Abstract
The fungi on Meju are known to play an important role as degrader of macromolecule of soybeans. In order to elucidate the origin of fungi on traditional Meju, mycobiota of the air both inside and outside traditional Meju fermentation rooms was examined. From 11 samples of air collected from inside and outside of 7 Meju fermentation rooms, 37 genera and 90 species of fungi were identified. In outside air of the fermentation room, Cladosporium sp. and Cladosporium cladosporioides were the dominant species, followed by Cladosporium tenuissimum, Eurotium sp., Phoma sp., Sistotrema brinkmannii, Alternaria sp., Aspergillus fumigatus, Schizophyllum commune, and Penicillium glabrum. In inside air of the fermentation room, Cladosporium sp., Aspergillus oryzae, Penicillium chrysogenum, Asp. nidulans, Aspergillus sp., Cla. cladosporioides, Eurotium sp., Penicillium sp., Cla. tenuissimum, Asp. niger, Eur. herbariorum, Asp. sydowii, and Eur. repens were collected with high frequency. The concentrations of the genera Aspergillus, Eurotium, and Penicillium were significantly higher in inside air than outside air. From this result and those of previous reports, the origin of fungi present on Meju was inferred. Of the dominant fungal species present on Meju, Lichtheimia ramosa, Mucor circinelloides, Mucor racemosus, and Scopulariopsis brevicaulis are thought to be originated from outside air, because these species are not or are rarely isolated from rice straw and soybean; however, they were detected outside air of fermentation room and are species commonly found in indoor environments. However, Asp. oryzae, Pen. polonicum, Eur. repens, Pen. solitum, and Eur. chevalieri, which are frequently found on Meju, are common in rice straw and could be transferred from rice straw to Meju. The fungi grow and produce abundant spores during Meju fermentation, and after the spores accumulate in the air of fermentation room, they could influence mycobiota of Meju fermentation in the following year. This could explain why concentrations of the genera Aspergillus, Eurotium, and Penicillium are much higher inside than outside of the fermentation rooms.
Keywords: Air, Fungi, Meju, Mycobiota, Origin
Meju is the important raw material for traditional Korean Jangryu (the singular form, Jang) such as Ganjang, Doenjang, and Gochujang [1]. Jangryu are useful and important sauces in Korean cuisine. Moreover, Jangryu have been reported to have health benefits such as antioxidative activity, antithrombotic effects, cholesterol-lowering effects, mutation suppression effects, and antitumor activities [2]. The taste and quality of Jangryu are decided by the Meju used to make them [3]. Various microorganisms are associated with Meju fermentation, because traditional Meju is naturally fermented [3]. In particular, fungi, which produce various enzymes and degrade macromolecules in soybeans, are important microorganisms in Meju fermentation [3,4]. The fungi Aspergillus oryzae, Eurotium chevalieri, E. repens, Lichtheimia ramosa, Mucor circinelloides, M. racemosus, Penicillium polonicum, P. solitum, and Scopulariopsis brevicaulis occur commonly on Meju [4,5,6,7,8].
In this study, we investigated the origins of the fungi on traditional Meju fermentation. This knowledge would help to control of fungi that are present during Meju fermentation, as some fungi on Meju are helpful for fermentation, but others are simply contaminants or toxigenic [2,9]. Possible sources of the fungi on traditional Meju include rice straw, soybeans, and the air inside and outside the fermentation room. The fungi present on rice straw and soybean have been previously reported [10,11]. Therefore, the aims of this study are to (1) identify the composition of the mycobiota in the air both inside and outside Meju fermentation room; (2) compare it with those of traditional Meju, rice straw, and soybeans; and (3) presume the origin of the fungi on traditional Meju.
