Summary
TOR and PKA signaling pathways control eukaryotic cell growth and proliferation. TOR activity in model fungi, such as Saccharomyces cerevisiae, responds principally to nutrients, e.g., nitrogen and phosphate sources, which are incorporated into the growing cell mass; PKA signaling responds to the availability of the cells' major energy source, glucose. In the fungal commensal and pathogen, Candida albicans, little is known of how these pathways interact. Here, the signal from phosphorylated ribosomal protein S6 (P-S6) was defined as a surrogate marker for TOR-dependent anabolic activity in C. albicans. Nutritional, pharmacologic, and genetic modulation of TOR activity elicited corresponding changes in P-S6 levels. The P-S6 signal corresponded to translational activity of a GFP reporter protein. Contributions of four PKA pathway components to anabolic activation were then examined. In high glucose concentrations, only Tpk2 was required to upregulate P-S6 to physiologic levels, whereas all four tested components were required to downregulate P-S6 in low glucose. TOR was epistatic to PKA components with respect to P-S6. In many host niches inhabited by C. albicans, glucose is scarce, with protein being available as a nitrogen source. We speculate that PKA may modulate TOR-dependent cell growth to a rate sustainable by available energy sources, when monomers of anabolic processes, such as amino acids, are abundant.
Keywords: Candida albicans, TOR, PKA, phosphorylated ribosomal protein S6, growth, nutritional signaling
Introduction
Candida albicans is a major human fungal commensal and opportunistic pathogen whose bloodstream infections are associated with up to 50% mortality (Wisplinghoff et al., 2004). Its primary lifestyle is as a commensal, colonizing the gastrointestinal tract and oral and vaginal mucous membranes of 2 - 70% of healthy individuals (Odds, 1988; Cannon & Chaffin, 1999). Cell-mediated immunity confers protection against mucosal infection, e.g., of the oropharyngeal and esophageal mucosa. Neutrophils prevent bloodstream infections in healthy individuals but immunosuppression, presence of plastic catheters in the bloodstream or body cavities, and other disruptions of host defenses can result in infections of the bloodstream and of deep organs (Odds, 1988; Kim & Sudbery, 2011).
The ability of C. albicans to survive and thrive in different niches within the host contributes to its success as a commensal and pathogen. The organism is exposed to distinct environmental conditions at each niche and its ability to withstand host defenses and proliferate depends on its ability to sense and respond to these external stimuli (Barelle et al., 2006; Biswas et al., 2007). For instance, in a late-phase oral candidiasis infection model, C. albicans was shown to up-regulate glucose- and nitrogen-starvation response genes, which suggests that the cell is exposed to low glucose and poor nitrogen sources; in contrast, the organism did not appear to up-regulate these genes upon liver tissue invasion (Wilson et al., 2009). In a reconstituted oral epithelium model, as well as in oral mucosa-infecting cells of AIDS patients, C. albicans up-regulated genes encoding components of the glyoxylate pathway, glucose transport, and gluconeogenesis, suggesting a response to unavailability of glucose (Zakikhany et al., 2007); these results were consistent with previous findings revealing the central importance of the glyoxylate cycle in the metabolism of C. albicans cells ingested by macrophages (Lorenz & Fink, 2001; Lorenz et al., 2004). Unavailability of glucose and utilization of alternative carbon sources can significantly affect the cell wall architecture and stress resistance of the fungus (Ene et al., 2012), and its interaction with the host (Ene et al., 2013). Candida's flexibility in mounting responses specific to occupied niches within the host may be a major factor in its ability to colonize very different mucosal surfaces, and to cause infection in a diverse range of tissues and organs (Wilson et al., 2009). How C. albicans is able to respond to different nutritional conditions in these distinct niches is an important aspect of its remarkable adaptability.
In eukaryotic cells, the TOR (Target of Rapamycin) pathway is a major signaling pathway that regulates cell growth and proliferation in response to nutrient availability (Loewith & Hall, 2011). The main component of the TOR pathway is the Tor kinase, a highly conserved serine/threonine kinase belonging to the phosphatidylinositol kinase-related protein kinase (PIKK) family. Tor1 was first identified as the target of the anti-fungal and immunosuppressive agent rapamycin (Heitman et al., 1991). The kinase forms part of two protein complexes, Tor complexes 1 and 2 (TORC1 and TORC2), with the former being sensitive to rapamycin (Loewith et al., 2002). TORC2, containing partially overlapping subunits with TORC1, regulates spatial and temporal aspects of cell growth. As TORC2 activities are not addressed in this work, further references to TOR signaling will refer to activity of the Tor complex 1.
In S. cerevisiae, TOR negatively regulates expression of genes important during nitrogen and carbon limitation by sequestering transcription factors in the cytoplasm under nutrient-rich conditions (Beck & Hall, 1999). Rapamycin treatment or nitrogen starvation inhibits Tor kinase, consequently leading to inhibition of anabolic processes, such as protein synthesis and cell proliferation, and induction of catabolic processes, such as autophagy (Loewith & Hall, 2011).
TOR inhibition by rapamycin blocks initiation of translation and results in cell cycle arrest at G1 (Barbet et al., 1996; Berset et al., 1998). TOR in mammalian cells (mTOR) activates protein synthesis by phosphorylating the kinase (S6K) for ribosomal protein S6 (Rps6 or S6) (Hay & Sonenberg, 2004; Wei & Zheng, 2011). The S6K ortholog in Saccharomyces cerevisiae, Sch9, is a direct substrate of TOR and regulates initiation of translation (Urban et al., 2007). TOR has also been shown to regulate phosphorylation of S6 in Schizosaccharomyces pombe (Nakashima et al., 2010).
Ribosomal protein S6 was found over 30 years ago to be the only ribosomal phosphoprotein in rapidly growing, regenerating rat liver cells, and up to 6 phosphorylation states of S6 were identified, suggesting 5 potential phospho-acceptor serine residues (Gressner & Wool, 1974). These authors speculated that “the phosphorylation of S6 (e.g., the increase in the number of phosphorylated derivatives) is causally, or fortuitously, related to the acceleration of protein synthesis that occurs during hepatic regeneration” (Gressner & Wool, 1974). The same authors reported two years later that cyclic AMP increases the levels of S6 phosphorylation in these cells (Gressner & Wool, 1976), suggesting that a connection may exist between the energy repletion and S6 phosphorylation states of a cell. Although a large body of work in intervening years has not revealed how the phosphorylation state of S6 is mechanistically linked to translational activity (Ruvinsky et al., 2005; Meyuhas, 2008), S6 phosphorylation was shown to be a critical downstream component of mTOR signaling in regulation of cell size (Ruvinsky et al., 2005).
P-S6 is used as a readout of mTOR activity in oncologic reasearch. In chronic myelogenous leukemia (CML), S6 was found to be constitutively phosphorylated downstream of the oncogenic fusion protein characteristic of this disease, the kinase Bcr-Abl, and of mTOR (Ly et al., 2003). For use in combination with an important chemotherapeutic anti-CML agent, the specific Bcr-Abl inhibitor imatinib (Gleevec), Zhang et al. examined the ability of a novel therapeutic compound to impair S6 phosphorylation as a readout of its potential anti-leukemic effect (Zhang et al., 2008). Analogously, the level of S6 phosphorylation in soft tissue sarcomas was used to predict a tumor response in patients treated with an experimental targeted mTOR inhibitor, with high levels of phosphorylated S6 predicting high mTOR activity in the sarcoma and a subsequent good response to the experimental drug (Iwenofu et al., 2008). S6 in malignant cells showed increased phosphorylation and, hence, the S6 phosphorylation state of pancreatic cancer cells was used as a readout of mTORC1 activation in a high-throughput RNAi screen conducted to identify anti-cancer drug targets (Papageorgiou & Avruch, 2012).
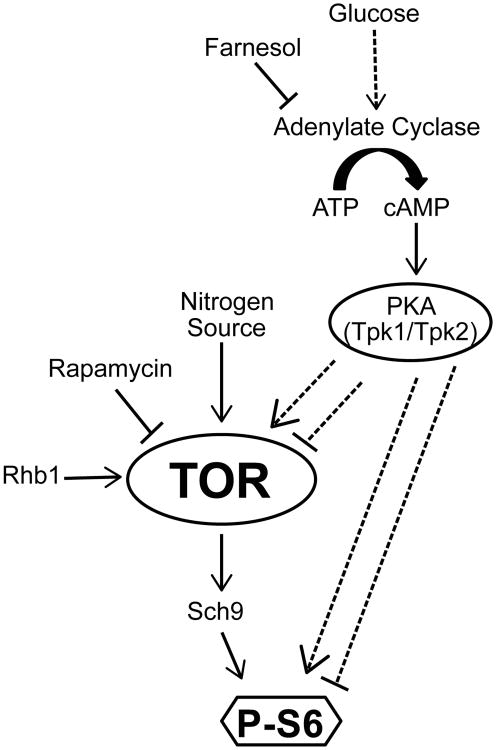
In addition to the TOR pathway, the cyclic AMP (cAMP)/Protein Kinase A (PKA) pathway is an important signaling pathway responding to nutritional cues. Similar to the effect of TOR inhibition, inactivation of cAMP/PKA pathway signaling represses cell cycle progression as well as cell growth (Dechant & Peter, 2008). The central regulator in this pathway is the adenylate cyclase, Cyr1 (Cdc35), which converts ATP into the second messenger cAMP. Cyr1 activation by numerous upstream signals results in increased cAMP levels (Biswas et al., 2007; Dechant & Peter, 2008; Zaman et al., 2008; Hall et al., 2009). Upon cAMP-binding to the inhibitory PKA subunit Bcy1, the catalytic subunits, encoded by TPK1 and TPK2 in C. albicans, are activated. Among the effectors of PKA activation are transcriptional regulators of processes such as morphogenesis, glycolysis, and virulence, e.g., Efg1 and Flo8 (Hall et al., 2009; Hogan & Sundstrom, 2009).
We established an assay to monitor TOR activity in C. albicans by measuring cellular levels of phosphorylated ribosomal protein S6 (P-S6) using an antibody against phosphorylated targets of the mammalian TOR pathway component, Akt kinase. We found pharmacologic and genetic TOR disruptions to inhibit phosphorylation of S6 without affecting levels of total S6.
We further observed correlation between P-S6 levels and translation of a heterologous green fluorescent protein (GFP) reporter regulated by a doxycycline-inducible promoter. P-S6 levels also responded to the quality of the nitrogen source, and to the availability of the preferred carbon source, glucose. The PKA pathway modulated the response to glucose, as mutants deleted in either catalytic subunit of PKA did not appropriately down-regulate P-S6 in glucose-limiting conditions. In addition, the mutant deleted in one subunit, Tpk2, failed to up-regulate P-S6 in abundant glucose. Transcriptional regulators downstream of PKA also participated in down-regulating P-S6. Our results were consistent with the PKA pathway regulating P-S6 by acting either upstream of, or parallel to the TOR pathway.
This study suggests that PKA modulates TOR-controlled cell growth, as monitored by a surrogate marker P-S6, to ensure not only availability of building blocks of anabolic processes such as translation, but also presence of sufficient energy sources to complete biosynthetic activity, once initiated.
Results
An anti-AKT target antibody recognizes C. albicans phosphorylated ribosomal protein S6
To examine the response of C. albicans cells to nutritional repletion versus starvation, a downstream target of TOR signaling was examined as a readout of anabolic activity. A major component of the mTOR pathway, S6K, phosphorylates ribosomal protein S6 during active growth (Hay & Sonenberg, 2004; Huang & Manning, 2008). We hypothesized that the S6 phosphorylation state could be monitored to reflect the activity level of the TOR pathway.
An antibody directed against phosphorylated targets of mammalian Akt kinase, a component of mammalian TOR signaling, was used to probe extracts of C. albicans cells grown in rich medium. In the carboxy-terminus of C. albicans S6 protein, 4 of 6 amino acid residues, centered on serine 233, correspond to the Akt target sequence ((Rust & Thompson, 2011); http://www.kinexus.ca/pdf/graphs_charts/ProteinSerKinaseSpecificity.pdf, accessed May 10 2015). Consistent with findings in the fission yeast Schizosaccharomyces pombe (Nakashima et al., 2010), a band with a molecular weight of ∼35 kDa appeared in protein extracts from actively growing cells, which approximately corresponds to the molecular weight of C. albicans Rps6 (27 kD as calculated by the ProtParam Tool on the ExPASy Bioinformatics Resource Portal (Gasteiger et al., 2005)). Upon treatment of cell extract with λ protein phosphatase, this band disappeared, indicating that it represented a phosphorylated protein (Fig. 1A).
Fig. 1.
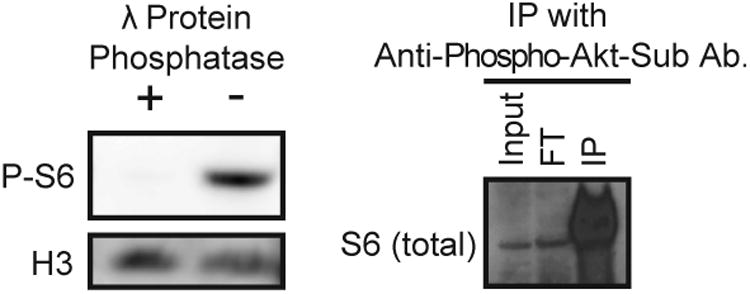
Anti-phosphorylated Akt substrate rabbit polyclonal antibody recognizes Candida albicans phosphorylated S6 (P-S6).
