Abstract
Aedes aegypti is an anautogenous mosquito that must blood feed on a vertebrate host to produce and lay a clutch of eggs. The rockpool mosquito, Georgecraigius atropalpus, is related to A. aegypti but is a facultatively autogenous species that produces its first clutch of eggs shortly after emerging without blood feeding. Consumption of a blood meal by A. aegypti triggers the release of ovary ecdysteroidogenic hormone (OEH) and insulin-like peptide 3 (ILP3) from the brain, which stimulate egg formation. OEH and ILP3 also stimulate egg formation in G. atropalpus but are released at eclosion independently of blood feeding. These results collectively suggest that blood meal dependent release of OEH and ILP3 is one factor that prevents A. aegypti from reproducing autogenously. Here, we examined two other factors that potentially inhibit autogeny in A. aegypti: teneral nutrient reserves and the ability of OEH and ILP3 to stimulate egg formation in the absence of blood feeding. Measures of nutrient reserves showed that newly emerged A. aegypti females had similar wet weights but significantly lower protein and glycogen reserves than G. atropalpus females when larvae were reared under identical conditions. OEH stimulated non-blood fed A. aegypti females to produce ecdysteroid hormone and package yolk into oocytes more strongly than ILP3. OEH also reduced host seeking and blood feeding behavior, yet females produced few mature eggs. Overall, our results indicate that multiple factors prevent A. aegypti from reproducing autogenously.
Keywords: neuropeptide, autogeny, reproduction, oogenesis, hormone, endocrine
1. Introduction
Insects usually package large amounts of yolk into their eggs, which provides the nutrients needed for embryonic development. Juvenile hormone (JH) stimulates the fat body to produce yolk proteins in many species (Riddiford, 2012; Marchal et al., 2014), whereas ecdysteroid hormone (ECD) stimulates yolk formation in some Diptera following consumption of a protein source like carrion, excrement or blood by an adult female (Browne, 2001; Attardo et al., 2005). Hematophagy has evolved multiple times in the Diptera including in the family Culicidae (mosquitoes) (Black and Kondratieff, 2005). Blood feeding has also led to many dipterans being important vectors of disease-causing vertebrate pathogens (Browne, 2001; Wiegmann et al., 2011).
Mosquitoes are a monophyletic group that is comprised of ~3500 species in 44 genera and two subfamilies (Reidenbach et al., 2009). Most mosquitoes are anautogenous, which means adult females must consume at least one blood meal for every clutch of eggs they produce and lay (Clements, 1992; Briegel, 2003). Regulation of egg formation by anautogenous species is best understood in the yellow fever mosquito, Aedes aegypti (subfamily Culicinae, Tribe Aedini) where blood feeding triggers the release of two types of neurohormones, ovary ecdysteroidogenic hormone (OEH) and insulin-like peptides (ILPs), from medial neurosecretory cells in the brain. Prior studies establish that OEH and one ILP family member (ILP3) stimulate the ovaries to produce ECD, which induces the fat body to produce vitellogenin (VG) and other yolk proteins that are secreted into circulation (Attardo et al., 2005, Brown et al. 2008; Dhara et al. 2013; Vogel et al., 2015). ILP3 also stimulates the midgut to express trypsin-like proteases that digest the blood meal while amino acid sensing through the target of rapamycin (TOR) pathway enhances OEH, ILP and ECD activity (Gulia-Nuss et al., 2011; Roy et al., 2007, 2011). Oocytes in the ovary then package yolk followed by chorion formation to produce mature eggs that females fertilize and oviposit (Clements, 1992). JH does not directly regulate yolk production in A. aegypti but a rise in JH titer following adult emergence affects tissue competency, which enhances the number and size of eggs females produce (Hernández-Martinez et al., 2007, 2015; Perez-Hedo et al., 2013; Zou et al., 2013; Clifton et al., 2011, 2014).
A few mosquitoes have evolved to produce eggs without blood feeding, which is referred to in the literature as autogeny (Clements, 1963, 1992; Briegel, 2003; Attardo et al., 2005). Obligately autogenous mosquitoes include species in three genera (Malaya, Topomyia, Toxorhynchites) that appear to never blood feed, while select species in other genera are referred to as facultatively autogenous because they produce a first clutch of eggs autogenously but thereafter may blood feed to produce additional clutches of eggs (O’Meara, 1985; Clements, 1992). These patterns indicate that facultative and obligate autogeny has evolved multiple times in the Culicidae from different anautogenous ancestors (Rioux et al., 1975; Reidenbach et al., 2009; Gulia-Nuss et al., 2012). The physiological and molecular mechanisms regulating autogeny in contrast remain unclear. Genetic studies suggest autogeny is a monofactorial trait in some species (O’Meara and Craig, 1969; Gwadz, 1970; O’Meara and Krasnick, 1970; O’Meara, 1972; Masler et al., 1980), while other species exhibit complex or multigenic modes of inheritance (Spielman, 1957, 1971; O’Meara, 1972, 1985; Trpis, 1978; Mori et al., 2008). Autogeny has also been linked to enhanced nutrient acquisition, adult size, and alterations in the endocrine control of egg formation relative to anautogenous species (Clements, 1963, 1992; Van Handel, 1976; Fuchs et al., 1980; Masler et al., 1980, Birnbaum, et al., 1984; Lea, 1970; Kelly et al., 1981, 1984; Ma et al., 1984; Mogi et al., 1995; Hugo et al., 2003; Ahmed, 2013).
The rockpool mosquito, Georgecraigious atropalpus (Culicinae, Aedini) is of interest because it is closely related to A. aegypti and facultatively autogenous, which results in females always producing a first clutch of eggs a few days after emergence without blood feeding (Hudson, 1970; Masler et al., 1983; Bowen et al., 1994). We recently reported that G. atropalpus females release OEH and ILPs shortly after adult emergence, which is then followed by the ovaries producing ECD, the fat body synthesizing yolk, and oocytes packaging yolk to produce a clutch of mature eggs that females lay 3–4 days post-emergence (PE) (Gulia-Nuss et al., 2012). These results indicate that egg formation in G. atropalpus is very similar to A. aegypti with the key exception that OEH and ILP secretion occurs shortly after eclosion and independently of blood feeding. This study also showed that the first clutch laid by G. atropalpus contains on average 100 eggs, which is only slightly lower than the number of eggs (~120) laid by similarly reared A. aegypti females after a blood meal (Brown et al., 2008; Wen et al., 2010; Gulia-Nuss et al., 2012). In contrast, JH does not appear to promote competency in G. atropalplus because: 1) little or no JH biosynthesis is detectable in newly emerged adult females, and 2) decapitation immediately after eclosion, which ablates the source of JH, followed by injection of OEH stimulates formation of the same number of eggs as produced by non-decapitated females (Telang et al., 2007; Gulia-Nuss et al., 2012).
Overall, these results suggest blood meal dependent release of OEH and ILP3 is one factor that prevents A. aegypti from reproducing autogenously. In this study, we examined two other factors that potentially inhibit autogeny in A. aegypti: teneral nutrient reserves and the ability of OEH and ILP3 to stimulate egg formation in the absence of blood feeding. Our results indicate that A. aegypti females emerge with lower teneral reserves than G. atropalpus, They also show that OEH and ILPs stimulate non-blood fed A. aegypti to package yolk into oocytes but females produce few mature eggs.
2. Materials and methods
2.1. Mosquitoes
The UGAL strain of A. aegypti and Bass Rock strain of G. atropalpus were maintained in an insectary under a 16 h light: 8 h dark photoperiod and a temperature of 27° C (Telang et al., 2006). Both species were reared in aluminum pans at a density of ~150 larvae per 0.5 L of distilled water and fed a mixture of powdered rat chow (LabDiet): lactalbumin (Sigma): torula yeast (Sigma) (1:1:1) (Coon et al., 2014). Adults were held in plexiglass cages after emerging from the pupal stage (= day 1 post-emergence (PE)), and provided water continuously. Some adult females were also provided a 5% sucrose solution continuously or the second day after emergence for some experiments. Females were blood fed using an anesthetized rat (UGA Animal Use Protocol A2010-6-094). This protocol was approved by The University of Georgia Institutional Animal Care and Use Committee, which oversees and provides veterinary care, maintains an Assurance of Compliance with the US Public Health Service and is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and licensed by the US Department of Agriculture. To obtain unmated females, pharate pupae were sexed by size, and the larger female pupae were held separately from males in cages for adult emergence. Otherwise females emerging with males mated on day 1 PE as confirmed by the presence of sperm in the spermatheca.
2.2 Hormones
Recombinant A. aegypti long OEH was produced and purified as described (Gulia-Nuss et al., 2012; Dhara et al., 2013). A. aegypti ILP3 was synthesized by CPC Scientific Inc. (90% purity, Sunnyvale, CA), and exhibited bioactivity identical to previous reports (Brown et al., 2008). Both peptides were diluted from frozen stock aliquots and injected in 0.5 µl of saline into an adult female. The ECD, 20-hydroxyecdysone (20ECD) (Sigma), was solubilized in absolute ethanol as a 5 µg/µl stock and further diluted in ethanol prior to use. A stock of juvenile hormone III (JHIII) (200 pmol/µl) (Sigma) was prepared in acetone.
