Abstract
Multilocus sequence typing analysis was applied to determine the genotypes of 147 (137 clinical and 10 environmental) Cryptococcus neoformans and three clinical Cryptococcus gattii isolates from 1993 to 2014 in Korea. Among the 137 clinical isolates of C. neoformans, the most prevalent genotype was ST5 (n = 131), followed by ST31 (n = 5) and ST127 (n = 1). Three C. gattii strains were identified as ST57, ST7, and ST113. All environmental isolates were identified as C. neoformans with two genotypes, ST5 (n = 7) and ST31 (n = 3). Our results show that C. neoformans isolates in Korea are genetically homogeneous, and represent a close genetic relationship between clinical and environmental isolates.
Keywords: Cryptococcus gattii, Cryptococcus neoformans, Genotype, Multilocus sequence typing
The Cryptococcus neoformans/Cryptococcus gattii species complex is the causative agent of cryptococcosis, especially in immunodeficient hosts. Both species contain a number of genetically diverse subgroups and differ in their ecology and epidemiology [1,2]. C. neoformans comprises two varieties and two serotypes (A and D), whereas C. gattii comprises two serotypes (B and C) [1]. C. neoformans has been associated worldwide with pigeon excreta and is responsible for more than 90% of the cases of cryptococcal infections [1,3]. Until recently, C. gattii was considered restricted to the tropical and subtropical climate zones and thought to be related mainly to Eucalyptus trees [4]. However, a recent outbreak of molecular type VGII of C. gattii in the Pacific Northwest of North America, a temperate area, indicated an environmental shift of this species [5,6].
Due to the number of genetically diverse subgroups within each serotype, several molecular typing methods, including PCR fingerprinting, amplified fragment length polymorphism, PCR-restriction fragment length polymorphism (RFLP) analysis of the orotidine monophosphate pyrophosphorylase (URA5) gene, random amplification of polymorphic DNA analysis, multilocus microsatellite typing, and multilocus sequence typing (MLST) have been developed to study the molecular epidemiology of C. neoformans and C. gattii [7,8,9,10]. Based on genetic differences, eight distinct molecular types have been recognized in two species: VNI and VNII (C. neoformans var. grubii, serotype A); VNIII (serotype AD, hybrid); VNIV (C. neoformans var. neoformans, serotype D); and VGI, VGII, VGIII, and VGIV (C. gattii, serotypes B and C). Within C. neoformans var. grubii, VNI is the most prevalent molecular type worldwide, especially in Asian countries, whereas VGI has been the most prevalent type of infection due to C. gattii [10,11]. Further genetic analysis has divided the VGII genotype into three subtypes: VGIIa, VGIIb, and VGIIc [12]. Among the molecular typing methods, MLST has shown a high degree of intraspecies discriminatory power as well as reproducibility between different laboratories. This method can be used to trace the putative origin of pathogenic populations [8,13]. Recently, a standardized MLST scheme for genotyping of the C. neoformans/C. gattii species complex has been established by the International Society of Human and Animal Mycoses (ISHAM) working group to enable the standardization and acquisition of global genotypic data [10]. Our previous data showed that the predominant genotype of 46 clinical C. neoformans isolates from 2008 to 2012 by MLST analysis was VNI-ST5 (95.7%), and the remaining genotype was VNI-ST31 (4.3%) [14]. Most C. neoformans isolates in the Far East Asian countries Korea, Japan, and China belong to VNI-ST5 with low genetic diversity. However, minor MLST genotypes identified within populations represent a greater genetic diversity among these countries [15,16,17]. Although considerable data on the global epidemiology of the C. neoformans/C. gattii species complex are available, information on the molecular epidemiology of Korean strains remains limited. In this study, we investigated the genotypes of the previously studied and untyped C. neoformans and C. gattii isolates from clinical and environmental sources using MLST, and compared them with the genotypic diversity among the Korean strains of the C. neoformans/C. gattii species complex.
