Abstract
A series of combretastatin A-4 (CA-4) analogues have been prepared from (Z)-substituted diarylacrylonitriles (1a–1p) obtained in a two-step synthesis from appropriate arylaldehydes and acrylonitriles. The resulting 4,5-disubstituted 2H-1,2,3-triazoles were evaluated for their anti-cancer activities against a panel of 60 human cancer cell lines. The diarylacrylonitrile analogue 2l exhibited the most potent anti-cancer activity in the screening studies, with GI50 values of <10 nM against almost all the cell lines in the human cancer cell panel and TGI values of <10nM against cancer cell lines SF-539, MDA-MB-435, OVCAR-3 and A498. Furthermore, in silico docking studies of compounds 2l, 2e and 2h within the active site of tubulin were carried out in order to rationalize the mechanism of anti-cancer properties of these compounds. From the in silico studies, compound 2e was predicted to have better affinity for the colchicine binding site on tubulin compared to compounds 2l and 2h. Analogue 2e was also evaluated for its anti-cancer activity by colony formation assay against 9LSF rat gliosarcoma cells and afforded an LD50 of 7.5 nM. A cell cycle redistribution assay using analogue 2e was conducted to further understand the mechanism of action of these CA-4 analogues. From this study, analogues 2e and 2l were the most potent anti-cancer agents in this structural class, and were considered lead compounds for further development as anti-cancer drugs.
Keywords: Novel 4,5-disubstituted 2H-1,2,3-triazoles; Anti-cancer activity; Gliosarcoma cells; In silico docking studies; Cell cycle redistribution assay
Graphical Abstract
1. Introduction
Cancer is the second most life threatening disease after cardiovascular disease, affecting more than six million people per year worldwide. Although, significant research has done to date to treat cancer, there is still a lack of effective chemotherapeutic treatment to cure it completely with minimal side effects. Also, considerable effort has been put into identifying molecules with anti-cancer properties from both natural and synthetic sources. More than 60% of the anti-cancer drugs currently available are from natural sources [1]. The search for potent semi synthetically derived anti-cancer agents from the parent natural products continues to be an important part of drug discovery process. Anti-mitotic agents are a major class of cytotoxic drugs for the treatment of cancer and drugs that target microtubule/tubulin dynamics are widely used in cancer chemotherapy [2].
There are three major binding sites for tubulin; i.e. the vinca, taxane and colchicine domains. Vinca alkaloids, such as vincristine and vinblastine, bind to the vinca domain inhibiting the assembly of microtubule structures and arresting mitosis [3]. Paclitaxel acts at the taxane domain stabilizing microtubules and interfering with the normal breakdown of microtubules during mitosis [4]. Our area of interest focused on the colchicine binding site. Colchicine binds to tubulin and inhibits microtubule polymerization. Anti-mitotic agents such as combretastatin A-4 (CA-4) bind at the colchicine domain of tubulin, and have received much attention in recent years; CA-4P, the water soluble phosphate salt of CA-4 is currently in phase III clinical trial for anaplastic thyroid cancer, and is also in phase II trials for polypoidal choroidal vasculopathy and neovascular age-related macular degeneration [5, 6].
CA-4 is classified as a cis-stilbene originating from the South African willow tree combretum caffrum. CA-4 functions as a microtubule targeting agent interfering with microtubule dynamics and perturbs the mitotic cycle [7]. When compared to colchicine, the vascular disrupting effects of CA-4 are well below the maximum tolerable dose with fewer side effects in vivo [8]. However, CA-4 suffers from stability issues because of its tendency to undergo cis-trans double bond isomerism in solution. CA-4 is a cis-configured stilbene which is readily converted to the thermodynamically more stable, but less potent trans-isomer [9]. Extensive studies have been conducted in attempts to stabilize the cis configuration by replacing the olefinic double bond with heterocyclic ring systems such as β-lactam, azetidone, thiazole, tetrazole, imidazole, pyrazole, oxazolone, triazole, furanone moieties [10–15].
In the work described herein, we report on the synthesis of series of novel cis-constrained 4,5-disubstituted 2H-1,2,3-triazole analogues of CA-4. The 2H-1,2,3-triazole ring system was designed to not only halt cis-trans isomerization, but also improve the drug likeness of the resulting CA-4 analogue, along with providing scope for further structural diversification. Evaluation of these novel triazole analogues of CA-4 against a panel of 60 human tumor cell lines has been performed, along with cell cycle redistribution assays. The molecular mechanism responsible for the of the anti-cancer activity of the three most potent molecules, 2e, 2h and 2l, has been investigated by performing molecular docking studies with the target molecule, tubulin.
