Abstract
The use of statins for bone regeneration is a promising and growing area of research. Statins, originally developed to treat high cholesterol, are inhibitors of the enzyme 3-hydroxy-3-methylglutaryl, the rate-limiting enzyme of the mevalonate pathway. Because the mevalonate pathway is responsible for the synthesis of a wide variety of important biochemical molecules, including cholesterol and other isoprenoids, the effects of statins are pleiotropic. In particular, statins can greatly affect the process of bone turnover and regeneration via effects on important cell types, including mesenchymal stem cells, osteoblasts, endothelial cells, and osteoclasts. Statins have also been shown to have anti-inflammatory and antimicrobial properties that may be useful since infection can derail normal bone healing. This review will explore the pleiotropic effects of statins, discuss the current use of statins for bone regeneration, particularly with regard to biomaterials-based controlled delivery, and offer perspectives on the challenges and future directions of this emerging area of bone tissue engineering.
Keywords: tissue engineering, osteogenesis, statin, HMG-CoA reductase, local delivery
INTRODUCTION
Statin drugs have become a mainstay in the treatment of high cholesterol since the discovery in the 1970s that molecules produced by Penicillium citrinum, called citrinin and compactin (mevastatin), are potent inhibitors of an important enzyme in the cholesterol production pathway, 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA reductase) [1,2]. Though the earliest HMG-CoA reductase inhibitors were never marketed due to adverse effects seen in animals, it was not long until another naturally derived statin, lovastatin, was derived from Aspergillus terreus in 1979 by Merck researchers and found to have an acceptable toxicity profile. Since the discovery of the naturally occurring lovastatin, six additional statins have been introduced to the market. Two of these are semi-synthetic (simvastatin and pravastatin) and four are synthetic (fluvastatin, atorvastatin, rosuvastatin, and pitavastatin) [1,2]. Cerivastatin, another synthetic statin, was withdrawn from market in 2001 due to concerns over side effects.
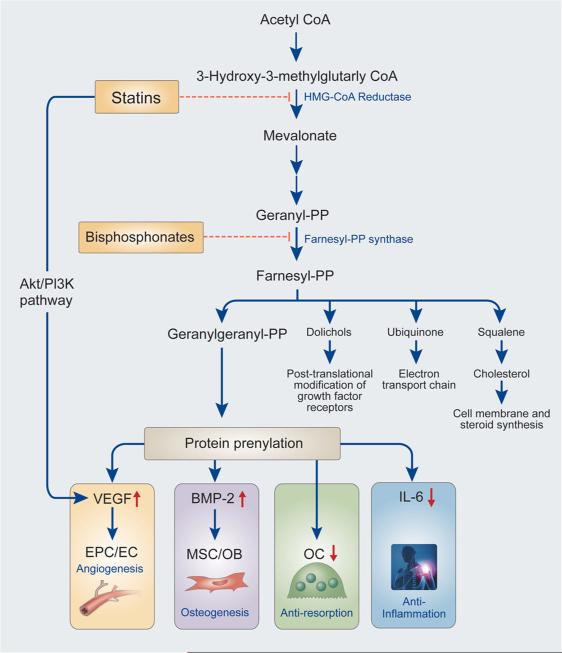
The initial impetus behind the search for HMG-CoA reductase inhibitors was the fact that this enzyme is the rate-limiting enzyme in the mevalonate pathway, which is responsible for the production of non-sterol and sterol isoprenoids, most notably cholesterol. As hypercholesterolemia is associated with adverse cardiovascular events, this enzyme was an obvious target for therapeutics. As seen in Fig. 1, the mevalonate pathway can be inhibited at a number of points, most notably at the conversion of HMG-CoA to mevalonic acid by statins and at the conversion of dimethylallyl pyrophosphate to geranyl pyrophosphate by bisphosphonates [3].
Figure 1.
A simplified flowchart of the biochemical effects of statins [3]. Statins inhibit the rate-limiting enzyme, HMG-CoA reductase, of the mevalonate pathway, which is primarily responsible for the production of steroid and non-steroid isoprenoids. In addition, statins can activate the AKT1/PI3K pathway, leading to some similar downstream effects as the inhibition of HMB-CoA reductase. Important downstream effects of statin administration are shown with blue arrows. The exact effects of statins can vary based on the specific statin and its concentration. PP, pyrosphosphate; BMP-2, bone morphogenetic protein-2; VEGF, vascular endothelial growth factor; PI3K, phosphatidylinositol-3 kinase; MSC, mesenchymal stem cell; OB, osteoblast; EPC, endothelial progenitor cell; EC, endothelial cell; OC, osteoclast.
Given the variety of biomolecules produced by the mevalonate pathway, it comes as little surprise that statins may have pleiotropic effects that extend beyond the expected cardioprotective properties. In particular, statins have begun to gain traction as a pro-osteogenic molecule after initial reports that lovastatin can stimulate the production of important osteogenic growth factors and reports of modest improvement in clinical parameters for osteoporotic patients receiving statins. Given these two important pieces of evidence, many laboratories have begun to investigate statins for use in bone regeneration and tissue engineering applications, particularly through acellular biomaterials-based local delivery strategies. However, the recent literature in this area has had varied results. In this review, the wide range of potential uses for statins will be discussed as well as the increasing relevance statins may have to the field of bone regeneration and tissue engineering.
PLEIOTROPIC EFFECTS OF STATINS
Anti-inflammatory and immunomodulatory properties of statins
Recent evidence in the literature suggests that part of the protective effects of statins with regard to cardiovascular events may be related to anti-inflammatory and immunomodulatory effects of statins. In 1995, a prospective trial of cardiac transplant patients randomized to receive either pravastatin or no statin post-transplant revealed that after 1 year, the pravastatin group had significantly lower cholesterol levels, less frequent rejection accompanied by hemo-dynamic compromise, better survival, and lower in cidence of coronary vasculopathy [4]. This study, as well as other reports that statins can significantly reduce the risk of stroke and vasculopathy post-transplant, supported the hypothesis that statins have an effect beyond simply decreasing circulating cholesterol levels and spurred interest into investigation of the anti-inflammatory effects of statins [5,6]. In vitro studies have shown that statins can reduce the production of inflammatory cytokines and modulate the ability of the immune system to recognize and attack allotransplanted cells [7,8]. Prospective trials such as the PRINCE (pravastatin inflammation/CRP evaluation) trial further support the anti-inflammatory properties of statins by showing that median C-reactive protein, a blood marker of inflammation, levels were reduced 16.9% at 24 weeks in patients taking pravastatin (P < 0.001) [9]. Additionally, a recent randomized, double-blind, multi-center trial indicates that patients on high-dose atorvastatin have less inflammation in atherosclerotic plaques than patients on low-dose atorvastatin [10].
While statins were initially developed for use in reducing cardiovascular events, their putative anti-inflammatory and immunomodulatory properties make them appealing for a wide variety of pathologies in diverse fields. Since many neurologic disorders are affected by lipid metabolism and inflammation, statins are being investigated for their possible neuroprotective properties with regard to diseases such as persistent seizures and Alzheimer's disease [11–13]. In the field of orthopedics, statins are being investigated as a possible solution to the destruction of cartilage by inflammation, as seen in osteoarthritis. While some studies support the use of statins to reduce inflammation, leading to increased survival of chondrocytes, another study has noted detrimental effects of atorvastatin and pravastatin with regard to the development of osteoarthritis [14–17]. When used in bone applications, locally applied simvastatin has been found to cause increased inflammation in a dose-dependent manner [18,19]. However, this effect may be a particular effect of simvastatin and not of statins in general. Subramanian et al. have found that patients on high-dose rosuvastatin (80 mg/day) had reduced periodontal inflammation compared to patients on low-dose rosuvastatin (10 mg/day) [20].
Use of statins for osteoporosis
Osteoporosis and low bone mass affect almost 80% of elderly women, as well over 40% of elderly men [21]. The disease is characterized by a decrease in bone mineral density (BMD), causing ‘brittle’ bones, but its effects are seen in the increased frequency of non-pathological fractures that are caused by the weakening of the bones [22]. Bisphosphonates are a class of drugs that are used currently to prevent the loss of bone mass [1]. Although bisphosphonates have seen clinical efficacy in a variety of pathologies, such as cancer metastases, multiple myeloma, Paget's disease of the bone, and osteoporosis, they can also cause serious side effects, most notably osteonecrosis of the jaw [1]. Given the high affinity of bisphosphonates for bone, some hypothesize that the accumulation of intravenously administered bisphosphonates in bone can lead to later necrosis of soft tissue and bone in the mandible and maxilla, especially following dental procedures [1,23,24]. As seen in Fig. 1, bisphosphonates inhibit an enzyme in the mevalonate pathway downstream of HMG-CoA reductase, giving rise to the hypothesis that statins could also be beneficial in pathologies related to loss of bone mass, such as osteoporosis, while reducing the potential for later osteonecrosis. In vitro studies have indicated that statins have direct anti-osteoclast activity, interfering with osteoclast function, although in vivo conclusions are conflicting [25–28].
When studying osteoporosis, two factors may be used to judge the severity of the disease: BMD and fracture risk. Various clinical studies have indicated that patients who had used statins to treat hyper-cholesterolemia experienced a beneficial effect in fighting osteoporosis. In the study of statins’ effects on osteoporosis, BMD and fracture risk have been examined in systemic statin users, both in cross-sectional retrospective studies and in controlled randomized trials. Within the literature, there have been widely varying opinions on the efficacy of statins to increase BMD and reduce fracture risk.