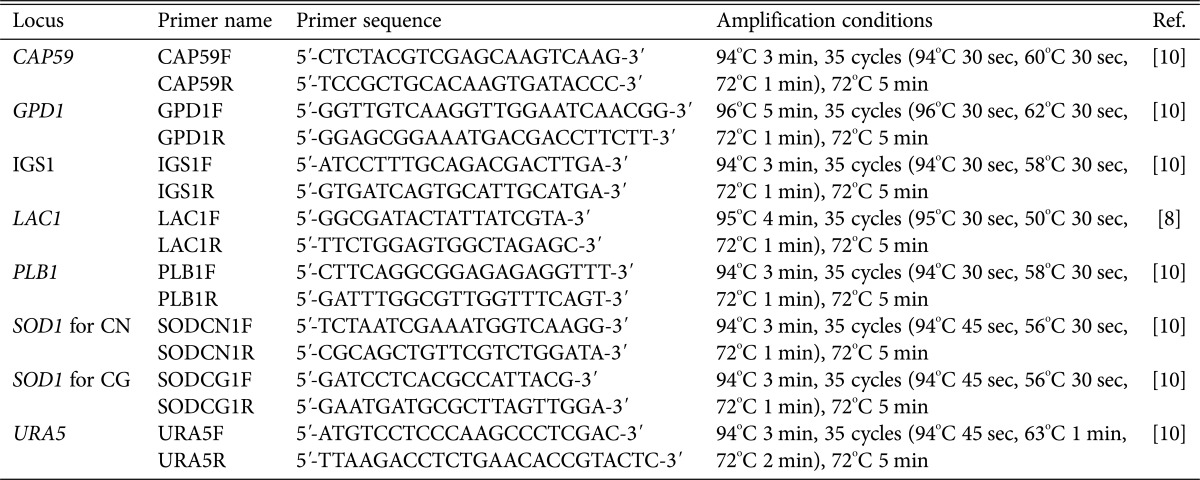
A total of 140 clinical isolates of Cryptococcus species were obtained from medical centers located in the Seoul (n = 117) and Busan (n = 23) areas between 1993 and 2014, and 10 environmental Cryptococcus species were isolated from pigeon droppings in Seoul (n = 3) and Busan (n = 7) during the same period, as previously described [18]. The identification of species strain was determined using the canavanine-glycine-bromothymol blue agar test [19] and serotyping was performed using a slide agglutination kit (Crypto Check; Iatron Laboratories, Tokyo, Japan), following the manufacturer's instructions. The reference strains used in this study were as follows: WM148 (serotype A, VNl); WM626 (serotype A, VNll); WM628 (serotype AD, VNlll); WM629 (serotype D, VNlV); WM179 (serotype B, VGl); WM178 (serotype B, VGll); WM175 (serotype B, VGlll); and WM779 (serotype C, VGlV). Genomic DNA was extracted from each isolate using a Qiagen DNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. Mating typing and molecular typing by URA5-RFLP were performed as previously described [20,21]. MLST analysis was performed according to an ISHAM consensus scheme of seven unlinked genetic loci (CAP59, GPD1, LAC1, PLB1, SOD1, URA5, and IGS1). DNA from each isolate was amplified by PCR in a 20 µL reaction volume for each of the seven MLST loci by using the primers (Table 1) and protocols as described previously [8,10]. Each locus was subsequently sequenced using the Applied Biosystems 3730 sequencer with the BigDye Terminator Cycle Sequencing Kit v3.1 (Applied Biosystems, Foster City, CA, USA). Alleles at each locus were assigned numbers (allele types) upon comparison with those identified in the global collection, resulting in a 7-digit profile for each isolate. Each unique allelic profile was concatenated and assigned a sequence type (ST) according to the MLST scheme (http://mlst.mycologylab.org). The phylogenetic tree from the combined DNA sequences of the 7 genes was inferred using neighbor joining by MEGA 5.2 software [22].
Table 1. Primers used for MLST analysis of Cryptococcus neoformans (CN) and C. gattii (CG) isolates.
MLST, multilocus sequence typing; Ref., reference.