2. Results and discussion
2.1. Drug synthesis
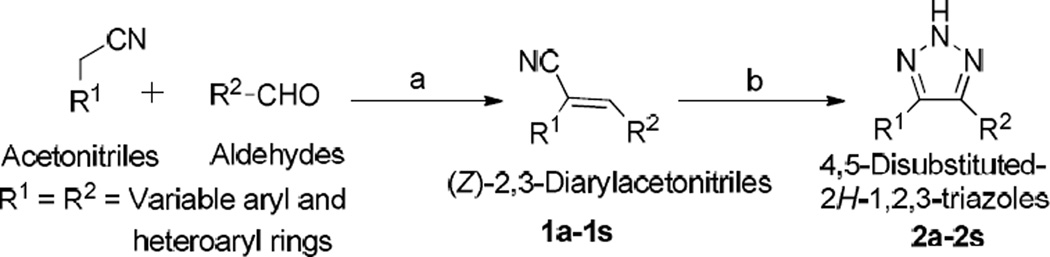
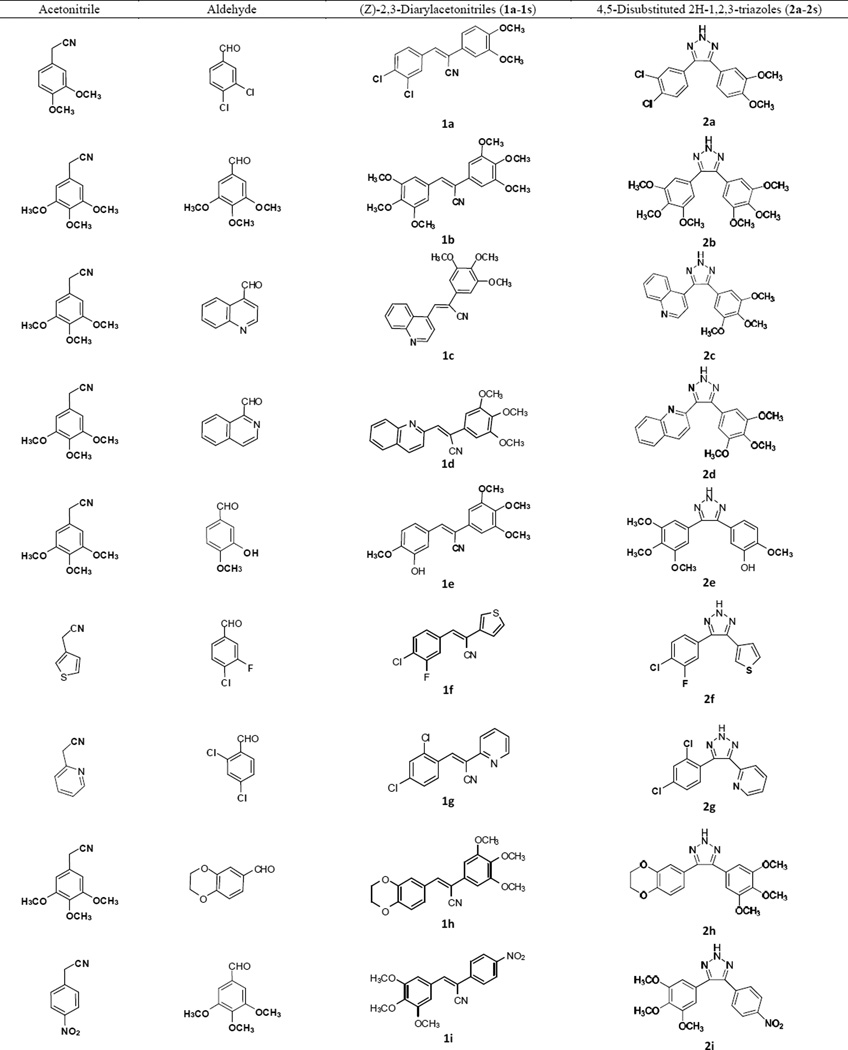
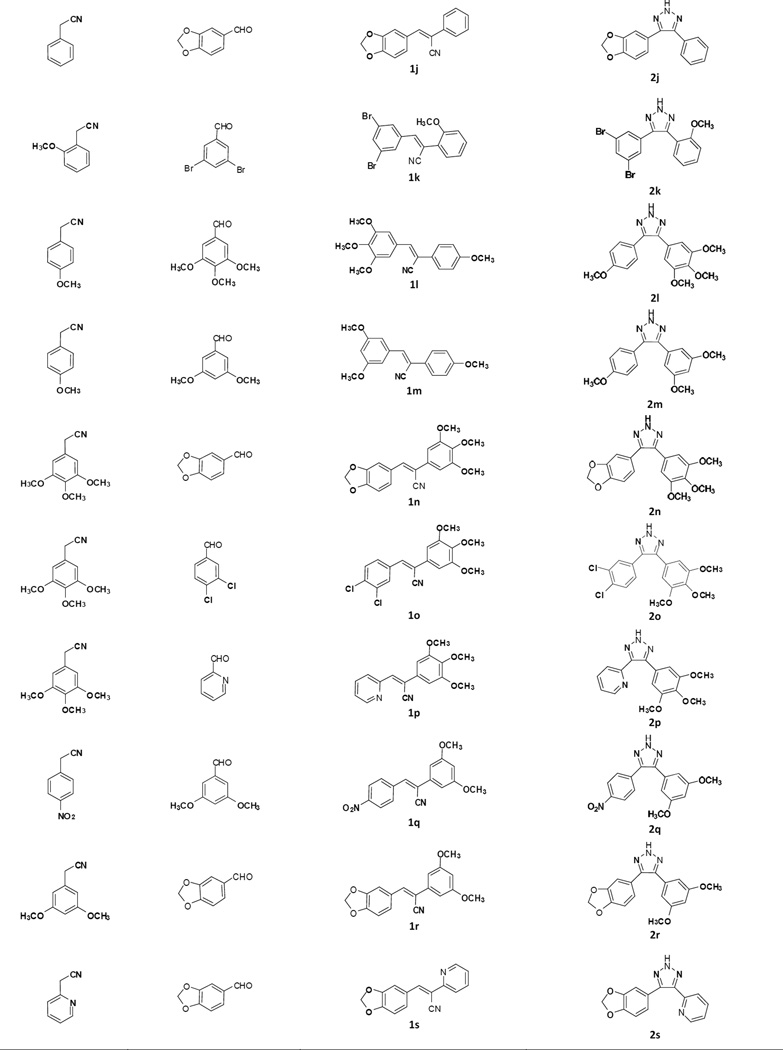
The general procedure for the synthesis of the 4,5-disubstituted 2H-1,2,3-triazole CA-4 analogues is illustrated in Scheme 1. In the first step, a series of (Z)-substituted diarylacrylonitrile analogues were synthesized by reacting substituted benzyl carbaldehydes with their corresponding substituted phenylacetonitriles in 5% NaOMe in methanol. The reaction mixture was stirred at room temperature for 2–3 hours, during which time the desired product precipitated out of the solution. The resulting product was then filtered, washed with water, and dried to yield the final compound; yields ranged from 70–95 % (Scheme 1) [16].
Scheme 1.
Synthesis of 4,5-disubstituted 2H-1,2,3-triazoles; reagents and conditions: (a) 5% NaOMe, MeOH, reflux. (b) NaN3, NH4Cl, DMF/H2O.
In the second step, 4,5-disubstituted-2H-1,2,3-triazoles were obtained by refluxing a mixture of the (Z)-2,3-diarylacrylonitrile (1a–1s) from step 1 with NaN3, and NH4Cl in a mole ratio of 1:3:3 in 10:1 volumes of DMF/H2O for 5–12 h. The reaction mixture was monitored by TLC, and when the starting material had completely disappeared, cold water was added and the mixture stirred over 10–15 min, during which the final product precipitated out and was filtered off, washed with water and dried. In cases where there was an absence of a precipitate, the product was extracted into ethyl acetate, the organic extract washed with copious amounts of water, and the resulting organic liquor evaporated to dryness on a rotary evaporator. The residue obtained was purified by flash column chromatography to afford the corresponding triazole (Scheme 1) [17]. The structure and purity of the triazole derivatives were verified by 1H, 13C-NMR spectroscopy, high resolution mass spectroscopy and X-ray crystallography [17–20].
Based on previous SAR studies on cis constrained CA-4 analogues, triazole analogues initially chosen for synthesis contained the 3,4,5-trimethoxyphenyl moiety (Ring A) and a variably substituted aryl or heteroaryl moiety (Ring B) [7, 21]. In later structural modifications we introduced halogeno, nitro and hydroxyl functionalities ring A.
2.2. Biological Evaluation
2.2.1. Anti-cancer activity against a panel of NCI 60 human cancer cells
The sulforhodamine B (SRB) assay procedure described by Rubinstein et al. was used to screen the CA-4 analogues 2a–2s against a panel of 60 human tumor cell lines [22]. Growth inhibitory or cytotoxic effects were measured by percentage growth (PG), which is proportional to optical density (OD) [23, 24]. OD measurements of SRB-derived color prior to and 48 hrs after exposure of cells to the test compound or vehicle control were recorded. Ten compounds (2c–2e, 2g, 2j, 2l–2n, 2r, and 2s) were initially identified as “hits” after screening at all the analogues at 10−5 M concentration. These single concentration results are presented in the supplementary data section. The screening protocol utilized to identify a hit was 60% growth inhibition at 10−5 M in at least eight cell lines from the panel of 60 cell lines. Compounds that met these criteria were then selected for a complete dose response study at five different concentrations, viz. 10−4 M, 10−5 M, 10−6 M, 10−7 M and 10−8 M. Six of the preliminary hits (2d, 2g, 2h, and 2l–2n) were evaluated in the full dose-response studies had effective GI50 and TGI (Total growth inhibition) values against a variety of human tumor cell lines (Table 2). All the compounds have LD50 values >100 µM against most of the human cancer cell lines, indicating that the compounds are anti-proliferative.
Table 2.
Growth inhibition (GI50/µM)a data of 2d, 2m, 2l, 2n and 2g against a panel of 60 human cancer cells.
| Panel/cell line |
2d (µM) |
2m (µM) |
2l (µM) |
2h (µM) |
2n (µM) |
2g (µM) |
|---|---|---|---|---|---|---|
| Leukemia | ||||||
| CCRF-CEM | 0.037 | 0.032 | <0.01 | 0.034 | 0.28 | 3.38 |
| HL-60(TB) | 0.030 | 0.023 | <0.01 | 0.019 | 0.20 | 5.75 |
| K-562 | 0.026 | <0.01 | <0.01 | <0.01 | 0.05 | 5.50 |
| MOLT-4 | 0.068 | 0.048 | 0.012 | 0.040 | 0.57 | 4.23 |
| RPMI-8226 | 0.037 | 0.037 | <0.01 | 0.033 | 0.33 | 10.7 |
| SR | 0.021 | <0.01 | <0.01 | <0.01 | 0.04 | 5.43 |
| Lung Cancer | ||||||
| A549/ATCC | 0.054 | 0.037 | <0.01 | 0.023 | 0.29 | 4.85 |
| HOP-62 | 0.047 | 0.025 | <0.01 | 0.018 | 0.34 | 5.32 |
| HOP-92 | 0.099 | <0.01 | <0.01 | <0.01 | 0.12 | 3.28 |
| NCI-H23 | 0.065 | 0.038 | <0.01 | 0.048 | 0.77 | 9.13 |
| NCI-H460 | 0.038 | 0.034 | <0.01 | 0.025 | 0.34 | 3.67 |
| Colon Cancer | ||||||
| COLO 205 | 0.228 | 0.149 | 0.029 | 0.027 | 0.31 | 2.89 |
| HCC-2998 | 0.230 | 0.046 | 0.024 | 0.039 | >100 | 9.16 |
| HCT-116 | 0.040 | <0.01 | <0.01 | <0.01 | 0.18 | 2.89 |
| HCT-15 | 0.037 | <0.01 | <0.01 | <0.01 | 0.13 | 2.93 |
| HT29 | 0.214 | 0.048 | <0.01 | 0.025 | 0.39 | 4.56 |
| KM12 | 0.035 | 0.030 | <0.01 | <0.01 | 0.07 | 6.87 |