2.3 Wet weights and measures of teneral nutrient reserves
Wet weights of A. aegypti and G. atropalpus were determined by collecting adult females within 2 h of emergence from the pupal stage, freezing them, and then weighing three cohorts of each species (10 individuals per cohort) on an analytical balance (Metler). The weight of each cohort was then divided by ten to generate a per individual weight estimate. Protein, glycogen, and lipid levels were quantified by isolating in abdomen walls from newly emerged, non-blood fed females of each species. In brief, two females were dissected per sample in saline to remove the gut, followed by transfer of abdomen walls with attached fat body to microfuge tubes where they were homogenized in 100 µl of water (protein assay) or 100 µl of Na2SO4 with 200 µl of methanol, and stored at −80° C. After centrifugation (12,000 × g, 4° C), supernatants were then used to determine total protein, glycogen and lipid amounts as previously described (Brown et al., 2008; Gulia-Nuss et al., 2012).
2.4 Ecdysteroid and vitellogenin detection
Non-blood fed females were injected with OEH (20 pmol), ILP3 (20 pmol), or saline (negative control), and ovaries from these females were dissected 24, 48 and 72 h later to measure ECD production. Triplicate samples of ovary pairs from two females treated as above were incubated for 6 h in 60 µl of Sf900 SFM II medium (Invitrogen) alone, which provided amino acids for activation of TOR pathway signaling. Medium (50 µl) was then collected and used in an established radioimmunoassay (RIA) to measure the amount of ECD present (Brown et al., 2008). A total of nine replicates per treatment were analyzed.
For detection of VG, abdomen walls with attached fat body were dissected from mosquitoes treated as above (two per sample) and transferred to a 1:1 mixture of water and Laemmli sample buffer without reducing agent (Gulia-Nuss et al., 2012). Following homogenization, the extracts were held in a water bath (90° C, 10 min), centrifuged, and loaded (0.1 tissue equivalent per lane) on a 4–20% Tris-HCl gel (BioRad Criterion) for electrophoresis. The separated proteins were then transferred to nitrocellulose (Protran 0.2 µm, Whatman), probed with a rabbit antibody to A. aegypti VG (R2, 1: 100,000), and visualized using a peroxidase-conjugated goat anti-rabbit secondary antibody (Sigma; 1: 20,000) and chemiluminescent substrate (ECL Advance kit, GE Healthcare) (Gulia-Nuss et al., 2012).
2.5 Egg formation and blood feeding bioassays
Cohorts of non-blood fed females 1–5 days PE were injected with a single dose of OEH (20 pmol), ILP3 (20 pmol) or both (20 pmol OEH, 20 pmol ILP3). Some females were dissected 24 h post-injection to determine the number of oocytes in one ovary that contained yolk and the amount of yolk per oocyte. Both parameters were determined as previously described by examining one ovary per female under a stereomicroscope fitted with an ocular micrometer (Brown et al., 2008; Gulia-Nuss et al., 2012). The amount of yolk per oocyte in a given female was determined by measuring the length of yolk (µm) in each oocyte from one ovary. This yielded mean and median measures of yolk for all oocytes in both ovaries. Other females were held individually in small plastic cages for 5 days post-treatment with an oviposition site to determine whether any laid eggs. All females were then dissected in saline to determine the total number of oocytes in one ovary that contained yolk and the amount of yolk per oocyte. We also recorded how many mature eggs were present in the ovary, which we formally defined as being surrounded by a chorion and containing >300 µm of yolk. Cohorts of non-blood fed females were injected with OEH (20 pmol) or saline on day 2 PE. Two to five females from each group were dissected 24 h post-treatment to determine the number of oocytes per ovary that contained yolk as above while the remainder were segregated into groups of 10 that were held for 48 h post-treatment in small plastic cages with a screen. These females were then exposed to an anesthetized rat. The proportion of females injected with OEH or saline that blood fed after 20 min was recorded. In a third assay, newly emerged females were placed in the cages and presented an anesthetized rat for blood feeding at 8, 15, 24 or 48 h PE followed by determination of the proportion of females that blood fed after 20 min. For all blood-feeding assays, females were scored as having blood fed if their abdomen was distended and blood was visible in the midgut.
2.6. Data analysis
All data were analyzed using the JMP 11.0 statistical platform (SAS, Cary, NC, USA). Mosquito weights and teneral reserves were examined by t-test, while the effects of OEH and ILP3 on ECD production were examined by 1-way ANOVA followed by the Tukey-Kramer multiple comparison procedure with treatment serving as the independent variable. The effect of OEH and ILP3 on the number of oocytes in ovaries containing yolk and the amounts of yolk per oocyte were initially examined by 1-way ANOVA followed by analysis of the residuals for skewness using a Shapiro-Wilks test. The data were then reanalyzed by nonparametric Kruskal-Wallis tests. The relationship between number of oocytes with yolk and the amount of yolk in oocytes was examined by linear regression with or without log transformation. Blood feeding bioassays were analyzed by contingency table analysis.
3. Results
3.1. A. aegypti females emerge with lower teneral nutrient reserves than G. atropalpus
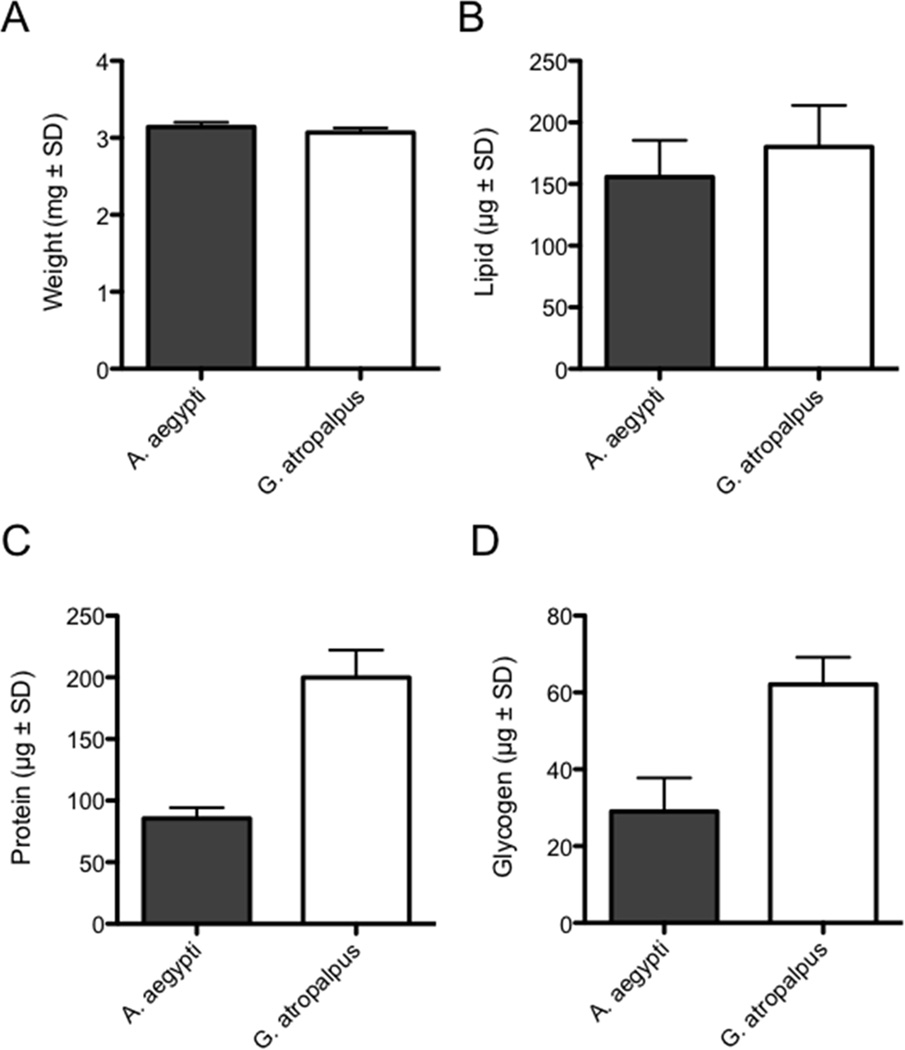
We reared UGAL A. aegypti and Bass Rock G. atropalpus larvae under conditions that produce adults of maximum average size for these strains (Telang et al., 2006; Gulia-Nuss et al., 2012). Comparing newly emerged adult females of each species to one another indicated that average wet weights were nearly identical (Fig. 1A). When nutrient levels in the fat body were measured, total lipids were also similar (Fig. 1B). Protein and glycogen stores in contrast were significantly lower in A. aegypti, which suggested that teneral reserves were overall higher in G. atropalpus (Fig. 1C, D.
