Summary
Better understanding of the immunological components and their interactions necessary to prevent or control Mycobacterium tuberculosis (Mtb) infection in humans is critical for tuberculosis (TB) vaccine development strategies. While the contributory role of humoral immunity in the protection against Mtb infection and disease is less defined than the role of T cells, it has been well established for many other intracellular pathogens. Here we update and discuss the increasing evidence and the mechanisms of B cells and antibodies in the defense against Mtb infection. We posit that B cells and antibodies have a variety of potential protective roles at each stage of Mtb infection, and postulate that such roles should be considered in the development strategies for TB vaccines and other immune-based interventions.
Keywords: Tuberculosis, immunity, antibodies, immunoglogulins, B-lymphocytes
Introduction
A more complete picture of the correlates of protection against M. tuberculosis (Mtb) infection and disease is critical for effective vaccine development. An estimated third of the world’s population is infected with Mtb but merely ~10% develop the disease, active tuberculosis (TB) during their lifetime (1). The immunological components and their interactions necessary to prevent or control Mtb infection in humans remain incompletely understood. The currently available M. bovis Bacillus Calmette-Guerin (BCG) vaccine, based on an attenuated M. bovis strain, has been in use for many decades but does not prevent Mtb infection and provides insufficient protection against disease (reviewed in (2, 3)). Many promising vaccines have been and are currently being developed (reviewed in (4)) but efficacy in humans remains to be proven. In 2013, there were an estimated 9 Million people who developed TB, and around 1.5 Million died from the disease emphasizing the urgency for better vaccines and other preventive measures (1).
Tuberculosis vaccine development strategies have been guided by the paradigm that protection against intracellular pathogens is based on cell-mediated immunity (CMI) while humoral immunity is relevant in the defense against extracellular pathogens. We note that almost two decades ago Rook and Hernandez-Pando (5) wrote: ‘Almost all the current review literature on the mechanism of immunity to tuberculosis states that antibody plays no role. We have found no evidence for this statement. Although antibody alone is certainly not sufficient, it may well be necessary”, at least in some hosts. Although cell-mediated immune mechanisms, predominantly based on T cells and mononuclear phagocytes, are the cornerstone in the defense against Mtb at most stages of the infection (reviewed in (6-11)), increasing evidence over the past years suggests that innate (12-14) and humoral immunity also play a role (reviewed in (15-18). Furthermore, the interactions and complementing effects between the different arms of the immune system will likely be needed for optimal protection against infection and development of disease.
Although antibodies (Abs) were previously believed to have little role in the defense against intracellular pathogens that view has changed in recent decades (reviewed in (19)). Abs to intracellular pathogens can mediate protection through various mechanisms extending from classical functions such opsonization and complement activation to non-classical functions such as signaling through Fc receptors (FcR) and modulation of the inflammatory host response (reviewed in (19-21)). In fact, the plethora of Ab functions against intracellular pathogens is likely to remain elusive unless specifically studied. A good example for this is the tremendous variety of Ab mechanisms which we have demonstrated in our experimental in vivo and in vitro studies with C. neoformans (reviewed in (22)). Humoral immunity as well as the synergistic effects between humoral and other arms of the immune system have become apparent for the protection against many intracellular pathogens (reviewed in (19, 23)). The list of intracellular pathogens that have been shown to be vulnerable to humoral immunity is long and has kept growing over the past decade (Table 1). As of today, it includes Gram negative bacteria such as Salmonella (24, 25), Yersinia (26-29), Ehrlichia (30, 31), Brucella (32-34), Coxiella (35, 36), Legionella (37-39), Neisseria (40, 41), and Chlamydia spp. (42-44); Gram positive bacteria such as Listeria spp. (45); fungi such as Cryptococcus (reviewed in (22)) and Histoplasma (46, 47) spp.; and parasites such as Plasmodium (48-52), Leishmania (53-55), and Toxoplasma (56, 57) spp. (57). More importantly, for several of these organisms vaccines with protective efficacy based on either Abs alone or the combination of humoral and CMI have been or are being developed (Table 1). We here review and update the evidence, functions and mechanisms of B cells and Abs in the protection against Mtb infection and disease, and discuss their potentially critical interactions with other arms of the immune system. Just as for other intracellular pathogens, we believe that these data should also be taken into consideration for TB vaccine development strategies.
Table 1.
A selection of studies demonstrating a role for B cells and antibodies in the protection against intracellular pathogens
| Pathogen | Disease | Mechanisms & Effects | Ref |
|---|---|---|---|
|
| |||
| Gram negative bacteria | |||
|
| |||
|
Salmonella enterica
serovar Typhi |
Typhoid | Live oral vaccine is protective against typhoid in humans and induces anti-LPS IgG that correlates significantly with increased opsonophagocytosis of S. enterica |
(25) |
|
| |||
| Neisseria meningitidis | Meningitis | Nasal vaccination of mice with surface exposed protein NadA of N. meningitidis serotype B induces strong cellular responses (Th1&Th2) and bactericidal Abs in the respiratory tract |
(41) |
|
| |||
| Yersinia pestis | Plague | mAb against LcrV promotes PMN phagocytosis of Y.
pestis |
(26) |
| Protection of mice against pneumonic plague by intranasal vaccination with inactivated Y. pestis is partially dependent on FcRs and can be transferred to naive mice with immune sera |
(27) |
||
| Vaccine induced antibodies against Y. pestis antigens F1 and LcrV protect mice against pneumonic plague |
(28, 29) | ||
|
| |||
| Ehrlichia chaffeensis | Ehrlichiosis | MAb against an outer membrane protein of E. chaffeensis
protects mice against E. chaffeensis infection |
(31) |
|
| |||
| Brucella abortus | Brucellosis | MAb targeting a polysaccharide protects mice against B.
abortus infection, likely through macrophage killing of opsonized B. abortus |
(34) |
|
| |||
| Coxiella burnetti | Q fever | Ab against Coxiella membrane protein/invasin OmpA inhibit internalization of Coxiella by non-phagocytic cells |
(35) |
| mAb against Coxiella phase I lipopolysaccharide protects mice against Coxiella infection by increasing macrophage phagocytosis via FcR |
(36) | ||
|
| |||
| Francisella tularensis | Tularemia | Intranasal immunization of F. tularensis-mAb immune complexes enhances IgA production, protects mice against infection, and is FcyR dependent |
(163) |
| MAb opsonizes F. tularensis and leads to FcR-dependent enhancement of T cells and dendritic cells |
(121, 164) | ||
|
| |||
| Legionella pneumophilia | Legionnaire's disease/pneumonia |
Engagement of FcR by Abs results in lysosomal fusion | (38) |
| Mice immunized with single B cell antigens or antigen combinations were protected against L. pneumophilia infection |
(39) | ||
|
| |||
| Chlamydia ssp. | Trachoma, urethritis, pelvic inflammatory disease, lymphogranuloma venerum, pneumonia |
IgG2a and IgA enhance rapid Th1 response via FcR | (165) |
| Abs enhance Th1 activation | (42) | ||
| Vaccination of koalas with chlamydial outer membrane protein induces specific neutralizing antibodies, and protects koalas against infection by eliciting strong cellular and humoral immunity |
(43) | ||
|
| |||
| Gram positive bacteria | |||
|
| |||
| Listeria monocytogenes | Meningitis, gastroenteritis, sepsis |
MAb against listeriolysin O reduces intracellular growth of L. monocytogenes and blocks passage from phagosome to cytosol |
(45) |
|
| |||
| Fungi | |||
|
| |||
|
Cryptococcus
neoformans |
Meningitis, pneumonia |
IgG1 mAb against the capsular polysaccharide GXM of C.
neoformans enhanced granulomatous inflammation in the lungs of C. neoformans infected mice, prolonged survival and reduced CFU |
(166) |
| IgG switch variants of an mAb against the GXM protect mice against C. neoformans independent of complement |
(167) | ||
| MAb against GXM induces increases of granulocytes among lung leukocytes, cytokine responses and may function by down-gegulating the inflammatory response in C. neoformans infected mice |
(168) | ||
| MAb against C. neoformans inhibits the release of capsular antigen |
(169) | ||
| Human IgG2 & IgG4 but not IgG1 & IgG4 protect mice against C. neoformans infection in passive transfer studies |
(170) | ||
| Binding of mAb against GXM to C. neoformans alters gene expression and modulates fungal metabolism |
(94) | ||
| Binding of protective mAbs against GXM impairs yeast budding |
(171) | ||
|
| |||
| Histoplasma capsulatum | Histoplasmosis | MAbs against a surface protein of H. capsulatum protect mice against H. capsulatum infection and were associated phagocytosis and lung cytokine levels |
(46) |
| Opsonization of H. capsulatum with mAb against a surface protein reduced the ability of H. capsulatum to regulate the phagosomal pH and resulted in more efficient T cell activation |
(47) | ||
|
| |||
| Parasites | |||
|
| |||
| Plasmodium falciparum | Malaria | Passive transfer of mAbs and imune sera inhibit liver- stage infection of P. falciparum in mice |
(51) |
| Vaccination with a fusion protein of circumsporozoite induces Ab-mediated protection against malaria in mice |
(50) | ||
| Introduction of genes encoding the mAb against the surface circumsporozoite protein protected mice against malaria |
(49) | ||
|
| |||
| Leishmania major | Leishmaniasis | IgE immune complexes via binding to the FcεRII-CD23 promote killing of L. major by inducing nitric oxide synthase in human macrophages |
(55) |
| L. amazonensis | B cells promote macrophage killing of L. amazonensis | (54) | |
| Non-specific IgG2a soluble immune complexes activate infected macrophages via the FcRy-common chain to kill of L. amazonensis in a NADPH oxidase dependent process |
(53) | ||
|
| |||
| Toxoplasma gondii | Toxoplasmosis | B cell deficient mice are more susceptible to T. gondii
infection that wild type mice and can be protected by passive transfer of immune sera |
(56) |
| IgE/FcεRII-CD23 mediated killing of intracellular T. gondii
by human macrophages |
(57) | ||
mAb: monoclonal antibody
Supporting Data for a Role of B cells and Abs in the Defense Against Mtb Infection
The limited appreciation of Abs as being a heterogeneous group of proteins that vary in protective and non-protective functions combined with the paradigm that they have little to no role in the defense against intracellular pathogens and the contradictory results of older serum transfer studies (58), has made it challenging to make a convincing case for their role in the defense against Mtb. The evidence for a role of B cells and Abs in the defense against Mtb infection was recently reviewed by us in detail (15-18), and is summarized and updated in Table 2. An important element of this evidence includes studies from various groups demonstrating that the passive transfer of monoclonal Abs (mAbs) against some Mtb antigens, ranging from surface-associated polysaccharides to proteins (59-66), as well as human polyclonal IgG or homologous immune sera (67-69), improve the outcome of mycobacterial infection in mice (Table 2). However, these studies have various limitations (reviewed in (15, 16), and well-designed in vitro and in vivo studies are warranted to expand such experiments and further identify the mechanisms of potentially protective Abs and their efficacy in various animal models. Other critical parts of the evidence are observational and experimental studies in humans and animal models showing inverse relationships between titers of Abs against certain mycobacterial antigens and susceptibility to infection and disease in humans and animals (70-73); and studies demonstrating increased TB susceptibility in animal models with deficits in B cell function and humoral immunity (74-79) (Table 2). Just as for mAbs, further research in these areas is now needed to elucidate the underlying mechanisms of these results.