| SW-620 | 0.041 | 0.030 | <0.01 | <0.01 | 0.11 | 4.40 |
| CNS Cancer | ||||||
| SF-268 | 0.503 | 0.177 | <0.01 | 0.069 | >100 | 7.36 |
| SF-295 | 0.014 | 0.011 | <0.01 | <0.01 | 0.10 | 3.01 |
| SF-539 | 0.020 | 0.014 | <0.01 | <0.01 | 0.18 | na |
| SNB-75 | 0.016 | 0.014 | <0.01 | <0.01 | 0.08 | 1.58 |
| U251 | 0.037 | 0.038 | <0.01 | 0.020 | 0.30 | 5.35 |
| Melanoma | ||||||
| LOX IMVI | 0.065 | 0.076 | <0.01 | 0.018 | 0.55 | 4.83 |
| M14 | 0.022 | <0.01 | <0.01 | <0.01 | na | 14.3 |
| MDA-MB-435 | <0.01 | <0.01 | <0.01 | <0.01 | 0.09 | 2.20 |
| SK-MEL-2 | 0.027 | 0.056 | <0.01 | <0.01 | 0.02 | 6.52 |
| SK-MEL-28 | >100 | na | na | >100 | 0.24 | 5.39 |
| SK-MEL-5 | 0.013 | 0.027 | <0.01 | <0.01 | >100 | 7.89 |
| UACC-62 | 0.157 | <0.01 | nd | >100 | >100 | 3.35 |
| Ovarian Cancer | ||||||
| IGROV1 | 0.065 | 0.051 | <0.01 | 0.033 | >100 | 5.94 |
| OVCAR-3 | 0.011 | 0.024 | <0.01 | <0.01 | 0.43 | 3.38 |
| OVCAR-4 | 0.077 | na | <0.01 | 0.034 | 0.08 | 3.04 |
| NCI/ADR-RES | 0.023 | <0.01 | <0.01 | <0.01 | 0.08 | 3.92 |
| SK-OV-3 | 0.075 | 0.046 | <0.01 | <0.01 | 0.49 | 2.72 |
| Renal Cancer | ||||||
| 786-0 | 0.043 | 0.015 | <0.01 | <0.01 | 0.62 | 2.75 |
| A498 | 0.033 | <0.01 | <0.01 | 0.010 | 0.34 | 3.18 |
| ACHN | 0.081 | 0.145 | <0.01 | <0.01 | 0.71 | 1.75 |
| CAKI-1 | 0.043 | 0.050 | <0.01 | <0.01 | 0.32 | 8.42 |
| UO-31 | 0.092 | 0.020 | <0.01 | <0.01 | 0.66 | 6.84 |
| Prostate Cancer | ||||||
| PC-3 | 0.046 | 0.042 | <0.01 | 0.018 | 0.26 | 4.50 |
| DU-145 | 0.027 | 0.045 | <0.01 | 0.025 | 0.35 | 1.41 |
| Breast Cancer | ||||||
| MCF7 | 0.027 | 0.025 | <0.01 | <0.01 | 0.08 | 2.58 |
| MDA-MB-231/ATCC | 0.094 | 0.046 | <0.01 | 0.044 | 0.53 | 5.89 |
| HS 578T | na | 0.041 | <0.01 | 0.668 | 0.44 | 11.6 |
| MDA-MB-468 | 0.035 | 0.023 | <0.01 | 0.015 | 0.23 | 3.20 |
na: Not analyzed, nd; not determined.
GI50: 50% growth inhibition, concentration of drug resulting in a 50% reduction in net cell growth as compared to cell numbers on day 0.
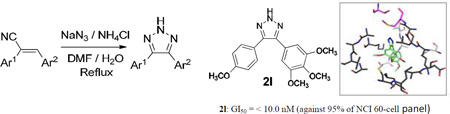
Analogue 2l had impressive GI50 values of less than 10 nM against almost all of the 60 cancer cell lines in the panel, except for melanoma cancer cell line UACC-62, and colon cancer cell lines COLO 205 and HCC-2998. Compound 2l also showed potent anti-proliferative activity with TGI values of <10 nM against CNS cancer cell line SF-539, melanoma cell MDA-MB-435, ovarian cancer cell line OVCAR-3, and renal cancer cell line A498.
Analogue 2m exhibited potent growth inhibitory activity with GI50 <10nM against leukemia cancer cell lines K-562 and SR, non-small cell lung cancer cell line HOP-92, colon cancer cell lines HCT-116 and HCT-15, melanoma cancer lines M14 and UACC-62, ovarian cancer cell line NCI/ADR-RES, and renal cancer cell line A498. Melanoma cancer cell line MDA-MB-435 appeared to be the most sensitive to the growth inhibitory effects of 2m, exhibiting a TGI value of 10 nM.
Analogue 2h also showed potential growth inhibitory properties with GI50 <10 nM against leukemia cancer cell lines K-562 and SR, non-small cell lung cancer cell line HOP-92, colon cancer cell lines HCT-116, HCT-15, KM-12, and SW-620, CNS cancer cell lines SF-295, SF-539, and SNB-75, melanoma cancer lines M14, MDA-MB-435, and SK-MEL-2, ovarian cancer cell lines NCI/ADR-RES, and SK-OV-3, renal cancer cell lines A498, ACHN, CAKI-1, and UO-31, and breast cancer cell line MCF-7.
From the above screening studies, analogue 2l was considered to be a lead candidate, since it exhibited very promising GI50 and TGI values against a wide variety of both hematological and solid tumor cell types. However, in silico tubulin docking studies carried out with the triazole analogues revealed slightly better tubulin binding affinity for compound 2e compared to 2l. Unfortunately, analogue 2e, which is the triazole-derived form of the CA4 molecule, was not selected for the 60 human cancer cell screen. We therefore evaluated 2e for its anti-cancer activity by colony formation assay against 9LSF rat gliosarcoma cells.
2.2.2 Colony formation assay results for 2e utilizing 9LSF rat gliosarcoma cells
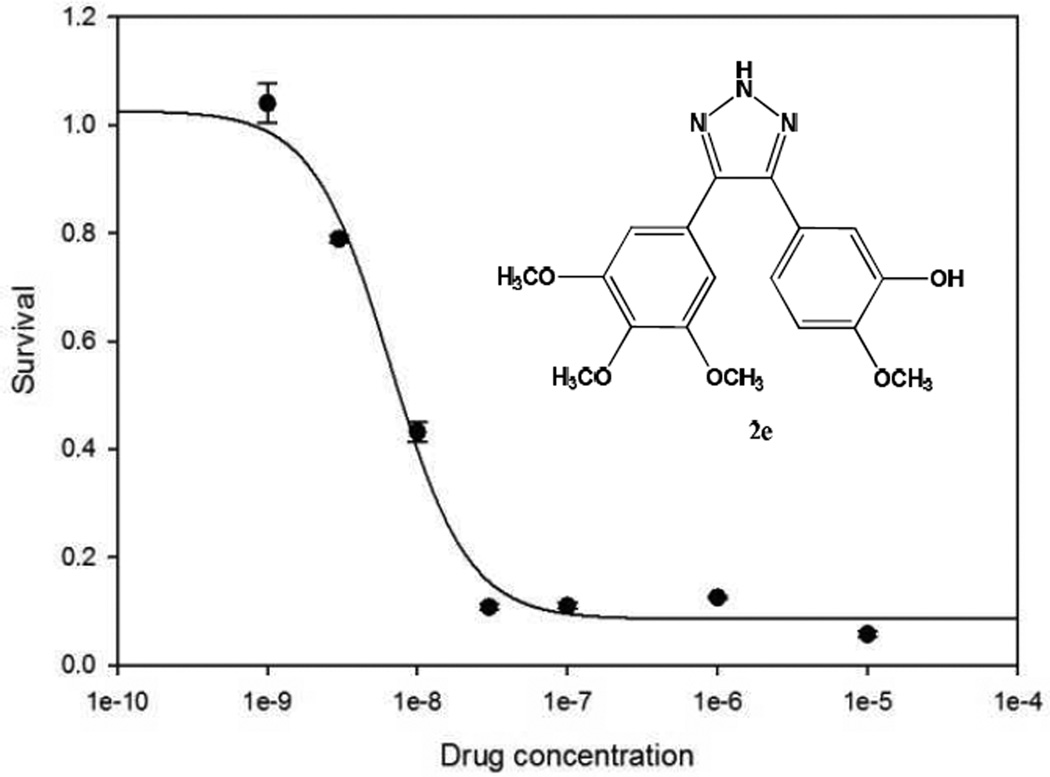
9LSF cells were acquired from the laboratory of Dennis Deen, Ph.D. (Brain Tumor Research Center, University of California, San Francisco). Cells were exposed to 2e at concentrations of 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 1 µM, or 10 µM for 24 hours prior to seeding for the colony formation assay. Cells were then trypsinized, counted, seeded into 25 cm2 flasks, and incubated at 37°C to form colonies [25]. All conditions were seeded in triplicate. Flasks that were plated with less than 50,000 cells were previously plated with 50,000 lethally irradiated A549 cells to serve as feeders [26]. Flasks were removed from incubation when colonies were large enough to count (>50 cells). Colonies were then fixed and stained with crystal violet, rinsed, allowed to dry, and counted. The protocol was run in triplicate, and results averaged, with error representing SEM. The LD50 value for 2e was determined to be 7.5 nM using SigmaPlot 11.
