Abstract
Mounting research evidence demonstrates a significant negative impact of circadian disruption on human health. Shift work, chronic jet lag and sleep disturbances are associated with increased incidence of metabolic syndrome, and consequently result in obesity, type 2 diabetes and dyslipidemia. Here, these associations are reviewed with respect to liver metabolism and disease.
KEY WORDS: Circadian rhythm, Liver, Metabolic syndrome, Type 2 diabetes
Abbreviations: ARC, arcuate nucleus; BMAL1, brain and muscle ARNT-like 1; CAR, constitutive androstane receptor; CLOCK, circadian locomotor output cycles kaput; CRY, cryptochrome; CYP7A1, cholesterol 7α-hydroxylase; CYPs, cytochrome P450 enzymes; DBP, D-site binding protein; E-box, enhance box; EMT, emergency medical technician; FAA, food anticipatory activity; FASPS, familial advanced sleep-phase syndrome; FEO, food entrainable oscillator; FOXO3, forkhead box O3; FXR, farnesoid-X receptor; GLUT2, glucose transporter 2; HDAC3, histone deacetylase 3; HIP, hypoxia inducing protein; HLF, hepatic leukemia factor; LDL, low-density lipoprotein; LRH1, liver receptor homolog 1; NAD+, nicotinamide adenine dinucleotide; PER, period; RHT, retinohypothalamic tract; RORα, retinoid-related orphan receptor α; RORE, ROR-response element; SCN, suprachiasmatic nucleus; SHP, small heterodimer partner; SIRT1, sirtuin 1; TEF, thyrotroph embryonic factor; TGR5, G protein-coupled bile acid receptor; TTFL, transcriptional translational feedback loop
Graphical abstract
Shift work, chronic jet lag, and sleep disturbances are associated with increased incidence of metabolic syndrome, and consequently result in obesity, Type 2 diabetes and dyslipidemia. Here, these associations are reviewed with respect to liver metabolism and disease.
1. Introduction
Circadian rhythms (Latin, circa: “approximate”; dies: “day”) refer to physiological processes that occur with a repeating period of approximately 24 h and ensure that internal physiology is synchronized with the external environment. Circadian rhythms are ubiquitously present in prokaryotes, fungi, algae, plants and mammals. Temporal organization within an organism is critical for maintenance of homeostasis as well as adaptation to changing environmental conditions. In mammals, this organization is generated and maintained endogenously by the biological clock, the suprachiasmatic nucleus (SCN), a heterogeneous paired cluster of about 20,000 neurons located in the hypothalamus of the brain. Circadian rhythms are defined by three basic properties: 1) they exist endogenously under constant conditions in the absence of resetting cues (for instance, in constant darkness) and oscillate with a period of approximately 24 h, 2) they are temperature- compensated, such that the period of the rhythm remains stable over a physiological range of temperatures, and 3) they are capable of entrainment, or synchronization, by external cues, such that timing of rhythms can be adjusted to match the external environment in a manor favorable to the organism1, 2. These properties result in endogenous stable rhythms that maintain basic homeostasis and also ensure adaptable physiological responses to the changing environmental photoperiod.
By way of the retinohypothalamic tract that connects the eye to the SCN3, 4, daily light/dark cues (i.e., the rotation of the earth every 24 h) are the main entraining agents, or Zeitgebers (German: “time giver”) that synchronize the clock to the external environment. However, non-photic cues such as social interaction, food, or exercise can also serve as Zeitgebers that change or reset the timing of the clock5. These Zeitgebers provide input to the SCN, which then processes the information and, through complex neurological pathways, ultimately influences behavioral, hormonal, and biochemical outputs that synchronize peripheral tissues to central timing (Fig. 1A).
Figure 1.
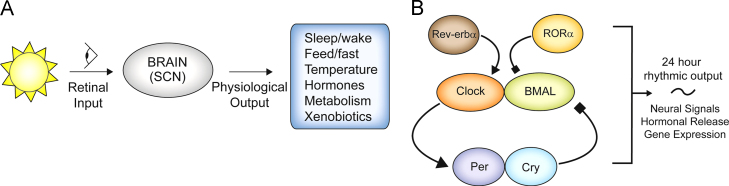
(A) Environmental signals perceived via the retinohypothalamic tract (RHT) by the biological clock, the suprachiasmatic nucleus (SCN) are the most prominent clock resetting agents. The SCN integrates photic and nonphotic signals to produce rhythmic outputs resulting in circadian regulation of locomotor activity, food intake, body temperature, hormonal release, and peripheral and xenobiotic metabolism. (B) Diagram depicting the transcriptional translational feedback loop (TTFL) that composes the molecular biological clock in almost all mammalian tissue types. Clock proteins CLOCK and BMAL1 heterodimerize to induce transcription of PER and CRY genes. PER1/2 and CRY1/2 proteins bind to E-box elements in the BMAL1 promoter to inhibit PER/CRY transcription via negative feedback. Additional regulatory clock components REV-ERBα and RORα positively and negatively regulate CLOCK/BMAL transcription, respectively, through binding to ROR-response elements in the BMAL1 promoter. This feedback loop takes approximately 24 h to complete and is the molecular basis for the mammalian biological clock that produces rhythmic outputs of neural and hormonal signals and gene transcripts.
At the molecular level, in both brain and peripheral tissues, clock outputs are generated in a cell-autonomous manner by the transcriptional translational feedback loop (TTFL) consisting of clock genes whose protein products oscillate to induce or suppress transcription of other clock genes, resulting in both positive and negative feedback loops6. Briefly, protein products of the core clock genes Clock (circadian locomotor output cycles kaput) and Bmal1 (brain and muscle ARNT-like 1) heterodimerize, translocate to the nucleus, and bind to E-box promoter sequences of target core clock genes Per1 and 2 (Period) and Cry1 and 2 (Cryptochrome) to initiate transcription. PER and CRY proteins translocate to the nucleus and interact with CLOCK/BMAL1 to inhibit their own transcription. The PER/CRY complex is eventually tagged for degradation via phosphorylation by casein kinase, which releases CLOCK/BMAL1 from suppression; this feedback loop takes approximately 24 h to complete. An additional regulatory loop exists whereby the nuclear receptors retinoid-related orphan receptor α (RORα) and REV-ERBα compete for the ROR response element (RORE) binding site in the Bmal1 promoter to activate or repress its transcription, respectively7 (Fig. 1B). The TTFL exists in almost all mammalian cells, including heart, liver, pancreas, muscle and white adipose tissue, in whole tissue, tissue explants and even persists in cell culture, and represents a mechanism by which peripheral tissue physiology can be entrained to central timing originating from the SCN.
