐CM model and its comparison to hAdultV‐CM. However, we consider the presentation of the AL hiPSC‐CM simulations useful, especially in the perspective of multicellular aggregates (e.g. multielectrode array and field potential).
By revealing differences and similarities in the sensitivity of hiPSC‐CMs and hAdultV‐CM to ionic current block, our analysis aims to help in the interpretation of further in vitro assays. We therefore expect the results of our investigations to support the currently developing synergy between in vitro and in silico approaches in the field of pharmacology and facilitate further development and use of hiPSC‐CMs as a powerful in vitro technology for pharmacological applications in drug development and prescreening, which can be extended to take into consideration patient‐ and pathology‐specificity.
hiPSC technology
Human‐induced pluripotent stem cell‐derived cardiomyocytes are likely to revolutionize drug testing as their production and use is more convenient and practical than harvesting human adult cells for in vitro studies, especially for non‐diseased subjects. Further, they could contribute to the reduction, refinement and replacement of animal experimentation.
Human‐induced pluripotent stem cell‐derived cardiomyocytes have already proved their value as in vitro models for investigations into specific pathologies (Moretti et al., 2010; Egashira et al., 2012; Kujala et al., 2012; Terrenoire et al., 2013), and due to their patient‐specificity, in identifying drugs for specific diseases (Itzhaki et al., 2011, 2012; Matsa et al., 2011; Yazawa et al., 2011; Jung et al., 2012), even though none of them has yet been administered to patients (Knollmann, 2013). Further advances in the development and application of the hiPSC technology for personalized therapies, however, require an in‐depth and systematic evaluation of hiPSC‐CMs and their maturity with a quantitative comparison with hAdultV‐CM, which can be facilitated by in silico investigations such as the one presented in this study.
The majority of the most recently published datasets shows not only a high variability in the proposed APs (Table 3), but also immature phenotypes, for example, characterized by spontaneous APs and depolarized MDP. This leads to the still‐open question “how similar are hiPSC‐CMs to adult CMs?” and our study provides the first systematic study to aid in the characterization of similarities and differences.
Table 3.
Comparison between four datasets to show the variability inherent with VL hiPSC‐CM measurements
| Ma2011 | Moretti2010 | Lahti2011 | Ma2013 | |
|---|---|---|---|---|
| Rate (beats min‐1) | 35 ± 2 | 68 ± 3 | 72 ± 6 | 69 ± 11 |
| MDP (mV) | −76 ± 1 | −64 ± 2 | −63 ± 1 | −61 ± 1 |
| APD90 (ms) | 415 ± 22 | 381 ± 35 | 314 ± 18 | 434 ± 31 |
VL, ventricular‐like; hiPSC‐CM, human‐induced pluripotent stem cell‐derived cardiomyocyte; APD, action potential duration; MDP, maximum diastolic potential.
Ma2011 (Ma et al., 2011) includes data from control hiPSC‐CMs, used to record currents (INa, ICaL, If, Ito, IKr, IKs and IK1), spontaneous APs and response to selective blocks of INa, ICaL, IKr and IKs. Moretti2010 (Moretti et al., 2010) includes AP (spontaneous and stimulated) and current data (IKs and IKr) from control and patient cells to study the effects of LQT1. Lahti2011 (Lahti et al., 2012) shows IKr and the spontaneous APs from control and patient hiPSC‐CMs for LQT2 assessment. Ma2013 (Ma et al., 2013) focuses on control and patient hiPSC‐CMs to characterize the effects of LQT3, analysing APs and the fast and late INa.
Effect of ionic current block on hiPSC‐CMs APs
Selective ionic current block was simulated by reducing the maximum conductance, using a simple pore block model. To investigate dose‐dependency of the effects, four degrees of block were applied for each current. Previous studies (Romero et al., 2009, 2011; Pueyo et al., 2010; Christophe, 2013; Zemzemi et al., 2013) have followed a similar approach to systematically investigate the sensitivity of AP biomarkers to specific ion channel changes. This approach was chosen because (i) selective blocks are important for cardiac drug discovery and (ii) it allows a controlled and systematic comparison between different cell types or models, which is a preliminary step before extending the analysis to more complex drug effects including multichannel actions.