2.2.3 Cell cycle redistribution using 2e
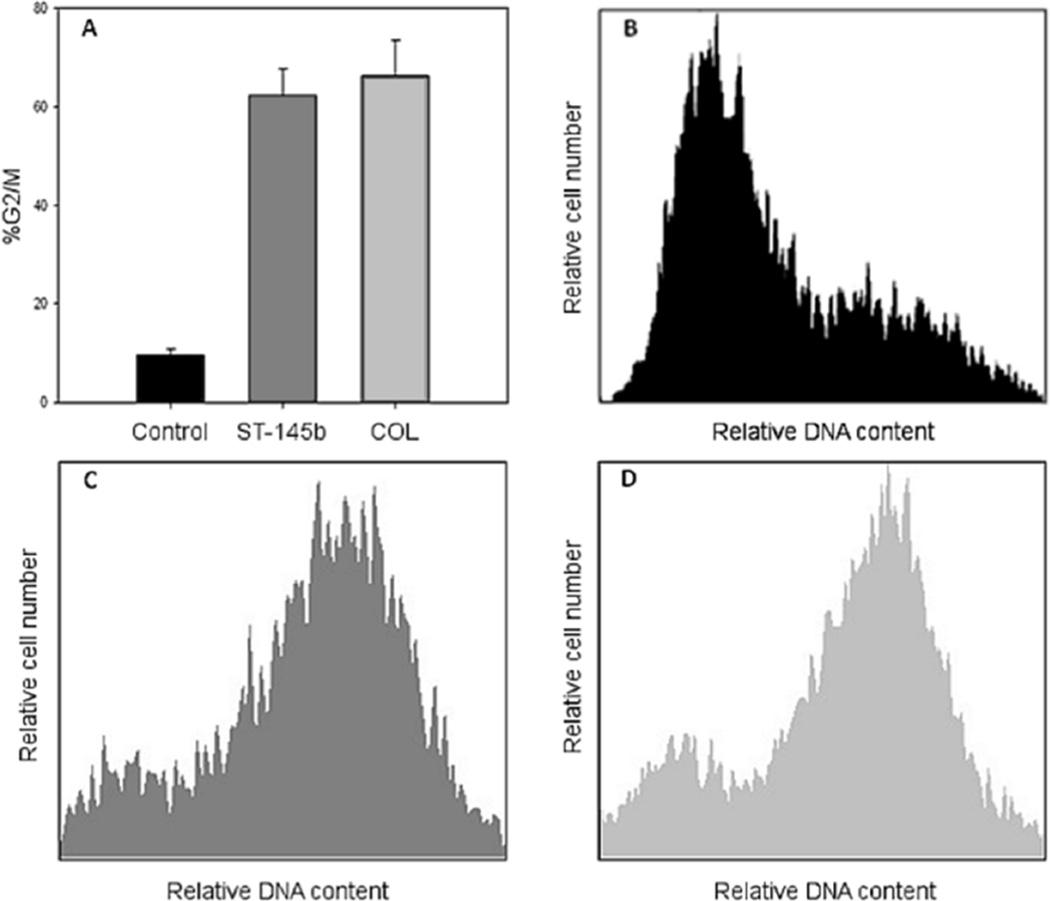
9LSF cells were exposed to 50 nM 2e or 50 nM colchicine (COL) for 24 hrs in vitro, along with controls. Cells were then trypsinized, counted, rinsed with PBS, and fixed using ice-cold 70% EtOH in PBS at 106 cells/mL. Samples were stored at 4°C prior to flow cytometry analysis; 106 cells from each condition were then pelleted and rinsed thrice with PBS. Samples were resuspended in 1 mL PBS, to which 1 µL propidium iodide (PI, 12.5 mg/mL in DMSO) was added. Samples were exposed to PI for 2 minutes at room temperature, then immediately analyzed by flow cytometry (Cell Lab Quanta SC, Beckman Coulter, Inc., Brea, CA.) The protocol was run in triplicate. Cell cycle distributions were analyzed using FCS Express 3 (De Novo Software, Glendale, CA).
3. In silico molecular docking studies
Two analogues (2h and 2l) from the 60 human cancer cell panel screens, and analogue 2e, GI50 <10 nM) were chosen for molecular docking studies utilizing the available crystal structure of tubulin, in order to understand the possible mechanism of their action as inhibitors of tubulin polymerization. Atomic coordinates for tubulin (PDB 1SA0) were downloaded from the protein structure database (www.rcsb.org/pdb). Colchicine was removed from the coordinate file and the coordinates of only chains A and B, corresponding to a α,β-tubulin heterodimer were used for the docking studies. Atomic coordinates for all compounds were generated using MarvinSketch (ChemAxon), and both the ligand and target protein coordinate files were prepared for docking using the Dock Prep module in the UCSF-Chimera package ((http://www.cgl.ucsf.edu/chimera). Docking was performed using SwissDock (http://www.swissdock.ch/), based on the docking algorithm EADock DSS [27]. Docking was performed using protocols established in our previous studies [16]. Use of the most exhaustive and unbiased option in SwissDock ensured the sampling of the maximum number of binding modes for each molecule. The best hits based on the SwissDock FullFitness scoring function (FF) from three repeated docking runs were considered further.
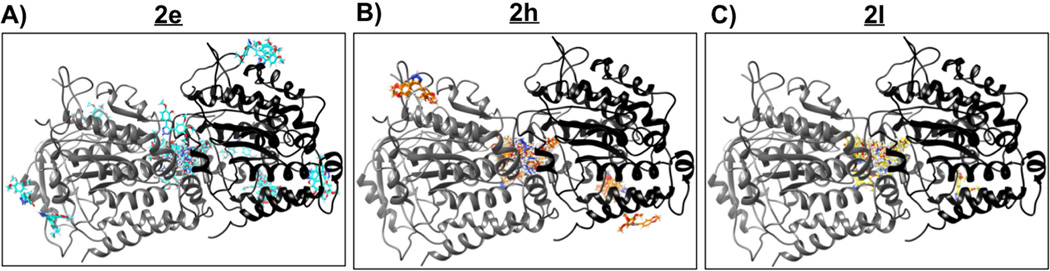
All three compounds docked almost exclusively to the colchicine-binding pocket on the α,β-tubulin heterodimer. This indicates that the mode of inhibition of tubulin polymerization by these three molecules is similar to that of colchicine and CA-4, and that the introduction of a triazole ring in place of the cis-olefinic bond still retains potent tubulin binding properties. Among the three molecules examined, 2e had the most number of ‘outlier’ poses that docked in non-colchicine binding, surfaceexposed pockets. However, 86% of the docked poses were still localized at the colchicine-pocket. In the case of 2h and 2l, this number was 95% and 99%, respectively (Fig. 4).
Fig. 4.
Docking poses of molecules 2e, 2h and 2l bound to tubulin. Subunits α and β of the tubulin heterodimer are shown as gray and black cartoons respectively. All the docking poses generated by Swissdock for molecules 2e, 2h and 2l are shown in panels A), B) and C) respectively. As seen clearly, majority of the poses bind to the ‘colchicine-binding’ pocket located at the interface of the two tubulin subunits for all three molecules. 2e shows the most, while 2l shows the least number of ‘outlier’ poses.