Central-to-peripheral synchronization provides a means for organs and tissues to function with maximal efficiency (for instance, in preventing metabolic futile cycles during feeding and fasting). It is thought that desynchronization of this timing, due to shift work, chronic jet lag, or mental health disorders that affect sleep quality and timing such as depression and schizophrenia, can contribute to the development of disease conditions. Circadian disruption has been significantly linked to increased incidence of cardiovascular events, gastrointestinal diseases, and metabolic syndrome, and in 2007 the International Agency for Research on Cancer designated shift work as a Class 2A probable human carcinogen8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19. Within the liver, approximately 10% of the transcriptome is rhythmically expressed, including genes involved in regulation of glucose, lipid and nutrient homeostasis, and bile acid synthesis and metabolism. Recently, a genome-wide analysis in mouse liver revealed several thousand CLOCK protein binding sites, most of which exhibited day-night variations in CLOCK occupancy, suggesting extensive and wide-reaching metabolic regulatory functions for CLOCK and other clock components20. Basic and clinical research continues to provide mounting evidence for a critical link between circadian homeostasis and human health; here, we review these connections with respect to liver metabolism and metabolic disease.
2. Rhythms in liver metabolism
2.1. Bile acids
Circadian regulation plays a large role in liver metabolism, as glucose, bile acids, lipids and cholesterol are all subject to timed circadian control. Bile acids are amphipathic molecules that mediate absorption of dietary fats, nutrients and vitamins. Bile acids are synthesized from cholesterol exclusively in the liver by the rate-limiting enzyme cholesterol 7α-hydroxylase (CYP7A1) and are stored in the gallbladder until they are released postprandially. Synthesis of bile acids accounts for the majority of cholesterol catabolism in humans and the process is tightly regulated (by nutrient availability, nuclear receptors, and negative feedback from bile acids themselves) to control cholesterol and lipid homeostasis21. Bile acids are also under circadian regulation to synchronize with periods of feeding and fasting, and CYP7A1 exhibits a well-documented rhythm of mRNA expression in rodents (peaking early in the dark phase when nocturnal animals become active) and enzyme activity in human serum (measured as the bile acid intermediate 7α-hydroxy-4-choleston-3-one, or C4)22, 23, 24. Likewise, cholesterol synthesis exhibits a rhythm in rodents that is synchronized with the timing of food-intake25, while in humans, the rhythms of cholesterol and bile acid synthesis are out of phase26. Strict regulation of hepatic metabolism through circadian-regulated hepatobiliary pathways plays an important role in maintaining maximally efficient nutrient use and storage.
As mentioned above, bile acid metabolism is in part regulated by nuclear and membrane receptors. Farnesoid-X receptor (FXR) and liver receptor homolog 1 (LRH) play a role in regulating Cyp7a1 gene expression, while pregnane-X receptor, vitamin D receptor and the G protein-coupled bile acid receptor GPBAR1 (TGR5) are receptors of which bile acids are ligands. FXR modulates the negative feedback mechanism of bile acid-mediated suppression of CYP7A1 activity by induction of the negative co-repressor small heterodimer partner (SHP). When bile acids are increased in the liver, they activate FXR, which recruits SHP to block LRH-mediated induction of Cyp7a1 gene transcription, thus preventing de novo induction of bile acid synthesis. We have previously shown that Cyp7a1 transcription is induced by food, glucose and insulin likely through acetylation of the Cyp7a1 promoter, while fasting dampens and refeeding induces CYP7A1 expression, respectively27. Per1/2 double knockout mice have elevated serum and liver bile acids and elevated serum hepatic enzymes levels, indicative of liver damage28. Due to the critical role of bile acids in maintaining glucose and cholesterol homeostasis, disrupted circadian regulation of bile acid gene regulation or homeostasis may contribute to the hyperlipidemic and/or gastrointestinal phenotypes observed in shift workers.
Recently it was reported that the clock gene Rev-erbα regulates Cyp7a1 gene expression in a positive fashion, possibly by binding to the Shp promoter to prevent transcription and resulting in de-repression of CYP7A1 by SHP in a time-dependent manner29. REV-ERBα may also represent a link between metabolism and peripheral rhythms, as heme, a cofactor for several proteins involved in cellular metabolism, was identified as the natural ligand for REV-ERBα30. Rev-erbα transcription is positively regulated through RORα-binding to the RevDR2 direct repeat site, and transcription is inhibited when REV-ERBα itself binds to the same site in its own gene promoter31, 32. Heme binding to REV-ERBα suppresses gluconeogenic gene expression in the liver33, while Rev-erbα/β double knockout mice have increased serum glucose and triglycerides compared to wild type, as well as fragmented locomotor activity and reduced circadian period34. Administration of synthetic REV-ERBα ligands to obese mice resulted in weight loss, reduced lipogenic gene expression and improved glucose and lipid regulation35. Taken together, this evidence may point to a new important role for REV-ERBα as a critical circadian mediator of not just bile acids, but also lipid and glucose metabolism in the liver.
2.2. Glucose
Maintenance of glucose tolerance is critical for physiological function of nearly all cell types, and brain and red blood cells nearly exclusively use glucose as fuel. As mentioned, almost all mammalian cell types contain a functional molecular clock, including liver, muscle, and adipose tissue. Within the liver, glucose uptake, gluconeogenesis and glycogenolysis represent the main pathways by which nutrient homeostasis is maintained over daily periods of feeding and fasting. Glucagon and insulin, synthesized in and released from pancreatic α and β cells, respectively, regulate these pathways, and daily plasma rhythms in these hormones have been identified in rodents and humans36, 37. Impairment of this regulation, particularly insulin, can lead to type 2 diabetes and insulin resistance, which further results in hyperglycemia and mismanaged glucose utilization.
Plasma glucose exhibits a circadian rhythm in concentration, with peak levels occurring near the onset of activity in rodents and humans. The SCN appears to play a role in this phenomenon, as SCN-lesioned rats failed to produce glucose concentration rhythms in response to either ad libitum or scheduled feeding38. Likewise, glucose tolerance also exhibits a circadian rhythm and follows the pattern of plasma glucose concentration, and is likely driven by a 24-h-rhythm in insulin sensitivity, which is also absent in SCN-lesioned rats39. Several mouse knockout models also demonstrated a significant role of the SCN in maintenance of glucose homeostasis–Clock mutant mice are hyperphagic, obese, and hyperglycemic40. Also, it was recently shown that insulin can regulate liver Clock gene expression via the transcription factor forkhead box O3 (FOXO3), suggesting close interactions between the peripheral clock and the critical homeostatic actions of insulin41. Likewise, Bmal1 null mice have hypoinsulinemia and glucose intolerance, as well as abnormal locomotor and feeding behaviors42. Recently, one study demonstrated glucose intolerance in pancreas-specific Bmal1 knockout mice, which have normal insulin content but impaired release due to the lack of a functional pancreatic clock43, suggesting that circadian control of insulin may be the driving factor in maintaining glucose homeostasis throughout periods of feeding and fasting.
Liver-specific Bmal1 knockout mice have disrupted circadian function within hepatocytes, but normal locomotor activity and normal central and peripheral clock function within the SCN and muscle tissue, respectively. These mice have reduced GLUT2 (glucose transporter 2) expression in the liver and subsequently, fasting hypoglycemia, reduced liver glycogen and increased glucose clearance44. These results seem paradoxical, as whole-body Bmal1 null mice present with hyperglycemia and weight gain. However, this may simply indicate that liver-specific impairment of the circadian clock results in glucose-related defects that may be masked in whole-body Bmal1 knockout mice, which have additional impairments in activity, feeding behaviors and insulin secretion.