Initially, observational studies generally reported an overall beneficial effect of statins on combating osteoporosis. Several studies have suggested that statins may increase BMD in a broad selection of patients, alone and in combination with hormone replacement therapy and other treatments, though many of these studies do not reach significance and advocate for further randomized controlled trials in order to establish a causal relationship [29–34]. However, positive effects were not seen in fluvastatin users [35,36], indicating that not all statins have the same effects on bone turnover and resorption, and many observational studies reported no significant effect on BMD [37,38]. The latter studies primarily focused on postmenopausal women, finding that the statins had no effect in reducing bone loss in these patients, perhaps indicating that statins are better suited towards prevention of osteoporosis rather than as a general, systemic treatment. In addition, controlled clinical studies tended to cautiously report more positive effects of statins for improving BMD, although some report neutral effects as well [39–43]. Effects of statins in these types of studies can be difficult to evaluate because bone mass loss often occurs within the setting of comorbidities, such as Type 2 diabetes mellitus and hormonal disturbances. It is likely that any positive effects of statins may be more beneficial for certain patient populations. Of note, several of these studies have suggested that there may be different effects seen with hydrophobic statins, such as simvastatin, compared to hydrophilic statins, such as pravastatin [36,39].
Many cross-sectional studies in several population types have investigated whether statin users have a reduced risk of fracture. Although there continues to be dissent among the reports, with many finding that statins had no effect on fracture risk [37,44], the majority of studies found that statins led to a reduced risk of fracture as high as 71%; however, there is a large range of reduced fracture risks, with the lowest seeing no reduction in risk [32,45–49]. The beneficial effects were not seen in pravastatin and fluvastatin [50], though combining statins with hormone replacement therapy again showed positive results [51]. Furthermore, increased accumulated statin use corresponded to a subsequent decrease in the risk of fracture [46–48]. Due to the necessity of a long time frame in order to accurately assess the fracture risk in statin users, fewer controlled studies have been performed to examine the effects of statins on fracture frequency, although the few that have been performed have not seen any marked benefits from statin use in regard to reduced fracture risk [50,52].
Extensive reviews and meta-analyses of the literature regarding the use of statins for osteoporosis have been conducted by others [53], and a representative summary of important studies can be found in Table 1.
Table 1.
A summary of conclusions on the effects of statins on indicators of osteoporosis. The sources are sorted by indicator measured as well as the type of study that was performed. Studies included in the various subsection under statin type evaluated statins as a general drug class and did not distinguish the differences in results between specific types.
| Statin type | Study method | Measured observable | General results | References | Comments |
|---|---|---|---|---|---|
| Various | Observational | BMD | Positive | [31] [29] [30] [34] [33] |
Improved BMD and lower levels of bone turnover, especially when combined with HRT |
| Prospective | No Effect | [37] [38] |
No significant effect on BMD in postmenopausal women | ||
| Observational | Fracture Risk | Positive | [32] [49] [51] |
Reduced risk of fracture, synergistic effects with HRT | |
| [47] [46] [55] [45] |
Reduced risk of fracture compared to non-statin lipid-lowering agents | ||||
| No Effect | [54] | Increased incidence rate compared to beta-blockers | |||
| Prospective | [37] [44] |
No significant effect on incidence of clinical fractures in postmenopausal women | |||
| Simvastatin | Observational | Fracture Risk | Positive | [48] | Reduced risk of fracture |
| Prospective | BMD | [39] [40] [42] [36] |
Reduced bone resorption and increased BMD and bone formation markers in osteopenic and osteoporotic patients, including postmenopausal women | ||
| No Effect | [43] | No significant effect on BMD in postmenopausal women | |||
| Atorvastatin | Prospective | BMD | Positive | [41] [36] |
Improvement in BMD over bisphosphonates alone |
| Fracture Risk | No Effect | [52] | No improvement of the relative risk ratio over two years | ||
| Pravastatin | Observational | Fracture Risk | No Effect | [47] [46] |
Non-pravastatin statin use was associated with a reduced risk of fracture (up to 0.52) compared against other lipid-lowering drugs; pravastatin use showed no beneficial effects |
| Prospective | [50] [52] |
No improvement of the relative risk ratio, possible negative effects | |||
| Fluvastatin | Prospective | BMD | No Effect | [55] [50] [36] |
No reduction in bone loss or increase bone growth in postmenopausal women with osteoporosis |
Because of the inconclusive nature of the present literature, some have raised the question of whether the reduced risk of fracture was a result of the ‘healthy drug-user effect’, which in this context asserts that patients who use a cholesterol-reducing drug like statins would in general work to have better health, which could lead to a false positive in regard to statins’ protective effects for bone [54]. In order to assess this risk, independent studies were conducted that examined statin users compared to users of other lipid-lowering drugs, and although there was one dissenting study [54], statin users were found to have a reduced risk of fracture compared to users of non-statin lipid-lowering drugs [45,47,55].
A cautiously positive trend in favor of the use of statins in prevention of osteoporosis can be found in the current literature, although the existence of conflicting reports necessitates further studies. Aside from the ‘healthy drug-user effect’, it may be possible that the dosage used for cardiovascular effects is suboptimal or that the limited concentration of statins available to bone via oral delivery is inadequate to limit the onset and effects of osteoporosis. This hypothesis inspires further study of statins in concentrated, local delivery systems within the context of bone tissue engineering.
Antimicrobial properties of statins
Given that the search for statins began as a screen of fungi metabolites that would inhibit HMG-CoA reductase, it is perhaps unsurprising that statins can display antimicrobial properties [2]. A major challenge in bone regeneration is the healthy integration of scaffolds that facilitate bone tissue regeneration into the injured area. Often, opportunistic bacterial, fungal, and viral infections complicate healing and become even harder to treat when they form biofilms that are more difficult to fight with traditional systemic antibiotic treatment, which may not reach the affected area in high enough doses. As the number of strains of bacteria that are resistant to traditional antibiotics increases, new treatments for infection are becoming necessary. Recently, there has been evidence that various statins have antimicrobial properties that, when combined with their other potentially helpful effects, make them a useful tool for bone tissue engineering.
Sepsis
Due to the anti-inflammatory effects that spurred the use of statins for treatment of heart disease, it has been of interest as to whether the drugs could have positive benefits for sepsis patients who also suffer from severe systemic inflammation. A review of clinical evidence, comprised primarily of retrospective studies, indicates that statin use assists in the treatment of patients with sepsis [56]. Additionally, during a randomized double-blind placebo-controlled trial, high doses of atorvastatin (40 mg/day) were seen to reduce the severity of sepsis by preventing its progression; however, it did not significantly reduce mortality rates [57]. This trend was observed in other clinical studies, where statin use was associated with improved 28-day mortality rates, but no statistically significant improvement was seen in 90-day mortality rates [58,59]. Additionally, statin use was not observed to affect IL-6 levels, a critical inflammation marker for sepsis, although there was a lower baseline in previous statin users [59]. However, there is a strong indication that statins can lower IL-6 levels by blocking isoprenoids, thereby blocking post-translational lipid modifications essential to the immune response and production of IL-6 [60–63]. More evidence is needed to definitively determine if statins affect IL-6 levels, a subject of some current trials.
Antiviral and fungicidal
Additionally, various statins have been shown to have antiviral and fungicidal effects. Through their inhibition of HMG-CoA, statins help to impede the creation of lipid rafts that carry the hepatitis C virus (HCV). Many statins, especially fluvastatin with simvastatin and atorvastatin exhibiting moderate activity, have a synergistic effect with PEGylated inter-feron, a staple of HCV management, thereby assisting in the treatment of HCV [64,65].
Candida albicans is an opportunistic human pathogenic fungus; lovastatin was seen to have a minimum inhibitory concentration (MIC) of 16 μg/mL and showed strong fungistatic effects against C. albicans [66]. Moreover, several statins, especially simvastatin and fluvastatin, were seen to act synergistically with various antifungals, showing strong fungicidal effects in vitro [67,68].
Antibacterial
Antibacterial effects of statins have also been found [69]. Statin therapy has been shown as beneficial in the treatment of bacteremia caused by Staphylococcus aureus, possibly by interfering with the inflammation caused by the S. aureus α-toxin [70,71]. Additionally, prolonged simvastatin therapy had a dose-dependent protective effect against Streptococcus.pneumoniae and reduced the bacterial concentration in the lungs, but mortality rates were equivalent to controls in an in vitro study in rats [72].
Further studies have been conducted to test the efficacy of a variety of statins, primarily simvastatin, against several clinically important bacteria. Atorvastatin and simvastatin were effective against various strains of bacteria, including methicillin-sensitive Staphylococcus aureus (MSSA), when dissolved in dimethyl sulfoxide (DMSO), although they were less effective against clinically isolated strains like methicillin-resistant Staphylococcus aureus (MRSA) [73]. When methanol was used as a solvent, simvastatin and fluvastatin treated both MSSA and MRSA, with simvastatin being the most effective against MSSA with an MIC of 29.2 mg/L, dissolved in methanol [73]. Simvastatin and atorvastatin were tested against S. aureus, Enterococcus faecium, Pseudomonas aeruginosa, and other bacteria in vitro. Simvastatin had an MIC of 32 μg/mL for S. aureus and 64 μg/mL for E. faecalis, but MICs of >128 μg/mL for all other strains; atorvastatin had an MIC of >128 μg/mL for all strains [74]. Although this suggests that the statins tested are not clinically relevant in systemic concentrations, higher, effective doses can be achieved by local delivery achievable in tissue engineering contexts.