In our study, VL hiPSC‐CMs and hAdultV‐CMs action potentials were simulated using the most comprehensive human models consistent with a large range of human data. Our new results showed a qualitatively similar response to current block between both cell types in several cases: (i) INa block induced a reduction of VMax, peak voltage and AP amplitude; (ii) ICaL block shortened the APD; (iii) IKr block prolonged the APD; (iv) Ito block increased the peak voltage; and (v) low sensitivity was found to IKs block. Nevertheless, quantitative differences were identified, such as (i) ICaL block shortened APD more in hiPSC‐CMs than in hAdultV‐CM and (ii) IK1 block elevated MDP in hiPSC‐CMs but not in hAdultV‐CM. The mechanisms underlying such similarities and differences were investigated and discussed in the following by using the AP models for specifically designed simulations, which provide a powerful tool to unravel the underlying ionic mechanisms.
Ionic mechanisms underlying hiPSC‐CM versus hAdultV‐CM similarities and differences
The first main difference we observed between VL hiPSC‐CM and hAdultV‐CM is the stronger APD reduction, induced by ICaL block, in the first model than in the second one. After testing many hypotheses, for example, the influence of the intracellular Na+ and Ca2+ concentrations, and removing one by one the currents from the hAdultV‐CM model to assess their contribution in case of ICaL block, we found that the different extent of APD shortening is mainly due to the different INaCa formulations and to the presence in hAdultV‐CM of INaL. Concerning INaCa, we performed the following in silico experiment: we transplanted the hiPSC‐CM formulation of INaCa into the hAdultV‐CM model, to evaluate its importance in the observed differences. Of note, this is one of the main advantages of in silico modelling, which allows in a relative short time to simulate phenomena and mechanisms too hard or time‐consuming to test in vitro. In this sense, the computational analysis offers an interesting mechanistic insight into why the response of the hiPSC‐CMs can be different from that of the adult cells: overexpression of INaCa can magnify the effects of ICaL block. Actually, INaCa overexpression in incompletely mature CMs, and thus in hiPSC‐CMs too, is still a matter of debate. Experiments in different animal models show the maximal INaCa density being reduced during maturation (Itoh et al., 2007). The same trend is also maintained in human cells, where the expression of INaCa is higher at the mid‐gestation than at the adult stage (Qu et al., 2000). In cardiomyocytes derived from human pluripotent cells, in particular embryonic, it is reported how the INaCa expression is larger than in fetal and adult cells (Liu et al., 2007; Li et al., 2013) and it was confirmed by the current measurements reported in Paci et al. (2012), where INaCa is shown to be greater at the early developmental stage (15–40 days). However, in Fu et al. (2010), INaCa was reported to be greater at 90 days than at 40 days post‐differentiation. Our simulation results point out the need for experimental data quantifying this aspect.
The second contribution to the preserved APD in hAdultV‐CM after ICaL block comes from INaL. Indeed, this current is included in the hAdultV‐CM model, whereas it is not in the hiPSC‐CM one, which is mainly based on the data of Ma et al. (2011), where no INaL was reported. The lack of this influx of positive charges delegates the support of the AP plateau phase to ICaL, whose block has stronger effects in VL hiPSC‐CM than in hAdultV‐CM. Because INaL has been reported in hiPSC‐CMs (Ma et al., 2013), in this case, simulation results point out the need for the experimental characterization of this current and its inclusion in the hiPSC‐CMs mathematical models.
The second main difference we observed between hiPSC‐CM and hAdultV‐CM is the extent of the effects of IK1 block, which confirms the critical role played by a non‐completely mature IK1 in stem cells‐derived CMs, in which instability of diastolic potential leads to spontaneous depolarization. Our results show that IK1 block results in significant effects on MDP and APD90 in VL hiPSC‐CM, whereas in hAdultV‐CM, MDP remains unaffected and APD90 prolongation is modest (<10%). We ascribe the greater sensitivity to IK1 blockade of VL hiPSC‐CM to a reduced reserve of repolarization (Carmeliet, 2006): in hiPSC‐CM, IKr is not able to carry out properly those compensatory mechanisms that in hAdultV‐CM allow the reduction of the IK1 block effects.
