Abstract
Alcoholism is a debilitating disorder that can take a significant toll on health and professional and personal relationships. Excessive alcohol consumption can have a serious impact on both drinkers and developing fetuses, leading to long-term learning impairments. Decades of research in laboratory animals and humans have demonstrated the value of eyeblink classical conditioning (EBC) as a well-characterized model system to study the neural mechanisms underlying associative learning. Behavioral EBC studies in adults with alcohol use disorders and in children with fetal alcohol spectrum disorders report a clear learning deficit in these two patient populations, suggesting alcohol-related damage to the cerebellum and associated structures. Insight into the neural mechanisms underlying these learning impairments has largely stemmed from laboratory animal studies. In this mini-review, we present and discuss exemplary animal findings and data from patient and neuroimaging studies. An improved understanding of the neural mechanisms underlying learning deficits in EBC related to alcoholism and prenatal alcohol exposure has the potential to advance the diagnoses, treatment, and prevention of these and other pediatric and adult disorders.
Keywords: alcoholism, ethanol, cerebellum, fetal alcohol spectrum disorders, eyeblink classical conditioning, associative learning
Introduction
Alcohol is one of the most widely abused substances in the world (1) and can have a major impact on health and professional and personal relationships. One reason for this negative societal impact is that excessive alcohol consumption often leads to long-term learning and memory impairments. In this mini-review, we will outline exemplary animal and human findings that guide our current understanding of how chronic alcohol exposure alters neural structure and function underlying a fundamental form of learning, eyeblink classical conditioning (EBC). Specifically, this mini-review will focus on alcohol use disorders (AUD) in adults and fetal alcohol spectrum disorders (FASD) in children.
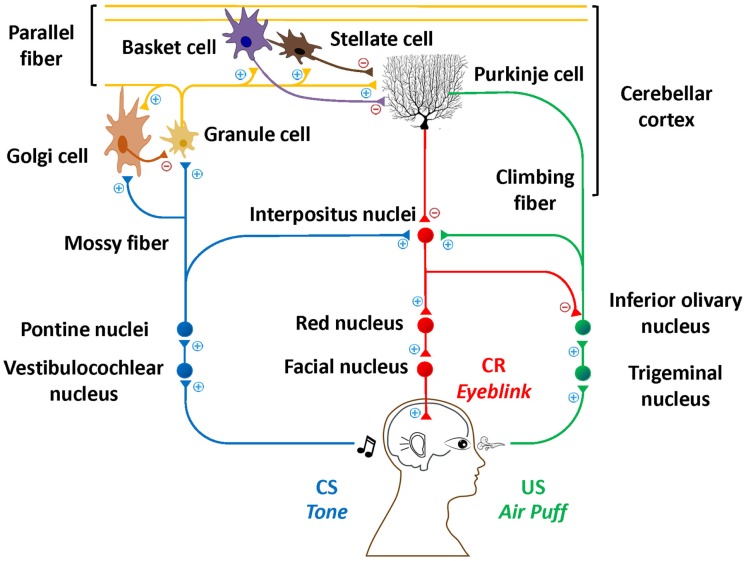
One area of the brain that is targeted in AUD and FASD is the cerebellum (2, 3). Although excessive alcohol consumption affects many other brain regions (4–6), this mini-review will focus on the cerebellum due to its critical involvement in EBC (7) and the particular vulnerability of the cerebellum to alcohol exposure (8, 9). This line of research has produced overwhelming evidence that the cerebellum and associated structures are critically important for EBC. Specifically, contributions from the cerebellar cortex, particularly in lateral lobule VI (10, 11), and cerebellar deep nuclei (12, 13) have been documented in both animals and humans. Figure 1 depicts this well-documented circuitry.
Figure 1.
Essential neural circuitry of eyeblink conditioning. Blue lines indicate the conditioned stimulus pathway. Green lines indicate the unconditioned stimulus pathway. Red lines indicate the conditioned response pathway. Excitatory and inhibitory synapses are represented by + and −, respectively.
Eyeblink classical conditioning involves the pairing of a neutral conditioned stimulus (CS; e.g., a tone) and an unconditioned stimulus (US; e.g., a corneal airpuff). The US is often a biologically salient stimulus sufficient to elicit an unconditioned response (UR; e.g., a blink). Following multiple CS–US pairings, an organism learns to produce a conditioned response (CR) in anticipation of the US presentation, suggesting that an association between the CS and US has been learned. EBC is a simple, yet elegant model of learning, which can already be assessed in humans by 5 months of age (14) and represents a foundation on which more complex learning is built (15, 16). Understanding the etiology of fundamental learning impairments that accompany alcohol-related disorders may have potential to foster new approaches to early diagnoses, intervention, and effective treatments and presents a model for studying effects of other pediatric and adult disorders as well as effects of other drugs or environmental contaminants.