Table 2.
Supporting data for a role of humoral immunity in the defense against Mtb infection
| Studies | Results | References |
|---|---|---|
|
| ||
| Passive transfer of mAbs and polyclonal IgG |
MAbs against AM, LAM, HBHA, 16 kDa α- crystalin and MPB83 improve the outcome of mycobacterial infection in mice |
(59-66) |
| Human polyclonal IgG or homologous immune sera improve the outcome of mycobacterial infection in mice |
(67-69) | |
| Transfer of immune sera from Mtb- infected mice reverses tissue neutrophilia in B cell deficient mice with TB |
(114) | |
|
| ||
| High Ab titer associated with reduced susceptibility |
AM-containing conjugate vaccine elicits Ab response that reduces susceptibility to infection |
(72, 172) |
| BCG as well as Mtb antigen-containing conjugate and DNA/RNA vaccines elicit cellular and humoral immune responses and improve outcome of infection |
(71, 72, 172-181) | |
|
| ||
| Increased susceptibility in hosts with deficits in humoral immunity |
Peak of childhood TB is temporally correlated with nadir in maternal Ab |
(182-184) |
| Lack of Abs against certain mycobacterial antigens is associated with TB dissemination in children and adults |
(70, 185-188) | |
| Lack of early humoral immune response in Mtb infected nonhuman primates predicts high likelihood for reactivation disease |
(73) | |
| B cell deficient mice are more susceptible to TB |
(74-76) | |
| Humans with pulmonary TB have lower peripheral blood B cell counts than subjects without or with Mtb infection but no disease |
(116, 117) | |
| Polymeric IgR-deficient mice loose mycobacterial antigen-specific IgA response in saliva and are more susceptible to respiratory BCG infection |
(79) | |
| IgA deficiency increases susceptibility to mycobacterial infection in mice |
(77, 78) | |
|
| ||
| Other | FcR-mediated phagocytosis promotes phagolysosomal fusion |
(80) |
| IgG bound to BCG enhances oxygen release in phagosomes and antimycobacterial activity of alveolar macrophages |
(82) | |
| Existence of mycobactericidal Abs | (97) | |
| FcR-mediated phagocytosis increases macrophage Ca2+ signaling and intracellular killing |
(81) | |
| Enhanced Mtb resistance of mice lacking the inhibitory FcyRIIB receptor is associated with a more robust polmonic Th1 response |
(74) | |
| Optimal BCG-induced Th1 response requires B cells |
(114) | |
| Higher FcyRIA expression in i) subjects with TB compared to LTBI; and ii) subjects pre compared to post antituberculous treatment |
(86) (87) |
|
mAb: monoclonal Ab; AM: arabinomannan; LAM: lipoarabinomannan; HBHA: heparin-binding haemaglutinin; Mtb: M. tuberculosis; Table modified and updated with permission from Achkar and Casadevall (15)
Ab Functions Contributing to the Protection Against Mtb
In considering the various mechanisms by which Abs could contribute to host defense against mycobacterial infection it may be worthwhile to separate them depending on whether the effect is direct or indirect.
Classical mechanisms of Ab action
A variety of studies have provided data supporting that several of the classical functions associated with Ab mediated immunity could potentially be effective against Mtb (Fig. 1). These include mechanisms such as opsonization and complement activation. Other direct mechanisms of Ab-mediated immunity such as toxin neutralization do not apply as Mtb does not produce toxins although it is conceivable that Ab binding and Ab-mediated clearance of mycobacterial antigens that are immunomodulatory would provide a comparable function. Ab-dependent cellular cytotoxicity (ADCC) could potentially enhance host resistance to Mtb but we are not aware of studies reporting this mechanism. With regards to opsonization, Ab-mediated phagocytosis has been shown to promote phagolysosomal fusion (80) and thus overcome the inhibition of lysosomal fusion, which is a major mechanism by which mycobacteria survive after ingestion by macrophages. In addition, FcγR engagement triggers signal transduction that results in increased macrophage Ca2+ and intracellular killing (81), and FcR engagement by Abs subverts the evasion of lysosomal degradation of BCG (38). Also indicative of a direct effect was the report that IgG coated compared to uncoated BCG resulted in an increased oxidative burst in the phagosomes of macrophages with enhanced mycobacterial killing (82). With regards to complement activation, TB patients with IgG2 (but not IgM) to LAM manifested increased complement activation on BCG (83). Similarly, human Ab (IgG > IgM) was reported to result in enhanced complement deposition on BCG (84), and human IgG was also reported to trigger complement activation resulting in increased phagocytosis of Mtb by macrophages (85).
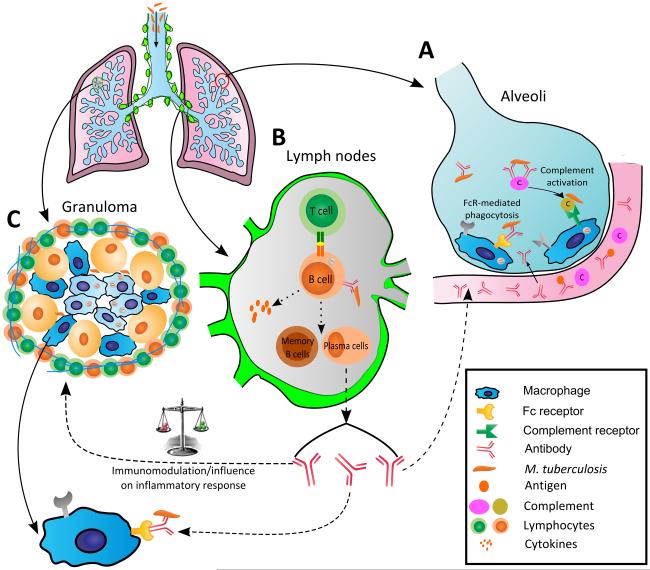
Fig. 1.
Scheme showing the pathogenesis of tuberculosis and steps where antibody (Ab) and B cells could conceivably provide protection based on literature reports of B cell and Ab action against M. tuberculosis (Mtb) infection. A) Ab could potentially affect the outcome of infection in the alveolar space through opsonization, complement activation, and FcR-mediated enhanced Mtb phagocytosis and killing. Ab could contribute defense mechanisms at the alveolar level during the initial state of infection when inflammation would increase serum permeability and allow the transfer of Abs and complement into the alveolar space; B) B cells located at the germinal center of lymphoid organs could play a role in the defense against infection through i) their function as antigen presenting cells and activation of T cells, ii) production of cytokines that could influence the development of the T helper response, and iii) production of Abs which could modulate various aspects of the innate and adaptive immune response, and clear immunomodulatory Mtb antigens by forming immune complexes; and C) Influence of B cells and Ab on the Abs on the inflammatory response, FcR-mediated phagocytosis and killing of extracellular Mtb within the cavity. Ab could affect granuloma formation through its intrinsic pro- and anti-inflammatory effects. Formation of better organized granulomas in the presence of Ab could potentially translate into reduced dissemination and local control of infection.
Non-classical mechanisms of Ab action
In recent years other mechanisms of Ab-mediated protection that could contribute to host defense against Mtb have been described (Fig. 1). The discovery of activating and inhibitory FcRs has added a rich new layer of possibilities to mechanisms for Ab action against Mtb. In this regard, modulation of the different receptors can have effects on macrophage function that affect their susceptibility to mycobacteria (74). For example, Mtb infection of mice lacking the activating FcγR γ-chain results in disease with more severe immunopathology that was associated with higher levels of IL-10 (74). Since TB is a disease characterized by an intense inflammatory response differences in the relative engagement of activating and inhibitory functions by the various Ab isotypes could have profound effects on whether the tissue response is effective at clearing mycobacteria or promotes mycobacterial persistence through immune damage. Indirect evidence to support such scenarios comes from the observation that individuals with TB had significantly higher gene expression of the activating FcγRIA (CD64) in whole blood than those with LTBI irrespective of HIV co-infection status (86). Consistent with this result, TB patients had a significant reduction in whole blood FcγRIA RNA expression after treatment relative to the levels before therapy was initiated (87). The FcγRIA receptor may be particularly important in the pathogenesis of TB because it is expressed in cells of the monocytic lineage, is involved in immune complex clearance, and its expression is induced by IFN-γ. Thus, these results provide intriguing hints for the importance of FcγR activation in the outcome of Mtb infection. Another receptor that has been potentially associated with resistance to Mtb is FcεRII-CD23. Activation of FcεRII-CD23 can trigger the killing of intracellular pathogens such as Leishmania major (55), and Toxoplasma gondii (57), and also enhance anti-mycobacterial activity (88).