Figure 1.
Wet weights and teneral nutrient reserves of newly emerged A. aegypti and G. atropalpus females. (A) Wet weights at emergence do not differ between species (n=3 cohorts of 10 adults for each species; t=1.4; P= 0.24). (B) Total lipid in abdomen walls also do not differ (n= 10 females for each species; t=1.70; P=0.11). (C) Total protein (n=10 for each species; t= 15.1; P<0.001) and (D) total glycogen (n= 10 for each species; t=9.0; P<0.001) in contrast are significantly higher at emergence in G. atropalpus.
3.2. OEH activates ECD and yolk protein synthesis in non-blood fed A. aegypti females
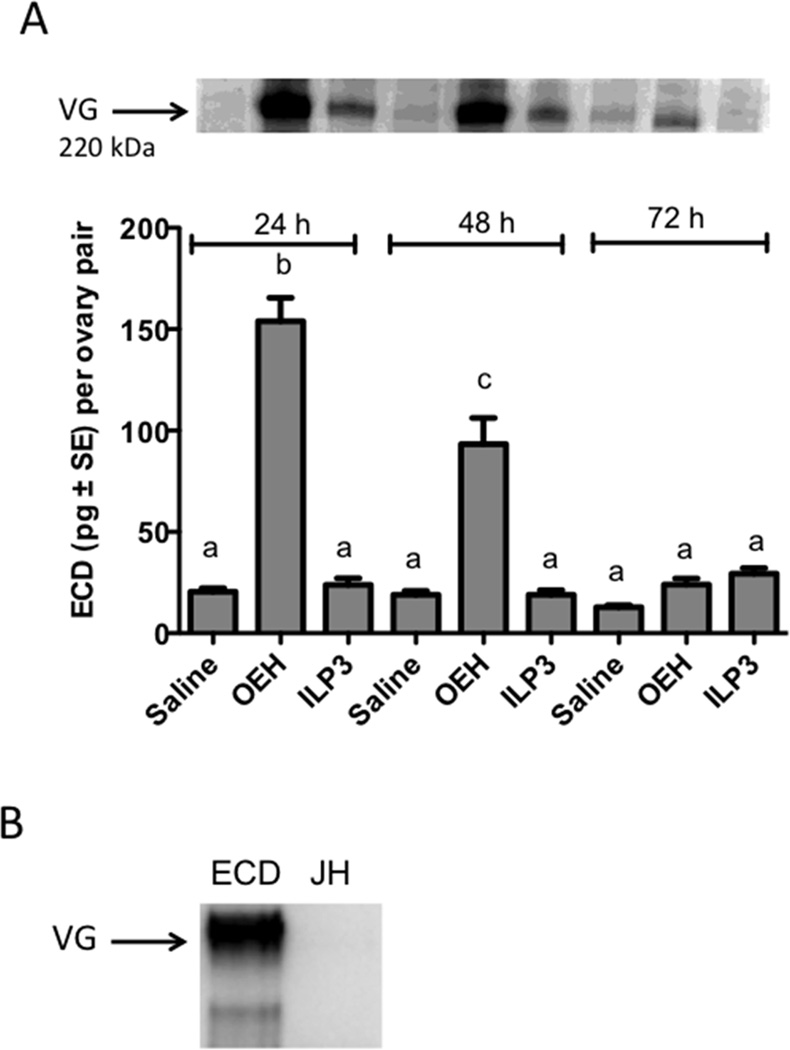
As previously noted, Bass Rock strain G. atropalpus females oviposit 3 to 5 days PE a clutch of ~100 eggs (Gulia-Nuss et al., 2012). In comparison, UGAL strain A. aegypti reared under the same conditions oviposit ~120 eggs 3 to 4 days after consuming a blood meal (Brown et al., 2008; Wen et al., 2010). Decapitation immediately after blood feeding ablates the endogenous source of OEH and ILPs in both species, resulting in no ECD or VG production, while injection of either OEH or ILP3 rescues these defects, which results in yolk uptake into oocytes (Brown et al., 2008; Gulia-Nuss et al., 2012; Dhara et al., 2013; Vogel et al., 2015). Thus, we next assessed whether OEH or ILP3 could induce a gonadotropic state in non-blood fed A. aegypti females. Females (day 1 PE) were injected with OEH (20 pmol), ILP3 (20 pmol) or saline (negative control), then 24, 48 and 72 h later, ovaries and body walls with fat body from these females were assessed for stimulation of ECD and VG production, respectively. Our results showed that a single dose of OEH stimulated the ovaries of non-blood fed females to produce a significant increase in ECD in vitro 24 and 48 h later, whereas injection of ILP3 or saline did not (Fig. 2A). Concurrently, immunoblotting detected a higher amount of vitellogenin (VG) in the fat body of females injected with OEH than ILP3, but we also noted that ILP3 injection resulted in detection of more VG than injection of saline (Fig. 2A). Injection of 20ECD (500 ng) into non-blood fed females also stimulated VG production by the fat body but topically treating non-blood fed females with JHIII (100 pmol) did not (Fig. 2B).
Figure 2.
OEH stimulates ecdysteroid (ECD) biosynthesis by the ovaries and vitellogenin production by fat bodies of non-blood fed A. aegypti. (A) Injection of OEH (20 pmol) more strongly stimulates the amount of ECD secreted by the ovaries than ILP3 (20 pmol) or saline at 24 and 48 h post-treatment (F8,72=60.6; P<0.0001). Different letters above the bars of the graph indicate means that significantly differ as determined by the Tukey-Kramer multiple comparison procedure (α = 0.05; n=9 per treatment). Above the graph is a representative immunoblot showing that OEH treatment also results in more VG production than ILP3 or saline. (B) Immunoblot showing that injection of ECD (500 ng) into non-blood fed A. aegypti also stimulates detectable levels of VG in the fat body after 24 h, whereas topical application of JHIII (100 pmol) does not.
3.3. OEH stimulates yolk uptake by oocytes in non-blood fed females but few mature eggs
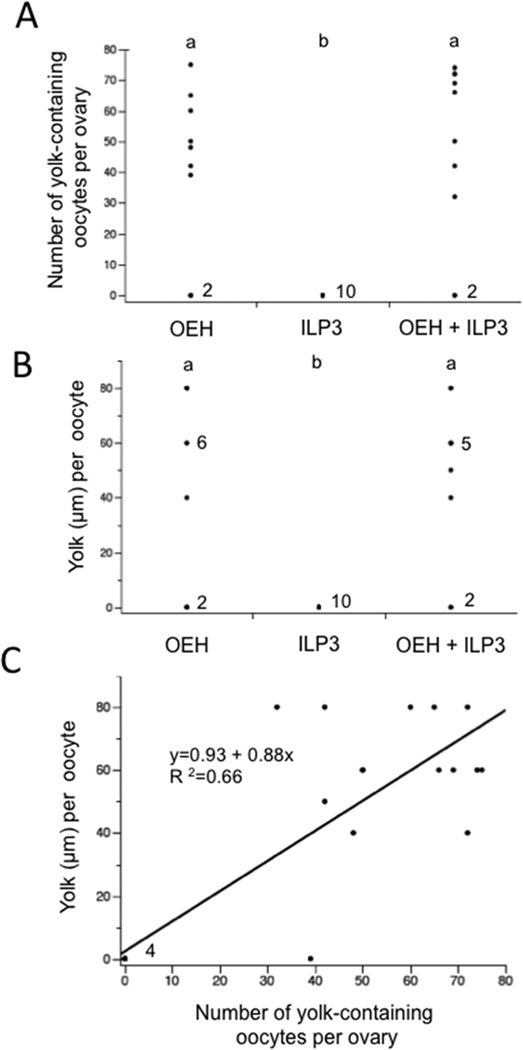
Since injection of OEH or ILP3 stimulated VG production in non-blood fed A. aegypti, we assessed whether these hormones could also induce yolk uptake into oocytes and formation of mature eggs that females lay. The first experiment we conducted was to inject females 1 day PE with OEH (20 pmol), ILP3 (20 pmol), or both followed 24 h later by dissection and inspection of the ovaries. This yielded information on whether yolk uptake into oocytes occurred within 24 h of treatment as occurs when blood-fed females are injected with each hormone following decapitation (Dhara et al., 2013). Results indicated that most females injected with OEH or OEH + ILP3 contained oocytes with yolk, whereas no yolk was present in oocytes of females injected with only ILP3 (Fig. 3A). Skew in the residuals generated by a one-way ANOVA together with a Shapiro-Wilks test (P= 0.004) also strongly indicated these data were non-normally distributed. We therefore used a nonparametric Kruskal-Wallis test to analyze treatment effects. This showed that females injected with OEH or OEH + ILP3 did not differ from one another in the number of oocytes with yolk they contained, but each significantly differed from females injected with only ILP3 where no oocytes contained yolk (Fig. 3A).
Figure 3.