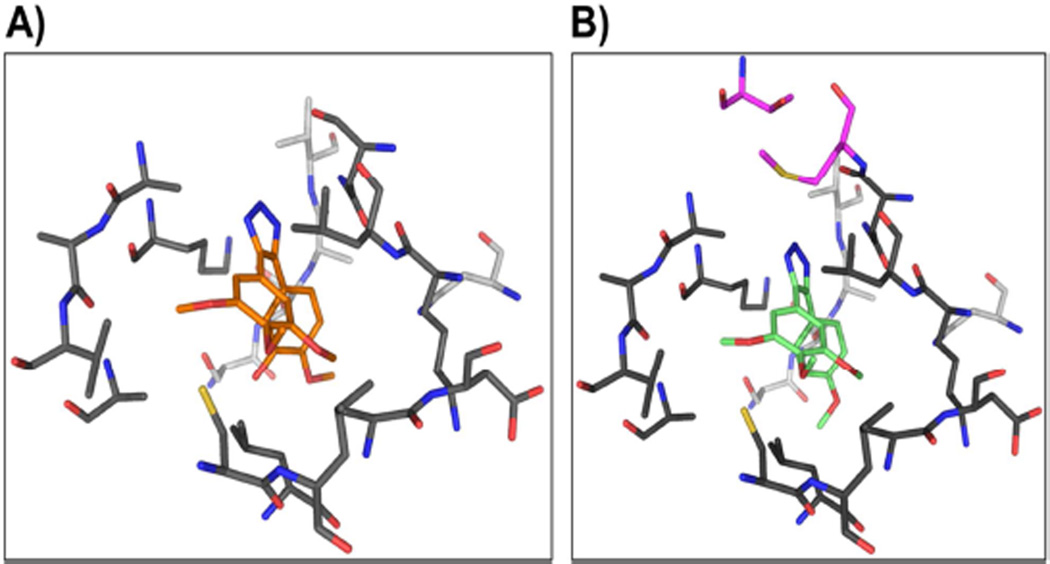
All three molecules were primarily stabilized through several van der Waals’ contacts with residues from the α- and β-subunits of tubulin. Compound 2e was found to interact with 13 β residues and 5 α residues of tubulin (Fig 5). In contrast, both molecules 2h and 2l, besides sharing all the contact residues of 2e, made additional contacts with Met259 and Thr314 of the β- subunit of tubulin (Fig 5B). This is reflected in the FF and ΔG scores of the three compounds (Table 3), with the FF score and free energy values for 2l and 2h being higher than that for 2e. Both scores followed the order: 2e < 2h < 2l.
Fig. 5.
Atomic contacts between tubulin and molecules 2e and 2l. A) 2e is shown as orange sticks, while residues of the α and β subunit of tubulin are in light and dark gray, respectively. B) 2l (green sticks) is shown bound at the colchicine-binding pocket of tubulin. The color scheme is the same as in A, while the residues of β-tubulin unique to 2l pocket are shown in magenta.
Table 3.
SwissDock statistics for the docking runs with compounds 2e, 2h and 2l (scores shown are averages from 3 docking runs).
| Comp | FF score (kcal/mol) | ΔG (kcal/mol) |
|---|---|---|
| 2l | −4224.7 | −8.4 |
| 2h | −4216.5 | −8.2 |
| 2e | −4211.4 | −7.9 |
4. Conclusions
A series of 4,5-disubstituted 2H-1,2,3-triazoles designed as CA4 analogues were synthesized from corresponding (Z)-substituted diarylacrylonitriles (1a–1s). The synthesis represents a facile and efficient reaction procedure for the preparation of 4,5-diaryl-2H-1,2,3-triazoles in modest to good yields. The resulting 4,5-disubstituted 2H-1,2,3-triazoles were evaluated for their anticancer properties against a panel of 60 human cancer cell lines. The diarylacrylonitrile analogue 2l exhibited the most potent anticancer activity in the cancer cell screening studies, with GI50 values of <10 nM against almost all the cell lines in the panel. Analogue 2l also exhibited promising TGI values of <10 nM against CNS cancer cell line SF-539, melanoma cell MDA-MB-435, ovarian cancer cell line OVCAR-3 and renal cancer cell line A498. Analogues 2m and 2h exhibited GI50 values <10nM against leukemia cell lines K-562, SR, non-small lung cancer cell line HOP-92, colon cancer cell lines HCT-116, and HCT-15, melanoma cancer lines M14, and UACC-62, ovarian cancer cell line NCI/ADR-RES and renal cancer cell line A498 Analogue 2e was evaluated for anti-cancer activity by colony formation assay against 9LSF rat gliosarcoma cells, and afforded an LD50 value of 7.5 nM. A cell cycle redistribution assay with 2e was also conducted to further understand the mechanism of action of this CA-4 analogue. In silico docking studies with compounds 2l, 2e and 2h within the active site of tubulin were carried out in order to rationalize the anticancer properties of these compounds. From these studies, compound 2e exhibited higher affinity for the colchicine binding site on tubulin compared to 2l and 2h. From this study, analogues 2e and 2l were considered lead compounds in this new structural class, and worthy of further development as anti-cancer agents.
5.0 Experimental Section
5.1. Chemistry
TLC experiments were carried out on pre-coated silica gel plates (F 254 Merck). 1H and 13C NMR spectra were recorded on a Varian 400 MHz spectrometer equipped with a Linux workstation running on vNMRj software. All spectra were phased, baseline was corrected where necessary, and solvent signals (CDCl3) were used as reference for both 1H and 13C spectra. HRMS data was obtained on an Agilent 6210 LCTOF instrument operated in multimode.
5.2. General Synthetic procedure for the synthesis of 4,5-disubstituted-2H-1,2,3-triazoles
In the first synthetic step, a series of (Z)-substituted diarylacrylonitrile analogues were synthesized by reacting substituted benzyl carbaldehydes with their corresponding substituted phenylacetonitriles in 5% NaOMe in methanol. The reaction mixture was stirred at room temperature for 2–3 hours for the reaction to complete and the final product to crash out of the solution. The precipitate is filtered, washed with water and dried to yield the final compounds with yields ranging from 70–95 % (Scheme 1) [16].
In the second synthetic step the 4,5-disubstituted-2H-1,2,3-triazoles were synthesized by refluxing a mixture of the (Z)-2,3-diarylacrylonitrile (1a–1s), NaN3, and NH4Cl in a mole ratio of 1:3:3 in 10:1 volumes of DMF/H2O for 5–12 h. The reaction was monitored by TLC. When the starting material had completely disappeared, cold water was added and the mixture was stirred over 10–15 min, during which time the final product precipitated out and was filtered off. In the absence of a precipitate, the product was extracted into ethyl acetate, the organic extract washed with copious amounts of water, and the resulting organic liquor evaporated to dryness on a rotavaporator. The residue obtained was purified by flash column chromatography to afford the corresponding triazole analogue of CA-4.
In the second synthetic step the 4,5-disubstituted-2H-1,2,3-triazoles were synthesized by refluxing a mixture of the (Z)-2,3-diarylacrylonitrile (1a–1s), NaN3, and NH4Cl in a mole ratio of 1:3:3 in 10:1 volumes of DMF/H2O for 5–12 h. The reaction was monitored by TLC. When the starting material had completely disappeared, cold water was added and the mixture was stirred over 10–15 min, during which time the final product precipitated out and was filtered off. In the absence of a precipitate, the product was extracted into ethyl acetate, the organic extract washed with copious amounts of water, and the resulting organic liquor evaporated to dryness on a rotavaporator. The residue obtained was purified by flash column chromatography to afford the corresponding triazole analogue of CA-4.
5.3. Analytical data of 4,5-disubstituted-2H-1,2,3-triazoles
5.3.1. 4-(3,4-dichlorophenyl)-5-(3,4-dimethoxyphenyl)-2H-1,2,3-triazole (2a)
1H NMR (400 MHz, CDCl3): δ 3.82 (s, 3H, -OCH3), δ 3.93 (s, 3H, -OCH3), 6.88 (d, J = 8 Hz, 1H, ArH), 7.06 (d, J = 12 Hz, 2H, ArH), 7.42 (s, 2H, ArH), 7.78 (s, 1H, ArH), 12.49 (bs, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 55.87, 55.96, 111.19, 121.31, 127.31, 129.80, 129.89, 130.54, 132.60, 132.86, 149.15, 149.73 ppm. HRMS (ESI): m/z calcd for C16H14Cl2N3O2 [M+H]+: 350.0463; found 350.0465.
5.3.2. 4,5-bis(3,4,5-trimethoxyphenyl)-2H-1,2,3-triazole (2b)
1H NMR (400 MHz, CDCl3): δ 3.76 (s, 12H, -OCH3), 3.88 (s, 6H, -OCH2), 6.84 (s, 4H, ArH), 12.50 (bs, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 56.08, 60.96, 105.57, 125.58, 138.28, 153.27 ppm. HRMS (ESI): m/z calcd for C20H24N3O6 [M+H]+: 402.1665; found 402.1668.
5.3.3. 4-(5-(3,4,5-trimethoxyphenyl)-2H-1,2,3-triazol-4-yl) quinolone (2c)
1H NMR (400 MHz, CDCl3): δ 3.47 (s, 6H, -OCH3), 3.81 (s, 3H, -OCH3), 6.65 (s, 2H, ArH), 7.50–7.52 (t, J = 8 Hz, 1H, ArH), 7.55 (d, J = 4.4 Hz, 1H, ArH), 7.77 (t, J = 1.6 Hz, 1H, ArH), 7.83 (d, J = 8 Hz, 1H, ArH), 8.28 (d, J = 8.8 Hz, 1H, ArH), 9.03 (d, J = 4.4 Hz, 1H, ArH) ppm. 13C NMR (100 MHz, CDCl3): δ 55.70, 60.85, 104.47, 122.68, 122.76, 124.69, 125.95, 126.66, 127.52, 129.42, 130.21, 138.21, 138.45, 148.15, 149.63, 149.69, 153.24 ppm. HRMS (ESI): m/z calcd for C20H19N4O3 [M+H]+: 363.1457; found 363.1460.