MATERIALS AND METHODS
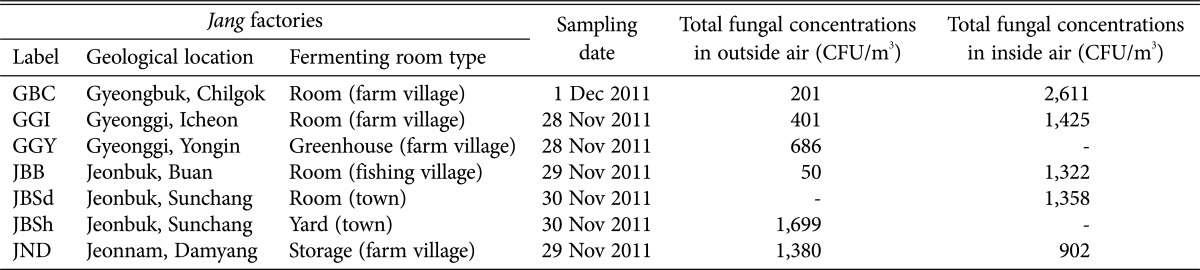
Eleven air samples were collected from inside (the fermentation room) and outside (surrounding Jang factory) of 7 Meju fermentation rooms during November 2011, before starting Meju production (Table 1). The samples were collected using a MAS-100 Eco air sampler (Merck, Darmstadt, Germany) with malt extract agar (MEA; for general fungi), dichloran rose bengal chloramphenicol agar (for general enumeration of fungi), dichloran 18% glycerol agar (DG18; for xerophilic fungi), and tryptic soy agar (for the genus Scopulariopsis). The air sampler put on the central floor of fermentation room or on the ground outside the Jang factory. And, 50 L, 100 L, and 500 L of air were collected from inside of fermentation room and 100 L, 500 L, and 1,000 L of air were collected from the outside of Jang factory. The plates were incubated at 25℃ in the dark for 3~5 days. The colonies grown on each plate were counted, and the average fungal concentrations of each plate were expressed as colony forming units per cubic meter (CFU/m3). The fungi grown on media were transferred into new MEA or DG18 media. After incubation for several days, the fungi were examined by light microscope for simple identification, transferred into MEA or DG18 slant for further examination, and then maintained at 4℃.
Table 1. Information about and fungal concentrations of air samples from Jang factories.
Molecular and morphological characteristics were used to identify the fungi via methods previously described in Kim et al. [11].
RESULTS AND DISCUSSION
As determined from 11 air samples from 7 Jang factories, the average concentrations of fungi inside and outside Meju fermentation rooms were 1,524 CFU/m3 and 736 CFU/m3 (Table 1), respectively, and they were identified into 37 genera and 90 species (Table 2). The fungal concentration in inside air of Meju fermentation room is similar or lower than that of green area in Seoul, Korea (average, 1,892 CFU/m3 in winter) [12] and is similar to that of low clean zone of other food product manufacturing plants (average, 2,600 CFU/m3) [13]. In this study, there was no significant difference of mycobiota according to Jang factories.
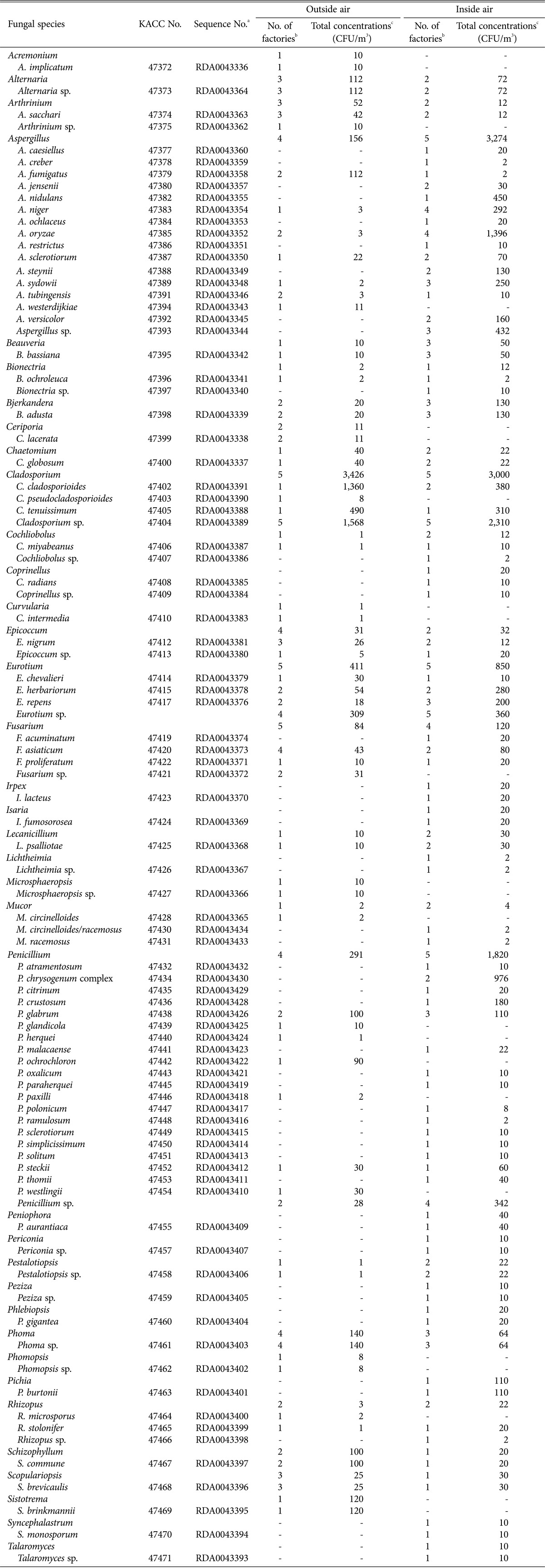
Table 2. List of fungal species from air of Meju fermentation room with their concentration in air.
