Abstract
BACKGROUND
High dietary intake of soy or selenium (Se) is associated with decreased risk of prostate cancer. Soy constituents and various chemical forms of Se have each been shown to downregulate expression of the androgen receptor (AR) and AR-regulated genes in the prostate. We hypothesized that downregulation of AR and AR-regulated genes by the combination of these dietary components would inhibit tumorigenesis in the TRansgenic Adenocarcinoma of Mouse Prostate (TRAMP) mouse.
METHODS
Male mice were exposed from conception to stock diets high or low in soy, with or without a supplement of Se-methylseleno-L-cysteine (MSC) in a 2 X 2 factorial design. Mice were sacrificed at 18 weeks. Prostate histopathology, urogenital tract (UGT) weight, hepatic activity of androgen-metabolizing enzymes, and expression of AR, AR-regulated, and AR-associated FOX family genes, in the dorsolateral prostate were examined.
RESULTS
High soy intake decreased activity of hepatic aromatase and 5α-reductase, expression of AR, AR-regulated genes, FOXA1, UGT weight, and tumor progression, and upregulated protective FOXO3. Supplemental MSC upregulated AKR1C14, which reduces 5α-dihydrotestosterone.
CONCLUSIONS
Soy is an effective pleiotropic dietary agent for prevention of prostate cancer. The finding of effects of soy on FOX family gene expression in animals is novel. Combination effects of supplemental MSC may depend upon the soy content of the basal diet to which it is added.
Keywords: methylselenocysteine, soy, androgen receptor, FOXO, TRAMP
INTRODUCTION
Prostate cancer is the most commonly diagnosed non-skin cancer and the second leading cause of cancer death among men in the United States (1). Its slow growth and long latency make prostate cancer an ideal candidate for chemoprevention by dietary or other means (2). Numerous dietary components, exerting their effects through multiple mechanisms, have shown promise of protection in epidemiological and preclinical studies (3), but when tested as single agents in randomized controlled trials (RCTs) few have proven beneficial (4).
Recent studies show that combinations of nutrients or phytochemicals can produce greater benefits than those food components used individually (5). In their recent update of the hallmarks of cancer, Hanahan and Weinberg noted the potential for cotargeting multiple molecules and processes in “mechanism-guided” combinations to achieve greater efficacy than targeting individual molecules or pathways with single agents (6).
High dietary intake, blood levels, or toenail concentrations of the essential trace element selenium (Se) have been correlated in preclinical models, in epidemiological studies, and in case-control studies with reduced risk of prostate cancer (7). Monomethylated forms of Se have been shown to decrease expression of the androgen receptor (AR), inhibit angiogenesis, induce apoptosis and affect multiple additional processes and pathways in ways that inhibit prostate cancer cell proliferation, tumor development and progression (8). The two randomized controlled trials of Se supplementation for prostate cancer risk reduction produced contrasting results. The Nutritional Prevention of Cancer Trial (NPCT) (9) showed significant chemopreventive efficacy of selenized yeast, containing a mixture of different forms of Se, in men in the lowest two tertiles of baseline plasma Se, with no exclusion of subjects based on PSA levels. In contrast, the failure of the Selenium and Vitamin E Cancer Prevention Trial (SELECT) (10) to show a protective effect of Se supplementation is likely due to the use of an ineffective, single chemical form of Se (selenomethionine), in men of high baseline Se status, selected for low baseline PSA values.
High intake of soy or soy components has also been associated with reduced prostate cancer risk in animals and in human studies (11). Some of the mechanisms by which soy compounds exert their protective effects are reported to be the same as those for Se e.g. reduction of AR expression (12). In addition, Se and soy may modify separate processes in ways that can potentiate the other’s effects. For example, genistein, a soy isoflavone, has been reported to reduce the expression of survivin (13). In a separate report, knockdown of survivin was shown to enhance the growth-inhibitory effects of Se in prostate cancer cell lines (14). Thus, genistein may enhance Se’s prostate cancer cell-killing by reduction of survivin. This is but one example of “mechanism-guided” combinations that merit further investigation.
We have previously shown changes, consistent with chemoprevention, in healthy rat prostates through the combined effects of a high dietary intake of Se and soy (15). In this study we sought to test the same diets for their effects on prostate cancer development and progression in the Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) model.
MATERIALS AND METHODS
Animals and Housing
All procedures related to animal care and use were approved by the Institutional Animal Care and Use Committee of Brigham Young University.
Male and female hemizygous C57BL/6 transgenic TRAMP mice (16), and male and female wild type FVB mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Hemizygous TRAMP males were bred with hemizygous TRAMP females to generate homozygous C57BL/6 TRAMP mice. Homozygous TRAMP males and females were then used as breeders, and mated with FVB mice to produce hemizygous C57BL/6 x FVB F1 TRAMP male pups that were used as subjects in this study. Producing C57BL/6 x FVB F1 TRAMP male pups by mating homozygous, rather than hemizygous, TRAMP breeders with wild type FBV mice obviated the need for PCR genotyping of all offspring, since all pups resulting from homozygous TRAMP X FVB crosses would be hemizygous. This also expedited the process of producing the needed number of animals, since all male pups produced, rather than only half, could be used as subjects. Dams were fed one of the four experimental diets (described below) for at least 30 d before mating to ensure that F1 animals used as subjects in this study would be exposed to their respective dietary treatments from conception (15). Dams continued consuming their respective diets through pregnancy and lactation. TRAMP male pups were weaned at 21 d to the diets consumed by their dams.
Diets
Mice were fed one of two pelleted basal stock diets, with or without a supplement of Se-methylseleno-L-cysteine (MSC), in a 2 X 2 factorial design. The first was a low-isoflavone (LIF) formulation containing no soy or alfalfa (Phytoestrogen Reduced Rodent Diet I, Zeigler Bros., Inc., Gardners, PA). The second stock diet was the high-isoflavone (HIF) 8604 formulation of Harlan Teklad (Madison, WI). All nutrients were provided in both diets at levels that met or exceeded the minimum recommendations of the AIN (17). Basal diets reportedly contained 0.34–0.45 mg Se/kg diet, which is higher than the minimum concentration (0.15 mg Se/kg diet) recommended by the AIN to maximize selenoprotein expression (17). Slight differences in Se concentration from batch to batch of both diets were due to minor variation of Se content in different lots of feedstuffs used to formulate the basal diets. Half of the diets were supplemented with 3.0 mg Se/kg diet as MSC (Product # BISEMC0501, Se-methylselenocysteine trituration 0.5%, Kelatron Corporation, Ogden, UT). A detailed analysis of these diets is included in our recent report (18).