For cardiotoxicity studies, the greater sensitivity of hiPSC‐CM to drug blocks could be regarded as a useful feature providing clearly detectable indications about how a specific compound affects the cell electrophysiology. However, as shown by our study and in vitro experiments (Ma et al., 2011; Knollmann, 2013), it is also an important indicator of the fact that the cells the Paci2013 model is based on, and more in general hiPSC‐CMs, were not behaving exactly like adult cells, thus particular care should be used when considering them as fully representative models of adult cells. This oversensitivity is something to be aware of, but not enough to dismiss hiPSC‐CMs as an inadequate model. We know indeed that every in vivo, in vitro or in silico model has advantages and disadvantages in the translation of its results to humans. If anything, this highlights the importance of further studies to achieve better and more effective maturation, characterization and possibly improve (e.g. IK1 overexpression) hiPSC‐CMs. The final goal is an integrated approach, in which compounds are tested in real hiPSC‐CMs, whereas in silico simulations with both hiPSC‐CM and hAdultV‐CM will help in interpreting and translating the results to real adult cells in vivo. Thus, decisions should be taken from data obtained by running in parallel in silico and in vitro experiments on hiPSC‐CMs and taking into account both sets of result. It will improve the degree of awareness of hiPSC‐CM descriptive power: if results are consistent, the confidence in them will be higher, otherwise the interpretation of the results should be treated with caution. This scenario also represents a typical field for the application of the new concept of model‐simulation‐experiment (MSE) system (Carusi et al., 2012). In this regard models, simulations and experiments are interlaced in a synergic assemblage, whose expressive power for the representation of biological mechanisms progressively grows, as the MSE system itself is constructed.
A further step for cardiotoxicity studies consists in analysing and integrating different levels of details into a multiscale approach. In fact, in order to get an overall understanding of arrhythmogenic mechanisms and how new compounds could affect them, the integration of measurements and simulations at the cellular, tissue, organ and even in vivo levels is essential.
Potential applications of our methodology are as follows: (i) in silico experiments aimed at evaluating stem cell‐derived CM maturity, for example, by replacing single or multiple ionic currents with their adult versions, in order to compare hiPSC‐CM models with hybrid or partially adult models; (ii) the investigation of the observed high in vitro variability by means of in silico models derived from patient‐specific and disease‐specific hiPSC‐CMs, for example exploiting specific populations of models (Britton et al., 2013; Gemmell et al., 2014); and (iii) the analysis of the already known differences between hiPSC‐CMs and hAdultV‐CMs, for example, assessing the impact of a reduced repolarization reserve on in vitro models of arrhythmogenic pathologies.
Future work and limitations
A comparison of published datasets highlights the surprisingly high variability in the APs measured during experiments in hiPSC‐CMs. In the light of such large variations in measurements and cell lines, the construction of in‐silico models requires an informed choice on the experimental data to be considered and integrated. In Paci et al. (2013a), the hiPSC‐CM models were based on the comprehensive dataset reported in Ma et al. (2011), giving only secondary consideration to additional datasets. Of note, this dataset is based on measurements from isolated iCell hiPSC‐CMs by Cellular Dynamics International; therefore, the Paci2013 model reproduces the electrophysiological behaviour of isolated hiPSC‐CMs from this specific commercial line. Other commercial hiPSC‐CMs lines are available (e.g. Cor.4U by Axiogenesis) and many more are produced in single laboratories, thus line‐to‐line variability is an issue. Moreover, hiPSC‐CM biomarkers are dependent on the measurement conditions, for example, isolated cell patch‐clamp versus microelectrode arrays on monolayers. Consequently, a possible limitation of the present study is the general applicability of our results to hiPSC‐CMs obtained in different laboratories and/or from different cell lines, suggesting that specific in silico models should be tailored for different hiPSC‐CM lines. However, it is worth noting that the Paci2013 hiPSC‐CM model already comprises the flexibility to reproduce datasets from cell lines different from the one used to develop the model. In Paci et al. (2013b, 2014), we showed how, with few changes in the maximal conductances of the ion currents, we reproduced different control APs. Moreover, integrating mutation‐specific data, mutant LQT1 and LQT3 APs were also properly reproduced. These results suggest that the intrinsic behaviour of the cells, and eventually their response to current blockers, could be more conserved than the more commonly measured AP features. Nevertheless, as reported earlier, this aspect needs to be investigated in more depth, for example, by exploiting the populations of models approach.