Non-blood fed A. aegypti package yolk into oocytes 24 h post-injection of OEH or OEH + ILP3. (A) Number of yolk-containing oocytes per female after treatment with OEH (20 pmol), ILP3 (20 pmol), or OEH + ILP3 (20 pmol of each) (n= 10 females per treatment) (χ2=13.1; df= 2; P= 0.002). Different letters above each treatment indicates the number of yolk-containing oocytes per female significantly differs (Wilcoxin Each Pair Comparison, α= 0.05). Each data point represents one female with exception of the 0 value where the number to the right for each treatment indicates the number females that had no yolk-containing oocytes in their ovaries. (B) Amount of yolk packaged per oocyte after treatment with OEH, ILP3, or AaOEH + ILP3 (χ2=12.7; df= 2; P= 0.002). Each data point indicates the median amount of yolk per oocyte for the oocytes in the ovary of each female. The numbers to the right of the 0 value for each treatment indicates the number females that had no yolk-containing oocytes in their ovaries, while the numbers to the right of the 60 µm value indicates the number of females where the median amount of yolk per oocyte was 60 µm. Statistical comparisons were performed as described in (A). (C) Amount of yolk per oocyte plotted against the number of yolk-containing oocytes per ovary from each female treated with OEH or OEH + ILP3. The linear fit of the data as indicated by the black line is highly significant (F1,18=35.5; P<0.0001).
We next considered the amount of yolk present per oocyte in each female. Fig. S1 presents the amount of yolk in oocytes of each ovary for 7 females injected with OEH. While a small amount of variation was found, yolk amounts per oocyte in each female were overall highly uniform. The micrographs in Fig. S1 show the ovaries from 2 females, which clearly illustrates the uniformity. The same trend was found for the ILP3 treatment, where no oocytes contained yolk, and the OEH + ILP3 treatment which resembled females injected with OEH only (data not shown). We therefore took the median amount of yolk per oocyte for each female and plotted it in Fig. 3B. Comparing these values non-parametrically as a function of treatment indicated that the effects of injecting OEH or OEH + ILP3 did not differ from one another but did significantly differ from the effects of ILP3 where no oocytes contained yolk. Lastly, we plotted the relationship between the number of oocytes with yolk per female and the median amount of yolk per oocyte by merging the data for individuals that were injected with OEH only or OEH + ILP3. This showed a significantly positive and linear relationship indicating that females with more yolk-containing oocytes also tended to package more yolk per oocyte (Fig. 3C).
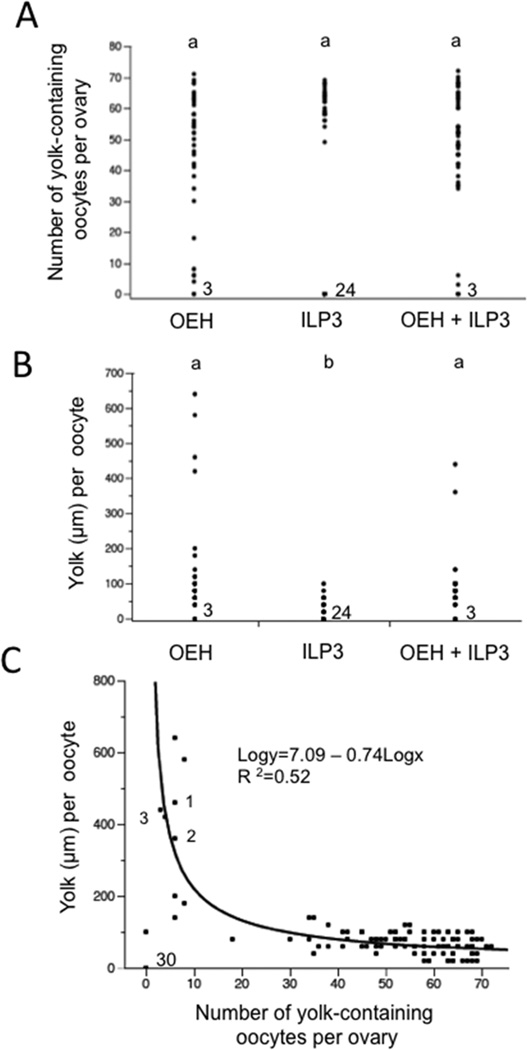
Having established that OEH or OEH + ILP3 treatment causes non-blood females to rapidly package yolk into oocytes, we next injected females 1 day PE with OEH, ILP3 or both but then held them for 5 days to assess whether any produced and laid mature eggs. Given evidence that JH release after adult emergence promotes reproductive competency in A. aegypti (Hernández-Martinez et al., 2007, 2015), we also injected females 2, 3 or 5 days PE as above and held them for 5 days to assess whether age influenced outcomes. No females treated with OEH (N=50) or ILP3 (N=50) laid eggs. From a total of 48 females injected with OEH + ILP3, 45 laid no eggs while 3 females injected at 1 day PE laid 12 eggs, 2 eggs and 1 egg respectively. We classified all of these oviposited eggs as mature because each had a chorion and contained ≥300 µm of yolk. Approximately half of these eggs also hatched into first instars when placed into water. Overall though, these results indicated that very few non-blood fed females laid any eggs after injection of OEH and/or ILP3. We therefore dissected all individuals on day 5 post-injection to determine the number of unlaid oocytes that contained yolk. As observed at 24 h post-injection, these data were non-normally distributed. Kruskal-Wallis tests, however, showed that females injected 1, 2, 3 or 5 days PE with OEH (χ2=2.26; df= 3; P= 0.52), ILP3 (χ2 =5.09; df= 3; P= 0.17), or OEH + ILP3 (χ2 =2.11; df= 3; P= 0.60) did not differ in the number of yolk-containing oocytes they contained. We therefore merged the data across female ages to analyze treatment effects. Plotting this merged data set showed that most females injected with OEH or OEH + ILP3 contained at least one oocyte with yolk while approximately half of the females injected with ILP3 contained no oocytes with yolk (Fig. 4A). On the other hand, formal data analysis found no differences among treatments in the number of yolk-containing oocytes per female. This was because several females injected with OEH or OEH + ILP3 contained only small numbers of yolk-containing oocytes (Fig. 4A).
Figure 4.
Females injected OEH or OEH + ILP3 package more yolk per oocyte after 5 days than females injected with only ILP3. (A) Number of yolk-containing oocytes per female after treatment with OEH (n=50 females), ILP3 (n=50 females), or OEH + ILP3 (n= 48 females) (χ2=4.0; df= 2; P= 0.14). Numbers to the right of the 0 value for each treatment indicates the number females that had no yolk-containing oocytes in their ovaries. (B) Amount of yolk packaged per oocyte after treatment with OEH, ILP3, or OEH + ILP3 (χ2 =66.8; df= 2; P< 0.001). Each data point indicates the median amount of yolk per oocyte for the oocytes in the ovary of each female. Statistical comparisons were performed as described in (A) while the numbers to the right of the 0 value for each treatment indicates the number females that had no yolk-containing oocytes in their ovaries. (C) Amount of yolk per oocyte plotted against number of oocytes with yolk per ovary for each treatment. The relationship is non-linear but log * log conversion of the data generated a highly significant fit as indicated by the black line (F1,115=121.6; P<0.0001). The number (30) next to 0 value at the bottom of (C) indicates the number of females that had no yolk-containing oocytes in their ovaries. The numbers 1, 2 and 3 next to three of the data points in (C) identify the three females that oviposited at least one egg (see Results).
As with the 24 h data, we also examined how OEH and/or ILP3 affected the amount of yolk per oocyte at 5 days post-treatment. Fig. S2 presents data for 14 females injected 1 day PE with OEH that were dissected after 5 days. For each female, the number of oocytes in each ovary that contained visible yolk is shown along with the amount of yolk per oocyte. Micrographs showing the ovaries of female 3, 4 and 12 are presented below these data. The amount of yolk per oocyte varied slightly within each female but overall these values were highly uniform relative to the variation between females (Fig. S2). The micrographs in Fig. S2 also clearly illustrate the variation between females by showing that the ovaries from female 3 and 12 contain a few oocytes with a uniformly large amount of yolk while most other oocytes contained no yolk. In contrast, the ovaries from female 4 contain several oocytes with a uniform amount of yolk, yet the amount of yolk per oocyte is also clearly less than in female 3 and 12 (Fig. S2). These same patterns were also observed in females that were injected with ILP3 or OEH + ILP3 treatment (data not shown).
