Significance
Organic compounds found in drinking water aquifers above the Marcellus Shale and other shale plays could reflect natural geologic transport processes or contamination from anthropogenic activities, including enhanced natural gas production. Using analyses of organic compounds coupled with inorganic geochemical fingerprinting, estimates of groundwater residence time, and geospatial analyses of shale gas wells and disclosed safety violations, we determined that the dominant source of organic compounds to shallow aquifers was consistent with surface spills of disclosed chemical additives. There was no evidence of association with deeper brines or long-range migration of these compounds to the shallow aquifers. Encouragingly, drinking water sources affected by disclosed surface spills could be targeted for treatment and monitoring to protect public health.
Keywords: natural gas extraction, high-volume hydraulic fracturing, groundwater, hydrophobic organic contaminants, transport mechanisms
Abstract
Hundreds of organic chemicals are used during natural gas extraction via high-volume hydraulic fracturing (HVHF). However, it is unclear whether these chemicals, injected into deep shale horizons, reach shallow groundwater aquifers and affect local water quality, either from those deep HVHF injection sites or from the surface or shallow subsurface. Here, we report detectable levels of organic compounds in shallow groundwater samples from private residential wells overlying the Marcellus Shale in northeastern Pennsylvania. Analyses of purgeable and extractable organic compounds from 64 groundwater samples revealed trace levels of volatile organic compounds, well below the Environmental Protection Agency’s maximum contaminant levels, and low levels of both gasoline range (0–8 ppb) and diesel range organic compounds (DRO; 0–157 ppb). A compound-specific analysis revealed the presence of bis(2-ethylhexyl) phthalate, which is a disclosed HVHF additive, that was notably absent in a representative geogenic water sample and field blanks. Pairing these analyses with (i) inorganic chemical fingerprinting of deep saline groundwater, (ii) characteristic noble gas isotopes, and (iii) spatial relationships between active shale gas extraction wells and wells with disclosed environmental health and safety violations, we differentiate between a chemical signature associated with naturally occurring saline groundwater and one associated with alternative anthropogenic routes from the surface (e.g., accidental spills or leaks). The data support a transport mechanism of DRO to groundwater via accidental release of fracturing fluid chemicals derived from the surface rather than subsurface flow of these fluids from the underlying shale formation.
Technological advances in high-volume hydraulic fracturing (HVHF) have led to the expansion of unconventional fossil fuel extraction in the United States over the past decade (1–3). Despite the clear economic and national security benefits associated with domestic fuel production, the colocation of industrial practices with residential areas raises concerns for public and environmental health (4–6). In particular, it is unclear whether the organic chemicals that are used in relatively small proportions (but potentially large volumes) and injected into deep shale formations can contaminate shallow drinking water aquifers. Several questions emerge. If organic chemicals are detected in groundwater, did they arrive via surface discharges, shallow subsurface pathways (e.g., leaking gas wells), or deep transport routes? Furthermore, are organic compounds present in groundwater derived from naturally occurring, geogenic sources or associated with industrial activities, such as HVHF? Finally, what are the chemical fingerprints that enable one to make this distinction?
Although few studies have examined the occurrence and origin of organic contaminants in groundwater in HVHF regions (7), the presence of light hydrocarbon gases (i.e., methane and ethane) and inorganic constituents has been investigated frequently. Osborn et al. (8) and Jackson et al. (9) demonstrated elevated methane levels within 1 km of unconventional gas wells over the Marcellus Shale. Further, Darrah et al. (10) showed that stray gas contamination in a subset of groundwater wells likely resulted from poor well integrity (i.e., casing and cementing issues). In contrast, inorganic chemical constituents (e.g., Cl− and Br−) in groundwater over the Marcellus Shale seem to reflect geogenic sources and provide evidence of hydraulic connectivity between shallow groundwaters and deeper formation brines on geological timescales in some areas in northeastern Pennsylvania (11–13). This deep-origin, saline groundwater has a chemical and isotopic fingerprint similar to the Marcellus brines (11) but distinct from Marcellus flowback water (14). However, it is unknown whether this deep saline water carries a unique organic chemical fingerprint of either geogenic or anthropogenic origin.
The same mechanistic approaches taken to source apportion methane and inorganic compounds have not yet been applied to organic compounds in groundwater. Although Gross et al. (15) reported surface spills of hydraulic fracturing fluids and wastes that could affect groundwater with organic chemicals in Colorado, and the Pennsylvania Department of Environmental Protection (PA DEP) has assessed multiple instances of local groundwater pollution by gas drilling operations (16), these studies relied on voluntary industry accident disclosure and did not probe for possible alternative exposure paths (i.e., through a broad geospatial sample set). In addition, they did not provide a detailed characterization of the water that could identify the dominant transport processes associated with the contamination (i.e., organic and inorganic markers, along with hydrocarbon composition, noble gas isotopes, and spatial distribution analysis). Recently, Llewellyn et al. (7) investigated a localized incident of stray gas groundwater pollution in Pennsylvania that exposed the potential for groundwater contamination from natural gas extraction practices, where the authors attributed the contamination to flow from HVHF wells through shallow subsurface pathways. Although critical and detailed, the targeted nature of this case study (i.e., sampling wells with documented contamination or close to contamination sites) precludes the identification of geologic transport mechanisms that may be occurring in the region. Thus, the mechanisms of organic chemical transport in groundwater associated with HVHF regions remain unclear.