In this study, we have considered single pore block models, and this approach has proved useful in previous studies (Brennan et al., 2009; Zemzemi et al., 2013). It includes important information on cardiac sensitivity to drug effects and can be extended to drugs with multichannel actions. In the case of hiPSC‐CMs, however, the situation is even more complex: indeed, for adult cells, studies have published IC50 for different drugs, and also for multiple channels (Mirams et al., 2011; Davies et al., 2012). At present, the same kind of information is not available for hiPSC‐CMs but our studies could be extended once comparable in vitro data become available for hiPSC‐CMs. Furthermore, our work focuses on the effect of ionic current block on AP biomarkers rather than EADs and those were observed only for very high degrees of IKr block in hAdultV‐CM. In the case of hiPSC‐CMs, the observed high variability reported in the literature also affects the presence of EADs. In particular, in the literature, the occurrence of EADs is reported to be variable (Hoekstra et al., 2012) and generally in specific stimulation conditions (e.g. very slow pacing rate such as 30 beats min‐1). Moreover, generally not all the cells tested showed the occurrence of EADs, but only a subset of them showed repolarization anomalies (Ma et al., 2011). Variations in the occurrence of EADs were observed not only in control cells but also in mutant hiPSC‐CMs derived from patients, for example, in conditions of impaired IKr by LQT2 syndrome: in Lahti et al. (2012), EADs were observed, but not by Bellin et al. (2013). It seems, therefore, that in order to reliably assess the occurrence of this kind of pro‐arrhythmia marker, the intercellular variability should also be taken into account in the computational models. Further studies can extend our hiPSC‐CMs models to investigate mechanisms of EADs generation. Finally, our modelling approach could also be extended to include more complex models of drug/ion channel interactions, specifically when drug‐induced effects on current kinetics need to be evaluated (Di Veroli et al., 2013; Romero et al., 2014).
Conclusions
This study presents the first application of hiPSC‐CM in silico models for the assessment and comparison of the effects of specific current block on hiPSC‐CM and hAdultV‐CM APs. Qualitatively, the response of all cell types was overall similar but an increased sensitivity was observed with the hiPSC‐CMs, specifically to ICaL and IK1 block. Quantitatively, we showed how the increased sensitivity of the hiPSC‐CMs was mainly due to the overexpression of INaCa in the case of ICaL block, whereas it was due to the reduced repolarization reserve in the case of IK1 block. Our results are potentially useful for developing synergistic approaches for pharmacological investigations, using combined in silico and in vitro hiPSC‐CM technologies for safety assessments and target identification during drug development.
Author contributions
M. P. conducted the experiments, analysed/interpreted data and wrote the article. J. H. designed the experiments, analysed/interpreted data and proofed/revised the article. B. R. designed the experiments, analysed/interpreted data, wrote the article and proofed/revised the article. S. S. designed the experiment, analysed/interpreted data and proofed/revised the article.
Conflict of interest
None.
Supporting information
Table S1a AP biomarkers changes induced by the different blockade levels in the stimulated ventricular‐like model.
Table S1b AP biomarkers percent variations with respect to the control AP induced by the different blockade levels in the stimulated ventricular‐like model.
Table S2a AP biomarkers changes induced by the different blockade levels in the stimulated atrial‐like model.
Table S2b AP biomarkers percent variations with respect to the control AP induced by the different blockade levels in the stimulated atrial‐like model.
Table S3a AP biomarkers changes induced by the different blockade levels in the adult ORd model (If not present in the ORd model).
Table S3b AP biomarkers percent variations with respect to the control AP induced by the different blockade levels in the adult ORd model.
Table S4 Comparison between spontaneous (gray) and stimulated (white) ventricular‐like hiPSC‐CM APs. Biomarkers are presented as percent variations with respect to the control AP induced by the different blockade levels in the stimulated ventricular‐like model.
Table S5 Comparison between spontaneous (gray) and stimulated (white) atrial‐like hiPSC‐CM APs. Biomarkers are presented as percent variations with respect to the control AP induced by the different blockade levels in the stimulated atrial‐like model.
Figure S1 Details of the effect of INa block on the AP upstroke.
Figure S2 Global comparison of the effect of ionic current blocks on the morphological action potential (AP) biomarkers simulated by the hAdultV‐CM model. The pacing rate is 60 bpm. Grey levels represent the percent variation of each biomarker (normalized in the interval [−1, 1]) for each block level. MDP: maximum diastolic potential, VMax: maximum upstroke velocity, APA: action potential amplitude, Peak: peak voltage, APDxx: action potential duration at XX% of repolarization.
Figure S3 If block effects on VL hiPSC‐CM and hAL hiPSC‐CM. In panels B and C the effects on MDP and Peak have been detailed, respectively.