A. Wild-type SC5314 cell lysates were treated with (“+”) or without (“-”) λ Protein Phosphatase at 30°C for 40 minutes. Samples were analyzed by Western blotting to monitor P-S6 and the loading control histone H3 with anti-phosphorylated-Akt-substrate antibody and anti-H3 antibody, respectively.
B. Proteins from wild-type SC5314 cell lysate were immuno-precipitated with anti-phosphorylated-Akt-substrate antibody. The lysate (“input”), flow-through (“FT”) (fraction unbound by the antibody), and immuno-precipitated (“IP”) samples were probed for total S6 with anti-S6 antibody.
The antibody was used to precipitate its targets from cell lysates, which were then probed with an antibody to total mammalian S6. A strong signal was observed at 35 kDa in the immuno-precipitated sample, indicating that the anti-total S6 antibody recognized the precipitated target of the antibody against phosphorylated Akt targets (Fig. 1B). The 35 kDa-band in the immuno-precipitated sample was excised from the gel and analyzed by mass spectrometry. Results showed that peptides from S6 were major components of the gel fragment contents (Table S1). Proteins around the same approximate size that directly interact with S6 were expected to also be present in the mass spectrometry sample. Indeed, the sample contained peptides from several other ribosomal proteins, as well as a mitochondrial membrane protein and a glycolytic enzyme (Table S1). Because the other precipitated ribosomal proteins are not known to be phosphoproteins, we concluded that the 35-kDa band recognized by the anti-phosphorylated-Akt-substrate antibody represented phosphorylated S6. The signal from the 35 kDa-band recognized by this antibody against phosphorylated Akt targets will be termed P-S6 in the following text for brevity.
Mammalian cells have 5 phospho-acceptor serine residues at the C terminus of S6 (Ruvinsky et al., 2005). In S6 of the model yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe, and in that of most other ascomycetous fungi, two of the homologous serine residues are conserved phosphorylation targets (Fig. S1); the C-terminal extension carrying three additional phospho-acceptor serine residues in mammalian S6 is not present in fungi (Meyuhas, 2008). Intriguingly, in C. albicans S6, one of the conserved serine residues is replaced with a glutamate, suggesting a phospho-mimetic substitution of one of the phospho-acceptor serines during C. albicans evolution. This substitution of glutamate for serine is conserved among close relatives of C. albicans belonging to the non-meiotic subclade within the CTG clade of Ascomycota, but not among the CTG clade members with a full sexual cycle (Fig. S1; (Fitzpatrick et al., 2006)). The glutamate substitution still permitted recognition of the C. albicans S6 epitope containing phosphorylated serine 233, by the anti-phosphorylated-Akt-substrate antibody that recognizes S. pombe P-S6 (Nakashima et al., 2010).
TOR modulation elicits corresponding changes in P-S6 levels
S6 interacts with mRNA, tRNA, and translation initiation factors and is thought to co-regulate translational activation under certain conditions (Nygard & Nilsson, 1990; Han et al., 2014). The phosphorylation state of S6 may reflect activity of cellular growth processes, as mice expressing non-phosphorylatable S6 are abnormally small and have altered insulin responses (Ruvinsky et al., 2005); however, the mechanism of this link is controversial (Ruvinsky & Meyuhas, 2006). The S6 kinase (S6K) is a well-characterized TOR effector in mammalian cells, and plays a key role in regulating translational activation. Recent work shows that TOR regulates S6 phosphorylation in S. pombe (Nakashima et al., 2010).
We examined the effect of modulating TOR activity on C. albicans P-S6 by Western blot analysis using the anti-phosphorylated-Akt-substrate antibody. TOR was pharmacologically inhibited by rapamycin treatment of cells actively growing in rich medium. When wild-type cells were exposed to rapamycin at a high concentration of 50 ngmL-1, P-S6 levels were at a barely detectable baseline (Fig. 2A). In contrast, a heterozygous mutant containing a rapamycin-resistant allele of TOR1, TOR1-1 (Cruz et al., 2001), showed robust P-S6 levels upon exposure to this rapamycin concentration. Rapamycin did not affect total S6 levels in wild-type or TOR1-1/TOR1 cells under these conditions, indicating that Tor inhibition had a post-translational effect on S6 (Fig. 2A).
Fig. 2.
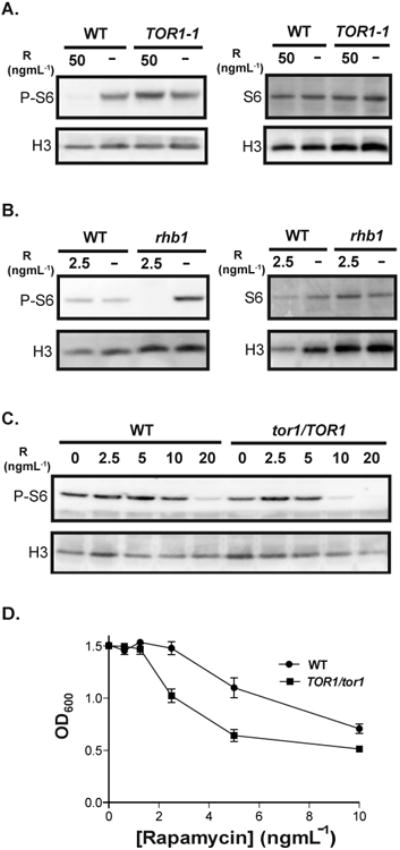
TOR inhibition by rapamycin affects P-S6 levels in C. albicans yeast cells.
A. Wild-type SC5314 and a rapamycin-resistant TOR1-1/TOR1 mutant were grown in YPD and treated with rapamycin (50 ngmL-1) for 1 hour at 30°C. The same volume of vehicle, 90% ethanol, was added to the control cultures (“-”). Cell lysates were probed for P-S6, total S6, and the loading control histone H3 with anti-phosphorylated-Akt-substrate, anti-S6, and anti-H3 antibody, respectively.
B. Wild-type SC5314 and a rapamycin-hypersensitive rhb1 (rhb1-/-) deletion mutant cells were treated with rapamycin (2.5 ngmL-1) and probed for P-S6, total S6, and H3, as described above.
C. Wild-type JKC1361 and isogenic TOR1 heterozygous (tor1/TOR1) JKC1345 cells were exposed to rapamycin at indicated concentrations and probed for P-S6, total S6, and H3, as described above.
D. The minimum inhibitory concentration (MIC) of rapamycin was monitored for wild-type JKC1361 and isogenic TOR1 heterozygous (tor1/TOR1) JKC1345 strain, using a modified standardized microdilution method adapted from the Clinical and Laboratory Standards Institute (CLSI) (CLSI, 2008). The OD600 of each strain was measured after 24 hours of growth in YPD containing two-fold dilutions of rapamycin at 30°C.
RHB1 encodes a plasma membrane-bound GTPase that activates Tor1 (Inoki et al., 2003; Tsao et al., 2009). An rhb1-/- deletion strain is hypersensitive to rapamycin (Tsao et al., 2009). At a low rapamycin concentration of 2.5 ngmL-1, the P-S6 level in the rhb1-/- strain was at a barely detectable baseline, whereas the level in rapamycin-treated wild-type cells remained unchanged relative to untreated cells (Fig. 2B). Similar to results obtained with the TOR1-1 cells, neither wild-type nor rhb1-/- cells showed a change in total S6 levels upon treatment with rapamycin for the duration of the experiment (Fig. 2B). Hence, in the examined time frame, TOR regulated S6 phosphorylation without having a discernible effect on total S6 protein levels.
Wild-type and isogenic tor1/TOR1 heterozygous deletion mutant strains were tested to compare their rapamycin-sensitivities with respect to P-S6. Cells actively growing in rich medium were exposed to rapamycin in two-fold concentration increments, beginning at 2.5 ngmL-1. The P-S6 level in tor1/TOR1 mutant cells dropped at a lower concentration of rapamycin (10 ngmL-1) than in wild-type cells (Fig. 2C). These results were consistent with the tor1/TOR1 heterozygous strain having a lower minimum inhibitory concentration (MIC) of rapamycin than that of an isogenic wild-type strain, as measured by monitoring OD600 in the presence of 2-fold increments of rapamycin concentration (Fig. 2D); the TOR1-1/TOR1 cells showed rapamycin-resistance under the same experimental conditions (data not shown).
P-S6 levels respond to the presence and quality of nitrogen sources
Cells were exposed to different nitrogen sources in defined minimal medium to physiologically modulate TOR activity, and the resulting effect on P-S6 was investigated. In contrast to cells grown in ammonium sulfate, the P-S6 signal was absent in cells grown in medium without a nitrogen source (Fig. 3A). When ammonium sulfate was added to the nitrogen-starved cultures, the P-S6 signal appeared in the re-fed cultures within the time window examined (30 minutes). In contrast, in cells that continued growth in nitrogen-free medium, P-S6 remained at barely detectable levels (Fig. 3A). The P-S6 signal therefore responded to modulation of TOR activity through nitrogen-starvation and -repletion.
Fig. 3.
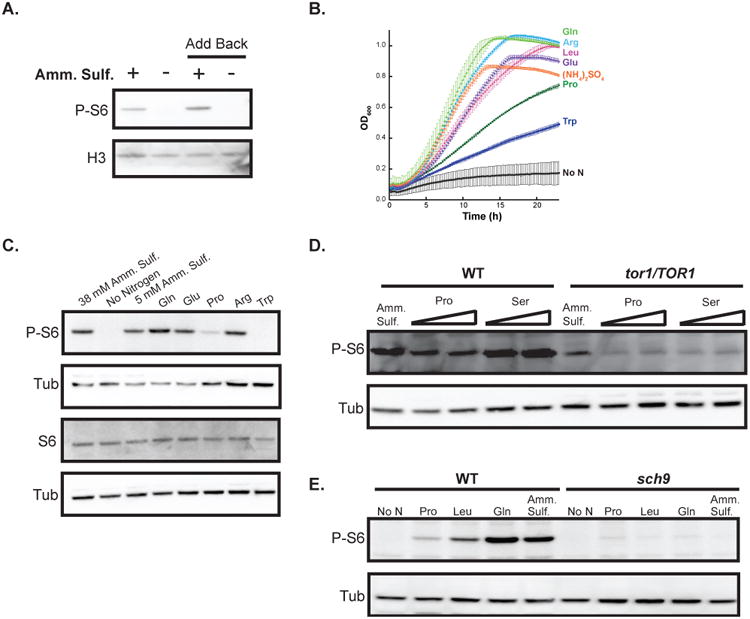
Preferred and less-favored nitrogen sources elicit congruent P-S6 and global growth responses.
A. Wild-type SC5314 cells were starved for nitrogen by growing in YNB containing glucose (2%) without ammonium sulfate (“-”) for 1 hour at 30°C; as a control, cells were grown in medium supplemented with ammonium sulfate (38 mM) (“+”). After 1 hour, ammonium sulfate was added to one culture of nitrogen-starved cells to a final concentration of 38 mM (“Add back “+””) and water was added to a second culture (“Add back “-””) as a control; all cells were then grown at 30°C for 30 minutes. Cell lysates were probed for P-S6 and H3.
B. Wild-type SC5314 cells were grown at 30°C without shaking in YNB with glucose (2%) containing 100 mM MES Buffer at pH 5, supplemented with ammonium sulfate (38 mM) or one of the following amino acids: glutamine, arginine, leucine, glutamate, proline, tryptophan (10 mM); a control sample was grown in the absence of a nitrogen source. The OD600 of the cells in the different culture conditions were monitored in 15-minute intervals for about 24 hours.
C. Exponentially-growing wild-type SC5314 cells were transferred to YNB containing glucose (2%) and supplemented with the indicated nitrogen sources: ammonium sulfate (5 or 38 mM), or one of the following amino acids glutamine, glutamate, proline, arginine, or tryptophan (10 mM); a culture was also set up in medium without a nitrogen source. All cultures were grown for 1 hour at 30°C and cell lysates were probed on separate blots for P-S6 (top) and total S6 (bottom); for the loading control, anti-tubulin antibody was used on both blots.
D. Exponentially-growing wild-type JKC1361 and isogenic TOR1 heterozygous (tor1/TOR1) JKC1345 strains were transferred to YNB containing glucose (2%) and supplemented with the indicated nitrogen sources: ammonium sulfate (38 mM) or proline (5 or 10 mM) or serine (5 or 10 mM) and grown for 1 hour at 30°C. Cell lysates were probed for P-S6 and tubulin.
E. Exponentially-growing wild-type JKC317 and isogenic sch9 deletion (sch9-/-) CCS3 strains were transferred to YNB containing glucose (2%) and supplemented with the indicated nitrogen sources: ammonium sulfate (5 mM) or one of the following amino acids proline, leucine, or glutamine (10 mM); cultures were also set up in medium containing no nitrogen source. All cultures were grown for 1 hour at 30°C and cell lysates were probed for P-S6 and tubulin.