To address this research gap, we sampled 64 private residential groundwater wells, ranging from 9–213 m deep, over a 3-y period (2012–2014) in northeastern Pennsylvania (n = 62) and in southern New York (n = 2) for analyses of GC-amenable organic compounds (Fig. 1). Fifty-nine samples were analyzed for volatile organic compounds (VOCs) and gasoline range organic compounds (GRO; defined as the hydrocarbons eluting between 2-methylpentane and 1,2,4-trimethylbenzene; approximately between nC6 and nC10), and 41 were also analyzed for diesel range organic compounds (DRO; defined as the hydrocarbons eluting between nC10 and nC28) (17). Analytical details are provided in SI Appendix. [Note that compounds included in the Environmental Protection Agency (EPA)-designated definitions of GRO and DRO are not necessarily gasoline or diesel derived]. A subset of these samples was analyzed using comprehensive two-dimensional gas chromatography (GC×GC) to evaluate whether compound-specific organic chemical fingerprints were associated with either HVHF activities or natural geologic processes. Complementary analyses of the inorganic chemical, methane stable isotope, and helium composition (i.e., [4He] and 3He/4He) were conducted to evaluate potential transport mechanisms for organic compounds into the shallow groundwater (i.e., from surface spills, leaky well casings, or communication with deep shale formations). Finally, we investigated the spatial distribution of disclosed surface spills, active shale gas wells, and groundwater samples with elevated GRO and DRO to determine whether there is an increased risk associated with the colocation of natural gas extraction activities with drinking water supplies over the Marcellus Shale.
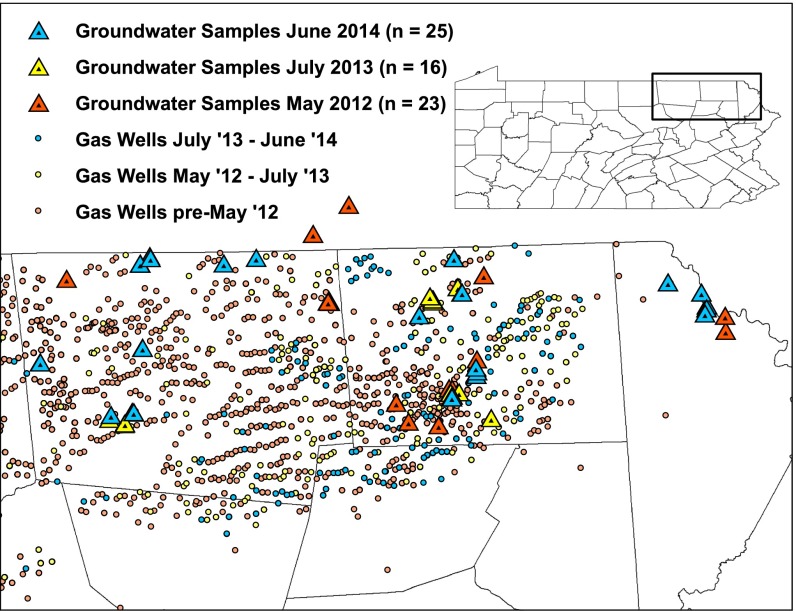
Fig. 1.
Shallow groundwater sample locations and the existing active shale gas wells at those times. Five samples were collected in December 2014 and included in the June 2014 data points. Shale gas well locations were obtained from the Pennsylvania Spatial Data Access.
Results and Discussion
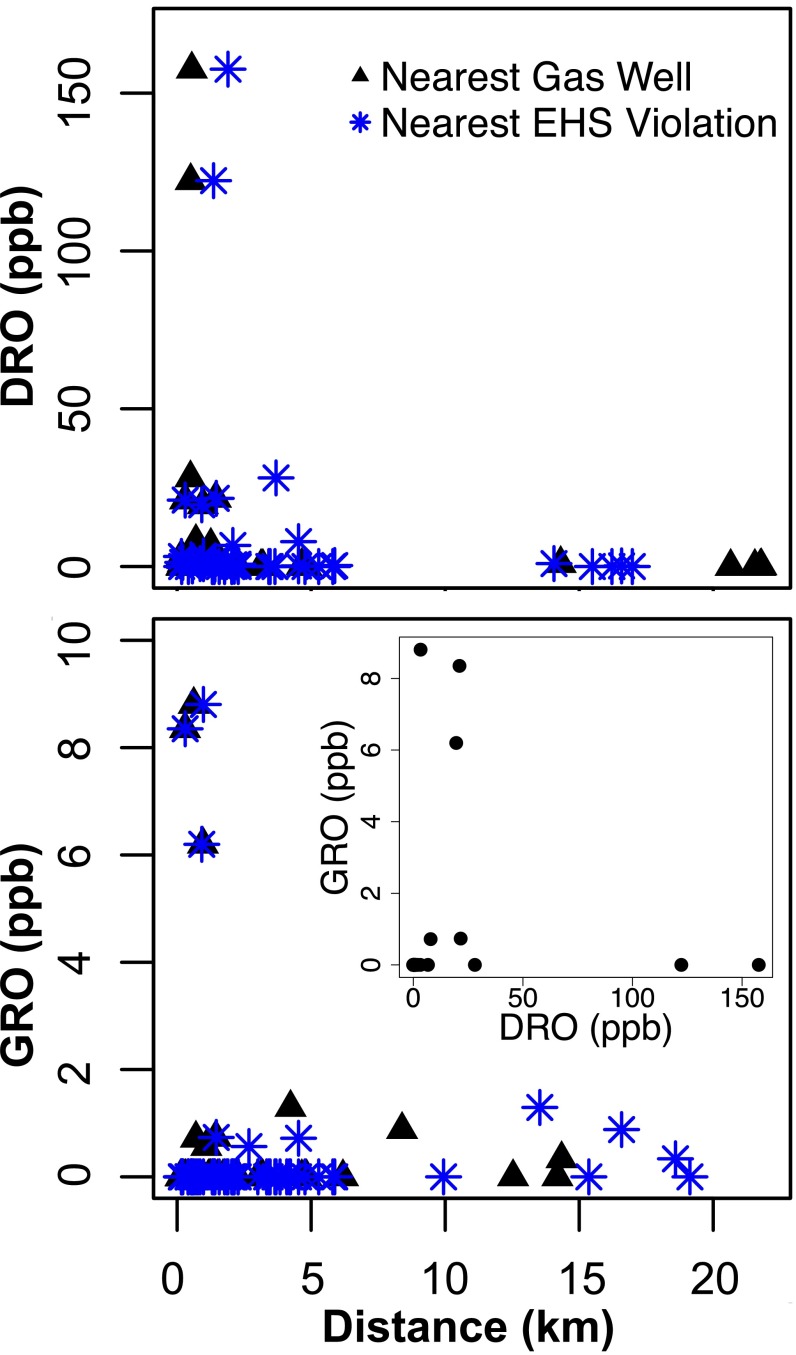
Trace levels of GRO and DRO compounds were detected in 9 of 59 (0–8.8 ppb total GRO) and 23 of 41 (0–157.6 ppb total DRO) groundwater samples, respectively. Although the highest concentrations of GRO and DRO were always detected within 1 km of active shale gas operations, this difference in concentration within 1 km (n = 21) and beyond 1 km (n = 20) from shale gas wells was only significantly higher in the case of DRO (Fig. 2; P = 0.01, Mann–Whitney U test; SI Appendix, Fig. S1). A sensitivity analysis indicated that the statistically significant difference in DRO levels persisted at 0.75–3.0 km (at 0.25-km intervals) from a gas well, as well as at 0.76 km away (the PA DEP’s suggested distance of evaluation; P < 0.01) (18). Notably, although the highest GRO- and highest DRO-containing samples all occurred within 1 km of a shale gas well, the elevated GRO and DRO were not cooccurring (Fig. 2, Inset; discussed below). Finally, trace levels (<1 ppb) of VOCs, including BTEX compounds (benzene, toluene, ethylbenzene, and xylenes), were detected in six samples (10%) at levels well below the EPA’s drinking water maximum contaminant level recommendations (19).