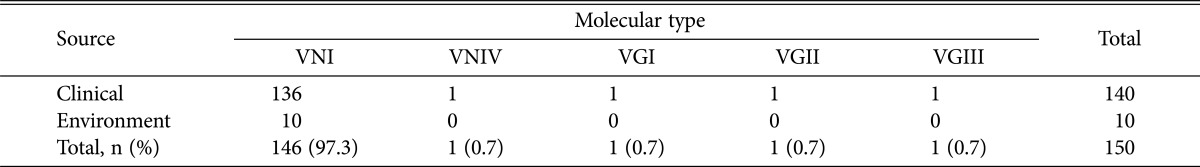
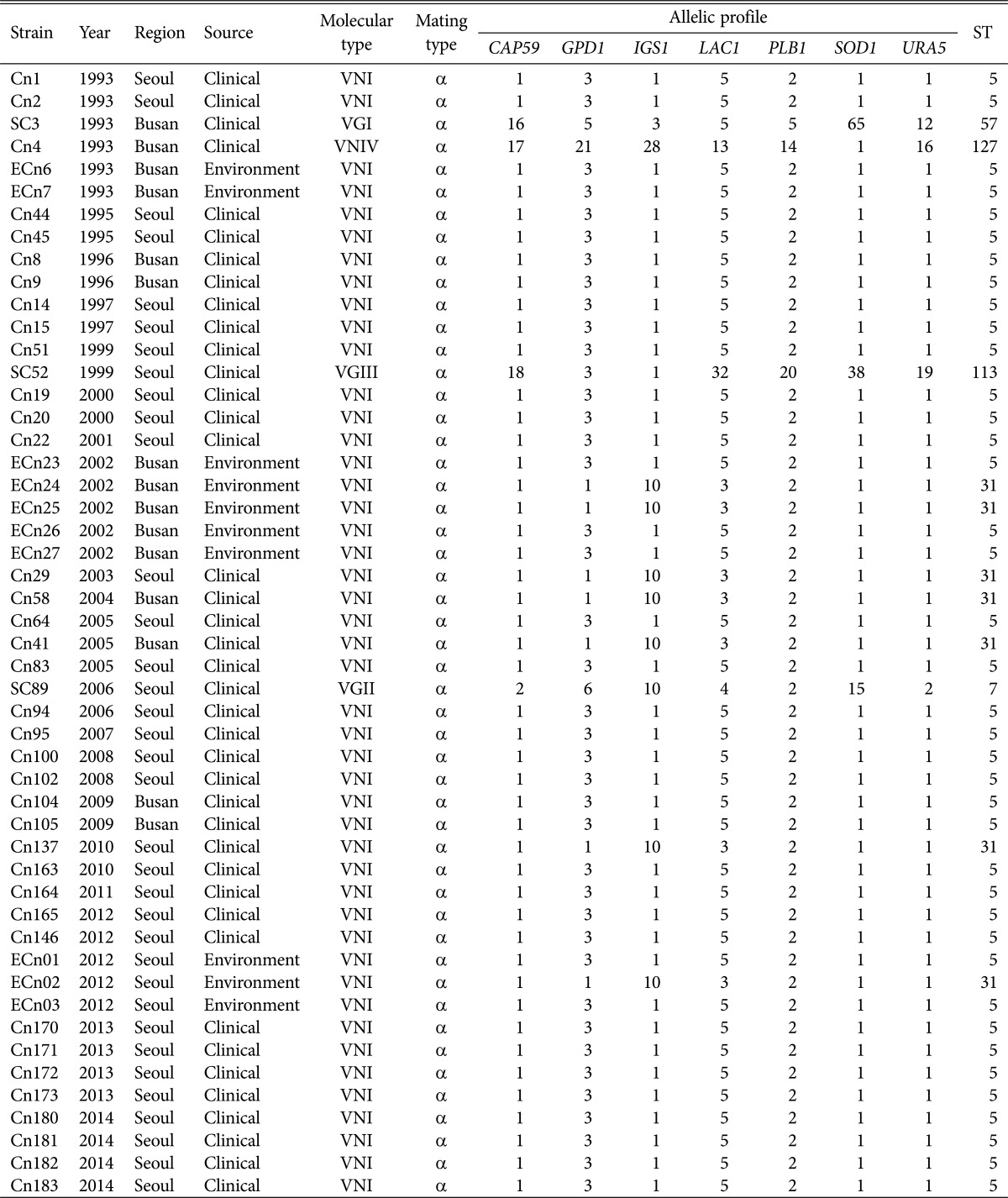
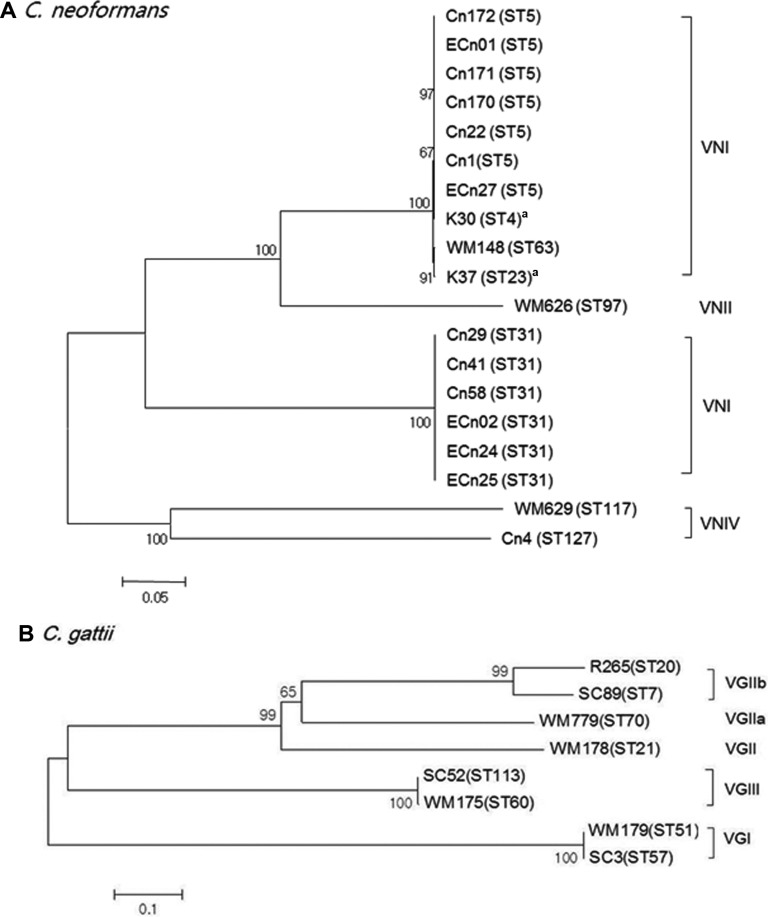
Among the 140 clinical strains of the C. neoformans/C. gattii species complex, 136 isolates were C. neoformans var. grubii serotype A, and one was C. neoformans var. neoformans serotype D. The remaining three were C. gattii serotype B. All environmental isolates were C. neoformans var. grubii serotype A. The most common molecular type by URA5-RFLP analysis was VNI among clinical isolates (97%) and environmental isolates (100%), and less frequent molecular types VNIV, VGI, VGII, and VGIII were also identified (Table 2). All isolates were mating type α. The MLST results and other information for the selected isolates are summarized in Table 3. MLST analysis divided the 137 C. neoformans isolates into three STs, including ST5 (96%, n = 131), ST31 (n = 5), and ST127 (n = 1). Ten environmental isolates were also identified as ST5 (n = 7) and ST31 (n = 3). Our data demonstrate that the clinical and environmental strains in Korea are genetically homogeneous, with most of the genotypes classified as ST5 that was also found to be the major type among the Chinese and Japanese populations [16,17]. However, the occurrence of the ST31 genotype in clinical and environmental isolates showed the genetic relatedness among the Asian countries including India and Thailand [11,23]. MLST data for three C. gattii isolates showed the presence of ST57, ST7, and ST113. A phylogenetic tree was constructed by the neighbor-joining method based on a concatenated data set from 7 loci for MLST (Fig. 1). The selected strains of C. neoformans showed three STs belonging to molecular types VNI and VNIV. Three C. gattii were STs belonging to molecular types VGI, VGII, and VGIII. On combining current and previous data, the