Abstract
Toll-like receptors (TLRs) recognize common microbial or host-derived macromolecules and have important roles in early activation of the immune system. Patients with primary immune deficiencies (PIDs) affecting TLR signaling can elucidate the importance of these proteins to the human immune system. Defects in interleukin-1 receptor-associated kinase (IRAK)-4 and myeloid differentiation factor 88 (MyD88) lead to susceptibility to infections with bacteria, while mutations in nuclear factor-κB essential modulator (NEMO) and other downstream mediators generally induce broader susceptibility to bacteria, viruses, and fungi. In contrast, TLR3 signaling defects are specific for susceptibility to herpes simplex virus type 1 (HSV-1) encephalitis. Other PIDs induce functional alterations of TLR signaling pathways, such as common variable immunodeficiency in which plasmacytoid dendritic cell (pDC) defects enhance defective responses of B cells to shared TLR agonists. Dampening of TLR responses is seen for TLRs 2 and 4 in chronic granulomatous disease (CGD) and X-linked agammaglobulinemia (XLA). Enhanced TLR responses, meanwhile, are seen for TLRs 5 and 9 in CGD, TLRs 4, 7/8, and 9 in XLA, TLRs 2 and 4 in hyper IgE syndrome, and for most TLRs in adenosine deaminase deficiency.
Keywords: Toll-like receptors, primary immune deficiency, human immunology, infection
Introduction
Toll-like receptors (TLRs) are expressed broadly by immune and non-immune cells and recognize both microbial and host-derived macromolecules, leading to early detection of infection and other potential dangers.1 Cells bearing these receptors include B and T cells, dendritic, endothelial and epithelial cells, fibroblasts, and macrophages.2 TLRs are germline-encoded and designed to bind conserved microbial structures, including cell wall subunits or nucleic acids and host inflammatory mediators. This broad reactively contrasts with the narrow antigen specificity of B and T cell receptors which are shaped through genetic rearrangement.3 TLR interactions with ligands stimulate innate host defense mechanisms promoting secretion of antimicrobial peptides and cytokines as well as expression of adhesion molecules; thus TLRs play a role in the initiation of long-lived adaptive immune responses by promoting antigen-presentation.4–6 The identification of PIDs impairing TLR signaling has contributed greatly to the understanding of the function of these receptors in humans.7 By focusing on PIDs due to genetic defects that directly or indirectly impair TLR signaling, and considering information gleaned from associations of TLR polymorphisms with infection, this review examines current understanding of how TLR and related signaling defects lead to susceptibility to infection in humans.
TLRs bridge innate and adaptive immunity
The discovery of TLRs and the identification of these receptors as sensory immunomodulators has been an important advancement in the field of immunology. The novel role of TLRs may explain how long term immunity can be achieved despite the fact that foreign antigen alone may be inadequate to illicit a lasting immune response.8 In recognition of this, Hoffman and Beutler, who first described TLRs in fruit flies, were awarded the 2011 Nobel Prize in Physiology or Medicine along with Ralph Steinman, who discovered the cell type that utilizes TLRs to initiate adaptive immunity, the dendritic cell (DC). Humans have 10 TLRs, 6 of which are extracellular (TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10) while 4 are expressed exclusively intracellularly (TLR3, TLR7, TLR8, and TLR9). These receptors have differing biologies in the detection of diverse pathogen-associated molecular patterns (PAMPs) and endogenous danger-associated molecular patterns (DAMPs).9, 10
A number of microbial and endogenous molecules have been identified as TLR ligands. TLR1 and TLR6 work in concert with TLR2 to detect di- and triacylated lipoproteins from Mycoplasma and other bacteria.11, 12 Numerous agonists for TLR2 have been reported, including lipoteichoic acid (Gram-positive bacteria), lipoarabinomannan (Mycobacteria), zymosan (fungi), as well as a number of envelop antigens from viruses.13–16 TLR4 is well known for its role as a sensor of lipopolysaccharide from Gram-negative bacteria, and has also been shown to bind heat-shock protein 60, the fusion protein of respiratory syncytial virus, and fungal mannan.17–20 A major agonist for TLR5 is flagellin, which is conserved among many microbial species.21 The intracellular TLRs TLR3, TLR7/8, and TLR9 sense double-stranded RNA, single-stranded RNA, and unmethylated (microbial) DNA oligonucleotides respectively.22–25 The ligand for TLR10 has not yet been identified, though roles for this receptor in the recognition of viral infection and in inflammatory regulation have been suggested.26, 27 Much of the work elucidating TLR agonists has utilized mouse models or cell lines over-expressing human proteins, which is an important caveat when considering the in vivo relevance to human immunity. Thus, PIDs impairing TLR signaling provide insightful models to corroborate findings of TLR function derived from experimental systems.
Overview of TLR function and NF-κB signaling
With the exception of TLR3 and specific modes of TLR4 signaling that utilize Toll/IL-1 receptor (TIR) domain-containing adaptor–inducing interferon-β (TRIF) and the TRIF-related adaptor molecule (TRAM), TLRs utilize myeloid differentiation factor 88 (MyD88) and the adaptor molecule IL-1 receptor-associated kinase (IRAK) complex to transduce intracellular signals (Fig. 1).28, 29 Upon binding ligand, TLR homo- or heterodimers (TLR1/TLR2 and TLR6/TLR2) are formed, which positions the intracellular TIR domains of these receptors such that subsequent signaling through adaptor and kinase proteins can occur.30 The major downstream effects of TLR signaling are activation of mitogen-activated protein kinase (MAPK) and nuclear factor of kappa light polypeptide gene enhancer in B cells (NF-κB) signal transduction pathways; these regulate cell differentiation, proliferation, and survival. Interferon regulatory factors IRF3 and IRF7 are also activated downstream of TLRs, regulating the production of type I interferons.31–33 Thus, TLRs have the potential to broadly impact the immune response. Quite surprisingly, however, broad immunological impairment is not the norm for PIDs that impair TLR signaling. Infection susceptibility due to genetic disruptions of TLR pathways in humans tends to be narrower than in mice with similar genetic abnormalities.34 The elucidation of PIDs affecting TLR signaling has highlighted the complexity and biological redundancy within these pathways, as well as indicated that there may be significant differences between human disease and animal models.
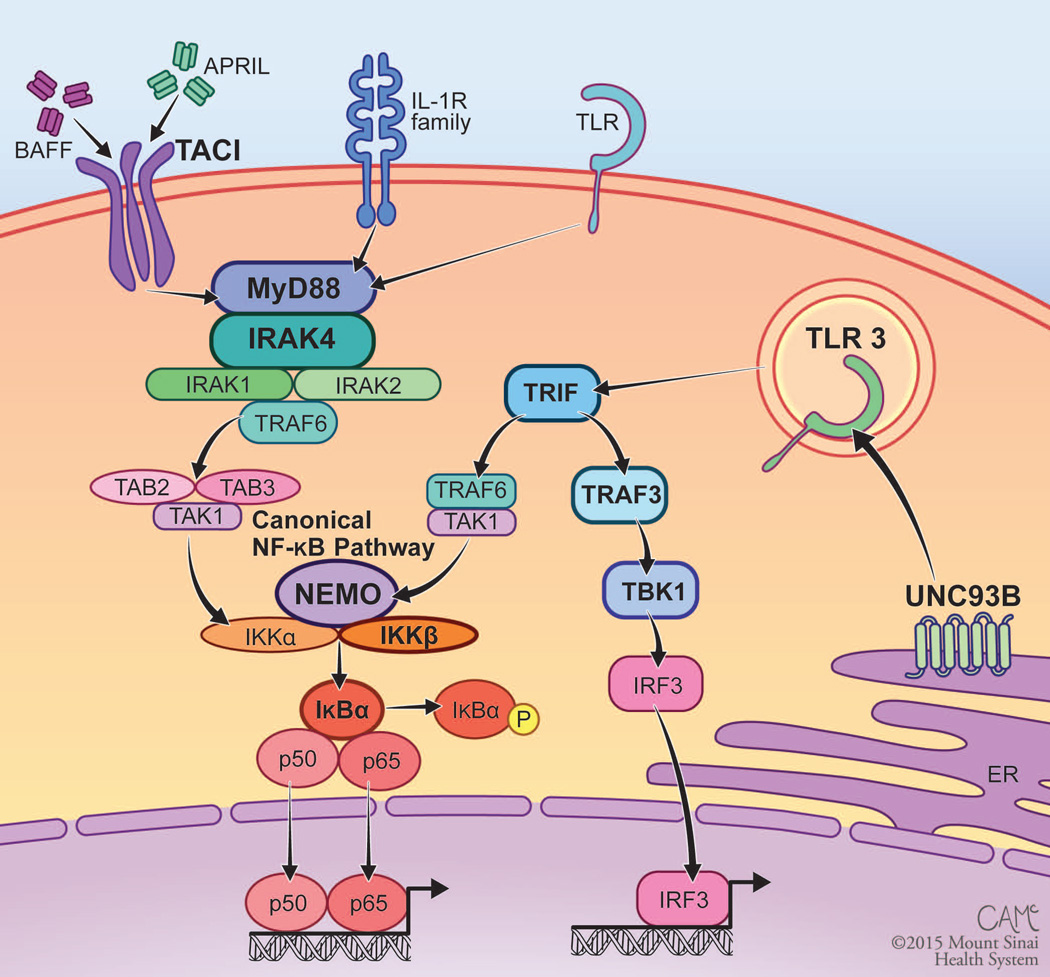
Figure 1.
TLR and NF-κB signaling disrupted by PID. The canonical NF-κB pathway is utilized by numerous immunologic receptors, including the IL-R family, TLRs, and TACI. Signal transduction is mediated through an IKK complex that results in phosphorylation and degradation of IκBα, allowing for nuclear localization of NF-κB. TLR3 signals through TRIF to both activate the canonical NF-κB pathway and induce type I interferon production through IRF3. Genes in which mutations cause known PID are in bold type. Figure courtesy of Courtney A. McKenna © 2015 Mount Sinai Health System.
NF-κB family members exist in their inactive form within the cytoplasm, either bound to inhibitory kappa B (IκB) proteins in the canonical pathway or sequestered by p100 in the non-canonical pathway.35 There are five NF-κB family proteins, NF-κB1 (p50), NF-κB2 (p52), RelA (p65), RelB, and c-Rel, which function as transcription factors through the formation of homo- or heterodimers.36 Canonical NF-κB signaling is mediated by the p50 subunit of NF-κB, while non-canonical NF-κB signaling utilizes p52, which is derived through the processing of p100. TLR activation induces phosphorylation of IκBs by the IκB kinase (IKK) complex, leading to translocation of canonical NF-κB dimers into the nucleus to initiate transcription (Figure 1). In contrast, the non-canonical NF-κB pathway mediates signaling through certain members of the tumor necrosis factor receptor superfamily, including B cell activating factor receptor (BAFF-R), CD40, lymphotoxin beta receptor (LTβR), and receptor activator of NF-κB (RANK).37
Human genetic defects impairing TLR and canonical NF-κB signaling
A number of PIDs affecting the canonical NF-κB pathway have been identified, all impairing TLRs (Table 1). These include inherited mutations in MYD88, IRAK4, NF-κB essential modulator (NEMO or inhibitor of NF-κB kinase subunit-γ, IKBKG), inhibitor of NF-κB kinase subunit-β (IKBKB), and NF-κB inhibitor-α (NFKBIA). Additionally, defects of TLR3 signaling, which does not utilize MyD88, have been defined, including genetic defects of TLR3, UNC93B1, Toll/IL-1R domain-containing adaptor inducing interferon (TRIF), TRAF3, and TANK-binding kinase 1 (TBK1). Homozygous mutations in caspase recruitment domain 11 (CARD11) and mucosa-associated lymphoid tissue 1 (MALT1) also impair canonical NF-κB activation in lymphocytes, but the impact upon TLR signaling is likely to be minimal, as these proteins exist downstream of antigen receptors rather than TLRs.38–40
Table I.