In normal humans, blood glucose and insulin levels in response to an oral glucose load vary over 24 h, with lower glucose response and higher insulin levels occurring in the morning, regardless of fasting duration, resulting in increased glucose tolerance in the morning compared to evening45, 46. The speculative causes of this variation include decreased nighttime glucose utilization, low late-day insulin secretion, and neurohormonal control of cortisol and other regulatory hormones47. In contrast, obese patients with type 2 diabetes were shown to have an inverted rhythm of glucose tolerance and insulin sensitivity, with increased sensitivity in the evening and night compared to morning37. An additional consequence of type 2 diabetes is known as the “dawn phenomenon”, whereby normal early morning release of counter-regulatory hormones (for instance, cortisol, growth hormone and epinephrine that function to oppose insulin and mobilize glucose) may cause increased blood glucose levels just before waking48, 49. The inverted rhythm of insulin sensitivity in these patients may be the cause of the elevated fasting blood glucose levels observed in the dawn phenomenon. Further study of the detrimental effects of glucose and insulin intolerance on normal functional peripheral rhythms will be necessary to provide better treatments for type 2 diabetes.
2.3. Lipids
The circadian clock plays a role in regulation of plasma and tissue lipids, including triglycerides, cholesterol and free fatty acids. Triglycerides, ingested from meals, are transported to the liver where they are either stored or utilized. During periods of fasting, adipose tissue is lipolysed to produce free fatty acids, which are also transported to the liver. It has been shown that high fat diets can affect peripheral clock function and suppress gene expression in mice50.
Feeding signals are important regulators of energy balance, peripheral circadian rhythms and feeding behavior. Imbalance caused by excess nutrients, circadian disruption, or both can lead to a feed-forward cycle by which excess fat disturbs peripheral rhythms of metabolic activity, which can lead to further imbalanced energy stores. Leptin is a circulating hormone primarily secreted by white adipose tissue that displays a circadian rhythm, and has been dubbed the “satiety hormone”. Leptin acts on several physiological levels by regulating sensation of hunger and metabolic energy use by binding to its receptor in the arcuate nucleus (ARC) in the hypothalamus, as well as receptors in the liver and other organs. Binding of leptin to its receptor signals a cellular cascade that ultimately promotes energy expenditure; decreased leptin signaling indicates an energy shortage and promotes food intake51. Circulating leptin displays a circadian rhythm in content52, independent of feeding time but dependent on the SCN. Obesity was associated with an overall increase in circulating leptin in humans (presumably due to increased fat mass), though the amplitude between nighttime peak and daytime nadir was significantly reduced compared to healthy controls53.
In addition to the effects on motivation to seek food (leptin-receptor signaling inhibited hedonic-based dopaminergic firing in reward areas of the brain in rodents54, while knockdown of leptin receptor signaling in dopamine-containing neurons of the ventral tegmental area resulted in increased locomotor activity, food-seeking behavior, and increased food intake in rodents55), leptin induces the JAK/STAT pathway that ultimately results in phosphorylated STAT3-mediated induction of transcription56. Studies suggest leptin mainly acts within the central nervous system, though this JAK/STAT effect is present in peripheral tissues. In pancreatic islets, leptin dose-dependently inhibited insulin secretion and mRNA transcript levels57, 58, while perfusion of leptin into livers of obese rats with a leptin-resistant phenotype led to decreased lipid-lowering effects, possibly via impaired activation of phosphoinositide 3-kinase59.
Several studies have utilized leptin- and/or leptin receptor-deficient rodents in attempts to determine the pathways involved in hormonal energy regulation. Mice with a leptin gene mutation (ob/ob mice) do not receive signals to the brain that indicate they have eaten enough, and as a result they are hyperphagic with hyperglycemia and hyperinsulinemia60. When compared to wild type mice, ob/ob mice also have intact SCN clock gene expression but abnormal peripheral clock gene expression, and these abnormalities were present prior to the onset of the metabolic phenotype61. It has also been shown that acetylation levels, indicative of transcriptional activation, of clock gene promoters were reduced in ob/ob mice62, which may partially contribute to the obese phenotype via disturbed clock function.
2.4. Epigenetic and posttranslational regulation of rhythms
Epigenetic regulatory mechanisms, including DNA methylation and histone modifications, participate heavily in the regulation of hepatic circadian rhythms63, 64. It was demonstrated that the methylation status of core clock genes was associated with obesity and other symptoms of metabolic syndrome, while type 2 diabetic patients exhibited hypermethylation of Per2 and subsequent decreased gene expression in pancreatic islets65, 66. CLOCK protein itself is a histone acetyltransferase that catalyzes the acetylation of both histone proteins and BMAL, which provides an implication for a possible role of the clock in the regulation of epigenetics67, 68. In addition, histone deacetylase 3 (HDAC 3), with REV-ERBα, was shown to be a key mediator of circadian lipid metabolism while deletion of HDAC3 led to hepatic steatosis69. Sirtuin1 (SIRT1), a mammalian histone deacetylase that requires nicotinamide adenine dinucleotide (NAD+) as a cofactor, acts as a cellular energy status sensor that responds to changing NAD+/NADH ratios. The HDAC activity of SIRT1 is regulated in a circadian manner. SIRT1 interacts with both CLOCK and BMAL1 proteins at the promoters of clock-controlled genes, may contribute to the rhythmic regulation of histone lysine acetylation in mouse liver70, and has been implicated as a regulatory link connecting the clock to cellular metabolism and energy use.
Proteomic and bioinformatic analyses, including analysis of posttranslational modifications of rhythmic proteins such as acetylation and methylation, represent extremely useful tools for the study of physiological rhythms. Like the rhythmic hepatic transcriptome, mass spectrometry-based analysis of the mouse liver proteome indicates that up to 20% of all hepatic proteins are subject to rhythmic control71. As mentioned earlier, a key regulatory step in the generation of endogenous rhythms is PER protein phosphorylation by casein kinase, which ultimately results in PER degradation. Mutation in the casein kinase binding site of the human PER2 protein or in casein kinase itself results in hypophosphorylation of PER2 which is thought to be the molecular basis of familial advanced sleep-phase syndrome (FASPS)72. FASPS is characterized by shortened and advanced endogenous circadian period, such that individuals exhibit extreme morning characteristics and have approximately 4–5 h advancement in sleep, body temperature and hormonal rhythms. Recent studies in mass spectrometry comprehensively identified phosphorylation sites in mPER2, and the results indicate that the dynamic cellular environment that influences protein stability and phosphorylation status plays a significant role in the generation and maintenance of rhythms at the molecular level73.
The circadian clock has also been implicated in the modulation of mitochondrial function via reversible protein acetylation. Lysine acetylation sites of proteins in mouse liver were analyzed over 24 h in Clock mutant mice and wild type littermates, and while a proportionately small number of sites remained oscillatory in Clock mutants (possibly due to a food entrainable oscillator, discussed in Section 3.2), it was shown that a significant number of proteins involved in metabolic pathways were rhythmically acetylated which were absent in Clock mutant mice. These results were also significantly correlated to already-existing data regarding the circadian metabolome74. Taken together, these data provide new insight into the critical regulatory interactions between peripheral clocks and modifications in post-transcription and post-translation that may direct cellular timing and possibly link clocks to cellular metabolism.