Study Design
Mice were killed at 18 weeks of age by decapitation. Urogenital tracts (UGTs), including bladder, seminal vesicles, prostate lobes, and a portion of the urethra were removed and weighed after which the dorsolateral prostate (DLP) was dissected and snap-frozen in liquid nitrogen for RNA/protein isolation or fixed in 4%-paraformaldehyde in PBS for 24 hours prior to histological processing. Livers were dissected then frozen in liquid nitrogen. Frozen samples were stored at −80°C until analyzed.
Enzyme Assays
Activity of hepatic cytosolic Se-dependent glutathione peroxidase 1 (EC 1.11.1.9; GPX1) and of glutathione S-transferase (EC 2.5.1.18; GST) was assayed to determine Se status. Mouse liver GPX1 activity was assayed by the coupled method of Lawrence and Burk, as we have previously reported (19) using 2.0 mmol/L reduced glutathione, and 0.25 mmol/L H2O2 as substrate. Cytosolic GST activity was measured by the method of Habig et al. using as substrate 1-chloro-2,4-dinitrobenzene as in our previous work (20). Other aliquots of mouse liver (5–10 mg of dissected tissue) were used for assay of aromatase and 5alpha-reductase activity, as described in our earlier studies (21).
Gene Expression
As in our previous work (15), we examined expression of genes identified in the data mining analysis of Zhang et al. (22) that met the following criteria: 1) dysregulated in human prostate cancer; 2) androgen-regulated; and 3) the androgen effect is opposed by Se. These included DHCR24 (24-dehydrocholesterol reductase), ACSL3 (acyl-CoA synthetase 3), and GUCY1A3 (guanylate cyclase alpha 3). In addition, we examined levels of AR itself and of AKR1C14, the enzyme which catalyzes reduction of 5α-dihydrotestosterone (DHT) to its corresponding, less potent 5α-androstane-3α, 17β-diol (commonly referred to as 3α-diol). We also examined expression of Forkhead box family proteins (FOXA1 and FOXO3) which were highlighted in the work of Zhang et al.
For immunoblots, equal quantities of protein from each lysate of individual DLPs were pooled (N =4 or 5 per dietary group). Fifty (50) μg of protein per pool were denatured in lithium dodecyl sulfate buffer (Bio-Rad, Hercules, CA) and subjected to electrophoresis on 4–12% Bis-Tris gels (Invitrogen Corp., Carlsbad, CA). Separated proteins were transferred onto nitrocellulose membranes (Bio-Rad) followed by blocking with 5% non-fat milk powder (w/v) in PBST (1 X PBS, 2% Tween 20). The membranes were probed with primary antibodies for AR, GUCY1A3, ACSL3, FOXA1, FOXO3, (Abcam, Cambridge, MA), DHCR24, and AKR1C14 (Santa Cruz Biotechnology Inc., Santa Cruz, CA), followed by probing with appropriate peroxidase-conjugated secondary antibodies (Abcam) and detection by enhanced chemiluminescence (Thermo Scientific, Rockford. IL). Densitometric analysis of bands on autoradiographs was done using ImageJ software (National Institutes of Health, Bethesda, MD).
Histopathology
Prostate tissues were processed and stained with hematoxylin and eosin (H&E) for histopathologic evaluation. Sections stained with H & E were examined by a board-certified pathologist (PU) and by a trained associate (TQ), who were blinded to the dietary treatments, and classified according to the grading scheme developed by Suttie et al. (23).
Statistical analysis
Statistical analysis of enzyme assays and protein levels was performed by Two Way Analysis of Variance (ANOVA) followed by Bonferroni’s pairwise comparisons (Systat Software, Inc., San Jose, CA). Due to the non-normal distribution of the UGT weights for each diet group ANOVA was not appropriate. Instead the Kruskal-Wallis test which uses rank order of samples to find statistical differences in non-normally distributed data sets was used. Pairwise comparisons were made using Dunn’s Test at P < 0.05 (Systat Software, Inc., San Jose, CA). Chi-squared analysis was used to test for differences between groups for the categorical data from histopathology scoring (Systat Software, Inc., San Jose, CA).
RESULTS
In our previous paper detailing the use of these diets in mice (18), measurements of food intake for the three days immediately preceding sacrifice showed no significant differences in food consumption among groups. Likewise, in this study we found no differences among groups in consumption of diet.
Enzyme Activity
There were no significant differences due to diet in the activity of hepatic GPX1 (main effect of MSC, P = 0.333; main effect of soy, P = 0.183; interaction, P = 0.089). This was expected as all the diets provided a concentration of Se higher than needed to maximize the activity of GPX1 in mouse liver. Likewise, activity of GST was unaffected by supplemental MSC, phytoestrogens, or the combination in TRAMP mice (main effect of MSC, P = 0.316; main effect of soy, P = 0.143; interaction, P = 0.211).
Activity of hepatic aromatase was significantly lower in mice fed HIF diets (main effect, P = 0.016; Fig. 1A). Main effects of MSC supplementation (P = 0.185) and of the interaction (P = 0.751) were not statistically significant. A similar pattern was observed for the effects of diet on 5α-reductase activity. Once again, HIF diets lowered enzyme activity compared to LIF diets (main effect, P = 0.016; Fig. 1B). In the case of 5α-reductase, the interaction between supplemental MSC and soy was also highly significant (P = 0.005). Supplemental MSC reduced enzyme activity in mice fed the LIF basal diet (P = 0.005) but not in animals consuming the HIF formulation (P = 0.195). Mean values for mice in the LIF+Se, HIF, and HIF+Se dietary groups were all significantly less (P < 0.02) than the mean for the LIF group.
Fig. 1.
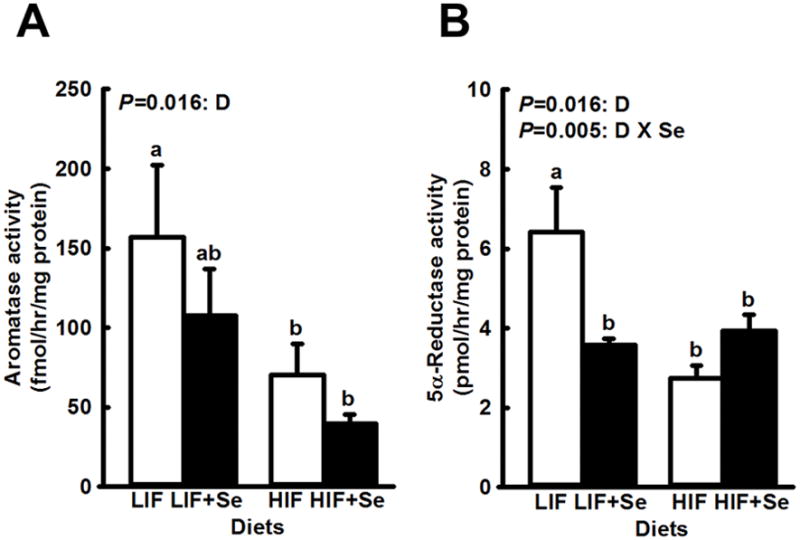
Activity of aromatase (A) and 5α-reductase (B) in livers of TRAMP mice fed diets low (LIF) or high (HIF) in soy, with or without added Se (3 mg/kg diet) as Se-methyl-L-selenocysteine. Bars and error bars show means + SDs (n=5/dietary group). P values show significance of main effects. For aromatase the main effect of diet (D) was statistically significant. For 5α-reductase the main effect of diet (D) and the diet X Se interaction (D x Se) were significant. Bars not sharing a common superscript are significantly different (P < 0.02).