Laboratory Animal Work
Structural Alterations (Mature Cerebellum)
There is extensive laboratory animal evidence showing that chronic intake of alcohol is associated with neuroanatomical changes in the cerebellum (17). A common observation is shrinkage of the cerebellum. In the adult rat, these volumetric reductions may be due to death and atrophy of cells in the Purkinje, granular, and molecular layers of the cerebellar cortex (18–21). In addition to degenerative changes in cell bodies, morphological changes to dendrites and axons have also been reported (22–24). Combined treatments of thiamine deficiency and alcohol exposure have led to axon terminal degeneration in the deep cerebellar nuclei, the sole output region for the cerebellum (25). Fewer synapses between parallel fibers and Purkinje cells (26) and a significant decrease in the number of dendritic microtubules have been found in alcohol-fed adult rats (27). At the molecular and cellular level, γ-aminobutyric acidA (GABAA) is altered by chronic alcohol consumption (28), whereas there is an overexpression of glutamate and a prolonged opening of mitochondrial permeability in the cerebellum following alcohol withdrawal (29).
Structural Alterations (Developing Cerebellum)
Cerebellar structural abnormalities also appear in the developing cerebellum as a result of excessive early alcohol exposure. This damaging effect appears to be sensitive to time of alcohol exposure as rats receiving alcohol on postnatal day 4 suffered up to 50% Purkinje cell loss, whereas later exposure (postnatal days 8/9) resulted in less severe (15%) cell loss (30, 31). Alcohol-related damage in granule cells has also been investigated and cell vulnerability again appears to be greatest early in development (postnatal days 4/5) (32, 33). The structural integrity of the cerebellar deep nuclei, a region believed to be crucially important for EBC memory formation and storage (7), has been shown to be susceptible to chronic alcohol consumption. Binge-like and moderate neonatal exposure to alcohol was sufficient to produce behavioral deficits in EBC associated with significant deep nuclear cell loss in adult rats (34, 35). During development, even a single exposure to alcohol introduced subcutaneously was sufficient to promote cellular apopotosis in the deep cerebellar nuclei (36).
Functional Differences (Mature Cerebellum)
Abnormal cerebellar functioning is another consequence of chronic alcohol exposure. Very little attention has been given to the chronic effects of alcohol on the cerebellum in adult laboratory animals. To the best of our knowledge, only one study to date has examined these effects. In mature mice, chronic alcohol consumption resulted in a decrease in simple and complex spike firing and an increase in complex spike duration and pause in Purkinje cells but no differences were detected in Golgi cell firing patterns (37).
Functional Differences (Developing Cerebellum)
Most of our current knowledge on the functional consequences of chronic alcohol exposure stems from work on the developing cerebellum. Following alcohol exposure during pregnancy, in vitro experiments using a long-term depression (LTD) induction protocol showed parallel fiber long-term potentiation (LTP) in cerebellar slices in alcohol-exposed juvenile mice but LTD in control mice (38). Furthermore, in vivo experiments showed that simple spike firing rates in Purkinje cells increased and showed faster oscillations of local field potentials in exposed mice relative to controls (38). These exposed mice also exhibited impaired EBC, further supporting the hypothesis that cerebellar LTD in Purkinje cells is crucial for the timing of eyeblink CRs (39). Interestingly, other in vitro electrophysiology experiments showed that alcohol exposure led to relatively greater inhibitory inputs to the Purkinje cells in the vermis (40). In the cerebellar deep nuclei, activity in the interpositus nucleus of the cerebellum was diminished and did not develop as rapidly in neonatal alcohol-exposed rats relative to controls during EBC (41, 42).
Learning Deficits
Since the cerebellum is vulnerable to chronic alcohol exposure and this structure plays a critical role in EBC, prolonged alcohol use is likely to result in learning deficits. Surprisingly, to date, there are no laboratory animal eyeblink conditioning studies investigating the role of chronic alcohol consumption in adulthood.
By contrast, there have been several animal studies on effects of pre- and neonatal exposure. Neonatal rats exposed to alcohol during the equivalent of the human third trimester showed learning deficits in standard delay EBC (43) as well as more complex EBC protocols, including trace conditioning, discrimination, and reversal learning (44, 45). The effects of alcohol on EBC also appear to be dose dependent, with higher dosages producing greater impairments (45, 46). Binge-like and even moderate exposure to alcohol during development produces EBC deficits that persist into adulthood, suggesting long-lasting permanent cerebellar damage (35, 47). This evidence is consistent with studies that report a significant correlation between learning and the number of deep cerebellar nuclear cells in alcohol-exposed rats (34). Finally, interventions to ameliorate neonatal alcohol-related learning deficits have been met with mixed results. MK-801 administration, choline supplementation, and a combination of exercise and environmental enrichment mitigate behavioral EBC deficits, suggesting neuroprotective or other ameliorative effects (48–50), whereas vitamin E did not reduce alcohol-related EBC deficits (51).
Human Work
Structural Alterations (Mature Cerebellum)
Consistent with laboratory animal findings, human data also indicate that chronic alcohol consumption has harmful effects on the structural integrity of the adult cerebellum (4, 52). Structural MRI has revealed gray matter reductions in the cerebellar hemispheres and vermis in AUDs (53). Furthermore, cerebellar gray matter volume loss was correlated with poor neuropsychological performance and early age of first drinking (54). Diffusion tensor imaging (DTI) showed that recovered AUDs had diminished white matter fibers relative to healthy controls, suggesting that impaired connectivity may partially mediate some of these behavioral deficits (55). Human histological studies report significant Purkinje cell loss in the cerebellar hemispheres and vermis as a result of years of alcohol abuse (9, 56, 57).