3. Circadian disruption and metabolic syndrome
3.1. Shift work
Millions of people work at night or work a rotating shift, employed as police, fire, and emergency medical technician (EMT) workers, doctors and nurses, truck drivers and pilots, just to name a few. They represent a significant portion of the population who are required to function at a time when humans have evolved to sleep, and are required to sleep when the SCN is promoting wakefulness. Also, technological advances have allowed for increased productivity in a 24 h society, which includes increased exposure to light at night, a relatively new phenomenon that may have adverse effects on human health75. In addition to fatigue-related safety issues, shift work has been associated with increased risk of breast cancer16, 17, 76, possibly linked to clock gene polymorphisms77 or suppression in melatonin production and signaling (which is normally increased at night) caused by light at night78. Cardiovascular disorders13, 79 and metabolic syndrome and obesity80, 81 have also been significantly linked to shift work and long-term exposure to light at night.
Within the context of metabolic disease, circadian disruption, sleep deprivation, and shift work are linked to hyperphagia, hyperinsulinemia, weight gain, and hypertriglyceridemia. In diabetes-prone hypoxia inducing protein (HIP) transgenic rats, a rotating light schedule resulted in accelerated development of type 2 diabetes and increased pancreatic β-cell apoptosis82. Wild type mice exposed to constant bright or dim light had increased body weight, reduced glucose tolerance, and ate more food during the daytime compared to controls housed under a normal light/dark schedule83. In a rodent model of shift work, mice forced to engage in daytime activity (by means of a slowly rotating activity wheel) for 5 weeks had decreased glucose tolerance, inverted clock gene expression and altered hepatic gene expression, and increased microvesicular steatosis84. In another model, 2 weeks of sleep restriction in mice, simulating shift work, resulted in suppression of core clock mRNA rhythms that preceded metabolic disruption85.
Several studies demonstrate that sleep restriction in healthy humans results in altered circulating levels of leptin86, 87, 88, and it is thought that loss of neurohormonal control of appetite and energy balance could be a contributing factor to the weight gain (though only partially due to overeating) associated with circadian disruption and shift work89. Night shift workers also have significantly decreased melatonin levels90 and elevated cortisol levels91, which have been shown to increase92 and suppress93 leptin, respectively. These complex hormonal pathways normally serve to finely regulate metabolism, and perturbation by even short-term shift work can lead to metabolic disarray and conflicting physiological signals. Finally, one study demonstrated that shifting the time of sleep (to simulate shift work) in a group of healthy volunteers resulted in increased inflammation and insulin insensitivity compared to the control group with normal bedtimes, despite both groups having slept the same numbers of hours94. These results highlight importance of studying the effects of disrupted rhythms on metabolism that may occur independently of sleep loss itself, possibly mediated through interactions of circulating hormones.
3.2. Restricted feeding
Related to shift work, studies involving timed food intake or restricted feeding have proven a valuable mechanism to determine how biological and cellular pathways in the periphery are regulated by the central clock95. Restricted feeding involves limiting the time or duration of food availability, or both, while controlling for caloric intake. Limiting feeding to the daytime in nocturnal animals effectively uncouples peripheral activity from what is dictated by the central clock and results in food anticipatory activity (FAA), which includes increased locomotor activity before the presentation of food as well as increased body temperature96. Induction of FAA is independent of the SCN and may be driven by a separate, yet still unidentified, food entrainable oscillator (FEO). Several neuronal clusters, or nuclei, may act as feeding centers in the brain, including the ARC, paraventricular nucleus, and dorsomedial hypothalamus, that participate in relaying signals to initiate eating behavior. Conflicting and inconclusive studies have yet to define a separate FEO, which may in fact represent multiple peripheral oscillators that all participate in generating FAA97.
When mice have access to a high fat diet only during the active dark phase, they are protected against diet-induced obesity and liver damage, and have reduced circulating leptin levels98. In addition, these mice have increased Cyp7a1 gene expression compared to controls whose expression profile was blunted by ad libitum high fat-feeding. Coupled with increased liver bile acids and decreased serum cholesterol, this is indicative of a shift toward cholesterol clearance. Conversely, restricting food to daylight hours in mice resulted in a phase-reversal of CYP7A1 expression and significantly elevated aspartate transaminase and alanine transaminase levels99. Several other studies have demonstrated the negative effects of daytime-restricted feeding in nocturnal rodents, including increased body weight and elevated and reversed circadian patterns of plasma leptin and ghrelin84, 100, 101. In addition, when caloric intake is not restricted, mice fed only during the inactive phase will consume more calories per day compared to night-fed controls102. Circadian regulation of leptin may play a role in mediating these effects, as ob/ob mice fed during the daytime only become more obese compared to controls when supplemented with rhythmic leptin administration, while daytime-fed mice receiving continuous administration of leptin via osmotic pump did not differ from control mice103.
However, several studies have also demonstrated neutral or even beneficial effects of time-restricted feeding in animals. A study by Sherman et al.104 demonstrated that long-term daytime restricted feeding of a high fat diet attenuated the normally disruptive effects of diet-induced obesity on the clock, including reducing body weight, cholesterol levels and markers of inflammation, and improving insulin sensitivity. The mechanism by which this occurs is unknown, though it was speculated that even though caloric intake was matched in ad libitum-fed controls, mice undergoing 4 h daytime restricted feeding were under fasting conditions for the remaining 20 h of each day, resulting in induction of fatty acid oxidation and catabolic pathways. In a separate study, it was shown that 8 h of daytime restricted feeding in Cry1/2 double knockout mice resulted in the rescue of circadian expression of several hundred genes that were previously arrhythmic, though this represented only a small subset of the total genes whose rhythmic expression was lost in the knockout phenotype105. In addition, a lipidomic analysis revealed that hepatic triglyceride levels still oscillated in Per1/2 double knockout mice, albeit with different phases of peak content, and that nighttime-restricted feeding reduced hepatic triglyceride content only in wild type mice106.
Much of the conflicting results likely stems from alterations in methods and animal models, though restricted feeding studies still represent a valuable tool that can be used to tease apart the effects of the endogenous clock versus the effects of food itself. Studies in humans are much less numerous and also give some conflicting results; the effects on body weight remain inconclusive, though the general consensus is that restricted feeding may provide some benefit toward reducing plasma lipids, improving insulin sensitivity and other metabolic risk factors107.
4. Chronopharmacology
In addition to the physiological effects of rhythms on hepatic metabolism, circadian rhythms must also be considered when developing drug plans or therapeutic interventions for disease treatment. Chronopharmacology is a branch of chronotherapy that applies the principles of circadian rhythms to determine the best timing of drug administration, which can affect the absorption, distribution, metabolism, and excretion of administered xenobiotics. Physiological influences such as gastric pH, hepatic and renal blood flow, serum hormone levels and liver enzyme activity exhibit circadian rhythms and can impact drug efficacy in a time-dependent manner. Chronopharmacology is currently utilized in the treatment of hypertension, asthma, and cancer, among other disorders. As an example, low-density lipoprotein (LDL) cholesterol-lowering statins, which inhibit the activity of HMG-CoA reductase, traditionally have been prescribed to be taken in the evening. The justification for this timing stems from the half-life of most statins being relatively short, coupled with the timing of peak cholesterol synthesis, which occurs in early morning108, 109. Awareness of rhythmic changes in efficacy, absorption or transport of a drug allows for improved drug development and decreased side effects.