We therefore took the median yolk per oocyte value for each female and plotted it in Fig. 4B. Comparing these values non-parametrically indicated that the effects of injecting OEH or OEH + ILP3 did not differ from one another but did significantly differ from the effects of ILP3. This was because several females injected with OEH or OEH + ILP3 contained a small number of oocytes with large amounts of yolk as shown in Fig. S2. Plotting the number of yolk-containing oocytes per female by the amount of yolk per oocyte after 5 days also revealed another key trend: namely, females with fewer yolk-containing oocytes packaged much larger amounts of yolk per oocyte than females with more yolk-containing oocytes (Fig. 4C). This negative, non-linear relationship was highly significant (Fig. 4C) and distinctly different from the positive relationship seen at 24 h post-treatment (see Fig. 3C). This relationship is also well illustrated in Fig. S2. All of the data points in Fig. 4C showing oocytes with >300 µm of yolk were from females injected with OEH or OEH + ILP3 (see Fig. 4B). All oocytes with >300 µm of yolk also had chorions. Taken together, these data indicated that most females after 5 days contained oocytes with <100 µm of yolk and no chorion. A few females treated with OEH or OEH + ILP3, however, produced a small number of mature eggs. These eggs were usually retained in the ovary, but three females oviposited some mature eggs. In contrast, the largest amount of yolk per oocyte in any female treated with ILP3 was 120 µm (Fig. 4B), which confirmed that ILP3 could stimulate VG biosynthesis in non-blood fed females (see Fig. 2A) that also results in yolk uptake into oocytes. However, this response was overall weaker than the response stimulated by OEH or OEH+ILP3.
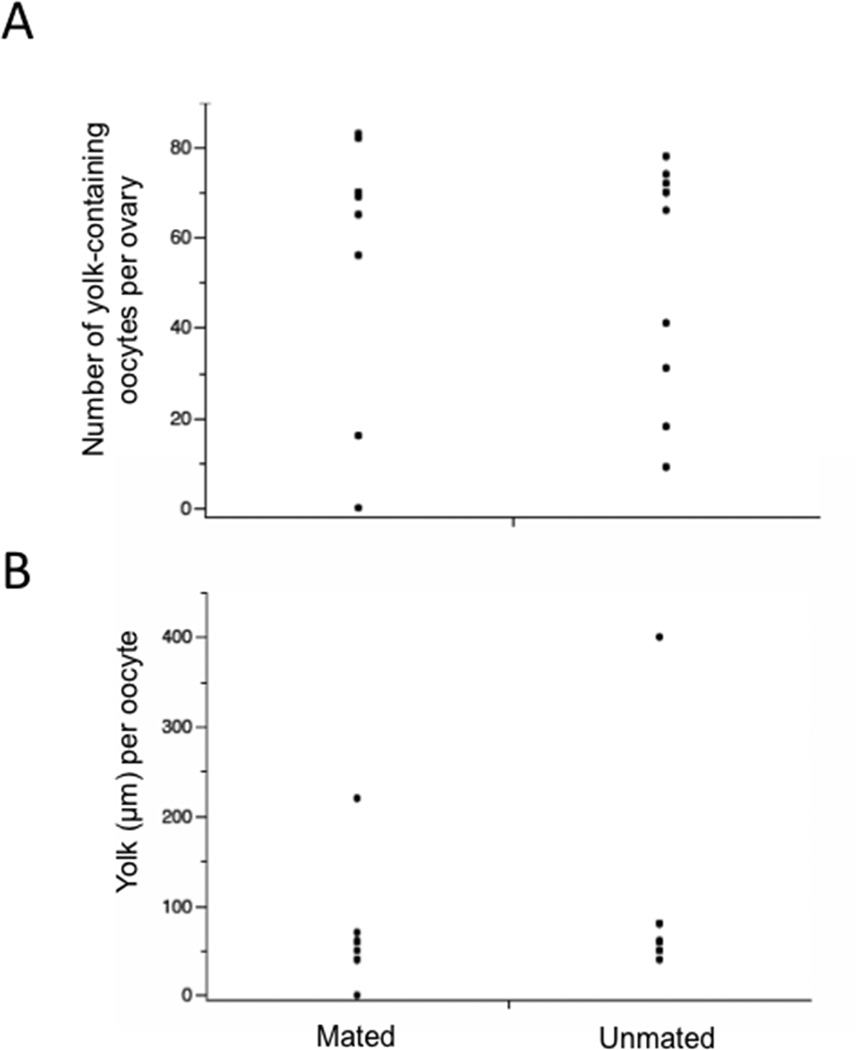
3.4. OEH stimulates yolk uptake by oocytes in mated and unmated non-blood fed females
Recent studies with A. aegypti show that males transfer JHIII to females during mating, which affects ovary development (Clifton et al., 2014), whereas Anopheles gambiae males transfer ECD to females that promote egg laying and loss of sexual receptivity (Gabrieli et al., 2014). In light of the important role these hormones and other male factors play in female behavior and oogenesis, we assessed whether mating status affected the ability of OEH to stimulate yolk packaging into oocytes. Females (1 day old) were injected with OEH (20 pmol) and after 5 days, examined for yolk deposition. The data showed that mating status had no significant effect on either the number of oocytes per ovary with yolk or the amount of yolk per oocyte (Fig. 5). The number of oocytes with yolk was similarly variable among mated and unmated females, whereas the amount of yolk per oocyte was fully uniform within individual mated and unmated females.
Figure 5.
Mating status has no effect on the number of oocytes with yolk (A) (t=0.43; P=0.67) or the amount of yolk per oocyte (B) (t=0.73; P=0.48) at 5 days post-injection of OEH (n=10 females for each treatment).
3.5. OEH reduces host seeking and blood feeding
Previous studies showed that blood feeding reduces host-seeking behavior by UGAL strain A. aegypti which results in few individuals consuming a second blood meal before oviposition (Klowden and Lea, 1979a,b; Klowden and Briegel, 1994). Reduced host-seeking behavior is also associated with two factors: distention of the midgut by the blood meal and release of neurohormones or other humoral factors into the hemolymph following blood feeding (Klowden and Lea, 1979 a,b ; Klowden, 1981; Klowden and Briegel, 1994; Liesch et al., 2013). Since OEH stimulated yolk deposition into oocytes in non-blood fed females with no associated distention of the midgut, we asked whether females also exhibited reduced host seeking and blood feeding behavior.
We first determined that no females at 8 h PE blood fed (N=200) within 20 min of exposure to an anesthetized rat. However, 40% of females blood fed (N=208) at 15 h PE, 61% of females blood fed (N=177) at 24 h PE, and 90% of females (N=200) blood fed at 48 h PE. This established that host seeking and blood feeding behavior develop in most females within the first 15 day of emergence as an adult. We therefore injected females at 24 h PE with either saline or OEH (20 pmol) and then two days post-injection exposed each cohort to an anesthetized rat for 20 min and counted blood feeding-females. Results showed that a significantly smaller percentage of females injected with OEH (39%, n=95) took a blood meal compared to females injected with saline (67%, N=70) (χ2 =12.1; df=1; P<0.0005). Dissection of 15 females from each cohort at the time of the bioassay also showed that no females injected with saline had any yolk-containing oocytes in their ovaries, while all females injected with OEH did.
4. Discussion
Detailed studies of A. aegypti conclusively show that egg formation is triggered by blood-meal dependent release of OEH and ILPs (Brown et al., 1998; Wen et al., 2010; Dhara et al., 2013), while the presence of OEH and ILP orthologs in all mosquito genomes examined to date (Marquez et al., 2011; Antonova et al., 2012; Vogel et al. 2013) suggest a conserved role for these neurohormones in regulating egg formation in other anautogenous species. That OEH and ILPs activate egg formation in G. atropalpus further suggests a factor underlying facultative autogeny in this species is a shift from OEH and ILP release being blood meal dependent to being blood meal independent (Gulia-Nuss et al., 2012).
OEH and ILPs are primarily synthesized in brain medial neurosecretory cells but are stored and released from the corpus cardiacum (CC), which is innervated by medial neurosecretory cell axons (Brown and Cao, 2001; Riehle et al., 2006). The factor(s) that stimulates release of OEH and ILPs from the CC is unknown but it is likely tied in some way to nutrient availability given that mosquitoes produce no eggs in the absence of nutrients acquired during the larval and/or adult stage. In the first part of this study, we show that G. atropalpus females emerge with larger protein and glycogen reserves than A. aegypti when larvae are reared under identical, nutrient-rich conditions. Similar adult sizes but larger teneral reserves were also found in an autogenous population of Aedes albopictus when compared to an anautogenous population (Chambers and Klowden, 1994). Thus, higher teneral reserves and associated nutrient sensing may play a role in stimulating release of OEH and ILPs in G. atropalpus. One option for nutrient sensing operative in mosquitoes and other insects is the TOR pathway, which assesses nutrient status directly by amino acid sensing or indirectly through the insulin-signaling pathway (Mirth and Riddiford, 2007; Roy and Raikhel, 2007). Our own results also show that disabling TOR and the insulin signaling pathway near fully inhibits egg formation in blood-fed A. aegypti (Gulia-Nuss et al. 2011). Another option for nutrient sensing identified in Drosophila is through neurons and/or enteroendocrine cells that relay information back to the central nervous system (Park and Kwon, 2011; Miyomoto et al. 2012).