rare isolates ST127, ST57, and ST113 were found to be unique to Korea. The ST7 type of C. gattii strain was identical to the less virulent Vancouver Island outbreak strain, VGIIb [12]. Previous studies suggested that the high prevalence of VNIc-M5 (VNI-ST5) among non-HIV infected persons in Korea may due to the cryptococcal ecology in Korea, and the possibility of the occurrence of VNIc-M5 in the environment [15]. In this study, we confirmed a strong linkage between clinical and environmental isolates of VNI-ST5 and VNI-ST31 genotypes, which might be the major causative genotypes of cryptococcosis in Korea. C. gattii infections are quite rare in temperate regions, especially Korea and Japan. Therefore, limited information on the molecular characteristics of the Korean C. gattii strain is available. Chen et al. [24] reported that nine C. gattii strains isolated from 1980 to 2006 were found to be the VGI molecular type, which is commonly isolated in South East Asia. Wu et al. [17] reported that two C. gattii were identified as ST44 (VGII) and ST159 (VGI) by MLST. In Asia, most genetic studies of the C. neoformans/C. gattii species complex have been performed in Thailand and India. The majority of the genotypes among the Thai cryptococcal isolates were ST4 and ST6 clones of C. neoformans molecular type VNI with 13 different STs, and ST7 of C. gattii molecular type VGII, the data of which indicated a significant genetic variation among different Asian regions [23]. Recently, increases in immunocompromised patients with malignant diseases and the elderly population in Korea have led to a higher incidence of invasive fungal infections such as cryptococcosis. Therefore, the clinical features and molecular epidemiology of the pathogenic C. neoformans/C. gattii species complex in Korea need to be continuously studied.