PIDs due to genetic defects of NF-κB and TLR signaling
| Genetic defect | Inheritance | Typical infections | Autoimmune features | Immunological features |
|---|---|---|---|---|
| MyD88 dependent | ||||
| IRAK-4, MyD88 | AR | Invasive and recurrent bacterial infections, most commonly S. pneumoniae, S aureus, and P. aeruginosa |
None reported | Impaired TLR and IL-1R responses, reduced IgM+IgD+CD27+ B cells, impaired TI IgM |
| NEMO (IKKγ) | XL | Bacterial, fungal, mycobacterial, and viral infections |
Arthritis, hemolytic anemia, inflammatory bowel disease-like colitis |
Impaired TLR and IL-1R responses, reduced T cell proliferative responses, impaired TI antibodies |
| IKKβ | AR | Bacterial, fungal, and viral infections |
None reported | Hypogammaglobulinemia, reduced CD45RO T cells, impaired T cell proliferative responses |
| IκBα | AD | Recurrent bacterial infections, pneumocystis pneumonia, chronic mucocutaneous candidiasis |
Colitis, recurrent diarrhea | Impaired TLR and TNF-α responses, reduced T cells |
| HOIL-1 | AR | Invasive bacterial infections | systemic autoinflammation, muscular amelopectinosis |
Impaired TLR responses, monocytes hyper responsive to IL-1β while fibroblasts are hyperresponsive, reduced memory B cells, impaired TI antibodies |
| MyD88 independent | ||||
| TBK1, TLR3, TRAF3, TRIF, UNC-93B |
AD or AR (TLR3, TRIF) AD (TBK1, TRAF3) AR (UNC-93B) |
HSV-1 encephalitis | None reported | Impaired TLR3 stimulation of type I interferon |
| Other | ||||
| TACI | AD with incomplete penetrance |
Recurrent bacterial infections | Autoimmunity and lymphoproliferation in monoallelic patients |
Hypogammagobulinemia, IgA deficiency, reduced isotype-switched memory B cells |
AD, autosomal dominant; AR, autosomal recessive; XL, X-linked recessive; TI, T independent
Amongst the best-described syndromes are autosomal recessive (AR) mutations in IRAK4 and MYD88, which impair signals via IL-1, TLR1, TLR2, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, and TIR domain containing adaptor protein (TIRAP)-dependent TLR4 signaling.41–44 These mutations also impair the activation of MAPK, NF-κB, and downstream cytokines in response to TLR ligands. Despite this seemingly broad immunologic loss, the spectrum of pathogens that cause infections in these patients is surprisingly narrow. Invasive infections (meningitis and sepsis) or non-invasive (cutaneous and respiratory infections), are mostly due to Streptococcus pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa.42, 45 These infections typically first occur before the age of 2 years, and re-occur in most undiagnosed survivors.46 Prophylactic antibiotics and vaccination are essential, and supplementation with immunoglobulin replacement therapy has been used in many cases. Interestingly, with increasing age, infections become limited to non-invasive forms, allowing for the discontinuation of prophylactic therapy in some patients.47 The reason for this clinical improvement is unclear. Despite the purported role of IRAK-4 and MyD88 in TLR-driven adaptive immune responses, circulating T cell numbers and T cell proliferation in response to antigen are normal.46
Based upon the important immunological role of TLR and canonical NF-κB signaling in host defense, the narrow spectrum of infectious disease in these subjects has been puzzling. Impairment of pneumococcal-specific IgG has been reported in a few subjects with IRAK4 mutations, but this finding does not always correlate with the occurrence of invasive bacterial infections.46, 48, 49 More recently, it was reported that circulating IgM+IgD+CD27+ B cells, which produce T-independent (TI) IgM responses against bacteria, are depleted in these patients, suggesting that the loss of this B cell subset may be clinically important.50, 51 Exploring this relationship further, we found that diminished TI IgM responses corresponds with inadequacy of the IgM+IgD+CD27+ B cell subset in IRAK-4 and MYD88 deficiencies, which may contribute to the susceptibility to bacterial infections.44 IgM+IgD+CD27+ B cells are thought to be a human corollary to the marginal zone B cell subset in mice, which mount rapid TI antibody responses that are required for host defense against bacteria.50, 52, 53 The importance of this B cell subset in bacterial infection could explain both the observed susceptibility to bacterial pathogens as well as the observed improvement with age. The TI IgG repertoire, which fully matures around adolescence, may ultimately compensate the impairment of TI IgM, lessening the frequency and severity of infections.54, 55 Though it is not yet clear why the IgM+IgD+CD27+ B cells and TI IgM responses would be more susceptible than other types of B cell-driven immunity to IRAK-4 or MyD88 deficiency, these findings may indicate that this B cell subset is more dependent upon IRAK-4– and MyD88-dependent signaling, perhaps through TACI or TLRs, for its activation and survival. Recently, a systems approach was utilized to further address the question of why IRAK-4 and MyD88 deficiencies predispose to a limited range of pathogens, revealing that while responses to purified TLR agonists are disrupted in these patients, only a narrow spectrum of transcriptional targets were impaired upon exposure to whole bacteria.56 This approach provides a limited panel of transcriptional candidates that will be tested further in the attempt to explain the confounding pattern of susceptibility in IRAK-4 and MYD88 deficiencies.
Mutations in IκBα and NEMO also impair MyD88-dependent and TRIF-dependent TLR signaling as well as T cell receptor, B cell receptor, and tumor necrosis factor receptor activation.57, 58 Given this broader role of IκBα and NEMO compared to that of IRAK-4 and MyD88, it is not surprising that the infectious susceptibility of these patients is also broader; infections with fungi, mycobacteria, and viruses, in addition to bacteria, are reported.59 Patients with IκBα and NEMO defects also may develop inflammatory bowel disease-like colitis and/or recurrent diarrhea. Additional manifestations of IκBα and NEMO defects include anhidrotic ectodermal dysplasia (EDA, abnormal teeth, hypohidrosis, and sparse hair), which along with the immunodeficiency syndrome is termed EDA-ID.60 IκBα mutations have been inherited in an autosomal dominant (AD) manner and have hypermorphic expression, as IκB proteins inhibit translocation of NF-κB to the nucleus. NEMO mutations are X-linked recessive (XL) and hypomorphic, as NEMO promotes IKK-mediated phosphorylation and removal of IκBs that allow for NF-κB translocation. Highlighting an important role for NF-κB signaling in B cell function, over 80% of patients with EDA-ID do not have measurable TI anti-carbohydrate antibody responses in the serum.36 While T cell numbers are generally normal, the T cell proliferative response to antigen is impaired in patients with EDA-ID. A wide spectrum of immunodeficiency phenotypes has been associated with NEMO mutations, including PID without EDA and infection susceptibility limited to mycobacterial disease.61–65 Treatment for EDA-ID patients usually includes vaccinations, antibiotic prophylaxis and immunoglobulin replacement therapy.48, 66 Bone marrow transplant has resulted in immune reconstitution in patients with both the AD and XL forms of EDA-ID, but there are concerns with engraftment and post-transplant complications that may make this intervention particularly challenging.67–71 Recently, four patients from two consanguineous families were described to have AR defects in IKKβ, which is incorporated with NEMO and IKKα in the IKK complex.72 They presented with childhood bacterial, fungal, and viral infections along with hypogammaglobulinemia and reduced effector T cell numbers and proliferation despite normal overall B and T cell counts.
TLRs are not the only immune receptors affected by genetic disruptions of canonical NF-κB signaling. The interleukin 1 receptor family (including IL-1R, IL-18R, and IL-33R) also signals through the canonical NF-κB pathway, and genetic defects of IRAK-4, MyD88, NEMO, and IκBα also impair these responses.47 Likewise, responses to TNF have been shown to be impaired in canonical NF-κB pathway defects. Transmembrane activator and CAML interactor (TACI), which is expressed by B cells, requires MyD88 and the canonical NF-κB pathway.73 TACI binds BAFF trimers as well as trimers of another B cell-activating cytokine, a proliferation inducing ligand (APRIL), to activate B cells in a T cell–independent manner.74 While the impact of genetic defects in the canonical NF-κB pathway upon TACI signaling have not been reported, defects in TACI are associated with antibody deficiency as well as a propensity for autoimmunity and lymphoproliferation.75 While both heterozygous and homozygous mutations in TACI can cause antibody deficiency, only monoallelic mutations appear to increase the risk of autoimmunity and lymphoproliferative complications.76 Curiously, unaffected family members may harbor the same heterozygous mutations in TACI and have B cell impairment in vitro, indicating that the relationship between TACI defects and PID is complex.77
AR deficiency of HOIL-1 (RBCK1), which is part of the linear ubiquitination chain assembly complex (LUBAC) and functions in the polyubiquination of NEMO and NF-κB activation, was recently described.78 These patients have susceptibility to invasive bacterial infections as well as systemic autoinflammation and muscular amylopectinosis, in which abnormal glycogen storage results in muscular deposition of glycogen. Notably, TLR signaling is impaired in fibroblasts and immortalized B cells derived from HOIL-1–deficient patients.78 However, monocytes from these patients are hyper-responsive to IL-1β while fibroblasts were found to be hyporesponsive to the same stimuli. This finding offers possible reconciliation for the concurrent immunodeficiency and hyperinflammatory phenotype seen in these patients, and further demonstrates the marked complexity of NF-κB signaling revealed by human PID. Total B and T cell counts do not appear to be reduced in HOIL-1–deficient patients, though memory B cell numbers and antibody responses to pneumococcal carbohydrates were reduced.78
Numerous PIDs affecting the TRIF-dependent pathway of TLR3 signaling have also been described, with mutations affecting TLR3, the endosomal TLR chaperone UNC-93B, TRIF, TRAF3, and TBK1 all resulting in early susceptibility to herpes simplex virus 1 (HSV-1) encephalitis.79–83 Even compared with the narrow spectrum of infections seen in some other defects of TLR signaling, the extent of pathogens seen in these patients with TRIF pathway defects is remarkably restricted, as infections have been limited to one specific pathogen (HSV-1) and one specific type of infection (encephalitis) in all cases. This suggests that the TLR3/TRIF-dependent pathway has a redundant immunological role in humans, with the exception of a required role in defense against HSV-1 in the central nervous system (CNS).84 Induced pluripotent stem cell-derived neurons and oligodendrocytes derived from TLR3 or UNC93B deficient patients were found to have impaired type I interferon production and heightened susceptibility to HSV-1 infection compared to controls.85 Thus, proteins functioning in TLR3/TRIF-mediated signaling may have a unique role in host defense against HSV-1, specifically by promoting protective type I interferon responses in the CNS.
TLR polymorphisms and infection susceptibility
Genome-wide association studies have uncovered numerous polymorphisms within TLRs in association with infectious and inflammatory disease.86 A complete review of these polymorphisms is beyond the scope of this article, and the reader is directed to excellent publications on this topic for further detail.86, 87 Below, we will consider single-nucleotide polymorphisms (SNPs) of particular relevance to TLR functions and infectious diseases, demonstrated experimentally or through known PID phenotypes. As is quite clear given the diversity of infections explored and populations tested, any conclusions derived from studies of TLR polymorphisms are potentially limited by small sample sizes, ethnic backgrounds, gender, environment, and polygenic influences.
