Abstract
This study reports for the first time, to our knowledge, descriptive epidemiological data for 18 invasive Candida isolates from Pakistan, including species identification and antifungal susceptibility against fluconazole, itraconazole, voriconazole, caspofungin, micafungin, anidulafungin and amphotericin. Risk factors for invasive candidiasis (IC) were determined for 96 patients from Karachi, Pakistan. In adults and neonates, Candida tropicalis (38 and 36 %, respectively) was the most common species, followed in adults by Candida parapsilosis (17.8 %), Candida glabrata (15.9 %) and Candida albicans (12.3 %). C. albicans (21 %) was the second most common in neonates. In children, C. albicans (31.9 %), C. tropicalis (26.4 %) and C. parapsilosis (19.4 %) were the most common. C. albicans IC was significantly associated with paediatric age [crude odds ratio (COR) 3.46, 95% confidence interval (CI) 1.63–7.32]. Rare species made up 17.5% of the total isolates studied. Resistance to fluconazole was seen in C. glabrata (15.0%) and Candida krusei (100.0%). Only one isolate (C. glabrata) was resistant to all three echinocandins. Low MICs of fluconazole for 98% (184/188) of isolates tested support its continued use as an empiric therapy for IC. Non-C. albicans IC was associated with the use of β lactam inhibitor combinations (COR 3.16, 95% CI 1.05–9.57). Use of healthcare devices was documented in 85.4% of IC patients, whilst 75.0% had been admitted to special care units. Surprisingly, 66.7% of patients with IC were not obviously immunosuppressed. The high frequency of modifiable risk factors in this population indicates that candidaemia can be reduced with stringent antibiotic and infection control measures. These data will be useful for empiric selection of antifungals in Karachi, and contribute to global assessments of antifungal resistance.
INTRODUCTION
Invasive candidiasis (IC) is a severe illness with a high mortality and increasing overall cost of management (Colombo et al., 2006; Hassan et al., 2009; Horn et al., 2009; Pfaller et al., 2011d). Advances in medical technology and therapeutics have resulted in increasing numbers of invasive fungal infections including IC (Diaz & Fell, 2004). Individuals considered at greatest risk of IC are premature infants, patients receiving broad-spectrum antibiotics or anti-cancer chemotherapy (Picazo et al., 2008) and those with immunodeficiencies such as haematopoietic stem-cell transplant or solid-organ transplant recipients, profound neutropenia or diabetes (Kontoyiannis et al., 2010; Pappas et al., 2010).
Globally, Candida albicans is the most frequently reported species; however, the emergence of non-C. albicans Candida species with resistance to fluconazole is of concern, highlighted by recent surveillance data (Pfaller et al., 2011d). As inappropriate initial therapy for IC can result in an adverse outcome, accurate identification of the species responsible for IC and knowledge of their corresponding susceptibility patterns are essential. It is also important to recognize populations at greater risk of IC in the healthcare setting. In the absence of a national surveillance system for IC in Pakistan, laboratory data are epidemiologically useful to identify susceptible patient populations, monitor trends and detect emerging resistance. Most of the clinical laboratories in Pakistan rely on phenotypic identification methods to report Candida species; however, current genotypic methods of identification are considered more reliable, especially for the less common species (Deak et al., 2010; Wang et al., 2008).
In this study, we determined the spectrum of invasive Candida isolates in samples collected from major cities of Pakistan, confirmed their identification to the species level with molecular methods and determined their susceptibility patterns. We also analysed risk factors associated with IC and have provided descriptive epidemiological information on these patients.
METHODS
Study background
The study was conducted in the clinical laboratory of the Aga Khan University Hospital (AKUH), Karachi, Pakistan. The laboratory has a national specimen collection network with more than 175 collection points in major cities and towns across the country. Samples are processed in the clinical laboratory based in Karachi. The laboratory data were collected from nine major cities in Pakistan, the majority from Karachi.
Definitions
IC was defined as the isolation of Candida species from normally sterile specimens, such as blood, cerebrospinal fluid, peritoneal fluid, pleural fluid, synovial fluid and bile, and from the tips of central venous catheters and from ventriculoperitoneal shunts. Patients were considered children if they were ≤14 years, and neonates if they were <30 days. Isolation of the same Candida species within 15 days was considered a single episode of IC. Sepsis was defined as presence of hypotension (systolic blood pressure <90 mm Hg or the need for inotropes), temperature <36 or >38 °C and a white blood cell count of <4000 or >10 000 mm−3. Admission to adult, paediatric or neonatal intensive care units was considered ‘special care stay’. Nosocomial infections were those acquired after >48 h of admission to, or within 90 days of discharge from, a healthcare facility. A central line-associated bloodstream infection (CLABSI) was considered if there was no other source of bloodstream infection except a central venous line. A β-lactam inhibitor combination (BLIC) was cephalosporin or penicillins combined with tazobactam, clavulanic acid or sulbactam. Patients on dialysis received either peritoneal or haemodialysis. Neutrophilia was defined as >70 % neutrophils on a peripheral blood smear, whilst neutropenia was defined as an absolute neutrophil count of <1000 mm−3. ‘Prior antifungal use’ was the administration of fluconazole, voriconazole, itraconazole or amphotericin B at least 24 h before collection of the specimen yielding Candida species. Healthcare devices included central venous lines [femoral, jugular or subclavian central venous catheters, haemodialysis catheters, peripherally inserted central catheter lines, portacath or Hickman lines], permanent or transient pacemakers, ventilators, abdominal or chest drains, peritoneal dialysis catheters, percutaneous nephrostomy tubes, suprapubic catheters, percutaneous endoscopic gastrostomy tubes, pancreatic duct stents, endoventricular drains, ventriculoperitoneal shunts and orthopaedic implants. ‘Complicated intra-abdominal infection’ was defined as abdominal infection spreading beyond the hollow viscus into the peritoneal cavity, resulting in peritonitis or intra-abdominal abscess. Community-acquired or healthcare-associated pneumonia or urinary tract infections were all included in ‘pneumonia’ or ‘urinary tract infection’, respectively. Total parenteral nutrition was the administration of amino acids and lipids via the intravenous route when enteral feeding was not possible or inadequate. ‘Immunosuppressed status’ was when the patient was receiving steroids, cyclosporine, azathioprine or anti-cancer chemotherapy, or was neutropenic, or was a cancer or transplant patient.