aThe Rural Development Administration (RDA) numbers are DNA sequence accession numbers from the Korean Agricultural Culture Collection (KACC). Readers can access the sequences at the KACC homepage (http://www.genebank.go.kr) using the corresponding KACC numbers.
bThe numbers indicates factories from which the species were isolated from 6 (outside air) and 5 (inside air) factories.
cThe numbers indicates the sum of the maximum concentrations of each factory among concentrations on each media.
In the 6 outside air samples, 27 genera and 50 species were found (Table 2). The concentration of the genus Cladosporium was significantly high, followed by those of Eurotium, Penicillium, Aspergillus, Sistotrema, and Schizophyllum were followed (Table 2). Cla. cladosporioides and Cladosporium sp. were the dominant species, followed by Cla. tenuissimum, Eurotium sp., Sistotrema brinkmannii, Asp. fumigatus, Schizophyllum commune, Phoma sp., and Alternaria sp. However, Cla. tenuissimum, Sis. brinkmannii, Asp. fumigatus, and Sch. commune were detected only in 1 or 2 factories. In addition, almost all the other fungi except Arthrinium sacchari, Epicoccum nigrum, and S. brevicaulis, which were detected in 3 Jang factories, were also detected only in 1 or 2 factories.
Of the 32 genera and 72 species from the 5 inside air samples, the genus Aspergillus, Cladosporium, Penicillium, and Eurotium were collected with high concentrations (Table 2). A. oryzae and Cladosporium sp. were the dominant species and were detected in 5 and 4 factories, respectively. In addition, Pen. chrysogenum (found in 2 factories), C. cladosporioides (2), Asp. niger (4), Eur. herbariorum (2), Aspergillus sp. (3), Penicillium sp. (4), and Asp. sydowii (3) were frequently detected in inside air of the fermentation rooms. Although Eur. repens, Pen. glabrum, Phoma sp., Bjerkandera adusta, and Beauveria bassiana were infrequently detected in inside air of the fermentation rooms, they were detected in 3 factories. The other species detected in inside air samples were detected in only 1 or 2 factories.
The mycobiota of outside and inside air of the fermentation rooms differed (Table 2), and fungi detected from inside air were more diverse. Seventy-two species were detected from the inside air, whereas 50 species were detected from the outside air. The species belong to the genus Alternaria, Arthrinium, Chaetomium, Cladosporium, Phoma, and Schizophyllum were present in higher concentrations in outside air than in inside air. In particular, Alternaria, Arthrinium, and Cladosporium were significantly higher. Therefore, these fungi present in the inside air may have come in from outside when the inside air was ventilated. However, many fungi belonging to the genus Aspergillus, Penicillium, and Eurotium were frequently detected in inside air but were rarely or not detected in outside air. These observations indicate that these fungi were not influenced by the outside mycobiota but may have been influenced by previous Meju fermentation, because they frequently occurred on Meju.