Structural Alterations (Developing Cerebellum)
As indicated above, animal models predict that alcohol exposure damages the developing cerebellum. These findings are also consistent with human studies: autopsy reports of children prenatally exposed to large quantities of alcohol describe malformations in the cerebellum characterized by reduced size and disorganization (58). In addition, cerebellar dysgenesis was reported in 10 of 16 FAS autopsies (59). Modern neuroimaging data agree with these observations, as exposed children had proportionately greater reductions in cerebellar cranial vault and volume (60, 61), including a 15% reduction in cerebellar volume in children with FAS (8). Specifically, significantly smaller cerebellar hemispheres and vermis were found in exposed relative to healthy children (62, 63). Differences in white matter integrity [lower fractional anisotropy (FA) and greater perpendicular diffusivity] between alcohol-exposed and non-exposed children have been identified in the middle cerebellar peduncles, fibers shown to be important in animal models of EBC (64, 65). Children with FAS also showed lower FA bilaterally in the superior peduncles. Finally, using in vivo (1) H magnetic resonance spectroscopy (MRS) to examine neurochemical differences in the cerebellar deep nuclei, Du Plessis et al. (66) found that prenatal alcohol exposure was associated with lower levels N-Acetylaspartate (NAA) and glycerophosphocholine + phosphocholine (Cho) and higher levels of glutamate plus glutamine (Glx).
Functional Differences (Mature and Developing Cerebellum)
Consistent with these structural findings, evidence from functional magnetic resonance imaging (fMRI) studies suggests fMRI brain activations are also affected by alcoholism. In a finger tapping task, AUD subjects tended to exhibit more extensive and bilateral cerebellar activation than healthy controls (67). Greater right superior cerebellar activity during a Sternberg working memory task was assessed in AUD subjects (68). In an auditory language task, AUD subjects showed greater fMRI activations in the cerebellar vermis, despite comparable behavioral performance to healthy controls (69). Children diagnosed with fetal alcohol syndrome (FAS) or partial FAS (PFAS) showed greater cerebellar activation in a working memory n-back task relative to healthy children (70). Rhythmic tapping elicited greater activation in children with FASD in crus I and vermis IV–V (71). This pattern of greater activation by adults and children may represent compensatory mechanisms during each task.
Learning Deficits
Similar to laboratory animals, humans also show alcohol-related deficits in EBC. Impaired standard delay eyeblink conditioning (CS and US co-terminate) was seen in amnesic Korsakoff patients and recovered, uncomplicated AUDs (72). These findings were extended to more complex conditioning protocols. During temporal discrimination, in which two distinct CSs with two different interstimulus intervals (ISI) were presented, AUDs’ peak CR latency at the long ISI was significantly shorter relative to healthy controls, demonstrating a deficit in adaptive CR timing (73). Trace conditioning is a procedure that incorporates a stimulus free period between offset of the CS and onset of the US. Naive AUDs showed learning deficits in trace conditioning, whereas AUDs previously trained in delay conditioning showed comparable trace conditioning to naive control subjects (74). AUDs who were successful at learning a delay discrimination protocol (i.e., learn that one CS predicts the US, whereas another CS predicts its absence) were impaired when the contingencies were reversed, suggesting an inability to learn new adaptive associations (75).
Similar to adults, children with FASD demonstrate remarkably consistent conditioning deficits. In a cross-sectional study comparing children with FASD, attention deficit hyperactive disorder (ADHD), dyslexia, and healthy controls, the children with FASD and dyslexia showed conditioning impairments relative to the healthy children and different patterns than those seen in children with ADHD (76). In the first prospective longitudinal study on EBC in children with FASD, Jacobson et al. (77) extended these findings by presenting additional trials (up to 150 trials) to 5-year-old children diagnosed with FAS, PFAS, heavily exposed non-syndromal (HE) children, and controls. Despite the additional training opportunity, none of the children with FAS met criterion for conditioning, whereas 75% of the controls did (77). In another cohort of school-aged children, 66.7% of the children with FAS failed to meet criterion on the delay task, and only 16.7% of the FAS and 21.4% of HE group met criterion for trace conditioning in comparison to 66.7% of healthy controls (78). Odds ratio data from a logistic regression analysis showed that the children with FAS were 7.7 times more likely to fail to meet criterion on the delay task compared with controls and 10.0 times more likely on the trace conditioning task. Similarly, the HE group was 5.1 times more likely to fail to meet criterion on delay and 7.3 times more likely on trace. In both the 5-year and school-age studies, IQ did not differentiate the children who reached criterion on delay and trace EBC from those who failed, indicating that it could not be a mediator of the effect of fetal alcohol exposure on performance on either EBC task; nor was ADHD responsible for the observed alcohol-related pattern of EBC impairment seen in the two cohorts. Collectively, these findings strongly support the view that prenatal alcohol exposure has deleterious effects on children’s ability to demonstrate successful EBC and thus has the potential to serve as a biobehavioral marker of prenatal alcohol impairment as well as a useful tool to assess the efficacy of an intervention (79).
Discussion
The damaging effects of alcoholism on the cerebellum and EBC have been well-documented in animal and human investigations. This mini-review summarizes some exemplary laboratory animal and human studies (see Table 1). Chronic, excessive alcohol consumption leads to neuroanatomical alterations in the adult and/or fetal cerebellum, including neuronal loss and white matter degradation. Alcohol exposure also triggers abnormal cerebellar activity as shown through electrophysiology and neuroimaging methodologies. The combination of these effects likely underlies the conditioning deficits seen by these two populations.