Figure S4 Assessment of the different contributions that sustain the AP in the hAdultV‐CM (ORd) model and comparison with full ICaL block in VL hiMPSC‐CM. Black, green and magenta traces refer to hAdultV‐CM hybridized with the VL hiPSC‐CM INaCa. The red trace refers to the hAdultV‐CM model with its original INaCa formulation and a 3.2‐fold increase in the maximal current.
Figure S5 Assessment of the repolarization reserve in VL hiPSC‐CM and hAdultV‐CM. A and B: comparison of different IK1 blocks on the APs and the underlying IKr. C: compensation of the IK1 block by doubling IKr (increment to 80 bpm of the pacing rate is needed to avoid spontaneous APs). D: the halved IKr and the consequent slower repolarization show how IKr compensates the absence of IK1 in adult cells.
Figure S6 Na+ concentration in conditions of INaCa block.
Figure S7 Ca2+ concentration in conditions of INaCa block. Peak of hAdultV‐CM Ca2+ transient equals to 0.022 mM.
Supporting info item
Acknowledgements
M. P. was financially supported by Tekes, Finland (decision: 40346/11) and by the Finnish Cultural Foundation, Central Fund, Finland. B. R. holds a Wellcome Trust Senior Research Fellowship in Basic Biomedical Science.
Paci, M. , Hyttinen, J. , Rodriguez, B. , and Severi, S. (2015) Human induced pluripotent stem cell‐derived versus adult cardiomyocytes: an in silico electrophysiological study on effects of ionic current block. British Journal of Pharmacology, 172: 5147–5160. doi: 10.1111/bph.13282.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al. (2013a). The Concise Guide to PHARMACOLOGY 2013/14: G protein‐coupled receptors. Br J Pharmacol 170: 1459–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al. (2013b). The Concise Guide to PHARMACOLOGY 2013/14: transporters. Br J Pharmacol 170: 1706–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellin M, Casini S, Davis RP, D'Aniello C, Haas J, Ward‐van Oostwaard D et al. (2013). Isogenic human pluripotent stem cell pairs reveal the role of a KCNH2 mutation in long‐QT syndrome. EMBO J 32: 3161–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan T, Fink M, Rodriguez B (2009). Multiscale modelling of drug‐induced effects on cardiac electrophysiological activity. Eur J Pharm Sci 36: 62–77. [DOI] [PubMed] [Google Scholar]
- Britton OJ, Bueno‐Orovio A, Van Ammel K, Lu HR, Towart R, Gallacher DJ et al. (2013). Experimentally calibrated population of models predicts and explains intersubject variability in cardiac cellular electrophysiology. Proc Natl Acad Sci U S A 110: E2098–E2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E (2006). Repolarization reserve in cardiac cells. J Med Biol Eng 26: 97–105. [Google Scholar]
- Carusi A, Burrage K, Rodríguez B (2012). Bridging experiments, models and simulations: an integrative approach to validation in computational cardiac electrophysiology. Am J Physiol Heart Circ Physiol 303: H144–H155. [DOI] [PubMed] [Google Scholar]
- Christophe B (2013). Simulation of early after‐depolarisation in non‐failing human ventricular myocytes: can this help cardiac safety pharmacology? Pharmacol Rep 65: 1281–1293. [DOI] [PubMed] [Google Scholar]
- Davies MR, Mistry HB, Hussein L, Pollard CE, Valentin J‐P, Swinton J et al. (2012). An in silico canine cardiac midmyocardial action potential duration model as a tool for early drug safety assessment. AJP ‐ Heart Circ Physiol 302: H1466–H1480. [DOI] [PubMed] [Google Scholar]
- Egashira T, Yuasa S, Suzuki T, Aizawa Y, Yamakawa H, Matsuhashi T et al. (2012). Disease characterization using LQTS‐specific induced pluripotent stem cells. Cardiovasc Res 95: 419–429. [DOI] [PubMed] [Google Scholar]
- Fu J‐D, Jiang P, Rushing S, Liu J, Chiamvimonvat N, Li RA (2010). Na+/Ca2+ exchanger is a determinant of excitation‐contraction coupling in human embryonic stem cell‐derived ventricular cardiomyocytes. Stem Cells Dev 19: 773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell P, Burrage K, Rodriguez B, Quinn TA (2014). Population of computational rabbit‐specific ventricular action potential models for investigating sources of variability in cellular repolarisation. PLoS One 9: e90112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan S, Greiser M, Harks E, Blaauw Y, van Hunnik A, Verheule S et al. (2006). AVE0118, blocker of the transient outward current (I(to)) and ultrarapid delayed rectifier current (I(Kur)), fully restores atrial contractility after cardioversion of atrial fibrillation in the goat. Circulation 114: 1234–1242. [DOI] [PubMed] [Google Scholar]