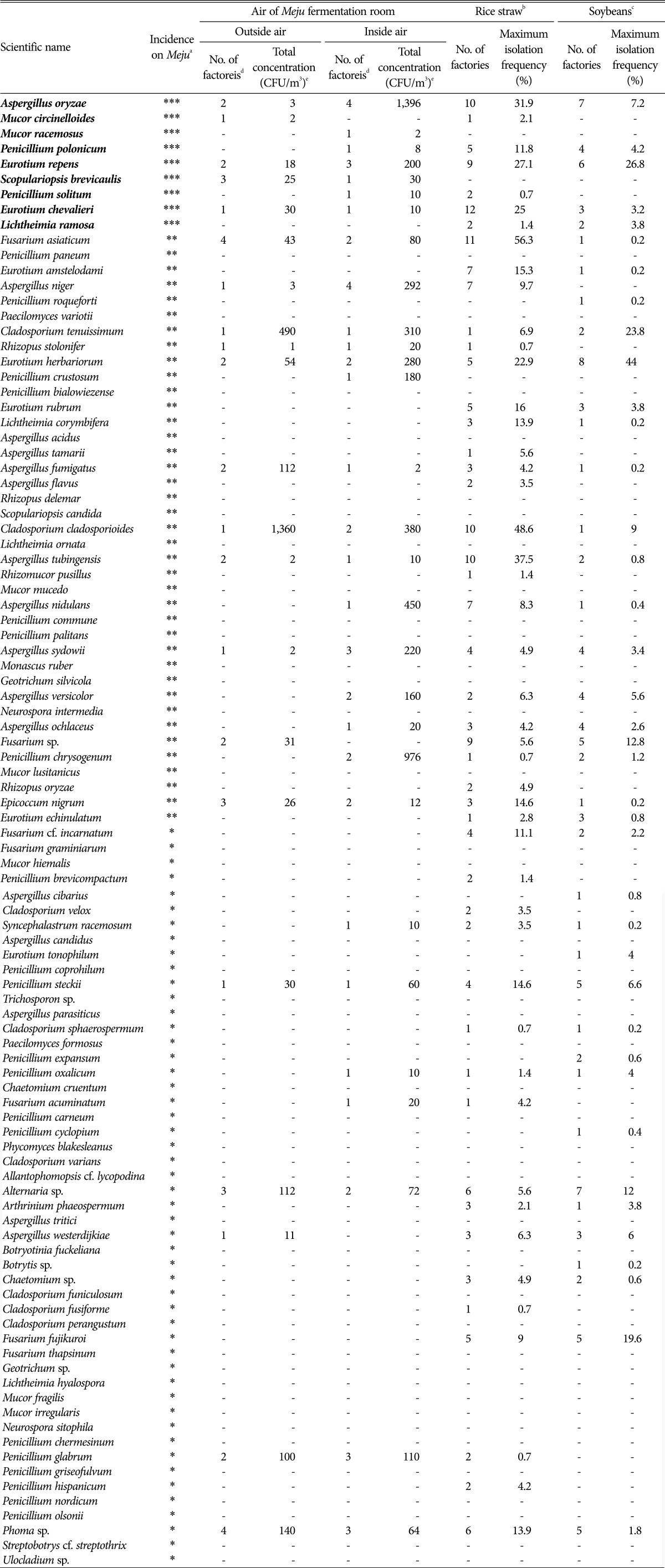
The species, Asp. oryzae, M. circinelloides, M. racemosus, Pen. polonicum, Eur. repens, Scopulariopsis brevicaulis, Pen. solitum, Eur. chevalieri, and L. ramosa were the main species from Meju (Table 3). A. oryzae, P. polonicum, E. repens, P. solitum, E. chevalieri, and F. asiaticum are detected on rice straw with high frequency but are rarely isolated from outside air of Jang factories. Therefore, most of these could be transferred from rice straw to Meju, and they grow and produce abundant spores during Meju fermentation, and then as their spores would accumulated inside air of fermentation room, they could influence mycobiota of Meju fermentation in the following year. This could explain why concentrations of Aspergillus, Eurotium, and Penicillium in inside air of Meju fermentation room is much higher than those of outside air.
Table 3. List of fungi on Meju and their isolation frequencies from air of Meju fermentation room, rice straw, and soybeans.
aThe species were isolated from Meju, with ***high frequency, **medium frequency, or *low frequency.
bThe number of factories indicates factories from which the species were isolated from 12 factories. Maximum isolation frequency indicates maximum isolation frequency (among 144 pieces of rice straw) among 9 different isolation conditions [8].
cThe number of factories indicates factories from which the species were isolated from 10 factories. Maximum isolation frequency indicates maximum isolation frequency (among 500 kernels) of untreated soybeans among 3 different media [9].
dThe number indicates factories from which the species were isolated from 6 (outside air) and 5 (inside air) factories.
eThe numbers indicate the sum of the maximum concentrations of each factory among concentrations on each media.
Lichtheimia ramosa, M. circinelloides, M. racemosus, and S. brevicaulis were rarely or not detected in rice straw and soybean. However, they were detected from outside air of Meju fermentation room, although their frequencies were not high, and they are generally known as common fungi in indoor environments [14]. Therefore, L. ramosa, M. circinelloides, M. racemosus, and S. brevicaulis on Meju might originate from outside (or inside) air of Meju fermentation room.