Risk factor study
Medical records of 96/183 patients (118/207 isolates) registered with AKUH were retrieved for a retrospective clinical data review. Risk factors, underlying disease and the course of clinical illness were recorded.
Yeast identification
During the study period (January 2006 to May 2009), 207 sterile specimens from 180 patients yielded Candida species. After excluding duplicates from the same episode of candidiasis, 188 isolates were included. Five patients had candidaemia with multiple species, whilst two had recurrence of IC with the same species. The specimens were 166 blood cultures, 15 sterile fluids (seven peritoneal, three pleural, three cerebrospinal fluid and two others), six central catheter tips and one ventricular shunt end tip. The geographical origin of these isolates was as follows: 134 were from AKUH, 34 were from other healthcare facilities in Karachi and 20 were from additional cities in Pakistan (seven from Lahore, four from Multan, three from Hyderabad, two from Quetta and one each from Peshawar, Rawalpindi, Bahawalpur and Rahimyar Khan). Species identification was based on conventional phenotypic characteristics: production of a germ tube, morphology on BBL BiGGY Agar (BD), growth with cycloheximide, urease production, morphology on cornmeal/Tween 80 agar and the identification profile generated using API 20C AUX (bioMe´rieux). Isolates were stored in glycerol phosphate buffer at −80 °C. Their identification was also confirmed using either a Luminex multianalyte profiling assay with the ITS2 target or DNA sequencing as described previously by Das et al. (2006) and Deak et al. (2010). Agreement between phenotypic and molecular identification was >90 % for common species such as C. albicans, Candida tropicalis and Candida parapsilosis.
Antifungal susceptibility testing
Antifungal susceptibility testing was performed by broth microdilution with fluconazole, itraconazole, voriconazole, posaconazole, anidulafungin, caspofungin and micafungin as described by the Clinical and Laboratory Standards Institute (CLSI, 2008) using frozen RPMI microbroth trays custom manufactured by TREK Diagnostics. Results were read visually after 24 h of incubation as the lowest concentration of drug that caused a significant decrease in growth compared with the control well. Recently approved although not yet published CLSI 24 h resistance breakpoints for fluconazole, voriconazole and the echinocandins were used (CLSI, 2008; Pfaller et al., 2010a, 2011a, c). C. albicans, C. tropicalis and C. parapsilosis with MICs ≥8 µg ml−1 and C. glabrata with an MIC ≥64 µg ml−1 were considered resistant to fluconazole, whilst Candida krusei was considered intrinsically resistant to fluconazole. C. albicans, C. tropicalis and C. parapsilosis with MICs ≥1 mg ml21 were considered resistant to voriconazole. C. albicans, C. tropicalis and C. krusei with MICs ≥1 µg ml−1 were considered resistant to caspofungin, micafungin and anidulafungin; C. para-psilosis with MICs ≥8 µg ml−1 was considered resistant to caspofungin, micafungin and anidulafungin; Candida glabrata with MICs ≥0.5 µg ml−1 was considered resistant to caspofungin and anidulafungin and with an MIC ≥0.25 µg ml−1 was resistant to micafungin. In the absence of clinical breakpoints, epidemiological cut-off values (ECVs) were used to interpret MICs as those conforming to wild-type or non-wild-type for a particular antifungal agent against a specific species. ECVs for fluconazole were 8 µg ml−1 for Candida guilliermondii, 4 µg ml−1 for Candida pelliculosa, 2 µg ml−1 for Candida lusitaniae and Candida orthopsilosis and 1 µg ml−1 for Candida kefyr. ECVs for voriconazole were 0.25 µg ml−1 for C. guilliermondii and C. pelliculosa, 0.03 µg ml−1 for C. lusitaniae, 0.06 µg ml−1 C. orthopsilosis and 0.015 µg ml−1 for C. kefyr (Pfaller et al., 2011b). Similarly, ECVs of anidulafungin were 4 µg ml−1 for C. guilliermondii, 2 µg ml−1 for C. lusitaniae and C. orthopsilosis, and 0.25 µg ml−1 for C. kefyr, and those for caspofungin were 2 µg ml−1 for C. guilliermondii, 1 µg ml−1 for C. lusitaniae, 0.5 µg ml−1 for C. orthopsilosis, 0.12 µg ml−1 for C. pelliculosa and 0.03 µg ml−1 for C. kefyr (Pfaller et al., 2011b). ECVs for micafungin were 2 µg ml−1 for C. guilliermondii, 1 µg ml−1 for C. orthopsilosis, 0.5 µg ml−1 for C. lusitaniae and 0.12 µg ml−1 for C. kefyr (Pfaller et al., 2011b). Amphotericin B MICs were obtained by Etest on RPMI agar by making a lawn with a 0.5 McFarland standard inoculum and incubating it for 24 h at 35 °C. The MICs of amphotericin and itraconazole were also interpreted according to ECVs (Pfaller et al., 2012). For amphotericin, the ECV for C. albicans, C. tropicalis, C. parapsilosis, C. glabrata, C. krusei, C. guilliermondii and C. lusitaniae was 2 µg ml−1. For itraconazole, ECVs were 0.12 µg ml−1 for C. albicans, 0.5 µg ml−1 for C. parapsilosis, C. tropicalis and C. lusitaniae, 1 µg ml−1 for C. krusei and C. guilliermondii and 2 µg ml−1 for C. glabrata.
Statistical analysis
Data were entered in Microsoft Access (2007) and transferred to SPSS version 19.0 for analysis. The frequencies of Candida species and their susceptibility profile and risk factors were determined. Patient characteristics associated with Candida species were evaluated using a χ2 test. The crude odds ratio (COR) and 95 % confidence interval (CI) were calculated for variables found to have significant associations.
RESULTS
Demographics, clinical characteristics and risk factors
A cross-sectional in vitro observational study was performed to determine the species spectrum in IC, together with the susceptibility profiles of the isolates and the risk factors associated with the disease. The demographics of 180 patients revealed a male/female ratio of 1.47. The mean age of 67 paediatric patients was 1.9 years (0 days–14 years), with 33 neonates. The mean age of 113 adults was 47.4 years (15–96 years), and 83 (73.5 %) of these were <65 years of age. The crude mortality rate was 52.1 %.