Another indirect mechanism by which Ab-mediated immunity can affect host susceptibility against Mtb is through the effects of Ab on CMI and the inflammatory response (Fig. 1). Evidence for the potential of Abs against Mtb to mediate synergy by the different immune arms in humans comes from the observation that BCG vaccinated individuals have Abs that enhance both innate and cell-mediated immunity to Mtb (71). Furthermore, Abs from individuals who have presumably been exposed to Mtb as a result of contact with TB patients block the proliferation of monocyte cultures after tuberculin stimulation if they have high but not low IgG to tuberculin (89). Abs are further involved in antigen clearance by forming Ag-Ab complexes that can then be disposed by cells of the reticuloendothelial system (90). For example, Abs to Mtb could potentially modify the outcome of infection by promoting the clearance of mycobacterial components that influence the immune response. In this regard, Abs to LAM have been shown to promote the clearance of this mycobacterial product that is known to have immunomodulatory effects which could promote disease by causing dysregulation of effective responses (90). Recent data further confirm the influence of mycobacterial surface-associated lipoproteins on the host’s response to Mtb. For example, the lipoproteins LprG and LpqH participate in regulating cell-mediated responses against Mtb (91), and LprG is further relevant for the surface expression of LAM on macrophages, which in turn is essential for the virulence of Mtb (92). Thus, it is not surprising that vaccination with Mtb membrane vesicles induces both cell-mediated and Ab responses against LprG and LpqH that protect mice against infection with Mtb (93). Again, further studies are critical to prove the causality of such associations, and to further study the specific mechanisms by which Abs against highly immunogenic antigens participate in the defense against Mtb infection.
Studies with other microorganisms suggest additional mechanisms by which Abs to Mtb could modify the outcome of the infection. For example, Abs to the fungus Cryptococcus neoformans and the Gram positive bacterium Streptococcus pneumonia can alter the metabolic state of these organisms by simply binding to their surfaces (94, 95). For C. neoformans Ab binding to the capsule altered lipid metabolism making the fungal cell more susceptible to antifungal agents (94). For S. pneumonia Ab binding led to agglutination that mimicked conditions where quorum sensing effects occurred leading to activation of competence and fratricidal mechanisms resulting in bacterial lysis (95). Direct Ab-mediated bactericidal effects have also been described for an Ab that binds outer surface protein B of Borrelia burgdorferi and disrupts the bacterial surface (96). Although these mechanisms have not been investigated with mycobacteria, the fact that they occur with such phylogenetically distant microbes suggests that Ab binding to the surface of Mtb could also modify the metabolic state of the microbe in a manner that is beneficial to the host and/or mediate direct antimicrobial effects. In this regard, an anti-idiotypic mAb mimicking the action of killer toxin was reported to directly kill mycobacteria (97).
Molecular characteristics of effective Abs against Mtb
Experience with other systems has established that the efficacy of Ab is determined by the structural characteristics of the immunoglobulin molecule, the amount of Ab available and the immunological state of the host (21). Hence, it is reasonable to expect that the efficacy of Ab-mediated immunity against Mtb will also depend on the same characteristics. Structural features of immunoglobulins that are effective against individual pathogens are generally gleaned from studies with mAbs. However, for Mtb only a few protective mAbs have been described and the database is too small to make firm statements. To date, four murine isotypes, IgM, IgG1, IgG3 and IgA have been represented among protective Abs against Mtb (59-66), suggesting that these constant regions can help mediate protection when coupled with variable regions that confer certain specificities. The amount of Ab is a critical parameter in determining whether protection occurs. With too little antibody there may be an insufficient amount to confer protection. However, too much Ab may be equally detrimental to efficacy once the amount is sufficient to produce a prozone effect (98). For Mtb there is evidence that prozone effects can occur (99), and there is currently no information available on the optimal Ab concentrations needed for protection. The complexity of these parameters together with the complexity of the response to Mtb suggests caution in concluding from negative experiments that a given mAb or Ab response is not protective unless the system is extensively explored.
Interactions of B Cells with Other Immunocytes in the defense against Mtb
Accumulating evidence suggest that B cells also play a role in the orchestration of an immune response against Mtb by interacting with various immune cells (reviewed in (17, 18) (Fig. 1). The functional versatility of B cells is remarkable and this lymphocyte subset can shape the development of an immune response through multiple mechanisms. B cells are proficient antigen presenting cells, a rich source of a wide array of cytokines, and the only generator of immunoglobulins. Each of these attributes can impact the differentiation and functions of a wide variety of immune cells such as T cells, neutrophils, macrophages, and dendritic cells (reviewed in (17, 18). Due to the highly active and dynamic interaction among various cells in the immune network, prediction of the relative contribution of a specific cell type is not currently possible or straightforward.
Interactions of B cells with macrophages
It has been observed that B cells exist in aggregates in the tuberculous lungs of a number of host species including humans, non-human primates and mice (100-104). Various immune cells are present in the aggregates, and immunophenotyping of these cells suggest that the structures represent ectopic germinal centers (GC) (75, 105). It is thus possible that in the GC-like aggregates, B cells interact with the other immune cells present to significantly regulate the local immune response. Because of the close proximity between B cells and macrophages in the tuberculous lungs of mice (101), and the regulation of this topological relationship by tumor necrosis factor-α (TNF) (106), a cytokine known to play an essential role in defense against Mtb, the implication of B cell-macrophage interaction will be discussed herein.
It is well established that macrophage subsets with distinct immunological functions can differentially regulate the immune response (107, 108). Indeed, evidence exists that M2 macrophages are conducive to microbial persistence (109) as well as tumor progression (110, 111). Relevant to tuberculosis, it has been shown that Nippostrongylus brasiliensis (a gastrointestinal nematode) infection in mice promotes Th2 immunity that in turn, compromises host defense against the tubercle bacillus through induction of the development of alternatively activated (M2) macrophages (112). One mechanism underlying M2 differentiation is based on the effect of B1 cells by way of IL-10 production (113). The role of this regulation in the progression of melanoma has been demonstrated in adoptive transfer experiments involving B1 cells (113). Whether B cells play a similar regulatory role in the M2 differentiation, thereby diminishing host defense against M. tuberculosis as observed in co-infection with N. brasiliansis remains to be determined.
Interactions of B cells with T cells
Emerging evidence indicates that B cells can also modulate T cell response to mycobacteria (Fig. 1). For example, the development of optimal BCG-induced Th1 response requires B cells to regulate T cell response in Mtb-infected mice during chronic infection, thereby modulating the level of immunopathology (114). B cells can further indirectly regulate T cell function through the production of Abs. Mice unable to signal through the inhibitory FcγRIIB receptor (FcγRIIB−/−), relative to wild-type, display enhanced resistance to the tubercle bacillus (74). The enhanced resistance in the FcγRIIB−/− mice is associated with a more robust pulmonic Th1 response, as assessed by the level of IFN-γ-producing CD4+ T cells (74). Together, these data suggest that B cells can regulate Th1 responses by the production of Abs, which, upon reacting with antigens, generate immune complexes that then initiate FcR signaling. Furthermore, immunohistochemical studies conducted on the lungs of Mtb-infected mice, non-human primates, and humans have revealed the presence of CXCR5+ T cells in the GC-like structures (104). This CXCR5+ T cell subset, a major interacting partner of B cells, may play a role in Mtb control by contributing to the formation of the lymphoid nodule and perhaps macrophage activation.
Interactions of B cells with Neutrophils
Mice deficient in B cells (the μMT strain) have provided further evidence that this lymphocyte is required for the development of optimal immune response to Mtb. B cell-deficiency results in enhanced tuberculous lung inflammation, tissue neutrophilia, and increased local production of IL-10 (75). The influence on a neutrophil phenotype is supported by the observation that B cell-deficiency is associated with neutrophils with enhanced mobility (115). This neutrophil phenotype can also adversely affect vaccine efficacy in B cell-deficient mice (114, 115). Tissue neutrophilia in B cell-deficient mice could be due to the ability of B cells to regulate the IL-17/neutrophilic response (114). Together, results of these latter two studies strongly suggest a role for B cells in modulating neutrophil function. The B cell-specificity for the phenotypes observed in the μMT strain is supported by the reversal of these aberrancies by B cell adoptive transfer experiments. In addition, the depletion of B cells in wild-type animals recapitulate the immunopathology and neutrophilia phenotypes observed in μMT mice (114). Worthy of note, the adoptive transfer studies revealed that in μMT tuberculous mice receiving B cells transplant reversal of the B cell-deficient phenotypes is associated with partial replenishment of serum Ig but not with homing of the transferred cells to the lungs (75). These latter results suggest that the ability of the transferred B cells to reverse the aberrant phenotypes observed in Mtb-infected μMT mice is at least partially due to the immune regulatory attributes of Abs, and that the humoral immune response in a tuberculous host significantly shapes the protective immunity against the tubercle bacillus. Indeed, administration of immune sera procured from Mtb-infected animals reverses tissue neutrophilia in tuberculous μMT mice (114).
Interestingly, humans with pulmonary TB have lower peripheral blood B cell counts than subjects without or with Mtb infection but no disease (116, 117). These observations are consistent and supportive of experimental studies demonstrating the role of B cells and Abs in the defense against Mtb infection. Collectively, the data suggest that B cells and Abs can also shape the immune response to Mtb through interaction with other immune cells. These interactions further highlight the complexity of the mechanisms underlying the development of an efficient protective immune response against Mtb, and as a result, the relative significance of specific immunological elements in host defense against the tubercle bacillus might be difficult to ascertain.
Role of Humoral Immunity at Different Stages of Mtb Infection
The relevance of defense mechanisms against Mtb infection has to be viewed in the context of the various states that lie between initial infection and established disease. In that respect, it is important to distinguish whether the goal of a vaccine or other immune-based intervention is a) to prevent or rapidly clear initial infection; b) to protect against the development of disease/reactivation in already latently infected individuals; or c) to treat already established disease. These goals will likely require different balances between involving the various elements of the immune defense, and Abs could have a different role at each level (Fig. 1). However, the diversity of Ab functions, the complex interplay between B-cells and other immunocytes, and the limitations of animal models (reviewed in (118)) pose major challenges to decipher the parts of the various immune arms and their specific balance relevant for optimal protection at each stage of infection. To complicate matters further, the heterogeneity of the human immune response as well as the level of immune competency of individuals (reviewed in (15, 119)) will have to be taken into account when developing vaccines and other immune-based interventions against Mtb infection.