TLR2 has been demonstrated to bind numerous conserved microbial antigens, including the cell wall from Gram-positive bacteria, Mycobacteria, and fungi, as well as envelope proteins from multiple viruses. Supporting the role of this TLR in the initiation of anti-bacterial immunity, the G2258A TLR2 variant has been correlated with asymptomatic bacteriuria in women.88 Other TLR2 polymorphisms were associated with aggressive periodontitis in one study, but not in another, and SNPs in this gene were also linked with nasopharyngeal bacterial carriage in infants as well as higher rates of Gram-positive bacterial infections in liver transplant patients.89–92 Supporting the role of TLR2 in the recognition of Mycobacteria, multiple reports link TLR2 SNPs with Mycobacteria susceptibility, both in leprosy and tuberculosis, corresponding with impaired TLR2 signaling.93–98 Additionally, the R753Q TLR2 polymorphism was associated with cytomegalovirus infection in liver transplant patients in two studies.99, 100
Suggestive of its role in recognizing viral nucleic acid, polymorphisms in TLR3 have been linked to susceptibility to viral infection. One TLR3 SNP was associated with hepatitis B virus infection.101 The L412F TLR3 polymorphism has been linked to both resistance against HIV infection in an Italian population and protection against acute liver transplant rejection in patients with hepatitis C virus (HCV) cirrhosis.102, 103 A polymorphism in the TLR3 promoter region was noted to predispose to HCV infection, however another study failed to find an association between two other TLR3 polymorphisms and chronic HCV infection.104, 105 While PIDs that impair TLR3 signaling have been exclusively associated with HSV-1 encephalitis, only one TLR3 polymorphism study has reported an association with HSV infection.106 In this study there was no association with severity of HSV disease, as the SNPs were found equally in symptomatic and asymptomatic individuals.
As TLR4 was one of the first TLR extensively studied and has the purported role of recognizing Gram-negative lipopolysaccharide as well as other bacterial, fungal, and viral antigens, many studies have looked for associations of polymorphisms in TLR4 with infection. Infections and sepsis with Gram-negative bacteria, RSV bronchiolitis, and disseminated candidiasis have all been linked to TLR4 polymorphisms.107–110 However, other studies have failed to associate the same TLR4 SNPs with sepsis.111–113 Similarly mixed results were found for mycobacterial infection, with some studies associating TLR4 polymorphisms with susceptibility to Mycobacteria while others did not.114–118 Numerous polymorphisms in the TLR4 promoter region were associated with urinary tract infections (UTIs) and impaired innate immune responses during UTIs, as measured by urinary IL-6, IL-8, and neutrophil numbers in a Swedish cohort.119
Other notable TLR SNPs include a TLR polymorphism in the ligand-binding domain of TLR5 (392STOP) that has been linked with increased susceptibility to Legionella pneumonia, impaired IL-6 response to flagellin, as well as an increased risk of UTIs in Caucasian women, but that did not correlate with Salmonella enterica serovar Typhi infection in a Vietnamese cohort.120, 121 Highlighting its putative role in sensing viral nucleic acid, a TLR7 polymorphism (c.32A>T) was found to be associated with chronic HCV infection and SNPs in TLR7 and TLR8 were associated with HCV infection in a Taiwanese cohort.122, 123
Functional alterations in TLR signaling
Curiously, altered responses to TLRs are seen in immune deficiencies in which the canonical components of TLR signaling do not bear known mutations (Table 2). Among the PIDs in this category, common variable immunodeficiency reveals the importance of crosstalk between distinct cell types when responding to shared TLR activators. Other PIDs, including chronic granulmatous disease and X-linked agammaglobulinemia, reveal the complexity of immune regulation, with defects highlighting positive and negative regulators of TLR signaling. While knockout mice have traditionally been used to unravel the complexity of immune pathways, human with PIDs provide opportunities for study of these pathways in context of the human immune system, whether confirming or altering the conclusions from animal models.
Table 2.
Changes reported for TLRs in PIDs with functional alterations of TLR signaling
| Immunodeficiency | Genetics | Phenotype | Cells: relevant TLRs | Details |
|---|---|---|---|---|
| Common variable immunodeficiency |
Unknown mutations; variable inheritance |
Decreased responses |
pDCs: TLR7 and TLR9 | Reduced secretion of IFN-α |
| B cells: TLR7 and TLR9 | Reduced TLR9 expression; reduced upregulation of costimulatory molecules; reduced proliferation; reduced cytokine secretion; reduced immunoglobulin secretion |
|||
| Normal responses |
pDCs: TLR3 | Normal upregulation of costimulatory molecules and secretion of IFN-α |
||
| Monocyte, NK, and DCs: TLR2, TLR4, TLR9 | Normal upregulation of costimulatory molecules | |||
| PBMCs: TLR1, TLR2, TLR3, TLR4, TLR5, TLR7/8, and TLR9 |
Normal secretion of TNF-α, IL-6, and IL-12 | |||
| Chronic Granulomatous Disease |
NADPH oxidase mutations; XL or AR |
Increased Responses |
Neutrophils: TLR4 | Increased TNF-α and IL-8 secretion |
| PBMCs: TLR2 and TLR4 | Increased TNF-α and IL-6 secretion | |||
| Decreased Responses |
Neutrophils: TLR5 and TLR9 | Reduced expression of TLR5 and TLR9; reduced phagocytosis of S. aureus |
||
| B cells: TLR9 | Antigen specific long term memory recall responses reduced |
|||
| X-Linked agammaglobulinemia |
Btk mutations; XL |
Decreased responses (some studies) |
Monocytes: TLR2 and TLR4 | Decreased secretion of TNF-α and IL-1β (but not IL-6, IL-8, and IL-10) |
| PBMCs: TLR2 and TLR4 | Reduced levels of phosphorylated p38 | |||
| Monocyte derived macrophages: TLR4 | Overexpressing btk restored defective TNF-α secretion (related kinase Tec strongly induced by culturing media) |
|||
| Monocyte derived DCs: TLR2, TLR3, TLR4, TLR7/8 |
Decreased DC maturation; decreased TNF-α (but not IL-6 or IL-12) secretion in one study; decreased TNF-α and IL-6 in response to TLR8 (but not other TLRs) in a second study |
|||
| Increased responses (some studies) |
Monocytes and DCs: TLR4 and TLR7/8 | Increased secretion of TNF-α and IL-6; increased secretion of IL-10 (DCs but not monocytes); intracellular cytokine levels similar to controls |
||
| PBMCs: TLR4 | Increased secretion of TNF-α, IL-1β, IL-6 and IL-10 | |||
| Monocyte derived DCs: TLR4 | Increased IL-10 (but not IL-12) secretion in one study and increased IL-6 secretion in another |
|||
| Normal responses |
pDCs: TLR3, TLR7/8, and TLR9 | Normal secretion of IFN-α | ||
| Neutrophils: TLR4 and TLR7/8 | Normal activation; normal oxidative burst | |||
| Hyper IgE syndrome | STAT3 mutations; AD |
Increased Responses |
PBMCs: TLR2 and TLR4 | Increased TNF-α and IL-12 secretion |
| Whole blood: TLR2 and TLR4 | Increased TNF-α and IL-12 secretion |
|||
| Adenosine deaminase deficiency |
ADA mutations; AR |
Decreased responses |
B cells: TLRs | Defective exclusion of autoreactive B cells during development (resemble IRAK4 or MyD88 deficient) |
| B cells: TLR7 and TLR9 | ADA inhibitors in healthy donor cells reduced upregulation of costimulatory molecules including CD86 (but not CD69); reduced phosphorylation of signaling components |
|||
| Increased responses |
Various: TLR2 and TLR4 | Adenosine agonists or cAMP in healthy donor cells reduced TNF-α and IL-12 secretion yet increased IL-10 |
||
| Altered | Various: TLR3 and TLR5 | Adenosone agonist-specific effects of increasing or decreasing IL-1β, IL-6, and IL-10 secretion in healthy donor cells |
AD, autosomal dominant; AR, autosomal recessive; XL, X-linked recessive; pDC, plasmacytoid dendritic cell; DC, conventional dendritic cell; PBMCs, peripheral blood mononuclear cells; NADPH, nicotinamide adenine dinucleotide phosphate; Btk, Bruton’s tyrosine kinase; STAT3, signal transducer and activator of transcription 3; ADA, adenosine deaminase.
TLR signaling in common variable immunodeficiency
Common variable immunodeficiency (CVID), the most common symptomatic PID, is a diagnosis of exclusion given to individuals with hypogammaglobulinemia and recurrent sinupulmonary infections who do not make specific antibodies following vaccination.124 With patients at increased risk of autoimmune, inflammatory, and malignant complications,125 CVID encompasses a heterogeneous group of patients, the large majority having no known underlying genetic cause.124 Given the hallmark of hypogammaglobulinemia, B cells have been heavily studied in this disorder, with defects reported in proliferation, class switch recombination, differentiation, and immunoglobulin (Ig) secretion. In addition, T cell cytopenia, clonality, and defects of (TLR-independent) cytokine production has been described,126 as have reduced numbers of dendritic cells127, 128 and in vivo increased activation of monocytes.129
Our group first reported defective B cell proliferation and Ig secretion in response to CpG motif containing nucleotides,130 although the mechanism was unknown as this preceded the discovery of mammalian TLR9.131 Following the characterization of human TLR9 and the discovery of powerful synthetic agonists, our group and others revisited responses of CVID patients to TLR9 stimulation, finding pervasive B cell defects in activation and in cytokine secretion when, notably, patients did not have mutations or variations in TLR9 (Fig. 2).132, 133
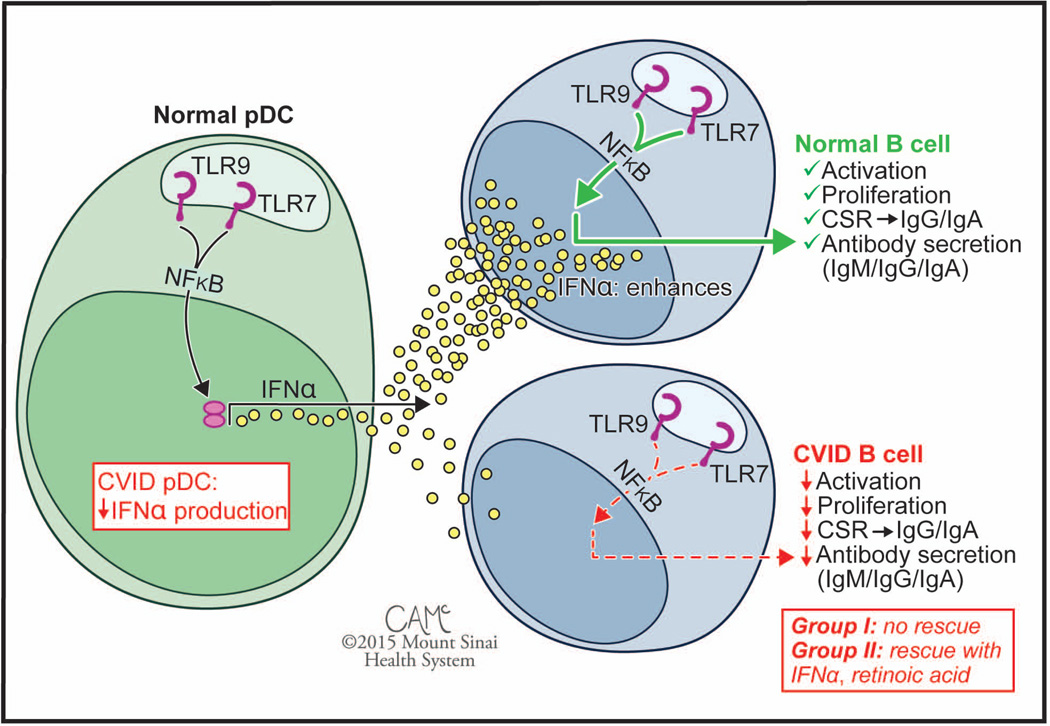
Figure 2.