Fig. 2.
DRO (Top) and GRO (Bottom) concentrations in shallow groundwater with respect to the distance from the nearest active shale gas well (black triangles) or gas well with an EHS violation (blue stars). DRO was significantly correlated (P = 0.01, Spearman correlation) with the distance to the nearest shale gas well and with the distance to the nearest EHS violation (P = 0.03). GRO was not correlated with distance to the nearest gas well (P = 0.42) or with the distance to the nearest EHS violation (P = 0.36). There was no correlation between GRO and DRO (Inset).
In this region, there are multiple potential sources of elevated DRO in groundwater, including (i) upward migration of naturally occurring, formation-derived organic compounds over geologic time; (ii) lateral transport of drilling muds, flowback, or produced fluids from faulty wells; (iii) leaking oil and gas waste containment ponds; (iv) input of organic contaminants from surface spills of either raw chemicals or residual fracturing fluids; and (v) leaking underground storage containers or local traffic. To evaluate these sources systematically, we used geochemical fingerprinting of inorganic constituents (i.e., Br/Cl ratios) (11), groundwater residence times (i.e., 4He concentration) (10, 13), and dissolved methane concentrations (8, 9), coupled with our GRO, DRO, and geospatial analysis.
Upward Migration from Deep Formation.
Warner et al. (11) demonstrated that shallow groundwater in some areas in northeastern Pennsylvania is saline with molar Br/Cl ratios similar to deeper Marcellus Formation water (designated “Type D” water), suggesting natural upward migration of deep saline water over geologic timescales. Using this inorganic fingerprinting approach, we found no statistical difference in GRO or DRO contents based on water type (SI Appendix, Fig. S2; GRO, P > 0.05; DRO, P > 0.05; Kruskal–Wallis test), suggesting that the increased GRO and DRO signals were not a result of upward migration of deeper, naturally occurring formation fluids. Furthermore, samples with elevated GRO (>5 ppb), but lower DRO (<50 ppb), which might be considered geogenic and shale derived, considering the distinct transport rates of each (i.e., retarded transport of higher-molecular-weight compounds due to slower diffusion through and higher sorptivity to porous media), were not found in Type D waters uniformly (i.e., two of three were not Type D).
Water migration from the Marcellus formation to shallow groundwater would also lead to enrichments in 4He and fractionation of air-saturated noble gases (i.e., 20Ne/36Ar) (10, 13). In contrast, we find that the highest concentration DRO and GRO samples occur in tritium-active groundwater (i.e., relatively young) and have the lowest 4He abundance (an integrated proxy for residence time; SI Appendix, Fig. S3) and, therefore, the lowest apparent crustal residence times (10). This suggests that contamination occurs in the younger groundwater systems. Consequently, our results indicate that connectivity with deep subsurface brines is not a dominant source of organic compounds in the shallow groundwater.
Lateral Transport from Faulty Wells.
An alternate source of organic compounds to shallow aquifers could be faulty gas well casings, as poor well integrity has been documented in gas wells targeting the Marcellus Shale (6, 9, 10, 20, 21). Llewellyn et al. (7) recently reported groundwater contamination of 2-n-butoxyethanol, whereas others documented stray gas contamination of light hydrocarbons (e.g., nC1–nC3) from poor well integrity (8–10). Also, previous studies suggested that hydraulic fracturing fluids and denser formation brines could migrate laterally, but on timescales longer than those typical of methane transport (5, 22, 23). Such migration through porous media from a well casing would result in elevated GRO with lower levels of DRO (due to the higher diffusivities and generally lower sorptivities of the lower-molecular-weight compounds), along with higher levels of methane and a salinity signature similar to that of flowback or produced waters. Methane abundance from paired samples or previous sampling campaigns showed no correlation with GRO or DRO (SI Appendix, Fig. S4), and the noble gas analysis provided no evidence for fugitive gas contamination in the elevated GRO and DRO samples [e.g., low air-saturated water abundances ([36Ar], [N2]), or 4He/CH4 (10)]. Furthermore, samples with elevated GRO (>5 ppb) had relatively low methane and Br− (<1 ppm for both). Thus, leaky well casings are an unlikely source of GRO compounds.
It is possible that a leaky well casing during slickwater injection could be a source of elevated GRO or DRO without commensurate brine or methane inputs in a relatively young well. If so, then one might expect some relationship between GRO or DRO occurrence in groundwater and the age of the nearest HVHF well if and only if chemical or bulk fluid transport times were fast relative to or on the same order as the well ages. To entertain the possibility of a well-age effect, we calculated time from the “spud date” (the drilling date of the nearest HVHF well) to sample collection. Well ages ranged broadly from 10 d to over 5 y with a fairly even temporal distribution, and levels of GRO and DRO were not correlated with the age of the well (DRO, P > 0.05; GRO, P > 0.05; SI Appendix, Fig. S5).