To examine global growth responses to individual nitrogen sources, proliferation rates of wild-type cells grown in different nitrogen sources were monitored. Cells grown in defined medium with preferred nitrogen sources, such as glutamine and arginine, grew more rapidly than cells grown in poorer nitrogen sources, such as proline and tryptophan (Fig. 3B; (Liao et al., 2008)). P-S6 levels corresponded to the growth rates of cells utilizing a specific nitrogen source; for instance, robust P-S6 levels were present in cells grown in medium with glutamine as a nitrogen source and substantially lower levels were detected in cells grown in medium with proline (Fig. 3C). Total S6 levels did not vary between cultures grown in different nitrogen sources under these conditions (Fig. 3C).
Lack of one TOR1 allele resulted in a reduction of P-S6 levels in cells grown in defined minimal media, irrespective of the nitrogen source (Fig. 3D). A low baseline level of P-S6 was seen in the tor1/TOR1 heterozygote, which did not appear to be affected by the quality of the nitrogen source. This result was consistent with haploinsufficiency of the tor1/TOR1 strain for P-S6 levels and for growth, as detected by the MIC, during TOR inhibition with rapamycin (Figs. 2C, D). Hence, both TOR1 alleles were required for physiologic regulation of S6 phosphorylation, and for normal resistance to rapamycin.
In S. cerevisiae, the AGC kinase Sch9 has been shown to be a direct TORC1 substrate, functioning as a kinase for S6 (Urban et al., 2007). We postulated that Sch9 could play a similar role in C. albicans and tested P-S6 levels in an sch9 deletion strain (Stichternoth et al., 2011) grown in different nitrogen sources. P-S6 levels in sch9-/- were barely detectable, irrespective of the nitrogen source (Fig. 3E); in contrast, robust P-S6 levels were detected in wild-type cells grown under the same conditions. These results were consistent with Sch9 being a major kinase of S6 in C. albicans. As observed with the tor1/TOR1 heterozygous strain, a low level of P-S6 was observed in the sch9 deletion strain under all tested conditions. The sch9 deletion strain was also hypersensitive to rapamycin with respect to growth, as determined by the MIC (Fig. 6A). From these results, we concluded that the phosphorylation state of S6 could be used as a surrogate marker for growth programs controlled by TOR.
Fig. 6.
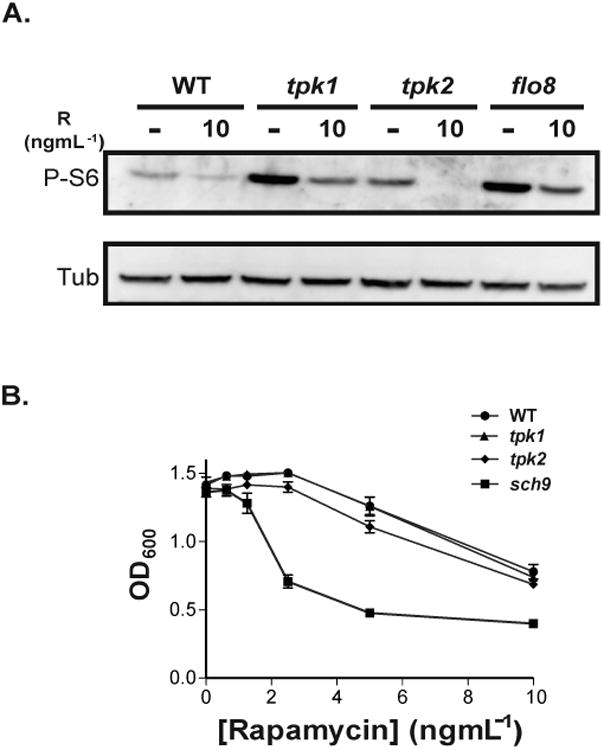
TOR inhibition decreases P-S6 and growth in wild-type as well as in Protein Kinase A (PKA) pathway mutant cells.
A. Exponentially growing wild-type JKC317 and isogenic strains with deletions in the PKA pathway components: tpk1 (tpk1-/-) (strain HPY300U), tpk2 (tpk2-/-) (strain HPY400U), and flo8 (flo8-/-) (strain JKC1467), were transferred to YNB (regular) containing glucose (0.3%) and grown for 1 hour at 30°C in the absence (“-“) or presence of rapamycin (10 ngmL-1); vehicle (90% ethanol) was added to the control cultures (“-”). Cell lysates were probed for P-S6 and tubulin.
B. The minimum inhibitory concentration (MIC) of rapamycin was determined for wild-type JKC317 and isogenic strains with deletions in sch9 (sch9-/-) (strain CCS3) or each of the PKA catalytic subunits: tpk1 (tpk1-/-) (strain HPY300U) or tpk2 (tpk2-/-) (strain HPY400U), using a modified standardized microdilution method as per the Clinical and Laboratory Standards Institute (CLSI) (CLSI, 2008). The OD600 of each strain was measured after 24 hours of growth in YPD containing two-fold dilutions of rapamycin at 30°C.
The P-S6 signal correlates with translation of a GFP reporter protein
Having shown that the P-S6 signal correlates with a predicted TOR activity state, we wanted to determine if it corresponds to directly observable translational activity. For this purpose, we used a heterologous green fluorescent protein (GFP), expressed from the inducible tetO promoter allowing for doxycycline-regulated transcription. Expression of the heterologous
GFP protein in this system was dependent only on the cells' translation rate
Cells were grown in rich medium without and with pharmacologic inhibition of translation. We used two drugs known to inhibit TOR, rapamycin and caffeine, and found that within 20 minutes after induction of GFP transcription by doxycycline, cells unexposed to inhibitors expressed robust levels of GFP protein with further increase over 10 to 20 additional minutes of incubation (Figs. 4A, B); these cells also had substantial P-S6 levels. Upon treatment with either drug, the doxycycline-treated cells contained low GFP levels and barely perceptible levels of P-S6. These findings indicated that the P-S6 signal was a highly sensitive readout of pharmacologic translational repression.
Fig. 4.
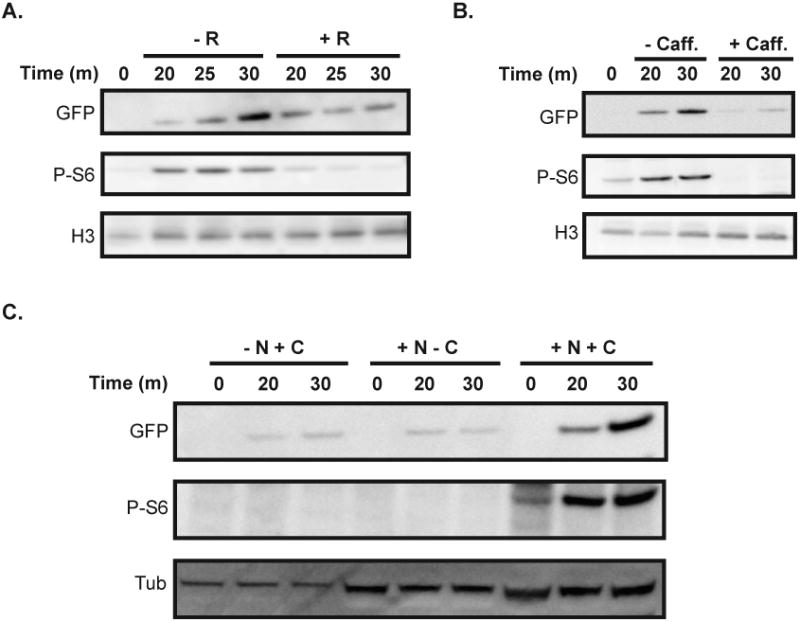
The signal from P-S6 corresponds to translational activity of a doxycycline-inducible GFP reporter.
A, B. Expression of GFP was induced in an exponentially-growing TETG25B strain with doxycycline (50 μgmL-1) in the presence and absence of rapamycin (50 ngmL-1) (A) or caffeine (20 mM) (B) for the indicated times in YPD at 30°C. Cell lysates were probed for GFP, P-S6, and H3 using anti-GFP, anti-phosphorylated-Akt-substrate, and anti-H3 antibody respectively.
C. An inducible tetO-GFP expressing strain (TETG25B) was starved for nitrogen or carbon by growing in YNB (without ammonium sulfate/nitrogen source) containing glucose (2%) (“- N + C”) or YNB (regular) containing no glucose (“+ N – C”), respectively, for 8 hours at 30°C; a control culture was set up in YNB containing both ammonium sulfate (38 mM) and glucose (2%) (“+ N + C”). Doxycycline (50 μgmL-1) was then added to each culture to induce GFP expression for the indicated times and the levels of GFP, P-S6, and H3 proteins were monitored.
We next wanted to confirm a correlation between the P-S6 signal and physiologic translational activation or repression by nutritional signals. Cells were starved of nitrogen or carbon for 8 hours, and then treated with doxycycline to induce GFP transcription. Twenty and 30 minutes after addition of doxycycline, cells starved for either nitrogen or carbon contained low levels of GFP, indicating slow translation rates. These cells also displayed a very low P-S6 signal. In contrast, cells grown in medium with both nitrogen and carbon sources showed robustly increasing GFP levels with a parallel increase in P-S6 (Fig. 4C). We concluded that the P-S6 signal corresponded to translational activation or repression by physiologic signals, supporting the use of P-S6 as a readout of the anabolic state of the cell.
A signal from glucose, and PKA signaling, contributes to P-S6
In experiments correlating the P-S6 signal to GFP translation, we found both carbon and nitrogen sources to be necessary for translational activation. Because cellular growth requires energy as well as building blocks for biosynthetic processes, we examined the extent to which S6 phosphorylation depended on the availability of glucose. Wild-type cells exposed to 0.1% glucose (the concentration in normal human peripheral blood) did not show a detectable P-S6 signal (Fig. S2); in contrast, cells exposed to glucose at 0.3% showed a reproducible signal (Fig. 5). This response appeared highly sensitive to small increments in glucose concentration in the growth medium. No P-S6 was detectable in cells grown in media containing carbon sources such as galactose, maltose, or mannitol (Fig. S2), suggesting that the P-S6 signal is composed of responses to the presence of a preferred nitrogen source and to the preferred carbon source, glucose.
Fig. 5.
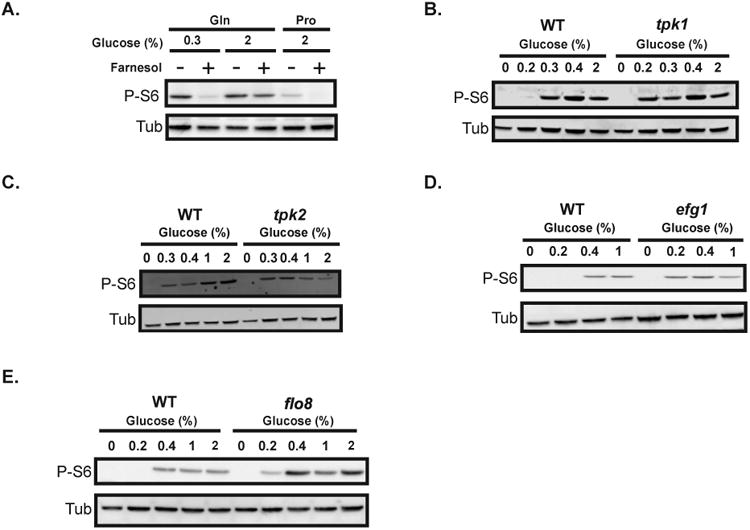
Glucose concentrations modulate levels of P-S6.
A. Exponentially-growing wild-type SC5314 cells were transferred to YNB (without ammonium sulfate) containing glucose at indicated concentrations (0.3 or 2%) and glutamine (10 mM) or proline (10 mM) as the nitrogen source. The cells were treated with farnesol (200 μM) (“+”) or equal volume of DMSO (“-”) as a control. All cultures were grown for 1 hour at 30°C. The lysates were probed for P-S6 and tubulin.
B-C. Exponentially-growing wild-type JKC317 and isogenic strains with single deletions in components of the PKA pathway were transferred to YNB with ammonium sulfate, containing the indicated glucose concentrations, and grown for 1 hour at 30°C. Cell lysates were probed for P-S6 and tubulin. The “0 time-point” represents cells prior to transfer into fresh media. Mutant strains contained deletions in either of the two catalytic subunits of PKA, TPK1 (tpk1-/-) (strain HPY300U) (B) or TPK2 (tpk2-/-) (strain HPY400U) (C); or deletions in the downstream PKA-regulated transcription factors EFG1 (efg1-/-) (strain DSC10) (D) or FLO8 deletion (flo8-/-) (strain JKC1467) (E).
We tested the effect of perturbing the cAMP/PKA pathway, known to respond to glucose availability, on P-S6. An inhibitor of C. albicans adenylate cyclase, the quorum-sensing sesquiterpene alcohol farnesol (Hornby et al., 2001; Ramage et al., 2002; Davis-Hanna et al., 2008; Hall et al., 2011), was used to inhibit PKA activity in wild-type cells. When cells exposed to farnesol in minimal medium containing glutamine and glucose (0.3%) were treated with farnesol, P-S6 was barely detectable. In contrast, farnesol treatment of cells in the same medium with a higher glucose level (2%) resulted in a modest decrease in P-S6 (Fig. 5A). The P-S6 level in cells grown in medium containing proline and glucose (2%) was drastically reduced upon farnesol treatment (Fig. 5A). Hence, lower baseline levels of P-S6 were reduced upon adenylate cyclase inhibition with farnesol, whereas the reduction was not discernible during maximal P-S6 induction (Fig. 5A). These results indicated that PKA signaling contributed to the P-S6 level in C. albicans.