Role of humoral immunity in preventing or rapidly clearing initial Mtb infection
Abs could play a substantial role in preventing or rapidly clearing initial Mtb infection. During its initial encounter with the host Mtb would still be in a predominantly extracellular phase where Abs could rapidly bind to Mtb and augment macrophage phagocytosis and killing through the engagement of FcR, a mechanism that has been effective in clearing other intracellular pathogens (Table 1). At this level pathophysiologic processes in the respiratory system have to be taken into particular consideration (reviewed in (120)). Although some doubts remain whether Abs could play a role at the alveolar level during initial infection (Orme I, Tuberculosis 2014, in press), systemic mAbs have shown to protect against other intracellular, respiratory pathogens. For example, intraperitoneal injection of mAb targeting a lipopolysaccharide of the Q fever producing facultative intracellular organism Coxiella burnetii has shown to protect mice against aerosolized infection (36). Also in accordance with these notions, vaccines based on the induction of Abs that promote phagocytosis of respiratory, intracellular pathogens such as Yersinia pestis and Francisella tularemia have recently been developed and shown protection against Plague and Tularemia (28, 29, 121). Furthermore, alveolar macrophages and epithelial cells produce several proteins of the classical and alternative pathways of complement (122, 123), and complement proteins have been detected in the bronchoalveolar lavage (BAL) fluid from various different mammalian species including humans (124-127). Thus, Abs activating complement (83) which would enhance phagocytosis and cellular recruitment further to the site of Mtb infection, could also contribute to the rapid clearing of Mtb.
Role of humoral immunity in containing Mtb infection
Mtb causes a spectrum of infectious states, and development of disease as well as disease manifestations are dependent on the immune competency of the host (reviewed in (119, 128-130). The role of the granuloma in containing Mtb infection is considered critical (131), although there are ongoing debates about this relevance (132-134). Abs could contribute to the control of Mtb infection and prevention of reactivation through various mechanisms at the granuloma level. For example, IgM was shown to enhance granuloma formation in BCG infected mice by promoting an early inflammatory response (135), a mechanism that could be relevant in both the immunologically naive and the latently infected host. Just as during the initial infection, FcR-mediated phagocytosis could also play a role in maintaining control over Mtb infection. This function would likely play a role when Mtb has left it’s predominantly "dormancy" state but has not yet reached a sufficient burden to cause disease. At this level, it would also be critical whether Abs function as pro- or anti-inflammatory proteins (reviewed in (15, 16)), which would depend on their ability to activate certain FcR with different beneficial or potentially harmful effects depending on the immune competency of the host.
Role of humoral immunity in improving disease manifestations
During disease, considerable amounts of Mtb are located in the extracellular space, especially in cavities and necrotic lesions (136, 137), and thus Abs could improve disease manifestations through their capability for FcR-mediated phagocytosis and killing of Mtb. Abs could further promote clearance of immunomodulatory antigens, such as polysaccharides and lipopolysaccharides which can hinder the development of an efficient immune response. Importantly, since Abs can be pro- and anti-inflammatory depending on their isotype and the type of receptor that they interact with (reviewed in (21)), the effect of Abs on disease manifestations could be very different depending on the type of Ab present. The damage-response framework posits that host damage is a function of the immune response producing a U-curve (138). Evidence supporting this view in animal models comes from experiments in zebra fish showing that control of mycobacterial infection requires the right balance between too little and tissue-damaging immune responses (139). Similar, in experimental Mtb infection of mice excessive pulmonary accumulation of the innate myeloid-derived suppressor cells resulted in TB lethality while targeted depletion ameliorated disease (140). In humans, such concept is supported by the beneficial effects of withdrawing anti-inflammatory drugs such as corticosteroids or TNF-alpha blocker in disease associated with drug-induced immunosuppression (141, 142) while such anti-inflammatory drugs are beneficial in disease manifestations associated with a profound and potentially fatal inflammatory response such as tuberculous pericarditis, pleurisy, meningitis, or pulmonary cavities (reviewed in (143-146). In this construct pro-inflammatory Abs are likely to be helpful to individuals with insufficient tissue response while the same Abs could be deleterious to individuals mounting a strong tissue response. Conversely, anti-inflammatory Ab responses could be helpful in down-regulating the florid tissue-damaging inflammatory response that accompanies pulmonary infection and produces the classical lesions of casseous necrosis. By this hypothesis such Abs would protect through reduced inflammation that in turn could promote clearance of mycobacteria from tissues and reduce person-to-person spread by preventing cavity formation. However, anti-inflammatory Ab responses would be expected to be deleterious in individuals with weak tissue responses, such as observed in milliary tuberculosis where an inadequate tissue response results in a high mycobacterial burden. Influencing the inflammatory balance will likely be particularly complex in certain forms of disseminated TB such as TB meningitis. This form of extrapulmonary TB results from hematogenous spread of Mtb likely due to a lack of sufficient immune and inflammatory response which could be worsened by anti-inflammatory effects, and yet steroids are presumed to be beneficial by reducing the sequelae of the local inflammatory response and have shown to reduce mortality (147-149). At this time we do not understand enough about the effects of Ab on the inflammatory response to predict if certain patients would benefit from specific immunomodulating Abs. Such knowledge and understanding would be especially critical when considering and developing post-exposure and therapeutic vaccines, as well as adjunctive Ab-based therapy, an approach that has been successful in other infectious and respiratory diseases such as those caused by Pseudomonas aeroginosa (150-152) and respiratory syncytial virus (153, 154), and might offer an alternative for difficult to treat multi-drug resistant TB cases.
View of Relevance for Abs in TB Vaccine and Treatment
The complexity of the mycobacterial pathogenesis, which involves intracellular and extracellular phases together with strong inflammatory responses that can contribute to tissue damage suggest that successful defense requires a layered approach where the various components of the immune system work synergistically to control the infection. Given the numerous observations that both Abs and B cells can contribute to host defense it is reasonable that future vaccine strategies include the goal of eliciting protective Ab responses in addition to inducing T cell immunity. In fact, it is possible that BCG vaccination mediates its modest protective effects by eliciting both T and B cell responses. Evidence for the importance of the B cell component comes from the observations that BCG was not effective in eliciting protective responses against Mtb in B cell-deficient CBA/xid mice (115). Furthermore B cells may also contribute by enhancing the T cell response given that these were impaired in when B cell-deficient μMt mice were vaccinated with BCG. Consequently there are both theoretical and experimental reasons to support the development of new vaccines that simultaneously elicit protective B and T cell responses including protective Abs. Since effective humoral responses require Abs of certain specificity and isotype it will be important that future vaccines include epitopes known to elicit protective Abs. Given the antigenic complexity of mycobacteria it is possible that for the full expression of Ab-mediated protection vaccines will need to elicit responses to multiple epitopes, such that those Abs can function in cooperative and synergistic manner. This has recently been demonstrated for HIV, where a broad diversity of neutralizing Abs has been isolated from HIV-infected patients with high Ab titers (155), and passive transfer of a combination of neutralizing Abs targeting different epitopes controlled HIV infection in humanized mice and SHIV infection in nonhuman primates more effectively that single Ab transfer (156, 157). One of the probably most promising targets to include in TB vaccines would be the mycobacterial capsular polysaccharide AM, as we and others have shown protection of mAbs targeting AM in passive transfer studies (61, 64), and have shown BCG comparable or superior protective efficacy of conjugate vaccines containing this antigen ((72) and Prados-Rosales, unpublished data).
Adjunctive Ab-based therapy would be another attractive option that could be particularly beneficial in difficult to treat TB cases. The benefit could lie in both improved cure rates as well as shortened therapy, and this approach might offer an attractive alternative for treating multi-drug resistant TB cases. Ab-based therapies are becoming more common, and have led to major improvements in the treatment of several malignancies and autoimmune diseases (reviewed in (158, 159)). This approach has had a slow development in infectious diseases (reviewed in (160)), but is currently gaining more ground in the treatment of diseases such as those caused by P. aeroginosa (150-152) and respiratory syncytial virus (153, 154), and could potentially revolutionize the treatment of HIV (156, 157). Given the unpredictable heterogeneity of polyclonal serum preparations, Ab-based therapy in TB, just as for other infections, would most likely have to be based on mAbs. Another aspect to consider is that combinations of mAbs targeting different epitopes could enhance (or decrease) their efficacy against infections (161, 162). These emerging properties will have to be further taken into consideration and their careful evaluation will be critical when developing Ab-based therapy against TB.
Summary and Conclusions
The majority of the TB vaccine field considers CMI the essential or even sole component of the immune response necessary for the defense against Mtb infection. The contributory role of B cells and Abs in the protection against Mtb infection is less defined than the role of T-cells. However, this role has been established for many intracellular pathogens by now, and the evidence that Abs can play an adjunctive or synergistic role with cellular immunity is a fact that should be considered in development strategies for TB vaccines and other immune-based interventions. The diversity of Ab functions, the complex interplay between B-cells and other immunocytes, the limitations of animal models, and the heterogeneity of the human humoral immune response to Mtb pose major challenges to decipher the parts of the various immune arms and their specific balance relevant for optimal protection at each stage of infection. Therefore, further studies of B cell and Ab functions in the defense against Mtb, their influence on the pathogenesis of the various states of infection, and their role in the outcome of mycobacterial infections are urgently needed.
Acknowledgements
This work was supported by funds from the National Institute of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID; AI-096213 to J.M.A., AI-063537 and AI-094745 to J.C., and AI-033774, AI-052733, AI-033142 to A.C.) and the National Heart, Lung, and Blood Institute (NHLBI; HL-059842 to A.C.), the Center for AIDS Research (CFAR) at the Albert Einstein College of Medicine (AI-51519; J.M.A.), the Aeras TB vaccine foundation (J.M.A. and A.C.) and the Food and Drug Administration (FDA; 1U18 FD004012/01 to J.M.A.). A.C. is also the recipient of a Bill & Melinda Gates Grand Challenge award and TB Vaccine Accelerator Program award. We thank Tingting Chen for her graphic assistance with the figure.