5.3.4. 2-(5-(3,4,5-trimethoxyphenyl)-2H-1,2,3-triazol-4-yl) quinolone (2d)
1H NMR (400 MHz, CDCl3): δ 3.78 (s, 6H, -OCH3), 3.91 (s, 3H, -OCH3), 7.08 (s, 2H, ArH), 7.56 (t, J = 14.8 Hz, 1H, ArH), 7.72 (t, J = 14.8 Hz, 1H, ArH), 7.83 (d, J = 6.4 Hz, 2H, ArH), 8.09 (d, J = 8.8 Hz, 1H, ArH), 8.17 (d, J = 8.4 Hz, 1H, ArH) ppm. 13C NMR (100 MHz, CDCl3): δ 56.08, 60.92, 106.21, 120.70, 125.57, 127.12, 127.56, 127.73, 127.68, 129.04, 130.20, 136.83, 138.50, 147.76, 153.19 ppm. HRMS (ESI): m/z calcd for C20H19N4O3 [M+H]+: 363.1457; found 363.1456.
5.3.5. 2-methoxy-5-(5-(3,4,5-trimethoxyphenyl)-2H-1,2,3-triazol-4-yl)phenol (2e)
1H NMR (400 MHz, CDCl3): δ 3.73 (s, 6H, -OCH3), 3.88 (s, 6H, -OCH3), 6.82 (d, J = 2 Hz, 3H, ArH), 7.04 (d, J = 8.4 Hz, 1H, ArH), 7.20 (s, 1H, ArH) ppm. 13C NMR (100 MHz, CDCl3): δ 55.92, 56.98, 56.07, 60.91, 60.98, 105.23, 110.63, 114.72, 120.61, 123.01, 125.74, 138.09, 145.66, 147.10, 153.23 ppm. HRMS (ESI): m/z calcd for C18H20N3O5 [M+H]+: 358.1403; found 358.1408.
5.3.6. 4-(4-chloro-3-fluorophenyl)-5-(thiophen-3-yl)-2H-1,2,3-triazole (2f)
1H NMR (400 MHz, CDCl3): δ 7.22 (d, J = 4.8 Hz, 1H, ArH), 7.32 (d, J = 8.4 Hz, 1H, ArH), 7.38–7.41 (m, 3H, ArH), 7.50 (d, J = 1.2 Hz, 1H, ArH), 10.5 (bs, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 116.17, 121.32, 124.56, 126.73, 127.01, 129.50, 130.75, 138.32, 141.30, 156.84, 159.31 ppm. HRMS (ESI): m/z calcd for C12H8N3FSCl [M+H]+: 280.0111; found 280.0118.
5.3.7. 2-(5-(2,4-dichlorophenyl)-2H-1,2,3-triazol-4-yl)pyridine (2g)
1H NMR (400 MHz, CDCl3): δ 7.26 (s, 1H, ArH), 7.39 (d, J = 8 Hz, 2H, ArH), 7.49–7.54 (m, 2H, ArH), 7.67(d, J = 6.8 Hz, 1H, ArH), 8.63 (s, 1H, ArH) ppm. 13C NMR (100 MHz, CDCl3): δ 121.68, 123.21, 127.27, 129.64, 132.76, 134.74, 135.52, 136.94, 149.45 ppm. HRMS (ESI): m/z calcd for C13H9N4Cl2 [M+H]+: 291.0204; found 291.0201.
5.3.8. 4-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-5-(3,4,5-trimethoxy phenyl)-2H-1,2,3-triazole (2h)
1H NMR (400 MHz, CDCl3): δ 3.77 (s, 6H, 2xOCH3), 3.89 (s, 3H, OCH3), 4.26–4.29 (m, 2xCH2), 6.84 (s, 2H, ArH), 6.88 (d, 1H, J = 8 Hz, Ar-H), 7.04–7.07 (dd, 1H, J = 2 and 8 Hz, 1H, Ar-H), 7.15 (d, 1H, Ar-H), 12.2 (bs, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 55.99, 60.99, 64.27, 105.24, 105.28, 117.42, 121.80, 138.18, 143.59, 144.09, 153.26 ppm. HRMS (ESI): m/z calcd for C19H20N3O5 [M+H]+: 370.1403; found 370.1398.
5.3.9. 4-(4-nitrophenyl)-5-(3,4,5-trimethoxyphenyl)-2H-1,2,3-triazole (2i)
1H NMR (400 MHz, DMSO-d6): δ 3.70–3.72 (d, 9H, -OCH3), 6.80 (s, 2H, ArH), 7.86 (s, 2H, ArH), 8.30 (d, J = 8.4 Hz, 2H, ArH) ppm. 13C NMR (100 MHz, DMSO-d6): 56.23, 61.06, 105.75, 123.91, 128.76, 137.57, 138.73, 147.50, 153.62 ppm. HRMS (ESI): m/z calcd for C17H17N4O5 [M+H]+: 357.1199; found 357.1199.
5.3.10. 4-(benzo[d][1,3]dioxol-5-yl)-5-phenyl-2H-1,2,3-triazole (2j)
1H NMR (400 MHz, CDCl3): δ 6.00 (s, 2H, -CH2), 6.81 (d, J = 8.8 Hz, 1H, ArH), 7.04 (d, J = 6.8 Hz, 2H, ArH), 7.39 (t, J = 5.2 Hz, 3H, ArH), 7.57 (m, J = 9.6 Hz, 1H, ArH) ppm. 13C NMR (100 MHz, CDCl3): δ 101.43, 108.77, 108.91, 122.46, 123.96, 128.41, 128.73, 128.88, 130.25, 142.29, 148.01, 148.11 ppm. HRMS (ESI): m/z calcd for C15H12N3O2 [M+H]+: 266.0930; found 266.0928.
5.3.11. 4-(3,5-dibromophenyl)-5-(2-methoxyphenyl)-2H-1,2,3-triazole (2k)
1H NMR (400 MHz, CDCl3): δ 3.76 (s, 3H, -OCH3), 7.04 (d, J = 4.8 Hz, 2H, ArH), 7.39–7.47 (m, 2H, ArH), 7.63 (d, J = 1.6 Hz, 1H, ArH), 7.71 (s, 2H, ArH), 12.2 (bs, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 55.51, 111.54, 121.21, 122.83, 129.17, 130.48, 131.29, 133.34, 133.42, 134.96, 156.51 ppm. HRMS (ESI): m/z calcd for C15H12N3O Br2 [M+H]+: 407.9347; found 407.9342.
5.3.12. 4-(4-methoxyphenyl)-5-(3,4,5-trimethoxyphenyl)-2H-1,2,3-triazole (2l)
1H NMR (400 MHz, CDCl3): δ 3.74 (s, 6H, -OCH3), 3.84 (s, 3H, -OCH3), 3.89 (s, 3H, -OCH3), 6.83 (s, 2H, ArH), 6.92 (d, J = 8.4 Hz, 2H, ArH), 7.52 (d, J = 8.0 Hz, 2H, ArH), 12.2 (bs, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 55.27, 55.96, 60.90, 105.14, 105.18, 114.03, 125.86, 129.79, 138.07, 153.27, 159.97 ppm. HRMS (ESI): m/z calcd for C18H20N3O4 [M+H]+: 342.1454; found 342.1448.
5.3.13. 4-(3,5-dimethoxyphenyl)-5-(4-methoxyphenyl)-2H-1,2,3-triazole (2m)
1H NMR (400 MHz, DMSO-d6): δ 3.68 (s, 6H, -OCH3), 3.78 (s, 3H, -OCH3), 6.5 (s, 1H, Ar-H), 6.64 (2H, Ar-H), 7.01 (d, 2H, J = 8 Hz, ArH), 7.43 (d, 2H, J = 8.8 Hz, Ar-H), HRMS (ESI): m/z calcd for C17H18N3O3 [M+H]+: 312.1348; found 312.1344.
5.3.14. 4-(benzo[d][1,3]dioxol-5-yl)-5-(3,4,5-trimethoxy phenyl)-2H-1,2,3-triazole (2n)
1H NMR (400 MHz, CDCl3): δ 3.77 (s, 6H, -OCH3), 3.89 (s, 3H, -OCH3), 4.26–4.29 (q, J = 12.4 Hz, 4H, ArH), 6.84 (s, 2H, ArH), 6.88 (d, J = 8 Hz, 1H, ArH), 7.04–7.07 (dd, J = 2 Hz, 8 Hz, 1H, ArH), 7.16 (d, J = 1.6 Hz, 1H, ArH), 7.26 (s, 1H, ArH) ppm. 13C NMR (100 MHz, CDCl3): δ 55.99, 56.08, 60.90, 60.99, 64.27, 64.47, 105.24, 105.28, 117.42, 121.80, 138.18, 143.59, 144.09, 153.26 ppm. HRMS (ESI): m/z calcd for C18H18N3O5 [M+H]+: 356.1246; found 356.1248.