In contrast, two lines of evidence strongly suggest teneral reserves alone do not explain why Bass Rock strain G. atropalpus produce eggs without blood feeding while UGAL strain A. aegypti do not. First, restricting the amount or quality of food consumed by G. atropalpus larvae reduces adult size and the average number of eggs per clutch, but it never prevents females from producing a first clutch of eggs autogenously (Telang and Wells, 2004; Telang et al., 2006). Second, several studies report low levels of autogeny in field populations of different Aedes species (Mori, 1979; Cui, 1982; O’Meara et al., 1993; Mori et al., 2008; Ahmed, 2013) including one report for A. aegypti collected from East Africa (Trpis, 1977). Autogeny in some of these studies also correlates with adult size, which the authors associate with larger nutrient reserves. No studies, however, show that enriching food quality during the larval stage promotes autogeny in A. aegypti or other Aedes species beyond individuals laying very small numbers of eggs relative to females that blood feed (Dimond et al., 1955; Lea et al., 1963; Trpis, 1977; Lea, 1982; Raikhel and Lea, 1982; Mori et al., 2008). Reducing the food provided to larval stage A. aegypti in contrast does consistently result in smaller adults that lay smaller clutches after a blood meal (Reyes-Villaneuva 2004; Scott et al. 1993a,b; Xue et al. 1995; Farjana and Tuno, 2013). Overall then, larval diet clearly affects adult size and the number of eggs per clutch G. atropalpus and A. aegypti females lay. However, it does not alter that Bass Rock G. atropalpus produces a first clutch of eggs autogenously, while A. aegypti does not. Thus, other factors besides nutrient sensing are likely involved in regulating OEH and ILP release from the CC.
Since UGAL strain A. aegypti never release OEH or ILPs without blood feeding, we asked in the second part of this study whether injection of OEH and/or ILP3 can artificially stimulate non-blood fed A. aegypti to produce eggs. Our previous results with G. atropalpus showed that A. aegypti OEH near fully restores egg maturation in decapitated females, while A. aegypti ILP3 is only marginally restorative (Gulia-Nuss et al., 2012). In this study, non-blood fed A. aegypti also responded more strongly to OEH than ILP3 as measured by ECD secretion, VG production and yolk uptake by oocytes. The level and duration of ECD production by ovaries from non-blood fed A. aegypti following a single dose of OEH was also comparable to the response shown by blood fed females (Dhara et al., 2013). Interestingly, treatment with the native ILP3 activates a modicum of VG production in non-blood fed females in the absence of detectable ECD production. This response is comparable to the low level of VG gene expression and protein detected in isolated A. aegypti fat bodies incubated with amino acids, which are a major component of mosquito hemolymph, with or without mammalian insulin (Roy et al., 2007; Roy and Raikhel, 2011 - Fig. 4C). Addition of ECD to the experimental medium used in these studies greatly stimulated VG gene expression, which likely is the same effect of ECD that is proeduced following OEH treatment of non-blood fed females. Although no yolk deposition was observed in the oocytes of ILP3 injected females a day afterwards, by five days, yolk was present in the oocytes of some females. Thus similar to G. atropalpus, OEH more strongly activates egg formation in non-blood A. aegypti than ILP3. Unlike G. atropalpus, however, only a small proportion of non-blood fed A. aegypti females produced mature eggs in response to OEH. This disparity may be due to A. aegypti females emerging with only half of the teneral protein and glycogen reserves present in G. atropalpus. It also could reflect an inability by A. aegypti females to convert available nutrient reserves to yolk following OEH stimulation.
Five days after injection of OEH or OEH+ILP3, the disparity between females with many oocytes having little yolk and those with a few mature oocytes is stark (Fig. 4; Fig. S2). Micrographs of the latter also show that numerous primary follicles containing oocytes with no yolk surround the few large oocytes that contain large amounts of yolk. Interestingly, the aforementioned study by Trpsis (1977) noted that an autogenous population of A. aegypti also initially deposited yolk into many oocytes, but after several days a few females contained small numbers of oocytes with large amounts of yolk, which were sometimes oviposited. An explanation for this phenomenon in our data set is that two to four days after injection of OEH or OEH+ILP3, some females resorb most of the oocytes that contain little yolk to allow a few oocytes to uptake sufficient yolk for maturity. During this period, ECD levels are sufficient to activate the maturation of secondary follicles into primary follicles seen five days later. We also note that an earlier study showed that physiological levels of ECD has the same effect in non-blood fed A. aegypti females (Beckemeyer and Lea, 1980). Taken together, results of this study corroborate earlier findings by Telang et al. (2006) that G. atropalpus accumulates larger nutrient reserves than A. aegypti during the larval stage. However, this study indicates that other factors also constrain autogenous reproduction by A. aegypti including blood meal dependent release of OEH and ILP3 and the response of ovaries to these hormones. Unlike Telang et al. (2006), which reports the correlation between teneral nutrient reserves and egg production or hormone biosynthesis (JH by the CA and ECD by the ovaries), this study experimentally examined the effects of OEH and ILPs on egg production by A. aegypti in the absence of a blood meal.
Consumption of a blood meal inhibits host seeking by A. aegypti and several other anautogenous species with gut distention and humoral factors both being implicated in causing this change in behavior (Klowden and Lea, 1978, 1979; Klowden and Briegel, 1994). A recent study identified head peptide as one factor underlying changes in host seeking behavior (Leisch et al., 2013), while results of this study suggest OEH itself impacts factors that affect host seeking and blood feeding behavior.
Supplementary Material
Highlights.
Anautogenous Aedes aegypti must blood feed to produce eggs.
Autogenous Georgecraigius atropalpus produces eggs with no blood meal.
We examined factors constraining autogeny by A. aegypti.
A. aegypti has lower teneral nutrient reserves than G. atropalpus.
Non-blood fed A. aegypti produce few eggs in response to neurohormones.
ACKNOWLEDGEMENTS
We thank Sarah Robertson for her assistance in maintaining the mosquito colony. This work was supported by a NIH grant RO1AI33108 to M. R. Strand and M. R. Brown.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmed AM. Mosquito autogeny in Aedes caspius (Diptera: Culicidae): alterations of larval nourishments reservation upon bacterial infection. Insect Sci. 2013;20:472–484. doi: 10.1111/j.1744-7917.2012.01544.x. [DOI] [PubMed] [Google Scholar]
- Antonova Y, Arik AJ, Moore W, Riehle MA, Brown MR. Insulin-like peptides: structure, signaling, and function. In: Gilbert LI, editor. Insect Endocrinology. New York: Elsevier/Academic Press; 2012. pp. 63–92. [Google Scholar]
- Attardo GM, Hansen IA, Raikhel AS. Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem. Mol. Biol. 2005;35:661–675. doi: 10.1016/j.ibmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Beckemeyer EF, Lea AO. Induction of follicle separation in the mosquito by physiological amounts of ecdysterone. Science. 1980;209:819–821. doi: 10.1126/science.209.4458.819. [DOI] [PubMed] [Google Scholar]
- Birnbaum MJ, Kelly TJ, Woods CW, Imberski RB. Hormonal regulation of ovarian ecdysteroid production in the autogenous mosquito, Aedes atropalpus. Gen. Comp. Endocrinol. 1984;56:9–18. doi: 10.1016/0016-6480(84)90055-8. [DOI] [PubMed] [Google Scholar]
- Black WC, IV, Kondratieff BC. Evolution of arthropod disease vectors. In: Marquardt WC, editor. Biology of Disease Vectors. 2nd edition. San Diego, CA: Elsevier Academic Press; 2005. pp. 9–23. [Google Scholar]
- Bowen MF, Davis EE, Haggart D, Romo J. Host-seeking behavior in the autogenous mosquito Aedes atropalpus. J. Insect Physiol. 1994;40:511–517. [Google Scholar]
- Briegel H. Physiological bases of mosquito ecology. J. Vector Ecol. 2003;28:1–11. [PubMed] [Google Scholar]