Table 1.
Effects of alcohol on cerebellar structure, function, and eyeblink conditioning reported in the literature.
| Animals |
Humans |
|||
|---|---|---|---|---|
| Reference | Comments | Reference | Comments | |
| Structural alterations | (32) | Purkinje and granule cell loss (D) | (9) | Purkinje cell volume loss (M) |
| (36) | Purkinje and deep cerebellar nuclear cell loss (D) | (8) | Cerebellar volume loss (D) | |
| (30) | Purkinje cell loss (lobules I–V, IX, and X) (D) | (63) | Hypoplasia of cerebellar vermis (D) | |
| (34, 35) | Deep cerebellar nuclear cell loss (D) | (54) | Cerebellar gray matter loss correlated with neuropsych. tests (M) | |
| (33) | Purkinje and granule cell loss (postnatal days 4–5) (D) | (55) | Diminished white matter fiber (M) | |
| (21) | Purkinje and granule cell loss (M) | (59) | Cerebellar dysgenesis in 10 of 16 FAS autopsies (D) | |
| (27) | Dendritic microtubules loss (M) | (58) | Cerebellar reduction and disorganization (D) | |
| (24) | Longer terminal dendritic segments in Purkinje cells (M) | (66) | Differences in cerebellar neurochemisiry (D) | |
| (25) | Deep cerebellar nuclear axon terminal degeneration (M) | (65) | Cerebellar peduncles damage (D) | |
| (18) | Granule cell loss (M) | (60, 61) | Reductions in cerebellar cranial vault and volume (D) | |
| (19, 22) | Longer and reduced Purkirje dendritic spines (M) | (57) | Cell loss in cerebellar vermis (M) | |
| (23) | Increased climbing fibers (M) | (62) | Cerebellar vermis volume reduction (D) | |
| (20) | Purkinje and granule cell loss (M) | (64) | Cerebellar peduncles damage (D) | |
| (26) | Fewer synapses between parallel fibers and Purkinje cells (M) | (53) | Cerebellar vermis gray matter deficits (M) | |
| (31) | Purkinje cell loss (postnatal days 4–5) (D) | (56) | Reduced Purkinje cell density in the vermis (M) | |
| Functional differences | (41) | No single-unit activity changes in cerebellar deep nuclei (D) | (69) | Greater fMRI activity in cerebellar vermis (M) |
| (40) | Greater inhibitory inputs to Purkinje cells (D) | (68) | Greater fMRI responses in lobule VI (M) | |
| (42) | Slower increases in deep nuclear activity (D) | (70) | Greater cerebellar fMRI activation (D) | |
| (37) | Purkinje cell firing differences (M) | (71) | Greater crus I and vermis IV–V activation (D) | |
| (38) | Purkinje cell firing differences (D) | (67) | More extensive cerebellar fMRI activation (M) | |
| Learning deficits | (44) | Impaired EBC discrimination learning (D) | (76) | Impaired delay EBC (D) |
| (34, 35, 47) | Impaired delay EBC (D) | (75) | Impaired EBC discrimination and reversal learning (M) | |
| (45) | Impaired trace EBC (D) | (77, 78) | Impaired delay and trace EBC (D) | |
| (43) | Impaired delay EBC (D) | (74) | Impaired trace EBC (M) | |
| (72, 73) | Impaired delay and temporal EBC discrimination (M) | |||
A summary of animal and human work investigating how excessive alcohol consumption affects the cerebellum and eyeblink conditioning. M and D indicate effects on the mature and developing cerebellum, respectively.
One limitation in this field of study is that alcohol affects multiple regions of the brain outside the cerebellum. Affected and connected areas may exert influences on cerebellar structures, making results difficult to interpret. Future work should consider the cerebellum as part of a larger network. This fundamental associative learning task is clinically relevant because it represents a foundation on which more complex learning is built. Studies of environmental exposures, such as alcohol, on EBC have the potential to provide new information about the EBC neural circuitry and behavioral performance and to elucidate vulnerable neural structures that are uniquely recruited during basic learning processes. A comparison of EBC and neuroimaging findings between adults with AUD and children with FASD to determine common neuroanatomical targets of alcohol abuse is an important goal. Moreover, EBC has the potential to identify impairment related to different exposures and in different pediatric and adult disorders, such as ADHD, schizophrenia, FASD, and AUD. This work could lead to assessment of degree of behavioral and cerebellar impairment in AUD and aid in early identification of fetal alcohol-affected children as well as assessment of efficacy of new interventions and treatments. Future interventions could involve the use of neuromodulatory tools, such as transcranial magnetic stimulation and transcranial direct current stimulation, as a way to alter brain activation in an effort to improve learning in AUD and FASD individuals. Finally, this learning model could also be used to identify at-risk individuals, thereby leading to effective prevention strategies.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by grants from the NIH/National Institute on Alcohol Abuse and Alcoholism (NIAAA; K01AA020873 to DC, R01AA018694 to JD, two supplements to RO1AA09524 to SJ, R01AA016781 to SJ, U01 AA014790 to SJ), and the Joseph Young, Sr., Fund, State of MI (to SJ and JJ).