Table 2. Distribution of the molecular types among Cryptococcus neoformans and C. gattii isolates in Korea.
Table 3. The allelic profiles and sequence types of selected Cryptococcus neoformans and C. gattii isolates.
ST, sequence type.
Fig. 1. Phylogenetic analysis of the Korean isolates and reference strains of Cryptococcus neoformans (A) and C. gattii based on neighbor-joining analysis of the concatenated seven International Society of Human and Animal Mycoses consensus multilocus sequence typing loci using the program MEGA 5.2. Numbers on each branch indicate the bootstrap values > 50%, based on 500 replications. aReference strains (Choi et al. [15]).
In conclusion, C. neoformans isolates in Korea are genetically homogeneous, with the majority of isolates belonging to ST5, and a strong linkage exists between clinical and environmental isolates. In addition, the occurrence of rare genotypes may serve as an important indicator of global epidemiologic analysis.
References
- 1.Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A. Cryptococcus from human pathogen to model yeast. Washington, DC: ASM Press; 2011. [Google Scholar]
- 2.Franzot SP, Hamdan JS, Currie BP, Casadevall A. Molecular epidemiology of Cryptococcus neoformans in Brazil and the United States: evidence for both local genetic differences and a global clonal population structure. J Clin Microbiol. 1997;35:2243–2251. doi: 10.1128/jcm.35.9.2243-2251.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazéra MS, Cavalcanti MA, Trilles L, Nishikawa MM, Wanke B. Cryptococcus neoformans var. gattii: evidence for a natural habitat related to decaying wood in a pottery tree hollow. Med Mycol. 1998;36:119–122. [PubMed] [Google Scholar]
- 4.Ellis DH, Pfeiffer TJ. Natural habitat of Cryptococcus neoformans var. gattii. J Clin Microbiol. 1990;28:1642–1644. doi: 10.1128/jcm.28.7.1642-1644.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datta K, Bartlett KH, Marr KA. Cryptococcus gattii: emergence in western North America: exploitation of a novel ecological niche. Interdiscip Perspect Infect Dis. 2009;2009:176532. doi: 10.1155/2009/176532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, Macdougall L, Boekhout T, Kwon-Chung KJ, Meyer W. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada) Proc Natl Acad Sci U S A. 2004;101:17258–17263. doi: 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer W, Marszewska K, Amirmostofian M, Igreja RP, Hardtke C, Methling K, Viviani MA, Chindamporn A, Sukroongreung S, John MA, et al. Molecular typing of global isolates of Cryptococcus neoformans var. neoformans by polymerase chain reaction fingerprinting and randomly amplified polymorphic DNA: a pilot study to standardize techniques on which to base a detailed epidemiological survey. Electrophoresis. 1999;20:1790–1799. doi: 10.1002/(SICI)1522-2683(19990101)20:8<1790::AID-ELPS1790>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Bovers M, Hagen F, Kuramae EE, Boekhout T. Six monophyletic lineages identified within Cryptococcus neoformans and Cryptococcus gattii by multi-locus sequence typing. Fungal Genet Biol. 2008;45:400–421. doi: 10.1016/j.fgb.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Illnait-Zaragozi MT, Martinez-Machín GF, Fernández-Andreu CM, Boekhout T, Meis JF, Klaassen CH. Microsatellite typing of clinical and environmental Cryptococcus neoformans var. grubii isolates from Cuba shows multiple genetic lineages. PLoS One. 2010;5:e9124. doi: 10.1371/journal.pone.0009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer W, Aanensen DM, Boekhout T, Cogliati M, Diaz MR, Esposto MC, Fisher M, Gilgado F, Hagen F, Kaocharoen S, et al. Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med Mycol. 