5.3.15. 4-(3,4-dichlorophenyl)-5-(3,4,5-trimethoxyphenyl)-2H-1,2,3-triazole (2o)
1H NMR (400 MHz, CDCl3): δ 3.79 (s, 6H, -OCH3), 3.91 (s, 3H, -OCH3), 6.77 (s, 2H, ArH), 7.46 (s, 2H, ArH), 7.82 (s, 1H, ArH) ppm. 13C NMR (100 MHz, CDCl3): δ 56.14, 60.98, 105.49, 127.51, 129.92, 130.57, 132.51, 132.77, 138.46, 153.47 ppm. HRMS (ESI): m/z calcd for C17H16Cl2N3O3 [M+H]+: 380.0569; found 380.0564.
5.3.16. 2-(5-(3,4,5-trimethoxyphenyl)-2H-1,2,3-triazol-4-yl)pyridine (2p)
1H NMR (400 MHz, CDCl3): δ 3.81 (s, 6H, -OCH3), 3.90 (s, 3H, -OCH3), 6.9 (s, 2H, ArH), 7.28 (d, J =12.8 Hz, 2H, ArH), 7.70 (s, 2H, ArH), 8.67 (s, 1H, ArH) ppm. 13C NMR (100 MHz, CDCl3): δ 56.10, 60.94, 106.04, 123.22, 123.46, 125.66, 137.06, 138.37, 149.36, 153.26 ppm. HRMS (ESI): m/z calcd for C16H17N4O3 [M+H]+: 313.1301; found 313.1298.
5.3.17. 4-(3,5-dimethoxyphenyl)-5-(4-nitrophenyl)-2H-1,2,3-triazole (2q)
1H NMR (400 MHz, CDCl3): δ 3.74 (s, 6H, -OCH3), 6.51 (d, J = 1.2 Hz, 1H, ArH), 6.62 (s, 2H, ArH), 7.8 (d, J = 8.4 Hz, 2H, ArH), 8.19 (d, J = 8.4 Hz, 2H, Ar-H) ppm. 13C NMR (100 MHz, CDCl3): δ 55.43, 101.28, 106.53, 123.85, 128.67, 137.08, 147.49, 161.13 ppm. HRMS (ESI): m/z calcd for C16H15N4O4 [M+H]+: 327.1093; found 327.1084.
5.3.18. 4-(benzo[d][1,3]dioxol-5-yl)-5-(3,5-dimethoxyphenyl)-2H-1,2,3-triazole (2r)
1H NMR (400 MHz, CDCl3): δ 3.75 (s, 6H, -OCH3), 5.99 (s, 2H, ArH), 6.48 (s, 1H, ArH), 6.73(d, J = 2.4 Hz, 2H, ArH), 6.82 (d, J = 8.8 Hz, 1H, ArH), 7.06 (d, J = 6.4 Hz, 2H, ArH), 12.1 (bs, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 55.34, 55.51, 101.05, 101.25, 106.12, 108.51, 108.80, 122.38, 131.91, 147.81, 147.98, 160.87 ppm. HRMS (ESI): m/z calcd for C17H16N3O4 [M+H]+: 326.1141; found 326.1133.
5.3.19. 2-(5-(benzo[d][1,3]dioxol-5-yl)-2H-1,2,3-triazol-4-yl)pyridine (2s)
1H NMR (400 MHz, CDCl3): δ 5.99 (s, 2H, -CH2), 6.83 (d, J = 8.4 Hz, 1H, ArH), 7.14 (d, J = 6 Hz, 2H, ArH), 7.27 (d, J = 8.4 Hz, 1H, ArH), 7.70 (d, J = 7.2 Hz, 2H, ArH), 8.69 (s, 1H, ArH) ppm. 13C NMR (100 MHz, CDCl3): δ 101.15, 101.25, 101.35, 108.52, 109.31, 109.34, 122.78, 123.30, 124.04, 137.02, 147.77, 148.06, 149.56 ppm. HRMS (ESI): m/z calcd for C14H11N4O2 [M+H]+: 267.0882; found 267.0879.
Supplementary Material
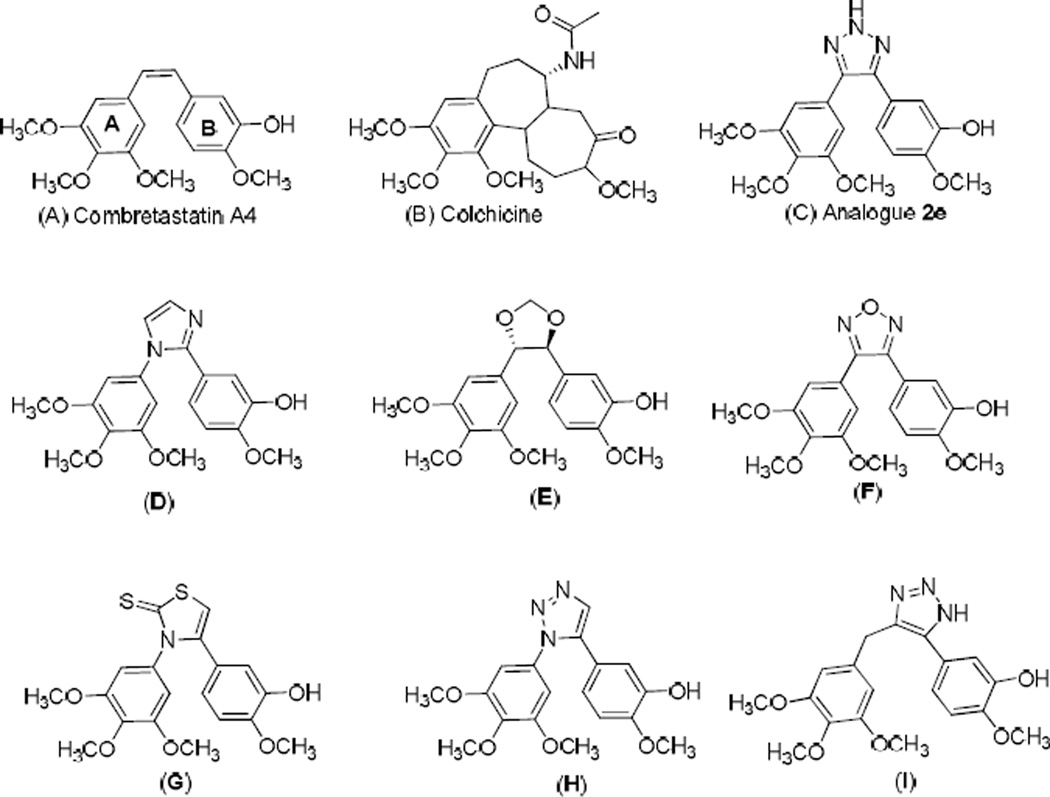
Fig. 1.
Structures of combretastatin A-4 (CA-4, A), colchicine (B), triazole analogue (2e, C) and reported anti-tubulin compounds (D–I).
Fig. 2.
Colony formation assay, 2e-treated 9LSF cells. Cells were exposed to 2e for 24 hours. Curve was fitted as four-parameter logistic curve (f1 = min + (max−min)/(1 + (x/EC50)^(−Hillslope))). Values represent mean ± SEM of 3 independent colony formation experiments.
Fig 3.
Cell cycle redistribution effects of 2e on 9L cells. (A) Percentage of cell population in G2/M phase in control cells versus those exposed to 50 µM 2e or 50 µM COL for 24 hrs. (B) Representative distribution of control cells. (C) Representative distribution of 2e-treated cells. (D) Representative distribution of COL-treated cells.
Table 1.
Synthesized (Z)-2,3-diarylacrylonitriles and 4,5-disubstituted-2H-1,2,3-triazoles from their corresponding acetonitrile and aldehyde precursors.
Highlights.
A variety of novel 4,5-disubstituted 2H-1,2,3-triazoles were synthesized from there corresponding (Z)-substituted diarylacrylonitriles.
The synthesized analogs were evaluated for their anti-cancer activity against NCI 60 human cancer cell lines.
Conducted in silico docking studies to rationalize the anti-cancer properties of the synthesized compounds.
Cell cycle redistribution assay was conducted to further understand the mechanism of action of the synthesized CA-4 analogues.
One of the analogue 2e showed promising anticancer activity with an LD50 value of 7.5 nM against 9LSF rat gliosarcoma cells.