References
- 1.World Health Organization. Global Status Report on Alcohol and Health [Online]. Geneva: WHO Press; (2014). [Google Scholar]
- 2.Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, et al. Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res (2003) 27:301–9. 10.1097/01.ALC.0000052584.05305.98 [DOI] [PubMed] [Google Scholar]
- 3.Norman AL, Crocker N, Mattson SN, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Dev Disabil Res Rev (2009) 15:209–17. 10.1002/ddrr.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) (2005) 180:583–94. 10.1007/s00213-005-2267-6 [DOI] [PubMed] [Google Scholar]
- 5.Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev (2011) 21:102–18. 10.1007/s11065-011-9163-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meintjes EM, Narr KL, Der Kouwe AJ, Molteno CD, Pirnia T, Gutman B, et al. A tensor-based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure. Neuroimage Clin (2014) 5:152–60. 10.1016/j.nicl.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem (2003) 10:427–55. 10.1101/lm.59603 [DOI] [PubMed] [Google Scholar]
- 8.Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol (2001) 43:148–54. 10.1017/S0012162201000299 [DOI] [PubMed] [Google Scholar]
- 9.Andersen BB. Reduction of Purkinje cell volume in cerebellum of alcoholics. Brain Res (2004) 1007:10–8. 10.1016/j.brainres.2004.01.058 [DOI] [PubMed] [Google Scholar]
- 10.Gerwig M, Dimitrova A, Kolb FP, Maschke M, Brol B, Kunnel A, et al. Comparison of eyeblink conditioning in patients with superior and posterior inferior cerebellar lesions. Brain (2003) 126:71–94. 10.1093/brain/awg011 [DOI] [PubMed] [Google Scholar]
- 11.Nolan BC, Freeman JH. Purkinje cell loss by OX7-saporin impairs acquisition and extinction of eyeblink conditioning. Learn Mem (2006) 13:359–65. 10.1101/lm.168506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavond DG, Lincoln JS, Mccormick DA, Thompson RF. Effect of bilateral lesions of the dentate and interpositus cerebellar nuclei on conditioning of heart-rate and nictitating membrane/eyelid responses in the rabbit. Brain Res (1984) 305:323–30. 10.1016/0006-8993(84)90438-4 [DOI] [PubMed] [Google Scholar]
- 13.Logan CG, Grafton ST. Functional anatomy of human eyeblink conditioning determined with regional cerebral glucose metabolism and positron-emission tomography. Proc Natl Acad Sci U S A (1995) 92:7500–4. 10.1073/pnas.92.16.7500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbert JS, Eckerman CO, Stanton ME. The ontogeny of human learning in delay, long-delay, and trace eyeblink conditioning. Behav Neurosci (2003) 117:1196–210. 10.1037/0735-7044.117.6.1196 [DOI] [PubMed] [Google Scholar]
- 15.Schmajuk NA, DiCarlo JJ. A neural network approach to hippocampal function in classical conditioning. Behav Neurosci (1991) 105:82–110. 10.1037/0735-7044.105.1.82 [DOI] [PubMed] [Google Scholar]
- 16.Stanton ME. Multiple memory systems, development and conditioning. Behav Brain Res (2000) 110:25–37. 10.1016/S0166-4328(99)00182-5 [DOI] [PubMed] [Google Scholar]
- 17.Jaatinen P, Rintala J. Mechanisms of ethanol-induced degeneration in the developing, mature, and aging cerebellum. Cerebellum (2008) 7:332–47. 10.1007/s12311-008-0034-z [DOI] [PubMed] [Google Scholar]
- 18.Tavares MA, Paula-Barbosa MM. Alcohol-induced granule cell loss in the cerebellar cortex of the adult rat. Exp Neurol (1982) 78:574–82. 10.1016/0014-4886(82)90075-9 [DOI] [PubMed] [Google Scholar]
- 19.Tavares MA, Paula-Barbosa MM, Gray EG. A morphometric Golgi analysis of the Purkinje cell dendritic tree after long-term alcohol consumption in the adult rat. J Neurocytol (1983) 12:939–48. 10.1007/BF01153343 [DOI] [PubMed] [Google Scholar]
- 20.Tavares MA, Paula-Barbosa MM, Cadete-Leite A. Chronic alcohol consumption reduces the cortical layer volumes and the number of neurons of the rat cerebellar cortex. Alcohol Clin Exp Res (1987) 11:315–9. 10.1111/j.1530-0277.1987.tb01315.x [DOI] [PubMed] [Google Scholar]
- 21.Oliveira SA, Chuffa LG, Fioruci-Fontanelli BA, Lizarte Neto FS, Novais PC, Tirapelli LF, et al. Apoptosis of Purkinje and granular cells of the cerebellum following chronic ethanol intake. Cerebellum (2014) 13:728–38. 10.1007/s12311-014-0591-2 [DOI] [PubMed] [Google Scholar]
- 22.Tavares MA, Paula-Barbosa MM, Gray EG. Dendritic spine plasticity and chronic alcoholism in rats. Neurosci Lett (1983) 42:235–8. 10.1016/0304-3940(83)90267-7 [DOI] [PubMed] [Google Scholar]
- 23.Tavares MA, Paula-Barbosa MM, Cadete-Leite A. Morphological evidence of climbing fiber plasticity after long-term alcohol intake. Neurobehav Toxicol Teratol (1986) 8:481–5. [PubMed] [Google Scholar]