Since the preceding well age argument relies on rapid fluid transport relative to the well ages, we note that typical bulk groundwater velocities are highly variable in the sampled aquifers and on the order of 0.1–8.2 km⋅y−1 (spanning velocities in alluvium to fractured bedrock aquifers) (24), and sorption-retarded transport velocities of the chemicals we detected (described below) would be on the order of 0.02–7.53 km⋅y−1 (∼2 mo–50 y to migrate a 1-km distance; SI Appendix). However, depending on the topography, hydraulic connectivity, and large pressure gradients experienced during injection (5), transport times could be faster than predicted by simple porous media transport models. For example, Llwellyn et al. (7) argue that fracturing fluids could be driven 1–3 km in a 2- to 3-y timeframe, which is reasonable for the fractured bedrock case. Although it is not possible to put an exact timeframe on the fluid transport under the hypothetical condition of leaky casing during slickwater injection, two conditions emerge: Either (i) chemical transport is slow and could not give rise to the elevated DRO compounds observed here (i.e., within 1 km and less than 2 y), or (ii) transport is faster and a relationship between DRO and well age could have been observed, which it was not. In either case, our data suggest that leaky wells are not a source of DRO to nearby groundwater wells.
Leaking Oil and Gas Waste Containment Ponds.
Following hydraulic fracturing, the flowback and produced waters are often stored in polymer-lined, open, waste-containment pits, which are demonstrated sources of contamination to surface water and groundwater in cases where the liner integrity was compromised (e.g., torn, ripped, folded, or other failure due to a physical breach that allowed fluid to pass unrestricted) (25). Although many of these pits have been phased out voluntarily, many were still in use at the time of our study. Unfortunately, Pennsylvania does not maintain a publically available database of the location of the polymer-lined containment pits, and no spatial analysis between elevated DRO or GRO levels and containment pits is possible.
Nonetheless, these pits were designed to allow volatile compounds to outgas and particles to settle (26), and the residual wastes are often highly saline with a high organic content (25, 27). Thus, leakage into groundwater from such containment basins would result in low GRO levels (due to volatile out gassing) and elevated DRO, such as observed in our samples (see SI Appendix for discussion of potential GRO/DRO fingerprints in groundwater and flowback water; SI Appendix, Fig. S8). Leaking from pits with compromised liners would give rise to elevated chloride and bromide in the high-DRO samples, which was not observed (SI Appendix, Fig. S6). Therefore, diffusive transport of DRO through uncompromised liners could give rise to the observed chemical composition of the groundwater. However, the types of compounds revealed in our compound-specific analysis (detailed below) have very long transport times through model polymers characteristic of such liners. Considering the fastest-possible transport, the compound would not migrate through a 4-mm liner to the soil interface after 4 y (only 2 × 10−27% of the water-side content would migrate to 1 mm depth after 4 y; model details provided in SI Appendix, Fig. S7). Over these long timescales, transport through intact pit liners could not have given rise the DRO observed in our samples. This implies that organic chemical transport through the liners was not the primary source of material in our samples.
Surface Spills of Hydraulic Fracturing Chemicals.
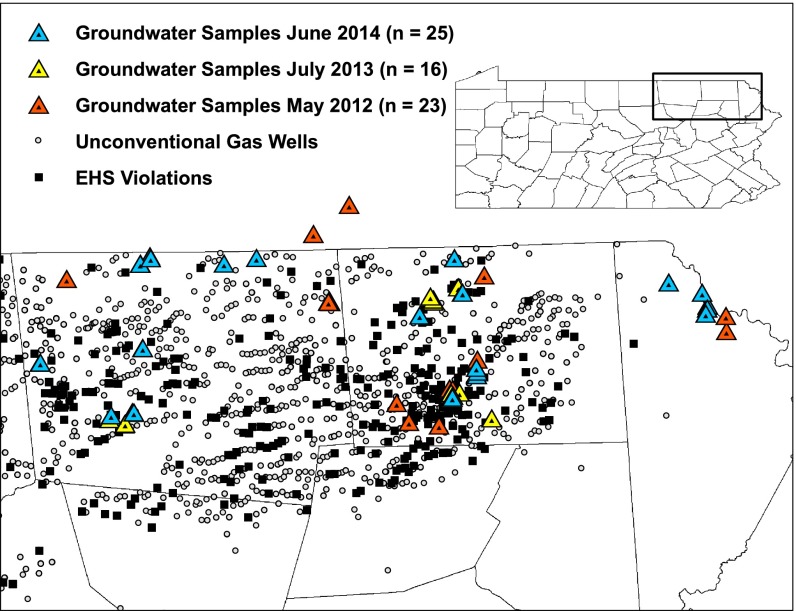
State databases are maintained for disclosed releases of hydraulic fracturing components at the surface, because these present a direct route for surface water and groundwater contamination. Surface releases could result in low GRO due to volatilization within weeks (28, 29), with elevated levels of DRO leaching to groundwater. Such elevated DRO with low GRO was observed in our samples, with higher DRO close to active shale gas wells (<1 km). To further evaluate the possible link between elevated DRO and proximity to the nearest well, we analyzed PA DEP violation reports. According to the PA DEP Oil and Gas Compliance Report, 5,791 violations were reported associated with 1,729 unconventional gas wells throughout the state between January 1, 2007 and June 1, 2014 (30) and classified as either “Administrative” or “Environmental Health & Safety” (EHS) violations. DRO concentrations were elevated significantly in groundwater samples in close proximity to EHS violations (P = 0.03, Spearman correlation; Fig. 3), but GRO concentrations were not (P = 0.36). Furthermore, DRO occurrence in samples within 2 km of an unconventional well with an EHS violation (n = 20) was statistically higher than in samples further away than 2 km (n = 21; P = 0.03, Mann–Whitney U test), whereas GRO did not show the same relationship (P = 0.92). Neither DRO nor GRO levels were significant at the 1- or 0.76-km cutoff distances, perhaps due to the fact that the distribution of shale gas wells with an EHS violation is spatially diffuse compared with individual shale gas well locations.
Fig. 3.