Xenobiotic metabolism and detoxification are performed by three classes of hepatic proteins–Phase I drug oxidation proteins, to which many cytochrome P450 enzymes (CYPs) belong, are typically enzymes involved in oxidation, reduction, and hydrolyzing reactions. An extensive list of CYPs displays a circadian rhythm of activity, including those involved in xenobiotic metabolism110, 111, and it has been shown that the molecular clock may control the basal circadian regulation of these CYPs via rhythmic expression of the PAR subfamily of bZIP transcription factors DBP (D-site binding protein), TEF (thyrotroph embryonic factor) and HLF (hepatic leukemia factor). When challenged with pentobarbital, triple knockout mice lacking these three transcription factors failed to demonstrate the increased nighttime clearance rate in the manner seen in wild type controls, and extensive transcriptome analysis concluded that PAR bZIP proteins contribute to circadian regulation of detoxification enzymes through direct transcriptional regulation and also indirectly via constitutive androstane receptor (CAR)112.
Phase II drug conjugation enzymes participate in drug conjugation and include sulfotransferase, methyltransferase, and glutathione-S-transferase. Phase III drug transporters passively or actively uptake or efflux xenobiotics in intestine, liver and other tissues. It has been shown that Phase I, II and III proteins exhibit rhythmic patterns of expression in mice, suggesting that transport and metabolism of nutrients and xenobiotics must be coordinated for maximal response113, 114. Interestingly, studies indicate involvement of RORα in the regulation of Phase I and Phase II enzymes, including several CYP enzymes and the sulfotransferase SULT2A1115, 116. Further study of the chronopharmacokinetics of drug detoxification enzymes will lead to more specialized treatments that produce less harmful side effects via lower dosages, in part by taking advantage of the timing of maximal efficiency of these proteins.
Chronotherapy and chronopharmacology are currently utilized in cancer treatments, aiming to minimize toxic side effects while maintaining effective treatment. In addition to targeting the tumor cell cycle at times that are advantageous for preventing cell proliferation, timing the administration of anti-cancer drugs can reduce the occurrence of negative side effects. Levi et al.117 demonstrated in colorectal cancer patients that nighttime administration of the anti-cancer drug 5-fluorouracil was more effective than constant-rate infusion of the same drug, with significant reduction in the percentage of patients hospitalized for toxicity coupled with a significant increase in the percentage of patients responding with a >50% reduction in tumor size. This may be due to the fact that the toxicity of 5-fluorouracil was found to be dependent on the activity of dihydropyrimidine dehydrogenase, which is expressed at peak levels during the night118. Further studies (and perhaps a push for personalized medicine) are necessary to ensure a balance in drug dosage and timing that is both maximally effective against disease targets while minimally affecting healthy cells. The continued study of liver chronopharmacology will likely play a significant role in the development of more effective drug treatments.
5. Conclusions
Circadian rhythms provide a relatively new perspective on hepatic function and metabolism, particularly with respect to disrupted rhythms and shift work. Circadian rhythms evolved in almost all living organisms, are present in nearly all mammalian tissues and, within the liver, serve to synchronize glucose, lipid, bile acid and xenobiotic metabolic timing (Fig. 2). However, our understanding of the homeostatic control exerted by the circadian system, in the liver and elsewhere in the body, is only partially realized. Even less understood is how disrupted timing leads to disease conditions in humans. Conflicting studies abound and mechanisms remain unknown, but the overarching evidence that circadian homeostasis is critical to human health, and conversely, that circadian disruption negatively affects health, cannot be ignored. Further studies that uncover the physiological means by which rhythms contribute to homeostatic health will lead to improved disease treatment and prevention. Chronopharmacology, chronotherapy and proteomic analyses represent unique and largely unexplored resources in the treatment and prevention of human diseases. In turn, treating human disease under the additional context of circadian timing will likely shed new informational light on the circadian regulation of peripheral mechanistic pathways of metabolism.
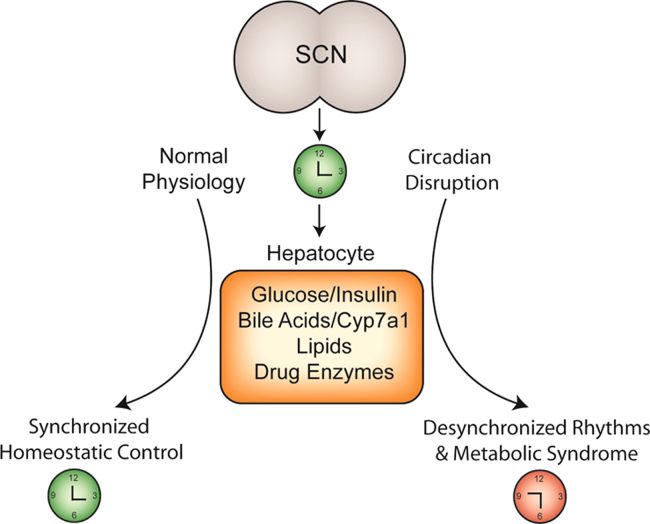
Figure 2.
The suprachiasmatic nucleus (SCN) generates endogenous biological rhythms, ensuring that internal physiology is synchronized with the external environment. Under normal conditions, rhythms in glucose and insulin, bile acids, lipids and drug enzymes contribute to homeostatic control of liver physiology. Under conditions of circadian disruption, including shift work, perturbations in these physiological rhythms result in desynchronized timing between SCN and the periphery and are associated with diabetes, obesity, and other symptoms of metabolic syndrome.