Cell type specific functional defects of TLR signaling in. CVID. While the normal pDC response to stimulation with TLR7 and TLR9 agonists is secretion of high levels of IFN-α, pDCs of subjects with CVID show defective responses. Similarly, while normal B cells are sensitive to stimulation with TLR7 and TLR9 agonists, patient B cells show defective responses. The B cell defect is correctable in less severely affected Group II patients with the addition of exogenous IFN-α or retinoic acid, but these fail to rescue B cells from Group I patients. Patients are subdivided into Group I (<0.55% SWMB, associated with non-infectious complications) and less severely affected Group II (>0.55% SWMB, associated with better clinical course). CVID, common variable immunodeficiency; SWMB, class switched memory B cells. Figure courtesy of Courtney A. McKenna © 2015 Mount Sinai Health System.
Intriguingly, both naïve CD27- and memory CD27+ B cells from patients showed reduced expression of TLR9 transcript and protein, suggesting a possible cause for the defective responses.132 Previous work had shown that TLR9 responsiveness increases following treatment with interferon alpha (IFN-α)134 and that, among peripheral blood mononuclear cells (PBMCs), plasmacytoid dendritic cells (pDCs) secrete high levels of IFN-α following TLR9 stimulation.135 When pDCs from subjects with CVID were examined, they were similarly found to have profound defects, with greatly reduced secretion of IFN-α following TLR9 stimulation, in spite of comparable expression of TLR9.132 A follow up report showed that supplementation of exogenous IFN-α restored TLR9 responsiveness of B cells, but only for subjects with less severely reduced frequency of class switched (CS) memory B cells.136 This was consistent with reports of higher levels of CS memory cells associated with better clinical course.137 In addition, pDCs and B cells from patients were found to be defective in responding to TLR7 and TLR7/8 stimulation,8 similarly showing rescue of the B cell phenotype with exogenous IFN-α, allowing B cells of patients with retained CS memory cells to proceed to class switch recombination and Ig secretion.136, 138
Of note, TLR signaling appears otherwise intact in CVID. IFN-α secretion by pDCs following stimulation through TLR3 mirrored controls,138 as did upregulation of activation markers on pDCs, conventional DCs, monocytes, and NK cells following stimulation through TLR2, TLR4, or TLR9.139 Furthermore, normal secretion of TNF-α, IL-6, and IL-12 from PBMCs stimulated through TLR1, TLR2 TLR3, TLR4, TLR5, TLR7, or TLR9 was seen in patients,138 as were levels of intracellular IL-12 in myeloid DCs stimulated through TLR9.128 Interestingly, a recent report did not find defects in pDC production of IFN-α following TLR9 stimulation,128 finding similar levels of intracellular IFN-α when gating on pDCs in PBMC cultures, unlike earlier studies examining isolated pDCs.132, 138 This discrepancy suggests either increased fragility of CVID pDCs, a pDC defect in IFNα secretion, but not intracellular accumulation, or else raises the possibility of correction of the pDC defect by a PBMC component. A tantalizing hint supporting involvement of additional cell types comes from a report of modest gains in TLR9-driven IgG secretion with the addition of exogenous retinoic acid,140 a vitamin A metabolite produced by some myeloid cells.141, 142 Collectively, these data support a view of functional aberrations of TLR7 and TLR9 signaling in pDCs and B cells of subjects with CVID, and highlight the importance of TLR-mediated cross talk between different cells in optimizing responses to shared activators.
TLR signaling in chronic granulomatous disease
Chronic granulomatous disease (CGD) is caused by genetic mutations in components of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system that produces reactive oxygen species (ROS) leading to the respiratory burst of phagocytes. In the absence of a functional system, neutrophils are incapable of killing phagocytosed bacteria and fungus, leaving patients at increased risk of recurrent pyogenic infections.124 Other immune aberrations in patients include inflammatory disorders, largely restricted to GI tract, that are thought to be independent of infection.124, 143 The most common form of CGD is caused by mutations in X-chromosome CYBB, affecting males but largely sparing their carrier mothers, and inactivating both copies of autosomal components of NADPH oxidase will similarly cause CGD.124 The first report assessing TLRs in CGD focused on patient neutrophils, finding them to secrete increased levels of TNF-α and IL-8 following LPS stimulation.144 Similar findings were later reported in PBMCs, with increased TNF-α and IL-6 secretion following treatment with TLR2 or TLR4 agonists (Fig. 3, in blue).145 This study further showed that the TLR induced activation of NF-κB was independent of ROS,145 strongly suggesting that ROS are needed for appropriate dampening of responses to these TLRs. The following year, microarray analysis of patient and control monocytes provided further support that the immune system of CGD patients excessively responded to TLR4 stimulation, with additional hypersensitivity seen at baseline as well.146 The authors linked the absence of ROS to increased expression of NF-κB subunits, seen both basally and following stimulation, suggesting that this loss endowed patient cells to produce higher levels of proinflamatory cytokines.146 As such, the defects in NADPH oxidase may result in increased TLR2 and TLR4 inflammatory responses contributing to the GI inflammation seen in these patients.
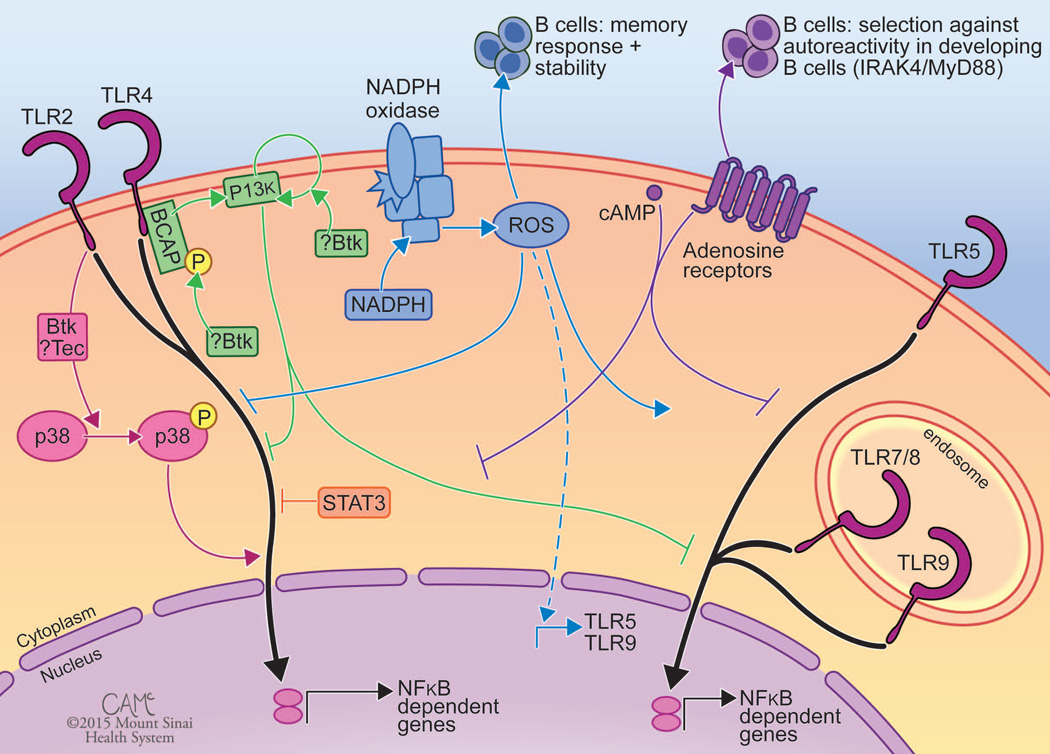
Figure 3.
The functional alterations in TLR signaling seen in various PIDs highlight crosstalk with regulatory pathways. TLR signaling is shown in black, with interacting pathways shown in color. Pink: Some studies of XLA patients demonstrate a role for BTK and related kinase Tec in activating MAPK pathways and amplifying responses to TLR2 and TLR4. Green: Other studies of XLA suggest a role for BTK in activating or amplifying PI3K signaling to dampen TLR responses. Blue: As illustrated by CGD, NADPH oxidase–induced ROS are important in dampening inflammation following TLR2 and TLR4 activation, promoting expression of TLR5 and TLR9 (dotted line) and responses to TLR5 and TLR9 agonists, and long term memory responses in B cells. Orange: AD-HIES patients indicate that STAT3 can dampen TLR2 and TLR4 signaling. Purple: Findings from ADA-SCID patients conjecture a role of cAMP and adenosine accumulation in inhibiting TLR signaling. CGD, chronic granulomatous disease; NADPH = nicotinamide adenine dinucleotide phosphate. ROS, reactive oxygen species; XLA, X-linked agammaglobulineima; BTK, Bruton’s tyrosine kinase; MAPK, mitogen activated protein kinase; BCAP, B cell adaptor for PI3K; PI3K, phosphoinositide 3 kinase. AD-HIES, autosomal dominant hyper IgE syndrome; STAT3, signal transducer and activator of transcription 3; ADA, adenosine deaminase; SCID, severe combined immunodeficiency; ERT, enzyme replacement therapy; cAMP, cyclic adenosine monophosphate. Figure courtesy of Courtney A. McKenna © 2015 Mount Sinai Health System.
Further assessment of TLRs in CGD found that TLR5 and TLR9, but not TLRs 1, 2, or 4, were expressed at lower levels by patient neutrophils (Fig. 3, in blue).147 These changes were independent of infectious history, and were seen in the mRNA transcript as well as in intracellular and, for TLR5, cell surface protein expression.147 Of note, TLR5 expression correlated with the extent of NADPH oxidase function, and inhibition of this system in neutrophils from healthy controls reduced the expression of TLR5 and TLR9 in these cells.147 As such, NADPH oxidase or resultant ROS may be important for proper expression of TLR5 and TLR9 in neutrophils, with absence resulting in impaired responses to stimulation by these TLR ligands and less efficient phagocytosis.147 The NADPH oxidase system was further shown to be important in the context of TLR9 stimulation in B cells, with defects in long term antibody memory responses recently reported in CGD patients.148 The authors linked deficient ROS production in B cells to reduced frequencies of memory B cells in patients, with a milder phenotype seen in carriers of CGD mutations (Fig. 3, in blue).148 In the setting of previous measles vaccination, authors demonstrated the functional implications of defective NADPH oxidase by showing that patients with CGD had age-dependent reductions in serum anti-measles antibody levels, concomitant with reduced induction of measles-specific antibody secreting cells (ASCs) downstream of TLR9 activation.148 While as earlier study found influenza-specific ASCs to be similar between patents and controls,149 some participants in this earlier study were on immunosuppressive therapy that could have altered their antibody responses. This study did not assess expression of TLR9 in CGD B cells, thus it remains unknown whether NADPH oxidase might promote TLR9 expression in B cells, as it does in neutrophils.147 If this were the case, reduced expression of TLR9 might link the defects in NADPH oxidase and ROS production to promotion of long-term B cell memory. In concert, these results highlight the importance of NADPH oxidase and ROS in regulating TLR signaling, and further demonstrate the this regulation can be TLR specific, with NADPH promoting responses downstream of TLRs 5 and 9147, 148 yet dampening responses downstream of TLRs 2 and 4.144–146 Future work to understand the mechanism remains, yet these results suggest that modulation of ROS might be useful, whether to possibly ameliorate CGD autoinflammatory disorders or possibly as a way to treat antibody based autoimmune syndromes.150, 151
TLR signaling in X-Linked agammaglobulinemia
X-Linked agammaglobulinemia (XLA), the first human PID described, was reported in 1952 by Col. Bruton.152 Brutons tyrosine kinase (BTK), a member of the Tec family of tyrosine kinases, was identified in 1993 as the gene mutated in XLA,153 and the critical dependence of B cells on BTK activation downstream of the B cell receptor (BCR) was reported shortly thereafter.154, 155 A role for Btk in other cells was quickly identified with a report that Xid mice, which have a point mutation in Btk and a milder phenotype than human patients, had disrupted microfilarial clearance.156 With BTK expression found in all hematopoietic cells with the exception of T cells, study of BTK functions in myeloid cells were undertaken, including studies of its role downstream of TLR signaling. However, studies of freshly isolated PBMCs from patients found conflicting results, reporting either defective157 or increased158 cytokine secretion following activation through various TLRs. Through mechanistic studies, BTK has been shown to interact with various pathways, both activating and inhibitory, such that a deficiency could cause either suppression or hyperactivation of immune responses. As such, these discrepant outcomes may reflect the preferential activation characteristics of the different cells, agonists, and putative inhibitors used in the various reports, with both informing the understanding of human TLR signaling pathways.