Many experiments testing the antimicrobial effects of statins use pure methanol as a solvent; this study found that simvastatin in 100% methanol performed only marginally better than pure methanol, and simvastatin in 5% methanol (pH adjusted to improve solubility) was more than an order of magnitude less effective [75]. This indicates that the use of methanol and other organic solvents for testing of low-water-soluble statins may confound testing; however, this could be improved by pH adjustment that improves statin solubility in water. Additionally, it was recently found that simvastatin in 2.5% DMSO, a solvent solution which did not exert any antibacterial effects by itself, was found to kill S.pneumonia and Moraxella catarrhalis in a dose-dependent manner with an MIC of 15 μg/mL [76]. This suggests that further tests are needed with non-organic solvents to assess the interference that occurs from the antimicrobial effects of many solvents. Additionally, an interesting topic of study is the assessment of any synergistic effects of statins with conventional antibiotics, of which there is limited information in the literature.
STATINS, BONE REGENERATION, AND BONE TISSUE ENGINEERING
The canonical tissue engineering paradigm involves using a combination of biomaterial scaffolds, cells, and/or bioactive factors to augment or stimulate regeneration of lost tissue [77]. Traditional replacement of missing tissue has been accomplished through transplantation of tissue from a donor. However, transplantation has serious drawbacks, including suboptimal tissue quality, potential for disease transmission, and inadequate donor volume to meet patient specific and population needs [77]. The goal, therefore, of tissue engineering is to replace tissue transplantation with implantation of constructs that stimulate endogenous regeneration and repair. While the traditional paradigm focuses heavily on the use of cell-seeded constructs, there are several practical and regulatory challenges associated with the translation of cell-based therapies and products into the clinic [78]. Because of these challenges, there is increased interest in acellular, biomaterials-based delivery of pro-osteogenic drugs for bone regeneration. Controlled delivery of shelf-stable small molecules, such as statins, that can stimulate endogenous cells to produce functional bone is particularly attractive because it circumvents important regulatory issues such as cell implantation and practical issues of implementation and clinical use, resulting in the development of a shelf-stable and relatively low cost therapeutic for bone regeneration.
Stimulation of growth factor expression
For bone tissue engineering applications, much of the literature regarding regeneration of bone has focused on the delivery of bone morphogenetic proteins (BMPs) and vascular endothelial growth factor (VEGF), two growth factors that have been widely investigated for use in the regeneration of bone [79–81]. However, these factors are expensive, have a relatively short half-life, and can be difficult to process into scaffolds and delivery vehicles due to the 3D conformation required for bioactivity. Thus, small molecule drugs that can be processed into sustained delivery vehicles and that can stimulate endogenous cells to increase production of BMPs and/or VEGF are valuable in the tissue engineering field.
The effects of statins on VEGF production are beginning to be elucidated. Simvastatin, atorvastatin, and cerivastatin, but not pravastatin, have been found to augment VEGF mRNA expression in osteoblastic cells in vitro [82]. This study investigated the effect of concentration and found that 1–10 μM simvastatin, 10–100 μM atorvastatin, and 0.1–1 μM cerivastatin were able to stimulate increased expression of VEGF mRNA. A study of ischemic hind limb injury in normocholesterolemic mice showed that systemic administration of cerivastatin (6 mg/kg/day for 3 days) resulted in increased collateral blood flow to the limb compared to saline controls [83]. These effects were also seen in a hind limb ischemia mouse model with administration of simvastatin (0.20 mg/kg/day for 21 days) and mesenchymal stem cells (MSCs) [84]. The stimulation of VEGF production by statins is dependent not only on statin type but also on cell type. Frick et al. reported that atorvastatin, simvastatin, and lovastatin in the range of 1–10 μM have reduced basal levels of VEGF production in human vascular smooth muscle cells and microvascular endothelial cells while increasing VEGF synthesis in human umbilical vein endothelial cells in vitro [85]. In addition, a concentration-dependent effect was seen in which low concentrations of statins displayed pro-angiogenic activity in umbilical vein endothelial cells while high concentrations of statin inhibited angiogenesis, despite increased levels of VEGF synthesis [85]. Weis et al. also describe this biphasic effect of statins on VEGF production using cerivastatin and atorvastatin with microvascular endothelial cells [86]. As with any pro-angiogenic factor, there are associated concerns with neovascularization that can lead to problems such as retinopathy, though currently available studies do not indicate that therapeutic doses for cholesterol control have a deleterious effect [87,88]. As seen in Fig. 1, the increased production of VEGF seen with statin administration in vitro is likely mediated by the Akt/phosphatidylinositol-3 kinase pathway [89,90].
In 1999, Mundy et al. reported that from a screen of over 30 000 compounds from a collection of natural products, lovastatin was the only compound in the collection that increased the expression of BMP-2 in an immortalized murine osteoblast cell line [91]. Following this discovery, many investigators have sought to clarify the influence of statins on the production of BMPs and elucidate the mechanism behind the observed expression [92,93]. Evidence suggests that the increased production of BMP-2 is mediated via the Rho pathway [94, 95]. Some studies have also found that not all statins stimulate the production of BMP-2 to equal extent. Sugiyama et al. studied the stimulation of BMP-2 promoter in human osteosarcoma cells treated with compactin, simvastatin, and pravastatin, and found that only compactin and simvastatin were capable of stimulating the BMP-2 promoter [96]. Additionally, the treatment of different cell types with the same statin results in differential promotion of BMP-2 expression. These results were further elaborated upon by Kupcsik et al. by looking at the effects of pravastatin, lovastatin, and simvastatin on clinically relevant human MSCs [97]. Upregulation of BMP-2 mRNA expression was seen in lovastatin- and simvastatin-treated groups, but not in pravastatin-treated groups. A hypothesis for this difference is that water-soluble statins such as pravastatin do not localize heavily within the cell since they must enter the cell via active transport, whereas hydrophobic statins such as lovastatin, simvastatin, and compactin can passively diffuse through the cell membrane [96,97]. However, a study utilizing another water-soluble statin, rosuvastatin, found that this statin was able to induce osteoblastic differentiation of rat MC3T3-E1 cells, indicating that perhaps specific transporter proteins influence the suitability of a particular statin for osteogenesis in addition to water solubility and hydrophobicity [98]. Interestingly, BMP-2 may act as a tumor suppressor for some colorectal cancers, and there is some epidemiologic evidence that suggests that long-term use of statins may decrease the incidence of colorectal cancer [99,100].
In vivo bone regeneration and osseous integration
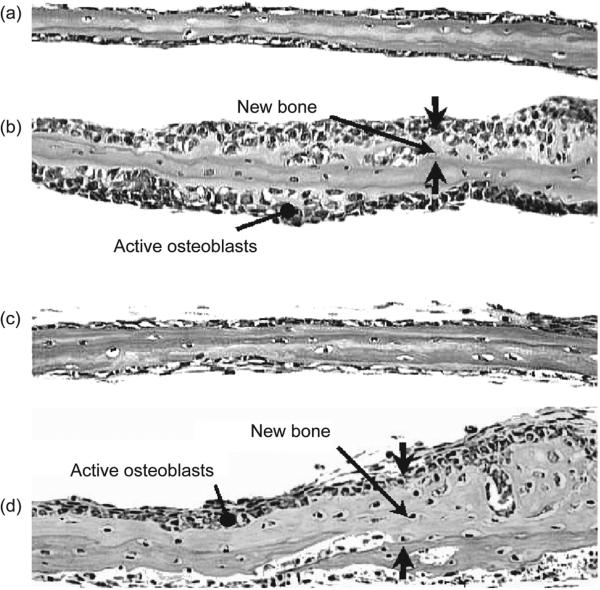
After the discovery that lovastatin could promote the expression of BMP-2, Mundy et al. injected lovastatin and simvastatin subcutaneously over the calvaria of mice and found marked increases in the amount of bone formed in treated mice, as shown in Fig. 2 [91].
Figure 2.
Histologic images from Mundy et al. of explanted murine treated with 1 μM simvastatin for four or seven days. Panels 1 and 2 show calvaria treated with control media or simvastatin, respectively, for four days. Panels 3 and 4 show calvaria treated with control media or simvastatin, respectively, for seven days. Simvastatin-treated calvaria displayed increased new bone formation compared to control, as well as an increase in mature osteoblasts. This figure is reprinted from [91] with permission.
Since then, many studies have examined whether statins can aid in the healing of fractures and bone defects. While many animal studies support the hypothesis that orally administered statins can enhance fracture healing and bone regeneration, other studies have questioned whether the increase in BMP-2 expression translates to increased bone healing [101,102].
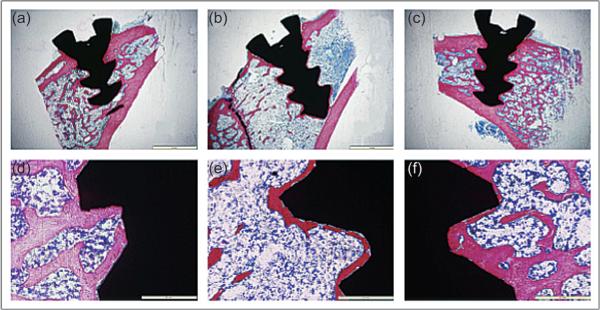
Closely related to the applications of fracture and defect repair, statins are being investigated for their potential to enhance osseous integration of implanted materials through systemic delivery and local delivery. This is of particular interest in both the orthopedic and craniofacial fields, as metallic implants are common modes of fixation and reconstruction. Masuzaki et al. found that fluvastatin-loaded microparticles injected at a distant site enhanced the osteogenesis around titanium rods implanted into a rat tibia [103]. This delivery technique was unique in that it utilized a local delivery vehicle to achieve systemic delivery through a non-parenteral and non-intravenous route. Similarly, Ayukawa et al. found that intraperitoneally delivered systemic simvastatin also enhances the integration of titanium rods in rat tibiae [104]. As seen in Fig. 3, Du et al. applied tibial screws in ovariectomized rats with osteoporotic bone changes and showed that oral administration of simvastatin is capable of improving osseointegration of the screws compared to ovariectomized rats not receiving simvastatin [105].