Acknowledgments
This work was supported by National Institutes of Health Grants (No. DK096784 to Jessica M. Ferrell and Nos. DK44442 and DK58379 to John Y.L. Chiang).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Aschoff J. Circadian rhythms in man. Science. 1965;148:1427–1432. doi: 10.1126/science.148.3676.1427. [DOI] [PubMed] [Google Scholar]
- 2.Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Cassone VM, Speh JC, Card JP, Moore RY. Comparative anatomy of the mammalian hypothalamic suprachiasmatic nucleus. J Biol Rhythms. 1988;3:71–91. doi: 10.1177/074873048800300106. [DOI] [PubMed] [Google Scholar]
- 4.Moore RY, Card JP. Visual pathways and the entrainment of circadian rhythms. Ann NY Acad Sci. 1985;453:123–133. doi: 10.1111/j.1749-6632.1985.tb11805.x. [DOI] [PubMed] [Google Scholar]
- 5.Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biol Rev. 1996;71:343–372. doi: 10.1111/j.1469-185x.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 6.Harmer SL, Panda S, Kay SA. Molecular bases of circadian rhythms. Annu Rev Cell Dev Biol. 2001;17:215–253. doi: 10.1146/annurev.cellbio.17.1.215. [DOI] [PubMed] [Google Scholar]
- 7.Guillaumond F, Dardente H, Giguère V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20:391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- 8.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I. Night-shift work and risk of colorectal cancer in the Nurses׳ health study. JNCI J Natl Cancer Inst. 2003;95:825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 9.Zhen Lu W, Ann Gwee K, Yu Ho K. Functional bowel disorders in rotating shift nurses may be related to sleep disturbances. Eur J Gastroenterol Hepatol. 2006;18:623–627. doi: 10.1097/00042737-200606000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Portaluppi F, Tiseo R, Smolensky MH, Hermida RC, Ayala DE, Fabbian F. Circadian rhythms and cardiovascular health. Sleep Med Rev. 2012;16:151–166. doi: 10.1016/j.smrv.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Konturek PC, Brzozowski T, Konturek SJ. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol. 2011;62:139–150. [PubMed] [Google Scholar]
- 12.Nojkov B, Rubenstein JH, Chey WD, Hoogerwerf WA. The impact of rotating shift work on the prevalence of irritable bowel syndrome in nurses. Am J Gastroenterol. 2010;105:842–847. doi: 10.1038/ajg.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietroiusti A, Neri A, Somma G, Coppeta L, Iavicoli I, Bergamaschi A. Incidence of metabolic syndrome among night-shift healthcare workers. Occup Environ Med. 2010;67:54–57. doi: 10.1136/oem.2009.046797. [DOI] [PubMed] [Google Scholar]
- 15.Esquirol Y, Bongard V, Mabile L, Jonnier B, Soulat JM, Perret B. Shift work and metabolic syndrome: respective impacts of job strain, physical activity, and dietary rhythms. Chronobiol Int. 2009;26:544–559. doi: 10.1080/07420520902821176. [DOI] [PubMed] [Google Scholar]
- 16.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. JNCI J Natl Cancer Inst. 2001;93:1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 17.Grundy A, Richardson H, Burstyn I, Lohrisch C, SenGupta SK, Lai AS. Increased risk of breast cancer associated with long-term shift work in Canada. Occup Environ Med. 2013;70:831–838. doi: 10.1136/oemed-2013-101482. [DOI] [PubMed] [Google Scholar]
- 18.Straif K, Baan R, Grosse Y, Secretan B, Ghissassi FE, Bouvard V. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8:1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 19.Lecarpentier Y, Claes V, Duthoit G, Hébert JL. Circadian rhythms, Wnt/β-catenin pathway and PPAR alpha/gamma profiles in diseases with primary or secondary cardiac dysfunction. Front Physiol. 2014;5:429. doi: 10.3389/fphys.2014.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshitane H, Ozaki H, Terajima H, Du NH, Suzuki Y, Fujimori T. CLOCK-controlled polyphonic regulation of circadian rhythms through canonical and noncanonical E-boxes. Mol Cell Biol. 2014;34:1776–1787. doi: 10.1128/MCB.01465-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiang JY. Regulation of bile acid synthesis. Front Biosci. 1998;3:d176–d193. doi: 10.2741/a273. [DOI] [PubMed] [Google Scholar]
- 22.Yamada M, Nagatomo J, Setoguchi Y, Kuroki N, Higashi S, Setoguchi T. Circadian rhythms of sterol 12α-hydroxylase, cholesterol 7α-hydroxylase and DBP involved in rat cholesterol catabolism. Biol Chem. 2000;381:1149–1153. doi: 10.1515/BC.2000.142. [DOI] [PubMed] [Google Scholar]
- 23.Kovář J, Leníček M, Zimolová M, Vítek L, Jirsa M, Piťha J. Regulation of diurnal variation of cholesterol 7α-hydroxylase (CYP7A1) activity in healthy subjects. Physiol Res. 2010;59:233–238. doi: 10.33549/physiolres.931753. [DOI] [PubMed] [Google Scholar]
- 24.Noshiro M, Usui E, Kawamoto T, Kubo H, Fujimoto K, Furukawa M. Multiple mechanisms regulate circadian expression of the gene for cholesterol 7α-hydroxylase (Cyp7α), a key enzyme in hepatic bile acid biosynthesis. J Biol Rhythms. 2007;22:299–311. doi: 10.1177/0748730407302461. [DOI] [PubMed] [Google Scholar]
- 25.Edwards PA, Muroya H, Gould RG. In vivo demonstration of the circadian thythm of cholesterol biosynthesis in the liver and intestine of the rat. J Lipid Res. 1972;13:396–401. [PubMed] [Google Scholar]
- 26.Gälman C, Angelin B, Rudling M. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology. 2005;129:1445–1453. doi: 10.1053/j.gastro.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Li T, Matozel M, Boehme S, Kong B, Nilsson LM, Guo G. Overexpression of cholesterol 7α-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology. 2011;53:996–1006. doi: 10.1002/hep.24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma K, Xiao R, Tseng HT, Shan L, Fu L, Moore DD. Circadian dysregulation disrupts bile acid homeostasis. PLoS One. 2009;4:e6843. doi: 10.1371/journal.pone.0006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duez H, van der Veen JN, Duhem C, Pourcet B, Touvier T, Fontaine C. Regulation of bile acid synthesis by the nuclear receptor REV-ERBα. Gastroenterology. 2008;135:689–698. doi: 10.1053/j.gastro.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 30.Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat Struct Mol Biol. 2007;14:1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delerive P, Chin WW, Suen CS. Identification of Reverbα as a novel RORα target gene. J Biol Chem. 2002;277:35013–35018. doi: 10.1074/jbc.M202979200. [DOI] [PubMed] [Google Scholar]
- 32.Raspè E, Mautino G, Duval C, Fontaine C, Duez H, Barbier O. Transcriptional regulation of human REV-ERBα gene expression by the orphan nuclear receptor retinoic acid-related orphan receptor α. J Biol Chem. 2002;277:49275–49281. doi: 10.1074/jbc.M206215200. [DOI] [PubMed] [Google Scholar]
- 33.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA. REV-ERBα, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 34.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT. Regulation of circadian behaviour and metabolism by REV-ERBα and REV-ERBβ. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solt LA, Wang YJ, Banerjee S, Hughes T, Kojetin DJ, Lundasen T. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiter M, La Fleur SE, van Heijningen C, van der Vliet J, Kalsbeek A, Buijs RM. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes. 2003;52:1709–1715. doi: 10.2337/diabetes.52.7.1709. [DOI] [PubMed] [Google Scholar]