To test whether P-S6 down-regulation is a response specific to nutrient stress, we examined the P-S6 signal during exposure to cell wall stress. When we induced the Protein Kinase C (PKC)/cell wall integrity (CWI) pathway with compounds that stress the cell wall, such as micafungin and calcofluor white, no discernible effect on P-S6 was observed (Fig. S4). All the tested drugs up-regulated levels of the phosphorylated MAP kinase Mkc1, indicating activation of the CWI pathway. Rapamycin treatment resulted in a sharp decrease in P-S6 (Figs. 2, S4) and an increase in phosphorylated Mkc1 (Fig. S4; (Tsao et al., 2009)). Hence, unlike the TOR and PKA pathways, perturbing the PKC/CWI pathway did not affect P-S6 under our tested conditions, and nutrient repletion versus –starvation seemed to be the key parameter driving P-S6 regulation.
Inhibition of PKA signaling with farnesol down-regulated P-S6 (Fig. 5A). To examine the contribution of individual components of the PKA pathway to this response, we tested the effects on P-S6 of genetically disrupting catalytic subunits of PKA, expecting that loss of subunits would decrease the P-S6 signal. However, in both tpk1-/- and the tpk2-/- deletion strains, P-S6 was detected in medium containing low concentrations of glucose (e.g., 0.2% and 0.3%), whereas P-S6 was lower in wild-type cells growing in these low glucose concentrations (Figs. 5B, C; S3A, B). No discernible difference in P-S6 between wild-type and tpk1-/- mutant strains was observed at higher glucose levels (Figs. 5B, S3A). However, in contrast to the tpk1-/- deletion strain, at higher glucose concentrations (e.g., 1 and 2%), tpk2-/- cells generated a weaker P-S6 signal relative to wild-type cells (Figs. 5C, S3B). The moderate increase in P-S6 at low glucose observed in PKA mutants was unexpected, but consistently reproducible. These results suggested that Tpk1 and Tpk2 both contribute to down-regulation of P-S6 in low glucose, and only Tpk2 is required to up-regulate P-S6 in high glucose concentrations (Figs. 5B, C; S3A, B).
The transcriptional regulator Efg1 acts downstream of PKA in C. albicans to control numerous cellular processes including filamentous growth and biofilm formation (reviewed in (Biswas et al., 2007)). To assess whether Tpk1 and/or Tpk2 affect S6 phosphorylation via Efg1, wild-type and an efg1-/- deletion mutant cells were exposed to increasing glucose concentrations. A higher P-S6 level was detected in the mutant at a low glucose concentration (e.g., 0.2%) than in the wild-type strain (Figs. 5D, S3C). At high glucose concentrations (e.g., 1%), the P-S6 level in the efg1-/- mutant strain was comparable to that in the wild-type strain, similar to results obtained with the tpk1-/- mutant strain (Figs. 5B, D; S3A, C). These results are consistent with the effect of Tpk1 on P-S6 depending on genes regulated by Efg1, but do not preclude the possibility of Tpk2 also acting via Efg1 on P-S6 in low glucose conditions.
Another transcription factor, Flo8, regulates a subset of Efg1-regulated genes (Cao et al., 2006; Hogan & Sundstrom, 2009). To test if Efg1 acts via Flo8 to regulate P-S6, P-S6 in a flo8-/- deletion mutant was monitored. An increase in P-S6 in the mutant strain compared to the wild type was observed in all glucose concentrations examined, indicating that Flo8 was required for down-regulation of P-S6 regardless of glucose availability (Figs. 5E, S3D), and therefore acted at least partially independent of Efg1 under these conditions.
PKA acts upstream of, or parallel to TOR, with respect to P-S6 modulation in low glucose
Rapamycin inhibits TOR by binding to, and blocking, Tor1 kinase in a complex with the FK506-binding protein (Heitman et al., 1991). Inhibition of Tor1 kinase activity by rapamycin decreases the P-S6 signal (Fig. 2) whereas eliminating either one of the PKA catalytic subunits increases the P-S6 signal at low glucose concentrations (Figs. 5B, C; S3A, B). If PKA effects on S6 phosphorylation occur downstream of Tor1, e.g., by modulating the S6 kinase, deletion of TPK1 or TPK2 is predicted to rescue the effect of Tor1 inhibition on P-S6. To examine the epistatic relationship between Tor1 and PKA perturbation in low glucose, we inhibited Tor1 with rapamycin in wild-type and in tpk1-/-, tpk2-/-, and flo8-/- cells (Fig. 6A). In each of the mutant strains, the P-S6 signal was increased compared to the wild-type strain when Tor1 was active. When Tor1 was blocked by rapamycin, P-S6 levels decreased in the PKA mutants as in the wild-type, indicating that Tor1 acts on P-S6 downstream of, or parallel to, PKA under these specific conditions.
PKA subunit deletion, tpk1-/- and tpk2-/-, strains were not hypersensitive to growth inhibition by rapamycin as determined by measurement of their MICs (Fig. 6B), nor were deletion mutants in EFG1 and FLO8 (data not shown). In contrast, the MIC of rapamycin for the sch9-/- deletion strain was considerably decreased (Fig. 6B). Therefore, the epistatic relationship of Tor1 to PKA pathway mutants with respect to P-S6 modulation was not due to global rapamycin hypersensitivity of these mutants.
Discussion
The TOR and cAMP/PKA pathways are two major nutrient-sensing pathways in eukaryotes, regulating processes such as cell growth and proliferation (Dechant & Peter, 2008; Loewith & Hall, 2011). TOR signaling has been shown to be important in morphogenesis, nutrient-sensing, and virulence of C. albicans (Cruz et al., 2001; Liao et al., 2008; Bastidas et al., 2009; Zacchi et al., 2010; Lu et al., 2011; Su et al., 2013). PKA signaling in C. albicans is of central importance in regulating morphogenesis and virulence, with important new findings continuing to emerge (Piispanen et al., 2013; Lindsay et al., 2014). Although both TOR and PKA respond to nutritional cues, the former is thought to sense nitrogen availability and the latter to respond to carbon sources. In the budding yeast S. cerevisiae, these pathways have overlapping targets in biological processes, such as protein synthesis and autophagy (Dechant & Peter, 2008; Zaman et al., 2008). Despite numerous lines of evidence linking them in S. cerevisiae (Pedruzzi et al., 2003; Santhanam et al., 2004; Schmelzle et al., 2004; Zurita-Martinez & Cardenas, 2005; Stephan et al., 2009; Ramachandran & Herman, 2011), a clear understanding of how these two important signaling pathways communicate remains elusive, suggesting that the two pathways are likely to interact at several levels.
We used an antibody developed against mammalian targets of Akt kinase, which has been shown to recognize phosphorylated S6 in S. pombe (Nakashima et al., 2010), to begin probing potential cross-talk between TOR and PKA signaling in C. albicans (Figs. 1-3). The phosphorylation state of the ribosomal protein S6 has been linked to anabolic processes such as translation, cell size, and cell proliferation in plants, yeast, and mammalian cells (Ruvinsky & Meyuhas, 2006; Boex-Fontvieille et al., 2013; Han et al., 2014). The phospho-acceptor serine residues of eukaryotic S6 are exposed on the small ribosomal subunit surface and therefore accessible to S6 kinases (Rabl et al., 2011), but how S6 phosphorylation potentially regulates translational activity remains unknown. Although S6 contacts both the A-site and the P-site in the assembled ribosome (Metspalu et al., 1978) and can be cross-linked to mRNA (Takahashi & Ogata, 1981), the phospho-acceptor serine residues of S6 are at least 130 Å from the decoding center on the back of the 40S subunit and therefore unlikely to directly interact with mRNAs or tRNAs, suggesting an indirect effect of their phosphorylation on translational activity (Rabl et al., 2011). Independently of the lack of mechanistic understanding, correlation of S6 phosphorylation with general anabolic activity has pragmatically been used in oncologic research to assess mTOR dependence of malignant tumors (Iwenofu et al., 2008) and to screen for chemotherapeutic drug targets (Zhang et al., 2008; Papageorgiou & Avruch, 2012).
S6K1 is directly phosphorylated by mammalian TOR. Activated S6K1, in turn, phosphorylates several targets, including ribosomal protein S6 (Dann et al., 2007; Powers, 2007). The S6K1 ortholog in S. cerevisiae, Sch9, is a direct substrate for TOR (Urban et al., 2007; Loewith & Hall, 2011; Kingsbury et al., 2014). Extrapolating from these findings in mammalian systems and in model yeasts, we used P-S6 as a surrogate marker for the activity of anabolic programs induced by TOR signaling.
We pharmacologically and physiologically inhibited Tor1 kinase, using rapamycin and nitrogen limitation respectively, and found that the P-S6 signal depends on TOR activity (Figs. 2-3). The levels of total S6 remained the same under our experimental conditions, indicating post-translational regulation of P-S6 by TOR.
A tor1/TOR1 heterozygous strain growing in rich medium had increased rapamycin sensitivity with respect to P-S6 levels, suggesting haploinsufficiency in cells lacking one TOR1 allele (Fig. 2C), which has been observed in other C. albicans mutants (Köhler & Fink, 1996; Uhl et al., 2003; Bharucha et al., 2011). Similarly, tor1/TOR1 cells were haploinsufficient for rapamycin-resistance with respect to global growth, as demonstrated by their decreased MIC (Fig. 2D). In minimal media, the haploinsufficiency phenotype of tor1/TOR1 cells was more pronounced: even in a preferred nitrogen source, the P-S6 signal in the heterozygous strain was weaker than in the wild-type strain (Fig. 3D). Hence, physiologic gene dosage of TOR1 is important for the cell to maintain normal P-S6 levels (Figs. 2-3). Low levels of P-S6 were present in the heterozygote when grown in different nitrogen sources suggesting that a baseline level of P-S6 may be maintained in the cell irrespective of TOR activity.
In S. cerevisiae, as noted above, Sch9 is the homolog of mammalian S6K, and is a direct substrate of TORC1 (Urban et al., 2007; Loewith & Hall, 2011). If CaSch9 is an S6 kinase in C. albicans, a prediction that follows is that P-S6 levels would be low in its absence. Indeed, P-S6 levels in an sch9-/- deletion strain (Stichternoth et al., 2011) were barely detectable in our assay (Fig. 3E). Moreover, the sch9-/- deletion strain was hypersensitive to rapamycin in rich medium (Fig. 6B). S6 regulation via TOR phosphorylation of a major S6K (Sch9) may therefore be evolutionarily conserved in C. albicans. Further work will be required to test if C. albicans Sch9 is also a direct substrate of Tor1.
Biosynthetic processes, such as translation, need the requisite monomeric building blocks and an efficient energy source to fuel their polymerization. Activating protein synthesis in the presence of high amino acid levels, but limiting amounts of an efficient carbon source, would expose the cell to the risk of having insufficient energy to complete the process and to be left with large numbers of partially-synthesized polypeptides. Experiments directly correlating translation of a heterologous gene, GFP, with the P-S6 signal, demonstrated that translational activation depends on the concurrent availability of a carbon and a nitrogen source (Fig. 4C).
Glucose is the preferred carbon and energy source of most cells (Towle, 2005; Sabina & Brown, 2009). Organisms have evolved intricate mechanisms for sensing and acquiring this important substrate from the environment and for mounting an appropriate metabolic response based on its availability. TOR signaling is known to respond to presence of glucose for induction of hexose transporters in S. cerevisiae, and inhibition of TOR signaling with rapamycin represses expression of a low-affinity glucose transporter, HXT1 (Hardwick et al., 1999; Tomas-Cobos et al., 2005). In the model plant Arabidopsis thaliana, energy production from glucose, but not from other carbon sources, is a key regulator of TOR activity (Xiong et al., 2013).
We grew wild-type cells in different carbon sources and consistently observed detectable P-S6 only in cells grown in moderate-to-high glucose (≥0.3%) (Figs. 5, S2). In both S. cerevisiae and C. albicans, the PKA pathway plays a major role in glucose signaling, although key differences exist in how the pathway in the two organisms detects upstream signals (Lorenz & Heitman, 1997; Maidan et al., 2005; Sabina & Brown, 2009). When adenylate cyclase was inhibited by treatment with the auto-regulatory sesquiterpene alcohol farnesol, P-S6 levels dropped; this drop was rescued by increasing the glucose level (Fig. 5A). When the nitrogen source was switched from glutamine to the less-preferred proline, farnesol treatment resulted in decreased P-S6 even in the presence of high glucose. Hence, the TOR and PKA pathways appear to collaborate in P-S6 regulation, titrating it in response to availability of nitrogen and carbon sources, as both are required for successful completion of translation (Fig. 4).