References
- 1.World Health Organization . Global tuberculosis report 2014. Geneva, Switzerland: 2014. http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.Colditz GA, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. Jama. 1994;271:698–702. [PubMed] [Google Scholar]
- 3.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 4.Andersen P, Kaufmann SH. Novel Vaccination Strategies against Tuberculosis. Cold Spring Harbor perspectives in medicine. 2014:4. doi: 10.1101/cshperspect.a018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rook GA, Hernandez-Pando R. The pathogenesis of tuberculosis. Annu Rev Microbiol. 1996;50:259–284. doi: 10.1146/annurev.micro.50.1.259. [DOI] [PubMed] [Google Scholar]
- 6.Lewinsohn DA, Gold MC, Lewinsohn DM. Views of immunology: effector T cells. Immunological reviews. 2011;240:25–39. doi: 10.1111/j.1600-065X.2010.00997.x. [DOI] [PubMed] [Google Scholar]
- 7.Ottenhoff TH. New pathways of protective and pathological host defense to mycobacteria. Trends Microbiol. 2012;20:419–428. doi: 10.1016/j.tim.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Ottenhoff TH, Kaufmann SH. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog. 2012;8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakai S, Mayer-Barber KD, Barber DL. Defining features of protective CD4 T cell responses to Mycobacterium tuberculosis. Curr Opin Immunol. 2014;29:137–142. doi: 10.1016/j.coi.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behar SM, Carpenter SM, Booty MG, Barber DL, Jayaraman P. Orchestration of pulmonary T cell immunity during Mycobacterium tuberculosis infection: Immunity interruptus. Semin Immunol. 2014 doi: 10.1016/j.smim.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst JD. The immunological life cycle of tuberculosis. Nature reviews Immunology. 2012;12:581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 12.Verrall AJ, Netea MG, Alisjahbana B, Hill PC, van Crevel R. Early clearance of Mycobacterium tuberculosis: a new frontier in prevention. Immunology. 2014;141:506–513. doi: 10.1111/imm.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassidy JP, Martineau AR. Innate Resistance to Tuberculosis in Man, Cattle and Laboratory Animal Models: Nipping Disease in the Bud? Journal of comparative pathology. 2014 doi: 10.1016/j.jcpa.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Natarajan K, Kundu M, Sharma P, Basu J. Innate immune responses to M. tuberculosis infection. Tuberculosis (Edinb) 2011;91:427–431. doi: 10.1016/j.tube.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Achkar JM, Casadevall A. Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell host & microbe. 2013;13:250–262. doi: 10.1016/j.chom.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Achkar JM, Chan J, Casadevall A. Role of B Cells and Antibodies in Acquired Immunity against Mycobacterium tuberculosis. Cold Spring Harbor perspectives in medicine. 2014 doi: 10.1101/cshperspect.a018432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan J, et al. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Seminars in Immunology. 2014 doi: 10.1016/j.smim.2014.10.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozakiewicz L, Phuah J, Flynn J, Chan J. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Adv Exp Med Biol. 2013;783:225–250. doi: 10.1007/978-1-4614-6111-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casadevall A, Pirofski LA. A reappraisal of humoral immunity based on mechanisms of antibody-mediated protection against intracellular pathogens. Adv Immunol. 2006;91:1–44. doi: 10.1016/S0065-2776(06)91001-3. [DOI] [PubMed] [Google Scholar]
- 20.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nature reviews Immunology. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 21.Casadevall A, Pirofski LA. A new synthesis for antibody-mediated immunity. Nat Immunol. 2012;13:21–28. doi: 10.1038/ni.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casadevall A, Pirofski L. Insights into mechanisms of antibody-mediated immunity from studies with Cryptococcus neoformans. Current molecular medicine. 2005;5:421–433. doi: 10.2174/1566524054022567. [DOI] [PubMed] [Google Scholar]
- 23.Igietseme JU, Eko FO, He Q, Black CM. Antibody regulation of Tcell immunity: implications for vaccine strategies against intracellular pathogens. Expert review of vaccines. 2004;3:23–34. doi: 10.1586/14760584.3.1.23. [DOI] [PubMed] [Google Scholar]
- 24.Wahid R, Simon R, Zafar SJ, Levine MM, Sztein MB. Live oral typhoid vaccine Ty21a induces cross-reactive humoral immune responses against Salmonella enterica serovar Paratyphi A and S. Paratyphi B in humans. Clin Vaccine Immunol. 2012;19:825–834. doi: 10.1128/CVI.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahid R, Zafar SJ, McArthur MA, Pasetti MF, Levine MM, Sztein MB. Live oral Salmonella enterica serovar Typhi vaccines Ty21a and CVD 909 induce opsonophagocytic functional antibodies in humans that cross-react with S. Paratyphi A and S. Paratyphi B. Clin Vaccine Immunol. 2014;21:427–434. doi: 10.1128/CVI.00786-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowan C, Philipovskiy AV, Wulff-Strobel CR, Ye Z, Straley SC. Anti-LcrV antibody inhibits delivery of Yops by Yersinia pestis KIM5 by directly promoting phagocytosis. Infect Immun. 2005;73:6127–6137. doi: 10.1128/IAI.73.9.6127-6137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar D, Kirimanjeswara G, Metzger DW. Intranasal administration of an inactivated Yersinia pestis vaccine with interleukin-12 generates protective immunity against pneumonic plague. Clin Vaccine Immunol. 2011;18:1925–1935. doi: 10.1128/CVI.05117-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyer JL, et al. Protective immunity against a lethal respiratory Yersinia pestis challenge induced by V antigen or the F1 capsular antigen incorporated into adenovirus capsid. Human gene therapy. 2010;21:891–901. doi: 10.1089/hum.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galen JE, et al. A bivalent typhoid live vector vaccine expressing both chromosomal and plasmid-encoded Y. pestis antigens fully protects against murine lethal pulmonary plague infection. Infect Immun. 2014 doi: 10.1128/IAI.02443-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li JS, Winslow GM. Survival, replication, and antibody susceptibility of Ehrlichia chaffeensis outside of host cells. Infect Immun. 2003;71:4229–4237. doi: 10.1128/IAI.71.8.4229-4237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li JS, et al. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J Immunol. 2001;166:1855–1862. doi: 10.4049/jimmunol.166.3.1855. [DOI] [PubMed] [Google Scholar]
- 32.Gomez G, Adams LG, Rice-Ficht A, Ficht TA. Host-Brucella interactions and the Brucella genome as tools for subunit antigen discovery and immunization against brucellosis. Frontiers in cellular and infection microbiology. 2013;3:17. doi: 10.3389/fcimb.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann EM, Houle JJ. Contradictory roles for antibody and complement in the interaction of Brucella abortus with its host. Critical reviews in microbiology. 1995;21:153–163. doi: 10.3109/10408419509113538. [DOI] [PubMed] [Google Scholar]
- 34.Winter AJ, Duncan JR, Santisteban CG, Douglas JT, Adams LG. Capacity of passively administered antibody to prevent establishment of Brucella abortus infection in mice. Infect Immun. 1989;57:3438–3444. doi: 10.1128/iai.57.11.3438-3444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez E, Cantet F, Fava L, Norville I, Bonazzi M. Identification of OmpA, a Coxiella burnetii protein involved in host cell invasion, by multi-phenotypic high-content screening. PLoS Pathog. 2014;10:e1004013. doi: 10.1371/journal.ppat.1004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng Y, Schoenlaub L, Elliott A, Mitchell WJ, Zhang G. Characterization of a Lipopolysaccharide-Targeted Monoclonal Antibody and Its Variable Fragments as Candidates for Prophylaxis against the Obligate Intracellular Bacterial Pathogen Coxiella burnetii. Infect Immun. 2014;82:4530–4541. doi: 10.1128/IAI.01695-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blander SJ, Horwitz MA. Vaccination with the major secretory protein of Legionella induces humoral and cell-mediated immune responses and protective immunity across different serogroups of Legionella pneumophila and different species of Legionella. J Immunol. 1991;147:285–291. [PubMed] [Google Scholar]
- 38.Joller N, et al. Antibodies protect against intracellular bacteria by Fc receptor-mediated lysosomal targeting. Proc Natl Acad Sci U S A. 2010;107:20441–20446. doi: 10.1073/pnas.1013827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber SS, et al. Identification of protective B cell antigens of Legionella pneumophila. J Immunol. 2012;189:841–849. doi: 10.4049/jimmunol.1200794. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal S, Vasudhev S, DeOliveira RB, Ram S. Inhibition of the classical pathway of complement by meningococcal capsular polysaccharides. J Immunol. 2014;193:1855–1863. doi: 10.4049/jimmunol.1303177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowe F, et al. Mucosal vaccination against serogroup B meningococci: induction of bactericidal antibodies and cellular immunity following intranasal immunization with NadA of Neisseria meningitidis and mutants of Escherichia coli heat-labile enterotoxin. Infect Immun. 2004;72:4052–4060. doi: 10.1128/IAI.72.7.4052-4060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hafner LM, Wilson DP, Timms P. Development status and future prospects for a vaccine against Chlamydia trachomatis infection. Vaccine. 2014;32:1563–1571. doi: 10.1016/j.vaccine.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 43.Khan SA, et al. Vaccination of koalas (Phascolarctos cinereus) with a recombinant chlamydial major outer membrane protein adjuvanted with poly I:C, a host defense peptide and polyphosphazine, elicits strong and long lasting cellular and humoral immune responses. Vaccine. 2014 doi: 10.1016/j.vaccine.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 44.Igietseme JU, Ward ME. Chlamydia update. Expert review of vaccines. 2004;3:639–642. doi: 10.1586/14760584.3.6.639. [DOI] [PubMed] [Google Scholar]
- 45.Edelson BT, Unanue ER. Intracellular antibody neutralizes Listeria growth. Immunity. 2001;14:503–512. doi: 10.1016/s1074-7613(01)00139-x. [DOI] [PubMed] [Google Scholar]