Figure 3.
Histologic images from Du et al. showing the osseous integration of screw-shaped titanium implants in the tibiae of non-ovariectomized rats (a, d), ovariectomized rats (b, e), and ovariectomized rats receiving 5 mg/kg/day simvastatin orally post-surgery (c, f) at 84 days post-implantation. Non-ovariectomized rats and ovariectomized rats receiving simvastatin showed significantly thicker new bone formation compared to ovariectomized rats receiving no simvastatin. This figure is reprinted from [105] with permission.
However, conflicting studies on the efficacy of statins with respect to osseointegration can also be found. Pauly et al. conducted a study in which rats received retrograde nailing of the femur with titanium implants coated with simvastatin for local release and reported that coated implants displayed decreased fixation strength compared to uncoated controls [106]. Additionally, another osteoporotic rat model showed no discernible advantage of local release from a simvastatin-coated titanium tibial implant, though a bisphosphonate-coated implant showed improved osseointegration over control [107]. While the models used are not precisely comparable, the discrepancy in results may be attributable in part to the disparity in total dosage provided to the animals. In the studies where positive effect of either fluvastatin or simvastatin was noted, whether locally delivered or orally delivered, the total dosage was determined by weight and supplied of the order of milligrams [103,105,108]. In the studies where negative or no effect was seen, simvastatin was supplied of the order of micrograms per implant, approximately a 1000-fold decrease compared to the studies that showed a positive effect [106,107].
Local release
The demonstrated potential of statins to promote bone regeneration has spurred an interest in the development of local delivery strategies for statins. Local delivery is of interest for a number of reasons. First, systemic administration of statins can result in rare but serious side effects, most notably liver toxicity, myositis (inflammation of the muscle), and rhabdomyolysis (severe muscle inflammation and damage) [109,110]. Second, local delivery allows an adequate dosage to be delivered to the desired area without relying on systemic administration, which depending on the situation may be hindered by impaired vascularity. Local administration may lessen the possibility of widespread muscle and liver damage and obviate any unintended effects of statin usage given its pleiotropic effects while providing therapeutic benefit to the delivery area. Additionally, the concentrations needed to take advantage of the antimicrobial nature of statins would be difficult to achieve with systemic delivery, but are feasible with local delivery. Local delivery vehicles for small molecule drugs can face several challenges. Most importantly, small molecular weight drugs tend to release more quickly than large molecular weight drugs since the mesh size has less of an impact on small drugs than large drugs, such as proteins and peptides. However, biomaterials engineers can attempt to manipulate release rate through choosing and modifying material parameters such as hydrophilicity/hydrophobicity, rate of degradation, mode of degradation, crosslinking density, and swelling.
Hydrogels are a popular method of local delivery for bone applications, and modifications of these systems for statin delivery have been investigated. Hydrogels are a heterogeneous group of biomaterials and can be either naturally derived or synthetic. Hydrogels such as collagen, gelatin, and poly(ethylene glycol) have the distinct advantage of being able to swell with water, making them very easy to load with drugs. In addition, modifications can be performed in order to impart desired characteristics, such as degradation or retention of bioactive factors. Benoit et al. synthesized a novel fluvastatin-releasing hydrogel with fluvastatin covalently tethered via lactic acid linkages into a poly(ethylene glycol) hydrogel and released by degradation of lactic acid into the surrounding area [111]. This system allowed for tuning of release rate by modulation of lactic acid repeats, and released fluvastatin tested on human MSCs resulted in greater BMP-2 production and mineralization [111]. This work was further elaborated upon by introducing heparin domains to sequester BMP-2 in order to more closely mimic the native extracellular matrix (ECM) surrounding bone and enhance the rate of stem cell differentiation [112]. Zou et al. have investigated an injectable, dual-hydrogel delivery system with simvastatin-loaded gelatin microparticles incorporated into a bulk phase of carboxymethylcellulose loaded with clodronate, a bisphosphonate. By utilizing two distinct hydro-gels for each drug, temporal spacing of drug delivery was achieved, allowing the bisphosphonate to first inhibit resorption of bone followed by stimulation of osteogenesis by statins [113]. A gelatin delivery system has been used to deliver simvastatin to a femoral fracture in vivo and been found to be effective at potentiating the regeneration of bone and blood flow in mice [114]. Collagen sponges have been used to deliver water-soluble statins. Alam et al. delivered pravastatin locally to a nasal bone defect in rabbits and found that statin-treated animals showed similar amounts of BMP-2-positive cells compared to rhBMP-2-treated rabbits [115]. Monjo et al. delivered rosuvastatin to critical size rabbit tibial defects via collagen sponge and found a dose-dependent increase in BMP-2 mRNA expression in cells surrounding the implant area as well as new bone formation in rosuvastatin-treated defects not seen in sham animals [116]. Jeon et al. sought to further refine the use of statins for bone regeneration by developing a cellulose acetate phthalate/Pluronic F-127 sintered-microparticle multi-layered construct for alternating release of parathyroid hormone (PTH) and simvastatin [117]. This strategy takes advantage of the interesting property that while continuous release of PTH results in bone resorption, intermittent delivery of PTH results in increased trabecular bone volume and improved BMD, biomechanical strength, and microarchitectural parameters in vivo, likely mediated through triggering of the osteogenic genes insulin-like growth factor 1 as well as BMPs [118,119]. Jeon et al. demonstrated that alternative release of PTH and simvastatin resulted in additive effects of the two therapies rather than synergistic, though they acknowledge that further optimization of dosage and timing could be performed [117].
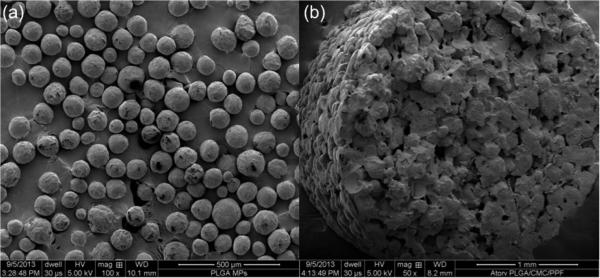
Ceramics and synthetic polymers are also being investigated as carriers for statins. Ceramic bone cements are readily available in FDA-approved formulations and provide a framework upon which bone can grow. In some cases, it is resorbable, allowing native bone to slowly replace it, and has been well characterized as a delivery vehicle for other small molecule drugs such as antibiotics. Simvastatin loaded into calcium phosphate cements has been shown to enhance both the osteogenic potential and degradation rates of these materials [120–122]. Similarly, simvastatin-loaded calcium sulfate cement has been shown to increase bone regeneration in a rat calvarial defect at eight weeks compared to non-simvastatin treated rats, despite the induction of a robust inflammatory response [123]. Simvastatin-loaded hydroxyapatite has also been characterized, though the slow degradation rate of this ceramic limits the bone-forming potential [120]. Synthetic polymers are another alternative to naturally derived polymers and have the advantage of being highly modifiable and tunable, though they also lack natural environmental cues to direct tissue growth. Poly(lactic-co-glycolic acid) (PLGA) and poly(lactic acid) are common synthetic polymer carriers for statins, either as microparticles or a membrane [103,124–126]. Stein et al. combined hydrogel and poly(lactic acid) to locally deliver simvastatin to the lateral aspect of the rat mandible and investigated dose response, finding that a low dose applied locally could achieve bone growth while avoiding the inflammation that occurs with administration of high doses [19]. Pauly et al. utilized simvastatin-loaded poly(lactic acid) to coat Kirschner wires for stabilization of closed rat tibial fractures and found comparable healing with application of simvastatin-coated wires compared to rhBMP-2-coated wires [127]. Simvastatin-loaded electrospun poly(ε-caprolactone) has also been investigated for regeneration of cranial defects [128]. In this study, scaffolds that displayed a burst release of simvastatin did not increase bone regeneration, but scaffolds with sustained release had significantly increased bone regeneration over both untreated controls and burst released statin-loaded scaffolds [128]. For load-bearing applications, stronger and slower degrading polymers could potentially be used, such as poly(propylene fumarate) (PPF) [129]. These polymers can be made porous to facilitate loading with drug-loaded microparticles so that the construct acts as a scaffold for bone growth while releasing osteogenic factors for recruited stem cells. Fig. 4 shows scanning electron micrographs of atorvastatin-loaded PLGA microparticles and a composite scaffold composed of PPF made porous using a hydrogel porogen and loaded with atorvastatin-loaded PLGA microparticles.
Figure 4.
Scanning electron micrographs of (a) PLGA microparticles loaded with 30 wt% atorvastatin and (b) a cross-section of a dried composite scaffold comprising 40 wt% atorvatastin-loaded PLGA microparticles, 40 wt% of the porogen carboxymethylcellulose hydrogel (9 wt% carboxymethylcellulose), and 20 wt% PPF on a wet basis imaged prior to leaching of the porogen. In (b), the distribution of drug-loaded PLGA microparticles and porogen can be appreciated. Note that the scale bar in (a) is 500 μm and in (b) is 1 mm.