The first reports using human cells found decreased TLR responsiveness with mutated BTK, proposing a role for the kinase in activating proinflammatory pathways. After stimulation with TLR2 or TLR4 agonists, XLA monocytes were found to secrete less TNF-α and IL-1β relative to cells from healthy controls, a defect not seen for secretion of IL-6, IL-8, and IL-10 (Fig. 3, in pink).157, 159 BTK phosphorylation, a sign of activation, was reported following TLR stimulation,157, 160 making a link between TLR pathways and BTK more likely, and a mechanism of direct interaction was proposed when overexpression and yeast two-hybrid studies showed BTK interactions with TIR domains of several TLRs as well as their signal adaptors Mal (also known as TIRAP) and IRAK-1.160 Signaling studies of patient PBMCs found normal IκBα phosphorylation and degradation, however, thereby strongly suggesting that BTK was not involved in the canonical NF-κB signaling cascade downstream of TLRs.157, 159, 161 Instead, patient PBMCs were found to have lower levels of phosphorylated p38, linking BTK to activation of MAPK pathways.157, 159 To determine whether BTK could phosphorylate p38 directly, authors overexpressed BTK in monocyte derived macrophages (moMs), showing increased cytokine secretion and p38 phosphorylation in cells overexpressing btk.157, 159 Interestingly, the M-CSF used to mature moMs strongly induced the expression of Tec, a closely related tyrosine kinase, which might have contributed to restoring LPS induced TNF-α secretion in patient cells (Fig. 3, in pink).157 As such, while overexpressed BTK can activate MAPK pathways downstream of TLR activation, this function might be more generally attributable to Tec family kinases. Several studies have similarly reported decreased cytokine secretion in monocyte derived DCs (moDCs) of XLA patients.162, 163 While these studies did not examine signaling, they nevertheless suggest that the involvement of Tec family members in promoting cytokine secretion downstream of TLR activation might be a general hallmark of these pathways.
Other studies, meanwhile, found increased cytokine secretion in the absence of BTK, in line with elevated levels of TNF-α, IL-1β, and IL-6 in patient serum.164 Our group found primary patient monocytes to secrete increased levels of TNF-α and IL-6, but not IL-10, when stimulated through TLR4 or TLR7/8, while patient myeloid DCs (mDCs) showed higher secretion of all three cytokines (Figure 3, in green).161 In line with these findings, a study examining PBMCs from 13 XLA patients found increased secretion of TNF-α, IL-1β, IL-6 and IL-10 in response to LPS.158 Increases in cytokine secretion were similarly seen among LPS stimulated moDCs, with one study reporting increased IL-10 but similar IL-12 levels,165 and another study reporting increases of IL-6.166 TLR hyperresponsiveness was not seen in pDCs, however, as these cells responded to TLR7/8 or TLR9 stimulation by secreting IFN-α to levels resembling controls.132, 161 As such, TLR7/8 responses were perturbed in some cells but not others, suggesting that BTK modulates TLR signaling without being directly downstream of these receptors. As patient monocytes showed increased cytokine secretion yet comparable intracellular cytokine levels, BTK could instead work to slow signaling kinetics.161 In agreement, TLR4 signaling was intact in the setting of increased responsiveness, inducing comparable IκB, p38, and ERK phosphorylation,161 findings also seen in another study of XLA patients that reported unchanged intracellular cytokine levels (without commenting on secreted cytokines).167 Work in Xid mice and cell lines treated with LFM-13A, a non-specific BTK inhibitor,161 showed that BTK could phosphorylate Mal, inducing its degradation.168 While LPS stimulated XLA cells showed a trend towards reduced Mal degradation,161 this mechanism is expected to be specific for TLRs 2 and 4168 and thus cannot account for increased responses to TLR7/8 agonists. An alternative mechanism, for which there is only indirect evidence, involves BTK dampening TLR signaling by enhancing PI3K activation, a known function of BTK downstream of BCR activation (Fig. 3, in green).169 Downstream of TLRs, PI3K can be activated by TIR containing B cell adaptor for PI3K (BCAP), a novel inhibitory TLR signaling adaptor.170 Intriguingly, a BCAP-deficient murine macrophage cell line showed enhanced early cytokine secretion following TLR stimulation,170 reminiscent of BTK deficient cells from XLA patients. While it is not known whether BTK amplifies TLR-dampening PI3K responses in myeloid cells, or whether BTK might even phosphorylate and activate BCAP in these cells, such roles would explain the increased responsiveness to TLR agonists seen in XLA.
Lastly, in concordance with a view of a functional modulation of TLR signaling, studies of neutrophils from subjects with XLA found normal activation and oxidative burst induction following TLR stimulation.167, 171 These findings are perhaps expected as patients on Ig replacement are not at increased risk of overwhelming infections, instead having increased risk of chronic and low grade infections, largely viral in nature.172, 173 As such, XLA represents a PID with cell-type restricted alterations in responses to TLR agonists.
TLR signaling in hyper IgE syndrome and adenosine deaminase deficiency
While the functions of TLR signaling have been extensively studied for each of the above PIDs, aberrations downstream of TLRs have also been reported hyper IgE syndrome (HIES) and adenosine deaminase (ADA) deficiency leading to severe combined immunodeficiency (SCID). Patients with HIES have recurrent skin and pulmonary abscesses, often without signs of inflammation, as well as with chronic eczema, eosinophilia, and increased serum IgE.124 The more common AD form of the disorder is caused by a dominant negative (DN) mutation in one copy of signal transducer and activator of transcription 3 (STAT3).174 Prior to identification of the genetic cause of HIES, two studies assessed TLR function in small groups of patients in order to determine whether TLR defects underlie this disorder. Instead of defective TLRs, both studies found increased responsiveness to TLR2 or TLR4 stimulation, with the first study reporting a trend towards increased TNF-α and IL-12 secretion from PBMCs of six patients175 and the second study finding increased TNF-α and IL-12 secretion from whole blood of four patients (Fig. 3, in orange).176 A later study of 25 patients confirmed these findings, showing increased TNFα secretion from patient PBMCs following TLR2 or TLR4 stimulation.177 While these early studies did not report STAT3 as a causative gene, the phenotype of TLR hyper-responsiveness was later confirmed in patients with DN-STAT3 mutations.178 While studies directly examining the mechanisms underlying TLR hyper responsiveness in HIES patients have not been performed, Stat3 deficient murine immune cells have been reported to have higher secretion of Th1 cytokines, such as TNF-α, after TLR stimulation.179, 180 With rising interest in blocking STAT3 in tumor immunotherapy, as reviewed in,181 AD-HIES patients serve as a cautionary reminder of the importance of determining optimal levels of STAT3 inhibition.
Deficiency of ADA, which accounts for 11% of newborn-screening detected SCID cases,182 is a T cell–negative, B cell–negative, and NK cell–negative form of SCID with AR inheritance. In addition to deaminating adenosine, ADA metabolizes lymphotoxic dATP and S-adenosyl homocysteine,183–185 and enzyme replacement therapy (ERT) can prevent recurrent infections in patients.186 Bone marrow transplant and/or gene therapy (BMT/GT) remains the preferred therapy as it fully corrects immune defects.187 In contrast, B cells from patients undergoing ERT still showed defects in tolerance reminiscent of IRAK4- and MyD88-deficient patients,188 prompting study of how ADA modulates TLR signaling in B cells. Using ADA inhibitors, human B cells were found to have defective phosphorylation of Syk, BTK, and PLCγ2 downstream of TLR signaling, with cells failing to upregulate activation markers including CD86 following TLR stimulation.188 Murine studies have shown that adenosine can block TLR4-mediated phosphorylation of IκB in splenic B cells,189 either directly or through anti-inflammatory effects of cyclic adenosine monophosphate (cAMP), which similarly accumulates in the absence of ADA (Fig. 3, in purple). As the signaling pathways of adenosine and cAMP are complex, the findings summarized in an extensive review are presented here.190 Both adenosine receptor agonists and cAMP generally dampen TLR signaling, reducing TLR4 induced TNF-α and IL-12, yet increasing IL-10 secretion. Similar modulation was seen downstream of TLR2, with adenosine agonists reducing TNF-α secretion. Agonist-specific modulation was noted for TLR3- and 5-dependent IL-1β, IL-6, and IL-10 secretion, however, making the effects of adenosine stimulation less conclusive. In context of the defects noted in patients undergoing ADA ERT,188 cell-intrinsic ability to degrade adenosine is likely important for fine-tuning TLR signaling, with further study needed to clarify the underlying mechanism.
Concluding remarks
Recent years have seen a dramatic increase in the identification of genetic causes for PIDs, leading to the elucidation of many essential immunologic processes in humans.191, 192 PIDs impairing TLR signaling have illustrated the significance of these pathways for a surprisingly narrow spectrum of infectious diseases in humans. Further research utilizing these PIDs as model systems to test TLR function will undoubtedly expand our understanding of these important receptors and related signaling pathways in humans, and may ultimately explain why defects in these vital immune regulatory proteins manifest as a remarkably small variety of infections clinically. Likewise, further efforts to delineate novel defects of TLR and related signaling pathways will continue to enhance our understanding of human immunology, specifically at the crossroads between innate and adaptive immunity. Additional PIDs, meanwhile, reveal the complexity of TLR regulation, both in terms of collaboration between cell types and in fine-tuning the intensity and duration of responses to TLR stimulation within a responding cell. Further study of such deficiencies could help identify the significant pathways intersecting with TLR signaling, with such understating potentially providing targeted treatment options for PIDs as well as for more common autoimmune and inflammatory disorders.
Acknowledgements
The authors wish to thank Courtney A. McKenna, Mount Sinai Health System, for creating the figures and to thank Scott Justus for reviewing the manuscript text.