- 46.Nosanchuk JD, Steenbergen JN, Shi L, Deepe GS, Jr., Casadevall A. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J Clin Invest. 2003;112:1164–1175. doi: 10.1172/JCI19361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi L, et al. A monoclonal antibody to Histoplasma capsulatum alters the intracellular fate of the fungus in murine macrophages. Eukaryotic cell. 2008;7:1109–1117. doi: 10.1128/EC.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cutts JC, Powell R, Agius PA, Beeson JG, Simpson JA, Fowkes FJ. Immunological markers of Plasmodium vivax exposure and immunity: a systematic review and meta-analysis. BMC medicine. 2014;12:150. doi: 10.1186/s12916-014-0150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deal C, Balazs AB, Espinosa DA, Zavala F, Baltimore D, Ketner G. Vectored antibody gene delivery protects against Plasmodium falciparum sporozoite challenge in mice. Proc Natl Acad Sci U S A. 2014;111:12528–12532. doi: 10.1073/pnas.1407362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizutani M, et al. Baculovirus-Vectored Multistage Plasmodium vivax Vaccine Induces Both Protective and Transmission-Blocking Immunities against Transgenic Rodent Malaria Parasites. Infect Immun. 2014;82:4348–4357. doi: 10.1128/IAI.02040-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sack BK, et al. Model for in vivo assessment of humoral protection against malaria sporozoite challenge by passive transfer of monoclonal antibodies and immune serum. Infect Immun. 2014;82:808–817. doi: 10.1128/IAI.01249-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright KE, et al. Structure of malaria invasion protein RH5 with erythrocyte basigin and blocking antibodies. Nature. 2014 doi: 10.1038/nature13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibson-Corley KN, et al. An In Vitro Model of Antibody-Enhanced Killing of the Intracellular Parasite Leishmania amazonensis. PLoS One. 2014;9:e106426. doi: 10.1371/journal.pone.0106426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibson-Corley KN, Boggiatto PM, Mukbel RM, Petersen CA, Jones DE. A deficiency in the B cell response of C57BL/6 mice correlates with loss of macrophage-mediated killing of Leishmania amazonensis. International journal for parasitology. 2010;40:157–161. doi: 10.1016/j.ijpara.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vouldoukis I, et al. The killing of Leishmania major by human macrophages is mediated by nitric oxide induced after ligation of the Fc epsilon RII/CD23 surface antigen. Proc Natl Acad Sci U S A. 1995;92:7804–7808. doi: 10.1073/pnas.92.17.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang H, Remington JS, Suzuki Y. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J Immunol. 2000;164:2629–2634. doi: 10.4049/jimmunol.164.5.2629. [DOI] [PubMed] [Google Scholar]
- 57.Vouldoukis I, Mazier D, Moynet D, Thiolat D, Malvy D, Mossalayi MD. IgE mediates killing of intracellular Toxoplasma gondii by human macrophages through CD23-dependent, interleukin-10 sensitive pathway. PLoS One. 2011;6:e18289. doi: 10.1371/journal.pone.0018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glatman-Freedman A, Casadevall A. Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin Microbiol Rev. 1998;11:514–532. doi: 10.1128/cmr.11.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balu S, et al. A novel human IgA monoclonal antibody protects against tuberculosis. J Immunol. 2011;186:3113–3119. doi: 10.4049/jimmunol.1003189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chambers MA, Gavier-Widen D, Hewinson RG. Antibody bound to the surface antigen MPB83 of Mycobacterium bovis enhances survival against high dose and low dose challenge. FEMS Immunol Med Microbiol. 2004;41:93–100. doi: 10.1016/j.femsim.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Hamasur B, Haile M, Pawlowski A, Schroder U, Kallenius G, Svenson SB. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab') fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2004;138:30–38. doi: 10.1111/j.1365-2249.2004.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lopez Y, et al. Induction of a protective response with an IgA monoclonal antibody against Mycobacterium tuberculosis 16kDa protein in a model of progressive pulmonary infection. International journal of medical microbiology : IJMM. 2009;299:447–452. doi: 10.1016/j.ijmm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Pethe K, et al. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature. 2001;412:190–194. doi: 10.1038/35084083. [DOI] [PubMed] [Google Scholar]
- 64.Teitelbaum R, et al. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci U S A. 1998;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams A, et al. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology. 2004;111:328–333. doi: 10.1111/j.1365-2567.2004.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buccheri S, et al. Prevention of the post-chemotherapy relapse of tuberculous infection by combined immunotherapy. Tuberculosis (Edinb) 2009;89:91–94. doi: 10.1016/j.tube.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 67.Guirado E, et al. Passive serum therapy with polyclonal antibodies against Mycobacterium tuberculosis protects against post-chemotherapy relapse of tuberculosis infection in SCID mice. Microbes Infect. 2006;8:1252–1259. doi: 10.1016/j.micinf.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 68.Olivares N, et al. The effect of the administration of human gamma globulins in a model of BCG infection in mice. Tuberculosis (Edinb) 2006;86:268–272. doi: 10.1016/j.tube.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Roy E, et al. Therapeutic efficacy of high-dose intravenous immunoglobulin in Mycobacterium tuberculosis infection in mice. Infect Immun. 2005;73:6101–6109. doi: 10.1128/IAI.73.9.6101-6109.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Costello AM, et al. Does antibody to mycobacterial antigens, including lipoarabinomannan, limit dissemination in childhood tuberculosis? Trans R Soc Trop Med Hyg. 1992;86:686–692. doi: 10.1016/0035-9203(92)90192-f. [DOI] [PubMed] [Google Scholar]
- 71.de Valliere S, Abate G, Blazevic A, Heuertz RM, Hoft DF. Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect Immun. 2005;73:6711–6720. doi: 10.1128/IAI.73.10.6711-6720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamasur B, et al. Mycobacterium tuberculosis arabinomannan-protein conjugates protect against tuberculosis. Vaccine. 2003;21:4081–4093. doi: 10.1016/s0264-410x(03)00274-3. [DOI] [PubMed] [Google Scholar]
- 73.Kunnath-Velayudhan S, et al. Proteome-scale antibody responses and outcome of Mycobacterium tuberculosis infection in nonhuman primates and in tuberculosis patients. J Infect Dis. 2012;206:697–705. doi: 10.1093/infdis/jis421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maglione PJ, Xu J, Casadevall A, Chan J. Fc gamma receptors regulate immune activation and susceptibility during Mycobacterium tuberculosis infection. J Immunol. 2008;180:3329–3338. doi: 10.4049/jimmunol.180.5.3329. [DOI] [PubMed] [Google Scholar]
- 75.Maglione PJ, Xu J, Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J Immunol. 2007;178:7222–7234. doi: 10.4049/jimmunol.178.11.7222. [DOI] [PubMed] [Google Scholar]
- 76.Vordermeier HM, Venkataprasad N, Harris DP, Ivanyi J. Increase of tuberculous infection in the organs of B cell-deficient mice. Clin Exp Immunol. 1996;106:312–316. doi: 10.1046/j.1365-2249.1996.d01-845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buccheri S, et al. IL-4 depletion enhances host resistance and passive IgA protection against tuberculosis infection in BALB/c mice. Eur J Immunol. 2007;37:729–737. doi: 10.1002/eji.200636764. [DOI] [PubMed] [Google Scholar]
- 78.Rodriguez A, et al. Role of IgA in the defense against respiratory infections IgA deficient mice exhibited increased susceptibility to intranasal infection with Mycobacterium bovis BCG. Vaccine. 2005;23:2565–2572. doi: 10.1016/j.vaccine.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 79.Tjarnlund A, et al. Polymeric IgR knockout mice are more susceptible to mycobacterial infections in the respiratory tract than wild-type mice. Int Immunol. 2006;18:807–816. doi: 10.1093/intimm/dxl017. [DOI] [PubMed] [Google Scholar]
- 80.Armstrong JA, Hart PD. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975;142:1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malik ZA, Denning GM, Kusner DJ. Inhibition of Ca(2+) signaling by Mycobacterium tuberculosis is associated with reduced phagosome-lysosome fusion and increased survival within human macrophages. J Exp Med. 2000;191:287–302. doi: 10.1084/jem.191.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suga M, Tanaka F, Muranaka H, Nishikawa H, Ando M. Effect of antibacterial antibody on bactericidal activities of superoxide and lysosomal enzyme from alveolar macrophages in rabbits. Respirology. 1996;1:127–132. doi: 10.1111/j.1440-1843.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 83.Hetland G, Wiker HG, Hogasen K, Hamasur B, Svenson SB, Harboe M. Involvement of antilipoarabinomannan antibodies in classical complement activation in tuberculosis. Clin Diagn Lab Immunol. 1998;5:211–218. doi: 10.1128/cdli.5.2.211-218.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carroll MV, Lack N, Sim E, Krarup A, Sim RB. Multiple routes of complement activation by Mycobacterium bovis BCG. Mol Immunol. 2009;46:3367–3378. doi: 10.1016/j.molimm.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 85.Manivannan S, Rao NV, Ramanathan VD. Role of complement activation and antibody in the interaction between Mycobacterium tuberculosis and human macrophages. Indian journal of experimental biology. 2012;50:542–550. [PubMed] [Google Scholar]
- 86.Sutherland JS, et al. Differential gene expression of activating Fcgamma receptor classifies active tuberculosis regardless of human immunodeficiency virus status or ethnicity. Clin Microbiol Infect. 2014;20:O230–238. doi: 10.1111/1469-0691.12383. [DOI] [PubMed] [Google Scholar]
- 87.Cliff JM, et al. Distinct phases of blood gene expression pattern through tuberculosis treatment reflect modulation of the humoral immune response. J Infect Dis. 2013;207:18–29. doi: 10.1093/infdis/jis499. [DOI] [PubMed] [Google Scholar]