Locations of EHS violations associated with unconventional gas well operations as reported by PA DEP Oil and Gas Reporting website (30).
Groundwater well depth could also provide information on the nature of the flow path of the compounds detected in our samples. For example, because vertical transport times are long, a deep or shallow source might give rise to a depth-dependent concentration gradient. There was no statistically significant difference between DRO or GRO concentrations in the shallowest (<100 m) or deepest (>100 m) sampled wells (DRO, P = 0.57; GRO, P = 0.89; Mann–Whitney U test), or at any other depth cutoff (50, 75, or 125 m), and neither GRO nor DRO was correlated with depth (SI Appendix, Fig. S9). This could be an artifact of the scale and spatial resolution of the sampling effort. Designed to cover a large area (∼7,400 km2) and constrained by well access, the groundwater samples were separated by widely varying lateral distances (7 ± 15 and 11 ± 18 km for groundwater wells containing detectable DRO and GRO, respectively). As a result, any point source or spatially constrained “plume” of organic material could conceivably affect only a small population of groundwater wells, obfuscating any effect of well depth on the GRO or DRO concentration. Nevertheless, the samples with the highest GRO and DRO are found in groundwater wells less than 100 m deep.
Leaking Underground Storage Tanks or Local Traffic.
We also explored the hypothesis that leaking underground storage tanks that typically contain gasoline, diesel, or fuel oil for both domestic and industrial use could provide a significant source of GRO and/or DRO. Leaking tank incident data obtained from PA DEP (31) showed no spatial correlations with DRO (P = 0.95, Spearman correlation) or GRO (P = 0.81) in the groundwater samples (SI Appendix, Fig. S10). In addition, the chemicals identified in the compound-specific analysis are not commonly stored in underground storage containers and are distinct from the chromatographic fingerprints of gasoline, diesel, or hydraulic fluids (SI Appendix, Fig. S11). Indeed, were these materials present, they would be readily obvious via our analytical methods (detection limits near 100 pg⋅L−1 or parts per quadrillion). Their absence implies that leaking underground storage tanks were not a source of material to the groundwater. Similarly, if local truck traffic were a source, then one might expect a distinct chemical fingerprint and correlation with distance to the nearest road. No such fingerprints (SI Appendix, Fig. S11) or correlations existed (DRO, P = 0.78; GRO, P = 0.63), suggesting that traffic was not responsible for the DRO observed in the studied groundwater.
Organic Chemical Fingerprinting via GC×GC Time-of-Flight MS.
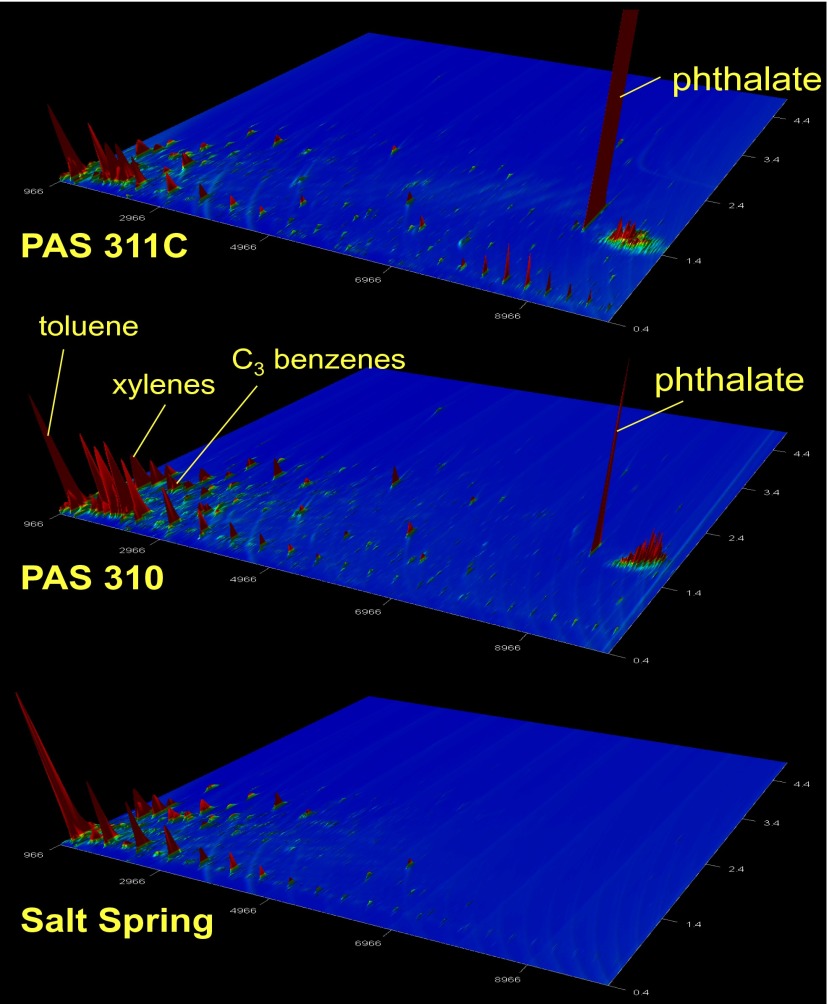
To further evaluate the source of elevated DRO compounds, we conducted a compound-specific investigation using GC×GC with time-of-flight MS. In particular, a subset (n = 12) of groundwater liquid–liquid extracts was analyzed, including those with the highest DRO levels, along with a field blank and one natural salt spring in Susquehanna County, Pennsylvania, that is typically used to indicate the composition of natural gas and brine-rich fluids derived from shale rock sources in the northern Appalachian Basin (7–12). The samples with the highest DRO (n = 2) contained bis(2-ethylhexyl) phthalate (Fig. 4), whereas the salt spring, lower-DRO samples (n = 8) and field blank did not (SI Appendix, Fig. S12). Fatty acid phthalate esters [including bis(2-ethylhexyl) phthalate] are used in drilling and in hydraulic fracturing fluids (32), and bis(2-ethylhexyl) phthalate has been reported in Marcellus Shale, Barnett Shale, and Denver–Julesburg basin flowback waters (27, 32–34), in runoff and surface waters following an incident at a gas well site (35), and in multiple residential groundwater wells in Dimock, Pennsylvania, where the EPA has identified contamination directly from hydraulic fracturing operations (discerned by the onset timing of the contamination) (36).