Further clinical information was available on 96 patients seen at AKUH (Tables 1 and 2). In this group, C. albicans IC was significantly associated with children and neonates (COR 3.46, 95% CI 1.63–7.32) and not with adults (COR 0.29, CI 0.14–0.61), whilst C. glabrata was associated with adults (COR 6.44, 95 % CI 1.45–28.69) and not with the paediatric group (COR 0.16, 95 % CI 0.04–0.69). However, the number of neonates with available clinical information was too small to compare separately for the presence of other risk factors.
Table 1.
Demographics and clinical characteristics of patients with IC. Statistically significant differences (P,0.05) within age groups are shown in bold. COR and 95 % CI have been shown for significant associations only. –, Difference not statistically significant; NA, not applicable.
| Variable | All cases (%) | Children | Adults | P value | COR (95% CI) |
|---|---|---|---|---|---|
| Mean age in years (range) | 30.48 (0–96) | NA | NA | NA | NA |
| No. of males (%) | 107 (59.4) | NA | NA | NA | NA |
| No. of children (%) | 67 (37.2) | NA | NA | NA | NA |
| Presence of underlying risk factors in 96 medical records reviewed |
NA | NA | NA | NA | |
| Any malignancy | 21 (21.9) | 4 (18.2) | 17 (23.0) | – | – |
| Haematological malignancy | 6 (6.3) | 2 (9.1) | 4 (5.4) | – | – |
| CLABSI | 24 (25.0) | 5 (22.7) | 19 (25.7) | – | – |
| Chronic liver disease | 9 (9.4) | 0 (0.0) | 9 (12.2) | – | – |
| Complicated intra-abdominal infection | 21 (21.9) | 2 (9.1) | 19 (25.7) | – | – |
| Central venous catheters | 64 (66.7) | 14 (63.6) | 50 (67.6) | – | – |
| Diabetes | 19 (19.8) | 0 (0.0) | 19 (25.7) | – | – |
| Dialysis | 17 (17.7) | 2 (9.1) | 15 (20.3) | – | – |
| Immunosuppressed status | 32 (33.3) | 6 (27.3) | 26 (35.1) | – | – |
| Neutropenia | 9 (9.4) | 3 (13.6) | 6 (8.1) | – | – |
| Nosocomial IC | 88 (91.6) | 19 (67.9) | 69 (84.1) | – | – |
| Pneumonia | 28 (29.2) | 6 (27.3) | 22 (29.8) | – | – |
| Prior antifungal use | 19 (19.8) | 3 (13.6) | 16 (21.6) | – | – |
| Sepsis | 68 (70.8) | 15 (68.2) | 53 (71.6) | – | – |
| Stay in special care units | 72 (75.0) | 14 (63.6) | 58 (78.4) | – | – |
| Total parenteral nutrition use | 21 (21.9) | 9 (40.9) | 12 (16.2) | 0.040 | 3.58 (1.25–10.23) |
| Transplant | 2 (2.1) | 0 (0.0) | 2 (2.7) | – | – |
| Use of BLIC | 44 (45.8) | 3 (13.6) | 41 (55.4) | 0.001 | 0.13 (0.35–0.47) |
| Use of carbapenems | 51 (53.1) | 15 (68.2) | 36 (48.6) | – | – |
| Use of cephalosporins | 36 (37.5) | 14 (63.6) | 22 (29.8) | 0.004 | 4.14 (1.52–11.26) |
| Use of healthcare devices | 82 (85.4) | 16 (72.7) | 66 (89.2) | – | – |
| Use of vancomycin | 40 (41.7) | 5 (22.7) | 35 (47.3) | 0.040 | 0.33 (0.11–0.98) |
| Urinary tract infection | 19 (19.8) | 2 (9.1) | 17 (23.0) | – | – |
| Crude mortality | 50 (52.1) | 10 (45.5) | 40 (54.1) | – | – |
Table 2.
Association of risk factors with the most prevalent Candida species A P value of <0.05 is considered statistically significant.
| Species | Risk factor | n/Total* | Frequency (%) | P value | COR (95% CI) |
|---|---|---|---|---|---|
| C. tropicalis (n=30) | Chronic liver disease | 6/9 | 66.7 | 0.017 | 5.16 (1.19–22.33) |
| Dialysis | 10/17 | 58.8 | 0.007 | 4.21 (1.42–12.55) | |
| Sepsis | 26/68 | 38.2 | 0.021 | 3.71 (1.16–11.92) | |
| C. albicans (n=20) | Paediatric age | 23/70 | 32.9 | 0.001 | 3.46 (1.64–7.32) |
| Adult age | 14/113 | 12.4 | 0.001 | 0.29 (0.14–0.61) | |
| Use of BLIC | 5/44 | 11.4 | 0.036 | 0.32 (0.10–0.96) | |
| C. parapsilosis (n=13) | CLABSI | 7/24 | 29.2 | 0.010 | 4.53 (1.35–15.25) |
| C. glabrata (n=11) | Adult age | 18/113 | 15.9 | 0.015 | 6.44 (1.45–28.69) |
| Paediatric age | 2/70 | 2.9 | 0.015 | 0.16 (0.04–0.69) | |
| Diabetes | 6/19 | 31.6 | 0.002 | 5.08 (1.21–21.34) | |
| Carbapenem use | 2/51 | 3.9 | 0.014 | 0.18 (0.03–0.95) |
n/Total is the number of that particular species found in the total number of patients with the risk factor.
Among these patients, overall use of carbapenems and BLICs was documented in 53 and 46% of cases, respectively. Paediatric IC, including neonates, was significantly associated with increased use of cephalosporins (COR 4.14, 95 % CI 1.52–11.26) and decreased use of BLICs (COR 0.13, 95% CI 0.35–0.47) and vancomycin (COR 0.33, 95 % CI 0.11–0.98) compared with adults.
In 91.6 % of cases, IC was acquired nosocomially. Use of healthcare devices of central venous catheters, ventilators and abdominal and pleural cavity drains was documented in 85.4 % of patients with IC, whilst 75.0 % of these individuals had spent time in special/intensive care units. Administration of total parenteral nutrition was also found to be significantly higher among children and neonates (COR 3.58, 95 % CI 1.25–10.23). Using the study criteria, 66.7 % of the study population were not overtly immunosuppressed.