Deletion mutants of each PKA catalytic subunit isoform, Tpk1 and Tpk2, contained increased P-S6 in low glucose relative to wild-type cells (Figs. 5B, C; S3A, B). At high glucose concentrations (1 and 2%), lack of either isoform had different effects: tpk1-/- had wild-type P-S6 levels whereas tpk2-/- had lower P-S6 levels relative to wild-type cells (Figs. 5B, C; S3A, B). Hence, the two isoforms have distinct roles in modulating P-S6.
Multiple studies have demonstrated distinct and overlapping functions of tpk1 and tpk2 isoforms in C. albicans (Bockmuhl et al., 2001; Park et al., 2005; Giacometti et al., 2009; Hogan & Sundstrom, 2009; Giacometti et al., 2011; Fanning et al., 2012; Schaekel et al., 2013). Although an essential requirement for growth can be fulfilled by one allele of either isoform, Tpk2, and specifically its catalytic domain, is needed for hyphal growth in liquid, and Tpk1 is required for hyphal growth on solid medium (Bockmuhl et al., 2001). In contrast, the N-terminal domain of Tpk2 is needed for agar invasion by yeast cells (Bockmuhl et al., 2001). Although Tpk2 is thought to be the more abundant isoform, Tpk1 is important for specific stress responses such as osmotic or oxidative stress (Giacometti et al., 2009), for normal resistance to the cell wall stressor caspofungin, and for normal biofilm formation and adherence on solid substrates such as silicone (Fanning et al., 2012). Moreover, Tpk2 has been shown to confer heat sensitivity in cells expressing decreased levels of Tpk1, presumably by interacting with inappropriate substrates (Giacometti et al., 2009). Tpk1 and Tpk2 also have opposing functions in glycogen accumulation (Souto et al., 2006; Giacometti et al., 2009). Thus, Tpk1 and Tpk2 may be performing distinct functions in allowing cells to down-regulate P-S6 in response to low glucose, as one isoform was not able to compensate for the absence of the other one. Another possibility is that a specific interaction of the two Tpk isoforms may be needed for the cell to respond appropriately to different stimuli. According to this model, in our study, the absence of either isoform disrupted mutual regulation between Tpk1 and Tpk2 activities with resulting dysregulation of the remaining isoform. Consequently, the mutant cells could not appropriately modulate P-S6 in response to low glucose availability. These results add to the known complexity of distinct functions of the catalytic PKA subunit isoforms in C. albicans.
A downstream effector of PKA, the transcriptional regulator Efg1, plays important roles in morphogenesis of C. albicans (Stoldt et al., 1997). During hyphal growth, Efg1 activates hyphae-specific genes, such as the cell wall protein genes and virulence factors HWP1 and ALS3 (Hogan & Sundstrom, 2009). In addition to activating transcription, Efg1 also represses genes involved in morphogenesis, depending on environmental cues and genetic backgrounds, and mutants in EFG1 have been shown to be defective in virulence (Lo et al., 1997; Biswas et al., 2007; Hogan & Sundstrom, 2009). P-S6 levels in the efg1-/- strain followed a pattern similar to that observed in the tpk1-/- mutant, with increased levels in low glucose (Figs. 5B, D; S3A, C). Hence, Tpk1 may act via Efg1 to down-regulate P-S6 levels in low glucose concentrations. In high glucose, lack of Tpk1 or Efg1 had no discernible effect on P-S6 modulation (Figs. 5B, D; S3A, C).
Efg1 regulates a subset of genes, such as HGC1, via another transcription factor, Flo8, and the two transcriptional regulators are thought to directly interact (Cao et al., 2006; Hogan & Sundstrom, 2009). Deletion of flo8 resulted in abnormally elevated P-S6 at all tested glucose concentrations (Figs. 5E; S3D). Hence, Flo8 appeared to restrain P-S6 irrespective of glucose levels, suggesting that Flo8 has a role independent of Efg1 in fine-tuning anabolic processes in the cell.
Of the PKA pathway components we examined, each gene was required to down-regulate P-S6 in low glucose. In contrast, only one of the four components, Tpk2, was unequivocally necessary to maximize P-S6 in high glucose. Because C. albicans in the human host more commonly finds itself in low-glucose than in high-glucose environments (Ene et al., 2012), we speculate that down-regulating anabolic processes during glucose limitation is at least as critical for overall fitness in C. albicans' diverse environments as up-regulating these processes during its abundance, as the cell apparently receives input from more signaling components when this energy source is scarce.
To examine if TOR activity is required for the effects of PKA subunits on P-S6, we tested if up-regulation of P-S6 in low glucose seen in the PKA subunit tpk1-/- and tpk2-/- mutants was sustained upon Tor1 inhibition by rapamycin. We found that P-S6 levels in tpk1-/- and tpk2-/-mutants were down-regulated to the same extent as in wild-type strains (Fig. 6A). These results were consistent with Tor1 being epistatic to or acting in parallel with the PKA catalytic subunits with respect to S6 phosphorylation. In contrast, PKA has been found to act upstream of, or parallel to Tor1 with respect to various processes regulated by both pathways in S. cerevisiae (Zurita-Martinez & Cardenas, 2005; Yorimitsu et al., 2007). The molecular mechanisms by which PKA and TOR signaling pathways intersect in C. albicans may differ with respect to each of their distinct target processes, such as anabolic activity or hyphal morphogenesis. They are also likely to differ from the mechanisms in model organisms, given the distinct lifestyle of C. albicans as a uniquely human host-adapted colonizer and occasional pathogen.
A single C. albicans strain will typically colonize its host at multiple sites, and become pathogenic only when loss of host defenses permits its invasion (Odds, 1984). Considering only its lifestyle as a commensal, the adaptability required of C. albicans is enormous, given the wide variety of nutritional conditions, their larger or smaller fluctuations at each site, and the differences between individual hosts, as reflected in the differences in bacterial microbiomes residing at each site (Zhou et al., 2014). For example, C. albicans colonizing the vagina experience a steady supply of glycogen and stable bacterial competitors with only slow fluctuations; in contrast, C. albicans colonizing the oral mucosa are confronted with wide rapid swings of availability of sugars and proteins, as well as constant introduction of new bacterial competitors in addition to the stable bacterial flora. In internal host compartments during invasive disease, C. albicans encounters a glucose concentration of ∼0.1% or ∼5.55 mM in extracellular fluid, i.e., blood plasma and interstitial fluid; in intracellular compartments, glucose concentration is even lower (Behjousiar et al., 2012). C. albicans must therefore flexibly respond to maximally utilize available resources (Askew et al., 2009) but should not commit to growth and replication when a requisite nutrient is scarce. In addition, when confronting live host cells, such as epithelial cells and macrophages, C. albicans has to adapt to host defense molecules like reactive oxygen and nitrogen species in addition to nutrient limitation (Lorenz & Fink, 2001; Lorenz et al., 2004; Barelle et al., 2006; Ramirez & Lorenz, 2007; Park et al., 2009). C. albicans strains, possessing a wide dynamic range of nutritional signaling and cross-talk between pathways that respond to different nutrients, are likely to be favored in the competition against other Candida strains and species, as well as against bacteria. For these reasons, decisions such as activation of translation are informed by a multiplicity of inputs including not just availability of amino acids, but also of the most efficient energy source, glucose. Disrupting the ability of C. albicans to optimize its growth and survival in the host will require more detailed understanding of these adaptive processes, and development of more simple methods to monitor their activity.
Experimental Procedures
Media and Buffers
YPD: 1% yeast extract, 2% peptone, 2% glucose.
YNB or YNB (regular): 3 mgmL-1 yeast nitrogen base (without amino acids or ammonium sulfate), 38 mM ammonium sulfate, 0.4 mM inositol.
Buffer S6: 50 mM Tris Cl pH 7.5, 150 mM NaCl, 5 mM EDTA, 10% Glycerol, 0.2% Nonidet P-40, 1 mM DTT, 1 mM PMSF, cOMplete Mini EDTA-free Protease Inhibitor Cocktail (Roche Applied Science), 0.1 mM sodium orthovanadate, 20 μM sodium glycerophosphate, 20 μM para-nitrophenyl phosphate, 20 μM sodium fluoride.
Strain Construction
JKC317 was constructed by transforming CAI-4 (Fonzi & Irwin, 1993) to uridine prototrophy with a BglII/PstI fragment of pLUBP. The IRO1 truncation of CAI-4 derivates was thereby also restored to wild type.
To make JKC917, an arginine and histidine auxotrophic strain containing tetR at the HIS1 locus, SN95 (Noble & Johnson, 2005) was transformed with pLC52 (Shen et al., 2008) and the FLP-NAT1 cassette was induced to recombine, eliminating the NAT1 selectable marker. JKC1345, containing a deletion of the TOR1 orf except for its 5′ 1143 bp, was made from JKC917 by transforming with a PCR product of fJK1142 and rJK1143 with template pFA-ARG4 (Gola et al., 2003). JKC1361 was the arginine prototrophic control for JKC1345 and was made by transforming JKC917 with a PCR product of fJK1184 and rJK1186 with genomic DNA of SC5214 as template.
JKC1467 was made by transforming CCF4 (Cao et al., 2006) to uridine prototrophy with a BglII/PstI fragment of pLUBP. As in JKC317, the IRO1 truncation of CAI-4 derivates was thereby also restored to wild type.
Immuno-precipitation and mass spectrometry
Wild-type SC5314 cells were grown over-night in YPD at 30°C, washed and resuspended in PBS, and diluted into fresh YPD to a starting OD600 of 1. The cells were allowed to grow for about 1.5 hours at 30°C.
Cells were washed in cold water and resuspended in buffer S6. After cell lysis using the MagNA Lyser (Roche Applied Science), anti-phospho (S/T) Akt substrate rabbit polyclonal antibody (Cell Signaling Technology, catalog # 9611) was added (1:25 dilution) to the lysate, followed by overnight incubation at 4°C with gentle rocking.
The lysate and antibody were then incubated with Protein G-Agarose beads (Roche Applied Science), which were pre-equilibrated in buffer S6, for about 3.5 hours at 4°C with gentle rocking. The beads were washed three times in buffer S6 and boiled for about 6 minutes in NuPAGE LDS Sample buffer (Life Technologies) containing DTT. The eluted immuno-precipitated proteins were then separated by SDS-PAGE. The gel band around 35 kDa was excised and analyzed by mass spectrometry at Taplin Mass Spectrometry Facility at Harvard Medical School (Boston, Massachusetts).
The immuno-precipitated proteins were also analyzed for (total) S6 by Western blotting (described below).
Phosphatase Treatment
Lysate from wild-type SC5314 (30 μg of protein) was incubated with λ Protein Phosphatase (800 units) (New England Biolabs), or an equal volume of water as a control, in NEBuffer for PMP and MnCl2 (1 mM) at 30°C for 40 minutes. Proteins in each sample were precipitated using tricholoroacetic acid and analyzed for P-S6 by Western blotting (described below).
Western Blotting
All cultures were grown and processed in parallel and blotted onto the same membrane in each experiment comparing different conditions (wild-type versus mutant, vehicle versus drug exposure, poor versus rich nutrients). All Western blot experiments were repeated at least twice for a total of at least 3 biological replicates.
Proteins in lysates were separated by SDS-PAGE and transferred to PVDF membranes, which were probed for P-S6 using anti-phospho (S/T) Akt substrate rabbit polyclonal antibody (Cell Signaling Technology, catalog # 9611). Total S6 was monitored using an anti-S6 sheep polyclonal antibody (R&D, catalog # AF5436). GFP was monitored using anti-GFP mouse monoclonal antibody (Roche, catalog # 11814460001). Anti-histone H3 rabbit polyclonal antibody (Cell Signaling Technology, catalog # 4499) or anti-tubulin rat monoclonal antibody (Abcam, catalog # ab6161) was used to monitor the loading controls. Secondary antibodies used were bovine anti-rabbit antibody (Santa Cruz Biotechnology, catalog # 2370) and goat anti-rat antibody (Abcam, catalog # ab97057), except for the blots shown in Figures 5B and 5C where IRDye® 680LT donkey anti-rabbit (Li-Cor, catalog # 926-68023) and IRDye® 800CW goat anti-rat (Li-Cor, catalog # 926-32219) were used. The blots were imaged either using Li-Cor Odyssey CLx Infrared Imaging System (Western blots shown in Figs. 5B, C) or the KODAK Image Station 4000MM (all other Western blots). The blots were quantitated by densitometry using ImageJ analysis software.
Monitoring Growth Rates
Wild-type SC5314 cells were resuspended and washed in PBS, followed by dilution, to a starting OD600 of 0.1, into YNB (without ammonium sulfate) containing 100 mM MES Buffer at pH 5, glucose (2%), and a single amino acid (10 mM) or ammonium sulfate (38 mM) as the nitrogen source. A control culture was also set up in medium without a nitrogen source. The OD600 of the cultures was monitored at 15-minute time intervals for ∼24 hours at 30°C without shaking on a TECAN infinite M200 plate reader.