Fig. 4.
GC×GC time-of-flight MS extracted ion (m/z 41) chromatograms of two shallow groundwater samples (PAS311C and PAS310) that contained bis(2-ethylhexyl) phthalate (labeled as phthalate) and the natural salt spring that did not.
Bis(2-ethylhexyl) phthalate is a ubiquitous chemical that is used in many industrial practices and materials, and it is difficult to attribute its presence solely to hydraulic fracturing activities. However, we present several lines of evidence that this particular phthalate is likely to be derived from HVHF activities. First, only our highest DRO samples contained bis(2-ethylhexyl) phthalate, suggesting that the compound was not derived from any step of our own analytical procedure. Second, if PVC pipes (known to contain phthalates and to be pervasive in water distribution systems) were a source of the bis(2-ethylhexyl) phthalate, then one would expect a widespread presence in the analyzed samples (n = 12). In contrast, it was only detected in the highest DRO samples (n = 2). Third, compound-specific analysis of the natural salt spring did not contain the phthalate. Thus, the presence of bis(2-ethylhexyl) phthalate likely reflects its presence in the contamination source and is not an artifact of our sampling or preparation protocol.
Curiously, this particular phthalate has relatively low aqueous solubility. In chemical disclosure databases, bis(2-ethylhexyl) phthalate is reportedly used in “perfball” form (i.e., it is transported and injected as a solid). A solubilized form of a phthalate could be derived from surface spills of flowback or produced waters, or transported through containment pit liners. However, the former would carry a brine signature, which was not observed in the high-DRO groundwater samples (SI Appendix, Fig. S6), and the latter has prohibitive transport timescales and could not give rise to the phthalate observed here (SI Appendix, Fig. S7). Consequently, our data suggest that some solubilized form of the phthalate (e.g., perfballs placed in a liquid carrier) is responsible for their appearance in shallow aquifers sampled in this study. Indeed, in all cases where the phthalate was detected, toluene was present as a cocontaminant. Further, because bis(2-ethylhexyl) phthalate is a disclosed additive in fracturing fluids, it is both (i) plausible that its presence in these samples is due to accidental surface releases of the parent fluids in the Marcellus region and (ii) reasonable given our statistical spatial analysis using the disclosed EHS violation database, as well as the complementary inorganic, methane, and helium abundance measurements. Nevertheless, one cannot rule out the possibility that the bis(2-ethylhexyl) phthalate is derived from some non-HVHF source and just coincidentally correlated with proximity to disclosed HVHF EHS violations.
Bis(2-ethylhexyl) phthalate is “reasonably anticipated to be a human carcinogen” (37). Due to the analytical challenges of obtaining clean blanks and ubiquitous industrial use, the environmental fate of phthalates has been understudied since their presumable first appearances after the advent of plastics in the 1970s (38–41). Detection of phthalates in environmentally derived samples, as well as their source apportionment, may have been overlooked out of fear of cross-contamination from other sources. However, if HVHF practices are using phthalates (which are disclosed, but not with great frequency), the environmental geochemistry community is challenged to develop robust methods to track and source apportion these materials. Careful efforts to avoid contamination (i.e., the use of precombusted, all-glass or metal materials) and accountability for all other potential local industrial sources will be critical.
Implications.
To our knowledge, this is the first study of its kind to evaluate, on a regional scale, different possible mechanistic sources of organic compounds detected in drinking water wells in the Marcellus region using complementary inorganic chemical analyses and residence time approximations. Based on the evaluation of different possible mechanisms, our data are consistent with a surface-derived source of organic compounds in the study area, possibly from releases of hydraulic fracturing materials near drill sites. The question arises: Is the spill rate associated with unconventional shale gas development worse than any other industrial chemical or energy extraction activity? Unfortunately, a quantitative comparison cannot be made due to the construct of the PA DEP disclosed violation reports, for which details are limited (42). Often, ambiguous language is used to describe the nature of the violation and volume estimates of reported releases are not provided. If volume data were available, an appropriate comparison of the environmental impacts of these releases could be calculated with the ratio of volume-of-spill to total-transported volume, as is done with crude oil [in 2014, 0.00007% of all oil transported by sea was spilled (43)]. Incorporating volumetric data on releases from natural gas operations would allow direct comparisons to other energy industries. Clearly, such a report relies on accurate self-reporting or more robust monitoring (44). Further, if the PA DEP required volume and chemical identity estimates, a more accurate assessment of the relative risks due to domestic energy extraction could be constructed.
Irrespective of the reporting nuances, it is clear that surface releases of fracturing fluids are usually accidental. Therefore, it is not necessarily the hydraulic fracturing process (i.e., the fluid injection) that can lead to groundwater contamination, but rather, the existence of the operation itself (i.e., the inherent risk associated with mechanical failure and human error in industrial practice). Domestic natural gas production necessitates colocation of residential areas with extraction facilities, and, like many industrial activities, the economic benefits come with some level of environmental and public health risk.
In summary, we show that some private residential groundwater wells contained trace concentrations of organic compounds (<200 ppb DRO) in close proximity to active shale gas wells and disclosed EHS violations. Surface sources are consistent with the presence of DRO compounds in groundwater with the lowest apparent groundwater residence times. We found no evidence for direct communication of deeper formation water or injected fracturing fluids with shallow drinking water wells due to upward migration from shale horizons. This result is encouraging, because it implies there is some degree of temporal and spatial separation between injected fluids and the drinking water supply. However, shallow groundwater should be monitored over longer timescales (45) in areas of enhanced fracturing activities [e.g., where preferential faults could enhance deep-to-surface communication (46)]. Future research should also focus on investigating chemical fingerprints of shale-derived organic matter via a careful comparison of raw fracturing fluids, flowback water, and geologic formation waters.