Dorsolateral Prostate Gene Expression
Western blots revealed lower levels of AR protein in DLPs of mice that were fed the HIF diets than those given LIF feed (Fig. 2A). Addition of MSC to LIF diets increase AR protein levels (P = 0.011) while MSC supplementation of HIF diets had no effect.
Fig. 2.
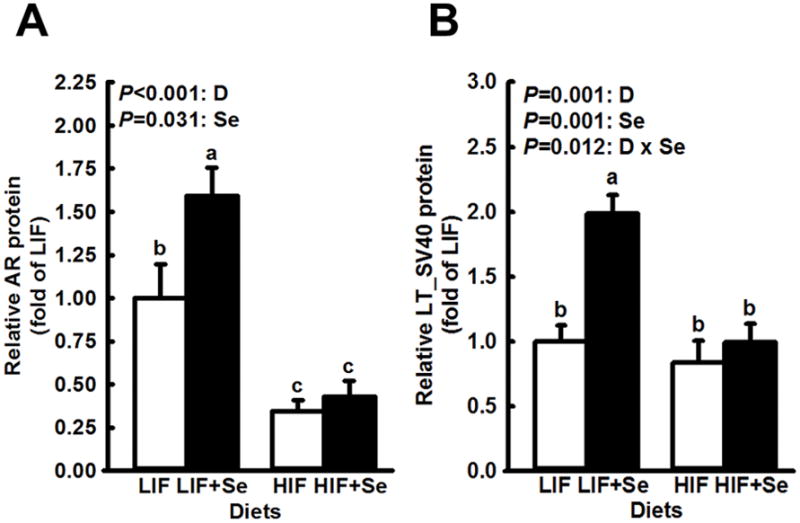
Expression of androgen receptor (AR) and SV40 Large T antigen in dorsolateral prostates of TRAMP mice fed diets low (LIF) or high (HIF) in soy, with or without added Se (3 mg/kg diet) as Se-methyl-L-selenocysteine. Bars and error bars show means + SDs. P values show significance of main effects. For AR (n=4 Western blots) the main effects of diet (D) and supplemental Se (Se) were statistically significant but the interaction of the two was not (P = 0.092). For Large T (n=5), both dietary components and their interaction had significant effects. Bars not sharing a common superscript are significantly different (P < 0.05).
Large T antigen expression, which drives tumorigenesis in this model, is under the control of AR. In this work the pattern of expression of Large T antigen (Fig. 2B) was similar to that of AR, including reduced expression in mice fed HIF diets, and an increased level in mice fed LIF+Se diets (P < 0.001). However, this difference did not correlate, directly or inversely, with differences in any other of the parameters measured, which suggests that variation in Large T antigen expression did not contribute significantly to observed differences in those parameters between dietary groups.
Androgen-regulated genes whose expression was significantly affected by supplemental MSC are shown in Fig. 3. For both GUCY1A3 and AKR1C14 supplemental MSC significantly increased expression (main effects: P = 0.006 and P < 0.001, respectively). Soy intake did not modify levels of these two proteins. In contrast, steady state levels of AR- associated FOXA1protein and AR-regulated DHCR24 (Fig. 4) were affected only by soy intake, which effect in each case was highly significant (P < 0.001). For AR-associated FOXO3 (Fig. 5) high soy consumption was associated with increased expression (P < 0.001), while MSC supplementation had the opposite effect (P = 0.009). Supplemental MSC significantly reduced FOXO3 levels in LIF-fed mice but not in HIF-fed subjects. However, the overall interaction fell short of statistical significance (P = 0.051).
Fig. 3.
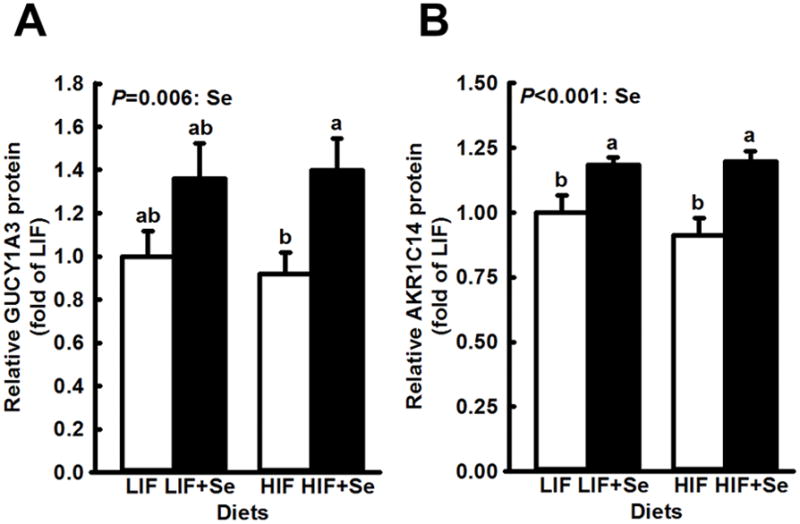
Modification by supplemental Se of expression of AR-regulated genes in dorsolateral prostates of TRAMP mice fed diets low (LIF) or high (HIF) in soy, with or without added Se (3 mg/kg diet) as Se-methyl-L-selenocysteine. Bars and error bars show means + SDs. P values show significance of main effects. Supplemental Se significantly increased expression of GUCY1A3 (n=5) and of AKR1C14 (n=5) when added to each basal diet. Expression was not affected by soy content of the basal diet, nor by the interaction of Se and soy. Bars not sharing a common superscript are significantly different (P < 0.05).
Fig. 4.