- Brown MR, Graf R, Swiderek KM, Fendley D, Stracker TH, Champagne DE, Lea AO. Identification of a steroidogenic neurohormone in female mosquitoes. J. Biol. Chem. 1998;273:3967–3971. doi: 10.1074/jbc.273.7.3967. [DOI] [PubMed] [Google Scholar]
- Brown MR, Cao C. Distribution of ovary ecdysteroidogenic hormone I in the nervous system and gut of mosquitoes. J. Insect Sci. 2001;1.3 doi: 10.1093/jis/1.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, Suderman RJ, Strand MR. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2008;105:5716–5721. doi: 10.1073/pnas.0800478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne LB. Quantitative aspects of the regulation of ovarian development in selected anautogenous Diptera: integration of endocrinology and nutrition. Entom. Exp. App. 2001;100:137–149. [Google Scholar]
- Chambers GM, Klowden MJ. Nutritional reserves of autogenous and anautogenous selected strains of Aedes albopictus (Diptera: Culicidae) J. Med. Entomol. 1994;31:554–560. doi: 10.1093/jmedent/31.4.554. [DOI] [PubMed] [Google Scholar]
- Clements AN. The Physiology of Mosquitoes. Oxford: Pergamon; 1963. [Google Scholar]
- Clements AN. The Biology of Mosquitoes, Vol. 1. London: Chapman & Hall; 1992. [Google Scholar]
- Clifton ME, Noriega FG. Nutrient limitation results in juvenile hormone-mediated resorption of previtellogenic ovarian follicles in mosquitoes. J. Insect Physiol. 2011;57:1274–1281. doi: 10.1016/j.jinsphys.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton ME, Correa S, Rivera-Perez C, Nouzova M, Noriega FG. Male Aedes aegypti mosquitoes use JH III transferred during copulation to influence previtellogenic ovary physiology and affect the reproductive output of female mosquitoes. J. Insect Physiol. 2014;64:40–47. doi: 10.1016/j.jinsphys.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon KL, Vogel KJ, Brown MR, Strand MR. Mosquitoes rely on their gut microbiota for development. Mol. Ecol. 2014;23:2727–2739. doi: 10.1111/mec.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui K-L. The autogeny of Aedes albopictus in the Guangzhou area. Acta Entomol. Sinica. 1982;25:256–259. [Google Scholar]
- Dhara A, Eum J-H, Robertson A, Gulia-Nuss M, Vogel KJ, Clark KD, Graf R, Brown MR, Strand MR. Ovary ecdysteroidogenic hormone functions independently of the insulin receptor in the yellow fever mosquito, Aedes aegypti. Insect Biochem. Molec. Biol. 2013;43:1100–1108. doi: 10.1016/j.ibmb.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimond JB, Lea AO, Brooks RF, DeLong DM. A preliminary note on some nutritional requirements for reproduction in female Aedes aegypti. Ohio J. Sci. 1955;55:209–211. [Google Scholar]
- Farjana T, Tuno N. Multiple blood feeding and host-seeking behavior in Aedes aegypti and Aedes albopictus (Diptera: Culicidae) J. Med. Entomol. 2013;50:838–846. doi: 10.1603/me12146. [DOI] [PubMed] [Google Scholar]
- Fuchs MS, Sundland BR, Kang SH. In vivo induction of ovarian development in Aedes atropalpus by a head extract from Aedes aegypti. Int. J. Invert. Repro. 1980;2:121–129. [Google Scholar]
- Gabrieli P, Kakani EG, Mitchell SN, Mameli E, Want EJ, Anton AM, Serrao A, Baldini F, Catteruccia F. Sexual transfer of the steroid hormone 20E induces the postmating switch in Anopheles gambiae. Proc. Natl. Acad. Sci. USA. 2014;111:16353–16358. doi: 10.1073/pnas.1410488111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulia-Nuss M, Robertson AE, Brown MR, Strand MR. Insulin-like peptides and the target of rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti. PloS ONE. 2011;6:e20401. doi: 10.1371/journal.pone.0020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulia-Nuss M, Eum J-H, Strand MR, Brown MR. Ovary ecdysteroidogenic hormone activates egg maturation in the mosquito, Georgecraigius atropalpus, after adult eclosion or a blood meal. J. Exp. Biol. 2012;215:3758–3767. doi: 10.1242/jeb.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwadz RW. Monofactorial inheritance of early sexual receptivity in the mosquito, Aedes atropalpus. Animal Behav. 1970;18:358–361. doi: 10.1016/s0003-3472(70)80048-3. [DOI] [PubMed] [Google Scholar]
- Hernandez-Martinez S, Mayoral JG, Li Y, Noriega FG. Role of juvenile hormone and allatotropin on nutrient allocation, ovarian development and survivorship in mosquitoes. J. Insect Physiol. 2007;53:230–234. doi: 10.1016/j.jinsphys.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Martinez S, Rivera-Perez C, Nouzova M, Noriega FG. Coordinated changes in JH biosynthesis and JH hemolymph titers in Aedes aegypti mosquitoes. J. Insect Physiol. 2015;72:22–27. doi: 10.1016/j.jinsphys.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A. Factors affecting egg maturation and oviposition by autogenous Aedes atropalpus (Diptera: Culicidae) Can. Entomol. 1970;12:939–950. [Google Scholar]
- Hugo LE, Kay BH, Ryan PA. Autogeny in Ochlerotatus vigilax (Diptera: Culicidae) from southeast Queensland, Australia. J. Med. Entomol. 2003;40:897–902. doi: 10.1603/0022-2585-40.6.897. [DOI] [PubMed] [Google Scholar]
- Kelly TJ, Fuchs MS, Kang SH. Induction of ovarian development in autogenous Aedes atropalpus by juvenile hormone and 20-hydroxyecdysone. Int. J. Invert. Repro. 1981;3:101–112. [Google Scholar]
- Kelly TJ, Birnbaum MJ, Woods CW, Borkovec AB. Effects of house fly oostatic hormone on egg development neurosecretory hormone action in Aedes atropalpus. J. Exp. Zool. 1984;229:491–496. [Google Scholar]
- Klowden MJ. Initiation and termination of host-seeking inhibition in Aedes aegypti during oocyte maturation. J. Insect Physiol. 1981;27:799–803. doi: 10.1016/0022-1910(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Klowden MJ, Lea AO. Blood meal size as a factor affecting continued host-seeking by Aedes aegypti (L.) Am. J. Trop. Med. Hyg. 1978;27:827–831. doi: 10.4269/ajtmh.1978.27.827. [DOI] [PubMed] [Google Scholar]
- Klowden MJ, Lea AO. Abdominal distention terminates subsequent host-seeking behaviour of Aedes aegypti following a blood meal. J. Insect Physiol. 1979a;25:583–585. doi: 10.1016/0022-1910(79)90073-8. [DOI] [PubMed] [Google Scholar]
- Klowden MJ, Lea AO. Effect of defensive host behavior on the blood meal size and feeding success of natural populations of mosquitoes (Diptera: Culicidae) J. Med. Entomol. 1979b;15:514–517. doi: 10.1093/jmedent/15.5-6.514. [DOI] [PubMed] [Google Scholar]
- Klowden MJ, Briegel H. Mosquito gonotrophic cycle and multiple feeding potential: contrasts between Anopheles and Aedes (Diptera: Culicidae) J. Med. Entomol. 1994;31:618–622. doi: 10.1093/jmedent/31.4.618. [DOI] [PubMed] [Google Scholar]
- Lea AO. Some relationships between environment corpora allata and egg maturation in aedine mosquitoes. J. Insect Physiol. 1963;9:793–809. [Google Scholar]
- Lea AO. Selection for autogeny in Aedes aegypti (Diptera: Culicidae) Ann. Entomol. Soc. Am. 1964;57:656–657. [Google Scholar]
- Lea AO. Endocrinology of egg maturation in autogenous and anautogenous Aedes taeniorhynchus. J. Insect Physiol. 1970;16:1689–1696. doi: 10.1016/0022-1910(70)90268-4. [DOI] [PubMed] [Google Scholar]
- Lea AO. Artifactual stimulation of vitellogenesis in Aedes aegypti by 20-hydroxyecdysone. J. Insect Physiol. 1982;28:173–176. [Google Scholar]
- Liesch J, Bellani LL, Vosshall LB. Functional and genetic characterization of neuropeptide Y-like receptors in Aedes aegypti. PLoS Negl. Trop. Dis. 2013;7:e2486. doi: 10.1371/journal.pntd.0002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Newton PB, He G, Kelly TJ, Hsu HT, Masler EP, Borkovec AB. Development of monoclonal antibodies for monitoring Aedes atropalpus vitellogenesis. J. Insect Physiol. 1984;30:529–536. [Google Scholar]
- Marchal E, Hult EF, Huang J, Pang Z, Stay B, Tobe SS. Methoprene-tolerant (Met) knockdown in the adult female cockroach, Diploptera punctate, completely inhibits ovarian development. PLoS One. 2014;9:e106737. doi: 10.1371/journal.pone.0106737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez AG, Pietri JE, Smithers HM, Nuss A, Antonova Y, Drexler AL, Riehle MA, Brown MR, Luckhart S. Insulin-like peptides in the mosquito Anopheles stephensi: identification and expression in response to diet and infection with Plasmodium falciparum. Gen. Comp. Endocrinol. 2011;173:303–312. doi: 10.1016/j.ygcen.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masler EP, Fuchs MS, Sage B, O’Connor JD. Endocrine regulation of ovarian development in the autogenous mosquito, Aedes atropalpus. Gen. Comp. Endocrinol. 1980;41:250–259. doi: 10.1016/0016-6480(80)90151-3. [DOI] [PubMed] [Google Scholar]