Materials and Methods
Shallow groundwater samples were collected in precombusted glass vials over three sampling campaigns from private residential groundwater wells. Wells were purged of stagnant water until stable readings of conductivity, pH, and temperature were recorded, upstream of any treatment system. The samples were fixed with acid then stored on ice until analysis within 14–28 d. For the organic compound analysis, light hydrocarbons were analyzed using standard purging and preconcentration techniques (see SI Appendix for details), whereas heavier hydrocarbons were concentrated via liquid–liquid extraction into organic solvents. Compounds and compound classes were quantified via gas chromatography with flame ionization detection and qualitatively identified with confirmed standards using GC-MS. A subset of liquid–liquid extracts was interrogated using comprehensive GC×GC with time-of-flight MS. Inorganic constituents were analyzed by methods detailed in Warner et al. (11). Methane was analyzed by methods detailed in Jackson et al. (9). Noble gases were analyzed by methods detailed in Darrah et al. (10, 13). Maps and spatial data analysis were prepared with ArcMap and all statistics were analyzed with the R statistical computing platform.
Supplementary Material
Acknowledgments
We thank Alissa White and Emma D’Ambro for their assistance and valuable discussion. This work was supported by Duke University’s Pratt School of Engineering and National Science Foundation (NSF) CBET Grant 1336702 and NSF EAGER Grant EAR-1249255.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1511474112/-/DCSupplemental.
References
- 1.Kerr RA. Energy. Natural gas from shale bursts onto the scene. Science. 2010;328(5986):1624–1626. doi: 10.1126/science.328.5986.1624. [DOI] [PubMed] [Google Scholar]
- 2.Malakoff D. Gas revolution. The gas surge. Introduction. Science. 2014;344(6191):1464–1467. doi: 10.1126/science.344.6191.1464. [DOI] [PubMed] [Google Scholar]
- 3.US Energy Information Administration . Drilling Productivity Report, March 2015. US Energy Information Administration; Washington, DC: 2015. [Google Scholar]
- 4.Howarth RW, Ingraffea A, Engelder T. Natural gas: Should fracking stop? Nature. 2011;477(7364):271–275. doi: 10.1038/477271a. [DOI] [PubMed] [Google Scholar]
- 5.Vengosh A, Jackson RB, Warner N, Darrah TH, Kondash A. A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ Sci Technol. 2014;48(15):8334–8348. doi: 10.1021/es405118y. [DOI] [PubMed] [Google Scholar]
- 6.Vidic RD, Brantley SL, Vandenbossche JM, Yoxtheimer D, Abad JD. Impact of shale gas development on regional water quality. Science. 2013;340(6134):1235009. doi: 10.1126/science.1235009. [DOI] [PubMed] [Google Scholar]
- 7.Llewellyn GT, et al. Evaluating a groundwater supply contamination incident attributed to Marcellus Shale gas development. Proc Natl Acad Sci USA. 2015;112(20):6325–6330. doi: 10.1073/pnas.1420279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osborn SG, Vengosh A, Warner NR, Jackson RB. Methane contamination of drinking water accompanying gas-well drilling and hydraulic fracturing. Proc Natl Acad Sci USA. 2011;108(20):8172–8176. doi: 10.1073/pnas.1100682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson RB, et al. Increased stray gas abundance in a subset of drinking water wells near Marcellus shale gas extraction. Proc Natl Acad Sci USA. 2013;110(28):11250–11255. doi: 10.1073/pnas.1221635110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darrah TH, Vengosh A, Jackson RB, Warner NR, Poreda RJ. Noble gases identify the mechanisms of fugitive gas contamination in drinking-water wells overlying the Marcellus and Barnett Shales. Proc Natl Acad Sci USA. 2014;111(39):14076–14081. doi: 10.1073/pnas.1322107111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warner NR, et al. Geochemical evidence for possible natural migration of Marcellus Formation brine to shallow aquifers in Pennsylvania. Proc Natl Acad Sci USA. 2012;109(30):11961–11966. doi: 10.1073/pnas.1121181109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llewellyn GT. Evidence and mechanisms for Appalachian Basin brine migration into shallow aquifers in NE Pennsylvania, USA. Hydrogeol J. 2014;22(5):1055–1066. [Google Scholar]
- 13.Darrah TH, et al. The evolution of Devonian hydrocarbon gases in shallow aquifers of the northern Appalachian Basin: Insights from integrating noble gas and hydrocarbon geochemistry. Geochimica et Cosmochimica Acta. 2015 doi: 10.1016/j.gca.2015.09.006. [DOI] [Google Scholar]
- 14.Warner NR, et al. New tracers identify hydraulic fracturing fluids and accidental releases from oil and gas operations. Environ Sci Technol. 2014;48(21):12552–12560. doi: 10.1021/es5032135. [DOI] [PubMed] [Google Scholar]
- 15.Gross SA, et al. Analysis of BTEX groundwater concentrations from surface spills associated with hydraulic fracturing operations. J Air Waste Manag Assoc. 2013;63(4):424–432. doi: 10.1080/10962247.2012.759166. [DOI] [PubMed] [Google Scholar]
- 16.Office of Oil and Gas Management . Oil and Gas Reports: Water Supply Determination Letters. Pennsylvania Department of Environmental Protection; Harrisburg PA: 2015. [Google Scholar]
- 17.US Environmental Protection Agency . Method 8015D Nonhalogenated Organics Using GC/FID. US Environmental Protection Agency; Washington, DC: 2003. [Google Scholar]
- 18. Oil and gas wells, The Pennsylvania Code, Chapter 78 (1987). Available at www.pacode.com/secure/data/025/chapter78/chap78toc.html. Accessed September 24, 2015.