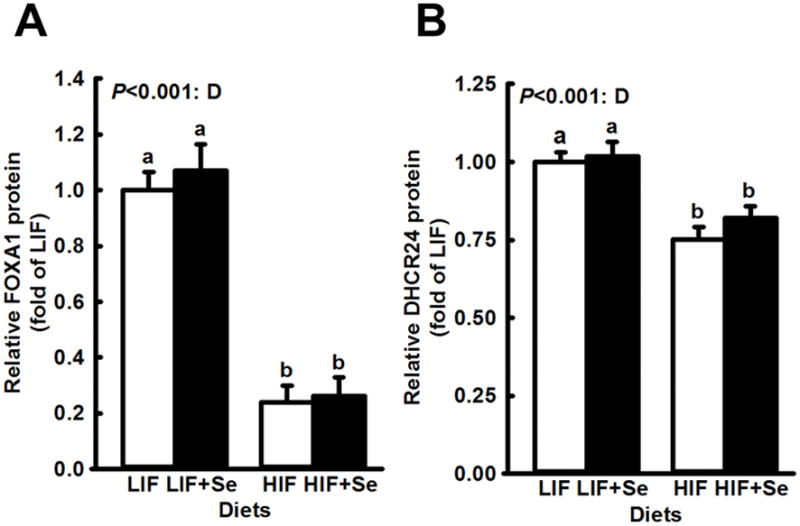
Determination by basal diet soy content of expression of genes in dorsolateral prostates of TRAMP mice fed diets low (LIF) or high (HIF) in soy, with or without added Se (3 mg/kg diet) as Se-methyl-L-selenocysteine. Bars and error bars show means + SDs. P values show significance of main effects. Mice fed HIF diets, with or without added Se, had significantly lower levels of both FOXA1 (n=4) and DHCR24 (n=8). Neither Se supplementation nor the interaction of Se and soy affected expression of these two proteins. Bars not sharing a common superscript are significantly different (P < 0.05).
Fig. 5.
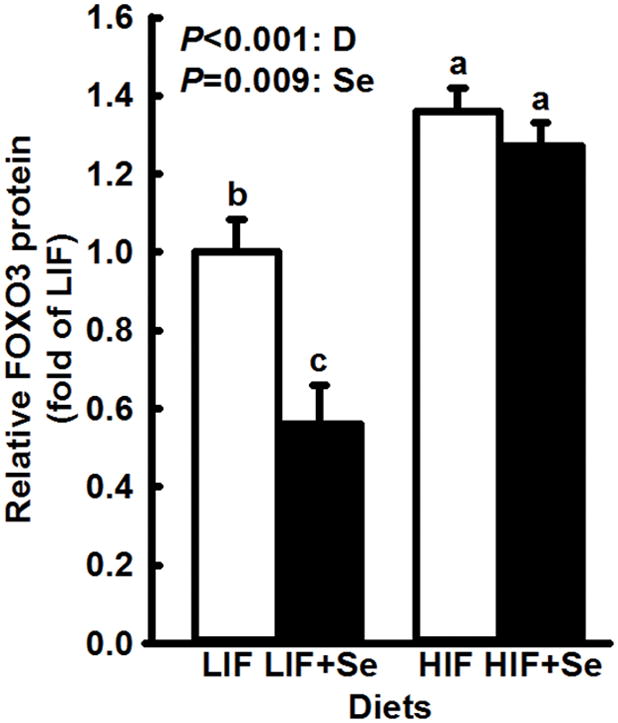
Opposite effects of Se and soy on expression of FOXO3 in dorsolateral prostates of TRAMP mice fed diets low (LIF) or high (HIF) in soy, with or without added Se (3 mg/kg diet) as Se-methyl-L-selenocysteine. Bars and error bars show means + SDs. P values show significance of main effects. Immunoblot analysis (n=3) showed a stimulatory effect (P < 0.001) of high soy intake on expression of FOXO3 while supplemental Se inhibited (P = 0.009) it. Bars not sharing a common superscript are significantly different (P < 0.05).
There were no significant differences in the expression of ACSL3 (data not shown).
Urogenital Tract Weight
The presence of one or more exceptionally large tumors in most groups at 18 weeks of age resulted in a non-normal distribution of UGT weights that could not be evaluated by ANOVA. Instead, the non-parametric, rank-sum based Kruskal-Wallis test was used to analyze differences between groups. This test revealed a significant effect of treatments on UGT weights (P < 0.05; Fig. 6). A post-hoc Dunn’s Test showed a significant difference between median values in the LIF and HIF dietary groups (P < 0.05).
Fig. 6.
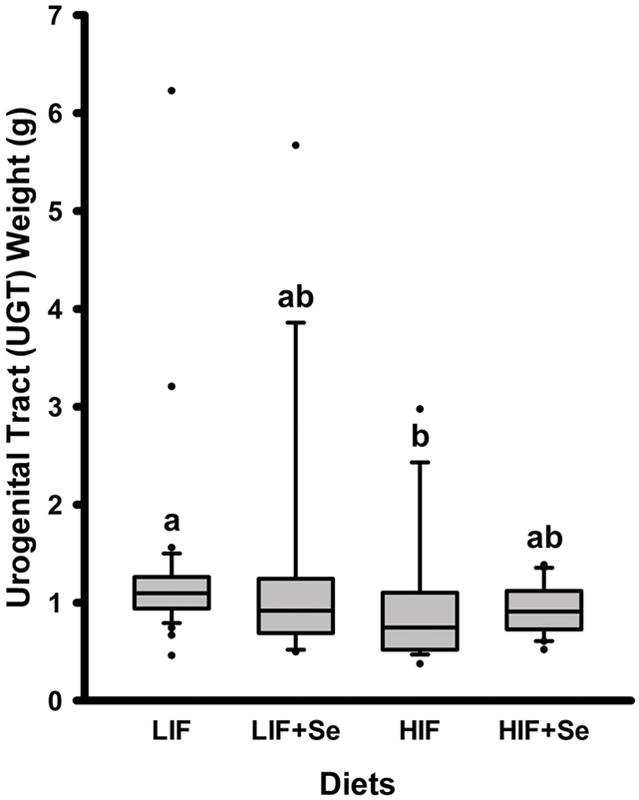
Urogenital tract weights of TRAMP mice fed diets low (LIF) or high (HIF) in soy, with or without added Se (3 mg/kg diet) as Se-methyl-L-selenocysteine. Groups not sharing a common superscript are significantly different (P < 0.05).
Histopathology
Striking differences in tumor progression due to diet are seen in Fig. 7. A Chi-squared analysis for categorical data showed a highly significant difference among dietary groups in (Chi-square = 17.51; P = 0.008) High intake of soy appeared to confer a protective effect. In 92% of animals fed the LIF diets DLPs were scored 5 (moderately-differentiated adenoma; the most advanced lesion observed) while only 20% of TRAMP mice given HIF diets were similarly scored. To assess the possible effect of supplemental MSC we compared unsupplemented animals (LIF and HIF combined) to MSC-supplemented mice (LIF+Se and HIF+Se combined). Among all unsupplemented animals, 61% were scored 5. Among all MSC-supplemented mice, 65% were scored 5. This negligible difference between all MSC-supplemented mice and all unsupplemented animals showed that the main effect of Se on progression of tumorigenesis was minimal. A similar analysis of soy effects showed that among all mice receiving soy-free diets (LIF and LIF+Se combined) 86% were scored 5, while among all mice receiving high-soy diets (HIF and HIF+Se combined) only 38% were scored 5. As noted for other parameters, the effect of supplemental MSC depended on the basal diet to which it was added. Fig. 7 shows that when added to the LIF basal formulation MSC modestly decreased the fraction of animals whose highest lesion score was 5. However, when added to the HIF basal diet MSC increased the percentage of mice whose lesions were scored 5.