- 88.Mossalayi MD, et al. CD23 mediates antimycobacterial activity of human macrophages. Infect Immun. 2009;77:5537–5542. doi: 10.1128/IAI.01457-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Encinales L, et al. Humoral immunity in tuberculin skin test anergy and its role in high-risk persons exposed to active tuberculosis. Mol Immunol. 2010;47:1066–1073. doi: 10.1016/j.molimm.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Glatman-Freedman A, Mednick AJ, Lendvai N, Casadevall A. Clearance and organ distribution of Mycobacterium tuberculosis lipoarabinomannan (LAM) in the presence and absence of LAM-binding immunoglobulin M. Infect Immun. 2000;68:335–341. doi: 10.1128/iai.68.1.335-341.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lancioni CL, et al. Mycobacterium tuberculosis lipoproteins directly regulate human memory CD4(+) T cell activation via Toll-like receptors 1 and 2. Infect Immun. 2011;79:663–673. doi: 10.1128/IAI.00806-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gaur RL, et al. LprG-Mediated Surface Expression of Lipoarabinomannan Is Essential for Virulence of Mycobacterium tuberculosis. PLoS Pathog. 2014;10:e1004376. doi: 10.1371/journal.ppat.1004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prados-Rosales R, et al. Mycobacterial membrane vesicles administered systemically in mice induce a protective immune response to surface compartments of Mycobacterium tuberculosis. mBio. 2014;5:e01921–01914. doi: 10.1128/mBio.01921-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McClelland EE, Nicola AM, Prados-Rosales R, Casadevall A. Ab binding alters gene expression in Cryptococcus neoformans and directly modulates fungal metabolism. J Clin Invest. 2010;120:1355–1361. doi: 10.1172/JCI38322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yano M, Gohil S, Coleman JR, Manix C, Pirofski LA. Antibodies to Streptococcus pneumoniae capsular polysaccharide enhance pneumococcal quorum sensing. mBio. 2011:2. doi: 10.1128/mBio.00176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Connolly SE, Thanassi DG, Benach JL. Generation of a complement-independent bactericidal IgM against a relapsing fever Borrelia. J Immunol. 2004;172:1191–1197. doi: 10.4049/jimmunol.172.2.1191. [DOI] [PubMed] [Google Scholar]
- 97.Conti S, et al. Mycobactericidal activity of human natural, monoclonal, and recombinant yeast killer toxin-like antibodies. J Infect Dis. 1998;177:807–811. doi: 10.1086/517815. [DOI] [PubMed] [Google Scholar]
- 98.Taborda CP, Rivera J, Zaragoza O, Casadevall A. More is not necessarily better: prozone-like effects in passive immunization with IgG. J Immunol. 2003;170:3621–3630. doi: 10.4049/jimmunol.170.7.3621. [DOI] [PubMed] [Google Scholar]
- 99.Schwebach JR. The carbohydrate surface of M. tuberculosis: antigenicity and antibody immunity. Albert Einstein College of Medicine; Bronx, New York: 2002. [Google Scholar]
- 100.Gonzalez-Juarrero M, Turner OC, Turner J, Marietta P, Brooks JV, Orme IM. Temporal and spatial arrangement of lymphocytes within lung granulomas induced by aerosol infection with Mycobacterium tuberculosis. Infect Immun. 2001;69:1722–1728. doi: 10.1128/IAI.69.3.1722-1728.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsai MC, et al. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8:218–232. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 102.Turner J, Frank AA, Brooks JV, Gonzalez-Juarrero M, Orme IM. The progression of chronic tuberculosis in the mouse does not require the participation of B lymphocytes or interleukin-4. Exp Gerontol. 2001;36:537–545. doi: 10.1016/s0531-5565(00)00257-6. [DOI] [PubMed] [Google Scholar]
- 103.Ulrichs T, et al. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. The Journal of pathology. 2004;204:217–228. doi: 10.1002/path.1628. [DOI] [PubMed] [Google Scholar]
- 104.Slight SR, et al. CXCR5(+) T helper cells mediate protective immunity against tuberculosis. J Clin Invest. 2013;123:712–726. doi: 10.1172/JCI65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kahnert A, Hopken UE, Stein M, Bandermann S, Lipp M, Kaufmann SH. Mycobacterium tuberculosis triggers formation of lymphoid structure in murine lungs. J Infect Dis. 2007;195:46–54. doi: 10.1086/508894. [DOI] [PubMed] [Google Scholar]
- 106.Chakravarty SD, et al. Tumor necrosis factor blockade in chronic murine tuberculosis enhances granulomatous inflammation and disorganizes granulomas in the lungs. Infect Immun. 2008;76:916–926. doi: 10.1128/IAI.01011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature reviews Immunology. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 108.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 109.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 110.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 111.Pollard JW. Trophic macrophages in development and disease. Nature reviews Immunology. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, Salgame P. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J Exp Med. 2011;208:1863–1874. doi: 10.1084/jem.20091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wong SC, et al. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol. 2010;40:2296–2307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- 114.Kozakiewicz L, et al. B cells regulate neutrophilia during Mycobacterium tuberculosis infection and BCG vaccination by modulating the interleukin-17 response. PLoS Pathog. 2013;9:e1003472. doi: 10.1371/journal.ppat.1003472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kondratieva TK, Rubakova EI, Linge IA, Evstifeev VV, Majorov KB, Apt AS. B cells delay neutrophil migration toward the site of stimulus: tardiness critical for effective bacillus Calmette-Guerin vaccination against tuberculosis infection in mice. J Immunol. 2010;184:1227–1234. doi: 10.4049/jimmunol.0902011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Corominas M, et al. B-lymphocytes and co-stimulatory molecules in Mycobacterium tuberculosis infection. Int J Tuberc Lung Dis. 2004;8:98–105. [PubMed] [Google Scholar]
- 117.Hernandez J, et al. Low number of peripheral blood B lymphocytes in patients with pulmonary tuberculosis. Immunological investigations. 2010;39:197–205. doi: 10.3109/08820130903586346. [DOI] [PubMed] [Google Scholar]
- 118.Flynn JL. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect. 2006;8:1179–1188. doi: 10.1016/j.micinf.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 119.Achkar JM, Jenny-Avital ER. Incipient and subclinical tuberculosis: defining early disease states in the context of host immune response. J Infect Dis. 2011;204(Suppl 4):S1179–1186. doi: 10.1093/infdis/jir451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schwander S, Dheda K. Human lung immunity against Mycobacterium tuberculosis: insights into pathogenesis and protection. Am J Respir Crit Care Med. 2011;183:696–707. doi: 10.1164/rccm.201006-0963PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bitsaktsis C, Babadjanova Z, Gosselin EJ. In Vivo Mechanisms Involved in Enhanced Protection Utilizing an FcR-Targeted Mucosal Vaccine Platform in a Bacterial Vaccine and Challenge Model. Infect Immun. 2014 doi: 10.1128/IAI.02289-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cole FS, Matthews WJ, Jr., Rossing TH, Gash DJ, Lichtenberg NA, Pennington JE. Complement biosynthesis by human bronchoalveolar macrophages. Clinical immunology and immunopathology. 1983;27:153–159. doi: 10.1016/0090-1229(83)90065-x. [DOI] [PubMed] [Google Scholar]
- 123.Strunk RC, Eidlen DM, Mason RJ. Pulmonary alveolar type II epithelial cells synthesize and secrete proteins of the classical and alternative complement pathways. J Clin Invest. 1988;81:1419–1426. doi: 10.1172/JCI113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Coonrod JD, Yoneda K. Complement and opsonins in alveolar secretions and serum of rats with pneumonia due to Streptococcus pneumoniae. Reviews of infectious diseases. 1981;3:310–322. doi: 10.1093/clinids/3.2.310. [DOI] [PubMed] [Google Scholar]
- 125.Giclas PC, King TE, Baker SL, Russo J, Henson PM. Complement activity in normal rabbit bronchoalveolar fluid. Description of an inhibitor of C3 activation. Am Rev Respir Dis. 1987;135:403–411. doi: 10.1164/arrd.1987.135.2.403. [DOI] [PubMed] [Google Scholar]
- 126.Watford WT, Ghio AJ, Wright JR. Complement-mediated host defense in the lung. American journal of physiology Lung cellular and molecular physiology. 2000;279:L790–798. doi: 10.1152/ajplung.2000.279.5.L790. [DOI] [PubMed] [Google Scholar]
- 127.Kolb WP, Kolb LM, Wetsel RA, Rogers WR, Shaw JO. Quantitation and stability of the fifth component of complement (C5) in bronchoalveolar lavage fluids obtained from non-human primates. Am Rev Respir Dis. 1981;123:226–231. doi: 10.1164/arrd.1981.123.2.226. [DOI] [PubMed] [Google Scholar]
- 128.Gideon HP, Flynn JL. Latent tuberculosis: what the host "sees"? Immunologic research. 2011;50:202–212. doi: 10.1007/s12026-011-8229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lawn SD, Wood R, Wilkinson RJ. Changing concepts of "latent tuberculosis infection" in patients living with HIV infection. Clin Dev Immunol. 2011:2011. doi: 10.1155/2011/980594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barry CE, 3rd, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saunders BM, Britton WJ. Life and death in the granuloma: immunopathology of tuberculosis. Immunol Cell Biol. 2007;85:103–111. doi: 10.1038/sj.icb.7100027. [DOI] [PubMed] [Google Scholar]
- 132.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rubin EJ. The granuloma in tuberculosis--friend or foe? N Engl J Med. 2009;360:2471–2473. doi: 10.1056/NEJMcibr0902539. [DOI] [PubMed] [Google Scholar]
- 134.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Russo RT, Mariano M. B-1 cell protective role in murine primary Mycobacterium bovis bacillus Calmette-Guerin infection. Immunobiology. 2010;215:1005–1014. doi: 10.1016/j.imbio.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 136.Grosset J. Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary. Antimicrobial agents and chemotherapy. 2003;47:833–836. doi: 10.1128/AAC.47.3.833-836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hoff DR, et al. Location of intra- and extracellular M. tuberculosis populations in lungs of mice and guinea pigs during disease progression and after drug treatment. PLoS One. 2011;6:e17550. doi: 10.1371/journal.pone.0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Casadevall A, Pirofski LA. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153:521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Knaul JK, et al. Lung-residing Myeloid-derived Suppressors Display Dual Functionality in Murine Pulmonary Tuberculosis. Am J Respir Crit Care Med. 2014 doi: 10.1164/rccm.201405-0828OC. [DOI] [PubMed] [Google Scholar]