- 19.US Environmental Protection Agency . National Primary Drinking Water Regulations. US Environmental Protection Agency; Washington, DC: 2009. [Google Scholar]
- 20.Davies RJ, et al. Oil and gas wells and their integrity: Implications for shale and unconventional resource exploration. Mar Pet Geol. 2014;56(0):239–254. [Google Scholar]
- 21.Considine TJ, Watson RW, Considine NB, Martin JP. Environmental regulation and compliance of Marcellus Shale gas drilling. Environ Geosci. 2013;20(1):1–16. [Google Scholar]
- 22.Harrison SS. Evaluating system for ground-water contamination hazards due to gas-well drilling on the Glaciated Appalachian Plateau. Ground Water. 1983;21(6):689–700. [Google Scholar]
- 23.Harrison SS. Contamination of aquifers by overpressuring the annulus of oil and gas wells. Ground Water. 1985;23(3):317–324. [Google Scholar]
- 24.Gelhar LW, Welty C, Rehfeldt KR. A critical review of data on field-scale dispersion in aquifers. Water Resour Res. 1992;28(7):1955–1974. [Google Scholar]
- 25.Ziemkiewicz PF, Quaranta JD, Darnell A, Wise R. Exposure pathways related to shale gas development and procedures for reducing environmental and public risk. J Nat Gas Sci Eng. 2014;16(0):77–84. [Google Scholar]
- 26.Rich AL, Crosby EC. Analysis of reserve pit sludge from unconventional natural gas hydraulic fracturing and drilling operations for the presence of technologically enhanced naturally occurring radioactive material (TENORM) New Solut. 2013;23(1):117–135. doi: 10.2190/NS.23.1.h. [DOI] [PubMed] [Google Scholar]
- 27.Hayes T. Sampling and Analysis of Water Streams Associated with the Development of Marcellus Shale Gas. Gas Technology Institute; Des Plaines, IL: 2009. [Google Scholar]
- 28.Serrano A, Gallego M, González JL, Tejada M. Natural attenuation of diesel aliphatic hydrocarbons in contaminated agricultural soil. Environ Pollut. 2008;151(3):494–502. doi: 10.1016/j.envpol.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Serrano A, Gallego M, González JL. Assessment of natural attenuation of volatile aromatic hydrocarbons in agricultural soil contaminated with diesel fuel. Environ Pollut. 2006;144(1):203–209. doi: 10.1016/j.envpol.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 30.Office of Oil and Gas Management 2015 Oil and Gas Reports: Oil and Gas Compliance Report (Pennsylvania Department of Environmental Protection, Harrisburg PA). Available at www.portal.state.pa.us/portal/server.pt/community/oil_and_gas_reports/20297. Accessed January 20, 2015.
- 31.Department of Environmental Protection 2015 Storage Tank Cleanup Locations (Pennsylvania Department of Environmental Protection, Harrisburg PA). Available at www.portal.state.pa.us/portal/server.pt/community/storage_tank_cleanup_program/20605/storage_tank_cleanup_locations/1053538. Accessed January 22, 2015.
- 32.Maguire-Boyle SJ, Barron AR. Organic compounds in produced waters from shale gas wells. Environ Sci Process Impacts. 2014;16(10):2237–2248. doi: 10.1039/c4em00376d. [DOI] [PubMed] [Google Scholar]
- 33.Ferrer I, Thurman EM. Chemical constituents and analytical approaches for hydraulic fracturing waters. Trends Analyt Chem. 2015;5:18–25. [Google Scholar]
- 34.Lester Y, et al. Characterization of hydraulic fracturing flowback water in Colorado: Implications for water treatment. Sci Total Environ. 2015;512-513(0):637–644. doi: 10.1016/j.scitotenv.2015.01.043. [DOI] [PubMed] [Google Scholar]
- 35.Justice J. U.S. Environmental Protection Agency Pollution/Situation Report, Statoil Eisenbarth Well Response – Removal Polrep. US Environmental Protection Agency; Washington, DC: 2014. [Google Scholar]
- 36.US Environmental Protection Agency 2015 Dimock Residential Groundwater Site. (US Environmental Protection Agency, Washington DC). Available at www.epa.gov/reg3hwmd/npl/PAN000306785.htm. Accessed January 22, 2015. [PubMed]
- 37.NTP (National Toxicology Program) 2014 Report on Carcinogens, Thirteenth Edition (US Department of Health and Human Services, Public Health Services, Research Triangle Park, NC). Available at ntp.niehs.nih.gov/pubhealth/roc/roc13/
- 38.Wade TL. 1974. Measurements of hydrocarbons, phthalic acid, and phthalic acid ester concentrations in environmental samples from the north Atlantic. M.S. thesis (Univ of Rhode Island, Kingston, RI)
- 39.Sanborn JR, Metcalf RL, Yu CC, Lu PY. Plasticizers in the environment: The fate of di-N-octyl phthalate (DOP) in two model ecosystems and uptake and metabolism of DOP by aquatic organisms. Arch Environ Contam Toxicol. 1975;3(2):244–255. doi: 10.1007/BF02220792. [DOI] [PubMed] [Google Scholar]
- 40.Hites RA, Biemann K. Water pollution: Organic compounds in the Charles River, Boston. Science. 1972;178(4057):158–160. doi: 10.1126/science.178.4057.158. [DOI] [PubMed] [Google Scholar]
- 41.Mayer FL, Stalling DL, Johnson JL. Phthalate esters as environmental contaminants. Nature. 1972;238(5364):411–413. doi: 10.1038/238411a0. [DOI] [PubMed] [Google Scholar]
- 42.Brantley SL, et al. Water resource impacts during unconventional shale gas development: The Pennsylvania experience. Int J Coal Geol. 2014;126(0):140–156. [Google Scholar]
- 43.US Energy Information Administration . World Oil Transit Chokepoints. US Energy Information Administration; Washington, DC: 2014. [Google Scholar]
- 44.Manda AK, Heath JL, Klein WA, Griffin MT, Montz BE. Evolution of multi-well pad development and influence of well pads on environmental violations and wastewater volumes in the Marcellus shale (USA) J Environ Manage. 2014;142(0):36–45. doi: 10.1016/j.jenvman.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Myers T. Potential contaminant pathways from hydraulically fractured shale to aquifers. Ground Water. 2012;50(6):872–882. doi: 10.1111/j.1745-6584.2012.00933.x. [DOI] [PubMed] [Google Scholar]
- 46.Davies RJ, Mathias SA, Moss J, Hustoft S, Newport L. Hydraulic fractures: How far can they go? Mar Pet Geol. 2012;37(1):1–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.