- 37.Boden G, Ruiz J, Urbain JL, Chen X. Evidence for a circadian rhythm of insulin secretion. Am J Physiol. 1996;271:E246–E252. doi: 10.1152/ajpendo.1996.271.2.E246. [DOI] [PubMed] [Google Scholar]
- 38.La Fleur SE, Kalsbeek A, Wortel J, Buijs RM. A suprachiasmatic nucleus generated rhythm in basal glucose concentrations. J Neuroendocrinol. 1999;11:643–652. doi: 10.1046/j.1365-2826.1999.00373.x. [DOI] [PubMed] [Google Scholar]
- 39.La Fleur SE, Kalsbeek A, Wortel J, Fekkes ML, Buijs RM. A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes. 2001;50:1237–1243. doi: 10.2337/diabetes.50.6.1237. [DOI] [PubMed] [Google Scholar]
- 40.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon EL. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaves I, van der Horst GT, Schellevis R, Nijman RM, Koerkamp MG, Holstege FC. Insulin-FOXO3 signaling modulates circadian rhythms via regulation of clock transcription. Curr Biol. 2014;24:1248–1255. doi: 10.1016/j.cub.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 42.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–124. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayer KH, Stamler J, Dyer A, Freinkel N, Stamler R, Berkson DM. Epidemiologic findings on the relationship of time of day and time since last meal to glucose tolerance. Diabetes. 1976;25:936–943. doi: 10.2337/diab.25.10.936. [DOI] [PubMed] [Google Scholar]
- 46.Aparicio NJ, Puchulu FE, Gagliardino JJ, Ruiz M, Llorens JM, Ruiz J. Circadian variation of the blood glucose, plasma insulin and human growth hormone levels in response to an oral glucose load in normal subjects. Diabetes. 1974;23:132–137. doi: 10.2337/diab.23.2.132. [DOI] [PubMed] [Google Scholar]
- 47.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18:716–738. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 48.Shih KC, Hsieh SH, Kwok CF, Hwu CM, Hsieh PS, Ho LT. Effect of growth hormone on dawn phenomenon in patients with type 2 diabetes. Growth Factors. 2013;31:66–73. doi: 10.3109/08977194.2013.772996. [DOI] [PubMed] [Google Scholar]
- 49.Porcellati F, Lucidi P, Bolli GB, Fanelli CG. Thirty years of research on the dawn phenomenon: lessons to optimize blood glucose control in diabetes. Diabetes Care. 2013;36:3860–3862. doi: 10.2337/dc13-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Muoio DM, Lynis Dohm G. Peripheral metabolic actions of leptin. Best Pract Res Clin Endocrinol Metab. 2002;16:653–666. doi: 10.1053/beem.2002.0223. [DOI] [PubMed] [Google Scholar]
- 52.Kalsbeek A, Fliers E, Romijn JA, la Fleur SE, Wortel J, Bakker O. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 2001;142:2677–2685. doi: 10.1210/endo.142.6.8197. [DOI] [PubMed] [Google Scholar]
- 53.Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Figlewicz DP, MacDonald Naleid A, Sipols AJ. Modulation of food reward by adiposity signals. Physiol Behav. 2007;91:473–478. doi: 10.1016/j.physbeh.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 56.Banks AS, Davis SM, Bates SH, Myers MG. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 57.Emilsson V, Liu YL, Cawthorne MA, Morton NM, Davenport M. Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes. 1997;46:313–316. doi: 10.2337/diab.46.2.313. [DOI] [PubMed] [Google Scholar]
- 58.Pallett AL, Morton NM, Cawthorne MA, Emilsson V. Leptin inhibits insulin secretion and reduces insulin mRNA levels in rat isolated pancreatic islets. Biochem Biophys Res Commun. 1997;238:267–270. doi: 10.1006/bbrc.1997.7274. [DOI] [PubMed] [Google Scholar]
- 59.Huang W, Dedousis N, Bhatt BA, O’Doherty RM. Impaired activation of phosphatidylinositol 3-kinase by leptin is a novel mechanism of hepatic leptin resistance in diet-induced obesity. J Biol Chem. 2004;279:21695–21700. doi: 10.1074/jbc.M401546200. [DOI] [PubMed] [Google Scholar]
- 60.Meinders AE, Toornvliet AC, Pijl H. Leptin. Neth J Med. 1996;49:247–252. doi: 10.1016/s0300-2977(96)00039-3. [DOI] [PubMed] [Google Scholar]
- 61.Ando H, Kumazaki M, Motosugi Y, Ushijima K, Maekawa T, Ishikawa E. Impairment of peripheral circadian clocks precedes metabolic abnormalities in ob/ob mice. Endocrinology. 2011;152:1347–1354. doi: 10.1210/en.2010-1068. [DOI] [PubMed] [Google Scholar]
- 62.Ishikawa-Kobayashi E, Ushijima K, Ando H, Maekawa T, Takuma M, Furukawa Y. Reduced histone H3K9 acetylation of clock genes and abnormal glucose metabolism in ob/ob mice. Chronobiol Int. 2012;29:982–993. doi: 10.3109/07420528.2012.706765. [DOI] [PubMed] [Google Scholar]
- 63.Mauvoisin D, Dayon L, Gachon F, Kussmann M. Proteomics and circadian rhythms: it׳s all about signaling! Proteomics. 2015;15:310–317. doi: 10.1002/pmic.201400187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Welberg L. Circadian rhythms: methylation mediates clock plasticity. Nat Rev Neurosci. 2014;15:206. doi: 10.1038/nrn3712. [DOI] [PubMed] [Google Scholar]
- 65.Volkmar M, Dedeurwaerder S, Cunha DA, Ndlovu MN, Defrance M, Deplus R. DNA methylation profiling identifies epigenetic dysregulation in pancreatic islets from type 2 diabetic patients. EMBO J. 2012;31:1405–1426. doi: 10.1038/emboj.2011.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milagro FI, Gómez-Abellán P, Campión J, Martínez JA, Ordovás JM, Garaulet M. CLOCK, PER2 and BMAL1 DNA methylation: association with obesity and metabolic syndrome characteristics and monounsaturated fat intake. Chronobiol Int. 2012;29:1180–1194. doi: 10.3109/07420528.2012.719967. [DOI] [PubMed] [Google Scholar]
- 67.Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450:1086–1090. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- 68.Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 69.Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O’Neill JS. Circadian orchestration of the hepatic proteome. Curr Biol. 2006;16:1107–1115. doi: 10.1016/j.cub.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 72.Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM. An hPER2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 73.Vanselow K, Kramer A. Role of phosphorylation in the mammalian circadian clock. Cold Spring Harb Symp Quant Biol. 2007;72:167–176. doi: 10.1101/sqb.2007.72.036. [DOI] [PubMed] [Google Scholar]
- 74.Masri S, Patel VR, Eckel-Mahan KL, Peleg S, Forne I, Ladurner AG. Circadian acetylome reveals regulation of mitochondrial metabolic pathways. Proc Natl Acad Sci USA. 2013;110:3339–3344. doi: 10.1073/pnas.1217632110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fonken LK, Nelson RJ. The effects of light at night on circadian clocks and metabolism. Endocr Rev. 2014;35:648–670. doi: 10.1210/er.2013-1051. [DOI] [PubMed] [Google Scholar]
- 76.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I. Rotating night shifts and risk of breast cancer in women participating in the nurses׳ health study. JNCI J Natl Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 77.Truong T, Liquet B, Menegaux F, Plancoulaine S, Laurent-Puig P, Mulot C. Breast cancer risk, nightwork, and circadian clock gene polymorphisms. Endocr Relat Cancer. 2014;21:629–638. doi: 10.1530/ERC-14-0121. [DOI] [PubMed] [Google Scholar]