Growth Conditions: Rapamycin-treatment
Cells were grown over-night in YPD at 30°C, washed and resuspended in PBS, and diluted into fresh YPD to a starting OD600 of 0.2. For the experiment with PKA pathway component mutants, the over-night cells were diluted into YNB with 2% glucose. The samples were incubated at 30°C for 4 hours. Appropriate volumes of rapamycin (LC Laboratories) were added after the 4 hour-incubation. Equal volumes of 90% ethanol were added to the control cultures without rapamycin. For the experiment with PKA pathway component mutants, prior to addition of rapamycin/90% ethanol, the cells were washed twice with 0.5× YNB (regular) with no carbon source and then resuspended into YNB with 0.3% glucose. All cultures were incubated for 1 hour at 30°C.
After washing with cold water, cells were resuspended in buffer S6 and lysed using the MagNA Lyser (Roche Applied Science). The lysates were analyzed for P-S6 and total S6 by Western blotting (described above).
Growth Conditions: Different nitrogen sources and/or glucose concentrations
Cells were grown over-night in YPD at 30°C, washed and resuspended in PBS, and diluted into YNB with glucose (2%) to a starting OD600 of 0.2. The diluted cells were incubated for 4 hours at 30°C and washed with 0.5× YNB (without ammonium sulfate) medium with glucose (1%) (experiments testing different nitrogen sources/nitrogen starvation) or with 0.5× YNB (regular) with no carbon source (experiments testing various glucose concentrations). When both glucose concentration and the nitrogen source needed to be switched (farnesol-treatment experiments), cells were washed with 0.5× YNB (without ammonium sulfate) and no carbon source.
To investigate the effect of different nitrogen sources on S6, after the 4-hour incubation and wash steps, cells were resuspended in YNB (without ammonium sulfate) containing a single amino acid (10 mM) or ammonium sulfate (5 or 38 mM) as the sole nitrogen source. Cultures were allowed to grow for 1 hour at 30°C and harvested.
For the nitrogen-starvation and re-feeding experiment, after the 4-hour incubation and wash steps, cells were resuspended in either YNB (regular) medium with glucose (2%) (1× culture) or YNB (without ammonium sulfate) medium with glucose (2%) (3× cultures) and incubated for 1 hour at 30°C. After 1 hour, ammonium sulfate was added to one of the cultures of YNB (without ammonium sulfate) medium with glucose (2%) to a final concentration of 38 mM; to another culture of YNB (without ammonium sulfate) medium with glucose (2%), an equal volume of water was added. All four cultures were grown for 30 minutes at 30°C and then harvested.
To test the effect of different glucose levels on S6, after the 4-hour incubation and wash steps, cells were resuspended in YNB (regular) containing different (indicated) concentrations of glucose as the sole carbon source. Cultures were allowed to grow for 1 hour at 30°C and harvested.
For experiments monitoring P-S6 in different nitrogen sources and varying glucose concentrations with and without farnesol, after the 4-hour incubation and wash steps, cells were resuspended in YNB (without ammonium sulfate) with glutamine (10 mM) and glucose (0.3 or 2%); or resuspended in YNB (without ammonium sulfate) with proline (10 mM) and glucose (2%); or resuspended in YNB (regular) with glucose (0.4%). Farnesol (Sigma-Aldrich) was added to one culture at each test condition to a concentration of 200 μM; equal volume of DMSO (vehicle) was added to a second culture at each test condition. All cultures were allowed to grow for 1 hour at 30°C and harvested.
During harvesting, cells were washed with cold water, followed by resuspension in buffer S6 and lysis using the MagNA Lyser (Roche Applied Science). The lysates were then analyzed for P-S6 or total S6 by Western blotting (described above).
Monitoring Translation of a Heterologous GFP Reporter
For experiments using rapamycin and caffeine treatments, TETG25B cells were grown over-night in YPD at 30°C, washed and resuspended in PBS, and diluted into fresh YPD to a starting OD600 of 0.2. After incubation for 4 hours at 30°C, doxycycline was added to a final concentration of 50 μgmL-1. Rapamycin or caffeine was added to a final concentration of 50 ngmL-1 and 20 mM respectively; equal volumes of 90% ethanol or water were added to the control cultures without rapamycin and caffeine respectively. The cultures were allowed to grow at 30°C in the dark and harvested at different indicated time points.
For experiments under nitrogen- or carbon-starving conditions, TETG25B cells were grown over-night in YPD at 30°C, washed and resuspended in PBS, and diluted to a starting OD600 of 0.2 into the different media: YNB (regular) with glucose (2%) or YNB (regular) with no carbon source, or YNB (without a nitrogen source) with glucose (2%). The cultures were grown at 30°C for about 8 hours before addition of doxycycline to a final concentration of 50 μgmL-1. The cells were allowed to continue growth at 30°C in the dark and harvested at the time points indicated.
During harvesting, cells were washed with cold water, followed by resuspension in buffer S6 and lysis using the MagNA Lyser (Roche Applied Science). The lysates were then analyzed for GFP, P-S6, H3, or tubulin by Western blotting (described above).
Measuring minimum inhibitory concentration (MIC) of Rapamycin
The CLSI microdilution method for monitoring susceptibility of yeast to antifungal agents (CLSI, 2008) was modified to measure the minimum inhibitory concentration (MIC) of rapamycin. Briefly, cells from YPD-agar plates were resuspended, washed in PBS and diluted in microtiter plates to an OD600 of 0.1 into fresh YPD broth containing two-fold serial dilutions of rapamycin. The plates were incubated at 30°C for 24 hours without shaking. After 24 hours, the OD600 was measured, after brief shaking, using a TECAN infinite M200 plate reader.
Supplementary Material
Fig. S1. The amino acid sequence of ribosomal protein Rps6 (S6) is highly conserved in fungi and animals. The conserved serine residues at the C-terminal region are boxed in red. One of these serine residues is replaced by a glutamate in C. albicans S6 and in its close relatives in the non-meiotic subclade within the CTG clade of Saccharomycotina.
Fig. S2. P-S6 responds to a high level of the preferred carbon source, glucose. Exponentially-growing wild-type SC5314 cells were transferred to YNB (regular) containing different carbon sources as indicated: glucose (“Gluc”) (0.1 or 2%), galactose (“Gal”) (2%), maltose (“Mal”) (2%), or mannitol (“Man”) (1%); a culture was also set up in medium without a carbon source. All cultures were grown for 1 hour at 30°C and cell lysates were probed on separate blots for P-S6 (top) and total S6 (bottom); tubulin was the loading control on both blots. The “0 time-point” indicates lysate from cells prior to transfer into media containing the different carbon sources.
Fig. S3. Exponentially-growing wild-type JKC317 and isogenic strains with single deletions in components of the PKA pathway were transferred to YNB (regular) containing different glucose concentrations and grown for 1 hour at 30°C. Cell lysates were analyzed by Western blotting and probed for P-S6 and tubulin (loading control). The P-S6 and tubulin signals were quantitated by densitometry using the ImageJ analysis software; the y-axis depicts the P-S6 signal normalized to the tubulin signal at each indicated glucose concentration (depicted on the x-axis). Mutant strains tested contained deletions in either of the two catalytic subunits of PKA, TPK1 (tpk1-/-) (strain HPY300U) (A) or TPK2 (tpk2-/-) (strain HPY400U) (B); or deletions in the downstream PKA-regulated transcription factors EFG1 (efg1-/-) (strain DSC10) (C) or FLO8 deletion (flo8-/-) (strain JKC1467) (D). WT is shown in orange and the mutants are shown in purple. The actual blots are shown in Figs. 5B-E.
Fig. S4. P-S6 does not appear to respond to drugs that disrupt cell wall integrity. Exponentially-growing wild-type SC5314 cells were exposed to indicated drugs rapamycin (20 ngmL-1), micafungin (“M”) (20 ngmL-1), calcofluor white (“CW”) (200 μgmL-1), and congo red (“CR”) (100 μgmL-1) (“+” samples) in YPD for 2 hours at 30°C in the dark. The control samples (“-”) were treated with the vehicles (90% ethanol for rapamycin, saline for micafungin, water/sodium hydroxide for calcofluor white, and water for congo red) instead of the drugs and grown under the same conditions. Cell lysates were probed for P-S6, phosphorylated Mkc1, and histone H3 with anti-phosphorylated-Akt-substrate antibody, anti-phospho-p44/42 MAPK (Erk1/2) (Thr 202/Tyr204) antibody, and anti-H3 antibody, respectively.
Table S1. Mass Spectrometry Identified Proteins (around 35 kDa) Immunoprecipitated using anti-Phospho(S/T) Akt Substrate Antibody.
Fig. 7.
A model depicting cross-talk between TOR and PKA pathways in C. albicans. Tor responds to the availability of nitrogen sources to control phosphorylation of S6 via the S6 kinase ortholog, Sch9. By a mechanism not yet elucidated, glucose availability causes increased adenylate cyclase activity and subsequent cAMP production, thereby activating the catalytic subunits Tpk1/Tpk2 of PKA. PKA modulates P-S6 levels, either independently of the TOR pathway or by acting on an upstream TOR pathway component. Dashed arrows indicate as yet undefined mechanisms.
Table 1.
Strains of Candida albicans used in this study.
| C. albicans Strain | Parent | Genotype | Source (Reference) |
|---|---|---|---|
| SC5314 | Patient isolate | Wild-type | (Fonzi & Irwin, 1993) |
| CAI-4 | SC5314 |
ura3∷λimm434/ura3∷λimm434 iro1∷λimm434/iro1∷λimm434 |
(Fonzi & Irwin, 1993) |
| JKC317 | CAI-4 |
URA3/ura3∷λimm434 IRO1/iro1∷λimm434 |
This work |
| JKC917 | SN95 |
his1/his1∷FRT-tetR arg4/arg4 URA3/ura3∷λimm434 IRO1/iro1∷λimm434 |
(Noble & Johnson, 2005); this work |
| JRB12 | SC5314 | TOR1-1/TOR1 | (Cruz et al., 2001) |
| CCT-D1 | SC5314 | rhb1∷FRT/rhb1∷FRT | (Tsao et al., 2009) |
| JKC1345 | JKC917 |
tor1∷ARG4/TOR1 his1/his1∷FRT-tetR arg4/arg4 URA3/ura3∷λimm434 IRO1/iro1∷λimm434 |
This work |
| JKC1361 | JKC917 |
his1/his1∷FRT-tetR ARG4/arg4 URA3/ura3∷λimm434 IRO1/iro1∷λimm434 |
This work |
| CCS3 | CAI-4 |
sch9∷hisG/sch9∷hisG ura3∷λimm434/ura3∷λimm434∷ URA3 |
(Stichternoth et al., 2011) |
| DSC10 | CAI-4 |
efg1∷hisG/efg1∷hisG URA3/ura3∷λimm434 IRO1/iro1∷λimm434 |
(Park et al., 2005) |
| HPY300U | CAI-4 |
tpk1∷hisG/tpk1∷hisG URA3/ura3∷λimm434 IRO1/iro1∷λimm434 |
(Park et al., 2005) |
| HPY400U | CAI-4 |
tpk2∷hisG/tpk2∷hisG URA3/ura3∷λimm434 IRO1/iro1∷λimm434 |
(Park et al., 2005) |
| CCF4 | CAI-4 |
flo8∷hisG/flo8∷hisG ura3∷λimm434/ura3∷λimm434 iro1∷λimm434/iro1∷λimm434 |
(Cao et al., 2006) |
| JKC1467 | CCF4 |
flo8∷hisG/flo8∷hisG URA3/ura3∷λimm434 IRO1/iro1∷λimm434 |
This work |
| TETG25B | CAI-4 |
ADH1/PADH1∷tetRinducible-tetO-GFP-URA3 ura3∷λimm434/ura3∷λimm434 |
(Park & Morschhauser, 2005) |
Table 2.
Plasmids used in this study.
| Plasmid | Description | Source (Reference) |
|---|---|---|
| pLUBP | URA3 in LITMUS28 | Gift from William A. Fonzi |
| pFA-ARG4 | ARG4 in pFA | (Gola et al., 2003) |
| pLC52 | his1∷tetR-FLP-CaNAT1 | (Shen et al., 2008) |
Table 3.