- 141.Tuberculosis associated with blocking agents against tumor necrosis factor-alpha--California, 2002-2003. MMWR Morb Mortal Wkly Rep. 2004;53:683–686. [PubMed] [Google Scholar]
- 142.Haanaes OC, Bergmann A. Tuberculosis emerging in patients treated with corticosteroids. European journal of respiratory diseases. 1983;64:294–297. [PubMed] [Google Scholar]
- 143.Lee CH, Wang WJ, Lan RS, Tsai YH, Chiang YC. Corticosteroids in the treatment of tuberculous pleurisy. A double-blind, placebo-controlled, randomized study. Chest. 1988;94:1256–1259. doi: 10.1378/chest.94.6.1256. [DOI] [PubMed] [Google Scholar]
- 144.Smego RA, Ahmed N. A systematic review of the adjunctive use of systemic corticosteroids for pulmonary tuberculosis. Int J Tuberc Lung Dis. 2003;7:208–213. [PubMed] [Google Scholar]
- 145.Ntsekhe M, Wiysonge C, Volmink JA, Commerford PJ, Mayosi BM. Adjuvant corticosteroids for tuberculous pericarditis: promising, but not proven. QJM. 2003;96:593–599. doi: 10.1093/qjmed/hcg100. [DOI] [PubMed] [Google Scholar]
- 146.Prasad K, Singh MB. Corticosteroids for managing tuberculous meningitis. The Cochrane database of systematic reviews. 2008:CD002244. doi: 10.1002/14651858.CD002244.pub3. [DOI] [PubMed] [Google Scholar]
- 147.Thwaites GE, et al. Serial MRI to determine the effect of dexamethasone on the cerebral pathology of tuberculous meningitis: an observational study. The Lancet Neurology. 2007;6:230–236. doi: 10.1016/S1474-4422(07)70034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thwaites GE, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741–1751. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 149.Dastur DK, Manghani DK, Udani PM. Pathology and pathogenetic mechanisms in neurotuberculosis. Radiol Clin North Am. 1995;33:733–752. [PubMed] [Google Scholar]
- 150.Francois B, et al. Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized,double-blind, placebo-controlled trial. Critical care medicine. 2012;40:2320–2326. doi: 10.1097/CCM.0b013e31825334f6. [DOI] [PubMed] [Google Scholar]
- 151.Lu Q, et al. Pharmacokinetics and safety of panobacumab: specific adjunctive immunotherapy in critical patients with nosocomial Pseudomonas aeruginosa O11 pneumonia. The Journal of antimicrobial chemotherapy. 2011;66:1110–1116. doi: 10.1093/jac/dkr046. [DOI] [PubMed] [Google Scholar]
- 152.Secher T, et al. The anti-Pseudomonas aeruginosa antibody Panobacumab is efficacious on acute pneumonia in neutropenic mice and has additive effects with meropenem. PLoS One. 2013;8:e73396. doi: 10.1371/journal.pone.0073396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 154.Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:415–420. doi: 10.1542/peds.2014-1665. [DOI] [PubMed] [Google Scholar]
- 155.Scheid JF, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 156.Klein F, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Barouch DH, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nature reviews Immunology. 2010;10:301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 159.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature reviews Immunology. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Saylor C, Dadachova E, Casadevall A. Monoclonal antibody-based therapies for microbial diseases. Vaccine. 2009;27(Suppl 6):G38–46. doi: 10.1016/j.vaccine.2009.09.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chow SK, Smith C, MacCarthy T, Pohl MA, Bergman A, Casadevall A. Disease enhacing antibodies improve the efficacy of bacterial toxin-neutralizing antibodies. Cell host & microbe. 2013 doi: 10.1016/j.chom.2013.03.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pohl MA, Rivera J, Nakouzi A, Chow SK, Casadevall A. Combinations of antibodies to anthrax toxin manifest emergent properties in neutralization assays. Infection and Immunity. 2013 doi: 10.1128/IAI.01328-12. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rawool DB, et al. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J Immunol. 2008;180:5548–5557. doi: 10.4049/jimmunol.180.8.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Iglesias BV, et al. Multiple mechanisms mediate enhanced immunity generated by mAb-inactivated F. tularensis immunogen. Immunol Cell Biol. 2013;91:139–148. doi: 10.1038/icb.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Moore T, et al. Fc receptor-mediated antibody regulation of T cell immunity against intracellular pathogens. J Infect Dis. 2003;188:617–624. doi: 10.1086/377134. [DOI] [PubMed] [Google Scholar]
- 166.Feldmesser M, Casadevall A. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J Immunol. 1997;158:790–799. [PubMed] [Google Scholar]
- 167.Shapiro S, et al. Immunoglobulin G monoclonal antibodies to Cryptococcus neoformans protect mice deficient in complement component C3. Infect Immun. 2002;70:2598–2604. doi: 10.1128/IAI.70.5.2598-2604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Feldmesser M, Mednick A, Casadevall A. Antibody-mediated protection in murine Cryptococcus neoformans infection is associated with pleotrophic effects on cytokine and leukocyte responses. Infect Immun. 2002;70:1571–1580. doi: 10.1128/IAI.70.3.1571-1580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Martinez LR, Moussai D, Casadevall A. Antibody to Cryptococcus neoformans glucuronoxylomannan inhibits the release of capsular antigen. Infect Immun. 2004;72:3674–3679. doi: 10.1128/IAI.72.6.3674-3679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Beenhouwer DO, Yoo EM, Lai CW, Rocha MA, Morrison SL. Human immunoglobulin G2 (IgG2) and IgG4, but not IgG1 or IgG3, protect mice against Cryptococcus neoformans infection. Infect Immun. 2007;75:1424–1435. doi: 10.1128/IAI.01161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cordero RJ, et al. Antibody binding to Cryptococcus neoformans impairs budding by altering capsular mechanical properties. J Immunol. 2013;190:317–323. doi: 10.4049/jimmunol.1202324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Glatman-Freedman A, et al. Antigenic evidence of prevalence and diversity of Mycobacterium tuberculosis arabinomannan. J Clin Microbiol. 2004;42:3225–3231. doi: 10.1128/JCM.42.7.3225-3231.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chang-hong S, Xiao-wu W, Hai Z, Ting-fen Z, Li-Mei W, Zhi-kai X. Immune responses and protective efficacy of the gene vaccine expressing Ag85B and ESAT6 fusion protein from Mycobacterium tuberculosis. DNA and cell biology. 2008;27:199–207. doi: 10.1089/dna.2007.0648. [DOI] [PubMed] [Google Scholar]
- 174.Giri PK, Verma I, Khuller GK. Enhanced immunoprotective potential of Mycobacterium tuberculosis Ag85 complex protein based vaccine against airway Mycobacterium tuberculosis challenge following intranasal administration. FEMS Immunol Med Microbiol. 2006;47:233–241. doi: 10.1111/j.1574-695X.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- 175.Grover A, Ahmed MF, Singh B, Verma I, Sharma P, Khuller GK. A multivalent combination of experimental antituberculosis DNA vaccines based on Ag85B and regions of difference antigens. Microbes Infect. 2006;8:2390–2399. doi: 10.1016/j.micinf.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 176.Huygen K, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nature medicine. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 177.Kohama H, et al. Mucosal immunization with recombinant heparin-binding haemagglutinin adhesin suppresses extrapulmonary dissemination of Mycobacterium bovis bacillus Calmette-Guerin (BCG) in infected mice. Vaccine. 2008;26:924–932. doi: 10.1016/j.vaccine.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 178.Niu H, et al. Construction and evaluation of a multistage Mycobacterium tuberculosis subunit vaccine candidate Mtb10.4-HspX. Vaccine. 2011;29:9451–9458. doi: 10.1016/j.vaccine.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 179.Palma C, et al. The LTK63 adjuvant improves protection conferred by Ag85B DNA-protein prime-boosting vaccination against Mycobacterium tuberculosis infection by dampening IFN-gamma response. Vaccine. 2008;26:4237–4243. doi: 10.1016/j.vaccine.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 180.Teixeira FM, et al. DNA vaccine using Mycobacterium bovis Ag85B antigen induces partial protection against experimental infection in BALB/c mice. Clin Vaccine Immunol. 2006;13:930–935. doi: 10.1128/CVI.00151-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xue T, et al. RNA encoding the MPT83 antigen induces protective immune responses against Mycobacterium tuberculosis infection. Infect Immun. 2004;72:6324–6329. doi: 10.1128/IAI.72.11.6324-6329.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cruz AT, Starke JR. Clinical manifestations of tuberculosis in children. Paediatr Respir Rev. 2007;8:107–117. doi: 10.1016/j.prrv.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 183.Donald PR, Marais BJ, Barry CE., 3rd Age and the epidemiology and pathogenesis of tuberculosis. Lancet. 2010;375:1852–1854. doi: 10.1016/S0140-6736(10)60580-6. [DOI] [PubMed] [Google Scholar]
- 184.Beyazova U, Rota S, Cevheroglu C, Karsligil T. Humoral immune response in infants after BCG vaccination. Tuber Lung Dis. 1995;76:248–253. doi: 10.1016/s0962-8479(05)80013-9. [DOI] [PubMed] [Google Scholar]
- 185.Boggian K, Fierz W, Vernazza PL. Infrequent detection of lipoarabinomannan antibodies in human immunodeficiency virus-associated mycobacterial disease. Swiss HIV Cohort Study. J Clin Microbiol. 1996;34:1854–1855. doi: 10.1128/jcm.34.7.1854-1855.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dayal R, et al. Serological diagnosis of tuberculosis. Indian J Pediatr. 2008;75:1219–1221. doi: 10.1007/s12098-008-0222-3. [DOI] [PubMed] [Google Scholar]
- 187.Gupta S, Bhatia R, Datta KK. Serological diagnosis of childhood tuberculosis by estimation of mycobacterial antigen 60-specific immunoglobulins in the serum. Tuber Lung Dis. 1997;78:21–27. doi: 10.1016/s0962-8479(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 188.Sada E, Ferguson LE, Daniel TM. An ELISA for the serodiagnosis of tuberculosis using a 30,000-Da native antigen of Mycobacterium tuberculosis. J Infect Dis. 1990;162:928–931. doi: 10.1093/infdis/162.4.928. [DOI] [PubMed] [Google Scholar]