Acknowledgements
We are grateful for support from NCI/NIH Grants CA 140409 (to P.A.C.) and CA 183895 (to R.L.E.), the UAMS Translational Research Institute (TRI), grant UL1TR000039 through the NIH National Center for Research Resources and National Center for Advancing Translational Sciences, the UAMS Department of Radiology the Arkansas Research Alliance (ARA), and to the NCI Developmental Therapeutic Program (DTP) for screening data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/….
Notes and references
- 1.Gordaliza M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol. 2007;9:767–776. doi: 10.1007/s12094-007-0138-9. [DOI] [PubMed] [Google Scholar]
- 2.Jordan MA. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem.: Anti-Cancer Agents. 2002;2:1–17. doi: 10.2174/1568011023354290. [DOI] [PubMed] [Google Scholar]
- 3.Hadfield JA, Ducki S, Hirst N, McGown AT. Tubulin and microtubules as targets for anticancer drugs. Prog. Cell. Cycle. Res. 2003;5:309–325. [PubMed] [Google Scholar]
- 4.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 5.Young SL, Chaplin DJ. Combretastatin-A4 phosphate: background and current clinical status. Expert. Opin. Investig. Drugs. 2004;13:1171–1182. doi: 10.1517/13543784.13.9.1171. [DOI] [PubMed] [Google Scholar]
- 6.Cooney MM, Ortiz J, Bukowski RM, Remick SC. Novel vascular targeting/disrupting agents: combretastatin-A4 phosphate and related compounds. Curr. Oncol. Rep. 2005;7:90–95. doi: 10.1007/s11912-005-0033-x. [DOI] [PubMed] [Google Scholar]
- 7.Tron GC, Pirali T, Sorba G, Pagliai F, Busacca S, Genazzani AA. Medicinal chemistry of combretastatin-A4: present and future directions. J. Med. Chem. 2006;49:3033–3044. doi: 10.1021/jm0512903. [DOI] [PubMed] [Google Scholar]
- 8.Tozer GM, Kanthou C, Parkins CS, Hill SA. The biology of the combretastatins as tumour vascular targeting agents. Int. J. Exp. Pathol. 2002;83:21–38. doi: 10.1046/j.1365-2613.2002.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh HP, Liou JP, Mahindroo N. Pharmaceutical design of anti-mitotic agents based on combretastatins. Curr. Pharm. Des. 2005;11:1655–1677. doi: 10.2174/1381612053764751. [DOI] [PubMed] [Google Scholar]
- 10.Carr M, Greene LM, Knox AJS, Lloyd DG, Zisterer DM, Meegan MJ. Lead identification of conformationally restricted β-lactam type combretastatin analogues: Synthesis, antiproliferative activity and tubulin targeting effects. Eur. J. Med. Chem. 2010;45:5752–5766. doi: 10.1016/j.ejmech.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 11.Banimustafa M, Kheirollahi A, Safavi M, Kabudanian Ardestani S, Aryapour H, Foroumadi A, Emami S. Synthesis and biological evaluation of 3-(trimethoxyphenyl)-2(3H)-thiazole thiones as combretastatin analogs. Eur. J. Med. Chem. 2013;70:692–702. doi: 10.1016/j.ejmech.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Beale TM, Bond PJ, Brenton JD, Charnock-Jones DS, Ley SV, Myers RM. Increased endothelial cell selectivity of triazole-bridged dihalogenated A-ring analogues of combretastatin A–1. Bioorg. Med. Chem. 2012;20:1749–1759. doi: 10.1016/j.bmc.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Demchuk DV, Samet AV, Chernysheva NB, Ushkarov VI, Stashina GA, Konyushkin LD, Raihstat MM, Firgang SI, Philchenkov AA, Zavelevich MP, Kuiava LM, Chekhun VF, Blokhin DY, Kiselyov AS, Semenova MN, Semenov VV. Synthesis and antiproliferative activity of conformationally restricted 1,2,3-triazole analogues of combretastatins in the sea urchin embryo model and against human cancer cell lines. Bioorg. Med. Chem. 2014;22:738–755. doi: 10.1016/j.bmc.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Shirai R, Takayama H, Nishikawa A, Koiso Y, Hashimoto Y. Asymmetric synthesis of antimitotic combretadioxolane with potent antitumor activity against multidrug resistant cells. Bioorg. Med. Chem. Lett. 1998;8:1997–2000. doi: 10.1016/s0960-894x(98)00344-8. [DOI] [PubMed] [Google Scholar]
- 15.Tron GC, Pagliai F, Del Grosso E, Genazzani AA, Sorba G. Synthesis and cytotoxic evaluation of combretafurazans. J. Med. Chem. 2005;48:3260–3268. doi: 10.1021/jm049096o. [DOI] [PubMed] [Google Scholar]
- 16.Penthala NR, Zong H, Ketkar A, Madadi NR, Janganati V, Eoff RL, Guzman ML, Crooks PA. Synthesis, anticancer activity and molecular docking studies on a series of heterocyclic trans-cyanocombretastatin analogues as antitubulin agents. Eur. J. Med. Chem. 2015;92:212–220. doi: 10.1016/j.ejmech.2014.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madadi NR, Penthala NR, Song L, Hendrickson HP, Crooks PA. Preparation of 4,5 disubstituted-2H-1,2,3-triazoles from (Z)-2,3-diaryl substituted acrylonitriles. Tetrahedron Lett. 2014;55:4207–4211. [Google Scholar]
- 18.Madadi NR, Penthala NR, Bommagani S, Parkin S, Crooks PA. Crystal structure of 4,5-bis-(3,4,5-tri-meth-oxy-phen-yl)-2H-1,2,3-triazole methanol monosolvate. Acta. Crystallogr. Sect. E: Struct. Rep. Online. 2014;70:o1128–o1129. doi: 10.1107/S1600536814020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penthala NR, Madadi NR, Bommagani S, Parkin S, Crooks PA. Comparison of crystal structures of 4-(benzo[b]thio-phen-2-yl)-5-(3,4,5-trimethoxyphenyl)-2H-1,2,3-triazole and 4-(benzo [b]thiophen-2-yl)-2-methyl-5-(3,4,5-trimethoxyphenyl)-2H-1,2,3-triaz ole. Acta. Crystallogr. Sect. E: Struct. Rep. Online. 2014;70:392–395. doi: 10.1107/S1600536814023095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penthala NR, Madadi NR, Janganati V, Crooks PA. L-Proline catalyzed one-step synthesis of 4,5-diaryl-2-1,2,3-triazoles from heteroaryl cyanostilbenes via [3+2] cycloaddition of azide. Tetrahedron Lett. 2014;55:5562–5565. doi: 10.1016/j.tetlet.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nam NH. Combretastatin-A4 analogues as anti-mitotic antitumor agents. Curr. Med. Chem. 2003;10:1697–1722. doi: 10.2174/0929867033457151. [DOI] [PubMed] [Google Scholar]
- 22.Rubinstein LV, Shoemaker RH, Paull KD, Simon RM, Tosini S, Skehan P, Scudiero DA, Monks A, Boyd MR. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J. Natl. Cancer. Inst. 1990;82:1113–1118. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- 23.Madadi NR, Penthala NR, Janganati V, Crooks PA. Synthesis and anti-proliferative activity of aromatic substituted 5-((1-benzyl-1 H-indol-3-yl) methylene)-1,3-dimethylpyri midine-2, 4, 6 (1H, 3H, 5H)-trione analogs against human tumor cell lines. Bioorg. Med. Chem. Lett. 2014;24:601–603. doi: 10.1016/j.bmcl.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madadi NR, Zong H, Ketkar A, Zheng C, Penthala NR, Janganati V, Bommagani S, Eoff RL, Guzman ML, Crooks PA. Synthesis and evaluation of a series of resveratrol analogues as potent anti-cancer agents that target tubulin. Med. Chem. Comm. 2015;6:788–794. doi: 10.1039/C4MD00478G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borrelli MJ, Stafford DM, Rausch CM, Bernock LJ, Freeman ML, Lepock JR, Corry PM. Diamide-induced cytotoxicity and thermotolerance in CHO cells. J. Cell, Physiol. 1998;177:483–492. doi: 10.1002/(SICI)1097-4652(199812)177:3<483::AID-JCP11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 26.Borrelli MJ, Thompson LL, Dewey WC. Evidence that the feeder effect in mammalian cells is mediated by a diffusible substance. Int. J. Hyperthermia. 1989;5:99–103. doi: 10.3109/02656738909140436. [DOI] [PubMed] [Google Scholar]
- 27.Grosdidier A, Zoete V, Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011;39:W270–W277. doi: 10.1093/nar/gkr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.