Table 2 shows the association of risk factors with isolation of the four most common Candida species. IC with C. tropicalis was significantly associated with the presence of chronic liver disease (COR 5.16, 95 % CI 1.19–22.33), sepsis (COR 3.71, 95 % CI 1.16–11.92) and the need for dialysis (COR 4.21, 95 % CI 1.42–12.55). Candidaemia with C. albicans was seen less frequently in cases where there was use of BLICs (COR 0.32, 95 % CI 0.10–0.96). Isolation of C. parapsilosis was higher with established CLABSI (COR 4.53, 95% CI 1.35–15.25) and C. glabrata from diabetic patients (COR 5.08, 95 % CI 1.21–21.34). The risk of isolation of C. glabrata was found to be low with the use of carbapenem antibiotics (COR 0.18, 95 % CI 0.03–0.95).
Species distribution
The most commonly isolated Candida species overall was C. tropicalis (32.5 %), followed by C. albicans (20.2 %) and C. parapsilosis (15.0 %). Rare species including C. guilliermondii, C. metapsilosis, C. orthopsilosis, C. viswanathii, C. pelliculosa, C. utilis, C. fabianii, C. rugosa, C. kefyr and two novel Candida species comprised 17.5 % of total IC isolates (Tables 3 and 4).
Table 3.
Invasive Candida species from Pakistan (188 isolates): frequencies and antifungal susceptibility profiles Caspofungin represents echinocandin susceptibilities (caspofungin, anidulafungin, micafungin). The uncommon Candida species included C. pelliculosa, C. viswanathii, C. utilis, C. kefyr, C. rugosa, C. fabianii and two novel Candida species.
| Species | n (%) | Antifungal | MIC range (µg ml−1) |
MIC50 (µg ml−1) |
MIC90 (µg ml−1) |
Resistant/non wild-type (%) |
|---|---|---|---|---|---|---|
| C. tropicalis | 61 (32.5) | Caspofungin | 0.015–0.12 | 0.03 | 0.06 | 0.0 |
| Fluconazole | 0.25–1.0 | 0.5 | 0.5 | 0.0 | ||
| Voriconazole | 0.015–0.12 | 0.015 | 0.06 | 0.0 | ||
| Itraconazole | 0.03–1.0 | 0.5 | 1.0 | 19.7 | ||
| Amphotericin B | 0.047–0.75 | 0.25 | 0.38 | 0.0 | ||
| C. albicans | 38 (20.2) | Caspofungin | 0.15–0.06 | 0.03 | 0.06 | 0.0 |
| Fluconazole | 0.12–2.0 | 0.25 | 1.0 | 0.0 | ||
| Voriconazole | 0.008–0.12 | 0.015 | 0.06 | 0.0 | ||
| Itraconazole | 0.06–0.5 | 0.12 | 0.25 | 23.7 | ||
| Amphotericin B | 0.012–0.125 | 0.064 | 0.094 | 0.0 | ||
| C. parapsilosis | 28 (15.0) | Caspofungin | 0.06–1.0 | 0.25 | 0.5 | 0.0 |
| Fluconazole | 0.25–4.0 | 0.5 | 1.0 | 0.0 | ||
| Voriconazole | 0.008–0.12 | 0.015 | 0.06 | 0.0 | ||
| Itraconazole | 0.06–0.5 | 0.12 | 0.5 | 0.0 | ||
| Amphotericin B | 0.016–0.125 | 0.06 | 0.125 | 0.0 | ||
| C. metapsilosis | 4 (2.1) | Caspofungin | 0.12–0.25 | – | – | – |
| Fluconazole | 1.0–2.0 | – | – | – | ||
| Voriconazole | 0.015 | – | – | – | ||
| Itraconazole | 0.12–0.5 | – | – | – | ||
| Amphotericin B | 0.032–0.38 | – | – | – | ||
| C. orthopsilosis | 3 (1.6) | Caspofungin | 0.12–0.25 | – | – | 0.0 |
| Fluconazole | 0.5 | – | – | 0.0 | ||
| Voriconazole | 0.015–0.03 | – | – | 0.0 | ||
| Itraconazole | 0.12–0.25 | – | – | – | ||
| Amphotericin B | 0.012–0.047 | – | – | – | ||
| C. glabrata | 20 (10.6) | Caspofungin | 0.03–0.5 | 0.03 | 0.06 | 5.0 |
| Fluconazole | 1.0–256.0 | 8.0 | 64.0 | 15.0 | ||
| Voriconazole | 0.06–4.0 | 0.25 | 1.00 | 5.0 | ||
| Itraconazole | 0.25–>16.0 | 0.5 | 1.3 | 10.0 | ||
| Amphotericin B | 0.06–0.5 | 0.25 | 0.5 | 0.0 | ||
| C. guilliermondii | 13 (6.9) | Caspofungin | 0.03–0.5 | 0.5 | 0.5 | 0.0 |
| Fluconazole | 2.0–4.0 | 2.0 | 4.0 | 0.0 | ||
| Voriconazole | 0.03–0.12 | 0.06 | 0.125 | 0.0 | ||
| Itraconazole | 0.25–1.0 | 0.5 | 0.5 | 0.0 | ||
| Amphotericin B | 0.016–0.064 | 0.03 | 0.06 | 0.0 | ||
| C. krusei | 4 (2.1) | Caspofungin | 0.12–0.25 | – | – | 0.0 |
| Fluconazole | 4.0–32.0 | – | – | 100.0 | ||
| Voriconazole | 0.12–0.25 | – | – | 0.0 | ||
| Itraconazole | 0.25–1.0 | – | – | 0.0 | ||
| Amphotericin B | 0.38–0.75 | – | – | 0.0 | ||
| C. lusitaniae | 4 (2.1) | Caspofungin | 0.25–0.5 | – | – | 0.0 |
| Fluconazole | 0.25–0.5 | – | – | 0.0 | ||
| Voriconazole | <0.008–0.008 | – | – | 0.0 | ||
| Itraconazole | 0.25–0.5 | – | – | 0.0 | ||
| Amphotericin B | 0.016–0.064 | – | – | 0.0 | ||
| Uncommon | 13 (6.9) | Caspofungin | 0.015–1.0 | 0.06 | 0.5 | – |
| Candida species | ||||||
| Fluconazole | 0.25–8.0 | 1.0 | 4.0 | –* | ||
| Voriconazole | <0.008–0.25 | 0.03 | 0.25 | – | ||
| Itraconazole | 0.03–0.5 | 0.5 | 0.5 | – | ||
| Amphotericin B | 0.016–0.5 | 0.094 | 0.5 | – |
One C. pelliculosa isolate was categorized as non-wild-type in respect to fluconazole, as the MIC of fluconazole fell beyond the wild-type range.