- Masler EP, Fuchs MS, Sage B, O’Connor JD. A positive correlation between oocyte production and ecdysteroid levels in adult Aedes. Physiol. Entomol. 1981;6:45–49. [Google Scholar]
- Masler EP, Whisenton LVR, Schlaeger DA, Kang SH, Fuchs MS. Chymotrypsin and trypsin levels in adult Aedes atropalpus and Toxorhynchites brevipalpus (Theobald) Comp. Biochem. Physiol. 1983;75B:435–440. [Google Scholar]
- Mirth CK, Riddiford LM. Size assessment and growth control: how adult size is determined in insects. Bioessays. 2007;29:344–355. doi: 10.1002/bies.20552. [DOI] [PubMed] [Google Scholar]
- Mogi M, Okazawa T, Sota T. Geographic pattern of autogeny and wing length in Aedes togoi (Diptera: Culicidae) Mosquito System. 1995;27:155–166. [Google Scholar]
- Mori A. Effects of larval density and nutrition on someattributes of immature and adult Aedes albopictus. Trop. Med. 1979;21:85–103. [Google Scholar]
- Mori A, Romero-Severson J, Black WC, IV, Severson DW. Quantitative trait loci determining autogeny and body size in the Asian tiger mosquito (Aedes albopictus) Heredity. 2008;101:75–82. doi: 10.1038/hdy.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–1125. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhout HF, Riddiford LM, Mirth C, Shingleton AW, Suzuki Y, Callier V. The developmental control of size in insects. Wiley Interdiscip. Rev. Dev. Biol. 2014;3:113–134. doi: 10.1002/wdev.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara GF. Gonotrophic interactions in mosquitoes – kicking the blood-feeding habit. Flor. Entomol. 1985;68:122–133. [Google Scholar]
- O’Meara GF. Polygenic regulation of fecundity in autogenous Aedes atropalpus. Ento. Exp. Applicata. 1972;15:81–89. [Google Scholar]
- O’Meara GF, Craig GB. Monofactoral inheritance of autogeny in Aedes atropalpus. Mosq. News. 1969;29:14–22. [Google Scholar]
- O'Meara GF, Krasnick GJ. Dietary and genetic control of the expression of autogenous reproduction in Aedes atropalpus (Coq.) (Diptera: Culicidae) J. Med. Entomol. 1970;7:328–334. doi: 10.1093/jmedent/7.3.328. [DOI] [PubMed] [Google Scholar]
- O'Meara GF, Larson VL, Mook DH. Blood feeding and autogeny in the peridomestic mosquito Aedes bahamensis (Diptera: Culicidae) J. Med. Entomol. 1993;30:378–383. doi: 10.1093/jmedent/30.2.378. [DOI] [PubMed] [Google Scholar]
- Park JH, Kwon JY. Heterogeneous expression of Drosophila gustatory receptors in enteroendocrine cells. PLoS One. 2011a;6:e29022. doi: 10.1371/journal.pone.0029022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Kwon JY. A systematic analysis of Drosophila gustatory receptor gene expression in abdominal neurons which project to the central nervous system. Mol. Cells. 2011b;32:375–381. doi: 10.1007/s10059-011-0128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Hedo M, Rivera-Perez C, Noriega FG. The insulin/TOR signal transduction pathway is involved in the nutritional regulation of juvenile hormone synthesis in Aedes aegypti. Insect Biochem. Mol. Biol. 2013;43:495–500. doi: 10.1016/j.ibmb.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikhel AS, Lea AO. Abnormal vitelline envelope induced by unphysiological doses of ecdysterone in Aedes aegypti. Physiol. Entomol. 1982;7:55–64. [Google Scholar]
- Reidenbach KR, Cook S, Bertone MA, Harbach RE, Wiegmann BM, Besansky NJ. Phylogenetic analysis and temporal diversification of mosquitoes (Diptera: Culicidae) based on nuclear genes and morphology. BMC Evol. Biol. 2009;9:298. doi: 10.1186/1471-2148-9-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Villanueva F. Egg development may require multiple bloodmeals among small Aedes aegypti (Diptera: Culicidae) field collected in northeastern Mexico. Fl. Entomol. 2004;87:630–632. [Google Scholar]
- Riddiford LM. How does juvenile hormone control insect metamorphosis and reproduction? Gen. Comp. Endocrinol. 2012;179:477–484. doi: 10.1016/j.ygcen.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Fan Y, Cao C, Brown MR. Molecular characterization and developmental expression of insulin-like peptides in the yellow fever mosquito, Aedes aegypti. Peptides. 2006;27:2547–2560. doi: 10.1016/j.peptides.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Rioux JA, Crost H, Pech-Perieres J, Guilvard E, Belmonte A. L’autogénèse chez les diptères autogénes. Ann. Parasit. Hum. Comp. 1975;50:134–140. [PubMed] [Google Scholar]
- Roy SG, Hansen IA, Raikhel AS. Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 2007;37:1317–1326. doi: 10.1016/j.ibmb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SG, Raikhel AS. The small GTPase Rheb is a key component linking amino acid signaling and TOR in the nutritional pathway that controls mosquito egg development. Insect Biochem. Mol. Biol. 2011;41:62–69. doi: 10.1016/j.ibmb.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TW, Chow E, Strickman D, Kittayapong P, Wirtz RA, L. Lorenz LH, Edman JD. Blood feeding patterns of Aedes aegypti (Diptera: Culicidae) collected in a rural Thai village. J. Med. Entomol. 1993a;30:922–927. doi: 10.1093/jmedent/30.5.922. [DOI] [PubMed] [Google Scholar]
- Scott TW, Clark GG, Lorenz LH, Amerasinghe PH, Reiter P, Edman JD. Detection of multiple blood feeding in Aedes aegypti (Diptera: Culicidae) during a single gonotrophic cycle using a histologic technique. J. Med. Entomol. 1993b;30:94–99. doi: 10.1093/jmedent/30.1.94. [DOI] [PubMed] [Google Scholar]
- Spielman A. The inheritance of autogeny in the Culex pipiens complex of mosquitoes. Amer. J. Hyg. 1957;65:404–425. doi: 10.1093/oxfordjournals.aje.a119878. [DOI] [PubMed] [Google Scholar]
- Spielman A. Bionomics of autogenous mosquitoes. Ann. Rev. Entomol. 1971;16:231–248. doi: 10.1146/annurev.en.16.010171.001311. [DOI] [PubMed] [Google Scholar]
- Telang A, Wells MA. The effect of larval and adult nutrition on successful autogenous egg production by a mosquito. J. Insect Physiol. 2004;50:677–685. doi: 10.1016/j.jinsphys.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Telang A, Li YP, Noriega FG, Brown MR. Effects of larval nutrition on the endocrinology of mosquito egg development. J. Exp. Biol. 2006;209:645–655. doi: 10.1242/jeb.02026. [DOI] [PubMed] [Google Scholar]
- Telang A, Frame L, Brown MR. Larval feeding duration affects ecdysteroid levels and nutritional reserves regulating pupal commitment in the yellow fever mosquito Aedes aegypti (Diptera: Culicidae) J. Exp. Biol. 2007;210:854–864. doi: 10.1242/jeb.02715. [DOI] [PubMed] [Google Scholar]
- Trpis M. Genetics of hematophagy and autogeny in the Aedes scutellaris complex (Diptera: Culicidae) J. Med. Entomol. 1978;15:73–80. doi: 10.1093/jmedent/15.1.73. [DOI] [PubMed] [Google Scholar]
- Van Handel E. The chemistry of egg maturation in the unfed mosquito Aedes atropalpus. J. Insect Physiol. 1976;22:521–522. doi: 10.1016/0022-1910(76)90170-0. [DOI] [PubMed] [Google Scholar]
- Vogel KJ, Brown MR, Strand MR. Phylogenetic investigation of peptide hormone and growth factor receptors in five dipteran genomes. Fron. Endocrin. 2013;4:193. doi: 10.3389/fendo.2013.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel KJ, Brown MR, Strand MR. Ovary ecdysteroidogenic hormone requires a receptor tyrosine kinase to activate egg formation in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2013;112:5057–5062. doi: 10.1073/pnas.1501814112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Gulia M, Clark KD, Dhara A, Crim JW, Strand MR, Brown MR. Two insulin-like peptide family members from the mosquito Aedes aegypti exhibit differential biological and receptor binding activities. Mol. Cell. Endocrinol. 2010;328:47–55. doi: 10.1016/j.mce.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegmann BM, Trautwein MD, Winkler IS, et al. Episodic radiations in the fly tree of life. Proc. Natl. Acad. Sci. USA. 2011;108:5691–5695. doi: 10.1073/pnas.1012675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue RD, Edman JD, Scott TW. Age and body size effects on blood meal size and multiple blood feeding by Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 1995;32:471–474. doi: 10.1093/jmedent/32.4.471. [DOI] [PubMed] [Google Scholar]
- Zou Z, Saha TT, Roy S, Shin SW, Backman TW, Girke T, White KP, Raikhel AS. Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc. Natl. Acad. Sci. USA. 2013;110:2173–2181. doi: 10.1073/pnas.1305293110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.