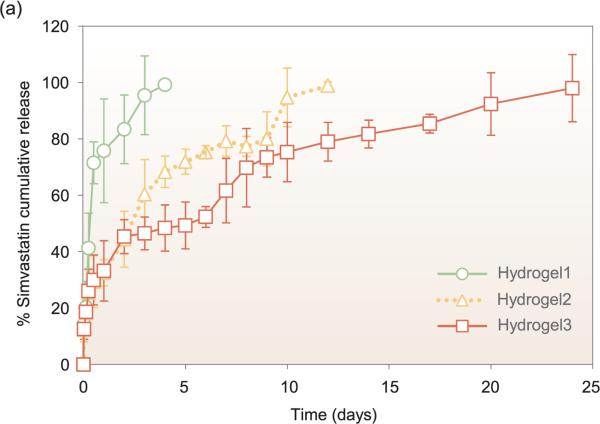
The optimal release kinetics of statins for bone regeneration have yet to be identified and in fact may differ based on type of statin. Release of hydrophobic statins from PLGA-based carriers appears to generally follow either zero-order release kinetics or an attenuated burst release followed by sustained release [103,125]. The low water solubility of hydrophobic statins combined with the hydrophobicity of PLGA may combine to extend the release of these drugs through low water penetration into the carrier and minimal solubilization of drug, though it is difficult to make any definitive conclusions due to the difference in geometries and release conditions between studies. Interestingly, hydrogels and hydrogel microparticle carriers can also show a moderate burst release (~40%) followed by sustained release despite the high water content of hydrogels, as seen in Fig. 5 [113,130].
Figure 5.
Biomaterial carriers and formulations for local release can significantly affect the release kinetics. Park et al. demonstrated that simvastatin loaded into hydrogels using a 1 mg/mL solution displays different release kinetics based on varying hydrogel stiffnesses. The hydrogel stiffnesses tested were 1800, 5800, and 8400 Pa for Hydro-gel1, Hydrogel2, and Hydrogel3, respectively. This figure is adapted from [130] with permission.
In contrast, release of the water-soluble statin rosuvastatin from an absorbable collagen sponge showed the typical burst release pattern, with 82% of loaded drug released during 24 h [116]. These distinct release patterns could affect the differentiation and proliferation of recruited stem cells and alter the activity of mature osteoblasts in the treated area via the amount and duration of release. The choice of appropriate polymer type will depend on the release kinetics determined to be the best for osteogenesis, with hydrogels generally releasing high concentrations of drug for shorter duration and polymers having a more tunable release profile.
Bone tissue engineering
Because canonical tissue engineering involves the use of a combination of scaffold, cells, and bioactive factors in order to stimulate the regeneration and repair of native tissue, there is currently a relative dearth of studies in this emerging niche using true tissue engineering scaffolds. Thus far, most researchers have relied primarily upon controlled acellular delivery of statins to act upon MSCs recruited through the natural inflammatory response. Benoit et al. have encapsulated human MSCs within a fluvastatin-releasing hydrogel with growth factor binding heparin domains and have shown that the use of MSCs within this construct is able to further augment MSC differentiation compared to fluvastatin release alone [112]. While acellular delivery is perhaps more desirable from the standpoint of immune response and ease of clinical translation, Benoit et al. have demonstrated that there are still clear benefits to the use of the traditional tissue engineering paradigm. As scaffolds that release statins in a controlled manner continue to be developed and characterized and the role of statins in the regenerative process becomes elucidated, the use of statins for tissue engineering scaffolds may become increasingly popular. As it stands currently, however, very few researchers have endeavored to use cellularized scaffolds with concomitant controlled release of statins either in vitro or in vivo.
Challenges and perspectives on future directions
It is clear that there are many challenges left to address in the emerging area of statin-stimulated bone regeneration. First, meaningful comparisons between types and effective concentrations of statins are difficult to elucidate from published in vitro and in vivo studies due to the differences in methodology and administration methods. With regard to type of statin, some efforts have been made to distinguish why different statins cause different effects, and there is some evidence that the answer may lie in the water solubility or hydrophobicity of the different types [96,97]. Another question that remains is to elucidate how other pleiotropic effects of statins could affect bone regeneration. For instance, initial inflammation is necessary for bone healing, but excessive inflammation is detrimental to bone regeneration [131,132]. It is possible that the anti-inflammatory properties of statins, which appear to differ based on specific type, may aid by limiting inflammation. Alternatively, given the effects that some statins have on angiogenesis, it is possible that the formation of blood vessels, another key process in bone healing, leads to the regeneration of bone. Most likely, the effect of statins on bone healing is a complex interaction of the many pleiotropic effects of statins that will require systematic experimentation to elucidate. Because of this, there remains concern that the effects of statins may be inconsistent and unpredictable, making this strategy unappealing for clinical translation.
The cells used to evaluate statins are not standardized and include a broad range of cell types, most commonly non-human primary cells and osteosarcoma MC3T3-E1 cell lines and less commonly, human primary cells [91,97,133]. The wide range of concentrations used in the studies published thus far are also indicative that each statin may have an optimal concentration for a particular cell type, and concentrations found to be optimal in a cell line such as MC3T3-E1 may not translate to human MSCs, which are the most relevant cell type for bone tissue engineering.
With regard to acellular controlled release of statins, further work still remains to be done to determine what type of release profile, burst release or sustained release, is optimal for bone regeneration. If sustained release is necessary, it will also be important to determine threshold dosages as well as the duration of delivery. Several of the studies discussed in this review drew conclusions regarding the efficacy of locally delivered statins for in vivo osseointegration and in vitro differentiation of cells. However, these conclusions were based on estimates of the appropriate dosage and timing parameters. Though some systematic experimentation to determine the optimal concentration of certain statins on different cell types has been performed [134,135], the effects of total dosage and release kinetics of local delivery systems on the in vitro differentiation of MSCs and bone regeneration in vivo should be addressed and optimized. The appropriate dosing for controlled release applications may be very difficult to determine given that many patients already receive statins systemically for cardioprotective purposes. However, given the conflicting results of prospective randomized trials regarding the use of statins for osteoporosis, systemic administration may or may not be of clinical significance for bone regeneration applications.
The use of statins could be expanded beyond acellular controlled release by introducing cells into statin-loaded constructs to investigate whether the co-delivery of cells and statins can stimulate earlier tissue regeneration in vivo. Dual delivery of statins with other biomolecules such as PTH or BMP-2 may be further investigated to determine whether synergistic effects on bone regeneration can be elicited. Furthermore, given the antimicrobial and antifungal properties of some statins, local release of statins may have some interesting effects in contaminated bone defects. These areas are ripe for investigation, and addressing any of these issues would greatly progress the field and further inform the utility of statins for bone regeneration and tissue engineering.
CONCLUSIONS
Statins show an impressive range of effects in the human body and can be leveraged for a wide variety of potential applications other than hyperlipidemia, including transplant medicine, infectious diseases, and neurologic disorders. In particular, statins show incredible promise as a therapeutic agent in the area of bone regeneration and bone tissue engineering. While systemic delivery of statins at current therapeutic doses has shown conflicting results with regard to prevention of osteoporosis, pre-clinical in vivo studies of local administration of statins from scaffolds demonstrate that local release appears to be an attractive solution to the problem of maintaining therapeutic doses at the affected area while minimizing undesired side effects. Further investigation will be required to determine appropriate local delivery concentrations to promote bone regeneration without inducing toxicity, as well as to assess the feasibility of using this molecule as a potential concomitant antimicrobial drug.
ACKNOWLEDGEMENTS
S.R.S. would like to acknowledge the support of the Baylor College of Medicine Medical Scientist Training Program. C.A.W. would like to acknowledge the support of the Monticello Foundation.
FUNDING
This work was supported by the National Institutes of Health (R01 AR048756, R01 AR057083) and the Armed Forces Institute of Regenerative Medicine (W81XWH-08-2-0032).