Table 4.
Susceptibilities and clinical details of rare Candida isolates Caspofungin represents echinocandin susceptibilities (caspofungin, anidulafungin, micafungin). The breakpoints for echinocandins and/or fluconazole were not established. CVC, Central venous catheter; HCC, hepatocellular carcinoma; PICC, peripherally inserted central catheter; TACE, transcatheter chemo-embolization; TPN, total parenteral nutrition.
| Species | Frequency (n) | Source | Patient characteristics | MIC range (µg ml−1) |
||||
|---|---|---|---|---|---|---|---|---|
| Caspofungin | Fluconazole | Voriconazole | Itraconazole | Amphotericin | ||||
| C. viswanathii | 3 | Blood culture | 28-Year-old male with renal transplant |
0.03–0.12 | 0.5–4.0 | 0.015–0.25 | 0.03–0.5 | 0.032–0.094 |
| 70-Year-old diabetic female | ||||||||
| C. pelliculosa | 3 | Two blood cultures, one CVC tip |
28-Day-old male (history not available) |
0.015–0.03 | 0.25–8.0 | <0.008–0.25 | 0.06–0.5 | 0.016–0.5 |
| 13-Year-old female with malignancy and CLABSI |
||||||||
| 43-Year-old male with CLABSI and complicated abdominal infection |
||||||||
| C. utilis | 2 | Blood cultures | Two neonates with late- onset neonatal sepsis |
0.015–0.5 | 1.0 | 0.03–0.06 | 0.25 | 0.032–0.047 |
| Novel Candida species | 2 | Blood cultures | 18-Month-old female with burn wound infection on TPN; 28-year-old male on chemotherapy; both received multiple antibiotics |
0.06–0.25 | 0.5–4.0 | 0.015 | 0.5 | 0.047–0.38 |
| C. fabianii | 1 | Blood culture | Neonate; clinical information not available |
0.03 | 0.5 | 0.03 | 0.5 | 0.094 |
| C. kefyr | 1 | Blood culture | 65-Year-old male; HCC, post-TACE with abdominal abscess and PICC line for chemotherapy |
0.12 | 0.5 | 0.03 | 0.5 | 0.38 |
| C. rugosa | 1 | Blood culture | 70-Year-old female with knee replacement, bed sores and Alzheimer’s disease |
1.0 | 1.0 | 0.008 | 0.5 | 0.75 |
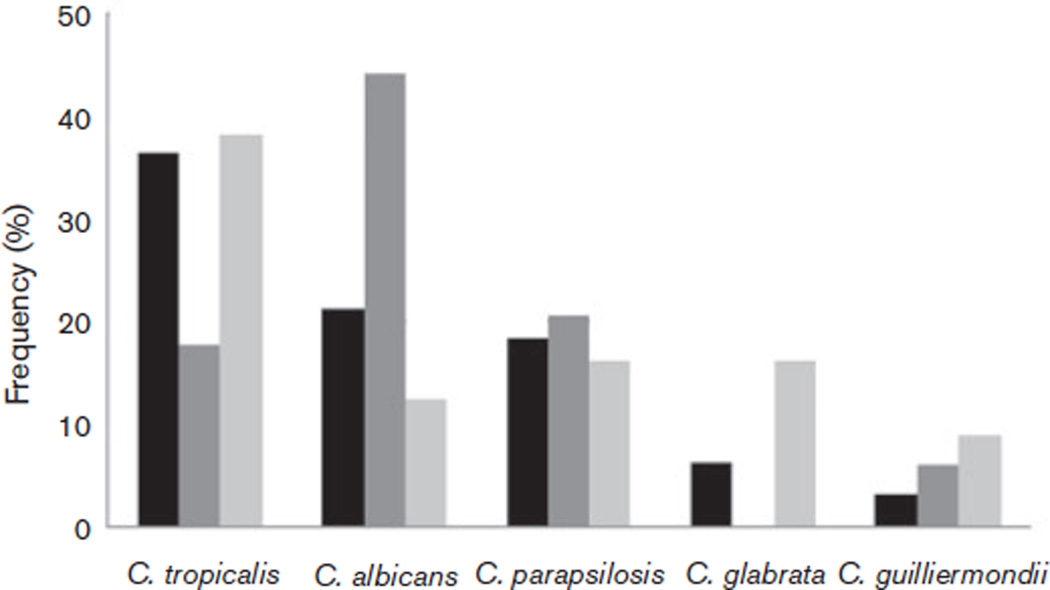
Adults, neonates and children displayed different species distribution profiles (Fig. 1). In adults and neonates, C. tropicalis (38 and 36 %, respectively) was common, whilst in children the predominant isolate was C. albicans (44 %). The isolation rates of C. glabrata were minimal (6 %) in neonates and 0 % in children.
Fig. 1.
Distribution of species based on the age of IC patients in this study. Black bars, neonates; dark grey bars, children; light grey bars, adults.
Antifungal susceptibility
Table 3 shows the MIC50 and MIC90 values, the MIC range and the percentage of resistant isolates. In general, most isolates were susceptible to the drugs tested. Resistance to fluconazole was seen in C. glabrata (15 %) and C. krusei (100 %). For the rare Candida species, the MIC90 values of fluconazole were ≤4 µg ml−1; however, one C. pelliculosa strain showed non-wild-type behaviour in respect to fluconazole with an MIC of 8 µg ml−1 (Tables 3 and 4). Non-wild-type behaviour in regard to itraconazole was seen among C. albicans (23.7 %), C. tropicalis (19.7 %) and C. glabrata (10.0 %) isolates (Table 3). Only one C. glabrata isolate was resistant to voriconazole. All isolates displayed amphotericin B MICs of <1.0 µg ml−1, well below the ECV of 2.0 µg ml−1. All isolates except one C. glabrata strain (caspofungin MIC of 0.5 µg ml−1, anidulafungin MIC of 1.0 µg ml−1 and micafungin MIC of 0.5 µg ml−1) were susceptible to echinocandins, as shown in Table 3.