- 78.Hill S, Blask D, Xiang S, Yuan L, Mao L, Dauchy R. Melatonin and associated signaling pathways that control normal breast epithelium and breast cancer. J Mammary Gland Biol Neoplasia. 2011;16:235–245. doi: 10.1007/s10911-011-9222-4. [DOI] [PubMed] [Google Scholar]
- 79.Puttonen S, Härmä M, Hublin C. Shift work and cardiovascular disease-pathways from circadian stress to morbidity. Scand J Work Environ Health. 2010;36:96–108. doi: 10.5271/sjweh.2894. [DOI] [PubMed] [Google Scholar]
- 80.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knutsson A. Health disorders of shift workers. Occup Med. 2003;53:103–108. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 82.Gale JE, Cox HI, Qian J, Block GD, Colwell CS, Matveyenko AV. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J Biol Rhythms. 2011;26:423–433. doi: 10.1177/0748730411416341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107:18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology. 2010;151:1019–1029. doi: 10.1210/en.2009-0864. [DOI] [PubMed] [Google Scholar]
- 85.Barclay JL, Husse J, Bode B, Naujokat N, Meyer-Kovac J, Schmid SM. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS One. 2012;7:e37150. doi: 10.1371/journal.pone.0037150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 88.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–656. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 89.Crispim CA, Waterhouse J, Dâmaso AR, Zimberg IZ, Padilha HG, Oyama LM. Hormonal appetite control is altered by shift work: a preliminary study. Metabolism. 2011;60:1726–1735. doi: 10.1016/j.metabol.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 90.Davis S, Mirick DK, Chen C, Stanczyk FZ. Night shift work and hormone levels in women. Cancer Epidemiol Biomarkers Prev. 2012;21:609–618. doi: 10.1158/1055-9965.EPI-11-1128. [DOI] [PubMed] [Google Scholar]
- 91.Manenschijn L, van Kruysbergen RG, de Jong FH, Koper JW, van Rossum EF. Shift work at young age is associated with elevated long-term cortisol levels and body mass index. J Clin Endocrinol Metab. 2011;96:E1862–E1865. doi: 10.1210/jc.2011-1551. [DOI] [PubMed] [Google Scholar]
- 92.Alonso-Vale MIC, Andreotti S, Peres SB, Anhê GF, das Neves Borges-Silva C, Neto JC. Melatonin enhances leptin expression by rat adipocytes in the presence of insulin. Am J Physiol Endocrinol Metab. 2005;288:E805–E812. doi: 10.1152/ajpendo.00478.2004. [DOI] [PubMed] [Google Scholar]
- 93.Wabitsch M, Bo Jensen P, Blum WF, Christoffersen CT, Englaro P, Heinze E. Insulin and cortisol promote leptin production in cultured human fat cells. Diabetes. 1996;45:1435–1438. doi: 10.2337/diab.45.10.1435. [DOI] [PubMed] [Google Scholar]
- 94.Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Damiola F, Minh NL, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mistlberger RE. Food-anticipatory circadian rhythms: concepts and methods. Eur J Neurosci. 2009;30:1718–1729. doi: 10.1111/j.1460-9568.2009.06965.x. [DOI] [PubMed] [Google Scholar]
- 97.Escobar C, Cailotto C, Angeles-Castellanos M, Delgado RS, Buijs RM. Peripheral oscillators: the driving force for food-anticipatory activity. Eur J Neurosci. 2009;30:1665–1675. doi: 10.1111/j.1460-9568.2009.06972.x. [DOI] [PubMed] [Google Scholar]
- 98.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu N, Yin L, Hanniman EA, Joshi S, Lazar MA. Negative feedback maintenance of heme homeostasis by its receptor, REV-ERBα. Genes Dev. 2009;23:2201–2209. doi: 10.1101/gad.1825809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity. 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Jr, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1071–R1079. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- 102.Bray MS, Ratcliffe WF, Grenett MH, Brewer RA, Gamble KL, Young ME. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int J Obes. 2013;37:843–852. doi: 10.1038/ijo.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arble DM, Vitaterna MH, Turek FW. Rhythmic leptin is required for weight gain from circadian desynchronized feeding in the mouse. PLoS One. 2011;6:e25079. doi: 10.1371/journal.pone.0025079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26:3493–3502. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- 105.Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci USA. 2009;106:21453–21458. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Adamovich Y, Rousso-Noori L, Zwighaft Z, Neufeld-Cohen A, Golik M, Kraut-Cohen J. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014;19:319–330. doi: 10.1016/j.cmet.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rothschild J, Hoddy KK, Jambazian P, Varady KA. Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr Rev. 2014;72:308–318. doi: 10.1111/nure.12104. [DOI] [PubMed] [Google Scholar]
- 108.Plakogiannis R, Cohen H. Optimal low-density lipoprotein cholesterol lowering—morning versus evening statin administration. Ann Pharmacother. 2007;41:106–110. doi: 10.1345/aph.1G659. [DOI] [PubMed] [Google Scholar]
- 109.Jones PJ, Schoeller DA. Evidence for diurnal periodicity in human cholesterol synthesis. J Lipid Res. 1990;31:667–673. [PubMed] [Google Scholar]
- 110.Tomalik-Scharte D, Suleiman AA, Frechen S, Kraus D, Kerkweg U, Rokitta D. Population pharmacokinetic analysis of circadian rhythms in hepatic CYP3A activity using midazolam. J Clin Pharmacol. 2014;54:1162–1169. doi: 10.1002/jcph.318. [DOI] [PubMed] [Google Scholar]
- 111.Košir R, Španinger K, Rozman D. Circadian events in human diseases and in cytochrome P450-related drug metabolism and therapy. IUBMB Life. 2013;65:487–496. doi: 10.1002/iub.1160. [DOI] [PubMed] [Google Scholar]
- 112.Gachon F, Olela FF, Schaad O, Descombes P, Schibler U. The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab. 2006;4:25–36. doi: 10.1016/j.cmet.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 113.Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–550. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- 114.Zhang YKJ, Yeager RL, Klaassen CD. Circadian expression profiles of drug-processing genes and transcription factors in mouse liver. Drug Metab Dispos. 2009;37:106–115. doi: 10.1124/dmd.108.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kang HS, Angers M, Beak JY, Wu XY, Gimble JM, Wada T. Gene expression profiling reveals a regulatory role for RORα and RORγ in phase I and phase II metabolism. Physiol Genomics. 2007;31:281–294. doi: 10.1152/physiolgenomics.00098.2007. [DOI] [PubMed] [Google Scholar]
- 116.Ou Z, Shi X, Gilroy RK, Kirisci L, Romkes M, Lynch C. Regulation of the human hydroxysteroid sulfotransferase (SULT2A1) by RORα and RORγ and its potential relevance to human liver diseases. Mol Endocrinol. 2013;27:106–115. doi: 10.1210/me.2012-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lévi F, Giacchetti S, Zidani R, Brezault-Bonnet C, Tigaud JM, Goldwasser F. Chronotherapy of colorectal cancer metastases. Hepatogastroenterology. 2001;48:320–322. [PubMed] [Google Scholar]
- 118.Harris BE, Song R, Soong SJ, Diasio RB. Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion. Cancer Res. 1990;50:197–201. [PubMed] [Google Scholar]