REFERENCES
- 1.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032–45. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endo A. A historical perspective on the discovery of statins. Proc Jpn Acad Ser B. 2010;86:484–93. doi: 10.2183/pjab.86.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov. 2007;6:541–55. doi: 10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- 4.Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–7. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 5.Wenke K, Meiser B, Thiery J, et al. Simvastatin reduces graft vessel disease and mortality after heart transplantation: a four-year randomized trial. Circulation. 1997;96:1398–402. doi: 10.1161/01.cir.96.5.1398. [DOI] [PubMed] [Google Scholar]
- 6.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–87. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 7.Iwata A, Shirai R, Ishii H, et al. Inhibitory effect of statins on inflammatory cytokine production from human bronchial epithelial cells. Clin Exp Immunol. 2012;168:234–40. doi: 10.1111/j.1365-2249.2012.04564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwak B, Mulhaupt F, Myit S, et al. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 9.Albert MA, Danielson E, Rifai N, et al. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 10.Tawakol A, Fayad ZA, Mogg R, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation. J Am Coll Cardiol. 2013;62:909–17. doi: 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 11.Lee J-K, Won J-S, Singh AK, et al. Statin inhibits kainic acid-induced seizure and associated inflammation and hippocampal cell death. Neurosci Lett. 2008;440:260–4. doi: 10.1016/j.neulet.2008.05.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordle A, Landreth G. 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors attenuate beta-amyloid-induced microglial inflammatory responses. J Neurosci. 2005;25:299–307. doi: 10.1523/JNEUROSCI.2544-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gouveia TLF, Scorza FA, Silva MJV, et al. Lovastatin decreases the synthesis of inflammatory mediators in the hippocampus and blocks the hyper-thermia of rats submitted to long-lasting status epilepticus. Epilepsy Behav. 2011;20:1–5. doi: 10.1016/j.yebeh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Chang C-H, Hsu Y-M, Chen Y-C, et al. Anti-inflammatory effects of hydrophilic and lipophilic statins with hyaluronic acid against LPS-induced inflammation in porcine articular chondrocytes. J Orthop Res. 2013;32:557–65. doi: 10.1002/jor.22536. [DOI] [PubMed] [Google Scholar]
- 15.Yudoh K, Karasawa R. Statin prevents chondrocyte aging and degeneration of articular cartilage in osteoarthritis (OA). Aging. 2010;2:990–8. doi: 10.18632/aging.100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadam UT, Blagojevic M, Belcher J. Statin use and clinical osteoarthritis in the general population: a longitudinal study. J Gen Intern Med. 2013;28:943–9. doi: 10.1007/s11606-013-2382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandebriel RJ, De Jong HJ, Gremmer ER, et al. Statins accelerate the onset of collagen type II-induced arthritis in mice. Arthritis Res Ther. 2012;14:R90. doi: 10.1186/ar3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thylin MR, McConnell JC, Schmid MJ, et al. Effects of simvastatin gels on murine calvarial bone. J Periodontol. 2002;73:1141–8. doi: 10.1902/jop.2002.73.10.1141. [DOI] [PubMed] [Google Scholar]
- 19.Stein D, Lee Y, Schmid MJ, et al. Local simvastatin effects on mandibular bone growth and inflammation. J Periodontol. 2005;76:1861–70. doi: 10.1902/jop.2005.76.11.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian S, Emami H, Vucic E, et al. High-dose atorvastatin reduces periodontal inflammation: a novel pleiotropic effect of statins. J Am Coll Cardiol. 2013;62:2382–91. doi: 10.1016/j.jacc.2013.08.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Looker AC, Borrud LG, Dawson-Hughes B, et al. Osteoporosis or low bone mass at the femur neck or lumbar spine in older adults: United States, 2005–2008. NCHS Data Brief 2012. 93:1–8. [PubMed] [Google Scholar]
- 22.Kanis JA, McCloskey EV, Johansson H, et al. A reference standard for the description of osteoporosis. Bone. 2008;42:467–75. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Reid IR, Bolland MJ, Grey AB. Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone. 2007;41:318–20. doi: 10.1016/j.bone.2007.04.196. [DOI] [PubMed] [Google Scholar]
- 24.Wei X, Pushalkar S, Estilo C, et al. Molecular profiling of oral microbiota in jawbone samples of bisphosphonate-related osteonecrosis of the jaw. Oral Dis. 2012;18:602–12. doi: 10.1111/j.1601-0825.2012.01916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher JE, Rogers MJ, Halasy JM, et al. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci USA. 1999;96:133–8. doi: 10.1073/pnas.96.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grasser WA, Baumann AP, Petras SF, et al. Regulation of osteoclast differentiation by statins. J Musculoskelet Neuronal Interact. 2003;3:53–62. [PubMed] [Google Scholar]
- 27.Staal A, Frith JC, French MH, et al. The ability of statins to inhibit bone resorption is directly related to their inhibitory effect on HMG-CoA reductase activity. J Bone Miner Res. 2003;18:88–96. doi: 10.1359/jbmr.2003.18.1.88. [DOI] [PubMed] [Google Scholar]
- 28.Hughes A, Rogers MJ, Idris AI, et al. A comparison between the effects of hydrophobic and hydrophilic statins on osteoclast function in vitro and ovariectomy-induced bone loss in vivo. Calcif Tissue Int. 2007;81:403–13. doi: 10.1007/s00223-007-9078-1. [DOI] [PubMed] [Google Scholar]
- 29.De Leo V, Morgante G, la Marca A, et al. Combination of statins and hormone replacement therapy in postmenopausal women is associated with increased bone mineral density. Gynecol Endocrinol. 2003;17:329–32. [PubMed] [Google Scholar]
- 30.Edwards CJ, Hart DJ, Spector TD. Oral statins and increased bone-mineral density in postmenopausal women. Lancet. 2000;355:2218–9. doi: 10.1016/s0140-6736(00)02408-9. [DOI] [PubMed] [Google Scholar]
- 31.Funkhouser HL, Adera T, Adler RA. Effect of HMG-CoA reductase inhibitors (statins) on bone mineral density. J Clin Densitom. 2002;5:151–8. doi: 10.1385/jcd:5:2:151. [DOI] [PubMed] [Google Scholar]
- 32.Pasco JA, Kotowicz MA, Henry MJ, et al. Statin use, bone mineral density, and fracture risk: Geelong osteoporosis study. Arch Intern Med. 2002;162:537–40. doi: 10.1001/archinte.162.5.537. [DOI] [PubMed] [Google Scholar]
- 33.Rejnmark L, Buus NH, Vestergaard P, et al. Statins decrease bone turnover in postmenopausal women: a cross-sectional study. Eur J Clin Invest. 2002;32:581–9. doi: 10.1046/j.1365-2362.2002.01024.x. [DOI] [PubMed] [Google Scholar]
- 34.Solomon DH, Finkelstein JS, Wang PS, et al. Statin lipid-lowering drugs and bone mineral density. Pharmacoepidemiol Drug Saf. 2005;14:219–26. doi: 10.1002/pds.984. [DOI] [PubMed] [Google Scholar]
- 35.Bjarnason NH, Riis BJ, Christiansen C. The effect of fluvastatin on parameters of bone remodeling. Osteoporos Int. 2001;12:380–4. doi: 10.1007/s001980170106. [DOI] [PubMed] [Google Scholar]
- 36.Hernández JL, Olmos JM, Romaña G, et al. Bone mineral density in statin users: a population-based analysis from a Spanish cohort. J Bone Miner Metab. 2013;32:184–91. doi: 10.1007/s00774-013-0481-6. [DOI] [PubMed] [Google Scholar]
- 37.LaCroix AZ, Cauley JA, Pettinger M, et al. Statin use, clinical fracture, and bone density in postmenopausal women: results from the Women's Health Initiative Observational Study. Ann Intern Med. 2003;139:97–104. doi: 10.7326/0003-4819-139-2-200307150-00009. [DOI] [PubMed] [Google Scholar]
- 38.Sirola J, Honkanen R, Kröger H, et al. Relation of statin use and bone loss: a prospective population-based cohort study in early postmenopausal women. Osteoporos Int. 2002;13:537–41. doi: 10.1007/s001980200070. [DOI] [PubMed] [Google Scholar]
- 39.Chuengsamarn S, Rattanamongkoulgul S, Suwanwalaikorn S, et al. Effects of statins vs non-statin lipid-lowering therapy on bone formation and bone mineral density biomarkers in patients with hyperlipidemia. Bone. 2010;46:1011–5. doi: 10.1016/j.bone.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 40.Lupattelli G, Scarponi AM, Vaudo G, et al. Simvastatin increases bone mineral density in hypercholesterolemic postmenopausal women. Metabolism. 2004;53:744–8. doi: 10.1016/j.metabol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Tanriverdi HA, Barut A, Sarikaya S. Statins have additive effects to vertebral bone mineral density in combination with risedronate in hypercholesterolemic postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2005;120:63–8. doi: 10.1016/j.ejogrb.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Montagnani A, Gonnelli S, Cepollaro C, et al. Effect of simvastatin treatment on bone mineral density and bone turnover in hypercholesterolemic postmenopausal women: a 1-year longitudinal study. Bone. 2003;32:427–33. doi: 10.1016/s8756-3282(03)00034-6. [DOI] [PubMed] [Google Scholar]
- 43.Rejnmark L, Buus HN, Vestergaard P, et al. Effects of simvastatin on bone turnover and BMD: a 1-year randomized controlled trial in postmenopausal osteopenic women. J Bone Miner Res. 2004;19:737–44. doi: 10.1359/JBMR.040209. [DOI] [PubMed] [Google Scholar]
- 44.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QRe-search database. Br Med J. 2010;340:c2197–7. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang PS, Solomon DH, Mogun H, et al. HMG-CoA reductase inhibitors and the risk of hip fractures in elderly patients. JAMA. 2000;283:3211–6. doi: 10.1001/jama.283.24.3211. [DOI] [PubMed] [Google Scholar]
- 46.Schoofs MW, Sturkenboom MC, van der Klift M, et al. HMG-CoA reductase inhibitors and the risk of vertebral fracture. J Bone Miner Res. 2004;19:1525–30. doi: 10.1359/JBMR.040607. [DOI] [PubMed] [Google Scholar]
- 47.Rejnmark L, Vestergaard P, Mosekilde L. Statin but not non-statin lipid-lowering drugs decrease fracture risk: a nation-wide case-control study. Calcif Tissue Int. 2006;79:27–36. doi: 10.1007/s00223-006-0024-4. [DOI] [PubMed] [Google Scholar]