- 24.Pentney RJ, Dlugos CA. Cerebellar Purkinje neurons with altered terminal dendritic segments are present in all lobules of the cerebellar vermis of ageing, ethanol-treated F344 rats. Alcohol Alcohol (2000) 35:35–43. 10.1093/alcalc/35.1.35 [DOI] [PubMed] [Google Scholar]
- 25.Phillips SC. Neuro-toxic interaction in alcohol-treated, thiamine-deficient mice. Acta Neuropathol (1987) 73:171–6. 10.1007/BF00693784 [DOI] [PubMed] [Google Scholar]
- 26.Tavares MA, Paula-Barbosa MM, Verwer RW. Synapses of the cerebellar cortex molecular layer after chronic alcohol consumption. Alcohol (1987) 4:109–16. 10.1016/0741-8329(87)90007-3 [DOI] [PubMed] [Google Scholar]
- 27.Paula-Barbosa MM, Tavares MA. Long term alcohol consumption induces microtubular changes in the adult rat cerebellar cortex. Brain Res (1985) 339:195–9. 10.1016/0006-8993(85)90645-6 [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, Fleming RL, Morrow AL. Ethanol regulation of gamma-aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol Ther (2004) 101:211–26. 10.1016/j.pharmthera.2003.12.001 [DOI] [PubMed] [Google Scholar]
- 29.Jung ME. Alcohol withdrawal and cerebellar mitochondria. Cerebellum (2015) 14:421–37. 10.1007/s12311-014-0598-8 [DOI] [PubMed] [Google Scholar]
- 30.Goodlett CR, Marcussen BL, West JR. A single day of alcohol exposure during the brain growth spurt induces brain weight restriction and cerebellar Purkinje cell loss. Alcohol (1990) 7:107–14. 10.1016/0741-8329(90)90070-S [DOI] [PubMed] [Google Scholar]
- 31.Thomas JD, Goodlett CR, West JR. Alcohol-induced Purkinje cell loss depends on developmental timing of alcohol exposure and correlates with motor performance. Brain Res Dev Brain Res (1998) 105:159–66. 10.1016/S0165-3806(97)00164-8 [DOI] [PubMed] [Google Scholar]
- 32.Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res (1990) 14:107–18. 10.1111/j.1530-0277.1990.tb00455.x [DOI] [PubMed] [Google Scholar]
- 33.Hamre KM, West JR. The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles. Alcohol Clin Exp Res (1993) 17:610–22. 10.1111/j.1530-0277.1993.tb00808.x [DOI] [PubMed] [Google Scholar]
- 34.Green JT, Tran T, Steinmetz JE, Goodlett CR. Neonatal ethanol produces cerebellar deep nuclear cell loss and correlated disruption of eyeblink conditioning in adult rats. Brain Res (2002) 956:302–11. 10.1016/S0006-8993(02)03561-8 [DOI] [PubMed] [Google Scholar]
- 35.Green JT, Arenos JD, Dillon CJ. The effects of moderate neonatal ethanol exposure on eyeblink conditioning and deep cerebellar nuclei neuron numbers in the rat. Alcohol (2006) 39:135–50. 10.1016/j.alcohol.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 36.Dikranian K, Qin YQ, Labruyere J, Nemmers B, Olney JW. Ethanol-induced neuroapoptosis in the developing rodent cerebellum and related brain stem structures. Brain Res Dev Brain Res (2005) 155:1–13. 10.1016/j.devbrainres.2004.11.005 [DOI] [PubMed] [Google Scholar]
- 37.Servais L, Bearzatto B, Delvaux V, Noel E, Leach R, Brasseur M, et al. Effect of chronic ethanol ingestion on Purkinje and Golgi cell firing in vivo and on motor coordination in mice. Brain Res (2005) 1055:171–9. 10.1016/j.brainres.2005.07.026 [DOI] [PubMed] [Google Scholar]
- 38.Servais L, Hourez R, Bearzatto B, Gall D, Schiffmann SN, Cheron G. Purkinje cell dysfunction and alteration of long-term synaptic plasticity in fetal alcohol syndrome. Proc Natl Acad Sci U S A (2007) 104:9858–63. 10.1073/pnas.0607037104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koekkoek SK, Hulscher HC, Dortland BR, Hensbroek RA, Elgersma Y, Ruigrok TJ, et al. Cerebellar LTD and learning-dependent timing of conditioned eyelid responses. Science (2003) 301:1736–9. 10.1126/science.1088383 [DOI] [PubMed] [Google Scholar]
- 40.Light KE, Hayar AM, Pierce DR. Electrophysiological and immunohistochemical evidence for an increase in GABAergic inputs and HCN channels in Purkinje cells that survive developmental ethanol exposure. Cerebellum (2015) 14(4):398–412. 10.1007/s12311-015-0651-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green JT, Johnson TB, Goodlett CR, Steinmetz JE. Eyeblink classical conditioning and interpositus nucleus activity are disrupted in adult rats exposed to ethanol as neonates. Learn Mem (2002) 9:304–20. 10.1101/lm.47602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindquist DH, Sokoloff G, Milner E, Steinmetz JE. Neonatal ethanol exposure results in dose-dependent impairments in the acquisition and timing of the conditioned eyeblink response and altered cerebellar interpositus nucleus and hippocampal CA1 unit activity in adult rats. Alcohol (2013) 47:447–57. 10.1016/j.alcohol.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanton ME, Goodlett CR. Neonatal ethanol exposure impairs eyeblink conditioning in weanling rats. Alcohol Clin Exp Res (1998) 22:270–5. 10.1111/j.1530-0277.1998.tb03649.x [DOI] [PubMed] [Google Scholar]