- Hamdam J, Sethu S, Smith T, Alfirevic A, Alhaidari M, Atkinson J et al. (2013). Safety pharmacology–current and emerging concepts. Toxicol Appl Pharmacol 273: 229–241. [DOI] [PubMed] [Google Scholar]
- Heist EK, Ruskin JN (2010). Drug‐induced arrhythmia. Circulation 122: 1426–1435. [DOI] [PubMed] [Google Scholar]
- Hoekstra M, Mummery CL, Wilde A a M, Bezzina CR, Verkerk AO (2012). Induced pluripotent stem cell derived cardiomyocytes as models for cardiac arrhythmias. Front Physiol 3: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Naito Y, Tomita M (2007). Simulation of developmental changes in action potentials with ventricular cell models. Syst Synth Biol 1: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki I, Maizels L, Huber I, Gepstein A, Arbel G, Caspi O et al. (2012). Modeling of catecholaminergic polymorphic ventricular tachycardia with patient‐specific human‐induced pluripotent stem cells. J Am Coll Cardiol 60: 990–1000. [DOI] [PubMed] [Google Scholar]
- Itzhaki I, Maizels L, Huber I, Zwi‐Dantsis L, Caspi O, Winterstern A et al. (2011). Modelling the long QT syndrome with induced pluripotent stem cells. Nature 471: 225–229. [DOI] [PubMed] [Google Scholar]
- Jung CB, Moretti A, Mederos y Schnitzler M, Iop L, Storch U, Bellin M et al. (2012). Dantrolene rescues arrhythmogenic RYR2 defect in a patient‐specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol Med 4: 180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannankeril P (2010). Drug‐induced long QT syndrome. Pharmacol Rev 62: 760–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knollmann BC (2013). Induced pluripotent stem cell‐derived cardiomyocytes: boutique science or valuable arrhythmia model? Circ Res 112: 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormos A, Nagy N, Acsai K, Váczi K, Ágoston S, Pollesello P et al. (2014). Efficacy of selective NCX inhibition by ORM‐10103 during simulated ischemia/reperfusion. Eur J Pharmacol 740: 539–551. [DOI] [PubMed] [Google Scholar]
- Kujala K, Paavola J, Lahti A, Larsson K, Pekkanen‐Mattila M, Viitasalo M et al. (2012). Cell model of catecholaminergic polymorphic ventricular tachycardia reveals early and delayed afterdepolarizations. PLoS One 7: e44660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti AL, Kujala VJ, Chapman H, Koivisto A‐P, Pekkanen‐Mattila M, Kerkela E et al. (2012). Model for long QT syndrome type 2 using human iPS cells demonstrates arrhythmogenic characteristics in cell culture. Dis Model Mech 5: 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leishman DJ, Beck TW, Dybdal N, Gallacher DJ, Guth BD, Holbrook M et al. (2012). Best practice in the conduct of key nonclinical cardiovascular assessments in drug development: current recommendations from the Safety Pharmacology Society. J Pharmacol Toxicol Methods 65: 93–101. [DOI] [PubMed] [Google Scholar]
- Li G, Feng J, Yue L, Carrier M (1998). Transmural heterogeneity of action potentials and Ito1 in myocytes isolated from the human right ventricle. Am J Physiol 275: H369–H377. [DOI] [PubMed] [Google Scholar]
- Li S, Chen G, Li RA (2013). Calcium signaling of human pluripotent stem cell‐derived cardiomyocytes. J Physiol 591: 5279–5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Fu JD, Siu CW, Li RA (2007). Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell‐derived cardiomyocytes: insights for driven maturation. Stem Cells 25: 3038–3044. [DOI] [PubMed] [Google Scholar]
- Ma D, Wei H, Zhao Y, Lu J, Li G, Binte N et al. (2013). Modeling type 3 long QT syndrome with cardiomyocytes derived from patient‐specific induced pluripotent stem cells. Int J Cardiol 168: 5277–5286. [DOI] [PubMed] [Google Scholar]