Fig. 7.
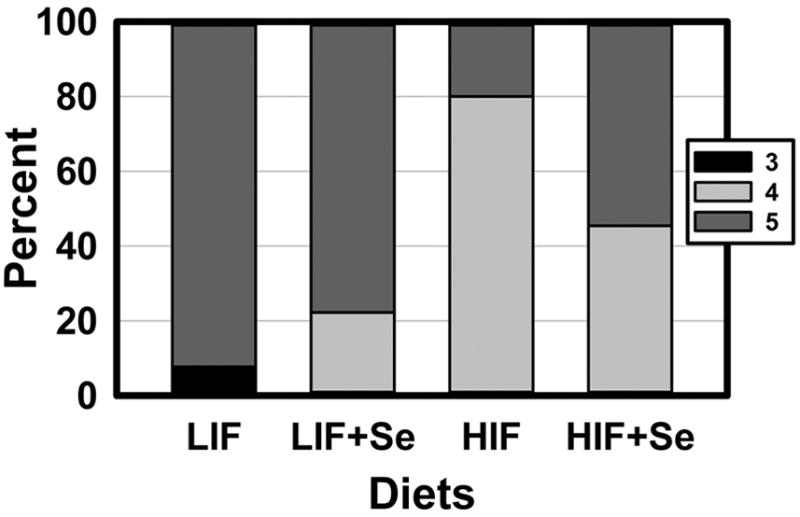
Histopathologic scoring of dorsolateral prostates of TRAMP mice fed diets low (LIF) or high (HIF) in soy, with or without added Se (3 mg/kg diet) as Se-methyl-L-selenocysteine, (n= 9–13). Bars indicate the percentage of animals in each group for whom the most advanced lesion was scored 3 (least advanced observed), 4, or 5 (most advanced seen). In no animals was the most advanced lesion scored 1, 2, or 6 (23). Chi-squared analysis for categorical data showed a highly significant difference among dietary groups in (Chi-square = 17.51; P = 0.008).
DISCUSSION
Pathological progression in the TRAMP mouse follows two pathways. Atypical hyperplasia of Tag develops primarily in the dorsolateral prostate but seldom progresses to adenocarcinoma. In contrast, neuroendocrine carcinomas develop most often in the ventral prostate and can become metastatic (24). From 40–100% of human castration-resistant prostate cancer demonstrates evidence of neuroendocrine differentiation, which is associated with poor prognosis (25). Interestingly, Se is reported to be most effective against advanced prostate cancer (26), which makes the TRAMP mouse a particularly appropriate model in which to study Se chemoprevention.
Human dietary intake is characterized by a variety of foods with multiple, complex interactions among nutrients and phytochemicals. This complexity is better modeled by stock diets than by semi-purified formulations. Our recent work showed that the composition of a basal diet may determine the effects of supplements added to it (18). We reasoned that adding MSC to different mouse stock diets, rather than semi-purified formulations, would better mimic MSC supplementation of human subjects consuming a wide variety of different foods. Perfect matching of stock diets in every nutrient is problematic. Different batches of dietary ingredients will vary from lot to lot in micronutrient composition, with corresponding variability in the finished formulations. Published nutrient composition data for stock diets are most often calculated, rather than measured values, and do not take into account losses in processing. As detailed in our previous report, differences between the two stock diets used in this work were reportedly 2–2.5 fold for vitamins D and E, and 10 fold for vitamin K. For the other 39 of the 42 macro- and micronutrients listed in product information sheets the reported difference in concentration was less than 50%. Few, if any, published studies have examined differences in intake of these nutrients – much less such small (2.5 fold or less) differences - on growth of prostate cancer cells in culture or in any animal model. In contrast, the measured difference between basal diets in isoflavone content was 60-fold, while the MSC-supplemented diets provided a concentration of Se that was roughly 8–10 fold higher than the unsupplemented basal diets. There is a wealth of literature showing the chemopreventive effects of soy constituents and Se individually in preclinical and human studies. Thus, it is highly likely that differences in this study between diets in enzyme activities, gene expression, UGT weights, and disease progression were the result of differences in Se and soy dietary concentrations, rather than minimal differences between diets in other nutrients.
Shortly after this work began, Wang et al. (27) published the results of their study in which TRAMP mice were fed semipurified diets and gavaged 5 times weekly with MSC or methylseleninic acid (MSA) at 3 mg Se/kg body weight. In that work, the two Se supplements showed equal, significant chemopreventive efficacy. Differences between this study and that of Wang et al. included the genetic background of TRAMP mice, the composition of the basal diets, and the quantity of Se given as a supplement. In this work, daily consumption of 5 g diet by a 25 g mouse would have provided a total of 15 μg supplemental Se as MSC, while in the study of Wang et al. a daily gavage of 3 mg Se/kg body weight would have supplied a 25 g mouse a 75 μg Se bolus.
More recent proteomic profiling of tissues from TRAMP mice used in the study by Wang et al. showed that MSA regulated expression of molecules involved in AR signaling but, surprisingly, MSC did not (28). The same group reported that MSC achieved its chemopreventive efficacy in spite of its induction of certain oncoproteins (29). Clearly, every single alteration of gene expression by MSC or any other agent does not have to be in the direction of protection in order for the agent to achieve an overall chemopreventive effect. In this work MSC increased AR expression in mice only when added to LIF diets. Other reports of MSC effects on AR expression in rodents are inconsistent (15,30,31). We recently reported that the composition of basal diets may determine the effects of supplemental MSC (18). The interactions of basal diet, chemical form of Se, quantity, and method of Se supplementation as determinants of AR expression require further work to thoroughly and carefully characterize.
The observation that increased Large T expression secondary to elevated AR in mice fed the LIF+Se diet did not significantly affect tumor growth or progression may possibly be explained by its mechanism of action. Large T antigen binds p53 and retinoblastoma proteins, which abolishes their tumor-suppressor functions. Such binding is necessary but insufficient by itself to initiate tumorigenesis, which requires other stochastic events to progress to adenocarcinoma (32). The level of Large T expression necessary to maximize binding of tumor suppressors in TRAMP mice is unknown. However, it is possible that maximum binding of tumor suppressors may have been achieved at the level of Large T expression seen in LIF, HIF, and HIF+Se groups, so that the higher level of Large T seen in LIF+Se-fed animals, would have achieved no further inhibition of tumor suppressor function.