DISCUSSION
This assessment of the epidemiology of IC in Pakistan showed C. tropicalis to be the species isolated most frequently from adult and neonatal IC patients (33 % of cases). This finding contrasts with reports from the USA, Europe, South America and the Far East, which have reported C. albicans as the most common species (Colombo et al., 2006; Falagas et al., 2010; Odds et al., 2007), with the most frequent non-C. albicans species being either C. parapsilosis in Southern Europe and South America or C. glabrata in the USA and UK. We found C. albicans to be the fourth most common Candida species among adult IC pathogens in Pakistan after C. tropicalis, C parapsilosis and C. glabrata. Our results support regional data from India showing C. tropicalis to be the most prevalent species in hospital-based surveys (Falagas et al., 2010; Kothari & Sagar, 2009). Studies from Singapore, Taiwan and Brazil have also reported C. tropicalis as the most common non-C. albicans species (Liu et al., 2010; Pereira et al., 2010; Tan et al., 2010). C. tropicalis has been reported as an emerging pathogen in hospital-based surveys of IC in Singapore and Hong Kong (Chai et al., 2007; Yap et al., 2009). Compared with data from the ARTEMIS disc diffusion surveillance, our isolation rates of C. tropicalis and C. guilliermondii were much higher than those reported from Africa, the Middle East and Asia Pacific (Pfaller et al., 2010a). The emergence of C. tropicalis causing IC in Indian studies has been attributed to increased fluconazole resistance and virulence, as portrayed by an increased rate of growth and the production of proteinases facilitating invasion (Basu et al., 2011; Kothavade et al., 2010). We did not find overt fluconazole resistance in Pakistani isolates of C. tropicalis, but the pathogenicity of this species requires further study. An association of C. tropicalis fungaemia with severity indicators such as sepsis (COR 3.71, 95 % CI 1.16–11.92; Table 2) and the need for dialysis (COR 4.21, 95 % CI 1.42–12.55; Table 2) supports our theory of increasing virulence in this species. It was also found more frequently in patients suffering from chronic liver disease (COR 5.16, 95 % CI 1.19–22.33), a result not particularly found in other studies but which possibly reflects increased C. tropicalis gastrointestinal carriage, leading to candidaemia in such patients (Kusne et al., 1994; Nucci & Colombo, 2007).
Our data showed a clear distinction between the spectrum of adult, neonatal and paediatric (age >1 month) IC pathogens, with C. albicans, C. tropicalis and C. parapsilosis being the three main paediatric IC agents. The age distribution of species in an international SENTRY survey for antifungal resistance showed C. albicans as the most common species isolated, irrespective of age group (Pfaller et al., 2010b). Conversely, our data showed a clear predominance of C. albicans (44 %) only in children, whilst in adults and neonates ~70 % of IC was caused by species other than C. albicans. This species distribution among the paediatric age group is supported by other reports published on the epidemiology of candidiasis in children (Ariff et al., 2011; Zaoutis, 2010). Differences in the epidemiology of IC in adults and children suggest that pressures, such as the spectrum of antibiotics used or gut colonization in Asian individuals, influencing the selection of non-C. albicans species in adults are not found as frequently in children. Although the distribution of species among neonates and adults appeared similar compared to that among children >1 month of age, a lack of clinical information unfortunately limited our ability to assess statistically the risk factors for neonates separately. Use of vancomycin and BLICs, an established risk factor for non-C. albicans species (Lin et al., 2005) and also significantly associated with non-C. albicans IC in this study (COR 0.32, 95 % CI 0.10–0.96; Table 2), was found to be significantly low at 22.7 and 13.6% in children and neonates, respectively (COR 0.33, 95 % CI 0.11–0.98 and COR 0.13, 95 % CI 0.35–0.47, respectively; Table 1).
The study of risk factors in our patients suggested that IC was mainly a nosocomial infection (91.6 % of cases), with most patients requiring intensive care and broad-spectrum parenteral antibiotic therapy. Although AKUH is a tertiary care hospital and a bone-marrow transplant centre, more than two thirds of the patients with IC in AKUH were without obvious immunocompromised status: only 9.4 % were neutropenic. Most studies of risk factors associated with IC are either hospital-based, intensive care unit or specific unit surveys (e.g. burns unit, transplant registry, etc.). A study from Greece that compared risk factors in immunocompetent and immunocompromised hosts (Dimopoulos et al., 2007) determined the risk factors to be the use of central venous catheters and urinary catheters, prolonged hospital stay and intra-abdominal infections in immunocompetent hosts and haematopoietic stem-cell transplant recipients, and neutropenia in immunocompromised patients. In hospital-based surveys, stay in special care units and infections with other nosocomial organisms were associated with high mortality, whilst central intravascular catheters and previous azole therapy were significantly associated with C. parapsilosis and C. krusei, respectively (Dimopoulos et al., 2007; Erdem et al., 2010; Ortega et al., 2011). Our study also found C. parapsilosis to be significantly associated with CLABSI (COR 4.53, 95 % CI 1.35–15.25; Table 2). Recently, the Infectious Disease Society of America has published guidelines for the prevention of intravascular catheter-related bloodstream infections, emphasizing barrier and aseptic precautions, clean sites of insertion and timely removal of central lines (O’Grady et al., 2011). In our patients, the high rates of modifiable risk factors, such as the use of central lines, staying in a special care unit and the use of broad-spectrum antibiotics, suggest that a significant proportion of IC may be preventable. Thus, adhering to the central line bundle and good antibiotic monitoring may effectively reduce IC rates, highlighting the importance of infection control.
The association of C. glabrata with diabetes is well established (Bader et al., 2004); however, the association of carbapenem use with a decreased risk of C. glabrata infection needs further investigation, as this may be due to the small sample size.
C. tropicalis, C. albicans, C. parapsilosis, C. glabrata and C. krusei comprised only 83 % of this collection, although they generally comprise >90 % of most surveillance collections (Pfaller & Diekema, 2007). Higher rates of recovery of uncommon species such as C. guilliermondii, C. lusitaniae and other rare species (17.5 % of the total IC isolates) in this study suggest that investment in molecular yeast identification methodology would be worthwhile. However, the phenotypic identification methods employed had good species agreement rates with molecular identification, with the exception of C. guilliermondii and rare Candida species, and may be used until molecular identification becomes more available in this country.
Resistance to azole antifungals, particularly fluconazole, in certain species has been increasing in recent years in the USA and Europe. Studies from Europe, South America and the USA reported low resistance rates to fluconazole and itraconazole before 2005 (Pfaller & Diekema, 2007), but data from the latter half of the decade show emerging resistance not only against azoles but also against echinocandins in nosocomial isolates (Pfaller et al., 2011d). Our isolates did not manifest high rates of fluconazole resistance except among C. glabrata and C. krusei isolates.