- Ma J, Guo L, Fiene SJ, Anson BD, Thomson JA, Kamp TJ et al. (2011). High purity human‐induced pluripotent stem cell‐derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. AJP ‐ Heart Circ Physiol 301: H2006–H2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsa E, Rajamohan D, Dick E, Young L, Mellor I, Staniforth A et al. (2011). Drug evaluation in cardiomyocytes derived from human induced pluripotent stem cells carrying a long QT syndrome type 2 mutation. Eur Heart J 32: 952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirams GR, Cui Y, Sher A, Fink M, Cooper J, Heath BM et al. (2011). Simulation of multiple ion channel block provides improved early prediction of compounds' clinical torsadogenic risk. Cardiovasc Res 91: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott‐Flügel L et al. (2010). Patient‐specific induced pluripotent stem‐cell models for long‐QT syndrome. N Engl J Med 363: 1397–1409. [DOI] [PubMed] [Google Scholar]
- Nagy N, Kormos A, Kohajda Z, Szebeni Á, Szepesi J, Pollesello P et al. (2014). Selective Na(+) /Ca(2+) exchanger inhibition prevents Ca(2+) overload‐induced triggered arrhythmias. Br J Pharmacol 171: 5665–5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara T, Virág L, Varró A, Rudy Y (2011). Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput Biol 7: e1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paci M, Hyttinen J, Aalto‐Setälä K, Severi S (2013a). Computational models of ventricular‐ and atrial‐like human induced pluripotent stem cell derived cardiomyocytes. Ann Biomed Eng 41: 2334–2348. [DOI] [PubMed] [Google Scholar]
- Paci M, Hyttinen J, Severi S (2013b). Computational modelling of LQT1 in human induced pluripotent stem cell derived cardiomyocytes. Comput Cardiol 40: 1239–1242. [Google Scholar]
- Paci M, Sartiani L, Lungo M, Jaconi M, Mugelli A, Cerbai E et al. (2012). Mathematical modelling of the action potential of human embryonic stem cell derived cardiomyocytes. Biomed Eng Online 11: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paci M, Severi S, Hyttinen J (2014). Computational modeling supports induced pluripotent stem cell‐derived cardiomyocytes reliability as a model for human LQT3. Comput Cardiol 41: 69–72. [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al. (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledgebase of drug targets and their ligands. Nucl. Acids Res. 42 (Database Issue): D1098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo E, Husti Z, Hornyik T (2010). Mechanisms of ventricular rate adaptation as a predictor of arrhythmic risk. AJP ‐ Heart Circ Physiol 298: H1577–H1587. [DOI] [PubMed] [Google Scholar]
- Qu Y, Ghatpande A, El‐Sherif N, Boutjdir M (2000). Gene expression of Na+/Ca2+ exchanger during development in human heart. Cardiovasc Res 45: 866–873. [DOI] [PubMed] [Google Scholar]
- Rodriguez B, Burrage K, Gavaghan D, Grau V, Kohl P, Noble D (2010). The systems biology approach to drug development: application to toxicity assessment of cardiac drugs. Clin Pharmacol Ther 88: 130–134. [DOI] [PubMed] [Google Scholar]
- Romero L, Carbonell B, Trenor B, Rodríguez B, Saiz J, Ferrero JM (2011). Systematic characterization of the ionic basis of rabbit cellular electrophysiology using two ventricular models. Prog Biophys Mol Biol 107: 60–73. [DOI] [PubMed] [Google Scholar]
- Romero L, Pueyo E, Fink M, Rodriguez B (2009). Impact of ionic current variability on human ventricular cellular electrophysiology. AJP Heart Circ Physiol 297: H1436–H1445. [DOI] [PubMed] [Google Scholar]
- Romero L, Trenor B, Yang P‐C, Saiz J, Clancy CE (2014). In silico screening of the impact of hERG channel kinetic abnormalities on channel block and susceptibility to acquired long QT syndrome. J Mol Cell Cardiol 72: 126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager PT, Gintant G, Turner JR, Pettit S, Stockbridge N (2014). Rechanneling the cardiac proarrhythmia safety paradigm: a meeting report from the Cardiac Safety Research Consortium. Am Heart J 167: 292–300. [DOI] [PubMed] [Google Scholar]
- Sartiani L, Bettiol E, Stillitano F, Mugelli A, Cerbai E, Jaconi ME (2007). Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells 25: 1136–1144. [DOI] [PubMed] [Google Scholar]