2009;47:561–570. doi: 10.1080/13693780902953886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khayhan K, Hagen F, Pan W, Simwami S, Fisher MC, Wahyuningsih R, Chakrabarti A, Chowdhary A, Ikeda R, Taj-Aldeen SJ, et al. Geographically structured populations of Cryptococcus neoformans variety grubii in Asia correlate with HIV status and show a clonal population structure. PLoS One. 2013;8:e72222. doi: 10.1371/journal.pone.0072222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrnes EJ, 3rd, Bildfell RJ, Frank SA, Mitchell TG, Marr KA, Heitman J. Molecular evidence that the range of the Vancouver Island outbreak of Cryptococcus gattii infection has expanded into the Pacific Northwest in the United States. J Infect Dis. 2009;199:1081–1086. doi: 10.1086/597306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cogliati M. Global molecular epidemiology of Cryptococcus neoformans and Cryptococcus gattii: an atlas of the molecular types. Scientifica (Cairo) 2013;2013:675213. doi: 10.1155/2013/675213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SH, Kim M, Joo SI, Hwang SM. Molecular epidemiology of clinical Cryptococcus neoformans isolates in Seoul, Korea. Mycobiology. 2014;42:73–78. doi: 10.5941/MYCO.2014.42.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi YH, Ngamskulrungroj P, Varma A, Sionov E, Hwang SM, Carriconde F, Meyer W, Litvintseva AP, Lee WG, Shin JH, et al. Prevalence of the VNIc genotype of Cryptococcus neoformans in non-HIV-associated cryptococcosis in the Republic of Korea. FEMS Yeast Res. 2010;10:769–778. doi: 10.1111/j.1567-1364.2010.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mihara T, Izumikawa K, Kakeya H, Ngamskulrungroj P, Umeyama T, Takazono T, Tashiro M, Nakamura S, Imamura Y, Miyazaki T, et al. Multilocus sequence typing of Cryptococcus neoformans in non-HIV associated cryptococcosis in Nagasaki, Japan. Med Mycol. 2013;51:252–260. doi: 10.3109/13693786.2012.708883. [DOI] [PubMed] [Google Scholar]
- 17.Wu SY, Lei Y, Kang M, Xiao YL, Chen ZX. Molecular characterisation of clinical Cryptococcus neoformans and Cryptococcus gattii isolates from Sichuan province, China. Mycoses. 2015;58:280–287. doi: 10.1111/myc.12312. [DOI] [PubMed] [Google Scholar]
- 18.Oh KS, Hwang SM. Isolation and characterization of Cryptococcus neoformans from environmental sources in Busan. Mycobiology. 2005;33:188–193. doi: 10.4489/MYCO.2005.33.4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon-Chung KJ, Polacheck I, Bennett JE. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var gattii (serotype B and C) J Clin Microbiol. 1982;15:535–537. doi: 10.1128/jcm.15.3.535-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaturvedi S, Rodeghier B, Fan J, McClelland CM, Wickes BL, Chaturvedi V. Direct PCR of Cryptococcus neoformans MATalpha and MATa pheromones to determine mating type, ploidy, and variety: a tool for epidemiological and molecular pathogenesis studies. J Clin Microbiol. 2000;38:2007–2009. doi: 10.1128/jcm.38.5.2007-2009.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E IberoAmerican Cryptococcal Study Group. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis. 2003;9:189–195. doi: 10.3201/eid0902.020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 23.Kaocharoen S, Ngamskulrungroj P, Firacative C, Trilles L, Piyabongkarn D, Banlunara W, Poonwan N, Chaiprasert A, Meyer W, Chindamporn A. Molecular epidemiology reveals genetic diversity amongst isolates of the Cryptococcus neoformans/C. gattii species complex in Thailand. PLoS Negl Trop Dis. 2013;7:e2297. doi: 10.1371/journal.pntd.0002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Varma A, Diaz MR, Litvintseva AP, Wollenberg KK, Kwon-Chung KJ. Cryptococcus neoformans strains and infection in apparently immunocompetent patients, China. Emerg Infect Dis. 2008;14:755–762. doi: 10.3201/eid1405.071312. [DOI] [PMC free article] [PubMed] [Google Scholar]