Aromatase (33) and 5α-reductase (34) are both aberrantly expressed in prostate cancer. Gao et al. (35) recently showed that MSA decreased aromatase gene expression and activity in cultured prostate cancer cells. Theirs was the first report of an effect of Se on aromatase in any model. Although a suggestive trend was observed in DLPs of mice in this study, the main effect of MSC supplementation was not statistically significant. The combination effect of supplemental MSC and HIF on activity of 5α-reductase is consistent with our previous report (15), which was the first to show an inhibitory effect of MSC supplementation on this enzyme in experimental animals.
Regulation of androgen-metabolizing enzymes by soy components has been studied in numerous models with varying results (36). In the TRAMP mouse genistein was shown to be protective against poorly differentiated tumor development when fed throughout life (37), but to promote tumorigenesis when introduced at a later age (38). However, no measurements of aromatase or 5α-reductase were made in those studies.
In this work dietary effects on gene expression were generally consistent with the chemoprevention shown in UGT weights (Fig. 6) and pathology scoring (Fig.7). In addition to AR, HIF diets also decreased expression of DHCR24 (Fig. 4B), an integral enzyme in the synthesis of cholesterol from desmosterol (39), which is AR-regulated in the prostate (40) where it scavenges reactive oxygen species (41). Reduction of DHCR24 by HIF diets may sensitize prostate cancer cells to apoptosis when challenged with oxidative stress, thus reducing tumor growth. Members of the AKR1C family are reductases with high specificity for steroid hormones including DHT. The enzyme AKR1C14 converts DHT to the less potent 3α-androstanediol (42). Thus, the increase in AKR1C14 seen in MSC-supplemented animals (Fig. 3B) may be protective.
Computer-assisted literature searches found only one previous study in which effects of soy constituents on FOX family transcription factors were examined in an animal model (43). FOXA1 binds near androgen response elements in the promoter regions of AR-regulated genes, facilitating AR binding and initiation of transcription (44). FOXA1 is up-regulated in androgen-sensitive and castration-resistant prostate cancer (CRPC) (45). Reduction of FOXA1 protein levels by HIF diets (Fig. 4A) may be one of the mechanisms by which soy down-regulates expression of AR and AR-regulated genes (44).
Forkhead box O family transcription factors were originally found at the site of chromosomal translocations in tumors which suggested that they may be tumor suppressors (46). FOXO1 has been shown to bind directly to the N-terminus domain of AR and induce its nuclear export and inactivation (47). Decreased protein levels of FOXO1 and FOXO3 are seen in localized prostate cancer, and even more so in CRPC (48). Higher levels of FOXO3 seen in animals fed HIF diets (Fig. 5) are novel observations and suggest possible mechanisms for down-regulation of AR.
This study is the first to show effects of dietary soy containing isoflavones in their natural blend and quantities on the expression of Forkhead box proteins in an animal model, and to suggest a possible mechanism for soy’s inhibition of AR. The effects of HIF diets in this study are internally consistent, showing reduced expression of FOXA1 (Fig. 4), FOXA1-dependent AR (Fig. 2), AR-regulated Large T antigen (Fig. 2) and DHCR24 (Fig. 4), and induced the protective FOXO3 (Fig 5). These observations in HIF-fed animals were closely correlated with the protective effect seen in reduced UGT weight (Fig. 6), and in a much smaller percentage of prostate tumors with high histopathology scores (Fig. 7).
As in our previous work (18), the effects of MSC added to basal diets in some cases depended upon the composition of those diets. Addition of MSC to LIF diets significantly reduced activity of 5α-reductase and expression of FOXO3, and increased expression of AR and Large T antigen, while supplementation of HIF diets with MSC did not affect these measurements. In contrast, MSC supplementation of HIF diets did significantly increase expression of GUCY1A3, which effect was not seen in MSC-supplemented, LIF-fed mice. The fact that the pattern of increased AR and Large T expression seen in MSC-supplemented LIF-animals, but not in HIF-fed mice, was not replicated in the expression of other AR-regulated genes strongly suggests that AR is not the only regulator of these genes. Effects of high soy intake and supplemental MSC on other regulators of AR expression and AR-regulated genes remain to be explored.
It is important to note that effects of Se supplementation seen in this study may be specific to MSC. The chemical form of Se determines its effects, which can vary widely among different forms of the element, as shown in the proteomic profiling studies of Zhang et al. (28,29). Likewise, although basal diets varied markedly in isoflavone content, which justifies their labeling as “LIF” and “HIF”, such designations are not intended to imply that all observed effects of high soy intake are due exclusively to its isoflavone content. It is possible that other bioactive components in soy may have contributed to its regulation of the processes examined in this study. That possibility remains the subject for future investigations.
In conclusion, when compared to a stock diet low in soy, consumption of diets high in soy decreased activity of hepatic aromatase and 5α-reductase, reduced expression of AR, AR-regulated genes, and AR-associated FOXA1, up-regulated protective FOXO3, and slowed tumor progression in TRAMP mice. These findings of soy effects on FOX family gene expression in animals are novel and suggest a mechanism by which soy may reduce expression of AR and AR-regulated, tumor-promoting genes. Consistent with our previous report, results from this study show that the chemopreventive effects of supplementation of a naturally-occurring food form of Se depend upon the composition of the basal diet to which it is added. The observation that supplemental MSC did little in this study to potentiate the chemopreventive effects of high soy intake suggests that there are more mechanisms shared by these two dietary components for their individual effects than there are mechanisms unique to each one. It is also consistent with the hypothesis that addition of a chemopreventive agent like MSC to a diet already high in protective compounds is less likely to provide further protection. To maximize the efficacy of Se supplementation, it should be targeted to those whose habitual diets are low in Prostate cancer-preventive phytochemicals and put them at highest risk of the disease.
Table I.