- Sauer AJ, Newton‐Cheh C (2012). Clinical and genetic determinants of torsade de pointes risk. Circulation 125: 1684–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RR (2013). Drug‐induced QT interval prolongation: does ethnicity of the thorough QT study population matter? Br J Clin Pharmacol 75: 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So PP‐S, Backx PH, Dorian P (2008). Slow delayed rectifier K+ current block by HMR 1556 increases dispersion of repolarization and promotes Torsades de Pointes in rabbit ventricles. Br J Pharmacol 155: 1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrenoire C, Wang K, Tung KWC, Chung WK, Pass RH, Lu JT et al. (2013). Induced pluripotent stem cells used to reveal drug actions in a long QT syndrome family with complex genetics. J Gen Physiol 141: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Gerlach U, Antzelevitch C (2003). HMR 1556, a potent and selective blocker of slowly activating delayed rectifier potassium current. J Cardiovasc Pharmacol 41: 140–147. [DOI] [PubMed] [Google Scholar]
- Di Veroli GY, Davies MR, Zhang H, Abi‐Gerges N, Boyett MR (2013). High‐throughput screening of drug‐binding dynamics to HERG improves early drug safety assessment. Am J Physiol Heart Circ Physiol 304: H104–H117. [DOI] [PubMed] [Google Scholar]
- Yazawa M, Hsueh B, Jia X, Pasca AM, Bernstein JA, Hallmayer J et al. (2011). Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471: 230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemzemi N, Bernabeu MO, Saiz J, Cooper J, Pathmanathan P, Mirams GR et al. (2013). Computational assessment of drug‐induced effects on the electrocardiogram: from ion channel to body surface potentials. Br J Pharmacol 168: 718–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1a AP biomarkers changes induced by the different blockade levels in the stimulated ventricular‐like model.
Table S1b AP biomarkers percent variations with respect to the control AP induced by the different blockade levels in the stimulated ventricular‐like model.
Table S2a AP biomarkers changes induced by the different blockade levels in the stimulated atrial‐like model.
Table S2b AP biomarkers percent variations with respect to the control AP induced by the different blockade levels in the stimulated atrial‐like model.
Table S3a AP biomarkers changes induced by the different blockade levels in the adult ORd model (If not present in the ORd model).
Table S3b AP biomarkers percent variations with respect to the control AP induced by the different blockade levels in the adult ORd model.
Table S4 Comparison between spontaneous (gray) and stimulated (white) ventricular‐like hiPSC‐CM APs. Biomarkers are presented as percent variations with respect to the control AP induced by the different blockade levels in the stimulated ventricular‐like model.
Table S5 Comparison between spontaneous (gray) and stimulated (white) atrial‐like hiPSC‐CM APs. Biomarkers are presented as percent variations with respect to the control AP induced by the different blockade levels in the stimulated atrial‐like model.
Figure S1 Details of the effect of INa block on the AP upstroke.
Figure S2 Global comparison of the effect of ionic current blocks on the morphological action potential (AP) biomarkers simulated by the hAdultV‐CM model. The pacing rate is 60 bpm. Grey levels represent the percent variation of each biomarker (normalized in the interval [−1, 1]) for each block level. MDP: maximum diastolic potential, VMax: maximum upstroke velocity, APA: action potential amplitude, Peak: peak voltage, APDxx: action potential duration at XX% of repolarization.
Figure S3 If block effects on VL hiPSC‐CM and hAL hiPSC‐CM. In panels B and C the effects on MDP and Peak have been detailed, respectively.
Figure S4 Assessment of the different contributions that sustain the AP in the hAdultV‐CM (ORd) model and comparison with full ICaL block in VL hiMPSC‐CM. Black, green and magenta traces refer to hAdultV‐CM hybridized with the VL hiPSC‐CM INaCa. The red trace refers to the hAdultV‐CM model with its original INaCa formulation and a 3.2‐fold increase in the maximal current.
Figure S5 Assessment of the repolarization reserve in VL hiPSC‐CM and hAdultV‐CM. A and B: comparison of different IK1 blocks on the APs and the underlying IKr. C: compensation of the IK1 block by doubling IKr (increment to 80 bpm of the pacing rate is needed to avoid spontaneous APs). D: the halved IKr and the consequent slower repolarization show how IKr compensates the absence of IK1 in adult cells.
Figure S6 Na+ concentration in conditions of INaCa block.
Figure S7 Ca2+ concentration in conditions of INaCa block. Peak of hAdultV‐CM Ca2+ transient equals to 0.022 mM.
Supporting info item