Oligonucleotides used in this study.
| Primer name | Sequence | Purpose |
|---|---|---|
| fJK1384 | ggtggtgcaatgggttatc | Confirm integration of pLUBP BglII/PstI fragment at 5′ end, forward primer |
| rJK1379 | ctaactcgttaaagattattc | Confirm integration of pLUBP BglII/PstI fragment at 5′ end, reverse primer |
| fJK1385 | ctaacatttctgctactgtcag | Confirm integration of pLUBP BglII/PstI fragment at 3′ end, forward primer |
| rJK1382 | gcttatgttcgagctcttgc | Confirm integration of pLUBP BglII/PstI fragment at 3′ end, reverse primer |
| fJK1142 | gttgaagaaatacaagtcccagaataacgagactgcaaataccgataagagtgccatatttaaaagcatagggttgatcgctttggaggttggcaatcaggaagcttcgtacgctgcaggtc | Delete TOR1 using pFA marker cassettes, forward primer |
| rJK1143 | atggcaatagctcttacaacaataatatatcaattctttatatttccctttataaaatagttacacataccatacttaacgacacatgacgatactcaacctctgatatcatcgatgaattcgag | Delete TOR1 using pFA marker cassettes, reverse primer |
| fJK1152 | catggatccttattgacgtac | Confirm deletion of TOR1 at 5′ end, forward primer |
| rJK1079 | gacctgcagcgtacgaagcttc | Confirm deletion of TOR1 at 5′ end, reverse primer |
| fJK1080 | ctcgaattcatcgatgatatcaga | Confirm deletion of TOR1 at 3′ end, forward primer |
| rJK1149 | cgctatagagaacttctgacc | Confirm deletion of TOR1 at 3′ end, reverse primer |
| fJK1184 | gaatccacaatcgtatatgaac | Amplify ARG4 for transformation to arginine prototrophy, forward primer |
| rJK1186 | gaatatagtgatgatgaggatg | Amplify ARG4 for transformation to arginine prototrophy, reverse primer |
| fJK1185 | gacatattgaccgacataattc | Confirm integration of ARG4 at 5′ end, forward primer |
| rJK1183 | gactctcattagagctcaacag | Confirm integration of ARG4 at 5′ end, reverse primer |
| fJK1200 | gacgttatggaaacttttgatt | Confirm integration of ARG4 at 3′ end, forward primer |
| rJK1199 | ggtagtctccgattatgattc | Confirm integration of ARG4 at 3′ end, reverse primer |
Acknowledgments
We thank Suzanne Noble, Gerald Fink, Joachim Morschhäuser, Joachim Ernst, William Fonzi, Joseph Heitman, Scott Filler, and Chung-Yu Lan for generous gifts of strains and plasmids. We thank members of the Dove, Watnick and Dvorin labs in the Division of Infectious Diseases, Boston Children's Hospital, for helpful discussions. We also thank Carol Kumamoto for fruitful discussions and helpful comments on the manuscript.
The Candida Genome Database is acknowledged for the sequence of ARG4 and TOR1 open reading frames. This work was supported by NIAID R21AI096054 and 1R01AI095305.
References
- Askew C, Sellam A, Epp E, Hogues H, Mullick A, Nantel A, Whiteway M. Transcriptional regulation of carbohydrate metabolism in the human pathogen Candida albicans. PLoS Pathog. 2009;5:e1000612. doi: 10.1371/journal.ppat.1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet NC, Schneider U, Helliwell SB, Stansfield I, Tuite MF, Hall MN. TOR controls translation initiation and early G1 progression in yeast. Mol Biol Cell. 1996;7:25–42. doi: 10.1091/mbc.7.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barelle CJ, Priest CL, Maccallum DM, Gow NA, Odds FC, Brown AJ. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell Microbiol. 2006;8:961–971. doi: 10.1111/j.1462-5822.2005.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastidas RJ, Heitman J, Cardenas ME. The protein kinase Tor1 regulates adhesin gene expression in Candida albicans. PLoS Pathog. 2009;5:e1000294. doi: 10.1371/journal.ppat.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck T, Hall MN. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature. 1999;402:689–692. doi: 10.1038/45287. [DOI] [PubMed] [Google Scholar]
- Behjousiar A, Kontoravdi C, Polizzi KM. In situ monitoring of intracellular glucose and glutamine in CHO cell culture. PLoS One. 2012;7:e34512. doi: 10.1371/journal.pone.0034512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berset C, Trachsel H, Altmann M. The TOR (target of rapamycin) signal transduction pathway regulates the stability of translation initiation factor eIF4G in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1998;95:4264–4269. doi: 10.1073/pnas.95.8.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha N, Chabrier-Rosello Y, Xu T, Johnson C, Sobczynski S, Song Q, et al. A large-scale complex haploinsufficiency-based genetic interaction screen in Candida albicans: analysis of the RAM network during morphogenesis. PLoS Genet. 2011;7:e1002058. doi: 10.1371/journal.pgen.1002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev. 2007;71:348–376. doi: 10.1128/MMBR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockmuhl DP, Krishnamurthy S, Gerads M, Sonneborn A, Ernst JF. Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol Microbiol. 2001;42:1243–1257. doi: 10.1046/j.1365-2958.2001.02688.x. [DOI] [PubMed] [Google Scholar]
- Boex-Fontvieille E, Daventure M, Jossier M, Zivy M, Hodges M, Tcherkez G. Photosynthetic control of Arabidopsis leaf cytoplasmic translation initiation by protein phosphorylation. PLoS One. 2013;8:e70692. doi: 10.1371/journal.pone.0070692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon RD, Chaffin WL. Oral colonization by Candida albicans. Crit Rev Oral Biol Med. 1999;10:359–383. doi: 10.1177/10454411990100030701. [DOI] [PubMed] [Google Scholar]
- Cao F, Lane S, Raniga PP, Lu Y, Zhou Z, Ramon K, et al. The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol Biol Cell. 2006;17:295–307. doi: 10.1091/mbc.E05-06-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Company, K.T.S.P. Protein-Ser/Thr Kinase Consensus Phosphorylation Site Specificity. Vol. 2015. Kinexus Bioinformatics Corporation; Canada: [Google Scholar]
- Cruz MC, Goldstein AL, Blankenship J, Del Poeta M, Perfect JR, McCusker JH, et al. Rapamycin and less immunosuppressive analogs are toxic to Candida albicans and Cryptococcus neoformans via FKBP12-dependent inhibition of TOR. Antimicrob Agents Chemother. 2001;45:3162–3170. doi: 10.1128/AAC.45.11.3162-3170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Davis-Hanna A, Piispanen AE, Stateva LI, Hogan DA. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol Microbiol. 2008;67:47–62. doi: 10.1111/j.1365-2958.2007.06013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechant R, Peter M. Nutrient signals driving cell growth. Curr Opin Cell Biol. 2008;20:678–687. doi: 10.1016/j.ceb.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Ene IV, Cheng SC, Netea MG, Brown AJ. Growth of Candida albicans cells on the physiologically relevant carbon source lactate affects their recognition and phagocytosis by immune cells. Infect Immun. 2013;81:238–248. doi: 10.1128/IAI.01092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ene IV, Adya AK, Wehmeier S, Brand AC, MacCallum DM, Gow NA, Brown AJ. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol. 2012;14:1319–1335. doi: 10.1111/j.1462-5822.2012.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning S, Xu W, Beaurepaire C, Suhan JP, Nantel A, Mitchell AP. Functional control of the Candida albicans cell wall by catalytic protein kinase A subunit Tpk1. Mol Microbiol. 2012;86:284–302. doi: 10.1111/j.1365-2958.2012.08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick DA, Logue ME, Stajich JE, Butler G. A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evol Biol. 2006;6:99. doi: 10.1186/1471-2148-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. Protein Identification and Analysis Tools on the ExPASy Server. In: Walker John M., editor. The Proteomics Protocols Handbook. Humana Press; 2005. [Google Scholar]
- Giacometti R, Kronberg F, Biondi RM, Passeron S. Catalytic isoforms Tpk1 and Tpk2 of Candida albicans PKA have non-redundant roles in stress response and glycogen storage. Yeast. 2009;26:273–285. doi: 10.1002/yea.1665. [DOI] [PubMed] [Google Scholar]
- Giacometti R, Kronberg F, Biondi RM, Passeron S. Candida albicans Tpk1p and Tpk2p isoforms differentially regulate pseudohyphal development, biofilm structure, cell aggregation and adhesins expression. Yeast. 2011;28:293–308. doi: 10.1002/yea.1839. [DOI] [PubMed] [Google Scholar]
- Gola S, Martin R, Walther A, Dunkler A, Wendland J. New modules for PCR-based gene targeting in Candida albicans: rapid and efficient gene targeting using 100 bp of flanking homology region. Yeast. 2003;20:1339–1347. doi: 10.1002/yea.1044. [DOI] [PubMed] [Google Scholar]
- Gressner AM, Wool IG. The phosphorylation of liver ribosomal proteins in vivo. Evidence that only a single small subunit protein (S6) is phosphorylated. J Biol Chem. 1974;249:6917–6925. [PubMed] [Google Scholar]
- Gressner AM, Wool IG. Effect of experimental diabetes and insulin on phosphorylation of rat liver ribosomal protein S6. Nature. 1976;259:148–150. doi: 10.1038/259148a0. [DOI] [PubMed] [Google Scholar]
- Hall RA, Cottier F, Muhlschlegel FA. Molecular networks in the fungal pathogen Candida albicans. Adv Appl Microbiol. 2009;67:191–212. doi: 10.1016/S0065-2164(08)01006-X. [DOI] [PubMed] [Google Scholar]
- Hall RA, Turner KJ, Chaloupka J, Cottier F, De Sordi L, Sanglard D, et al. The quorum-sensing molecules farnesol/homoserine lactone and dodecanol operate via distinct modes of action in Candida albicans. Eukaryot Cell. 2011;10:1034–1042. doi: 10.1128/EC.05060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Jaimovich A, Dey G, Ruggero D, Meyuhas O, Sonenberg N, Meyer T. Parallel measurement of dynamic changes in translation rates in single cells. Nat Methods. 2014;11:86–93. doi: 10.1038/nmeth.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick JS, Kuruvilla FG, Tong JK, Shamji AF, Schreiber SL. Rapamycin-modulated transcription defines the subset of nutrient-sensitive signaling pathways directly controlled by the Tor proteins. Proc Natl Acad Sci U S A. 1999;96:14866–14870. doi: 10.1073/pnas.96.26.14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Hogan DA, Sundstrom P. The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans. Future Microbiol. 2009;4:1263–1270. doi: 10.2217/fmb.09.106. [DOI] [PubMed] [Google Scholar]
- Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, et al. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol. 2001;67:2982–2992. doi: 10.1128/AEM.67.7.2982-2992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwenofu OH, Lackman RD, Staddon AP, Goodwin DG, Haupt HM, Brooks JS. Phospho-S6 ribosomal protein: a potential new predictive sarcoma marker for targeted mTOR therapy. Mod Pathol. 2008;21:231–237. doi: 10.1038/modpathol.3800995. [DOI] [PubMed] [Google Scholar]
- Jorgensen P, Tyers M. How cells coordinate growth and division. Curr Biol. 2004;14:R1014–1027. doi: 10.1016/j.cub.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Kim J, Sudbery P. Candida albicans, a major human fungal pathogen. J Microbiol. 2011;49:171–177. doi: 10.1007/s12275-011-1064-7. [DOI] [PubMed] [Google Scholar]
- Kinexus, The Systems Proteomics Company. Protein-Ser/Thr Kinase Consensus Phosphorylation Site Specificity. 2015 May 10; Retrieved from http://www.kinexus.ca/pdf/graphs_charts/ProteinSerKinaseSpecificity.pdf.