Summary of Proteins Quantified
| Designation | Protein | Description |
|---|---|---|
| AR | Androgen receptor | Nuclear hormone receptor that binds to androgen and induces transcription of AR-regulated genes |
| GUCY1A3 | Guanylate cyclase 1 subunit alpha | Catalyzes the production of cGMP in response to NO signaling; can induce VEGF expression |
| ACSL3 | Acyl-CoA synthetase long-chain family member 3 | Catalyzes the production of long-chain fatty acyl-CoA; inhibits anti-apoptotic fatty acid synthase (FAS). |
| DHCR24 | 24-dehydrocholesterol reductase (seladin-1) | Catalyzes synthesis of cholesterol from desmosterol; inhibits apoptotic caspase-3 |
| AKR1C14 | Aldo-keto reductase family 1 member C14 | Catalyzes conversion of DHT to 3α-androstanediol and androstenedione into testosterone. |
| FOXA1 | Forkhead box A1 | Transcription factor; involved in AR-mediated gene expression. |
| FOXO3 | Forkhead box O3 | Transcription factor; involved in up-regulation of p27, FasL, TRAIL, etc; also interacts with AR |
| LT_SV40 | SV40 Large T antigen | Abrogates p53, Rb tumor suppressor genes; facilitates tumor progression in TRAMP mice |
Footnotes
Disclosure: None of the authors have any conflicts of interest to disclose.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Syed DN, Khan N, Afaq F, Mukhtar H. Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol Biomarkers Prev. 2007;16:2193–2203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad A, Sakr WA, Rahman KM. Novel targets for detection of cancer and their modulation by chemopreventive natural compounds. Front Biosci (Elite Ed) 2012;4:410–425. doi: 10.2741/e388. [DOI] [PubMed] [Google Scholar]
- 4.Kumar N, Crocker T, Smith T, Pow-Sang J, Spiess PE, Connors S, Chornukur G, Dickinson SI, Bai W, Williams CR, Salup R, Fu W. Prostate cancer chemoprevention targeting high risk populations: model for trial design and outcome measures. J Cancer Sci Ther 2012. 2011 doi: 10.4172/1948-5956.s3-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan N, Adhami VM, Mukhtar H. Apoptosis by dietary agents for prevention and treatment of prostate cancer. Endocr Relat Cancer. 2010;17:R39–52. doi: 10.1677/ERC-09-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Dennert G, Zwahlen M, Brinkman M, Vinceti M, Zeegers MP, Horneber M. Selenium for preventing cancer. Cochrane Database Syst Rev. 2011:CD005195. doi: 10.1002/14651858.CD005195.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdulah R, Kobayashi K, Yamazaki C, Koyama H. Molecular targets of selenium in prostate cancer prevention (Review) Int J Oncol. 2011;39:301–309. doi: 10.3892/ijo.2011.1035. [DOI] [PubMed] [Google Scholar]
- 9.Duffield-Lillico AJ, Dalkin BL, Reid ME, Turnbull BW, Slate EH, Jacobs ET, Marshall JR, Clark LC. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91:608–612. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 10.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr, Baker LH, Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009;61:598–606. doi: 10.1080/01635580902825639. [DOI] [PubMed] [Google Scholar]
- 12.Lund TD, Munson DJ, Adlercreutz H, Handa RJ, Lephart ED. Androgen receptor expression in the rat prostate is down-regulated by dietary phytoestrogens. Reprod Biol Endocrinol. 2004;2:5. doi: 10.1186/1477-7827-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Montemayor MM, Otero-Franqui E, Martinez J, De La Mota-Peynado A, Cubano LA, Dharmawardhane S. Individual and combined soy isoflavones exert differential effects on metastatic cancer progression. Clin Exp Metastasis. 2010;27:465–480. doi: 10.1007/s10585-010-9336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Gao R, Dong Y, Gao L, Zhao Y, Zhao L, Zhao X, Zhang H. Survivin gene silencing sensitizes prostate cancer cells to selenium growth inhibition. BMC Cancer. 2010;10:418. doi: 10.1186/1471-2407-10-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legg RL, Tolman JR, Lovinger CT, Lephart ED, Setchell KD, Christensen MJ. Diets high in selenium and isoflavones decrease androgen-regulated gene expression in healthy rat dorsolateral prostate. Reprod Biol Endocrinol. 2008;6:57. doi: 10.1186/1477-7827-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gingrich JR, Barrios RJ, Foster BA, Greenberg NM. Pathologic progression of autochthonous prostate cancer in the TRAMP model. Prostate Cancer P D. 1999;2:70–75. doi: 10.1038/sj.pcan.4500296. [DOI] [PubMed] [Google Scholar]
- 17.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 18.Quiner TE, Nakken HL, Mason BA, Lephart ED, Hancock CR, Christensen MJ. Soy content of basal diets determines the effects of supplemental selenium in male mice. J Nutr. 2011;141:2159–2165. doi: 10.3945/jn.111.146498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen MJ, Cammack PM, Wray CD. Tissue specificity of selenoprotein gene expression in rats. J Nutr Biochem. 1995;6:367–372. doi: 10.1016/0955-2863(95)80004-v. [DOI] [PubMed] [Google Scholar]
- 20.Christensen MJ, Nelson BL, Wray CD. Regulation of glutathione S-transferase gene expression and activity by dietary selenium. Biochem Biophys Res Commun. 1994;202:271–277. doi: 10.1006/bbrc.1994.1923. [DOI] [PubMed] [Google Scholar]
- 21.Weber KS, Jacobson NA, Setchell KD, Lephart ED. Brain aromatase and 5alpha-reductase, regulatory behaviors and testosterone levels in adult rats on phytoestrogen diets. Proc Soc Exp Biol Med. 1999;221:131–135. doi: 10.1046/j.1525-1373.1999.d01-66.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Dong Y, Zhao H, Brooks JD, Hawthorn L, Nowak N, Marshall JR, Gao AC, Ip C. Microarray data mining for potential selenium targets in chemoprevention of prostate cancer. Cancer Genomics Proteomics. 2005;2:97–114. [PMC free article] [PubMed] [Google Scholar]
- 23.Suttie A, Nyska A, Haseman JK, Moser GJ, Hackett TR, Goldsworthy TL. A grading scheme for the assessment of proliferative lesions of the mouse prostate in the TRAMP model. Toxicol Pathol. 2003;31:31–38. doi: 10.1080/01926230390173842. [DOI] [PubMed] [Google Scholar]