- 48.Rejnmark L, Olsen M, Johnsen SR, et al. Hip fracture risk in statin users— a population-based Danish case-control study. Osteoporos Int. 2004;15:452–8. doi: 10.1007/s00198-003-1568-z. [DOI] [PubMed] [Google Scholar]
- 49.Chan KA, Andrade SE, Boles M, et al. Inhibitors of hydroxymethylglutarylcoenzyme A reductase and risk of fracture among older women. Lancet. 2000;355:2185–8. doi: 10.1016/S0140-6736(00)02400-4. [DOI] [PubMed] [Google Scholar]
- 50.Reid IR, Hague W, Emberson J, et al. Effect of pravastatin on frequency of fracture in the LIPID study: secondary analysis of a randomised controlled trial. Long-term intervention with pravastatin in ischaemic disease. Lancet. 2001;357:509–12. doi: 10.1016/s0140-6736(00)04042-3. [DOI] [PubMed] [Google Scholar]
- 51.Bakhireva LN, Shainline MR, Carter S, et al. Synergistic effect of statins and postmenopausal hormone therapy in the prevention of skeletal fractures in elderly women. Pharmacotherapy. 2010;30:879–87. doi: 10.1592/phco.30.9.879. [DOI] [PubMed] [Google Scholar]
- 52.Reid IR, Tonkin A, Cannon CP. Comparison of the effects of pravastatin and atorvastatin on fracture incidence in the PROVE IT-TIMI 22 trial— secondary analysis of a randomized controlled trial. Bone. 2005;37:190–1. doi: 10.1016/j.bone.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 53.Uzzan B, Cohen R, Nicolas P, et al. Effects of statins on bone mineral density: a meta-analysis of clinical studies. Bone. 2007;40:1581–7. doi: 10.1016/j.bone.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 54.Ray WA, Daugherty JR, Griffin MR. Lipid-lowering agents and the risk of hip fracture in a Medicaid population. Inj Prev. 2002;8:276–9. doi: 10.1136/ip.8.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scranton RE, Young M, Lawler E, et al. Statin use and fracture risk: study of a US veterans population. Arch Intern Med. 2005;165:2007–12. doi: 10.1001/archinte.165.17.2007. [DOI] [PubMed] [Google Scholar]
- 56.Falagas ME, Makris GC, Matthaiou DK, et al. Statins for infection and sepsis: a systematic review of the clinical evidence. J Antimicrob Chemother. 2008;61:774–85. doi: 10.1093/jac/dkn019. [DOI] [PubMed] [Google Scholar]
- 57.Patel JM, Snaith C, Thickett DR, et al. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS Trial). Crit Care. 2012;16:R231. doi: 10.1186/cc11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kruger P, Bailey M, Bellomo R, et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med. 2013;187:743–50. doi: 10.1164/rccm.201209-1718OC. [DOI] [PubMed] [Google Scholar]
- 59.Yende S, Milbrandt EB, Kellum JA, et al. Understanding the potential role of statins in pneumonia and sepsis. Crit Care Med. 2011;39:1871–8. doi: 10.1097/CCM.0b013e31821b8290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rezaie-Majd A, Maca T, Bucek RA, et al. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2002;22:1194–9. doi: 10.1161/01.atv.0000022694.16328.cc. [DOI] [PubMed] [Google Scholar]
- 61.Arnaud C, Burger F, Steffens S, et al. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol. 2005;25:1231–6. doi: 10.1161/01.ATV.0000163840.63685.0c. [DOI] [PubMed] [Google Scholar]
- 62.Loppnow H, Zhang L, Buerke M, et al. Statins potently reduce the cytokine-mediated IL-6 release in SMC/MNC cocultures. J Cell Mol Med. 2011;15:994–1004. doi: 10.1111/j.1582-4934.2010.01036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buhaescu I, Izzedine H. Mevalonate pathway: a review of clinical and therapeutical implications. Clin Biochem. 2007;40:575–84. doi: 10.1016/j.clinbiochem.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 64.Ikeda M, Abe K-I, Yamada M, et al. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44:117–25. doi: 10.1002/hep.21232. [DOI] [PubMed] [Google Scholar]
- 65.Ikeda M, Kato N. Life style-related diseases of the digestive system: cell culture system for the screening of anti-hepatitis C virus (HCV) reagents: suppression of HCV replication by statins and synergistic action with interferon. J Pharmacol Sci. 2007;105:145–50. doi: 10.1254/jphs.fm0070050. [DOI] [PubMed] [Google Scholar]
- 66.Gyetvai ÃG, Emri TS, Takács K, et al. Lovastatin possesses a fungistatic effect against Candida albicans, but does not trigger apoptosis in this opportunistic human pathogen. FEMS Yeast Res. 2006;6:1140–8. doi: 10.1111/j.1567-1364.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- 67.Nyilasi I, Kocsube S, Pesti M, et al. In vitro interactions between primycin and different statins in their effects against some clinically important fungi. J Med Microbiol. 2010;59:200–5. doi: 10.1099/jmm.0.013946-0. [DOI] [PubMed] [Google Scholar]
- 68.Nyilasi I, Kocsubé S, Krizsán K, et al. In vitro synergistic interactions of the effects of various statins and azoles against some clinically important fungi. FEMS Microbiol Lett. 2010;307:175–84. doi: 10.1111/j.1574-6968.2010.01972.x. [DOI] [PubMed] [Google Scholar]
- 69.Welsh A-M, Kruger P, Faoagali J. Antimicrobial action of atorvastatin and rosuvastatin. Pathology. 2009;41:689–91. doi: 10.3109/00313020903305860. [DOI] [PubMed] [Google Scholar]
- 70.Liappis AP, Kan VL, Rochester CG, et al. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis. 2001;33:1352–7. doi: 10.1086/323334. [DOI] [PubMed] [Google Scholar]
- 71.Pruefer D, Makowski J, Schnell M, et al. Simvastatin inhibits inflamma-tory properties of Staphylococcus aureus alpha-toxin. Circulation. 2002;106:2104–10. doi: 10.1161/01.cir.0000034048.38910.91. [DOI] [PubMed] [Google Scholar]
- 72.Boyd AR, Hinojosa CA, Rodriguez PJ, et al. Impact of oral simvastatin therapy on acute lung injury in mice during pneumococcal pneumonia. BMC Microbiol. 2012;12:73. doi: 10.1186/1471-2180-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jerwood S, Cohen J. Unexpected antimicrobial effect of statins. J Antimicrob Chemother. 2007;61:362–4. doi: 10.1093/jac/dkm496. [DOI] [PubMed] [Google Scholar]
- 74.Coban AY, Tekeli HO, Güney AK, et al. [Investigation of the in vitro antibacterial effects of statins]. Mikrobiyol Bul. 2010;44:161–3. [PubMed] [Google Scholar]
- 75.Matzneller P, Manafi M, Zeitlinger M. Antimicrobial effect of statins: organic solvents might falsify microbiological testing results. Int J Clin Pharmacol Ther. 2011;49:666–71. doi: 10.5414/cp201581. [DOI] [PubMed] [Google Scholar]
- 76.Bergman P, Linde C, Pütsep K, et al. Studies on the antibacterial effects of statins—in vitro and in vivo. Plos One. 2011;6:e24394. doi: 10.1371/journal.pone.0024394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee EJ, Kasper FK, Mikos AG. Biomaterials for tissue engineering. Ann Biomed Eng. 2013;42:323–37. doi: 10.1007/s10439-013-0859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Halme DG, Kessler DA. FDA regulation of stem-cell-based therapies. N Engl J Med. 2006;355:1730–5. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- 79.Street J, Bao M, Bunting S, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–61. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Young S, Patel ZS, Kretlow JD, et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15:2347–62. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patel ZS, Young S, Tabata Y, et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–40. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maeda T, Kawane T, Horiuchi N. Statins augment vascular endothelial growth factor expression in osteoblastic cells via inhibition of protein prenylation. Endocrinology. 2003;144:681–92. doi: 10.1210/en.2002-220682. [DOI] [PubMed] [Google Scholar]
- 83.Sata M, Nishimatsu H, Suzuki E, et al. Endothelial nitric oxide synthase is essential for the HMG-CoA reductase inhibitor cerivastatin to promote collateral growth in response to ischemia. FASEB J. 2001;15:2530–2. doi: 10.1096/fj.01-0415fje. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Zhang R, Li Y, et al. Simvastatin augments the efficacy of therapeutic angiogenesis induced by bone marrow-derived mesenchymal stem cells in a murine model of hindlimb ischemia. Mol Biol Rep. 2011;39:285–93. doi: 10.1007/s11033-011-0737-y. [DOI] [PubMed] [Google Scholar]
- 85.Frick M, Dulak J, Cisowski J, et al. Statins differentially regulate vascular endothelial growth factor synthesis in endothelial and vascular smooth muscle cells. Atherosclerosis. 2003;170:229–36. doi: 10.1016/s0021-9150(03)00299-5. [DOI] [PubMed] [Google Scholar]
- 86.Weis M, Heeschen C, Glassford AJ, et al. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–45. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 87.Zambarakji HJ, Nakazawa T, Connolly E, et al. Dose-dependent effect of pitavastatin on VEGF and angiogenesis in a mouse model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2006;47:2623–31. doi: 10.1167/iovs.05-0855. [DOI] [PubMed] [Google Scholar]
- 88.Zhang J, McGwin G. Association of statin use with the risk of developing diabetic retinopathy. Arch Ophthalmol. 2007;125:1096–9. doi: 10.1001/archopht.125.8.1096. [DOI] [PubMed] [Google Scholar]
- 89.Dimmeler S, Aicher A, Vasa M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–7. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–50. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 91.Mundy G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–9. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 92.Ruiz-Gaspa S, Nogues X, Enjuanes A, et al. Simvastatin and atorvastatin enhance gene expression of collagen type 1 and osteocalcin in primary human osteoblasts and MG-63 cultures. J Cell Biochem. 2007;101:1430–8. doi: 10.1002/jcb.21259. [DOI] [PubMed] [Google Scholar]
- 93.Mori M, Nishikawa T, Masuno K, et al. Statins: candidates for promoting bone formation via BMP-2. Oral Med Pathol. 2010;14:81–7. [Google Scholar]
- 94.Ohnaka K, Shimoda S, Nawata H, et al. Pitavastatin enhanced BMP-2 and osteocalcin expression by inhibition of Rho-associated kinase in human osteoblasts. Biochem Biophys Res Commun. 2001;287:337–42. doi: 10.1006/bbrc.2001.5597. [DOI] [PubMed] [Google Scholar]