REFERENCES
- 1.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature Immunology. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Frontiers in Immunology. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schatz DG, Ji Y. Recombination centres and the orchestration of V(D)J recombination. Nature Reviews Immunology. 2011;11:251–263. doi: 10.1038/nri2941. [DOI] [PubMed] [Google Scholar]
- 4.Uehara A, et al. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Molecular Immunology. 2007;44:3100–3111. doi: 10.1016/j.molimm.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Pegu A, et al. Human lymphatic endothelial cells express multiple functional TLRs. J Immunol. 2008;180:3399–3405. doi: 10.4049/jimmunol.180.5.3399. [DOI] [PubMed] [Google Scholar]
- 6.Schenten D, Medzhitov R. The control of adaptive immune responses by the innate immune system. Advances in Immunology. 2011;109:87–124. doi: 10.1016/B978-0-12-387664-5.00003-0. [DOI] [PubMed] [Google Scholar]
- 7.Wong T, et al. Human primary immunodeficiencies causing defects in innate immunity. Current Opinion in Allergy and Clinical Immunology. 2013;13:607–613. doi: 10.1097/ACI.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 8.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor symposia on quantitative biology. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Kono H, Rock KL. How dying cells alert the immune system to danger. Nature Reviews Immunology. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medzhitov R. Damage control in host-pathogen interactions. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15525–15526. doi: 10.1073/pnas.0908451106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin MS, et al. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu T, Kida Y, Kuwano K. A dipalmitoylated lipoprotein from Mycoplasma pneumoniae activates NF-kappa B through TLR1, TLR2, and TLR6. J Immunol. 2005;175:4641–4646. doi: 10.4049/jimmunol.175.7.4641. [DOI] [PubMed] [Google Scholar]
- 13.Lien E, et al. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. JBC. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 14.Drage MG, et al. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cellular immunology. 2009;258:29–37. doi: 10.1016/j.cellimm.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozinsky A, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Nat. Acad. Sci. USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bieback K, et al. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. Journal of Virology. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 18.Bulut Y, et al. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J Immunol. 2002;168:1435–1440. doi: 10.4049/jimmunol.168.3.1435. [DOI] [PubMed] [Google Scholar]
- 19.Kurt-Jones EA, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nature Immunology. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 20.Tada H, et al. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiology and Immunology. 2002;46:503–512. doi: 10.1111/j.1348-0421.2002.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 21.Gewirtz AT, et al. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 22.Alexopoulou L, et al. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 23.Diebold SS, et al. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 24.Heil F, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 25.Hemmi H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 26.Lee SM, et al. Toll-like receptor 10 is involved in induction of innate immune responses to influenza virus infection. Proc. Nat. Acad. Sci. USA. 2014;111:3793–3798. doi: 10.1073/pnas.1324266111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oosting M, et al. Human TLR10 is an anti-inflammatory pattern-recognition receptor. Proc. Nat. Acad. Sci. USA. 2014;111:E4478–E4484. doi: 10.1073/pnas.1410293111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto M, et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nature Immunology. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 30.Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunological Reviews. 2009;227:248–263. doi: 10.1111/j.1600-065X.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ysebrant de Lendonck L, Martinet V, Goriely S. Interferon regulatory factor 3 in adaptive immune responses. Cellular and Molecular Life Sciences. 2014;71:3873–3883. doi: 10.1007/s00018-014-1653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ning S, Pagano JS, Barber GN. IRF7: activation, regulation, modification and function. Genes and Immunity. 2011;12:399–414. doi: 10.1038/gene.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nature Cell Biology. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 34.von Bernuth H, et al. Experimental and natural infections in MyD88- and IRAK-4-deficient mice and humans. European journal of Immunology. 2012;42:3126–3135. doi: 10.1002/eji.201242683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun SC. The noncanonical NF-kappaB pathway. Immunological Reviews. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frans G, et al. Addressing diagnostic challenges in primary immunodeficiencies: laboratory evaluation of Toll-like receptor- and NF-kappaB-mediated immune responses. Critical Reviews in Clinical Laboratory Sciences. 2014;51:112–123. doi: 10.3109/10408363.2014.881317. [DOI] [PubMed] [Google Scholar]
- 37.Gardam S, Brink R. Non-Canonical NF-kappaB Signaling Initiated by BAFF Influences B Cell Biology at Multiple Junctures. Frontiers in Immunology. 2014;4:509. doi: 10.3389/fimmu.2013.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stepensky P, et al. Deficiency of caspase recruitment domain family, member 11 (CARD11), causes profound combined immunodeficiency in human subjects. The Journal of Allergy and Clinical Immunology. 2013;131:477–485. doi: 10.1016/j.jaci.2012.11.050. e471. [DOI] [PubMed] [Google Scholar]
- 39.Greil J, et al. Whole-exome sequencing links caspase recruitment domain 11 (CARD11) inactivation to severe combined immunodeficiency. The Journal of Allergy and Clinical Immunology. 2013;131:1376–1383. doi: 10.1016/j.jaci.2013.02.012. e1373. [DOI] [PubMed] [Google Scholar]
- 40.Jabara HH, et al. A homozygous mucosa-associated lymphoid tissue 1 (MALT1) mutation in a family with combined immunodeficiency. The Journal of Allergy and Clinical Immunology. 2013;132:151–158. doi: 10.1016/j.jaci.2013.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picard C, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 42.von Bernuth H, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto M, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 44.Maglione PJ, et al. IRAK-4- and MyD88- deficiencies impair IgM responses against T-independent bacterial antigens. Blood. 2014 doi: 10.1182/blood-2014-07-587824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ku CL, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. The Journal of Experimental Medicine. 2007;204:2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Picard C, et al. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine. 2010;89:403–425. doi: 10.1097/MD.0b013e3181fd8ec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Picard C, Casanova JL, Puel A. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkappaBalpha deficiency. Clinical Microbiology Reviews. 2011;24:490–497. doi: 10.1128/CMR.00001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ku CL, et al. IRAK4 and NEMO mutations in otherwise healthy children with recurrent invasive pneumococcal disease. Journal of Medical Genetics. 2007;44:16–23. doi: 10.1136/jmg.2006.044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borgers H, et al. Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens by multiplexed bead assay. Clinical Immunology. 2010;134:198–205. doi: 10.1016/j.clim.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nature reviews. Immunology. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weller S, et al. IgM+IgD+CD27+ B cells are markedly reduced in IRAK-4-, MyD88-, and TIRAP- but not UNC-93B–deficient patients. Blood. 2012;120:4992–5001. doi: 10.1182/blood-2012-07-440776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weller S, et al. Human blood IgM "memory" B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 54.Aucouturier P, et al. [Serum levels of IgG subclasses in the normal child. Evaluation by an immunoenzymatic method using monoclonal antibodies] Archives francaises de pediatrie. 1988;45:255–258. [PubMed] [Google Scholar]
- 55.Douglas RM, et al. Antibody response to pneumococcal vaccination in children younger than five years of age. The Journal of Infectious Diseases. 1983;148:131–137. doi: 10.1093/infdis/148.1.131. [DOI] [PubMed] [Google Scholar]
- 56.Alsina L, et al. A narrow repertoire of transcriptional modules responsive to pyogenic bacteria is impaired in patients carrying loss-of-function mutations in MYD88 or IRAK4. Nature Immunology. 2014;15:1134–1142. doi: 10.1038/ni.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doffinger R, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nature Genetics. 2001;27:277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 58.Courtois G, et al. A hypermorphic IkappaBalpha mutation is associated with autosomal dominant anhidrotic ectodermal dysplasia and T cell immunodeficiency. The Journal of Clinical Investigation. 2003;112:1108–1115. doi: 10.1172/JCI18714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Orange JS, et al. The presentation and natural history of immunodeficiency caused by nuclear factor kappaB essential modulator mutation. The Journal of Allergy and Clinical Immunology. 2004;113:725–733. doi: 10.1016/j.jaci.2004.01.762. [DOI] [PubMed] [Google Scholar]
- 60.Puel A, et al. Inherited disorders of NF-kappaB-mediated immunity in man. Current Opinion in Immunology. 2004;16:34–41. doi: 10.1016/j.coi.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 61.Hanson EP, et al. Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. The Journal of Allergy and Clinical Immunology. 2008;122:1169–1177. doi: 10.1016/j.jaci.2008.08.018. e1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orange JS, et al. Human nuclear factor kappa B essential modulator mutation can result in immunodeficiency without ectodermal dysplasia. The Journal of Allergy and Clinical Immunology. 2004;114:650–656. doi: 10.1016/j.jaci.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 63.Niehues T, et al. Nuclear factor kappaB essential modulator-deficient child with immunodeficiency yet without anhidrotic ectodermal dysplasia. The Journal of Allergy and Clinical Immunology. 2004;114:1456–1462. doi: 10.1016/j.jaci.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 64.Dai YS, et al. Characteristics of mycobacterial infection in patients with immunodeficiency and nuclear factor-kappaB essential modulator mutation, with or without ectodermal dysplasia. Journal of the American Academy of Dermatology. 2004;51:718–722. doi: 10.1016/j.jaad.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 65.Filipe-Santos O, et al. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. The Journal of Experimental Medicine. 2006;203:1745–1759. doi: 10.1084/jem.20060085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ku CL, et al. NEMO mutations in 2 unrelated boys with severe infections and conical teeth. Pediatrics. 2005;115:e615–e619. doi: 10.1542/peds.2004-1754. [DOI] [PubMed] [Google Scholar]
- 67.Dupuis-Girod S, et al. Successful allogeneic hemopoietic stem cell transplantation in a child who had anhidrotic ectodermal dysplasia with immunodeficiency. Pediatrics. 2006;118:e205–e211. doi: 10.1542/peds.2005-2661. [DOI] [PubMed] [Google Scholar]
- 68.Tono C, et al. Correction of immunodeficiency associated with NEMO mutation by umbilical cord blood transplantation using a reduced-intensity conditioning regimen. Bone Marrow Transplantation. 2007;39:801–804. doi: 10.1038/sj.bmt.1705658. [DOI] [PubMed] [Google Scholar]
- 69.Fish JD, et al. Challenges in the use of allogeneic hematopoietic SCT for ectodermal dysplasia with immune deficiency. Bone Marrow Transplantation. 2009;43:217–221. doi: 10.1038/bmt.2008.308. [DOI] [PubMed] [Google Scholar]
- 70.Permaul P, et al. Allogeneic hematopoietic stem cell transplantation for X-linked ectodermal dysplasia and immunodeficiency: case report and review of outcomes. Immunologic Research. 2009;44:89–98. doi: 10.1007/s12026-008-8085-2. [DOI] [PubMed] [Google Scholar]
- 71.Abbott JK, et al. Successful hematopoietic cell transplantation in patients with unique NF-kappaB essential modulator (NEMO) mutations. Bone Marrow Transplantation. 2014;49:1446–1447. doi: 10.1038/bmt.2014.157. [DOI] [PubMed] [Google Scholar]