All Candida species in our study had low amphotericin B MICs, reinforcing the reliability of amphotericin B as an empirical choice. Although all our isolates were echinocandin naïve, as caspofungin has only recently become available in Pakistan, one C. glabrata isolate showed resistance to echinocandins, suggesting that selection pressures may play a role in the future as echinocandin use increases. Regardless of this, where cost is not an issue and C. glabrata and C. krusei have been isolated, echinocandins can be considered good choices for directed antifungal therapy in Pakistan. There are, however, reports from other parts of the world of resistance among nosocomial C. glabrata isolates against echinocandins (Pfaller et al., 2011d; Zimbeck et al., 2010).
With the infrequent isolation of C. glabrata and C. krusei and fluconazole resistance rates of 0 % among other Candida species in this study, fluconazole may be considered a good empiric choice. Good antibiotic monitoring could save the use of echinocandins for confirmed cases of C. glabrata and C. krusei, keeping the cost of therapy and antifungal exposure minimal, as the costs attributable to candidaemia are already high (Hassan et al., 2009). As the clinical use of antifungals increases we may see the emergence of resistance, and accurate identification to the species level as well as routine antifungal susceptibility testing are likely to have greater implications. There is evidence of changes in epidemiology and increasing rates of azole resistance in developed countries, which advocates continuing surveillance for species and the antifungal susceptibility spectrum (Falagas et al., 2010; Pfaller et al., 2011d).
ACKNOWLEDGEMENTS
This study was supported through grants from the Joint Pakistan–US Academic and Research Program Higher Education Commission/ Ministry of Science and Technology/United States Agency for International Development (HEC/MoST/USAID). We would like to acknowledge Eszter Deak and Joyce Peterson from the Centers for Disease Control and Prevention, Atlanta, GA, USA, for their technical support. From AKUH, Karachi, Faisal Malik and Afsheen Ayaz provided help in data entry and statistical analysis and Dr Summiya Nizamuddin in the review of clinical records. The findings and conclusions of this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Abbreviations
- AKUH
Aga Khan University Hospital
- BLIC
β-lactam inhibitor combination
- CI
confidence interval
- CLABSI
central line-associated bloodstream infection
- COR
crude odds ratio
- ECV
epidemiological cut-off value
- IC
invasive candidiasis
REFERENCES
- Ariff S, Saleem AF, Soofi SB, Sajjad R. Clinical spectrum and outcomes of neonatal candidiasis in a tertiary care hospital in Karachi, Pakistan. J Infect Dev Ctries. 2011;5:216–223. doi: 10.3855/jidc.1232. [DOI] [PubMed] [Google Scholar]
- Bader MS, Lai SM, Kumar V, Hinthorn D. Candidemia in patients with diabetes mellitus: epidemiology and predictors of mortality. Scand J Infect Dis. 2004;36:860–864. doi: 10.1080/00365540410021126. [DOI] [PubMed] [Google Scholar]
- Basu S, Chakraborty D, Dey SK, Das S. Biological characteristics of nosocomial Candida tropicalis isolated from different clinical materials of critically ill patients at ICU. Int J Microbiol Res. 2011;2:112–119. [Google Scholar]
- Chai YA, Wang Y, Khoo AL, Chan FY, Chow C, Kumarasinghe G, Singh K, Tambyah PA. Predominance of Candida tropicalis bloodstream infections in a Singapore teaching hospital. Med Mycol. 2007;45:435–439. doi: 10.1080/13693780701385868. [DOI] [PubMed] [Google Scholar]
- CLSI. Approved Standard M27-A3. 3rd edn. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. [Google Scholar]
- Colombo AL, Nucci M, Park BJ, Nouér SA, Arthington-Skaggs B, da Matta DA, Warnock D, Morgan J& Brazilian Network Candidemia Study. Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. J Clin Microbiol. 2006;44:2816–2823. doi: 10.1128/JCM.00773-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Brown TM, Kellar KL, Holloway BP, Morrison CJ. DNA probes for the rapid identification of medically important Candida species using a multianalyte profiling system. FEMS Immunol Med Microbiol. 2006;46:244–250. doi: 10.1111/j.1574-695X.2005.00031.x. [DOI] [PubMed] [Google Scholar]
- Deak E, Etienne KA, Lockhart SR, Gade L, Chiller T, Balajee SA. Utility of a Luminex-based assay for multiplexed, rapid species identification of Candida isolates from an ongoing candidemia surveillance. Can J Microbiol. 2010;56:348–351. doi: 10.1139/w10-003. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Fell JW. High-throughput detection of pathogenic yeasts of the genus Trichosporon . J Clin Microbiol. 2004;42:3696–3706. doi: 10.1128/JCM.42.8.3696-3706.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos G, Karabinis A, Samonis G, Falagas ME. Candidemia in immunocompromised and immunocompetent critically ill patients: a prospective comparative study. Eur J Clin Microbiol Infect Dis. 2007;26:377–384. doi: 10.1007/s10096-007-0316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem I, Oguzoglu N, Ozturk Engin D, Ozgultekin A, Inan AS, Ceran N, Kaya F, Genc I, Goktas P. Incidence, etiology and risk factors associated with mortality of nosocomial candidemia in a tertiary care hospital in Istanbul, Turkey. Med Princ Pract. 2010;19:463–467. doi: 10.1159/000320305. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Roussos N, Vardakas KZ. Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: a systematic review. Int J Infect Dis. 2010;14:e954–e966. doi: 10.1016/j.ijid.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Hassan I, Powell G, Sidhu M, Hart WM, Denning DW. Excess mortality, length of stay and cost attributable to candidaemia. J Infect. 2009;59:360–365. doi: 10.1016/j.jinf.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang C-H, Webster KM. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48:1695–1703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW other authors. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- Kothari A, Sagar V. Epidemiology of Candida bloodstream infections in a tertiary care institute in India. Indian J Med Microbiol. 2009;27:171–172. doi: 10.4103/0255-0857.49440. [DOI] [PubMed] [Google Scholar]