In this study, all main species on Meju except L. ramosa were detected both inside and outside of fermentation room. However, L. ramosa is usually known as an indoor fungus [14]. After all, Meju could be provided with almost fungi from air in and out Meju fermentation room, which is used for Meju fermentation for more than one time.
Rice straw comes into direct contact with soybeans in Meju, and so the fungi on rice straw can move to Meju and grow on it. Therefore, this has a great influence on Meju mycobiota. Rice straw could provide Meju with useful fungi for fermentation but also could provide unwanted fungi such as Fusarium asiaticum. Traditional Meju fermentation is composed of drying at a low temperature (low temperature fermentation process) and fermenting at a high temperature and humidity (high temperature fermentation process) [4]. F. asiaticum usually originates from rice straw and grows on it during fermentation at low temperatures. Therefore, in order to avoid contamination of F. asiaticum, a method could be developed to use rice straw only during high temperature fermentation. Without rice straw, Meju might be provided with main fungi from both inside and outside of Meju fermentation room during the low temperature fermentation process, if the fermentation room is used for Meju fermentation more than once.
ACKNOWLEDGEMENTS
This work was partly supported by a research project (no. PJ00866601) of the National Academy of Agricultural Science (NAAS), Rural Development Administration, Republic of Korea.
References
- 1.Byun YG, Kim SH, Joo HK, Lee GS, Yim MH. Isolation and identification of protease producing bacteria, Bacillus subtillis YG-95 from the traditional Me-Ju and its production conditions. Agric Chem Biotechnol. 1998;41:342–348. [Google Scholar]
- 2.Han J, Kim HJ, Lee SS, Lee IS. Inhibitive effects of Meju extracts made with a single inoculum of the fungi isolated from the traditional Meju on the human leukemia cell line. Korean J Mycol. 1999;27:312–317. [Google Scholar]
- 3.Lee SS, Park KH, Choi KJ, Won SA. A study in hyphomycetous fungi found on maejus, a raw material of Korean traditional soysources. Korean J Mycol. 1993;21:247–272. [Google Scholar]
- 4.Hong SB, Kim DH, Lee M, Baek SY, Kwon SW, Houbraken J, Samson RA. Zygomycota associated with traditional Meju, a fermented soybean starting material for soy sauce and soybean paste. J Microbiol. 2012;50:386–393. doi: 10.1007/s12275-012-1437-6. [DOI] [PubMed] [Google Scholar]
- 5.Hong SB, Kim DH, Lee M, Baek SY, Kwon SW, Samson RA. Taxonomy of Eurotium species isolated from Meju. J Microbiol. 2011;49:669–674. doi: 10.1007/s12275-011-0376-y. [DOI] [PubMed] [Google Scholar]
- 6.Kim DH, Lee M, Baek SY, Lee JK, Samson RA, Hong SB. Identification and extracellular enzyme activities of Penicillium strains isolated from Meju. J Microbiol. (in press) [Google Scholar]
- 7.Lee SS. Meju fermentation for a raw material of Korean traditional soy products. Korean J Mycol. 1995;23:161–175. [Google Scholar]
- 8.Hong SB, Kim DH, Samson RA. Aspergillus associated with Meju, a fermented soybean starting material for traditional soy sauce and soybean paste in Korea. Mycobiology. doi: 10.5941/MYCO.2015.43.3.218. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi KS, Lee HJ, Kwon DJ. Physicochemical and microbiological properties of Korean traditional Meju. Korean J Food Preserv. 2009;16:217–222. [Google Scholar]
- 10.Kim DH, Kim SH, Kwon SW, Lee JK, Hong SB. Fungal diversity of rice straw for Meju fermentation. J Microbiol Biotechnol. 2013;23:1654–1663. doi: 10.4014/jmb.1307.07071. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Kim SH, Kwon SW, Lee JK, Hong SB. Mycoflora of soybeans used for Meju fermentation. Mycobiology. 2013;41:100–107. doi: 10.5941/MYCO.2013.41.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KY, Kim D. Distribution characteristics of airborne fungi in a partial area of Seoul city. J Environ Health Sci. 2012;38:407–414. [Google Scholar]
- 13.Gwak HJ, Lee HJ, Lee SH, Na HJ. Identification and concentration of airborne microbes in food manufacturing plants. J Food Hyg Saf. 2011;26:361–365. [Google Scholar]
- 14.Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B. Food and indoor fungi. Utrecht: CBS-KNAW Fungal Biodiversity Center; 2010. pp. 10–18. [Google Scholar]