- 44.Brown KL, Calizo LH, Goodlett CR, Stanton ME. Neonatal alcohol exposure impairs acquisition of eyeblink conditioned responses during discrimination learning and reversal in weanling rats. Dev Psychobiol (2007) 49:243–57. 10.1002/dev.20178 [DOI] [PubMed] [Google Scholar]
- 45.Murawski NJ, Jablonski SA, Brown KL, Stanton ME. Effects of neonatal alcohol dose and exposure window on long delay and trace eyeblink conditioning in juvenile rats. Behav Brain Res (2013) 236:307–18. 10.1016/j.bbr.2012.08.025 [DOI] [PubMed] [Google Scholar]
- 46.Brown KL, Calizo LH, Stanton ME. Dose-dependent deficits in dual interstimulus interval classical eyeblink conditioning tasks following neonatal binge alcohol exposure in rats. Alcohol Clin Exp Res (2008) 32:277–93. 10.1111/j.1530-0277.2007.00579.x [DOI] [PubMed] [Google Scholar]
- 47.Green JT, Rogers RF, Goodlett CR, Steinmetz JE. Impairment in eyeblink classical conditioning in adult rats exposed to ethanol as neonates. Alcohol Clin Exp Res (2000) 24:438–47. 10.1111/j.1530-0277.2000.tb02010.x [DOI] [PubMed] [Google Scholar]
- 48.Young BW, Sengelaub DR, Steinmetz JE. MK-801 administration during neonatal ethanol withdrawal attenuates interpositus cell loss and juvenile eyeblink conditioning deficits. Alcohol (2010) 44:359–69. 10.1016/j.alcohol.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas JD, Tran TD. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus (2012) 22:619–30. 10.1002/hipo.20925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamilton GF, Jablonski SA, Schiffino FL, St Cyr SA, Stanton ME, Klintsova AY. Exercise and environment as an intervention for neonatal alcohol effects on hippocampal adult neurogenesis and learning. Neuroscience (2014) 265:274–90. 10.1016/j.neuroscience.2014.01.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tran TD, Jackson HD, Horn KH, Goodlett CR. Vitamin E does not protect against neonatal ethanol-induced cerebellar damage or deficits in eyeblink classical conditioning in rats. Alcohol Clin Exp Res (2005) 29:117–29. 10.1097/01.ALC.0000150004.53870.E1 [DOI] [PubMed] [Google Scholar]
- 52.Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev (2007) 17:239–57. 10.1007/s11065-007-9038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sullivan EV, Deshmukh A, Desmond JE, Mathalon DH, Rosenbloom MJ, Lim KO, et al. Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Arch Gen Psychiatry (2000) 57:894–902. 10.1001/archpsyc.57.9.894 [DOI] [PubMed] [Google Scholar]
- 54.Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology (2007) 32:429–38. 10.1038/sj.npp.1301219 [DOI] [PubMed] [Google Scholar]
- 55.Chanraud S, Reynaud M, Wessa M, Penttila J, Kostogianni N, Cachia A, et al. Diffusion tensor tractography in mesencephalic bundles: relation to mental flexibility in detoxified alcohol-dependent subjects. Neuropsychopharmacology (2009) 34:1223–32. 10.1038/npp.2008.101 [DOI] [PubMed] [Google Scholar]
- 56.Torvik A, Torp S. The prevalence of alcoholic cerebellar atrophy. A morphometric and histological study of an autopsy material. J Neurol Sci (1986) 75:43–51. [DOI] [PubMed] [Google Scholar]
- 57.Phillips SC, Harper CG, Kril J. A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain (1987) 110(Pt 2):301–14. 10.1093/brain/110.2.301 [DOI] [PubMed] [Google Scholar]
- 58.Clarren SK, Alvord EC, Jr, Sumi SM, Streissguth AP, Smith DW. Brain malformations related to prenatal exposure to ethanol. J Pediatr (1978) 92:64–7. 10.1016/S0022-3476(78)80072-9 [DOI] [PubMed] [Google Scholar]
- 59.Clarren SK. Neuropathology and fetal alcohol syndrome. In: West JR, editor. Alcohol and Brain Development. New York, NY: Oxford University Press; (1986). p. 158–66. [Google Scholar]
- 60.Mattson SN, Riley EP, Jernigan TL, Ehlers CL, Delis DC, Jones KL, et al. Fetal alcohol syndrome: a case report of neuropsychological, MRI and EEG assessment of two children. Alcohol Clin Exp Res (1992) 16:1001–3. 10.1111/j.1530-0277.1992.tb01909.x [DOI] [PubMed] [Google Scholar]
- 61.Mattson SN, Riley EP, Jernigan TL, Garcia A, Kaneko WM, Ehlers CL, et al. A decrease in the size of the basal ganglia following prenatal alcohol exposure: a preliminary report. Neurotoxicol Teratol (1994) 16:283–9. 10.1016/0892-0362(94)90050-7 [DOI] [PubMed] [Google Scholar]
- 62.Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: size reduction in lobules I-V. Alcohol Clin Exp Res (1996) 20:31–4. 10.1111/j.1530-0277.1996.tb01039.x [DOI] [PubMed] [Google Scholar]