- Kingsbury JM, Sen ND, Maeda T, Heitman J, Cardenas ME. Endolysosomal membrane trafficking complexes drive nutrient-dependent TORC1 signaling to control cell growth in Saccharomyces cerevisiae. Genetics. 2014;196:1077–1089. doi: 10.1534/genetics.114.161646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler JR, Fink GR. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc Natl Acad Sci U S A. 1996;93:13223–13228. doi: 10.1073/pnas.93.23.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao WL, Ramon AM, Fonzi WA. GLN3 encodes a global regulator of nitrogen metabolism and virulence of C. albicans. Fungal Genet Biol. 2008;45:514–526. doi: 10.1016/j.fgb.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay AK, Morales DK, Liu Z, Grahl N, Zhang A, Willger SD, et al. Analysis of Candida albicans Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation. PLoS Genet. 2014;10:e1004567. doi: 10.1371/journal.pgen.1004567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HJ, Köhler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189:1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- Lorenz MC, Heitman J. Yeast pseudohyphal growth is regulated by GPA2, a G protein alpha homolog. EMBO J. 1997;16:7008–7018. doi: 10.1093/emboj/16.23.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz MC, Fink GR. The glyoxylate cycle is required for fungal virulence. Nature. 2001;412:83–86. doi: 10.1038/35083594. [DOI] [PubMed] [Google Scholar]
- Lorenz MC, Bender JA, Fink GR. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell. 2004;3:1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Su C, Wang A, Liu H. Hyphal development in Candida albicans requires two temporally linked changes in promoter chromatin for initiation and maintenance. PLoS Biol. 2011;9:e1001105. doi: 10.1371/journal.pbio.1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C, Arechiga AF, Melo JV, Walsh CM, Ong ST. Bcr-Abl kinase modulates the translation regulators ribosomal protein S6 and 4E-BP1 in chronic myelogenous leukemia cells via the mammalian target of rapamycin. Cancer Res. 2003;63:5716–5722. [PubMed] [Google Scholar]
- Maidan MM, De Rop L, Serneels J, Exler S, Rupp S, Tournu H, et al. The G protein-coupled receptor Gpr1 and the Galpha protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans. Mol Biol Cell. 2005;16:1971–1986. doi: 10.1091/mbc.E04-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metspalu A, Saarma M, Villems R, Ustav M, Lind A. Interaction of 5-S RNA, 5.8-S RNA and tRNA with rat-liver ribosomal proteins. Eur J Biochem. 1978;91:73–81. doi: 10.1111/j.1432-1033.1978.tb20938.x. [DOI] [PubMed] [Google Scholar]
- Meyuhas O. Physiological roles of ribosomal protein S6: one of its kind. Int Rev Cell Mol Biol. 2008;268:1–37. doi: 10.1016/S1937-6448(08)00801-0. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Sato T, Tamanoi F. Fission yeast TORC1 regulates phosphorylation of ribosomal S6 proteins in response to nutrients and its activity is inhibited by rapamycin. J Cell Sci. 2010;123:777–786. doi: 10.1242/jcs.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SM, Johnson AD. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot Cell. 2005;4:298–309. doi: 10.1128/EC.4.2.298-309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard O, Nilsson L. Kinetic determination of the effects of ADP-ribosylation on the interaction of eukaryotic elongation factor 2 with ribosomes. J Biol Chem. 1990;265:6030–6034. [PubMed] [Google Scholar]
- Odds FC. Ecology and epidemiology of Candida species. Zentralbl Bakteriol Mikrobiol Hyg A. 1984;257:207–212. [PubMed] [Google Scholar]
- Odds FC. Activity of cilofungin (LY121019) against Candida species in vitro. J Antimicrob Chemother. 1988;22:891–897. doi: 10.1093/jac/22.6.891. [DOI] [PubMed] [Google Scholar]
- Papageorgiou A, Avruch J. A genome-wide RNAi screen for polypeptides that alter rpS6 phosphorylation. Methods Mol Biol. 2012;821:187–214. doi: 10.1007/978-1-61779-430-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Myers CL, Sheppard DC, Phan QT, Sanchez AA, J EE, Filler SG. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell Microbiol. 2005;7:499–510. doi: 10.1111/j.1462-5822.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- Park H, Liu Y, Solis N, Spotkov J, Hamaker J, Blankenship JR, et al. Transcriptional responses of candida albicans to epithelial and endothelial cells. Eukaryot Cell. 2009;8:1498–1510. doi: 10.1128/EC.00165-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YN, Morschhauser J. Tetracycline-inducible gene expression and gene deletion in Candida albicans. Eukaryot Cell. 2005;4:1328–1342. doi: 10.1128/EC.4.8.1328-1342.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedruzzi I, Dubouloz F, Cameroni E, Wanke V, Roosen J, Winderickx J, De Virgilio C. TOR and PKA signaling pathways converge on the protein kinase Rim15 to control entry into G0. Mol Cell. 2003;12:1607–1613. doi: 10.1016/s1097-2765(03)00485-4. [DOI] [PubMed] [Google Scholar]
- Piispanen AE, Grahl N, Hollomon JM, Hogan DA. Regulated proteolysis of Candida albicans Ras1 is involved in morphogenesis and quorum sensing regulation. Mol Microbiol. 2013;89:166–178. doi: 10.1111/mmi.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers T. TOR signaling and S6 kinase 1: Yeast catches up. Cell Metab. 2007;6:1–2. doi: 10.1016/j.cmet.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Rabl J, Leibundgut M, Ataide SF, Haag A, Ban N. Crystal structure of the eukaryotic 40S ribosomal subunit in complex with initiation factor 1. Science. 2011;331:730–736. doi: 10.1126/science.1198308. [DOI] [PubMed] [Google Scholar]
- Ramachandran V, Herman PK. Antagonistic interactions between the cAMP-dependent protein kinase and Tor signaling pathways modulate cell growth in Saccharomyces cerevisiae. Genetics. 2011;187:441–454. doi: 10.1534/genetics.110.123372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002;68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez MA, Lorenz MC. Mutations in alternative carbon utilization pathways in Candida albicans attenuate virulence and confer pleiotropic phenotypes. Eukaryot Cell. 2007;6:280–290. doi: 10.1128/EC.00372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust HL, Thompson PR. Kinase consensus sequences: a breeding ground for crosstalk. ACS Chem Biol. 2011;6:881–892. doi: 10.1021/cb200171d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, et al. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19:2199–2211. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabina J, Brown V. Glucose sensing network in Candida albicans: a sweet spot for fungal morphogenesis. Eukaryot Cell. 2009;8:1314–1320. doi: 10.1128/EC.00138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam A, Hartley A, Duvel K, Broach JR, Garrett S. PP2A phosphatase activity is required for stress and Tor kinase regulation of yeast stress response factor Msn2p. Eukaryot Cell. 2004;3:1261–1271. doi: 10.1128/EC.3.5.1261-1271.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaekel A, Desai PR, Ernst JF. Morphogenesis-regulated localization of protein kinase A to genomic sites in Candida albicans. BMC Genomics. 2013;14:842. doi: 10.1186/1471-2164-14-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzle T, Beck T, Martin DE, Hall MN. Activation of the RAS/cyclic AMP pathway suppresses a TOR deficiency in yeast. Mol Cell Biol. 2004;24:338–351. doi: 10.1128/MCB.24.1.338-351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Cowen LE, Griffin AM, Chan L, Köhler JR. The Candida albicans pescadillo homolog is required for normal hypha-to-yeast morphogenesis and yeast proliferation. Proc Natl Acad Sci U S A. 2008;105:20918–20923. doi: 10.1073/pnas.0809147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto G, Giacometti R, Silberstein S, Giasson L, Cantore ML, Passeron S. Expression of TPK1 and TPK2 genes encoding PKA catalytic subunits during growth and morphogenesis in Candida albicans. Yeast. 2006;23:591–603. doi: 10.1002/yea.1377. [DOI] [PubMed] [Google Scholar]
- Stephan JS, Yeh YY, Ramachandran V, Deminoff SJ, Herman PK. The Tor and PKA signaling pathways independently target the Atg1/Atg13 protein kinase complex to control autophagy. Proc Natl Acad Sci U S A. 2009;106:17049–17054. doi: 10.1073/pnas.0903316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stichternoth C, Fraund A, Setiadi E, Giasson L, Vecchiarelli A, Ernst JF. Sch9 kinase integrates hypoxia and CO2 sensing to suppress hyphal morphogenesis in Candida albicans. Eukaryot Cell. 2011;10:502–511. doi: 10.1128/EC.00289-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoldt VR, Sonneborn A, Leuker CE, Ernst JF. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C, Lu Y, Liu H. Reduced TOR signaling sustains hyphal development in Candida albicans by lowering Hog1 basal activity. Mol Biol Cell. 2013;24:385–397. doi: 10.1091/mbc.E12-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Ogata K. Ribosomal proteins cross-linked to natural mRNA by UV irradiation of rat liver polysomes. J Biochem. 1981;90:1549–1552. doi: 10.1093/oxfordjournals.jbchem.a133624. [DOI] [PubMed] [Google Scholar]
- Tomas-Cobos L, Viana R, Sanz P. TOR kinase pathway and 14-3-3 proteins regulate glucose-induced expression of HXT1, a yeast low-affinity glucose transporter. Yeast. 2005;22:471–479. doi: 10.1002/yea.1224. [DOI] [PubMed] [Google Scholar]
- Towle HC. Glucose as a regulator of eukaryotic gene transcription. Trends Endocrinol Metab. 2005;16:489–494. doi: 10.1016/j.tem.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Tsao CC, Chen YT, Lan CY. A small G protein Rhb1 and a GTPase-activating protein Tsc2 involved in nitrogen starvation-induced morphogenesis and cell wall integrity of Candida albicans. Fungal Genet Biol. 2009;46:126–136. doi: 10.1016/j.fgb.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Uhl MA, Biery M, Craig N, Johnson AD. Haploinsufficiency-based large-scale forward genetic analysis of filamentous growth in the diploid human fungal pathogen C.albicans. EMBO J. 2003;22:2668–2678. doi: 10.1093/emboj/cdg256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J, Soulard A, Huber A, Lippman S, Mukhopadhyay D, Deloche O, et al. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol Cell. 2007;26:663–674. doi: 10.1016/j.molcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Wei Y, Zheng XF. Nutritional control of cell growth via TOR signaling in budding yeast. Methods Mol Biol. 2011;759:307–319. doi: 10.1007/978-1-61779-173-4_18. [DOI] [PubMed] [Google Scholar]
- Wilson D, Thewes S, Zakikhany K, Fradin C, Albrecht A, Almeida R, et al. Identifying infection-associated genes of Candida albicans in the postgenomic era. FEMS Yeast Res. 2009;9:688–700. doi: 10.1111/j.1567-1364.2009.00524.x. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- Xiong Y, McCormack M, Li L, Hall Q, Xiang C, Sheen J. Glucose-TOR signalling reprograms the transcriptome and activates meristems. Nature. 2013;496:181–186. doi: 10.1038/nature12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T, Zaman S, Broach JR, Klionsky DJ. Protein kinase A and Sch9 cooperatively regulate induction of autophagy in Saccharomyces cerevisiae. Mol Biol Cell. 2007;18:4180–4189. doi: 10.1091/mbc.E07-05-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchi LF, Gomez-Raja J, Davis DA. Mds3 regulates morphogenesis in Candida albicans through the TOR pathway. Mol Cell Biol. 2010;30:3695–3710. doi: 10.1128/MCB.01540-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakikhany K, Naglik JR, Schmidt-Westhausen A, Holland G, Schaller M, Hube B. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell Microbiol. 2007;9:2938–2954. doi: 10.1111/j.1462-5822.2007.01009.x. [DOI] [PubMed] [Google Scholar]
- Zaman S, Lippman SI, Zhao X, Broach JR. How Saccharomyces responds to nutrients. Annu Rev Genet. 2008;42:27–81. doi: 10.1146/annurev.genet.41.110306.130206. [DOI] [PubMed] [Google Scholar]
- Zhang M, Fu W, Prabhu S, Moore JC, Ko J, Kim JW, et al. Inhibition of polysome assembly enhances imatinib activity against chronic myelogenous leukemia and overcomes imatinib resistance. Mol Cell Biol. 2008;28:6496–6509. doi: 10.1128/MCB.00477-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Mihindukulasuriya KA, Gao H, La Rosa PS, Wylie KM, Martin JC, et al. Exploration of bacterial community classes in major human habitats. Genome Biol. 2014;15:R66. doi: 10.1186/gb-2014-15-5-r66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurita-Martinez SA, Cardenas ME. Tor and cyclic AMP-protein kinase A: two parallel pathways regulating expression of genes required for cell growth. Eukaryot Cell. 2005;4:63–71. doi: 10.1128/EC.4.1.63-71.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The amino acid sequence of ribosomal protein Rps6 (S6) is highly conserved in fungi and animals. The conserved serine residues at the C-terminal region are boxed in red. One of these serine residues is replaced by a glutamate in C. albicans S6 and in its close relatives in the non-meiotic subclade within the CTG clade of Saccharomycotina.
Fig. S2. P-S6 responds to a high level of the preferred carbon source, glucose. Exponentially-growing wild-type SC5314 cells were transferred to YNB (regular) containing different carbon sources as indicated: glucose (“Gluc”) (0.1 or 2%), galactose (“Gal”) (2%), maltose (“Mal”) (2%), or mannitol (“Man”) (1%); a culture was also set up in medium without a carbon source. All cultures were grown for 1 hour at 30°C and cell lysates were probed on separate blots for P-S6 (top) and total S6 (bottom); tubulin was the loading control on both blots. The “0 time-point” indicates lysate from cells prior to transfer into media containing the different carbon sources.
Fig. S3. Exponentially-growing wild-type JKC317 and isogenic strains with single deletions in components of the PKA pathway were transferred to YNB (regular) containing different glucose concentrations and grown for 1 hour at 30°C. Cell lysates were analyzed by Western blotting and probed for P-S6 and tubulin (loading control). The P-S6 and tubulin signals were quantitated by densitometry using the ImageJ analysis software; the y-axis depicts the P-S6 signal normalized to the tubulin signal at each indicated glucose concentration (depicted on the x-axis). Mutant strains tested contained deletions in either of the two catalytic subunits of PKA, TPK1 (tpk1-/-) (strain HPY300U) (A) or TPK2 (tpk2-/-) (strain HPY400U) (B); or deletions in the downstream PKA-regulated transcription factors EFG1 (efg1-/-) (strain DSC10) (C) or FLO8 deletion (flo8-/-) (strain JKC1467) (D). WT is shown in orange and the mutants are shown in purple. The actual blots are shown in Figs. 5B-E.
Fig. S4. P-S6 does not appear to respond to drugs that disrupt cell wall integrity. Exponentially-growing wild-type SC5314 cells were exposed to indicated drugs rapamycin (20 ngmL-1), micafungin (“M”) (20 ngmL-1), calcofluor white (“CW”) (200 μgmL-1), and congo red (“CR”) (100 μgmL-1) (“+” samples) in YPD for 2 hours at 30°C in the dark. The control samples (“-”) were treated with the vehicles (90% ethanol for rapamycin, saline for micafungin, water/sodium hydroxide for calcofluor white, and water for congo red) instead of the drugs and grown under the same conditions. Cell lysates were probed for P-S6, phosphorylated Mkc1, and histone H3 with anti-phosphorylated-Akt-substrate antibody, anti-phospho-p44/42 MAPK (Erk1/2) (Thr 202/Tyr204) antibody, and anti-H3 antibody, respectively.
Table S1. Mass Spectrometry Identified Proteins (around 35 kDa) Immunoprecipitated using anti-Phospho(S/T) Akt Substrate Antibody.