- 72.Mousallem T, et al. A nonsense mutation in IKBKB causes combined immunodeficiency. Blood. 2014;124:2046–2050. doi: 10.1182/blood-2014-04-571265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He B, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nature immunology. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vincent FB, et al. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine & Growth Factor Reviews. 2013;24:203–215. doi: 10.1016/j.cytogfr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang L, et al. Transmembrane activator and calcium-modulating cyclophilin ligand interactor mutations in common variable immunodeficiency: clinical and immunologic outcomes in heterozygotes. The Journal of Allergy and Clinical Immunology. 2007;120:1178–1185. doi: 10.1016/j.jaci.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salzer U, et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood. 2009;113:1967–1976. doi: 10.1182/blood-2008-02-141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martinez-Gallo M, et al. TACI mutations and impaired B-cell function in subjects with CVID and healthy heterozygotes. The Journal of of Allergy and Clinical Immunology. 2013;131:468–476. doi: 10.1016/j.jaci.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boisson B, et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nature Immunology. 2012;13:1178–1186. doi: 10.1038/ni.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang SY, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 80.Casrouge A, et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 81.Perez de Diego R, et al. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity. 2010;33:400–411. doi: 10.1016/j.immuni.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sancho-Shimizu V, et al. Herpes simplex encephalitis in children with autosomal recessive and dominant TRIF deficiency. The Journal of Clinical Investigation. 2011;121:4889–4902. doi: 10.1172/JCI59259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herman M, et al. Heterozygous TBK1 mutations impair TLR3 immunity and underlie herpes simplex encephalitis of childhood. The Journal of Experimental Medicine. 2012;209:1567–1582. doi: 10.1084/jem.20111316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo Y, et al. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. The Journal of Experimental Medicine. 2011;208:2083–2098. doi: 10.1084/jem.20101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lafaille FG, et al. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature. 2012;491:769–773. doi: 10.1038/nature11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Medvedev AE. Toll-like receptor polymorphisms, inflammatory and infectious diseases, allergies, and cancer. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2013;33:467–484. doi: 10.1089/jir.2012.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Netea MG, Wijmenga C, O'Neill LA. Genetic variation in Toll-like receptors and disease susceptibility. Nature Immunology. 2012;13:535–542. doi: 10.1038/ni.2284. [DOI] [PubMed] [Google Scholar]
- 88.Hawn TR, et al. Genetic variation of the human urinary tract innate immune response and asymptomatic bacteriuria in women. PloS One. 2009;4:e8300. doi: 10.1371/journal.pone.0008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takahashi M, et al. Toll-like receptor 2 gene polymorphisms associated with aggressive periodontitis in Japanese. The Open Dentistry Journal. 2011;5:190–194. doi: 10.2174/1874210601105010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Emingil G, et al. Toll-like receptor 2 and 4 gene polymorphisms in generalized aggressive periodontitis. Journal of Periodontology. 2007;78:1968–1977. doi: 10.1902/jop.2007.060360. [DOI] [PubMed] [Google Scholar]
- 91.Vuononvirta J, et al. Nasopharyngeal bacterial colonization and gene polymorphisms of mannose-binding lectin and toll-like receptors 2 and 4 in infants. PloS One. 2011;6:e26198. doi: 10.1371/journal.pone.0026198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee SO, et al. Toll-like receptor 2 polymorphism and Gram-positive bacterial infections after liver transplantation. Liver Transplantation. 2011;17:1081–1088. doi: 10.1002/lt.22327. [DOI] [PubMed] [Google Scholar]
- 93.Kang TJ, Chae GT. Detection of Toll-like receptor 2 (TLR2) mutation in the lepromatous leprosy patients. FEMS Immunology and Medical Microbiology. 2001;31:53–58. doi: 10.1111/j.1574-695X.2001.tb01586.x. [DOI] [PubMed] [Google Scholar]
- 94.Kang TJ, Lee SB, Chae GT. A polymorphism in the toll-like receptor 2 is associated with IL-12 production from monocyte in lepromatous leprosy. Cytokine. 2002;20:56–62. doi: 10.1006/cyto.2002.1982. [DOI] [PubMed] [Google Scholar]
- 95.Bochud PY, Hawn TR, Aderem A. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J. Immunol. 2003;170:3451–3454. doi: 10.4049/jimmunol.170.7.3451. [DOI] [PubMed] [Google Scholar]
- 96.Ben-Ali M, et al. Toll-like receptor 2 Arg677Trp polymorphism is associated with susceptibility to tuberculosis in Tunisian patients. Clinical and diagnostic laboratory Immunology. 2004;11:625–626. doi: 10.1128/CDLI.11.3.625-626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ogus AC, et al. The Arg753GLn polymorphism of the human toll-like receptor 2 gene in tuberculosis disease. The European Respiratory Journal. 2004;23:219–223. doi: 10.1183/09031936.03.00061703. [DOI] [PubMed] [Google Scholar]
- 98.Xiong Y, et al. R753Q polymorphism inhibits Toll-like receptor (TLR) 2 tyrosine phosphorylation, dimerization with TLR6, and recruitment of myeloid differentiation primary response protein 88. The Journal of Biological Chemistry. 2012;287:38327–38337. doi: 10.1074/jbc.M112.375493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kijpittayarit S, et al. Relationship between Toll-like receptor 2 polymorphism and cytomegalovirus disease after liver transplantation. Clin Infect Dis. 2007;44:1315–1320. doi: 10.1086/514339. [DOI] [PubMed] [Google Scholar]
- 100.Kang SH, et al. Homozygosity for the toll-like receptor 2 R753Q single-nucleotide polymorphism is a risk factor for cytomegalovirus disease after liver transplantation. The Journal of Infectious Disease. 2012;205:639–646. doi: 10.1093/infdis/jir819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Al-Qahtani A, et al. Toll-like receptor 3 polymorphism and its association with hepatitis B virus infection in Saudi Arabian patients. Journal of Medical Virology. 2012;84:1353–1359. doi: 10.1002/jmv.23271. [DOI] [PubMed] [Google Scholar]
- 102.Sironi M, et al. A common polymorphism in TLR3 confers natural resistance to HIV-1 infection. J Immunol. 2012;188:818–823. doi: 10.4049/jimmunol.1102179. [DOI] [PubMed] [Google Scholar]
- 103.Citores MJ, et al. Toll-like receptor 3 L412F polymorphism may protect against acute graft rejection in adult patients undergoing liver transplantation for hepatitis C-related cirrhosis. Transplantation Proceedings. 2011;43:2224–2226. doi: 10.1016/j.transproceed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 104.Medhi S, et al. Promoter region polymorphism & expression profile of toll like receptor-3 (TLR-3) gene in chronic hepatitis C virus (HCV) patients from India. The Indian journal of Medical Research. 2011;134:200–207. [PMC free article] [PubMed] [Google Scholar]
- 105.Askar E, et al. TLR3 gene polymorphisms and liver disease manifestations in chronic hepatitis C. Journal of Medical Virology. 2009;81:1204–1211. doi: 10.1002/jmv.21491. [DOI] [PubMed] [Google Scholar]
- 106.Svensson A, et al. Polymorphisms in Toll-like receptor 3 confer natural resistance to human herpes simplex virus type 2 infection. The Journal of General Virology. 2012;93:1717–1724. doi: 10.1099/vir.0.042572-0. [DOI] [PubMed] [Google Scholar]
- 107.Agnese DM, et al. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. The Journal of Infectious Diseases. 2002;186:1522–1525. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- 108.Lorenz E, et al. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Archives of Internal Medicine. 2002;162:1028–1032. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- 109.Tal G, et al. Association between common Toll-like receptor 4 mutations and severe respiratory syncytial virus disease. The Journal of Infectious Diseases. 2004;189:2057–2063. doi: 10.1086/420830. [DOI] [PubMed] [Google Scholar]
- 110.Van der Graaf CA, et al. Toll-like receptor 4 Asp299Gly/Thr399Ile polymorphisms are a risk factor for Candida bloodstream infection. European Cytokine Network. 2006;17:29–34. [PubMed] [Google Scholar]
- 111.Feterowski C, et al. Effects of functional Toll-like receptor-4 mutations on the immune response to human and experimental sepsis. Immunology. 2003;109:426–431. doi: 10.1046/j.1365-2567.2003.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakada TA, et al. Influence of toll-like receptor 4, CD14, tumor necrosis factor, and interleukine-10 gene polymorphisms on clinical outcome in Japanese critically ill patients. The Journal of Surgical Research. 2005;129:322–328. doi: 10.1016/j.jss.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 113.Jessen KM, et al. Common TNF-alpha, IL-1 beta, PAI-1, uPA, CD14 and TLR4 polymorphisms are not associated with disease severity or outcome from Gram negative sepsis. BMC Infectious Diseases. 2007;7:108. doi: 10.1186/1471-2334-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ferwerda B, et al. The toll-like receptor 4 Asp299Gly variant and tuberculosis susceptibility in HIV-infected patients in Tanzania. Aids. 2007;21:1375–1377. doi: 10.1097/QAD.0b013e32814e6b2d. [DOI] [PubMed] [Google Scholar]
- 115.Najmi N, et al. Human Toll-like receptor 4 polymorphisms TLR4 Asp299Gly and Thr399Ile influence susceptibility and severity of pulmonary tuberculosis in the Asian Indian population. Tissue Antigens. 2010;76:102–109. doi: 10.1111/j.1399-0039.2010.01481.x. [DOI] [PubMed] [Google Scholar]
- 116.Pulido I, et al. The TLR4 ASP299GLY polymorphism is a risk factor for active tuberculosis in Caucasian HIV-infected patients. Current HIV Research. 2010;8:253–258. doi: 10.2174/157016210791111052. [DOI] [PubMed] [Google Scholar]
- 118.Xue Y, et al. Toll-like receptors 2 and 4 gene polymorphisms in a southeastern Chinese population with tuberculosis. International journal of immunogenetics. 2010;37:135–138. doi: 10.1111/j.1744-313X.2009.00892.x. [DOI] [PubMed] [Google Scholar]
- 119.Ragnarsdottir B, et al. Toll-like receptor 4 promoter polymorphisms: common TLR4 variants may protect against severe urinary tract infection. PloS One. 2010;5:e10734. doi: 10.1371/journal.pone.0010734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hawn TR, et al. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. The Journal of Experimental Medicine. 2003;198:1563–1572. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hawn TR, et al. Toll-like receptor polymorphisms and susceptibility to urinary tract infections in adult women. PloS One. 2009;4:e5990. doi: 10.1371/journal.pone.0005990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schott E, et al. Association of TLR7 single nucleotide polymorphisms with chronic HCV-infection and response to interferon-a-based therapy. Journal of Viral Hepatitis. 2008;15:71–78. doi: 10.1111/j.1365-2893.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- 123.Wang CH, et al. TLR7 and TLR8 gene variations and susceptibility to hepatitis C virus infection. PloS One. 2011;6:e26235. doi: 10.1371/journal.pone.0026235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Al-Herz W, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Frontiers in Immunology. 2014;5:162. doi: 10.3389/fimmu.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clinical Immunology. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 126.Giovannetti A, et al. Unravelling the complexity of T cell abnormalities in common variable immunodeficiency. Journal of Immunology. 2007;178:3932–3943. doi: 10.4049/jimmunol.178.6.3932. [DOI] [PubMed] [Google Scholar]
- 127.Viallard JF, et al. Altered dendritic cell distribution in patients with common variable immunodeficiency. Arthritis Res Ther. 2005;7:R1052–R1055. doi: 10.1186/ar1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Taraldsrud E, et al. Common variable immunodeficiency revisited: normal generation of naturally occurring dendritic cells that respond to Toll-like receptors 7 and 9. Clin Exp Immunol. 2014;175:439–448. doi: 10.1111/cei.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barbosa RR, et al. Monocyte activation is a feature of common variable immunodeficiency irrespective of plasma lipopolysaccharide levels. Clin Exp Immunol. 2012;169:263–272. doi: 10.1111/j.1365-2249.2012.04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Branda RF, et al. B-cell proliferation and differentiation in common variable immunodeficiency patients produced by an antisense oligomer to the rev gene of HIV-1. Clin Immunol Immunopathol. 1996;79:115–121. doi: 10.1006/clin.1996.0058. [DOI] [PubMed] [Google Scholar]
- 131.Du X, et al. Three novel mammalian toll-like receptors: gene structure, expression, and evolution. European Cytokine Network. 2000;11:362–371. [PubMed] [Google Scholar]
- 132.Cunningham-Rundles C, et al. TLR9 activation is defective in common variable immune deficiency. Journal of Immunology. 2006;176:1978–1987. doi: 10.4049/jimmunol.176.3.1978. [DOI] [PubMed] [Google Scholar]