- Kothavade RJ, Kura MM, Valand AG, Panthaki MH. Candida tropicalis: its prevalence, pathogenicity and increasing resistance to fluconazole. J Med Microbiol. 2010;59:873–880. doi: 10.1099/jmm.0.013227-0. [DOI] [PubMed] [Google Scholar]
- Kusne S, Tobin D, Pasculle AW, Van Thiel DH, Ho M, Starzl TE. Candida carriage in the alimentary tract of liver transplant candidates. Transplantation. 1994;57:398–402. doi: 10.1097/00007890-199402150-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MY, Carmeli Y, Zumsteg J, Flores EL, Tolentino J, Sreeramoju P, Weber SG. Prior antimicrobial therapy and risk for hospital-acquired Candida glabrata and Candida krusei fungemia: a case-case-control study. Antimicrob Agents Chemother. 2005;49:4555–4560. doi: 10.1128/AAC.49.11.4555-4560.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-Y, Liao C-H, Chen Y-C, Chang S-C. Changing epidemiology of nosocomial bloodstream infections in 11 teaching hospitals in Taiwan between 1993 and 2006. J Microbiol Immunol Infect. 2010;43:416–429. doi: 10.1016/S1684-1182(10)60065-5. [DOI] [PubMed] [Google Scholar]
- Nucci M, Colombo AL. Candidemia due to Candida tropicalis: clinical, epidemiologic, and microbiologic characteristics of 188 episodes occurring in tertiary care hospitals. Diagn Microbiol Infect Dis. 2007;58:77–82. doi: 10.1016/j.diagmicrobio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA& other authors. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:e162–e193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds FC, Hanson MF, Davidson AD, Jacobsen MD, Wright P, Whyte JA, Gow NA, Jones BL. One year prospective survey of Candida bloodstream infections in Scotland. J Med Microbiol. 2007;56:1066–1075. doi: 10.1099/jmm.0.47239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega M, Marco F, Soriano A, Almela M, Martínez JA, López J, Pitart C, Mensa J. Candida species bloodstream infection: epidemiology and outcome in a single institution from 1991 to 2008. J Hosp Infect. 2011;77:157–161. doi: 10.1016/j.jhin.2010.09.026. [DOI] [PubMed] [Google Scholar]
- Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L& other authors. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50:1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- Pereira GH, Müller PR, Szeszs MW, Levin AS, Melhem MS. Five-year evaluation of bloodstream yeast infections in a tertiary hospital: the predominance of non-C. albicans Candida species. Med Mycol. 2010;48:839–842. doi: 10.3109/13693780903580121. [DOI] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Andes D, Diekema DJ, Espinel-Ingroff A, Sheehan D& CLSI Subcommittee for Antifungal Susceptibility Testing. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist Updat. 2010a;13:180–195. doi: 10.1016/j.drup.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Pfaller MA, Castanheira M, Messer SA, Moet GJ, Jones RN. Variation in Candida spp. distribution and antifungal resistance rates among bloodstream infection isolates by patient age: report from the SENTRY Antimicrobial Surveillance Program (2008- 2009) Diagn Microbiol Infect Dis. 2010b;68:278–283. doi: 10.1016/j.diagmicrobio.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Pfaller MA, Andes D, Arendrup MC, Diekema DJ, Espinel-Ingroff A, Alexander BD, Brown SD, Chaturvedi V, Fowler CL& other authors. Clinical breakpoints for voriconazole and Candida spp. revisited: review of microbiologic, molecular, pharmacodynamic, and clinical data as they pertain to the development of species-specific interpretive criteria. Diagn Microbiol Infect Dis. 2011a;70:330–343. doi: 10.1016/j.diagmicrobio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Pfaller MA, Castanheira M, Diekema DJ, Messer SA, Jones RN. Triazole and echinocandin MIC distributions with epidemiological cutoff values for differentiation of wild-type strains from non-wild-type strains of six uncommon species of Candida . J Clin Microbiol. 2011b;49:3800–3804. doi: 10.1128/JCM.05047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ, Andes D, Arendrup MC, Brown SD, Lockhart SR, Motyl M, Perlin DS& CLSI Subcommittee for Antifungal Testing. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updat. 2011c;14:164–176. doi: 10.1016/j.drup.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. Candida bloodstream infections: comparison of species distributions and antifungal resistance patterns in community-onset and nosocomial isolates in the SENTRY Antimicrobial Surveillance Program, 2008–2009. Antimicrob Agents Chemother. 2011d;55:561–566. doi: 10.1128/AAC.01079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Espinel-Ingroff A, Canton E, Castanheira M, Cuenca-Estrella M, Diekema DJ, Fothergill A, Fuller J, Ghannoum M& other authors. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B, flucytosine, and itraconazole and Candida spp. as determined by CLSI broth microdilution. J Clin Microbiol. 2012;50:2040–2046. doi: 10.1128/JCM.00248-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picazo JJ, González-Romo F, Candel FJ. Candidemia in the critically ill patient. Int J Antimicrob Agents. 2008;32(Suppl. 2):S83–S85. doi: 10.1016/S0924-8579(08)70005-0. [DOI] [PubMed] [Google Scholar]
- Tan TY, Tan AL, Tee NW, Ng LS, Chee CW. The increased role of non-albicans species in candidaemia: results from a 3-year surveillance study. Mycoses. 2010;53:515–521. doi: 10.1111/j.1439-0507.2009.01746.x. [DOI] [PubMed] [Google Scholar]
- Wang QM, Li J, Wang SA, Bai FY. Rapid differentiation of phenotypically similar yeast species by single-strand conformation polymorphism analysis of ribosomal DNA. Appl Environ Microbiol. 2008;74:2604–2611. doi: 10.1128/AEM.02223-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap HY, Kwok KM, Gomersall CD, Fung SC, Lam TC, Leung PN, Hui M, Joynt GM. Epidemiology and outcome of Candida bloodstream infection in an intensive care unit in Hong Kong. Hong Kong Med J. 2009;15:255–261. [PubMed] [Google Scholar]
- Zaoutis T. Candidemia in children. Curr Med Res Opin. 2010;26:1761–1768. doi: 10.1185/03007995.2010.487796. [DOI] [PubMed] [Google Scholar]
- Zimbeck AJ, Iqbal N, Ahlquist AM, Farley MM, Harrison LH, Chiller T, Lockhart SR. FKS mutations and elevated echinocandin MIC values among Candida glabrata isolates from U.S. population-based surveillance. Antimicrob Agents Chemother. 2010;54:5042–5047. doi: 10.1128/AAC.00836-10. [DOI] [PMC free article] [PubMed] [Google Scholar]