- 24.Chiaverotti T, Couto SS, Donjacour A, Mao JH, Nagase H, Cardiff RD, Cunha GR, Balmain A. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol. 2008;172:236–246. doi: 10.2353/ajpath.2008.070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lapuk AV, Wu C, Wyatt AW, McPherson A, McConeghy BJ, Brahmbhatt S, Mo F, Zoubeidi A, Anderson S, Bell RH, Haegert A, Shukin R, Wang Y, Fazli L, Hurtado-Coll A, Jones EC, Hach F, Hormozdiari F, Hajirasouliha I, Boutros PC, Bristow RG, Zhao Y, Marra MA, Fanjul A, Maher CA, Chinnaiyan AM, Rubin MA, Beltran H, Sahinalp SC, Gleave ME, Volik SV, Collins CC. From sequence to molecular pathology, and a mechanism driving the neuroendocrine phenotype in prostate cancer. J Pathol. 2012;227:286–297. doi: 10.1002/path.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Platz EA. Is prostate cancer prevention with selenium all in the genes? Cancer Prev Res (Phila) 2010;3:576–578. doi: 10.1158/1940-6207.CAPR-10-0072. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Bonorden MJ, Li GX, Lee HJ, Hu H, Zhang Y, Liao JD, Cleary MP, Lu J. Methyl-selenium compounds inhibit prostate carcinogenesis in the transgenic adenocarcinoma of mouse prostate model with survival benefit. Cancer Prev Res (Phila) 2009;2:484–495. doi: 10.1158/1940-6207.CAPR-08-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Wang L, Anderson LB, Witthuhn B, Xu Y, Lu J. Proteomic profiling of potential molecular targets of methyl-selenium compounds in the transgenic adenocarcinoma of mouse prostate model. Cancer Prev Res (Phila) 2010;3:994–1006. doi: 10.1158/1940-6207.CAPR-09-0261. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Wang L, Li G, Anderson LB, Xu Y, Witthuhn B, Lu J. Mouse prostate proteomes are differentially altered by supranutritional intake of four selenium compounds. Nutr Cancer. 2011;63:778–789. doi: 10.1080/01635581.2011.563029. [DOI] [PubMed] [Google Scholar]
- 30.Lee SO, Yeon Chun J, Nadiminty N, Trump DL, Ip C, Dong Y, Gao AC. Monomethylated selenium inhibits growth of LNCaP human prostate cancer xenograft accompanied by a decrease in the expression of androgen receptor and prostate-specific antigen (PSA) Prostate. 2006;66:1070–1075. doi: 10.1002/pros.20329. [DOI] [PubMed] [Google Scholar]
- 31.Lindshield BL, Ford NA, Canene-Adams K, Diamond AM, Wallig MA, Erdman JW., Jr Selenium, but not lycopene or vitamin E, decreases growth of transplantable dunning R3327-H rat prostate tumors. PLoS One. 2010;5:e10423. doi: 10.1371/journal.pone.0010423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dardick I, Ho J, Paulus M, Mellon PL, Mirels L. Submandibular gland adenocarcinoma of intercalated duct origin in Smgb-Tag mice. Lab Invest. 2000;80:1657–1670. doi: 10.1038/labinvest.3780176. [DOI] [PubMed] [Google Scholar]
- 33.Ellem SJ, Risbridger GP. Aromatase and prostate cancer. Minerva Endocrinol. 2006;31:1–12. [PubMed] [Google Scholar]
- 34.Hamilton RJ, Freedland SJ. 5-alpha reductase inhibitors and prostate cancer prevention: where do we turn now? BMC Med. 2011;9:105. doi: 10.1186/1741-7015-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao R, Zhao L, Liu X, Rowan BG, Wabitsch M, Edwards DP, Nishi Y, Yanase T, Yu Q, Dong Y. Methylseleninic acid is a novel suppressor of aromatase expression. J Endocrinol. 2012;212:199–205. doi: 10.1530/JOE-11-0363. [DOI] [PubMed] [Google Scholar]
- 36.Morrissey C, Watson RW. Phytoestrogens and prostate cancer. Curr Drug Targets. 2003;4:231–241. doi: 10.2174/1389450033491154. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Eltoum IE, Lamartiniere CA. Genistein chemoprevention of prostate cancer in TRAMP mice. J Carcinog. 2007;6:3. doi: 10.1186/1477-3163-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Touny LH, Banerjee PP. Identification of a biphasic role for genistein in the regulation of prostate cancer growth and metastasis. Cancer Res. 2009;69:3695–3703. doi: 10.1158/0008-5472.CAN-08-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waterham HR, Koster J, Romeijn GJ, Hennekam RC, Vreken P, Andersson HC, FitzPatrick DR, Kelley RI, Wanders RJ. Mutations in the 3beta-hydroxysterol Delta24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. American Journal of Human Genetics. 2001;69:685–694. doi: 10.1086/323473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonaccorsi L, Luciani P, Nesi G, Mannucci E, Deledda C, Dichiara F, Paglierani M, Rosati F, Masieri L, Serni S, Carini M, Proietti-Pannunzi L, Monti S, Forti G, Danza G, Serio M, Peri A. Androgen receptor regulation of the seladin-1/DHCR24 gene: altered expression in prostate cancer. Lab Invest. 2008;88:1049–1056. doi: 10.1038/labinvest.2008.80. [DOI] [PubMed] [Google Scholar]
- 41.Peri A, Danza G, Benvenuti S, Luciani P, Deledda C, Rosati F, Cellai I, Serio M. New insights on the neuroprotective role of sterols and sex steroids: the seladin-1/DHCR24 paradigm. Front Neuroendocrinol. 2009;30:119–129. doi: 10.1016/j.yfrne.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Velica P, Davies NJ, Rocha PP, Schrewe H, Ride JP, Bunce CM. Lack of functional and expression homology between human and mouse aldo-keto reductase 1C enzymes: implications for modelling human cancers. Mol Cancer. 2009;8:121. doi: 10.1186/1476-4598-8-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross AE, Marchionni L, Phillips TM, Miller RM, Hurley PJ, Simons BW, Salmasi AH, Schaeffer AJ, Gearhart JP, Schaeffer EM. Molecular effects of genistein on male urethral development. J Urol. 2011;185:1894–1898. doi: 10.1016/j.juro.2010.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Heul-Nieuwenhuijsen L, Dits NF, Jenster G. Gene expression of forkhead transcription factors in the normal and diseased human prostate. BJU Int. 2009;103:1574–1580. doi: 10.1111/j.1464-410X.2009.08351.x. [DOI] [PubMed] [Google Scholar]
- 45.Gerhardt J, Montani M, Wild P, Beer M, Huber F, Hermanns T, Muntener M, Kristiansen G. FOXA1 promotes tumor progression in prostate cancer and represents a novel hallmark of castration-resistant prostate cancer. Am J Pathol. 2012;180:848–861. doi: 10.1016/j.ajpath.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 46.Zanella F, Link W, Carnero A. Understanding FOXO, new views on old transcription factors. Curr Cancer Drug Targets. 2010;10:135–146. doi: 10.2174/156800910791054158. [DOI] [PubMed] [Google Scholar]
- 47.Ma Q, Fu W, Li P, Nicosia SV, Jenster G, Zhang X, Bai W. FoxO1 mediates PTEN suppression of androgen receptor N- and C-terminal interactions and coactivator recruitment. Mol Endocrinol. 2009;23:213–225. doi: 10.1210/me.2008-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang JY, Hung MC. A new fork for clinical application: targeting forkhead transcription factors in cancer. Clin Cancer Res. 2009;15:752–757. doi: 10.1158/1078-0432.CCR-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]