- 133.Escobar D, et al. Defective B cell response to TLR9 ligand (CpG-ODN), Streptococcus pneumoniae and Haemophilus influenzae extracts in common variable immunodeficiency patients. Cellular Immunology. 2010;262:105–111. doi: 10.1016/j.cellimm.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 134.Giordani L, et al. IFN-alpha amplifies human naive B cell TLR9-mediated activation and Ig production. J Leukoc Biol. 2009;86:261–271. doi: 10.1189/jlb.0908560. [DOI] [PubMed] [Google Scholar]
- 135.Krug A, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. European Journal of Immunology. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 136.Yu JE, et al. TLR-mediated B cell defects and IFN-α in common variable immunodeficiency. Journal of Clinical Immunology. 2012;32:50–60. doi: 10.1007/s10875-011-9602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sanchez-Ramon S, et al. Memory B cells in common variable immunodeficiency: clinical associations and sex differences. Clinical Immunology. 2008;128:314–321. doi: 10.1016/j.clim.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yu JE, et al. Toll-like receptor 7 and 9 defects in common variable immunodeficiency. The Journal of Allergy and Clinical Immunology. 2009;124:349–356. doi: 10.1016/j.jaci.2009.05.019. 356.e341–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Trujillo CM, et al. Quantitative and functional evaluation of innate immune responses in patients with common variable immunodeficiency. J Investig Allergol Clin Immunol. 2011;21:207–215. [PubMed] [Google Scholar]
- 140.Indrevær RL, et al. Retinoic acid improves defective TLR9/RP105-induced immune responses in common variable immunodeficiency-derived B cells. Journal of immunology. 2013;191:3624–3633. doi: 10.4049/jimmunol.1300213. [DOI] [PubMed] [Google Scholar]
- 141.Coleman MM, et al. Alveolar macrophages contribute to respiratory tolerance by inducing FoxP3 expression in naive T cells. Am J Respir Cell Mol Biol. 2013;48:773–780. doi: 10.1165/rcmb.2012-0263OC. [DOI] [PubMed] [Google Scholar]
- 142.Khare A, et al. Cutting edge: inhaled antigen upregulates retinaldehyde dehydrogenase in lung CD103+ but not plasmacytoid dendritic cells to induce Foxp3 de novo in CD4+ T cells and promote airway tolerance. Journal of Immunology. 2013;191:25–29. doi: 10.4049/jimmunol.1300193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Alimchandani M, et al. Gastrointestinal histopathology in chronic granulomatous disease: a study of 87 patients. Am J Surg Pathol. 2013;37:1365–1372. doi: 10.1097/PAS.0b013e318297427d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hatanaka E, et al. Hyperresponsiveness of neutrophils from gp 91phox deficient patients to lipopolysaccharide and serum amyloid A. Immunol Lett. 2004;94:43–46. doi: 10.1016/j.imlet.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 145.Bylund J, et al. Enhanced inflammatory responses of chronic granulomatous disease leukocytes involve ROS-independent activation of NF-kappa B. European Journal of Immunology. 2007;37:1087–1096. doi: 10.1002/eji.200636651. [DOI] [PubMed] [Google Scholar]
- 146.Brown KL, et al. ROS-deficient monocytes have aberrant gene expression that correlates with inflammatory disorders of chronic granulomatous disease. Clinical Immunology. 2008;129:90–102. doi: 10.1016/j.clim.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 147.Hartl D, et al. Dysregulation of innate immune receptors on neutrophils in chronic granulomatous disease. The Journal of Allergy and Clinical Immunology. 2008;121:375–382. doi: 10.1016/j.jaci.2007.10.037. e379. [DOI] [PubMed] [Google Scholar]
- 148.Cotugno N, et al. Defective B-cell proliferation and maintenance of long-term memory in patients with chronic granulomatous disease. The Journal of Allergy and Clinical Immunology. 2015;135:753–761. doi: 10.1016/j.jaci.2014.07.012. e2. [DOI] [PubMed] [Google Scholar]
- 149.Moir S, et al. Humans with chronic granulomatous disease maintain humoral immunologic memory despite low frequencies of circulating memory B cells. Blood. 2012;120:4850–4858. doi: 10.1182/blood-2012-05-430959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gelderman KA, et al. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12831–12836. doi: 10.1073/pnas.0604571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hultqvist M, et al. A new arthritis therapy with oxidative burst inducers. PLoS Med. 2006;3:e348. doi: 10.1371/journal.pmed.0030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.BRUTON OC. Agammaglobulinemia. Pediatrics. 1952;9:722–728. [Google Scholar]
- 153.Vetrie D, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 154.de Weers M, et al. B-cell antigen receptor stimulation activates the human Bruton's tyrosine kinase, which is deficient in X-linked agammaglobulinemia. The Journal of Biological Chemistry. 1994;269:23857–23860. [PubMed] [Google Scholar]
- 155.Aoki Y, Isselbacher KJ, Pillai S. Bruton tyrosine kinase is tyrosine phosphorylated and activated in pre-B lymphocytes and receptor-ligated B cells. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:10606–10609. doi: 10.1073/pnas.91.22.10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mukhopadhyay S, et al. Delayed clearance of filarial infection and enhanced Th1 immunity due to modulation of macrophage APC functions in xid mice. Journal of Immunology. 1999;163:875–883. [PubMed] [Google Scholar]
- 157.Horwood NJ, et al. Bruton's tyrosine kinase is required for lipopolysaccharide-induced tumor necrosis factor alpha production. The Journal of Experimental Medicine. 2003;197:1603–1611. doi: 10.1084/jem.20021845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.González-Serrano ME, et al. Increased pro-inflammatory cytokine production after lipopolysaccharide stimulation in patients with X-linked agammaglobulinemia. Journal of Clinical Immunology. 2012;32:967–974. doi: 10.1007/s10875-012-9706-z. [DOI] [PubMed] [Google Scholar]
- 159.Horwood NJ, et al. Bruton's tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. Journal of Immunology. 2006;176:3635–3641. doi: 10.4049/jimmunol.176.6.3635. [DOI] [PubMed] [Google Scholar]
- 160.Jefferies CA, et al. Bruton's tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor kappaB activation by Toll-like receptor 4. The Journal of Biological Chemistry. 2003;278:26258–26264. doi: 10.1074/jbc.M301484200. [DOI] [PubMed] [Google Scholar]
- 161.Marron TU, et al. Toll-like receptor 4-, 7-, and 8-activated myeloid cells from patients with X-linked agammaglobulinemia produce enhanced inflammatory cytokines. The Journal of Allergy and Clinical Immunology. 2012;129:184–190. doi: 10.1016/j.jaci.2011.10.009. e181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sochorová K, et al. Impaired Toll-like receptor 8-mediated IL-6 and TNF-alpha production in antigen-presenting cells from patients with X-linked agammaglobulinemia. Blood. 2007;109:2553–2556. doi: 10.1182/blood-2006-07-037960. [DOI] [PubMed] [Google Scholar]
- 163.Taneichi H, et al. Toll-like receptor signaling is impaired in dendritic cells from patients with X-linked agammaglobulinemia. Clinical Immunology. 2008;126:148–154. doi: 10.1016/j.clim.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 164.Danks L, et al. Elevated cytokine production restores bone resorption by human Btk-deficient osteoclasts. J Bone Miner Res. 2011;26:182–192. doi: 10.1002/jbmr.210. [DOI] [PubMed] [Google Scholar]
- 165.Gagliardi MC, et al. Bruton's tyrosine kinase defect in dendritic cells from X-linked agammaglobulinaemia patients does not influence their differentiation, maturation and antigen-presenting cell function. Clin Exp Immunol. 2003;133:115–122. doi: 10.1046/j.1365-2249.2003.t01-1-02178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lougaris V, et al. Bruton tyrosine kinase mediates TLR9-dependent human dendritic cell activation. The Journal of Allergy and Clinical Immunology. 2014;133:1644–1650. doi: 10.1016/j.jaci.2013.12.1085. e1644. [DOI] [PubMed] [Google Scholar]
- 167.Pérez de Diego R, et al. Bruton's tyrosine kinase is not essential for LPS-induced activation of human monocytes. The Journal of Allergy and Clinical Immunology. 2006;117:1462–1469. doi: 10.1016/j.jaci.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 168.Mansell A, et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nature Immunology. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 169.Saito K, et al. BTK regulates PtdIns-4,5-P2 synthesis: importance for calcium signaling and PI3K activity. Immunity. 2003;19:669–678. doi: 10.1016/s1074-7613(03)00297-8. [DOI] [PubMed] [Google Scholar]
- 170.Troutman TD, et al. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:273–278. doi: 10.1073/pnas.1118579109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Marron TU, et al. TLR signaling and effector functions are intact in XLA neutrophils. Clinical Immunology. 2010;137:74–80. doi: 10.1016/j.clim.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Quartier P, et al. Early and prolonged intravenous immunoglobulin replacement therapy in childhood agammaglobulinemia: a retrospective survey of 31 patients. J Pediatr. 1999;134:589–596. doi: 10.1016/s0022-3476(99)70246-5. [DOI] [PubMed] [Google Scholar]
- 173.Howard V, et al. The health status and quality of life of adults with X-linked agammaglobulinemia. Clinical Immunology. 2006;118:201–208. doi: 10.1016/j.clim.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 174.Minegishi Y, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 175.Renner ED, et al. No indication for a defect in toll-like receptor signaling in patients with hyper-IgE syndrome. Journal of Clinical Immunology. 2005;25:321–328. doi: 10.1007/s10875-005-4183-2. [DOI] [PubMed] [Google Scholar]
- 176.Hawn TR, et al. Hyper-IgE syndrome is not associated with defects in several candidate toll-like receptor pathway genes. Hum Immunol. 2005;66:842–847. doi: 10.1016/j.humimm.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 177.Yeganeh M, et al. Toll-like receptor stimulation induces higher TNF-alpha secretion in peripheral blood mononuclear cells from patients with hyper IgE syndrome. Int Arch Allergy Immunol. 2008;146:190–194. doi: 10.1159/000115886. [DOI] [PubMed] [Google Scholar]
- 178.Holland SM, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 179.Takeda K, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 180.Wang T, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 181.Lee H, et al. STAT3: a target to enhance antitumor immune response. Curr Top Microbiol Immunol. 2011;344:41–59. doi: 10.1007/82_2010_51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kwan A, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA. 2014;312:729–738. doi: 10.1001/jama.2014.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cohen A, et al. Deoxyadenosine triphosphate as a potentially toxic metabolite in adenosine deaminase deficiency. Proceedings of the National Academy of Sciences of the United States of America. 1978;75:472–476. doi: 10.1073/pnas.75.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hershfield MS, Krodich NM. S-adenosylhomocysteine hydrolase is an adenosine-binding protein: a target for adenosine toxicity. Science. 1978;202:757–760. doi: 10.1126/science.715439. [DOI] [PubMed] [Google Scholar]
- 185.Van De Wiele CJ, et al. Adenosine kinase inhibition promotes survival of fetal adenosine deaminase-deficient thymocytes by blocking dATP accumulation. The Journal of Clinical Investigation. 2002;110:395–402. doi: 10.1172/JCI15683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hershfield MS. PEG-ADA: an alternative to haploidentical bone marrow transplantation and an adjunct to gene therapy for adenosine deaminase deficiency. Hum Mutat. 1995;5:107–112. doi: 10.1002/humu.1380050202. [DOI] [PubMed] [Google Scholar]
- 187.Gaspar HB, et al. How I treat severe combined immunodeficiency. Blood. 2013;122:3749–3758. doi: 10.1182/blood-2013-02-380105. [DOI] [PubMed] [Google Scholar]
- 188.Sauer AV, et al. Defective B cell tolerance in adenosine deaminase deficiency is corrected by gene therapy. The Journal of Clinical Investigation. 2012;122:2141–2152. doi: 10.1172/JCI61788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Minguet S, et al. Adenosine and cAMP are potent inhibitors of the NF-kappa B pathway downstream of immunoreceptors. European Journal of Immunology. 2005;35:31–41. doi: 10.1002/eji.200425524. [DOI] [PubMed] [Google Scholar]
- 190.Power Coombs MR, et al. Adenosine modulates Toll-like receptor function: basic mechanisms and translational opportunities. Expert Rev Anti Infect Ther. 2011;9:261–269. doi: 10.1586/eri.10.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Conley ME, Casanova JL. Discovery of single-gene inborn errors of immunity by next generation sequencing. Current Opinion in Immunology. 2014;30C:17–23. doi: 10.1016/j.coi.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Milner JD, Holland SM. The cup runneth over: lessons from the ever-expanding pool of primary immunodeficiency diseases. Nature Reviews Immunology. 2013;13:635–648. doi: 10.1038/nri3493. [DOI] [PubMed] [Google Scholar]