- 95.Yoshikawa H, Yoshioka K, Nakase T, et al. Stimulation of ectopic bone formation in response to BMP-2 by Rho kinase inhibitor: a pilot study. Clin Orthop Relat Res. 2009;467:3087–95. doi: 10.1007/s11999-009-0976-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sugiyama M, Kodama T, Konishi K, et al. Compactin and simvastatin, but not pravastatin, induce bone morphogenetic protein-2 in human osteosarcoma cells. Biochem Biophys Res Commun. 2000;271:688–92. doi: 10.1006/bbrc.2000.2697. [DOI] [PubMed] [Google Scholar]
- 97.Kupcsik L, Meurya T, Flury M, et al. Statin-induced calcification in human mesenchymal stem cells is cell death related. J Cell Mol Med. 2009;13:4465–73. doi: 10.1111/j.1582-4934.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Monjo M, Rubert M, Ellingsen JE, et al. Rosuvastatin promotes osteoblast differentiation and regulates SLCO1A1 transporter gene expression in MC3T3-E1 cells. Cell Physiol Biochem. 2010;26:647–56. doi: 10.1159/000322332. [DOI] [PubMed] [Google Scholar]
- 99.Hardwick JCH, van den Brink GR, Bleuming SA, et al. Bone morpho-genetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology. 2004;126:111–21. doi: 10.1053/j.gastro.2003.10.067. [DOI] [PubMed] [Google Scholar]
- 100.Kodach LL, Bleuming SA, Peppelenbosch MP, et al. The effect of statins in colorectal cancer is mediated through the bone morphogenetic protein pathway. Gastroenterology. 2007;133:1272–81. doi: 10.1053/j.gastro.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 101.Skoglund B, Forslund C, Aspenberg P. Simvastatin improves fracture healing in mice. J Bone Miner Res. 2002;17:2004–8. doi: 10.1359/jbmr.2002.17.11.2004. [DOI] [PubMed] [Google Scholar]
- 102.Anbinder AL, Junqueira JC, Mancini MNG, et al. Influence of simvastatin on bone regeneration of tibial defects and blood cholesterol level in rats. Braz Dent J. 2006;17:267–73. doi: 10.1590/s0103-64402006000400001. [DOI] [PubMed] [Google Scholar]
- 103.Masuzaki T, Ayukawa Y, Moriyama Y, et al. The effect of a single remote injection of statin-impregnated poly (lactic-co-glycolic acid) microspheres on osteogenesis around titanium implants in rat tibia. Biomaterials. 2010;31:3327–34. doi: 10.1016/j.biomaterials.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 104.Ayukawa Y, Okamura A, Koyano K. Simvastatin promotes osteogenesis around titanium implants. Clin Oral Implants Res. 2004;15:346–50. doi: 10.1046/j.1600-0501.2003.01015.x. [DOI] [PubMed] [Google Scholar]
- 105.Du Z, Chen J, Yan F, et al. Effects of simvastatin on bone healing around titanium implants in osteoporotic rats. Clin Oral Implants Res. 2009;20:145–50. doi: 10.1111/j.1600-0501.2008.01630.x. [DOI] [PubMed] [Google Scholar]
- 106.Pauly S, Back DA, Kaeppler K, et al. Influence of statins locally applied from orthopedic implants on osseous integration. BMC Musculoskelet Disord. 2012;13:208. doi: 10.1186/1471-2474-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stadlinger B, Korn P, Tödtmann N, et al. Osseointegration of biochemically modified implants in an osteoporosis rodent model. Eur Cell Mater. 2013;25:326–40. doi: 10.22203/ecm.v025a23. [DOI] [PubMed] [Google Scholar]
- 108.Ayukawa Y, Yasukawa E, Moriyama Y, et al. Local application of statin promotes bone repair through the suppression of osteoclasts and the enhancement of osteoblasts at bone-healing sites in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:336–42. doi: 10.1016/j.tripleo.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 109.Sinzinger H, Wolfram R, Peskar BA. Muscular side effects of statins. J Cardiovasc Pharmacol. 2002;40:163. doi: 10.1097/00005344-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 110.Cohen DE, Anania FA, Chalasani N. An assessment of statin safety by hepatologists. Am J Cardiol. 2006;97:S77–81. doi: 10.1016/j.amjcard.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 111.Benoit DSW, Nuttelman CR, Collins SD, et al. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials. 2006;27:6102–10. doi: 10.1016/j.biomaterials.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 112.Benoit DSW, Collins SD, Anseth KS. Multifunctional hydrogels that promote osteogenic human mesenchymal stem cell differentiation through stimulation and sequestering of bone morphogenic protein 2. Adv Funct Mater. 2007;17:2085–93. doi: 10.1002/adfm.200700012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zou Y, Brooks JL, Talwalkar V, et al. Development of an injectable two-phase drug delivery system for sequential release of antiresorptive and osteogenic drugs. J Biomed Mater Res B. 2011;100:155–62. doi: 10.1002/jbm.b.31933. [DOI] [PubMed] [Google Scholar]
- 114.Fukui T, Ii M, Shoji T, et al. Therapeutic effect of local administration of low-dose simvastatin-conjugated gelatin hydrogel for fracture healing. J Bone Miner Res. 2012;27:1118–31. doi: 10.1002/jbmr.1558. [DOI] [PubMed] [Google Scholar]
- 115.Alam S, Ueki K, Nakagawa K, et al. Statin-induced bone morphogenetic protein (BMP) 2 expression during bone regeneration: an immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:22–9. doi: 10.1016/j.tripleo.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 116.Monjo M, Rubert M, Wohlfahrt JC, et al. In vivo performance of absorbable collagen sponges with rosuvastatin in critical-size cortical bone defects. Acta Biomater. 2010;6:1405–12. doi: 10.1016/j.actbio.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 117.Jeon JH, Puleo DA. Alternating release of different bioactive molecules from a complexation polymer system. Biomaterials. 2008;29:3591–8. doi: 10.1016/j.biomaterials.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–46. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang R, Edwards JR, Ko S-Y, et al. Transcriptional regulation of BMP2 expression by the PTH-CREB signaling pathway in osteoblasts. Plos One. 2011;6:e20780. doi: 10.1371/journal.pone.0020780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rojbani H, Nyan M, Ohya K, et al. Evaluation of the osteoconductivity of α-tricalcium phosphate, β-tricalcium phosphate, and hydroxyapatite combined with or without simvastatin in rat calvarial defect. J Biomed Mater Res A. 2011;98:488–98. doi: 10.1002/jbm.a.33117. [DOI] [PubMed] [Google Scholar]
- 121.Yin H, Li Y-G, Si M, et al. Simvastatin-loaded macroporous calcium phosphate cement: preparation, in vitro characterization, and evaluation of in vivo performance. J Biomed Mater Res A. 2012;100:2991–3000. doi: 10.1002/jbm.a.34228. [DOI] [PubMed] [Google Scholar]
- 122.Chou J, Ito T, Bishop D, et al. Controlled release of simvastatin from biomimetic β-TCP drug delivery system. Plos One. 2013;8:e54676. doi: 10.1371/journal.pone.0054676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nyan M, Sato D, Oda M, et al. Bone formation with the combination of simvastatin and calcium sulfate in critical-sized rat calvarial defect. J Pharmacol Sci. 2007;104:384–6. doi: 10.1254/jphs.sc0070184. [DOI] [PubMed] [Google Scholar]
- 124.Tai I-C, Fu Y-C, Wang, et al. Local delivery of controlled-release simvastatin/PLGA/HAp microspheres enhances bone repair. Int J Nanomedicine. 2013;8:3895–904. doi: 10.2147/IJN.S48694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nath SD, Son S, Sadiasa A, et al. Preparation and characterization of PLGA microspheres by the electrospraying method for delivering simvastatin for bone regeneration. Int J Pharm. 2013;443:87–94. doi: 10.1016/j.ijpharm.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 126.Wu Z, Liu C, Zang G, et al. The effect of simvastatin on remodelling of the alveolar bone following tooth extraction. Int J Oral Surg. 2008;37:170–6. doi: 10.1016/j.ijom.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 127.Pauly S, Luttosch F, Morawski M, et al. Simvastatin locally applied from a biodegradable coating of osteosynthetic implants improves fracture healing comparable to BMP-2 application. Bone. 2009;45:505–11. doi: 10.1016/j.bone.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 128.Pişkin E, Işoğlu IA, Bölgen N, et al. In vivo performance of simvastatin-loaded electrospun spiral-wound polycaprolactone scaffolds in reconstruction of cranial bone defects in the rat model. J Biomed Mater Res A. 2009;90:1137–51. doi: 10.1002/jbm.a.32157. [DOI] [PubMed] [Google Scholar]
- 129.Henslee AM, Spicer PP, Yoon DM, et al. Biodegradable composite scaffolds incorporating an intramedullary rod and delivering bone morphogenetic protein-2 for stabilization and bone regeneration in segmental long bone defects. Acta Biomater. 2011;7:3627–37. doi: 10.1016/j.actbio.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Park YS, David AE, Park KM, et al. Controlled release of simvastatin from in situ forming hydrogel triggers bone formation in MC3T3-E1 cells. AAPS J. 2012;15:367–76. doi: 10.1208/s12248-012-9442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Edwards CJ, Spector TD. Statins as modulators of bone formation. Arthritis Res. 2002;4:151–3. doi: 10.1186/ar399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thomas MV, Puleo DA. Infection, inflammation, and bone regeneration: a paradoxical relationship. J Dent Res. 2011;90:1052–61. doi: 10.1177/0022034510393967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maeda T, Matsunuma A, Kurahashi I, et al. Induction of osteoblast differentiation indices by statins in MC3T3-E1 cells. J Cell Biochem. 2004;92:458–71. doi: 10.1002/jcb.20074. [DOI] [PubMed] [Google Scholar]
- 134.Newman A, Clutterbuck RD, Powles RL, et al. Selective inhibition of primary acute myeloid leukaemia cell growth by lovastatin. Leukemia. 1994;8:274–80. [PubMed] [Google Scholar]
- 135.Newman A, Clutterbuck RD, Powles RL, et al. A comparison of the effect of the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors simvastatin, lovastatin and pravastatin on leukaemic and normal bone marrow progenitors. Leuk Lymphoma. 1997;24:533–7. doi: 10.3109/10428199709055590. [DOI] [PubMed] [Google Scholar]