- 63.Autti-Ramo I, Autti T, Korkman M, Kettunen S, Salonen O, Valanne L. MRI findings in children with school problems who had been exposed prenatally to alcohol. Dev Med Child Neurol (2002) 44:98–106. 10.1111/j.1469-8749.2002.tb00294.x [DOI] [PubMed] [Google Scholar]
- 64.Spottiswoode BS, Meintjes EM, Anderson AW, Molteno CD, Stanton ME, Dodge NC, et al. Diffusion tensor imaging of the cerebellum and eyeblink conditioning in fetal alcohol spectrum disorder. Alcohol Clin Exp Res (2011) 35:2174–83. 10.1111/j.1530-0277.2011.01566.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan J, Meintjes EM, Molteno CD, Spottiswoode BS, Dodge NC, Alhamud AA, et al. White matter integrity of the cerebellar peduncles as a mediator of effects of prenatal alcohol exposure on eyeblink conditioning. Hum Brain Mapp (2015) 36(7):2470–82. 10.1002/hbm.22785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du Plessis L, Jacobson JL, Jacobson SW, Hess AT, Van Der Kouwe A, Avison MJ, et al. An in vivo (1)H magnetic resonance spectroscopy study of the deep cerebellar nuclei in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res (2014) 38:1330–8. 10.1111/acer.12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parks MH, Morgan VL, Pickens DR, Price RR, Dietrich MS, Nickel MK, et al. Brain fMRI activation associated with self-paced finger tapping in chronic alcohol-dependent patients. Alcohol Clin Exp Res (2003) 27:704–11. 10.1111/j.1530-0277.2003.tb04408.x [DOI] [PubMed] [Google Scholar]
- 68.Desmond JE, Chen SH, Derosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage (2003) 19:1510–20. 10.1016/S1053-8119(03)00102-2 [DOI] [PubMed] [Google Scholar]
- 69.Chanraud-Guillermo S, Andoh J, Martelli C, Artiges E, Pallier C, Aubin HJ, et al. Imaging of language-related brain regions in detoxified alcoholics. Alcohol Clin Exp Res (2009) 33:977–84. 10.1111/j.1530-0277.2009.00918.x [DOI] [PubMed] [Google Scholar]
- 70.Diwadkar VA, Meintjes EM, Goradia D, Dodge NC, Warton C, Molteno CD, et al. Differences in cortico-striatal-cerebellar activation during working memory in syndromal and nonsyndromal children with prenatal alcohol exposure. Hum Brain Mapp (2013) 34:1931–45. 10.1002/hbm.22042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du Plessis L, Jacobson SW, Molteno CD, Robertson FC, Peterson BS, Jacobson JL, et al. Neural correlates of cerebellar-mediated timing during finger tapping in children with fetal alcohol spectrum disorders. Neuroimage Clin (2015) 7:562–70. 10.1016/j.nicl.2014.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGlinchey-Berroth R, Cermak LS, Carrillo MC, Armfield S, Gabrieli JD, Disterhoft JF. Impaired delay eyeblink conditioning in amnesic Korsakoff’s patients and recovered alcoholics. Alcohol Clin Exp Res (1995) 19:1127–32. 10.1111/j.1530-0277.1995.tb01590.x [DOI] [PubMed] [Google Scholar]
- 73.McGlinchey-Berroth R, Fortier CB, Cermak LS, Disterhoft JF. Temporal discrimination learning in abstinent chronic alcoholics. Alcohol Clin Exp Res (2002) 26:804–11. 10.1111/j.1530-0277.2002.tb02608.x [DOI] [PubMed] [Google Scholar]
- 74.McGlinchey RE, Fortier CB, Capozzi SM, Disterhoft JF. Trace eyeblink conditioning in abstinent alcoholic individuals: effects of complex task demands and prior conditioning. Neuropsychology (2005) 19:159–70. 10.1037/0894-4105.19.2.159 [DOI] [PubMed] [Google Scholar]
- 75.Fortier CB, Steffen EM, Lafleche G, Venne JR, Disterhoft JF, Mcglinchey RE. Delay discrimination and reversal eyeblink classical conditioning in abstinent chronic alcoholics. Neuropsychology (2008) 22:196–208. 10.1037/0894-4105.22.2.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coffin JM, Baroody S, Schneider K, O’Neill J. Impaired cerebellar learning in children with prenatal alcohol exposure: a comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex (2005) 41:389–98. 10.1016/S0010-9452(08)70275-2 [DOI] [PubMed] [Google Scholar]
- 77.Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, et al. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res (2008) 32:365–72. 10.1111/j.1530-0277.2007.00585.x [DOI] [PubMed] [Google Scholar]
- 78.Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, et al. Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol Clin Exp Res (2011) 35:250–64. 10.1111/j.1530-0277.2010.01341.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jacobson SW, Jacobson JL, Stanton ME, Meintjes EM, Molteno CD. Biobehavioral markers of adverse effect in fetal alcohol spectrum disorders. Neuropsychol Rev (2011) 21:148–66. 10.1007/s11065-011-9169-7 [DOI] [PMC free article] [PubMed] [Google Scholar]